In Vitro and Ex Vivo Evaluation of Nepafenac-Based Cyclodextrin Microparticles for Treatment of Eye Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nepafenac Eye Drop Preparation
2.3. Physicochemical Characterization
2.3.1. Particle Size Analysis
2.3.2. Zeta Potential and pH
2.3.3. Rheological Analysis
2.3.4. In Vitro Mucoadhesive Studies
2.4. Ocular Tolerance Test (HET-CAM assay)
2.5. In Vitro Cell Viability
2.6. Diffusion Assays
2.7. Ex Vivo Corneal and Scleral Permeability
2.8. Human Monocytes
2.8.1. Differentiation into Macrophages
2.8.2. Anti-inflammatory Activity
2.9. Statistical Analysis
3. Results and Discussion
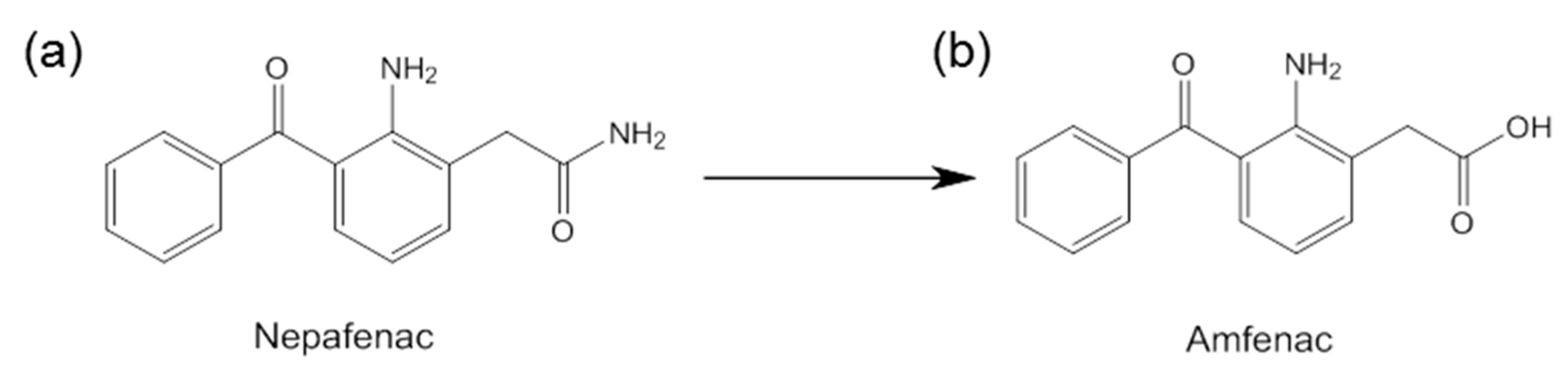
3.1. Solubility of Nepafenac Eye Drops and Their Characterization
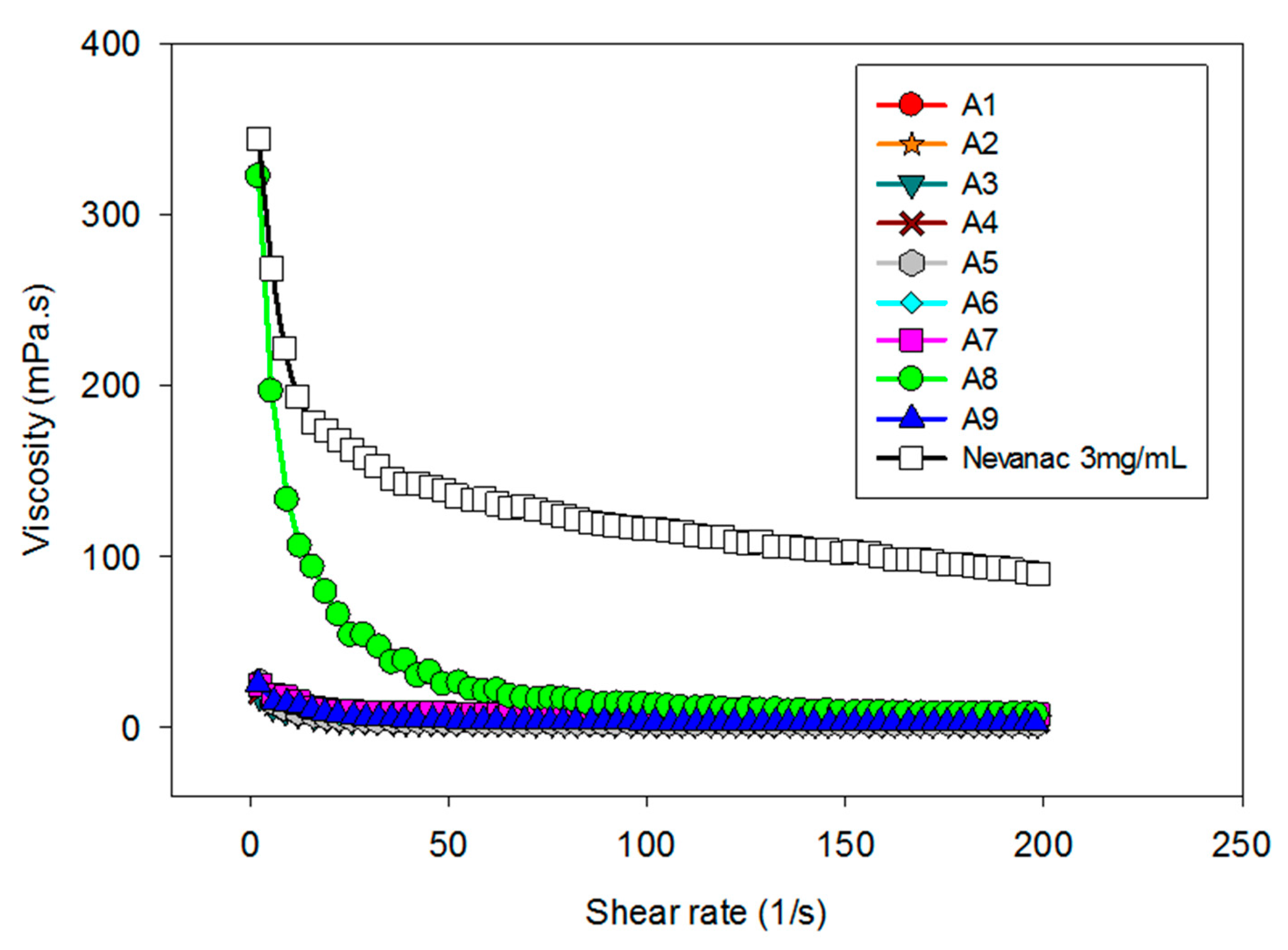
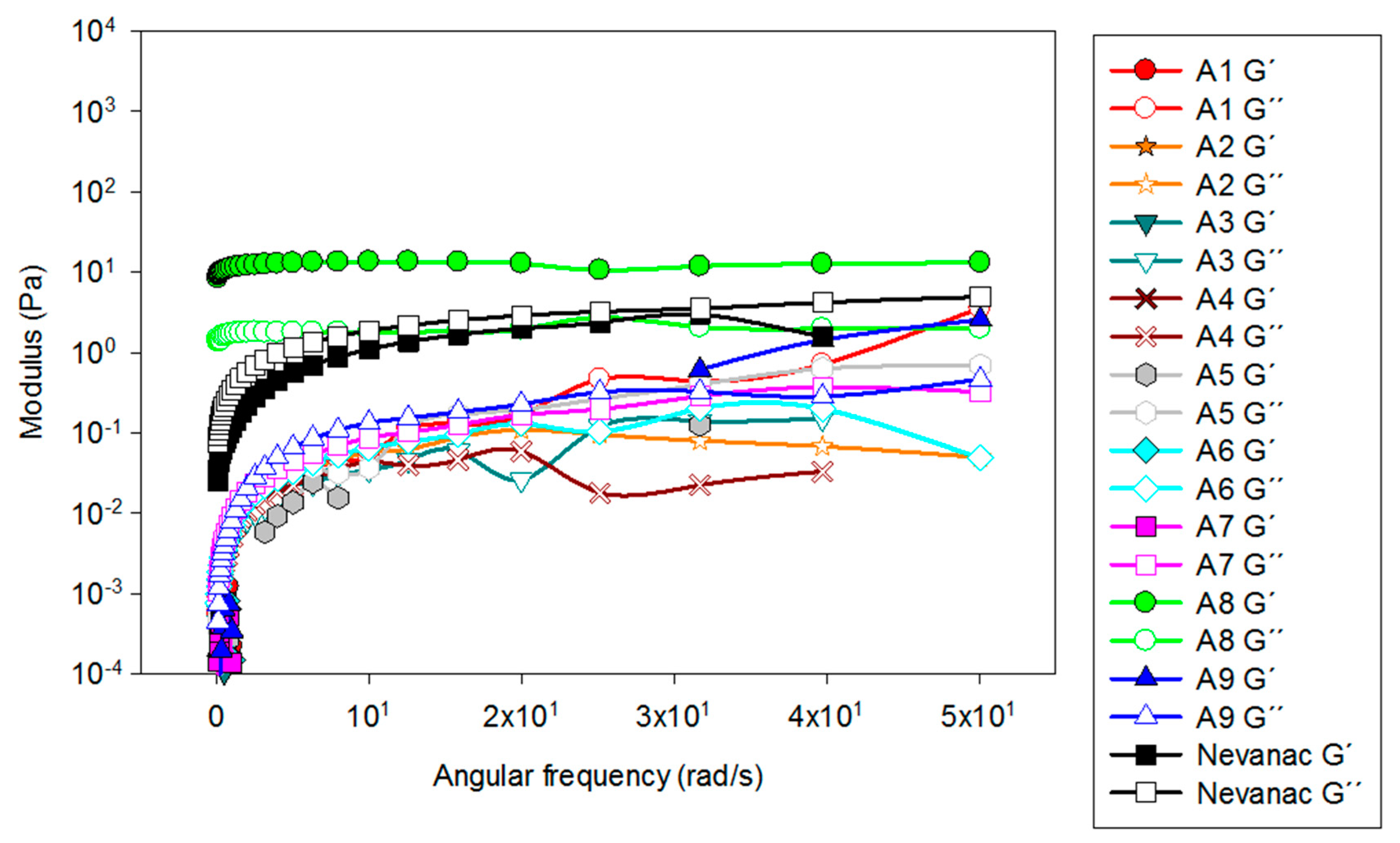
3.2. Rheological Characterization
3.3. Mucoadhesion Studies
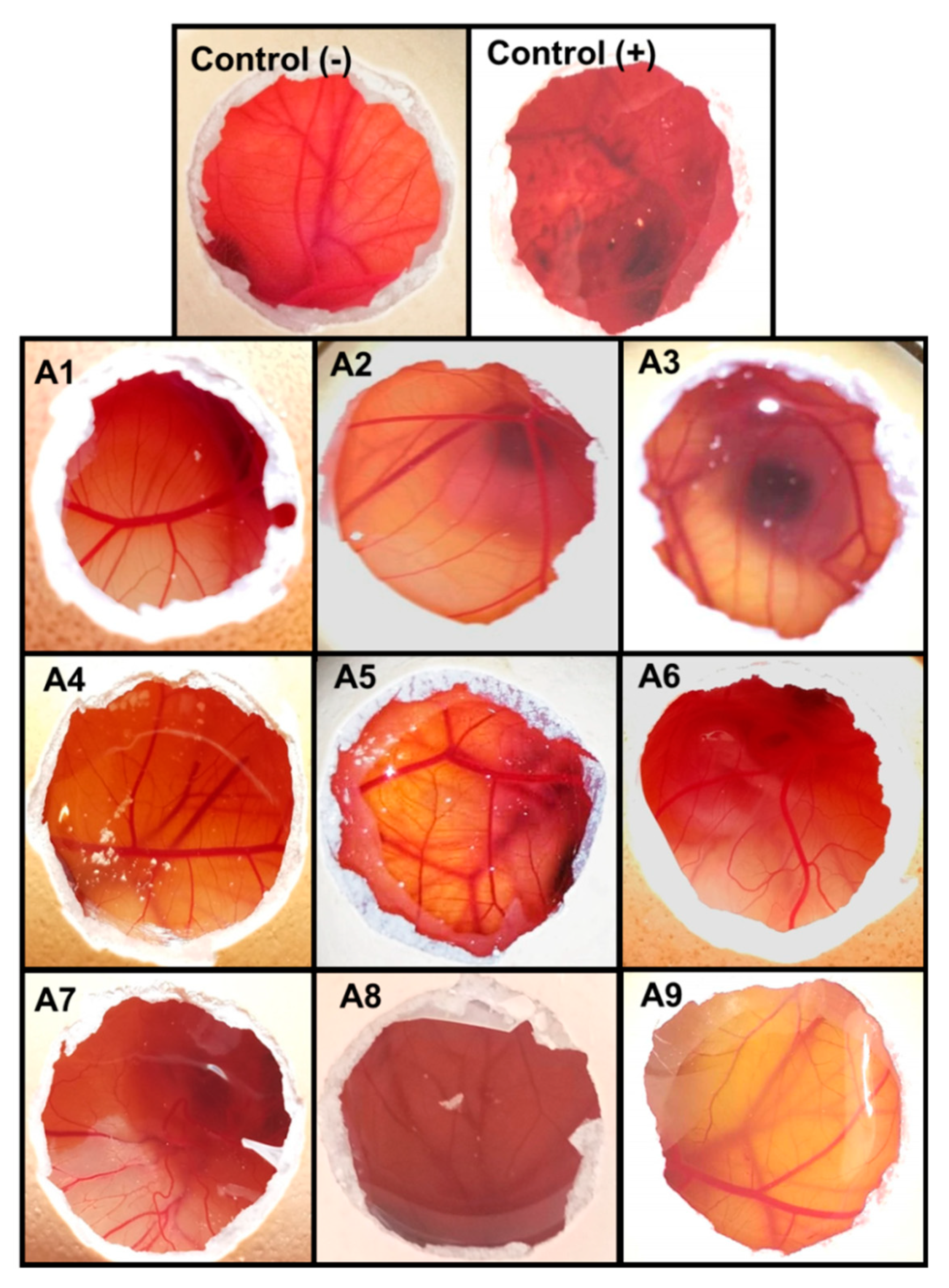
3.4. Ocular Irritancy Test (HET-CAM)
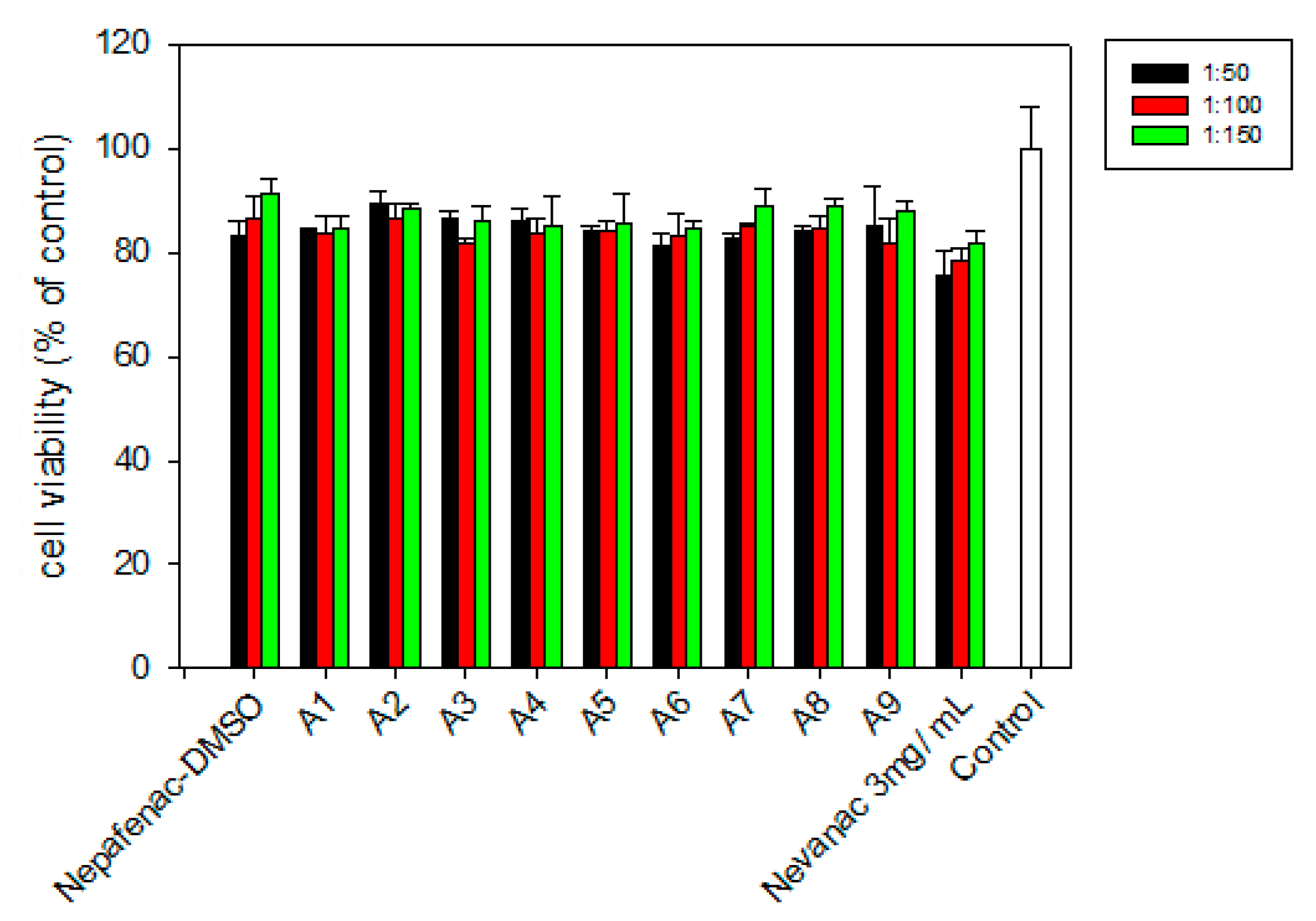
3.5. Cell Viability
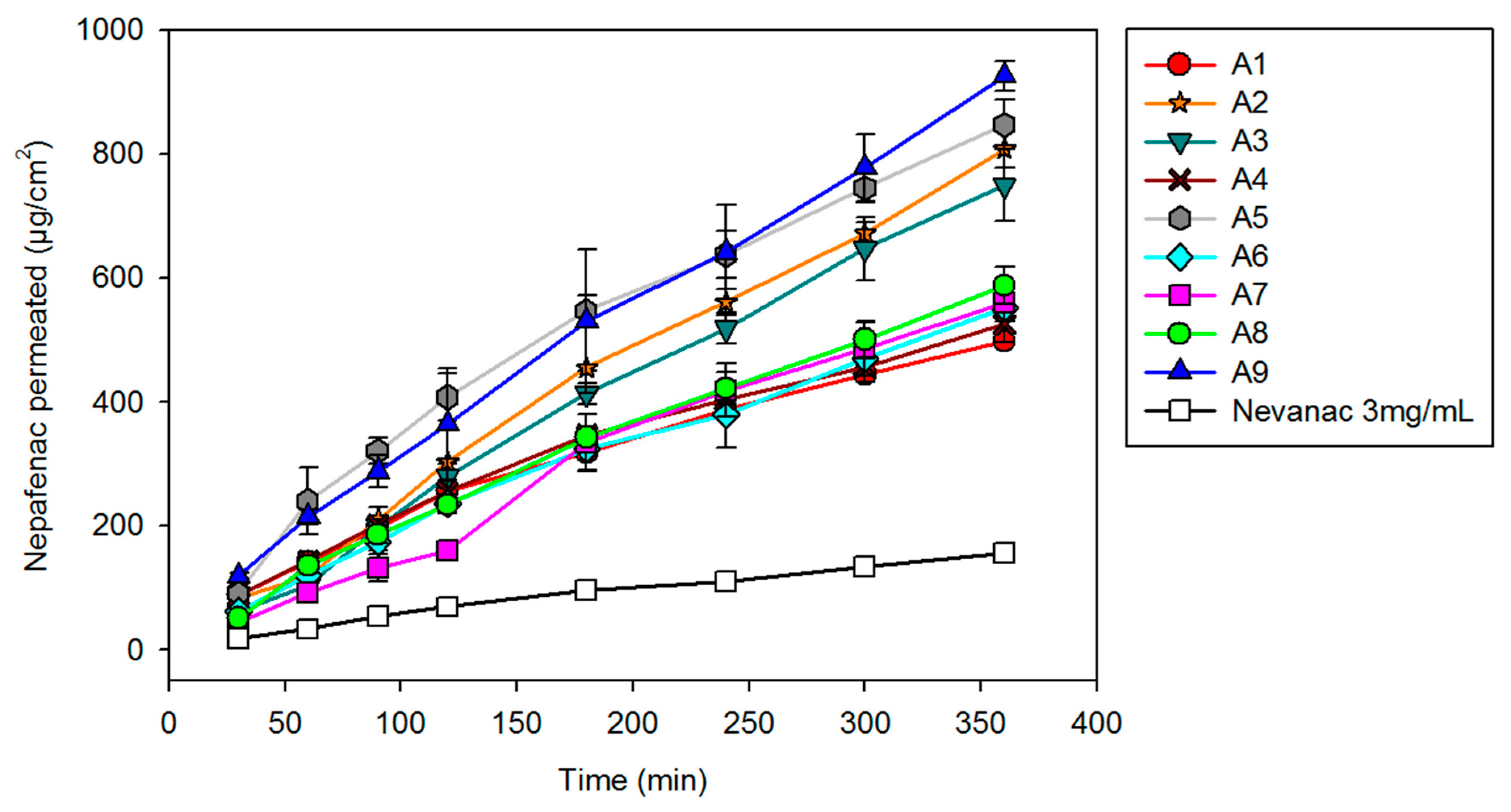
3.6. In Vitro Diffusion Studies
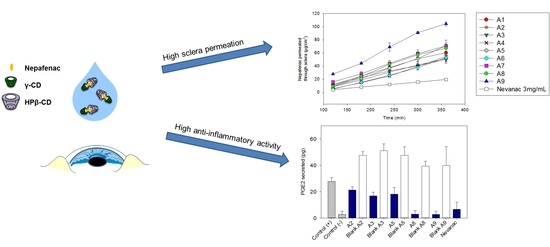
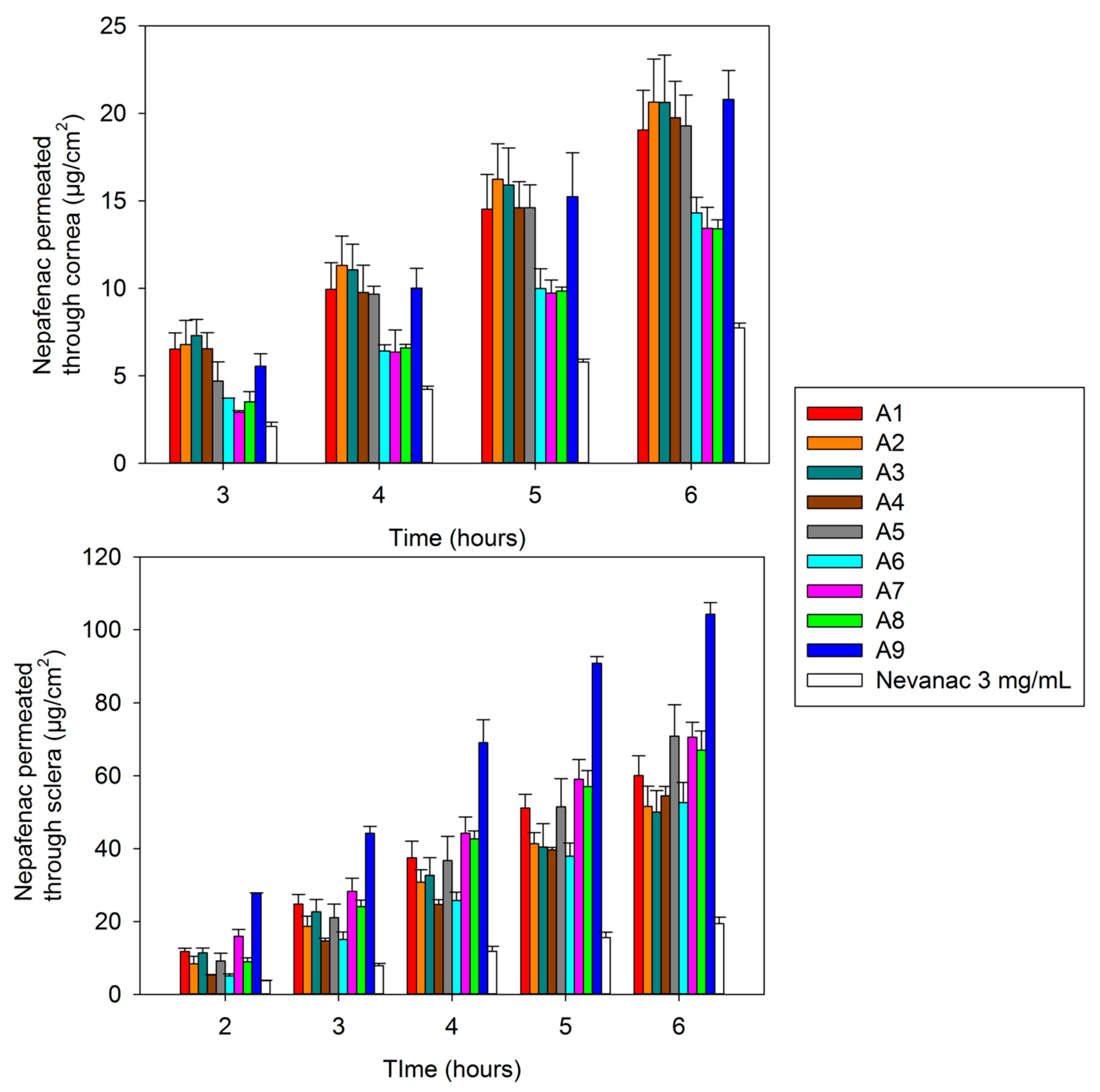
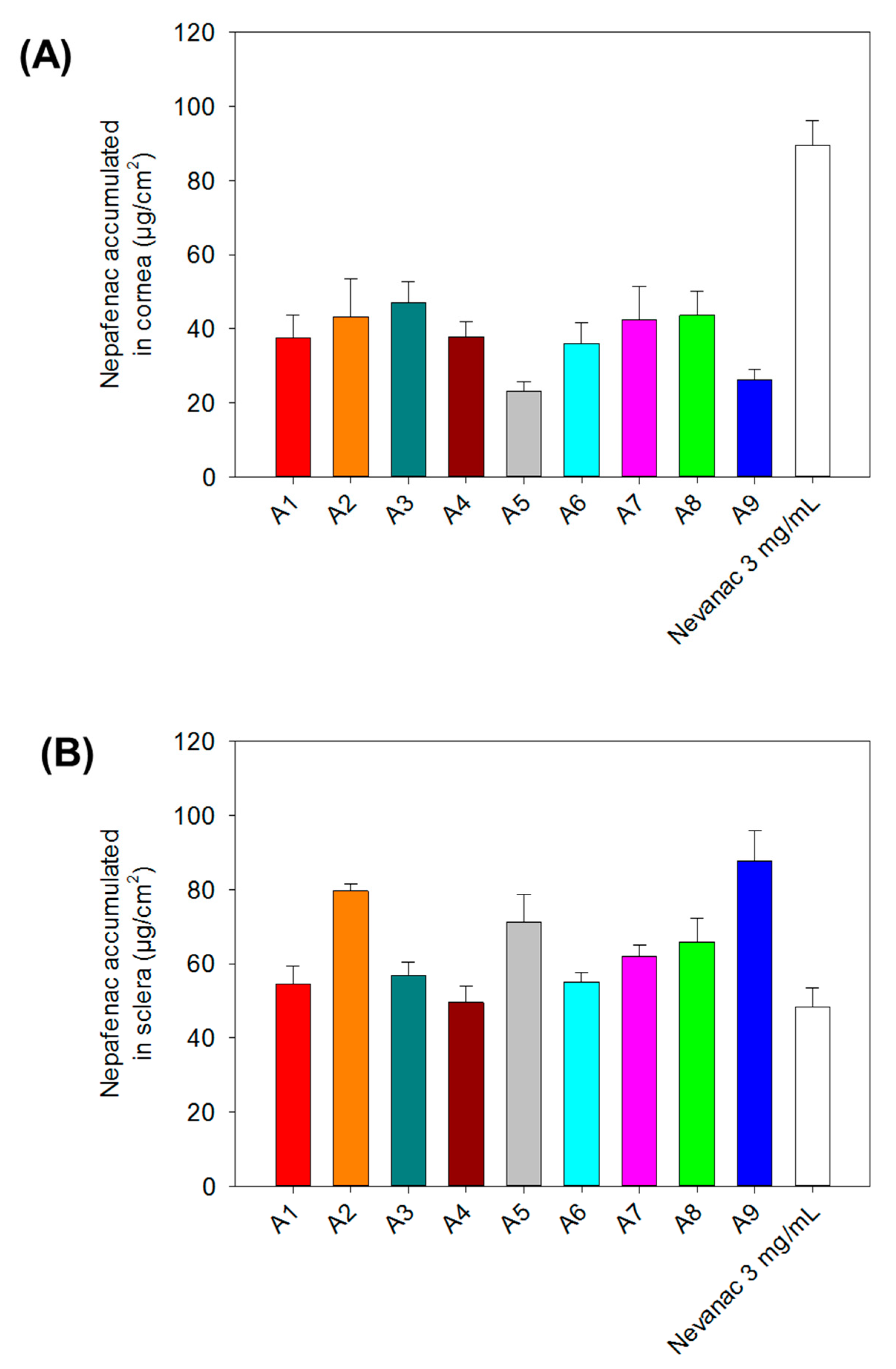
3.7. Ex Vivo Corneal and Scleral Permeability Studies
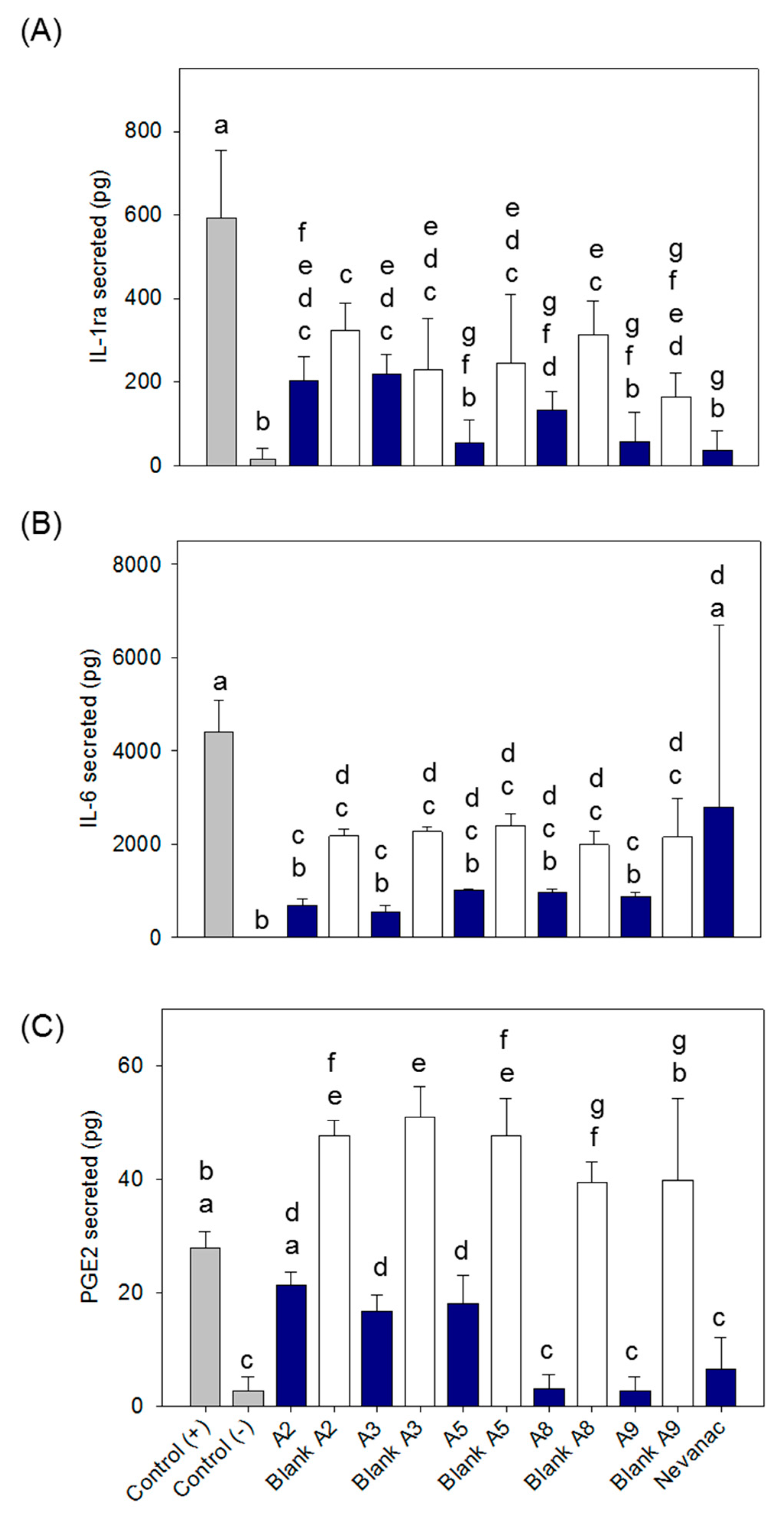
3.8. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Callegan, M.; Gregory-Ksander, M.; Willcox, M.; Lightman, S. Ocular inflammation and infection. Int. J. Inflamm. 2012, 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Red Eye. Available online: https://www.nhs.uk/conditions/red-eye/ (accessed on 28 August 2019).
- Rodrigues, E.B.; Farah, M.E.; Bottos, J.M.; Bom Aggio, F. Nonsteroidal anti-inflammatory drugs in the treatment of retinal diseases. Dev. Ophthalmol. 2016, 55, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.S.; Braga-Mele, R.; Donaldson, K.; Emerick, G.; Henderson, B.; Kahook, M.; Mamalis, N.; Miller, K.M.; Realini, T.; Shorstein, N.H.; et al. Cataract surgery and nonsteroidal antiinflammatory drugs. J. Cataract Refract. Surg. 2016, 42, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Egwuagu, C.E.; Sun, L.; Kim, S.H.; Dambuza, I.M. Ocular inflammatory diseases: Molecular pathogenesis and immunotherapy. Curr. Mol. Med. 2015, 15, 517–528. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Kessel, L.; Tendal, B.; Jorgensen, K.J.; Erngaard, D.; Flesner, P.; Andresen, J.L.; Hjortdal, J. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: A systematic review. Ophthalmology 2014, 121, 1915–1924. [Google Scholar] [CrossRef]
- Miller, K.; Fortun, J.A. Diabetic macular edema: Current understanding, pharmacologic treatment options, and developing therapies. Asia Pac. J. Ophthalmol. 2018, 7, 28–35. [Google Scholar] [CrossRef]
- Comstock, T.L.; Decory, H.H. Advances in corticosteroid therapy for ocular inflammation: Loteprednol etabonate. Int. J. Inflamm. 2012, 2012, 789623. [Google Scholar] [CrossRef]
- Rao, R.; Kumar, R.; Sarwal, A.; Sinha, V.R. Ocular Inflammation and NSAIDs: An Overview with Selective and Non-Selective cox Inhibitors. 2016. Available online: https://pdfs.semanticscholar.org/97ac/e3cb72c3af14ac8c72d0e397cb930b9f0bac.pdf (accessed on 28 August 2019).
- Gaynes, B.I.; Onyekwuluje, A. Topical ophthalmic NSAIDs: A discussion with focus on nepafenac ophthalmic suspension. Clin. Ophthalmol. 2008, 2, 355–368. [Google Scholar] [CrossRef]
- Guo, S.; Patel, S.; Baumrind, B.; Johnson, K.; Levinsohn, D.; Marcus, E.; Tannen, B.; Roy, M.; Bhagat, N.; Zarbin, M. Management of pseudophakic cystoid macular edema. Surv. Ophthalmol. 2015, 60, 123–137. [Google Scholar] [CrossRef] [PubMed]
- EMA. Nepafenac. Available online: https://www.ema.europa.eu/en/documents/overview/nevanac-epar-summary-public_en.pdf (accessed on 3 June 2019).
- Paulsamy, M.; Ponnusamy, C.; Palanisami, M.; Nackeeran, G.; Paramasivam, S.; Sugumaran, A.; Kandasamy, R.; Natesan, S.; Palanichamy, R. Nepafenac loaded silica nanoparticles dispersed in-situ gel systems: Development and characterization. Int. J. Biol. Macromol. 2018, 110, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Ram, J.; Bansal, R.; Pandav, S.S.; Gupta, A. Effect of topical ketorolac 0.4%, nepafenac 0.1%, and bromfenac 0.09% on postoperative inflammation using laser flare photometry in patients having phacoemulsification. J. Cataract Refract. Surg. 2015, 41, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.S.; Lehmann, R.P.; Walters, T.R.; Fong, R.; Christie, W.C.; Roel, L.; Nethery, D.; Sager, D.; Tsorbatzoglou, A.; Philipson, B.; et al. Once-daily nepafenac ophthalmic suspension 0.3% to prevent and treat ocular inflammation and pain after cataract surgery: Phase 3 study. J. Cataract Refract. Surg. 2014, 40, 203–211. [Google Scholar] [CrossRef]
- Chastain, J.E.; Sanders, M.E.; Curtis, M.A.; Chemuturi, N.V.; Gadd, M.E.; Kapin, M.A.; Markwardt, K.L.; Dahlin, D.C. Distribution of topical ocular nepafenac and its active metabolite amfenac to the posterior segment of the eye. Exp. Eye Res. 2016, 145, 58–67. [Google Scholar] [CrossRef]
- Kawahara, A.; Utsunomiya, T.; Kato, Y.; Takayanagi, Y. Comparison of effect of nepafenac and diclofenac ophthalmic solutions on cornea, tear film, and ocular surface after cataract surgery: The results of a randomized trial. Clin. Ophthalmol. 2016, 10, 385–391. [Google Scholar] [CrossRef][Green Version]
- Kleiter, M.; Malarkey, D.E.; Ruslander, D.E.; Thrall, D.E. Expression of cyclooxygenase-2 in canine epithelial nasal tumors. Vet. Radiol. Ultrasound. Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2004, 45, 255–260. [Google Scholar] [CrossRef]
- Kirkby, N.S.; Chan, M.V.; Zaiss, A.K.; Garcia-Vaz, E.; Jiao, J.; Berglund, L.M.; Verdu, E.F.; Ahmetaj-Shala, B.; Wallace, J.L.; Herschman, H.R.; et al. Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-κB and NFAT transcriptional pathways. Proc. Natl. Acad. Sci. USA 2016, 113, 434–439. [Google Scholar] [CrossRef]
- Ozcimen, M.; Sakarya, Y.; Goktas, S.; Sakarya, R.; Yener, H.I.; Bukus, A.; Demir, L.S. Effect of nepafenac eye drops on pain associated with pterygium surgery. Eye Contact Lens 2015, 41, 187–189. [Google Scholar] [CrossRef]
- Kim, S.J. Novel approaches for retinal drug and gene delivery. Transl. Vis. Sci. Technol. 2014, 3, 7. [Google Scholar] [CrossRef][Green Version]
- Shah, S.S.; Denham, L.V.; Elison, J.R.; Bhattacharjee, P.S.; Clement, C.; Huq, T.; Hill, J.M. Drug delivery to the posterior segment of the eye for pharmacologic therapy. Expert Rev. Ophthalmol. 2010, 5, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Franco, Y.L.; Zhou, Y.; Chen, J. Nanotechnology in retinal drug delivery. Int. J. Ophthalmol. 2018, 11, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Yi, J.; Lv, S.; Zhang, B. Ocular amphotericin B delivery by chitosan-modified nanostructured lipid carriers for fungal keratitis-targeted therapy. J. Liposome Res. 2017, 27, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Trabado, J.; Diebold, Y.; Sanchez, A. Designing lipid nanoparticles for topical ocular drug delivery. Int. J. Pharm. 2017, 532, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A. Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016, 89, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Karasawa, K.; Onodera, R.; Takeuchi, H. Feasibility of drug delivery to the eye’s posterior segment by topical instillation of PLGA nanoparticles. Asian J. Pharm. Sci. 2017, 12, 394–399. [Google Scholar] [CrossRef]
- Balguri, S.P.; Adelli, G.R.; Majumdar, S. Topical ophthalmic lipid nanoparticle formulations (SLN, NLC) of indomethacin for delivery to the posterior segment ocular tissues. Eur. J. Pharm. Biopharm. 2016, 109, 224–235. [Google Scholar] [CrossRef]
- Subrizi, A.; Del Amo, E.M.; Korzhikov-Vlakh, V.; Tennikova, T.; Ruponen, M.; Urtti, A. Design principles of ocular drug delivery systems: Importance of drug payload, release rate, and material properties. Drug Discov. Today 2019, 24, 1446–1457. [Google Scholar] [CrossRef]
- Srinivasarao, D.A.; Lohiya, G.; Katti, D.S. Fundamentals, challenges, and nanomedicine-based solutions for ocular diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1548. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef]
- Loftsson, T.; Stefansson, E. Cyclodextrins and topical drug delivery to the anterior and posterior segments of the eye. Int. J. Pharm. 2017, 531, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Johannsdottir, S.; Jansook, P.; Stefansson, E.; Loftsson, T. Development of a cyclodextrin-based aqueous cyclosporin a eye drop formulations. Int. J. Pharm. 2015, 493, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Johannsdottir, S.; Kristinsson, J.K.; Fulop, Z.; Asgrimsdottir, G.; Stefansson, E.; Loftsson, T. Formulations and toxicologic in vivo studies of aqueous cyclosporin a eye drops with cyclodextrin nanoparticles. Int. J. Pharm. 2017, 529, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Cyclolab. Available online: https://cyclolab.hu/userfiles/CD%20NEWS2018January_.pdf (accessed on 11 June 2019).
- Tsai, C.H.; Wang, P.Y.; Lin, I.C.; Huang, H.; Liu, G.S.; Tseng, C.L. Ocular drug delivery: Role of degradable polymeric nanocarriers for ophthalmic application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Khare, A.; Grove, K.; Pawar, P.; Singh, I. Mucoadhesive polymers for enhancing retention in ocular drug delivery. In Progress in Adhesion and Adhesives; Wiley-Scrivener: Beverly, MA, USA, 2015; Volume 13, pp. 451–484. [Google Scholar] [CrossRef]
- Lorenzo-Veiga, B.; Sigurdsson, H.H.; Loftsson, T. Nepafenac-loaded cyclodextrin/polymer nanoaggregates: A new approach to eye drop formulation. Materials 2019, 12, 229. [Google Scholar] [CrossRef]
- Akhter, S.; Anwar, M.; Siddiqui, M.A.; Ahmad, I.; Ahmad, J.; Ahmad, M.Z.; Bhatnagar, A.; Ahmad, F.J. Improving the topical ocular pharmacokinetics of an immunosuppressant agent with mucoadhesive nanoemulsions: Formulation development, in-vitro and in-vivo studies. Colloids Surf. B Biointerfaces 2016, 148, 19–29. [Google Scholar] [CrossRef]
- Campana-Seoane, M.; Peleteiro, A.; Laguna, R.; Otero-Espinar, F.J. Bioadhesive emulsions for control release of progesterone resistant to vaginal fluids clearance. Int. J. Pharm. 2014, 477, 495–505. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Fernandez-Villanueva, D.; Concheiro, A.; Alvarez-Lorenzo, C. Alpha-lipoic acid in soluplus((r)) polymeric nanomicelles for ocular treatment of diabetes-associated corneal diseases. J. Pharm. Sci. 2016, 105, 2855–2863. [Google Scholar] [CrossRef]
- Fernandez-Ferreiro, A.; Santiago-Varela, M.; Gil-Martinez, M.; Parada, T.G.; Pardo, M.; Gonzalez-Barcia, M.; Piñeiro-Ces, A.; Rodríguez-Ares, M.T.; Blanco-Mendez, J.; Lamas, M.J.; et al. Ocular safety comparison of non-steroidal anti-inflammatory eye drops used in pseudophakic cystoid macular edema prevention. Int. J. Pharm. 2015, 495, 680–691. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hirlekar, R.S.; Sonawane, S.N.; Kadam, V.J. Studies on the effect of water-soluble polymers on drug-cyclodextrin complex solubility. AAPS Pharmscitech. 2009, 10, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Vavia, P.R. Effect of hydrophilic polymer on solubilization of fenofibrate by cyclodextrin complexation. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 247–251. [Google Scholar] [CrossRef]
- Nepafenac. Available online: https://www.drugbank.ca/drugs/DB06802 (accessed on 28 August 2019).
- Saldias, C.; Velasquez, L.; Quezada, C.; Leiva, A. Physicochemical assessment of dextran-g-poly (varepsilon-caprolactone) micellar nanoaggregates as drug nanocarriers. Carbohydr. Polym. 2015, 117, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Shelley, H.; Rodriguez-Galarza, R.M.; Duran, S.H.; Abarca, E.M.; Babu, R.J. In situ gel formulation for enhanced ocular delivery of nepafenac. J. Pharm. Sci. 2018, 107, 3089–3097. [Google Scholar] [CrossRef]
- Jansook, P.; Muankaew, C.; Stefansson, E.; Loftsson, T. Development of eye drops containing antihypertensive drugs: Formulation of aqueous irbesartan/gammaCD eye drops. Pharm. Dev. Technol. 2015, 20, 626–632. [Google Scholar] [CrossRef]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.L.; Dinu-Pîrvu, C.E. Strategies for improving ocular drug bioavailability and corneal wound healing with chitosan-based delivery systems. Polym (Basel) 2018, 10, 1221. [Google Scholar] [CrossRef]
- Greaves, J.L.; Wilson, C.G. Treatment of diseases of the eye with mucoadhesive delivery systems. Adv. Drug Deliv. Rev. 1993, 11, 349–383. [Google Scholar] [CrossRef]
- Ivarsson, D.; Wahlgren, M. Comparison of in vitro methods of measuring mucoadhesion: Ellipsometry, tensile strength and rheological measurements. Colloids Surf. B Biointerfaces 2012, 92, 353–359. [Google Scholar] [CrossRef]
- Almeida, H.; Lobão, P.; Frigerio, C.; Fonseca, J.; Silva, R.; Quaresma, P.; Lobo, J.M.S.; Amaral, M.H. Development of mucoadhesive and thermosensitive eyedrops to improve the ophthalmic bioavailability of ibuprofen. J. Drug Deliv. Sci. Technol. 2016, 35, 69–80. [Google Scholar] [CrossRef]
- Brako, F.; Thorogate, R.; Mahalingam, S.; Raimi-Abraham, B.; Craig, D.Q.M.; Edirisinghe, M. Mucoadhesion of progesterone-loaded drug delivery nanofiber constructs. ACS Appl. Mater. Interfaces 2018, 10, 13381–13389. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ahn, H.S.; Kim, E.K.; Kim, T.I. Efficacy of sodium hyaluronate and carboxymethylcellulose in treating mild to moderate dry eye disease. Cornea 2011, 30, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.L.; Woods, S.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesive polysaccharides modulate sodium retention, release and taste perception. Food Chem. 2018, 240, 482–489. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.M.; Cairns, D. The hen’s egg chorioallantoic membrane (HET-CAM) test to predict the ophthalmic irritation potential of a cysteamine-containing gel: Quantification using Photoshop(R) and ImageJ. Int. J. Pharm. 2015, 490, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Hailian, Q.; Na, Y.; Kang, B.C.; Yoon, J.H.; Cho, E.Y.; Lee, M.; Kim, D.; Bae, S.; Seok, S.H.; et al. Exploration and comparison of in vitro eye irritation tests with the ISO standard in vivo rabbit test for the evaluation of the ocular irritancy of contact lenses. Toxicol. Vitr. 2016, 37, 79–87. [Google Scholar] [CrossRef]
- Hayashi, K.; Mori, T.; Abo, T.; Koike, M.; Takahashi, Y.; Sakaguchi, H.; Nishiyama, N. A tiered approach combining the short time exposure (STE) test and the bovine corneal opacity and permeability (BCOP) assay for predicting eye irritation potential of chemicals. J. Toxicol. Sci. 2012, 37, 269–280. [Google Scholar] [CrossRef]
- Scheel, J.; Kleber, M.; Kreutz, J.; Lehringer, E.; Mehling, A.; Reisinger, K.; Steiling, W. Eye irritation potential: Usefulness of the HET-CAM under the globally harmonized system of classification and labeling of chemicals (GHS). Regul. Toxicol. Pharmacol. RTP 2011, 59, 471–492. [Google Scholar] [CrossRef]
- Yu, S.; Tan, G.; Liu, D.; Yang, X.; Pan, W. Nanostructured lipid carrier (NLC)-based novel hydrogels as potential carriers for nepafenac applied after cataract surgery for the treatment of inflammation: Design, characterization and in vitro cellular inhibition and uptake studies. RSC Adv. 2017, 7, 16668–16677. [Google Scholar] [CrossRef]
- Aktaş, Y.; Ünlü, N.; Orhan, M.; İrkeç, M.; Atilla Hıncal, A. Influence of Hydroxypropyl β-Cyclodextrin on the Corneal Permeation of Pilocarpine. Drug Dev. Ind. Pharm. 2003, 29, 223–230. [Google Scholar] [CrossRef]
- Loch, C.; Zakelj, S.; Kristl, A.; Nagel, S.; Guthoff, R.; Weitschies, W.; Seidlitz, A. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur. J. Pharm. Sci. 2012, 47, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Amparo, F.; Dastjerdi, M.H.; Okanobo, A.; Ferrari, G.; Smaga, L.; Hamrah, P.; Jurkunas, U.; Schaumberg, D.A.; Dana, R. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: A randomized clinical trial. JAMA Ophthalmol. 2013, 131, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H. Roles of IL-6 in Ocular Inflammation: A Review. Ocul. Immunol. Inflamm. 2018, 26, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Doucette, L.P.; Walter, M.A. Prostaglandins in the eye: Function, expression, and roles in glaucoma. Ophthalmic Genet 2017, 38, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Miller, C.M.; Du, Y.; Zheng, L.; Mohr, S.; Ball, S.L.; Kim, M.; Jamison, J.A.; Bingaman, D.P. Topical Administration of Nepafenac Inhibits Diabetes-Induced Retinal Microvascular Disease and Underlying Abnormalities of Retinal Metabolism and Physiology. Diabetes 2007, 56, 373. [Google Scholar] [CrossRef]
- Calles, J.A.; López-García, A.; Vallés, E.M.; Palma, S.D.; Diebold, Y. Preliminary characterization of dexamethasone-loaded cross-linked hyaluronic acid films for topical ocular therapy. Int. J. Pharm. 2016, 509, 237–243. [Google Scholar] [CrossRef]
| Formulations |
|---|
| F2 = 1.0% (w/v) CMC |
| F3 = 2.0% (w/v) PVA + 1.0% (w/v) CMC+ 0.1% (w/v) Tyloxapol |
| F5 = 1.0% (w/v) PVP + 1.0% (w/v) CMC + 0.1% (w/v) HPMC |
| F9 = 2.0% (w/v) PVA + 1.0% (w/v) CMC + 0.1% (w/v) MC |
| Eye Drop Formulations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Component (% w/v) | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 |
| PVP | – | – | – | – | 1.0 | – | – | – | – |
| PVA | – | – | 2.0 | – | – | – | – | – | 2.0 |
| CMC | – | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | – | 1.0 | 1.0 |
| HPMC | – | – | – | – | 0.1 | – | – | – | – |
| MC | – | – | – | – | – | – | – | – | 0.1 |
| Tyloxapol | – | – | 0.1 | – | – | – | – | – | – |
| HA | 0.2 | – | – | 0.2 | – | – | – | 0.2 | – |
| SA | – | – | – | – | – | 0.4 | 0.4 | 0.4 | – |
| Formulation | Apparent Drug Solubility at 25 °C (mg/mL) | Dissolved Drug Content (%) | Zeta Potential (mV) | pH |
|---|---|---|---|---|
| A1 | 1.87 ± 0.03 | 62.33 | −10.9 ± 0.6 | 6.08 ± 0.23 |
| A2 | 1.95 ± 0.02 | 65.00 | −10.4 ± 0.3 | 6.18 ± 0.05 |
| A3 | 2.23 ± 0.01 | 74.33 | −6.4 ± 0.8 | 6.09 ± 0.03 |
| A4 | 1.91 ± 0.02 | 63.66 | −12.1 ± 1.4 | 6.21 ± 0.09 |
| A5 | 2.50 ± 0.02 | 83.33 | −7.8 ± 0.9 | 6.07 ± 0.02 |
| A6 | 1.89 ± 0.05 | 63.00 | −27.4 ± 1.7 | 6.13 ± 0.33 |
| A7 | 1.68 ± 0.01 | 56.00 | −14.4 ± 1.4 | 6.01 ± 0.23 |
| A8 | 1.95 ± 0.02 | 65.00 | −14.7 ± 1.2 | 6.16 ± 0.15 |
| A9 | 2.61 ± 0.02 | 87.00 | −6.9 ± 1.4 | 6.08 ± 0.05 |
| Peak Summary | ||
|---|---|---|
| Formulation | Size (d. nm) | Intensity (%) |
| A1 | 5880.0 | 73.3 |
| 2619.0 | 19.9 | |
| A2 | 5880.0 | 46.9 |
| 4300.0 | 46.8 | |
| 1953.0 | 6.3 | |
| A3 | 5590.0 | 96.3 |
| 827.0 | 3.7 | |
| A4 | 5870.0 | 100.0 |
| A5 | 3090.0 | 53.5 |
| 5950.0 | 42.0 | |
| 481.0 | 4.5 | |
| A6 | 5575.0 | 100.0 |
| A7 | 3380.0 | 65.1 |
| 5560.0 | 34.9 | |
| A8 | 4510.0 | 66.4 |
| 1572.0 | 33.6 | |
| A9 | 5510.0 | 77.1 |
| 3250.0 | 15.3 | |
| 340.0 | 7.6 | |
| Formulation | Mucoadhesive Strength (N) |
|---|---|
| A1 | 0.39 ± 0.15 |
| A2 | 0.54 ± 0.13 |
| A3 | 0.41 ± 0.04 |
| A4 | 0.56 ± 0.11 |
| A5 | 0.52 ± 0.02 |
| A6 | 0.39 ± 0.06 |
| A7 | 0.36 ± 0.08 |
| A8 | 0.47 ± 0.02 |
| A9 | 0.38 ± 0.06 |
| Nevanac 3 mg/mL | 0.67 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Veiga, B.; Diaz-Rodriguez, P.; Alvarez-Lorenzo, C.; Loftsson, T.; Sigurdsson, H.H. In Vitro and Ex Vivo Evaluation of Nepafenac-Based Cyclodextrin Microparticles for Treatment of Eye Inflammation. Nanomaterials 2020, 10, 709. https://doi.org/10.3390/nano10040709
Lorenzo-Veiga B, Diaz-Rodriguez P, Alvarez-Lorenzo C, Loftsson T, Sigurdsson HH. In Vitro and Ex Vivo Evaluation of Nepafenac-Based Cyclodextrin Microparticles for Treatment of Eye Inflammation. Nanomaterials. 2020; 10(4):709. https://doi.org/10.3390/nano10040709
Chicago/Turabian StyleLorenzo-Veiga, Blanca, Patricia Diaz-Rodriguez, Carmen Alvarez-Lorenzo, Thorsteinn Loftsson, and Hakon Hrafn Sigurdsson. 2020. "In Vitro and Ex Vivo Evaluation of Nepafenac-Based Cyclodextrin Microparticles for Treatment of Eye Inflammation" Nanomaterials 10, no. 4: 709. https://doi.org/10.3390/nano10040709
APA StyleLorenzo-Veiga, B., Diaz-Rodriguez, P., Alvarez-Lorenzo, C., Loftsson, T., & Sigurdsson, H. H. (2020). In Vitro and Ex Vivo Evaluation of Nepafenac-Based Cyclodextrin Microparticles for Treatment of Eye Inflammation. Nanomaterials, 10(4), 709. https://doi.org/10.3390/nano10040709