Synergistic Tribo-Activity of Nanohybrids of Zirconia/Cerium-Doped Zirconia Nanoparticles with Nano Lamellar Reduced Graphene Oxide and Molybdenum Disulfide
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
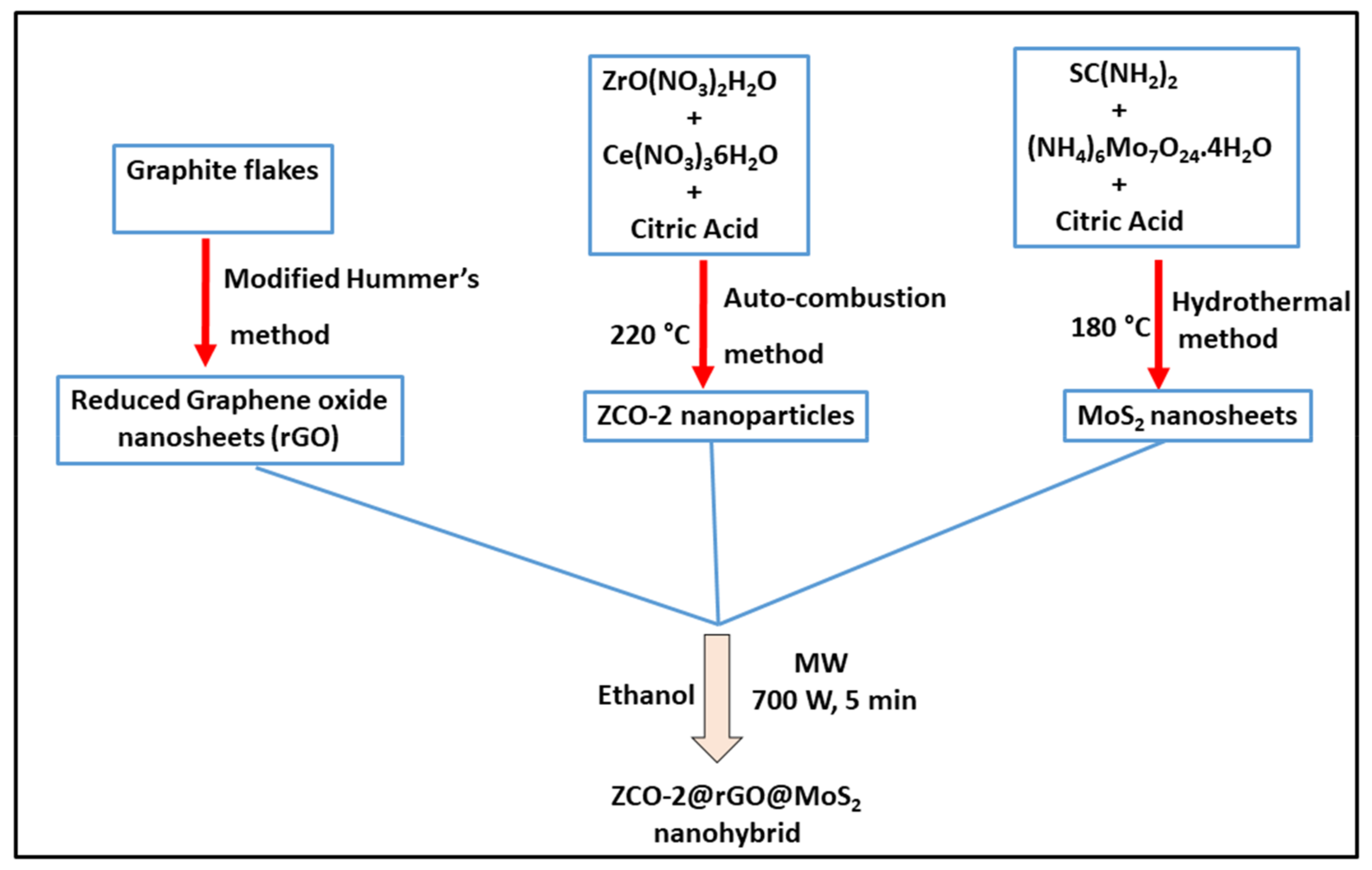
2.2. Synthesis of Nano-Additives
2.2.1. Preparation of Zirconia and Cerium-Doped Zirconia Nanoparticles, 10% (ZCO-1), 20% (ZCO-2), and 30% (ZCO-3)
2.2.2. Preparation of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO)
2.2.3. Preparation of MoS2 Nanosheets
2.2.4. Preparation of Binary Composites of ZrO2/ZCO-2 with Reduced Graphene Oxide
2.2.5. Preparation of Ternary Composites of ZrO2/ZCO-2, rGO and MoS2
2.3. Techniques Employed for Characterization of the Nano Additives
2.4. Tribological Tests
3. Results and Discussion
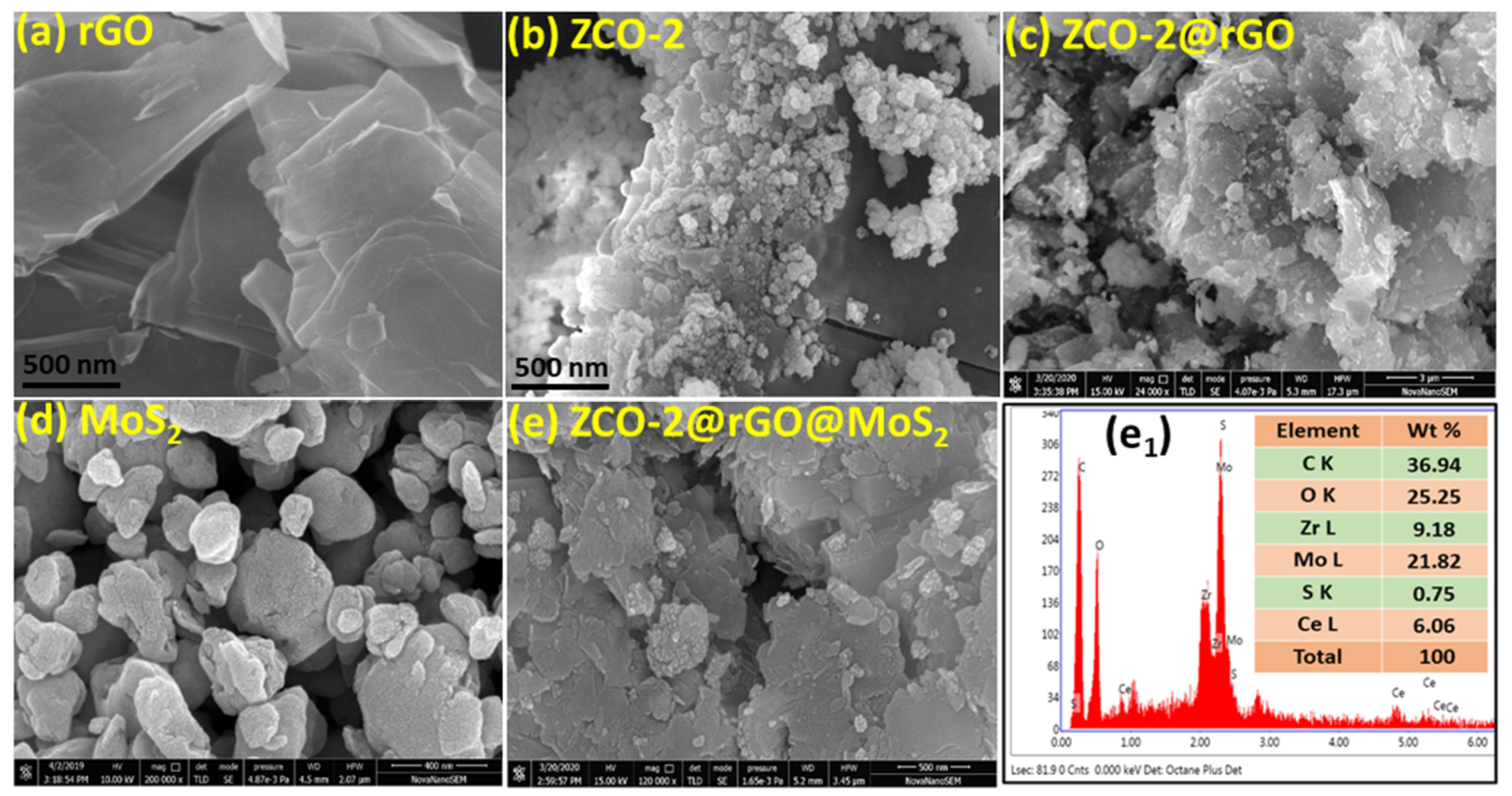
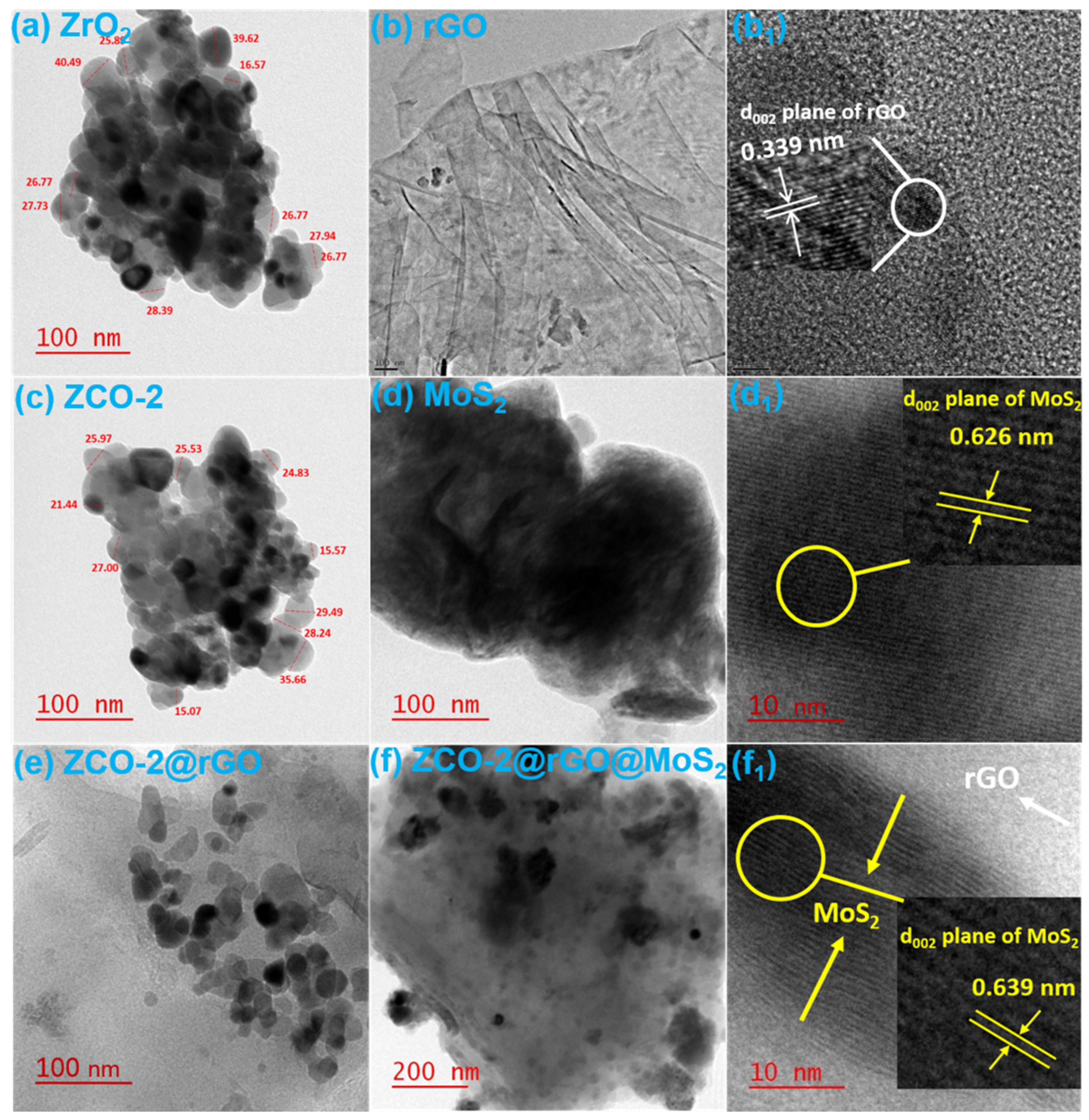
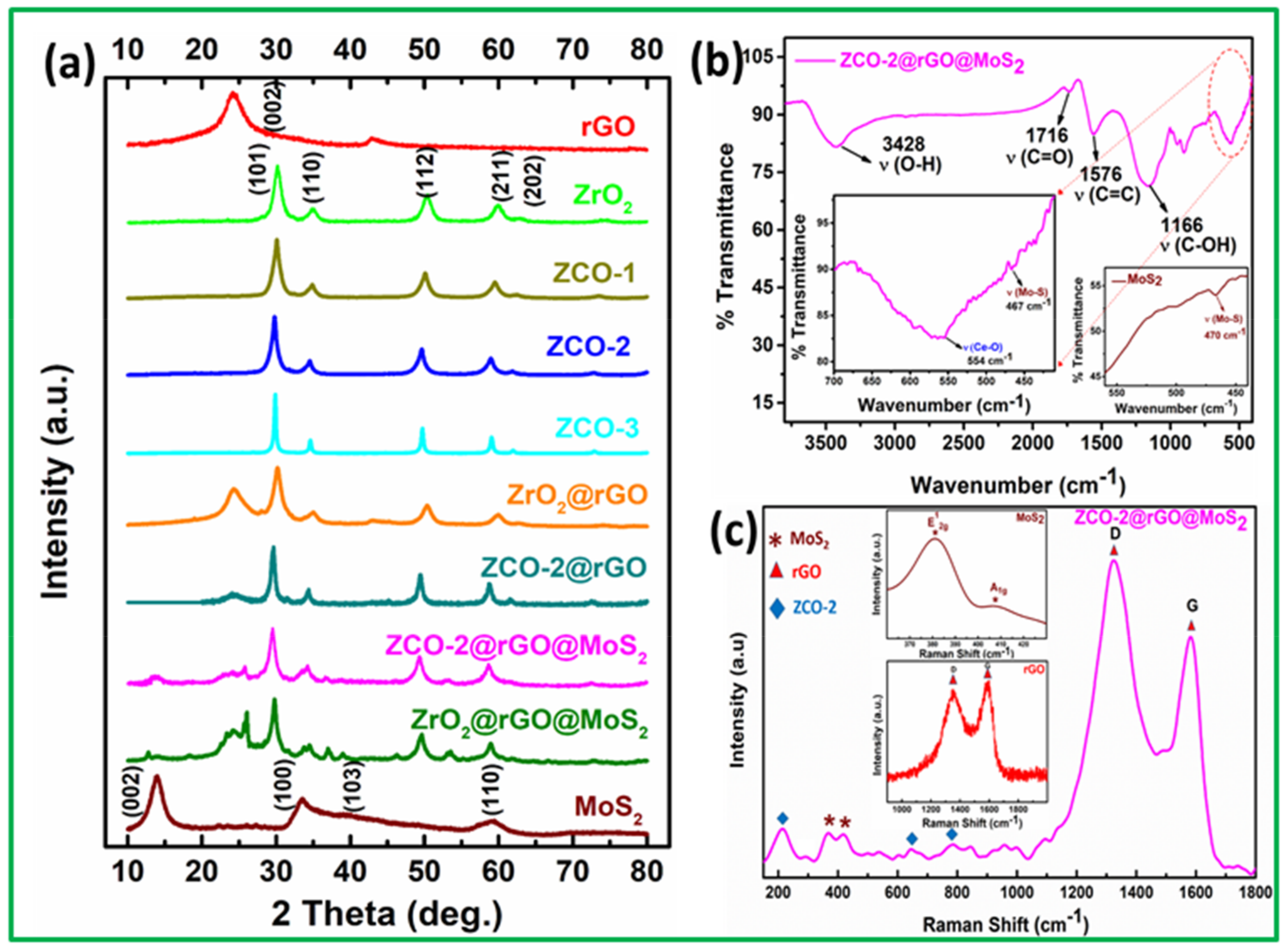
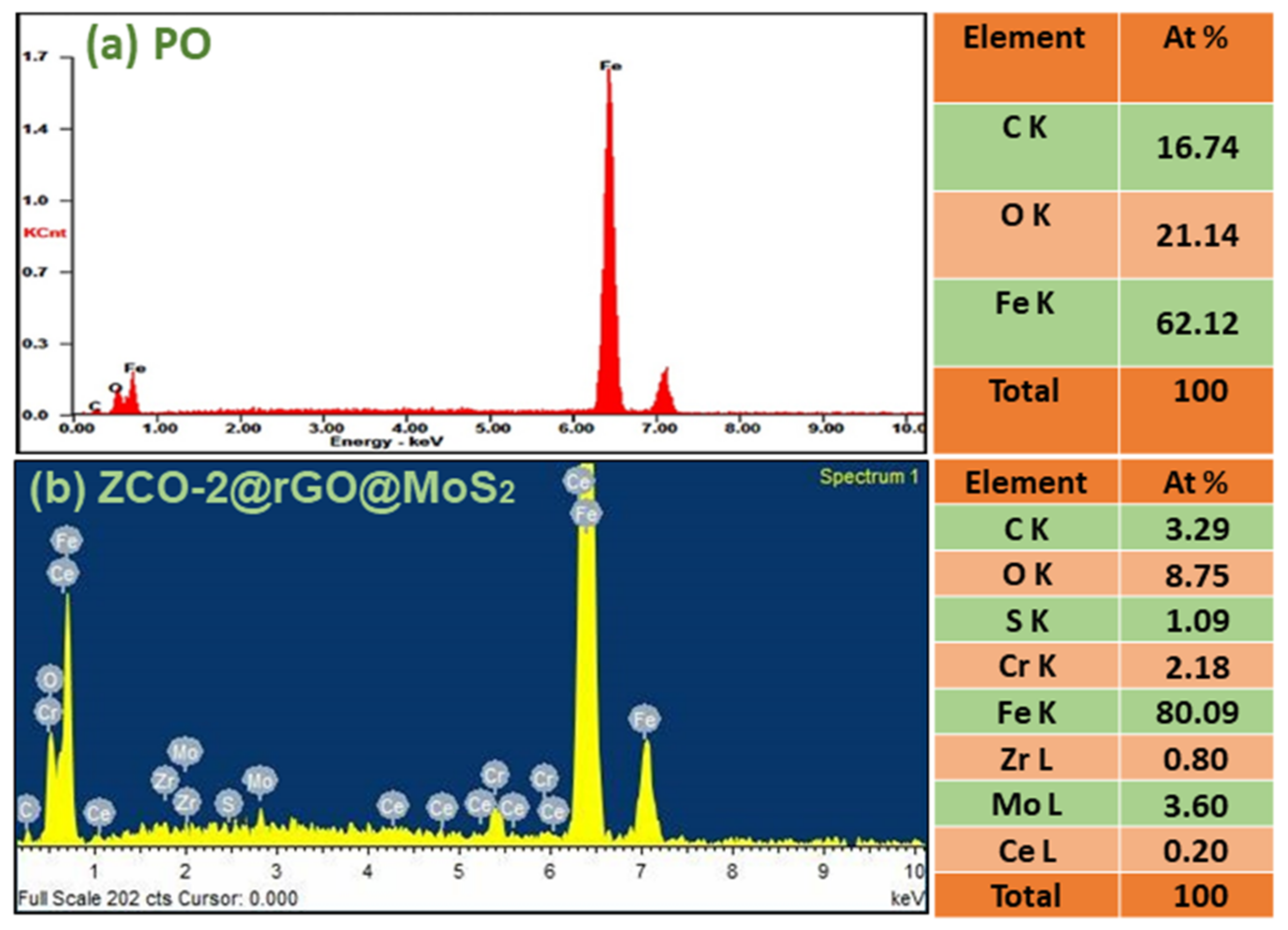
3.1. Structural and Morphological Features of Nano Additive
3.2. Assessment of Tribological Behaviour of Nano Additives in Paraffin Oil
3.2.1. Dispersion Stability
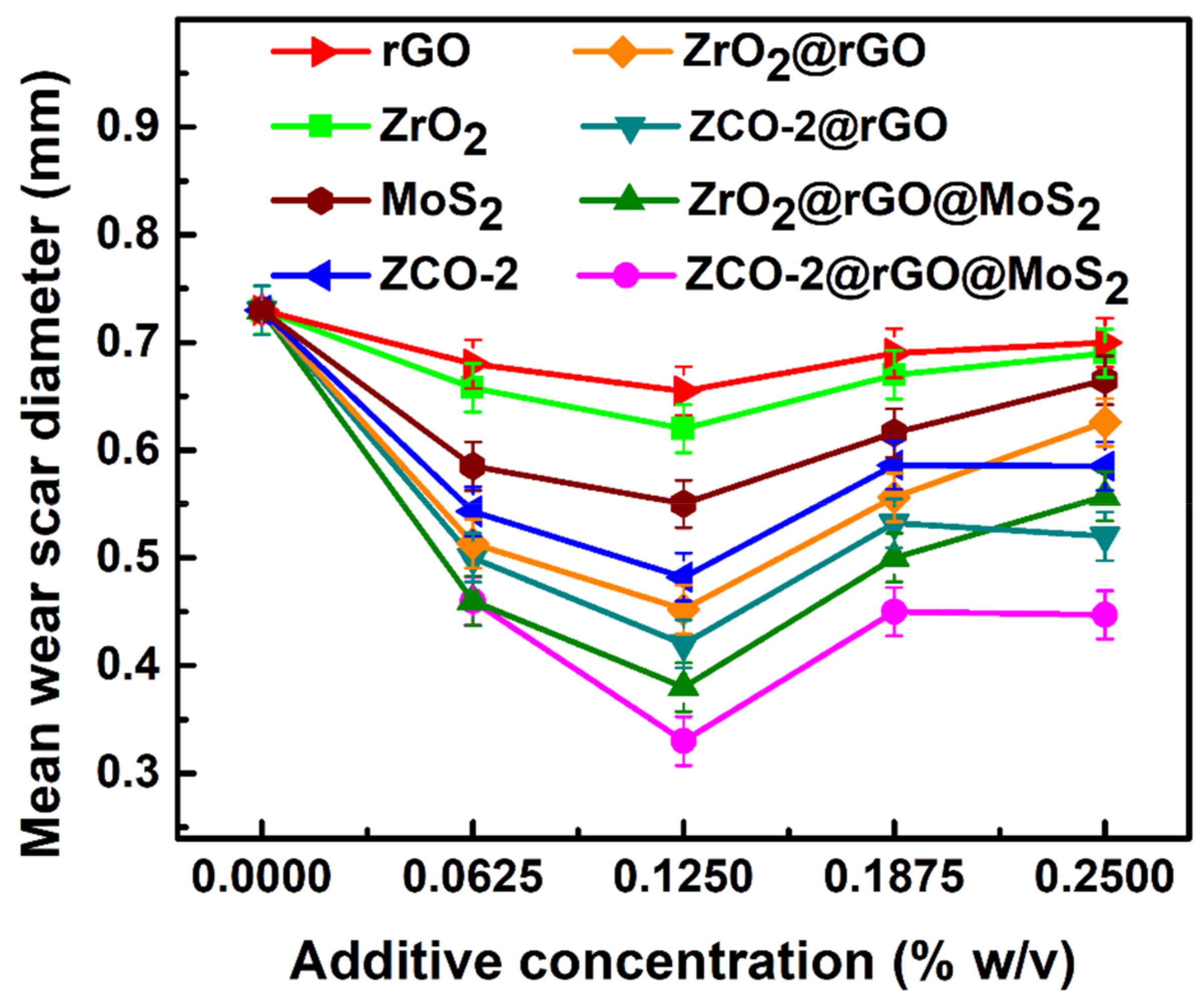
3.2.2. Optimization of Concentration of Additives
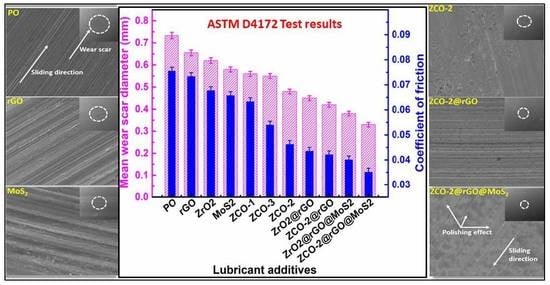
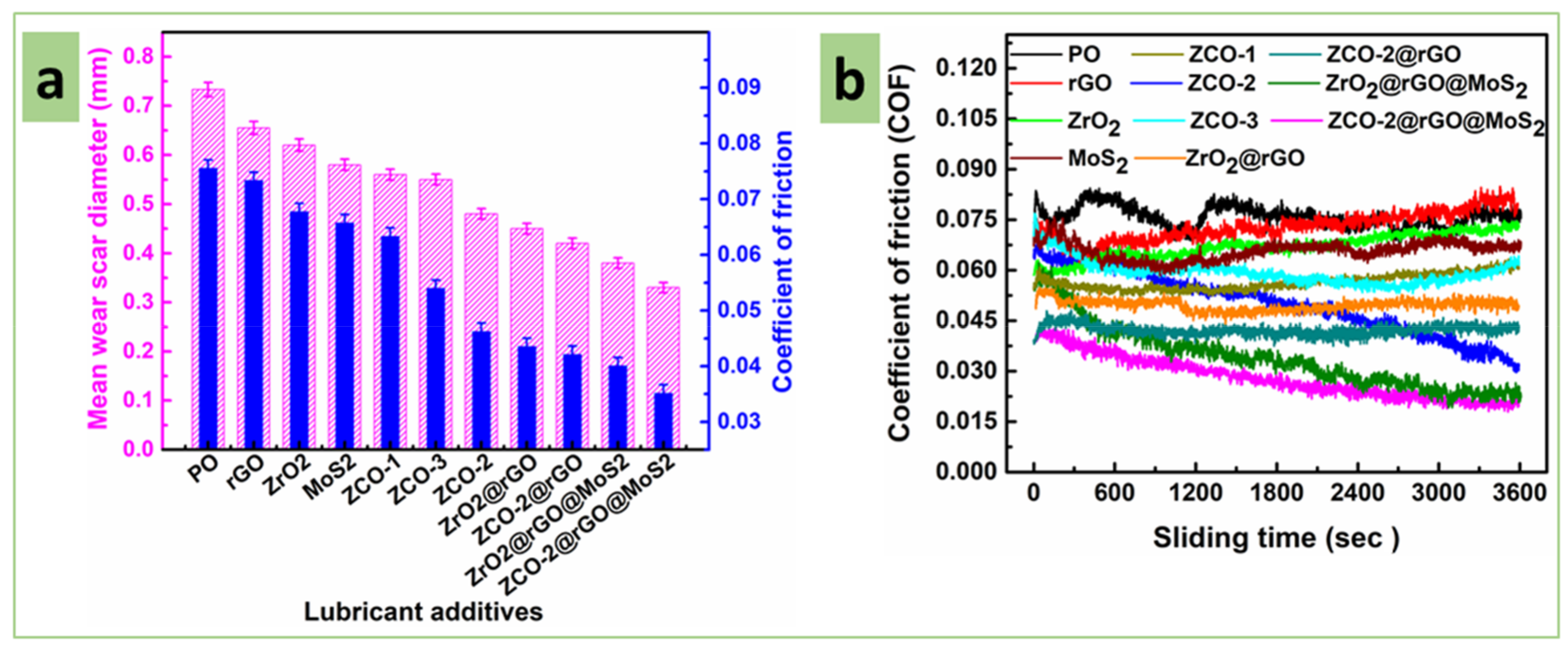
3.2.3. Friction and Wear-Reducing Properties
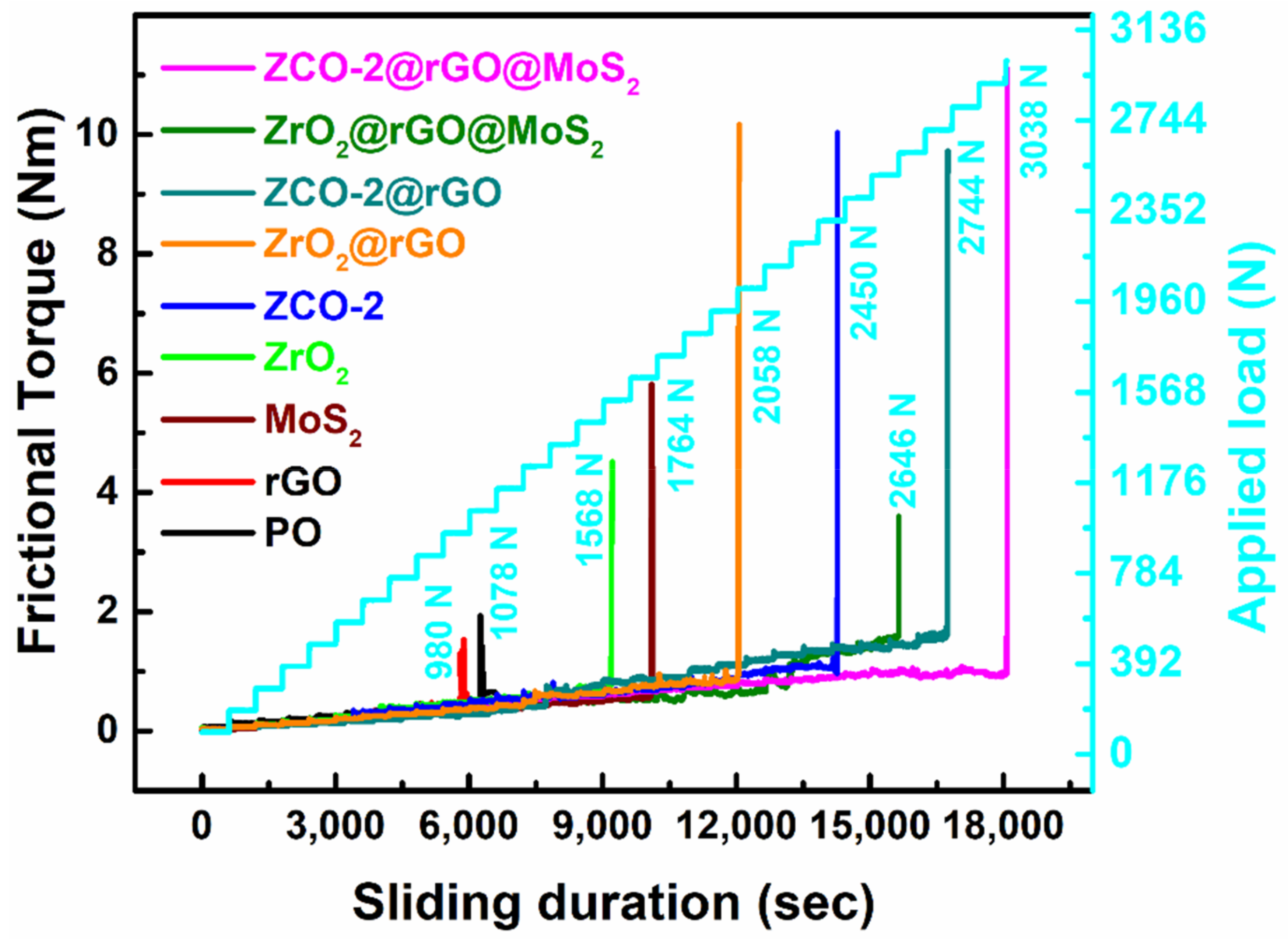
3.2.4. Load-Bearing Capacity
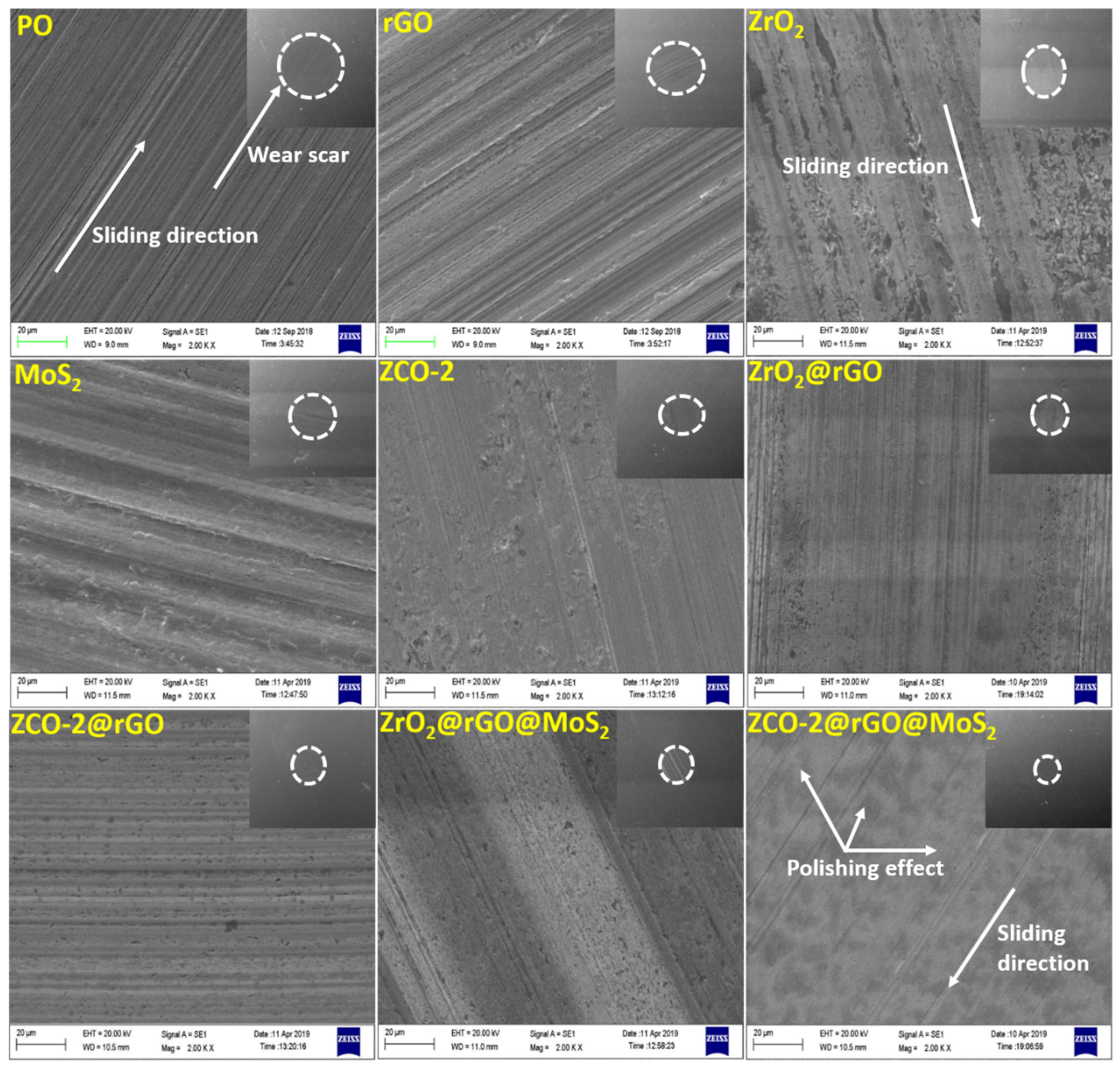
3.2.5. Morphological Features of the Worn Surface
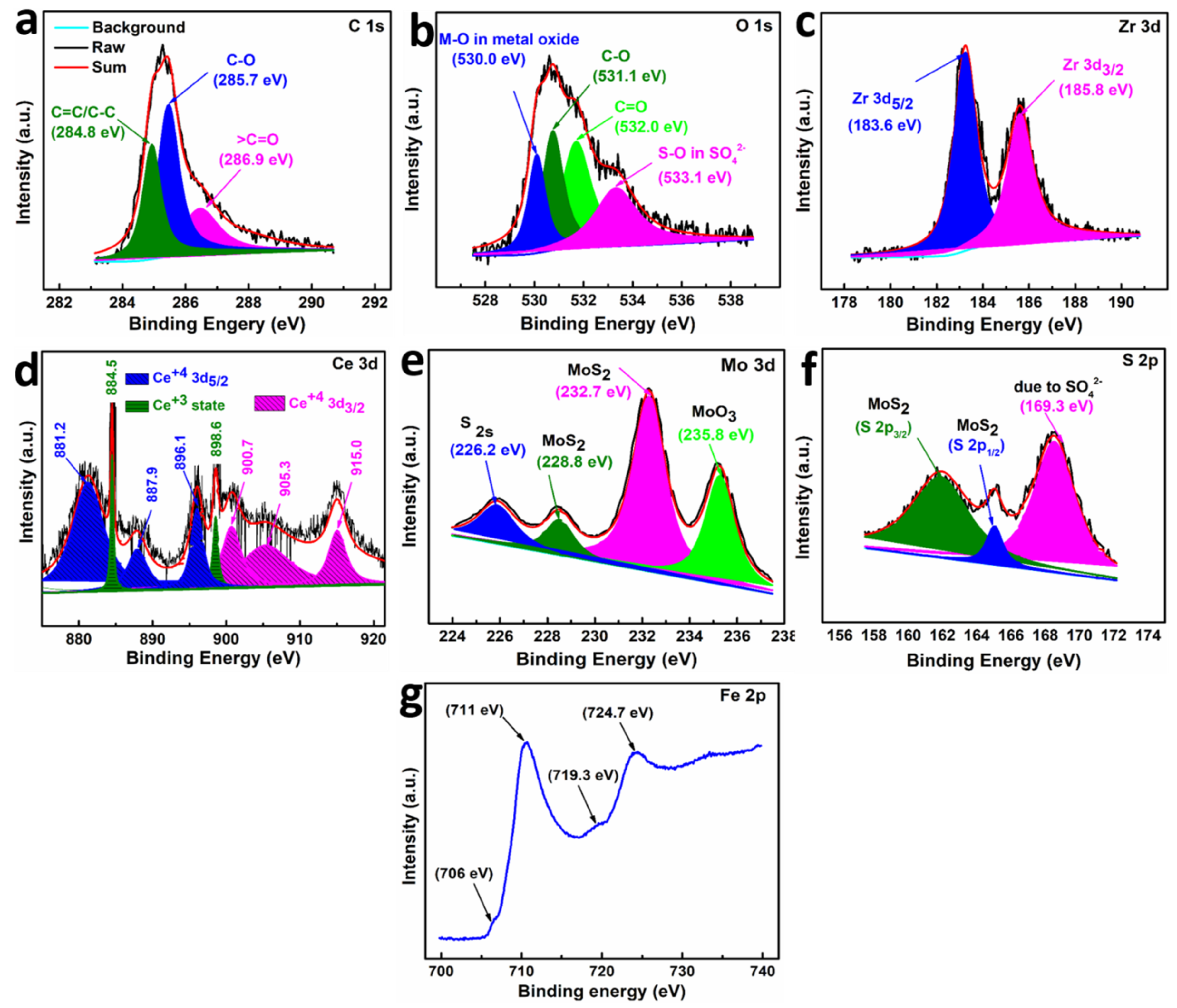
3.3. Tribo-Chemistry and Mechanism of Lubrication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Chouhan, A.; Mungse, H.P.; Sharma, O.P.; Singh, R.K.; Khatri, O.P. Chemically functionalized graphene for lubricant applications: Microscopic and spectroscopic studies of contact interfaces to probe the role of graphene for enhanced tribo-performance. J. Colloid Interface Sci. 2018, 513, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, H.; Kumari, S.; Khatri, O.P.; Tyagi, R. Copper matrix composites reinforced by rGO-MoS2 hybrid: Strengthening effect to enhancement of tribological properties. Compos. Part B Eng. 2019, 173, 106931. [Google Scholar] [CrossRef]
- Wang, L.; Han, W.; Ge, C.; Zhang, R.; Bai, Y.; Zhang, X. Covalent functionalized boron nitride nanosheets as efficient lubricant oil additives. Adv. Mater. Interfaces 2019, 6, 1901172. [Google Scholar] [CrossRef]
- Rawat, S.S.; Harsha, A.P.; Deepak, A.P. Tribological performance of paraffin grease with silica nanoparticles as an additive. Appl. Nanosci. 2019, 9, 305–315. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Approaches for Achieving Superlubricity in Two-Dimensional Materials. ACS Nano 2018, 12, 2122–2137. [Google Scholar] [CrossRef]
- Zhang, J.; Li, P.; Zhang, Z.; Wang, X.; Tang, J.; Liu, H.; Shao, Q.; Ding, T.; Umar, A.; Guo, Z. Solvent-free graphene liquids: Promising candidates for lubricants without the base oil. J. Colloid Interface Sci. 2019, 542, 159–167. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, J.; Chen, B.; Guo, S.; Li, J.; Li, C. One-step hydrothermal synthesis of reduced graphene oxide/zinc sulfide hybrids for enhanced tribological properties of epoxy coatings. Surf. Coat Tech. 2017, 326, 87–95. [Google Scholar] [CrossRef]
- Xie, H.; Dang, S.; Jiang, B.; Xiang, L.; Zhou, S.; Sheng, H.; Yang, T.; Pan, F. Tribological performances of SiO2/graphene combinations as water-based lubricant additives for magnesium alloy rolling. Appl. Surf. Sci. 2019, 475, 847–856. [Google Scholar] [CrossRef]
- Zhai, W.; Zhou, K. Nanomaterials in superlubricity. Adv. Funct. Mater. 2019, 29, 1806395. [Google Scholar] [CrossRef]
- Guo, Y.; Li, A.; Liu, S.; He, Q.; Kong, L.; Zhang, Y. Tribological properties of nanometer cerium oxide as additives in lithium grease. J. Rare Earths 2018, 36, 209–214. [Google Scholar]
- Yun, J.; Liu, X.; Liu, Q.; Wang, D.; Shen, T.; Peng, Z. Tribological properties and tribochemical analysis of nano-cerium oxide and sulfurized isobutene in titanium complex grease. Tribol. Int. 2016, 93, 332–346. [Google Scholar]
- Jia, X.; Huang, J.; Li, Y.; Yang, J.; Song, H. Monodisperse Cu nanoparticles@MoS2 nanosheets as a lubricant additive for improved tribological properties. Appl. Surf. Sci. 2019, 494, 430–439. [Google Scholar] [CrossRef]
- Song, W.; Yan, J.; Ji, H. Fabrication of GNS/MoS2 composite with different morphology and its tribological performance as a lubricant additive. Appl. Surf. Sci. 2019, 469, 226–235. [Google Scholar] [CrossRef]
- Hou, X.; Yang, C.; He, J.; Li, Z.; Zhang, Z. Preparation and tribological properties of lanthanum trifluoride nanoparticles-decorated graphene oxide nanosheets. Ind. Eng. Chem. Res. 2015, 54, 4773–4780. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, J.; Wang, L.; Xue, Q. Synergistic effect of hybrid carbon nanotube-graphene oxide as nanoadditive enhancing the frictional properties of ionic liquids in high vacuum. ACS Appl. Mater. Interfaces 2015, 7, 8592–8600. [Google Scholar] [CrossRef]
- Rastogi, R.B.; Kumar, D. Synthesis, characterization, and tribological evaluation of SDS-stabilized magnesium-doped zinc oxide (Zn0.88Mg0.12O) nanoparticles as efficient antiwear lubricant additives. ACS Sustain. Chem. Eng. 2016, 4, 3420–3428. [Google Scholar]
- Jaiswal, V.; Rastogi, R.B.; Kumar, R.; Singh, L.; Mandal, K.D. Tribological studies of stearic acid-modified CaCu2.9Zn0.1Ti4O12 nanoparticles as effective zero SAPS antiwear lubricant additives in paraffin oil. J. Mater. Chem. A 2014, 2, 375–386. [Google Scholar] [CrossRef]
- Li, D.; Xie, Y.; Yong, H.; Sun, D. Surfactant-assisted preparation of Y2O3-stabilized ZrO2 nanoparticles and their tribological performance in mineral and commercial lubricating oils. RSC Adv. 2017, 7, 3727–3735. [Google Scholar] [CrossRef]
- Kim, M.; Laine, R.M. One-step synthesis of core-shell (Ce0.7Zr0.3O2)x (Al2O3)1-x [(Ce0.7Zr0.3O2)@Al2O3] nanopowders via liquid-feed flame spray pyrolysis (LF-FSP). J. Am. Chem. Soc. 2009, 131, 9220–9229. [Google Scholar] [CrossRef]
- Philip, J.; Koshy, C. Surface morphology and stability analysis of ceria-based nanoparticles for its utilization as a lubricant additive. In International Conference on Communication and Signal Processing (ICCASP 2016); Atlantis Press: Paris, France, 2016; Volume 137, pp. 316–323. [Google Scholar]
- Su, Y.; Zhang, Y.; Song, J.; Hu, L. Novel approach to the fabrication of an alumina-MoS2 self-lubricating composite via the in situ synthesis of nanosized MoS2. ACS Appl. Mater. Interfaces 2017, 9, 30263–30266. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Srikanth, N.; Kong, L.B.; Zhou, K. Carbon nanomaterials in tribology. Carbon 2017, 119, 150–171. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, K. Recent progress on graphene-analogous 2D nanomaterials: Properties, modeling and applications. Prog. Mater. Sci. 2019, 100, 99–169. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Gao, S.; Chen, Q.; Peng, L.; Liu, K.; Wei, X. Superlubricity between MoS2 Monolayers. Adv. Mater. 2017, 29, 1701474. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.R.; Zhang, W.; Liu, Z.; Cheng, Z.L. A novel preparation method for MoS2 nanosheets with good tribology performance by the combination of expansion and freeze exfoliation. Ceram. Int. 2019, 45, 1730–1736. [Google Scholar] [CrossRef]
- Dietzel, D.; Brndiar, J.; Stich, I.; Schirmeisen, A. Limitations of structural superlubricity: Chemical bonds versus contact size. ACS Nano 2017, 11, 7642–7647. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, C. The synthesis of two-dimensional MoS2 nanosheets with enhanced tribological properties as oil additives. RSC Adv. 2018, 8, 9564–9573. [Google Scholar] [CrossRef]
- Chen, C.; Mei, W.; Yu, W.; Chen, X.; Zeng, L.; Tsang, Y.; Chao, Z.; Liu, X. Enhanced sunlight-driven photocatalytic property of Mg-doped ZnO nanocomposites with three-dimensional graphene oxide/MoS2 nanosheet composites. RSC Adv. 2018, 8, 17399–17409. [Google Scholar] [CrossRef]
- Paul, G.; Hirani, H.; Kuila, T.; Murmu, N.C. Nanolubricants dispersed with graphene and its derivatives: An assessment and review of the tribological performance. Nanoscale 2019, 11, 3458–3483. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, T.; Erdemir, A.; Li, Q. Tribology of two-dimensional materials: From mechanisms to modulating strategies. Mater. Today 2019, 26, 67–86. [Google Scholar] [CrossRef]
- Sinclair, R.C.; Suter, J.L.; Coveney, P.V. Graphene-graphene interactions: Friction, superlubricity, and exfoliation. Adv. Mater. 2018, 30, 1705791. [Google Scholar]
- Li, S.; Li, Q.; Carpick, R.W.; Gumbsch, P.; Liu, X.Z.; Ding, X.; Sun, J.; Li, J. The evolving quality of frictional contact with graphene. Nature 2016, 539, 541–545. [Google Scholar] [PubMed]
- Song, W.; Yan, J.; Ji, H. Tribological Study of the SOCNTs@MoS2 Composite as a lubricant additive: Synergistic effect. Ind. Eng. Chem. Res. 2018, 57, 6878–6887. [Google Scholar]
- Xu, Y.; Peng, Y.; Dearn, K.D.; Zheng, X.; Yao, L.; Hu, X. Synergistic lubricating behaviors of graphene and MoS2 dispersed in esterified bio-oil for steel /steel contact. Wear 2017, 343, 297–309. [Google Scholar]
- Song, H.; Wang, B.; Zhou, Q.; Xiao, J.; Jia, X. Preparation and tribological properties of MoS2/graphene oxide composites. Appl. Surf. Sci. 2017, 419, 24–34. [Google Scholar]
- Wu, P.R.; Feng, Y.M.; Ge, T.; Kong, Y.C.; Ma, Z.S.; Liu, Z.; Cheng, Z.L. An investigation on tribological properties of the chemically capped zinc borate(ZB)/MoS2 nanocomposites in oil. J. Ind. Eng. Chem. 2018, 63, 157–167. [Google Scholar]
- Wu, P.R.; Kong, Y.C.; Ma, Z.S.; Ge, T.; Feng, Y.M.; Liu, Z.; Cheng, Z.L. Preparation and tribological properties of novel zinc borate/MoS2 nanocomposites in grease. J. Alloys Compd. 2018, 740, 823–829. [Google Scholar]
- Zheng, X.; Hu, X.; Xu, Y.; Geng, J.; Peng, Y.; Olson, D. Tribological behavior of Fe3O4/MoS2 nanocomposites additives in aqueous and oil phase media. Tribol. Int. 2016, 102, 79–87. [Google Scholar]
- Liu, L.; Jiao, S.; Peng, Y.; Zhou, W. A Green design for lubrication: multifunctional system containing Fe3O4@MoS2 nanohybrid. ACS Sustain. Chem. Eng. 2018, 6, 7372–7379. [Google Scholar]
- Zheng, D.; Wu, Y.P.; Li, Z.Y.; Cai, Z.B. Tribological properties of WS2/graphene nanocomposites as lubricating oil additives. RSC Adv. 2017, 7, 14060–14068. [Google Scholar]
- Jaiswal, V.; Umrao, S.; Rastogi, R.B.; Kumar, R.; Srivastava, A. Synthesis, characterization, and tribological evaluation of TiO2-reinforced boron and nitrogen Co-doped reduced graphene oxide based hybrid nanomaterials as efficient antiwear lubricant additives. ACS Appl. Mater. Interfaces 2016, 8, 11698–11710. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Kumar, B.; Rastogi, R.B. Zinc oxide- and magnesium-doped zinc oxide-decorated nanocomposites of reduced graphene oxide as friction and wear modifiers. ACS Appl. Mater. Interfaces 2019, 11, 2418–2430. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Wang, J.; Yang, Z.; Wang, H.; Wang, Z.; Yang, S. Preparation of a highly effective lubricating oil additive-ceria/graphene composite. RSC Adv. 2014, 4, 47096–47105. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, J.; Wang, J.; Yang, Z.; Liu, S.; Wang, Z.; Yang, S. Preparation of a reduced graphene oxide/zirconia nanocomposite and its application as a novel lubricant oil additive. RSC Adv. 2015, 5, 91802–91812. [Google Scholar] [CrossRef]
- Rawat, S.S.; Harsha, A.P.; Agarwal, D.P.; Kumari, S.; Khatri, O.P. Pristine and alkylated MoS2 nanosheets for enhancement of tribological performance of paraffin grease under boundary lubrication regime. J. Tribol. 2019, 141, 072102. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wu, W.; Chen, J.; Chu, G.; Zou, H. Synthesis and characterizations of graphene-copper nanocomposites and their antifriction application. J. Ind. Eng. Chem. 2014, 20, 2043–2049. [Google Scholar] [CrossRef]
- Si, R.; Zhang, Y.W.; Li, S.J.; Lin, B.X.; Yan, C.H. Urea-based hydrothermally derived homogeneous nanostructured Ce1-xZrxO2 (x = 0–0.8) solid solutions: A strong correlation between oxygen storage capacity and lattice strain. J. Phys. Chem. B 2004, 108, 12481–12488. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, E.; Hu, K.; Xu, Y.; Hu, X. Formation of an adsorption film of MoS2 nanoparticles and dioctyl sebacate on a steel surface for alleviating friction and wear. Tribol. Int. 2015, 92, 172–183. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Wang, Y.; Wang, W.; Yan, L.; Luo, J. An investigation on the tribological properties of multilayer graphene and MoS2 nanosheets as additives used in hydraulic applications. Tribol. Int. 2016, 97, 14–20. [Google Scholar] [CrossRef]
- Tang, Z.; Li, S. A review of recent developments of friction modifiers for liquid lubricants (2007-present). Curr. Opin. Solid State Mater. Sci. 2014, 18, 119–139. [Google Scholar] [CrossRef]
- Uflyand, I.E.; Zhinzhilo, V.A.; Burlakova, V.E. Metal-containing nanomaterials as lubricant additives: State-of-the-art and future development. Friction 2019, 7, 93–116. [Google Scholar] [CrossRef]
- Battez, A.H.; González, R.; Viesca, J.L.; Fernández, J.E.; Fernández, J.D.; Machado, A.; Chou, R.; Riba, J. CuO, ZrO2 and ZnO nanoparticles as antiwear additive in oil lubricants. Wear 2008, 265, 422–428. [Google Scholar] [CrossRef]
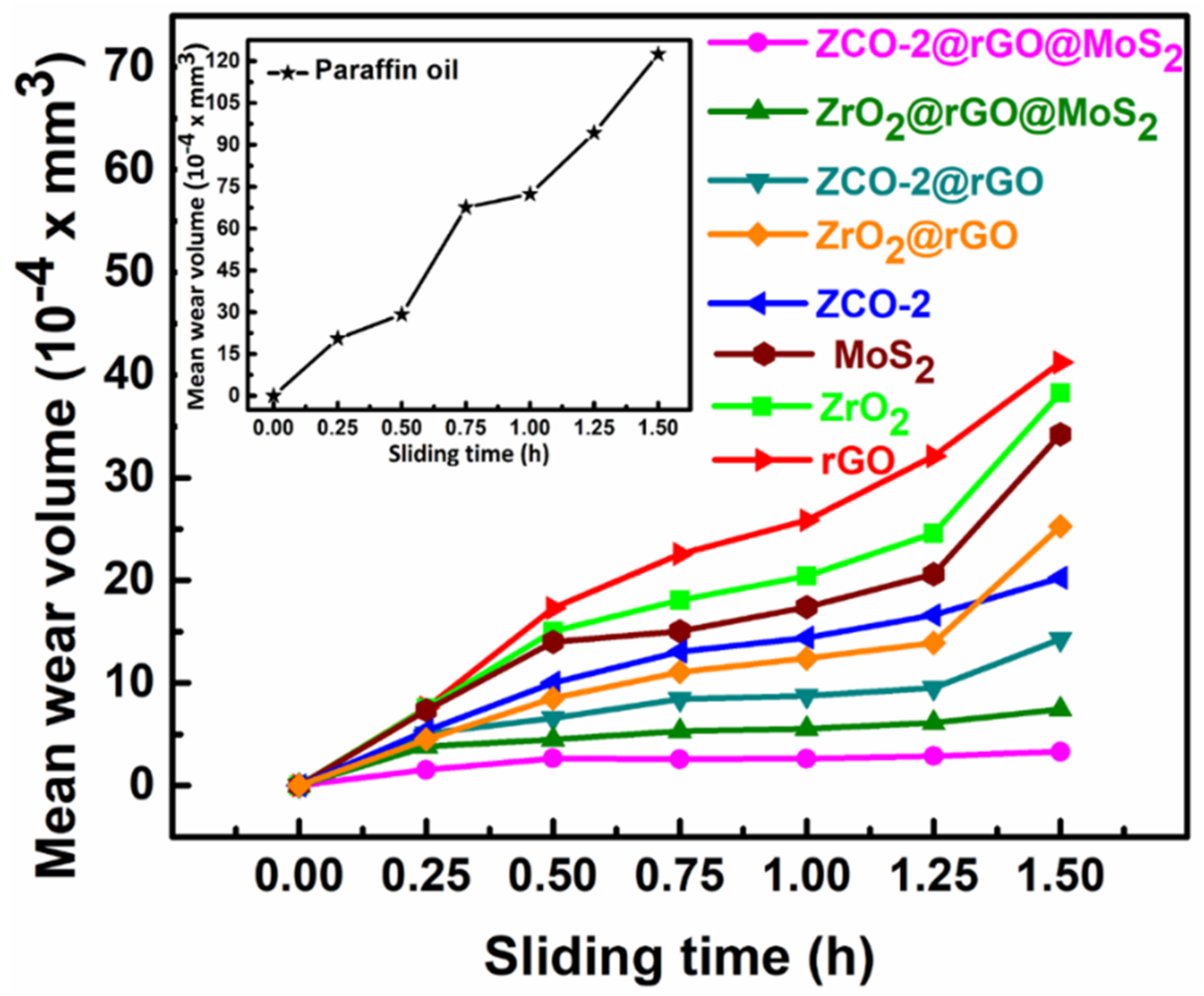

| S/N | Lubricants | Wear Rate (10−4 × mm3/h) | |
|---|---|---|---|
| Running-In | Steady-State | ||
| 1 | PO | 84.482 ± 3.556 | 53.326 ± 3.359 |
| 2 | rGO | 31.074 ± 2.292 | 18.784 ± 3.236 |
| 3 | ZrO2 | 24.664 ± 3.092 | 13.080 ± 2.125 |
| 4 | MoS2 | 20.724 ± 4.227 | 11.080 ± 0.970 |
| 5 | ZCO-2 | 17.524 ± 1.484 | 7.080 ± 0.969 |
| 6 | ZrO2@rGO | 14.864 ± 1.270 | 5.680 ± 0.162 |
| 7 | ZCO-2@rGO | 10.728 ± 2.321 | 2.140 ± 0.497 |
| 8 | ZrO2@rGO@MoS2 | 6.640 ± 2.102 | 1.600 ± 0.439 |
| 9 | ZCO-2@rGO@MoS2 | 3.543 ± 1.039 | 0.588 ± 0.263 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, D.K.; Shukla, N.; Kumar, B.; Singh, A.K.; Shahu, K.; Yadav, M.; Rhee, K.Y.; Rastogi, R.B. Synergistic Tribo-Activity of Nanohybrids of Zirconia/Cerium-Doped Zirconia Nanoparticles with Nano Lamellar Reduced Graphene Oxide and Molybdenum Disulfide. Nanomaterials 2020, 10, 707. https://doi.org/10.3390/nano10040707
Verma DK, Shukla N, Kumar B, Singh AK, Shahu K, Yadav M, Rhee KY, Rastogi RB. Synergistic Tribo-Activity of Nanohybrids of Zirconia/Cerium-Doped Zirconia Nanoparticles with Nano Lamellar Reduced Graphene Oxide and Molybdenum Disulfide. Nanomaterials. 2020; 10(4):707. https://doi.org/10.3390/nano10040707
Chicago/Turabian StyleVerma, Dinesh Kumar, Nivedita Shukla, Bharat Kumar, Alok Kumar Singh, Kavita Shahu, Mithilesh Yadav, Kyong Yop Rhee, and Rashmi Bala Rastogi. 2020. "Synergistic Tribo-Activity of Nanohybrids of Zirconia/Cerium-Doped Zirconia Nanoparticles with Nano Lamellar Reduced Graphene Oxide and Molybdenum Disulfide" Nanomaterials 10, no. 4: 707. https://doi.org/10.3390/nano10040707
APA StyleVerma, D. K., Shukla, N., Kumar, B., Singh, A. K., Shahu, K., Yadav, M., Rhee, K. Y., & Rastogi, R. B. (2020). Synergistic Tribo-Activity of Nanohybrids of Zirconia/Cerium-Doped Zirconia Nanoparticles with Nano Lamellar Reduced Graphene Oxide and Molybdenum Disulfide. Nanomaterials, 10(4), 707. https://doi.org/10.3390/nano10040707