Polypseudorotaxanes of Pluronic® F127 with Combinations of α- and β-Cyclodextrins for Topical Formulation of Acyclovir
Abstract
1. Introduction
2. Materials and Methods
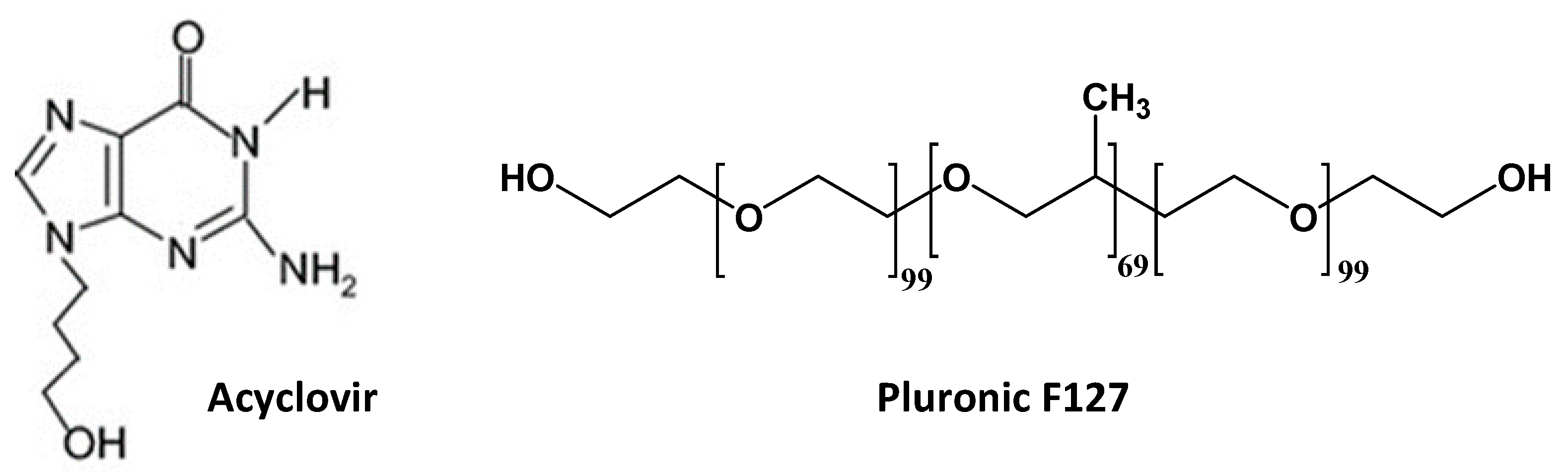
2.1. Materials
2.2. Gel Preparation
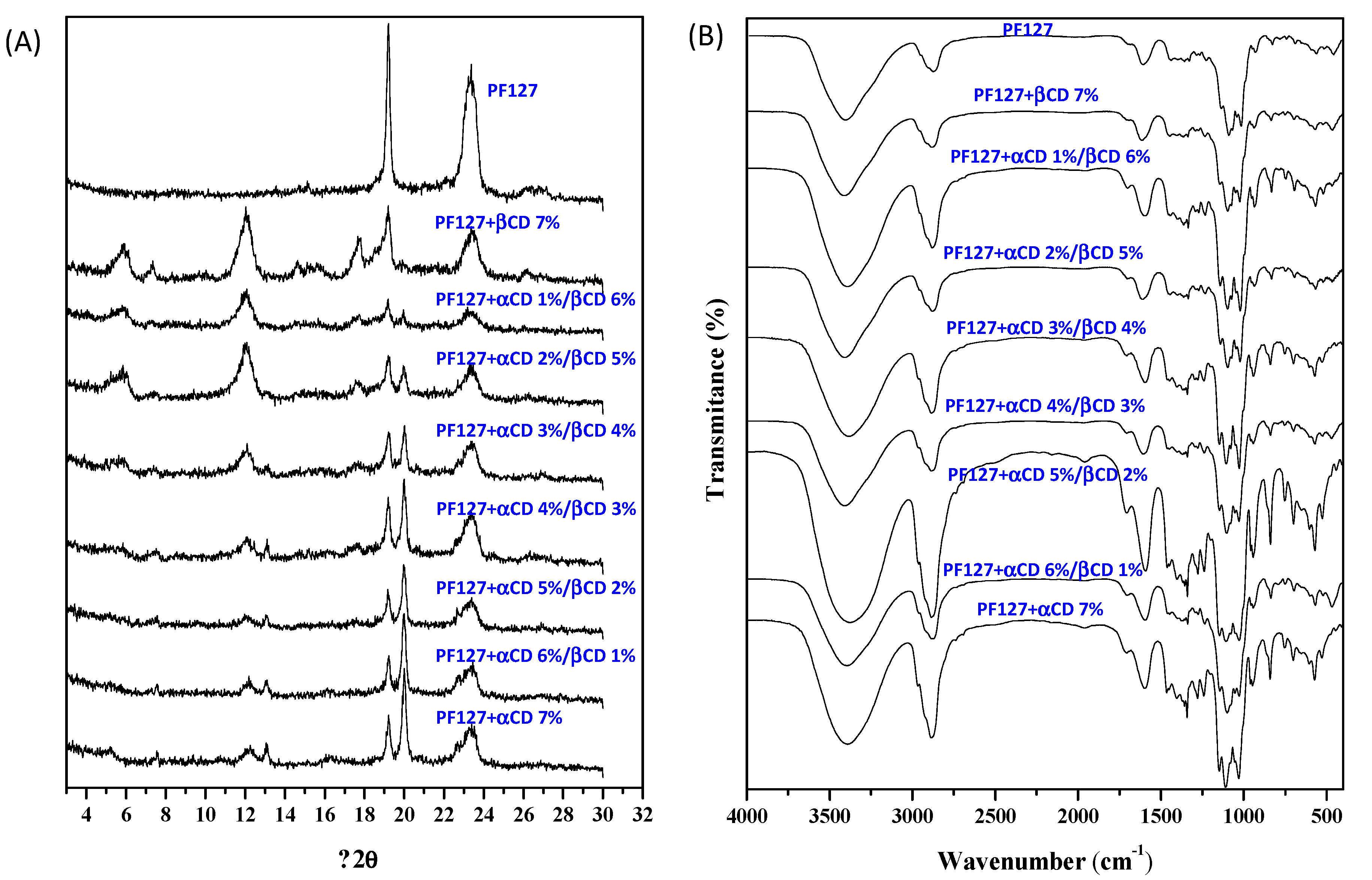
2.3. X-Ray Diffraction and FT-IR Spectroscopy
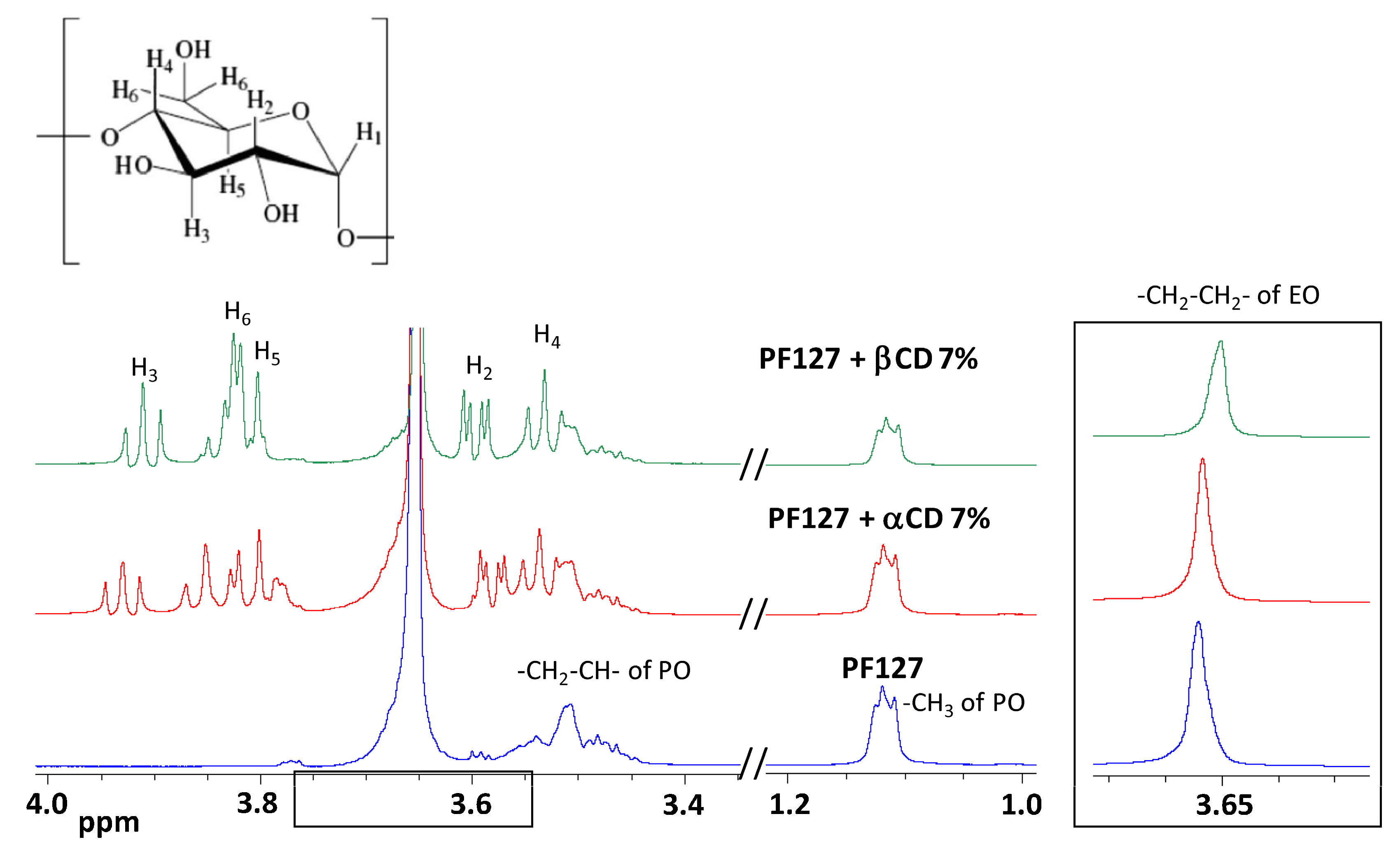
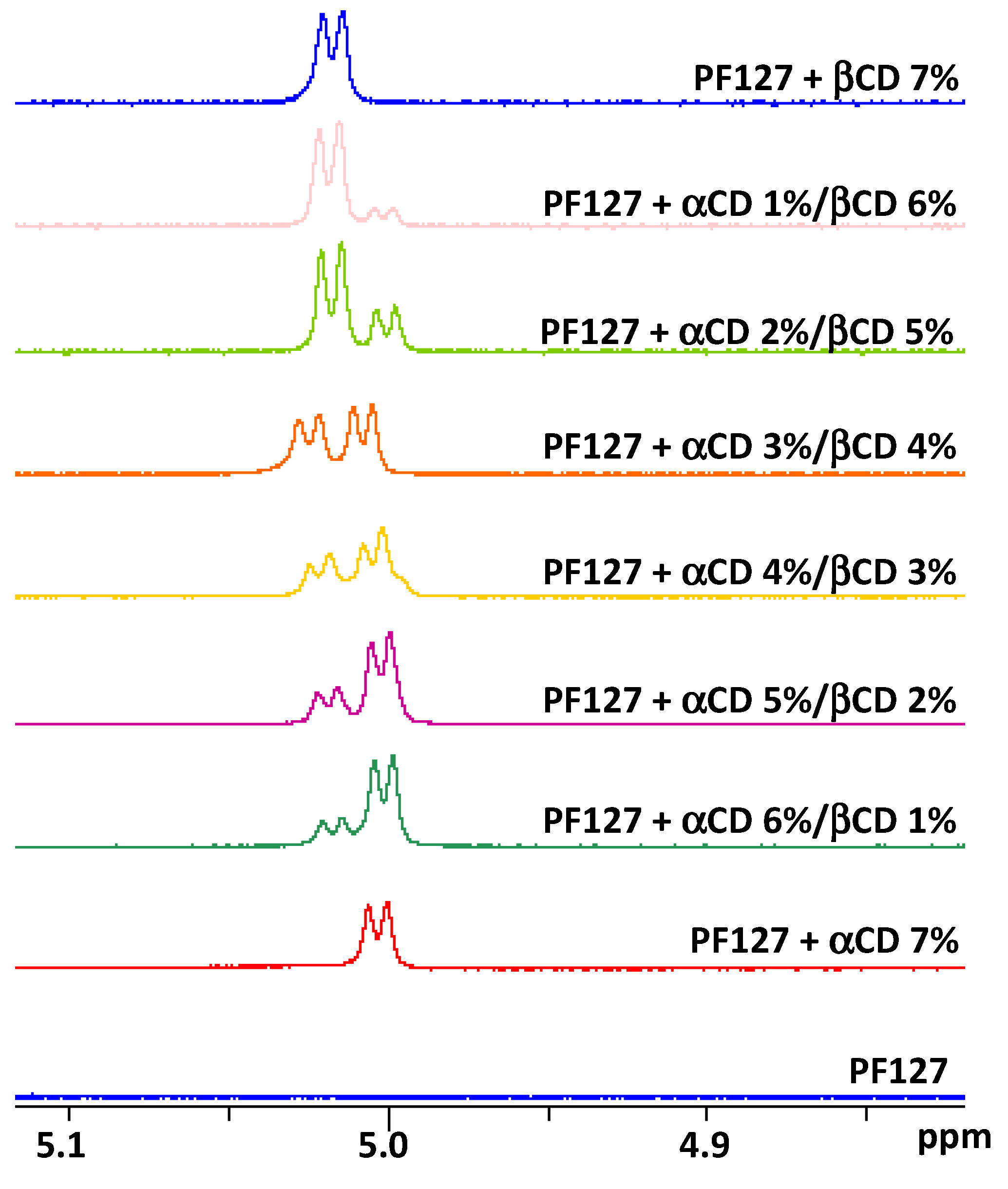
2.4. NMR Studies
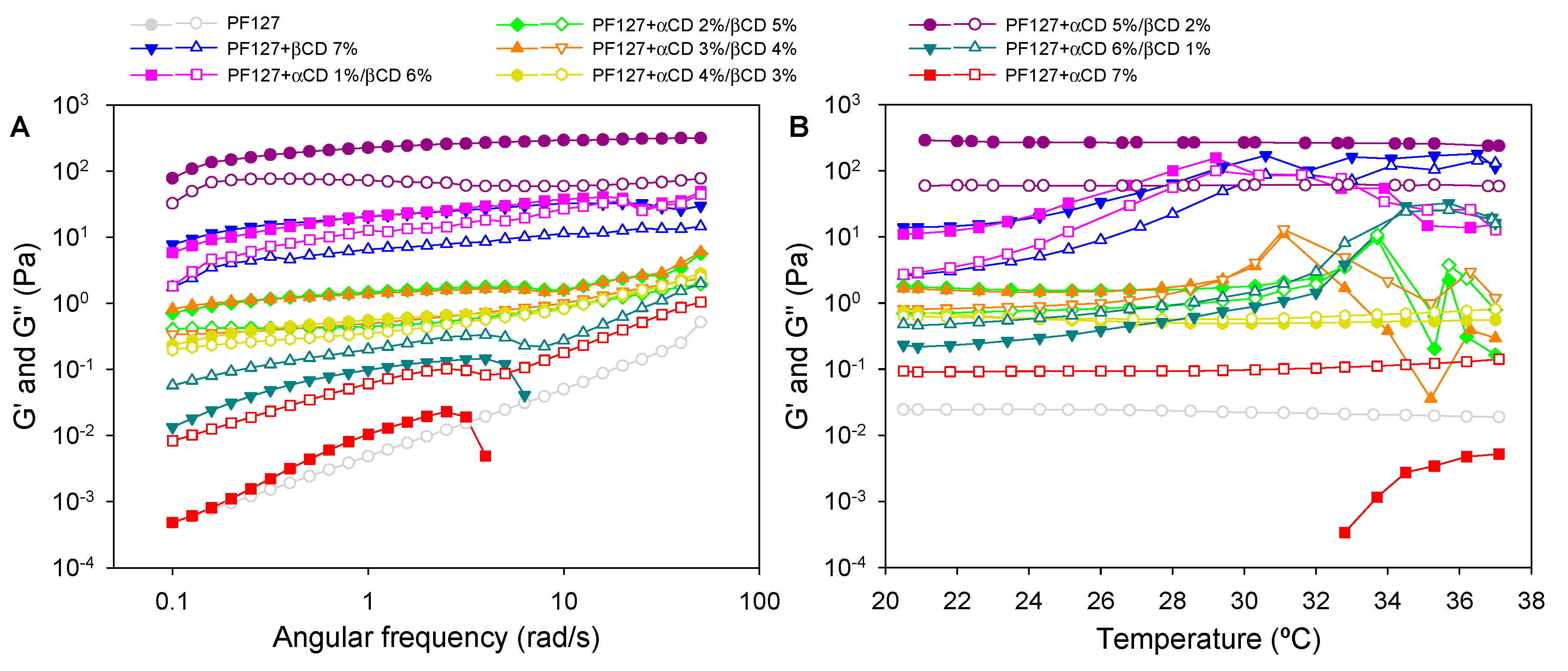
2.5. Rheological Studies
2.6. Phase Solubility Studies
2.7. HET-CAM Assay
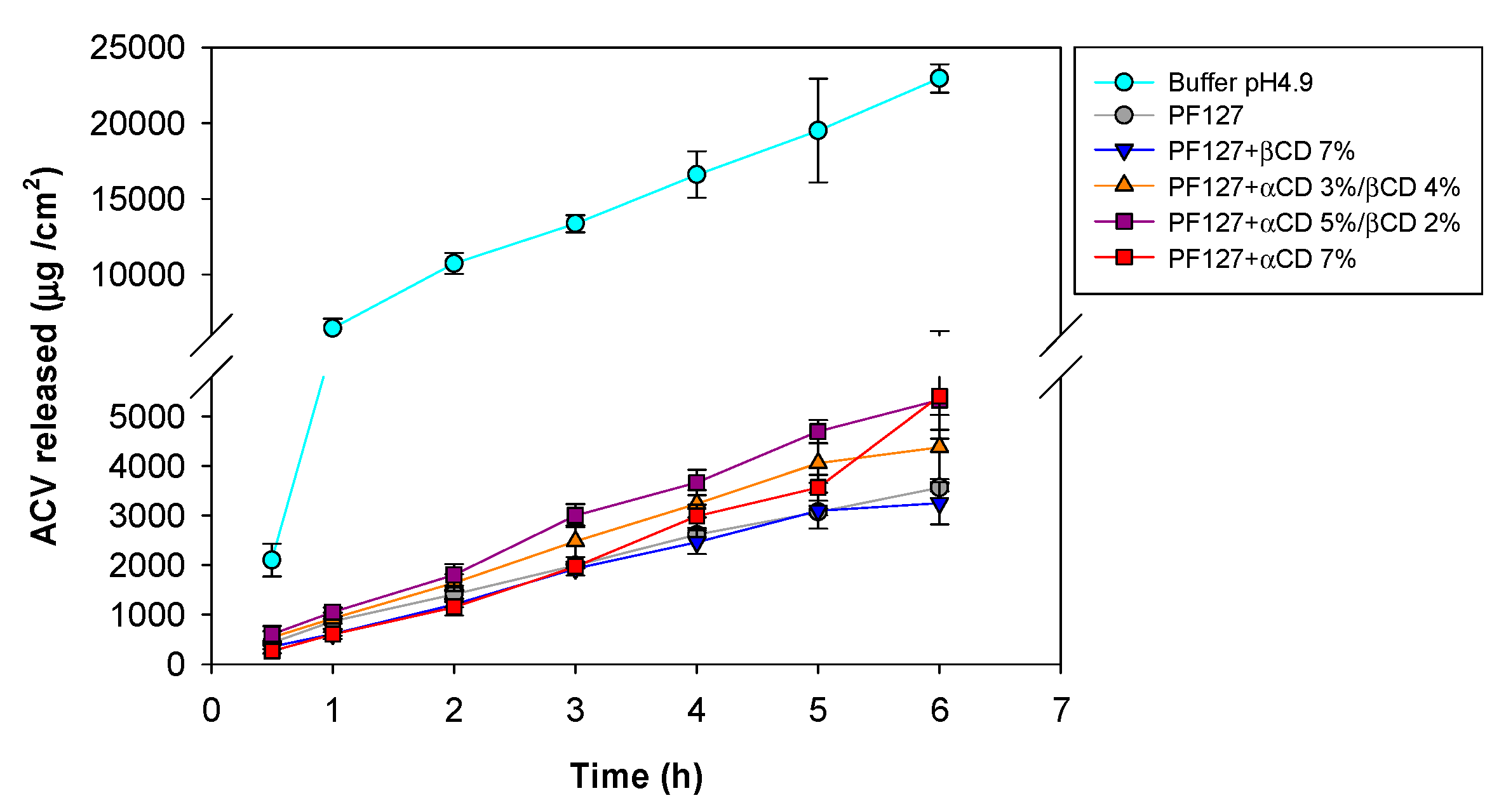
2.8. ACV Release Tests
2.9. Statistical Analysis
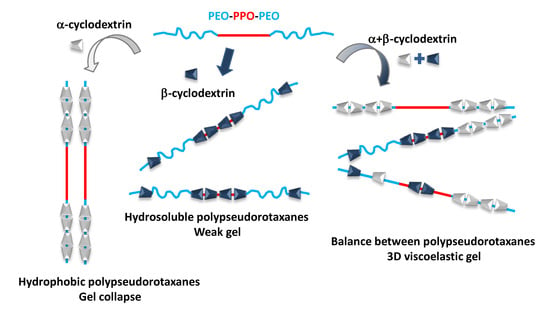
3. Results and Discussion
3.1. Polypseudorotaxanes Formation and Characterization in Solid State
3.2. NMR Studies
3.3. Rheological Properties
3.4. Phase Solubility Studies
3.5. HET-CAM Test
3.6. ACV Release Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soleimani, F.; Karimi, R.; Gharib, F. Thermodynamic studies on protonation constant of acyclovir at different ionic strengths. J. Solut. Chem. 2016, 45, 920–931. [Google Scholar] [CrossRef]
- Wagstaff, A.J.; Faulds, D.; Goa, K.L. Aciclovir: A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1994, 47, 153–205. [Google Scholar] [CrossRef]
- Roberts, J.K.; Stockmann, C.; Constance, J.E.; Stiers, J.; Spigarelli, M.G.; Ward, R.M.; Sherwin, C.M.T. Pharmacokinetics and pharmacodynamics of antibacterials, antifungals, and antivirals used most frequently in neonates and infants. Clin. Pharmacokinet. 2014, 53, 581–610. [Google Scholar] [CrossRef]
- Brigden, D.; Whiteman, P. The mechanism of action, pharmacokinetics and toxicity of acyclovir-A review. J. Infect. 1983, 6, 3–9. [Google Scholar] [CrossRef]
- Arnal, J.; Gonzalez-Alvarez, I.; Bermejo, M.; Amidon, G.L.; Junginger, H.E.; Kopp, S.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; et al. Biowaiver monographs for immediate release solid oral dosage forms: Aciclovir. J. Pharm. Sci. 2008, 97, 5061–5073. [Google Scholar] [CrossRef]
- Hassan, H.; Adam, S.K.; Othman, F.; Shamsuddin, A.F.; Basir, R. Antiviral nanodelivery systems: Current trends in acyclovir administration. J. Nanomater. 2016, 2016, 4591634. [Google Scholar] [CrossRef]
- Pamornpathomkul, B.; Ngawhirunpat, T.; Tekko, I.A.; Vora, L.; McCarthy, H.O.; Donnelly, R.F. Dissolving polymeric microneedle arrays for enhanced site-specific acyclovir delivery. Eur. J. Pharm. Sci. 2018, 121, 200–209. [Google Scholar] [CrossRef]
- Karolewicz, B.; Nartowski, K.; Pluta, J.; Górniak, A. Physicochemical characterization and dissolution studies of acyclovir solid dispersions with Pluronic F127 prepared by the kneading method. Acta Pharm. 2016, 66, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Bencini, M.; Ranucci, E.; Ferruti, P.; Trotta, F.; Donalisio, M.; Cornaglia, M.; Lembo, D.; Cavalli, R. Preparation and in vitro evaluation of the antiviral activity of the Acyclovir complex of a beta-cyclodextrin/poly(amidoamine) copolymer. J. Control. Release 2008, 126, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Ahmad, M.; Sarfraz, R.M.; Minhas, M.U. Development of acyclovir loaded β-cyclodextrin-g-poly methacrylic acid hydrogel microparticles: An in vitro characterization. Adv. Polym. Technol. 2016, 37, 21711. [Google Scholar] [CrossRef]
- Nair, A.B.; Attimarad, M.; Al-Dhubiab, B.E.; Wadhwa, J.; Harsha, S.; Ahmed, M. Enhanced oral bioavailability of acyclovir by inclusion complex using hydroxypropyl-β-cyclodextrin. Drug Deliv. 2014, 21, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.; Pedersen, J.S. Structural study on the micelle formation of poly (ethylene oxide)–poly (propylene oxide)–poly (ethylene oxide) triblock copolymer in aqueous solution. Macromolecules 1993, 26, 805–812. [Google Scholar] [CrossRef]
- He, C.; Kim, S.W.; Lee, D.S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control. Release 2008, 127, 189–207. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.; Agnely, F.; Chaumeil, J. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as functional excipients: Methods to enhance complexation efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Pose-Vilarnovo, B.; Rodriguez-Tenreiro, C.; dos Santos, J.F.R.; Vazquez-Doval, J.; Concheiro, A.; Alvarez-Lorenzo, C.; Torres-Labandeira, J.J. Modulating drug release with cyclodextrins in hydroxypropyl methylcellulose gels and tablets. J. Control. Release 2004, 94, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Simoes, S.M.N.; Veiga, F.; Torres-Labandeira, J.J.; Ribeiro, A.C.F.; Concheiro, A.; Alvarez-Lorenzo, C. Syringeable self-assembled cyclodextrin gels for drug delivery. Curr. Top. Med. Chem. 2014, 14, 494–509. [Google Scholar] [CrossRef]
- Zheng, Y.; Wyman, I.W. Supramolecular nanostructures based on cyclodextrin and poly (ethylene oxide): Syntheses, structural characterizations and applications for drug delivery. Polymers 2016, 8, 198. [Google Scholar] [CrossRef]
- Wenz, G.; Han, B.-H.; Müller, A. Cyclodextrin rotaxanes and polyrotaxanes. Chem. Rev. 2006, 106, 782–817. [Google Scholar] [CrossRef]
- Nogueiras-Nieto, L.; Alvarez-Lorenzo, C.; Sandez-Macho, I.; Concheiro, A.; Otero-Espinar, F.J. Hydrosoluble cyclodextrin/poloxamer polypseudorotaxanes at the air/water interface, in bulk solution, and in the gel state. J. Phys. Chem. B 2009, 113, 2773–2782. [Google Scholar]
- Perry, C.; Hebraud, P.; Gernigon, V.; Brochon, C.; Lapp, A.; Lindner, P.; Schlatter, G. Pluronic and beta-cyclodextrin in water: From swollen micelles to self-assembled crystalline platelets. Soft Matter 2011, 7, 3502–3512. [Google Scholar] [CrossRef]
- Lorenzo-Veiga, B.; Sigurdsson, H.H.; Loftsson, T.; Alvarez-Lorenzo, C. Cyclodextrin-amphiphilic copolymer supramolecular assemblies for ocular delivery of natamycin. Nanomaterials 2019, 9, 745. [Google Scholar]
- Taura, D.; Li, S.J.; Hashidzume, A.; Harada, A. Formation of side-chain hetero-polypseudorotaxane composed of alpha- and beta-cyclodextrins with a water-soluble polymer bearing two recognition sites. Macromolecules 2010, 43, 1706–1713. [Google Scholar] [CrossRef]
- Jansook, P.; Pichayakorn, W.; Muankaew, C.; Loftsson, T. Cyclodextrin–poloxamer aggregates as nanocarriers in eye drop formulations: Dexamethasone and amphotericin B. Drug Dev. Ind. Pharm. 2016, 42, 1446–1454. [Google Scholar] [CrossRef]
- Mondjinou, Y.A.; Hyun, S.H.; Xiong, M.; Collins, C.J.; Thong, P.L.; Thompson, D.H. Impact of mixed β-cyclodextrin ratios on pluronic rotaxanation efficiency and product solubility. ACS Appl. Mater. Interfaces 2015, 7, 23831–23836. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 6th ed.; Council of Europe: Strasbourg, France, 2007; p. 509.
- Schmolka, I.R. Artificial skin. I. Preparation and properties of Pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef]
- Griesinger, C.; Ernst, R.R. Frequency offset effects and their elimination in NMR rotating-frame cross-relaxation spectroscopy. J. Magn. Reson. 1987, 75, 261–271. [Google Scholar] [CrossRef]
- Hwang, T.L.; Shaka, A.J. Water suppression that works. Excitation sculpting using arbitrary waveforms and pulsed field gradients. J. Magn. Reson. 1995, 112, 275–279. [Google Scholar] [CrossRef]
- Cohen, Y.; Avram, L.; Frish, L. Diffusion NMR spectroscopy in supramolecular and combinatorial chemistry: An old parameter—New insights. Angew. Chem. Int. Ed. Engl. 2005, 44, 520–554. [Google Scholar] [CrossRef]
- Computer Aided Resonance Assignment. Available online: http://www.cara.nmr.ch/doku.php (accessed on October 2019).
- Iacovino, R.; Rapuano, F.; Caso, J.V.; Russo, A.; Lavorgna, M.; Russo, C.; Isidori, M.; Russo, L.; Malgieri, G.; Isernia, C. β-Cyclodextrin inclusion complex to improve physicochemical properties of pipemidic acid: Characterization and bioactivity evaluation. Int. J. Mol. Sci. 2013, 14, 13022–13041. [Google Scholar] [CrossRef] [PubMed]
- Iacovino, R.; Caso, J.V.; Rapuano, F.; Russo, A.; Isidori, M.; Lavorgna, M.; Malgieri, G.; Isernia, C. Physicochemical characterization and cytotoxic activity evaluation of hydroxymethylferrocene:β-Cyclodextrin inclusion complex. Molecules 2012, 17, 6056–6070. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Fernandez-Villanueva, D.; Concheiro, A.; Alvarez-Lorenzo, C. α-Lipoic acid in Soluplus® polymeric nanomicelles for ocular treatment of diabetes-associated corneal diseases. J. Pharm. Sci. 2016, 105, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, W.I. Analysis of data on the medicament release from ointments. J. Pharm. Sci. 1962, 51, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.M.; Veiga, F.; Ribeiro, A.C.; Figueiras, A.R.; Taboada, P.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular gels of poly-α-cyclodextrin and PEO-based copolymers for controlled drug release. Eur. J. Pharm. Biopharm. 2014, 87, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Bílková, E.; Sedlák, M.; Dvorak, B.; Ventura, K.; Knotek, P.; Benes, L. Prednisolone-α-cyclodextrin-star PEG polypseudorotaxanes with controlled drug delivery properties. Org. Biomol. Chem. 2010, 8, 5423–5430. [Google Scholar] [CrossRef]
- Simões, S.M.; Veiga, F.; Torres-Labandeira, J.J.; Ribeiro, A.C.; Sandez-Macho, M.I.; Concheiro, A.; Alvarez-Lorenzo, C. Syringeable pluronic-α-cyclodextrin supramolecular gels for sustained delivery of vancomycin. Eur. J. Pharm. Biopharm. 2012, 80, 103–112. [Google Scholar] [CrossRef]
- Segredo-Morales, E.; Martin-Pastor, M.; Salas, A.; Évora, C.; Concheiro, A.; Alvarez-Lorenzo, C.; Delgado, A. Mobility of water and polymer species and rheological properties of supramolecular polypseudorotaxane gels suitable for bone regeneration. Bioconjugate Chem. 2018, 29, 503–516. [Google Scholar] [CrossRef]
- Li, J.; Ni, X.P.; Zhou, Z.H.; Leong, K.W. Preparation and characterization of polypseudorotaxanes based on block-selected inclusion complexation between poly (propylene oxide)-poly (ethylene oxide)-poly (propylene oxide) triblock copolymers and alpha-cyclodextrin. J. Am. Chem. Soc. 2003, 125, 1788–1795. [Google Scholar] [CrossRef]
- Puig-Rigall, J.; Serra-Gómez, R.; Stead, I.; Grillo, I.; Dreiss, C.A.; González-Gaitano, G. Pseudo-polyrotaxanes of cyclodextrins with direct and reverse X-shaped block copolymers: A kinetic and structural study. Macromolecules 2019, 52, 1458–1468. [Google Scholar] [CrossRef]
- Lo Nostro, P.; Lopes, J.R.; Cardelli, C. Formation of cyclodextrin-based polypseudorotaxanes: Solvent effect and kinetic study. Langmuir 2001, 17, 4610–4615. [Google Scholar] [CrossRef]
- Caso, J.V.; Russo, L.; Palmieri, M.; Malgieri, G.; Galdiero, S.; Falanga, A.; Isernia, C.; Iacovino, R. Investigating the inclusion properties of aromatic amino acids complexing beta-cyclodextrins in model peptides. Amino Acids 2015, 47, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Vadnere, M.; Amidon, G.; Lindenbaum, S.; Haslam, J.L. Thermodynamic studies on the gel–sol transition of some pluronic polyols. Int. J. Pharm. 1984, 22, 207–218. [Google Scholar] [CrossRef]
- Wang, P.; Johnston, T.P. Kinetics of sol-to-gel transition for poloxamer polyols. J. Appl. Polym. Sci. 1991, 43, 283–292. [Google Scholar] [CrossRef]
- del Rosario, C.; Rodríguez-Évora, M.; Reyes, R.; Simões, S.; Concheiro, A.; Évora, C.; Alvarez-Lorenzo, C.; Delgado, A. Bone critical defect repair with poloxamine–cyclodextrin supramolecular gels. Int. J. Pharm. 2015, 495, 463–473. [Google Scholar] [CrossRef]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Soluplus micelles for acyclovir ocular delivery: Formulation and cornea and sclera permeability. Int. J. Pharm. 2018, 552, 39–47. [Google Scholar] [CrossRef]
- Plessing Rossel, C.; Sepúlveda Carreño, J.; Rodríguez-Baeza, M.; Alderete, J.B. Inclusion complex of the antiviral drug acyclovir with cyclodextrin in aqueous solution and in solid phase. Química Nova 2000, 23, 749–752. [Google Scholar] [CrossRef]
- Tavaszi, J.; Budai, P. The use of HET-CAM test in detecting the ocular irritation. Commun. Agric. Appl. Biol. Sci. 2007, 72, 137–141. [Google Scholar]
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.M.; Cairns, D. The hen’s egg chorioallantoic membrane (HET-CAM) test to predict the ophthalmic irritation potential of a cysteamine-containing gel: Quantification using Photoshop® and ImageJ. Int. J. Pharm. 2015, 490, 1–8. [Google Scholar] [CrossRef]
- Harness, J.C.; Elrub, Q.M.A.; Jung, J.O.; Koburger, T.; Assadian, O.; Dissemond, J.; Baguhl, R.; Papke, R.; Kramer, A. Irritative potency of selected wound antiseptics in the hen’s egg test on chorioallantoic membrane to predict their compatibility to wounds. Wound Rep. Reg. 2019, 27, 183–189. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Gomez-Amoza, J.L.; Martinez-Pacheco, R.; Souto, C.; Concheiro, A. Microviscosity of hydroxypropylcellulose gels as a basis for prediction of drug diffusion rates. Int. J. Pharm. 1999, 180, 91–105. [Google Scholar] [CrossRef]
- Krise, K.M.; Hwang, A.A.; Sovic, D.M.; Milosavljevic, B.H. Macro- and microscale rheological properties of poly (vinyl alcohol) aqueous solutions. J. Phys. Chem. B 2011, 115, 2759–2764. [Google Scholar] [CrossRef] [PubMed]
| Pluronic® F127 (%, w/w) | αCD (%, w/w) | βCD (%, w/w) | ACV (%, w/w) |
|---|---|---|---|
| 6.5 | 0 | 0 | 0/5 |
| 6.5 | 7 | 0 | 0/5 |
| 6.5 | 6 | 1 | 0/5 |
| 6.5 | 5 | 2 | 0/5 |
| 6.5 | 4 | 3 | 0/5 |
| 6.5 | 3 | 4 | 0/5 |
| 6.5 | 2 | 5 | 0/5 |
| 6.5 | 1 | 6 | 0/5 |
| 6.5 | 0 | 7 | 0/5 |
| Medium | ACV Apparent Solubility (mg/mL) |
|---|---|
| Buffer pH 4.9 | 27.4 (0.1) |
| PF127 2% | 18.9 (0.5) |
| PF127 3% | 19.7 (0.3) |
| PF127 4% | 18.8 (1.4) |
| PF127 6.5% | 16.7 (0.2) |
| PF127 8% | 14.8 (0.2) |
| PF127 13% | 12.6 (1.3) |
| αCD 7% | 22.5 (2.8) |
| αCD 6%/βCD 1% | 26.4 (1.1) |
| αCD 5%/βCD 2% | 24.7 (0.1) |
| αCD 4%/βCD 3% | 36.0 (0.1) |
| αCD 3%/βCD 4% | 26.7 (0.1) |
| αCD 2%/βCD 5% | 26.3 (0.1) |
| αCD 1%/βCD 6% | 25.9 (0.1) |
| βCD 7% | 21.7 (3.6) |
| Formulation | Peff (cm/s) | D (cm2/s) |
|---|---|---|
| Buffer pH 4.9 | 54.3 × 10−6 | 70.9 × 10−6 (17.8 × 10−6) |
| PF127 6.5% dispersion | 8.4 × 10−6 | 1.76 × 10−6 (0.09 × 10−6) |
| PF127 + βCD 7% | 8.6 × 10−6 | 1.77 × 10−6 (0.43 × 10−6) |
| PF127 + αCD 3%/βCD 4% | 10.7 × 10−6 | 2.95 × 10−6 (0.63 × 10−6) |
| PF127 + αCD 5%/βCD 2% | 12.5 × 10−6 | 4.24 × 10−6 (0.59 × 10−6) |
| PF127 + αCD 7% | 10.6 × 10−6 | 5.38 × 10−6 (0.13 × 10−6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Donato, C.; Iacovino, R.; Isernia, C.; Malgieri, G.; Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Polypseudorotaxanes of Pluronic® F127 with Combinations of α- and β-Cyclodextrins for Topical Formulation of Acyclovir. Nanomaterials 2020, 10, 613. https://doi.org/10.3390/nano10040613
Di Donato C, Iacovino R, Isernia C, Malgieri G, Varela-Garcia A, Concheiro A, Alvarez-Lorenzo C. Polypseudorotaxanes of Pluronic® F127 with Combinations of α- and β-Cyclodextrins for Topical Formulation of Acyclovir. Nanomaterials. 2020; 10(4):613. https://doi.org/10.3390/nano10040613
Chicago/Turabian StyleDi Donato, Cristina, Rosa Iacovino, Carla Isernia, Gaetano Malgieri, Angela Varela-Garcia, Angel Concheiro, and Carmen Alvarez-Lorenzo. 2020. "Polypseudorotaxanes of Pluronic® F127 with Combinations of α- and β-Cyclodextrins for Topical Formulation of Acyclovir" Nanomaterials 10, no. 4: 613. https://doi.org/10.3390/nano10040613
APA StyleDi Donato, C., Iacovino, R., Isernia, C., Malgieri, G., Varela-Garcia, A., Concheiro, A., & Alvarez-Lorenzo, C. (2020). Polypseudorotaxanes of Pluronic® F127 with Combinations of α- and β-Cyclodextrins for Topical Formulation of Acyclovir. Nanomaterials, 10(4), 613. https://doi.org/10.3390/nano10040613