Platinum and Iridium Oxide Co-modified TiO2 Nanotubes Array Based Photoelectrochemical Sensors for Glutathione
Abstract
1. Introduction
2. Experimental Section
2.1. Reagent and Apparatus
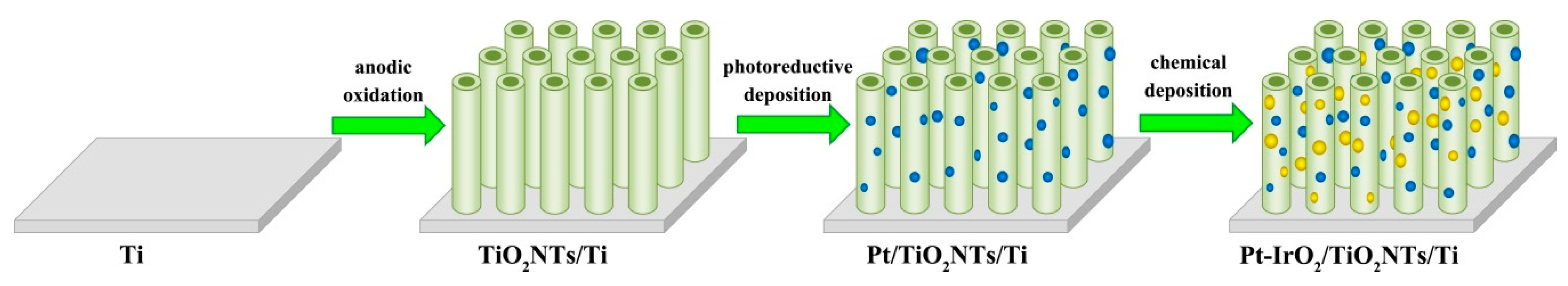
2.2. Preparation of TiO2NTs/Ti
2.3. Preparation of Pt-IrO2/TiO2NTs/Ti
2.4. Photoelectrochemical Measurement System
3. Results and Discussions
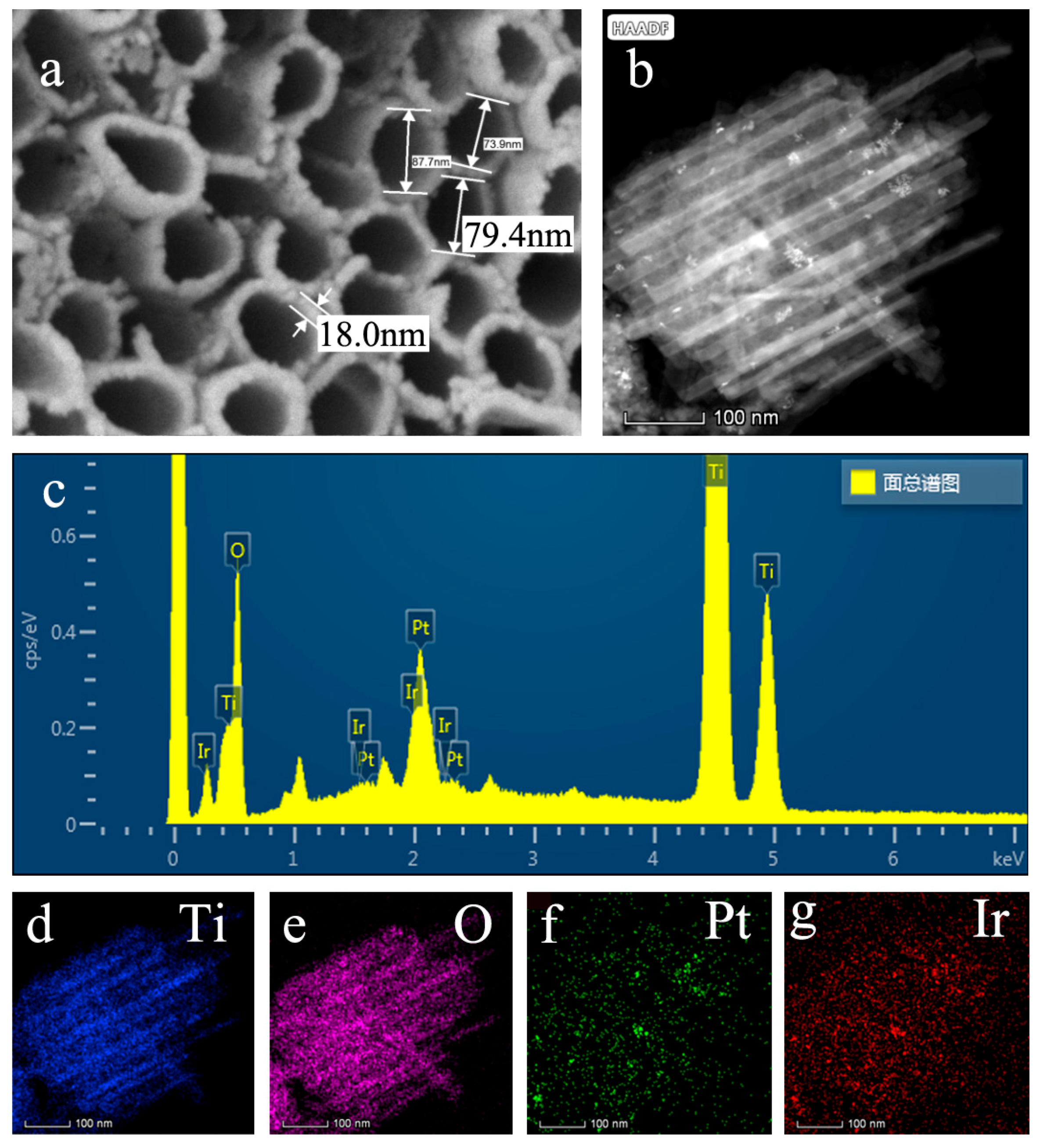
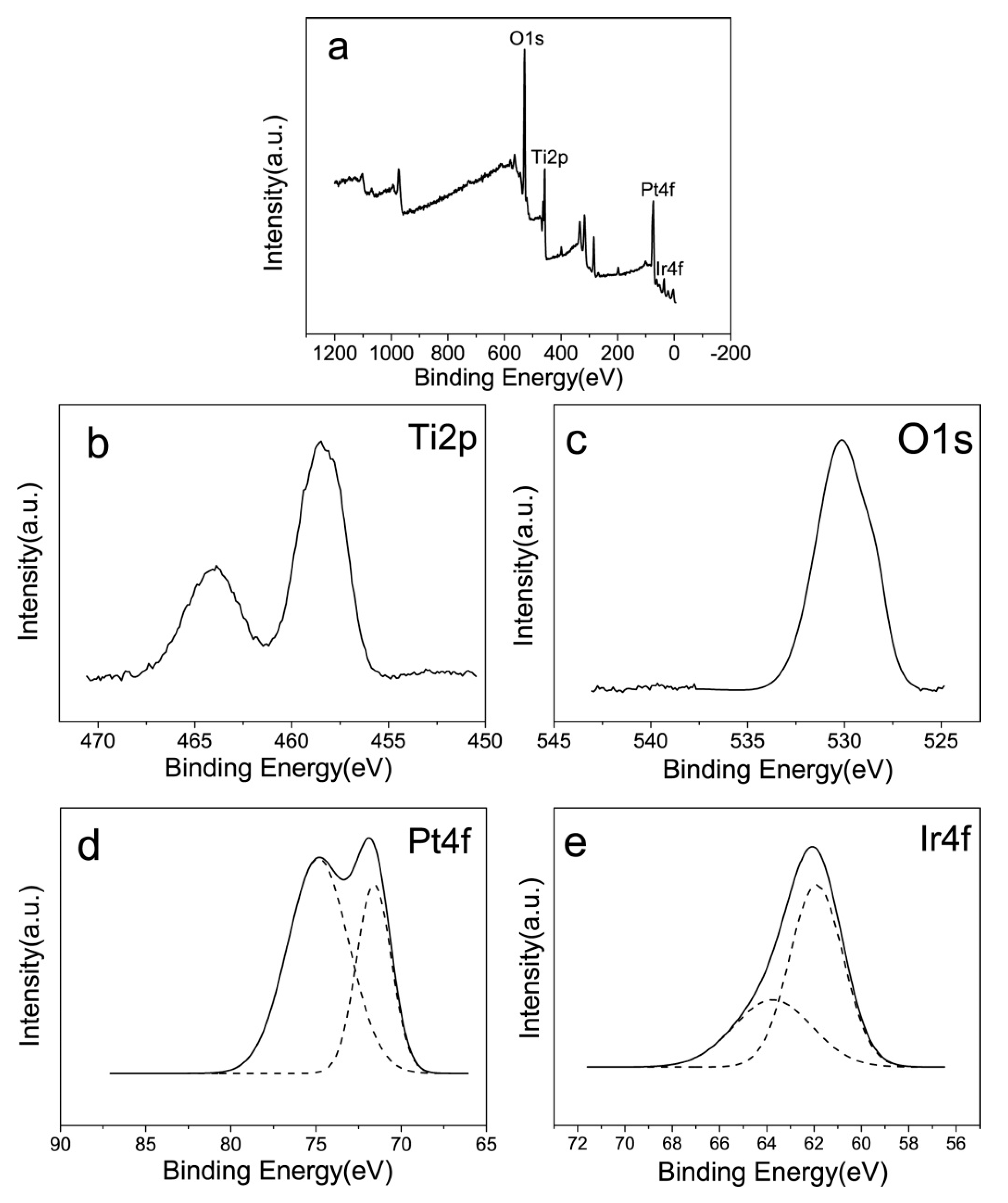
3.1. Microstructure Analysis
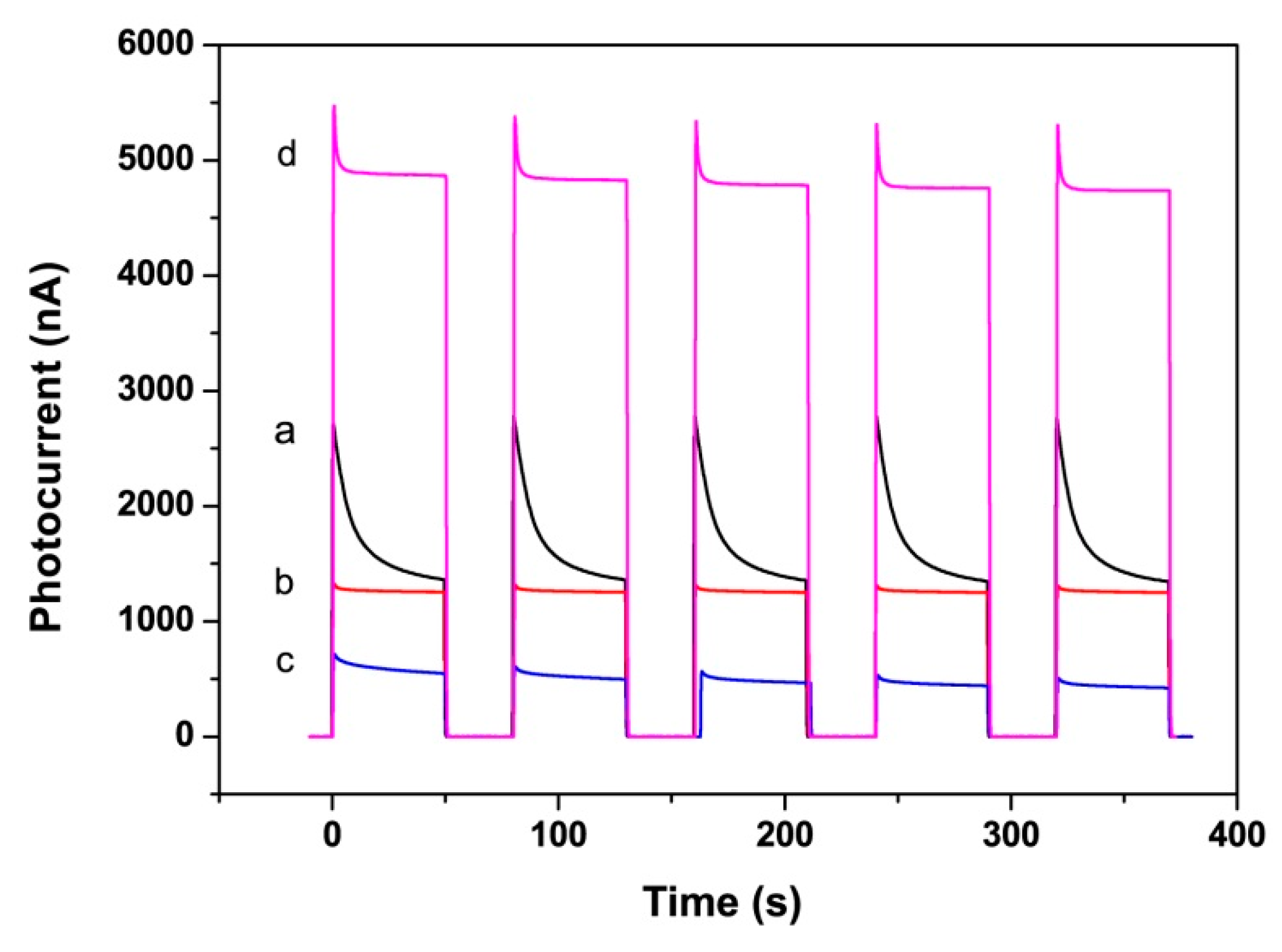
3.2. Photoelectrochemical Performance
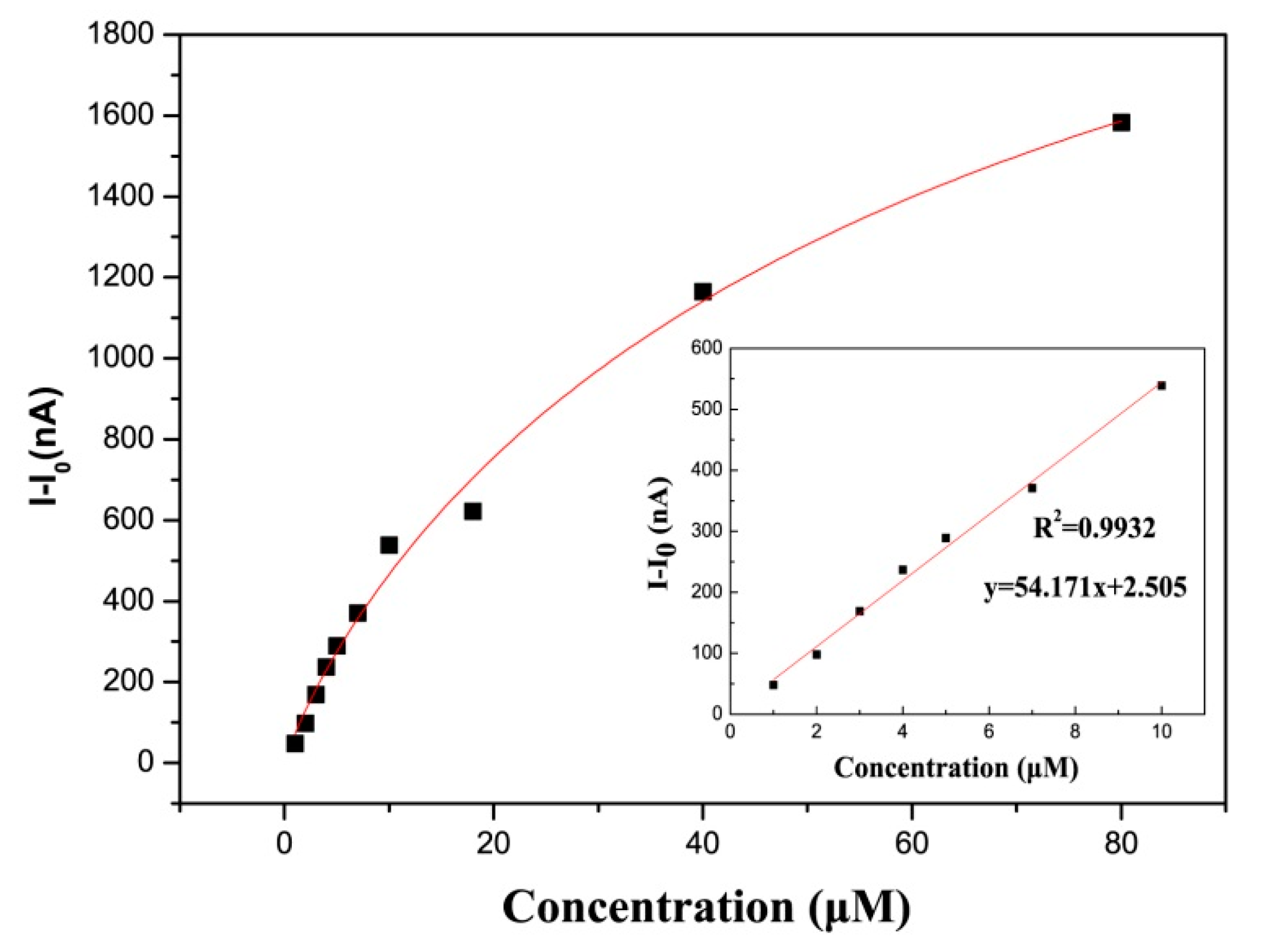
3.3. PEC biosensing Application for GSH
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Tu, L.; Zhao, S.; Liu, G.; Wang, Y.; Wang, Y.; Yue, Z. Fluorescent gold nanoclusters based photoelectrochemical sensors for detection of H2O2 and glucose. Biosens. Bioelectron. 2015, 67, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ruan, Y.F.; Zhang, L.B.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Nanochannels photoelectrochemical biosensor. Anal. Chem. 2018, 90, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.M.; Zhu, Y.C.; Liu, Y.L.; Qu, P.; Xu, M.T.; Shen, Q.; Zhao, W.W. Ferroelectric perovskite oxide@TiO2 nanorod heterostructures: Preparation, characterization, and application as a platform for photoelectrochemical bioanalysis. Anal. Chem. 2018, 90, 10803–10811. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.F.; Zhang, N.; Zhu, Y.C.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Photoelectrochemical bioanalysis platform of gold nanoparticles equipped perovskite Bi4NbO8Cl. Anal. Chem. 2017, 89, 7869–7875. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, N.; Zhao, W.W.; Jiang, D.C.; Xu, J.J.; Chen, H.Y. Polymer dots for photoelectrochemical bioanalysis. Anal. Chem. 2017, 89, 4945–4950. [Google Scholar] [CrossRef]
- Tu, L.; Liu, G.; Zhang, W.; Qin, J.; Yue, Z. Doped QDs based photoelectrochemical sensors for detection of H2O2 and Glucose. IEEE J. Sel. Top. Quantum. 2014, 20, 175–183. [Google Scholar] [CrossRef]
- Wu, S.; Song, H.; Song, J.; He, C.; Ni, J.; Zhao, Y.; Wang, X. Development of triphenylamine functional dye for selective photoelectrochemical sensing of cysteine. Anal. Chem. 2014, 86, 5922–5928. [Google Scholar] [CrossRef]
- Tee, S.Y.; Ye, E.; Pan, P.H.; Lee, C.J.; Hui, H.K.; Zhang, S.Y.; Koh, L.D.; Dong, Z.; Han, M.Y. Fabrication of bimetallic Cu/Au nanotubes and their sensitive, selective, reproducible and reusable electrochemical sensing of glucose. Nanoscale 2015, 7, 11190–11198. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Jiang, S.; Dai, H.; Sun, X.; Li, Y. Improved photoelectrochemical property of a nanocomposite NiO/CdS@ZnO photoanode for water splitting. Sol. Energy Mater. Sol. Cells 2015, 132, 40–46. [Google Scholar] [CrossRef]
- Wang, T.; Jin, B.; Jiao, Z.; Lu, G.; Ye, J.; Bi, Y. Electric field-directed growth and photoelectrochemical properties of cross-linked Au-ZnO hetero-nanowire arrays. Chem. Commun. 2015, 51, 2103–2106. [Google Scholar] [CrossRef]
- Okoth, O.K.; Yan, K.; Feng, J.; Zhang, J. Label-free photoelectrochemical aptasensing of diclofenac based on gold nanoparticles and graphene-doped CdS. Sens. Actuators B 2018, 256, 334–341. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Liu, X.; Li, X.A.; Huang, W. High-performance CdS–ZnS core–shell nanorod array photoelectrode for photoelectrochemical hydrogen generation. J. Mater. Chem. A 2015, 3, 535–541. [Google Scholar] [CrossRef]
- Zhu, J.; Huo, X.; Liu, X.; Ju, H. Gold nanoparticles deposited polyaniline-TiO2 nanotube for surface plasmon resonance enhanced photoelectrochemical biosensing. ACS Appl. Mater. Interfaces 2016, 8, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Gong, Z.; Yao, G.; Cheng, Y.; Zhang, M.; Lv, J.; Shi, S.; He, G.; Chen, X.; Sun, Z. Enhanced photocatalytic and photoelectrochemical properties of TiO2 nanorod arrays sensitized with CdS nanoplates. Ceram. Int. 2016, 42, 11716–11723. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, Y.; Wang, F.B.; Xia, X.H. Oriented self-assembled monolayer of Zn(II)-tetraphenylporphyrin on TiO2 electrode for photoelectrochemical analysis. Anal. Chem. 2019, 91, 2759–2767. [Google Scholar] [CrossRef]
- Tu, W.; Dong, Y.; Lei, J.; Ju, H. Low-potential photoelectrochemical biosensing using porphyrin-functionalized TiO2 nanoparticles. Anal. Chem. 2010, 82, 8711–8716. [Google Scholar] [CrossRef]
- Tang, J.; Kong, B.; Wang, Y.; Xu, M.; Wang, Y.; Wu, H.; Zheng, G. Photoelectrochemical detection of glutathione by IrO2-hemin-TiO2 nanowire arrays. Nano Lett. 2013, 13, 5350–5354. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.; Li, X.; Li, J. Biofunctional titania nanotubes for visible-light-activated photoelectrochemical biosensing. Anal. Chem. 2010, 82, 2253–2261. [Google Scholar] [CrossRef]
- Singh, S.C.; Swarnkar, R.K.; Gopal, R. Synthesis of Titanium Dioxide Nanomaterial by Pulsed Laser Ablation in Water. J. Nanosci. Nanotechnol. 2009, 9, 5367–5371. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Wang, L.; Han, J.; Feng, J.; Wang, X.; Su, D.; Hou, X.; Hou, J.; Liang, J.; Dou, S.X. Simultaneously efficient light absorption and charge transport of CdS/TiO2 nanotube array toward improved photoelectrochemical performance. Int. J. Hydrogen Energy 2019, 44, 30899–30909. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.; Kamat, P.V. Semiconductor-Metal composite nanostructures. To what extent do metal nanoparticles improve the photocatalytic activity of TiO2 films? J. Phys. Chem. B 2001, 105, 11439–11446. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Cao, S.-W.; Xue, C. Au/Pt nanoparticle-decorated TiO2 nanofibers with plasmon-enhanced photocatalytic activities for solar-to-fuel conversion. J. Phys. Chem. C 2013, 117, 25939–25947. [Google Scholar] [CrossRef]
- Chandrasekharan, N.; Kamat, P.V. Improving the photoelectrochemical performance of nanostructured TiO2 films by adsorption of gold nanoparticles. J. Phys. Chem. B 2000, 104, 10851–10857. [Google Scholar] [CrossRef]
- Akita, A.; Kobayashi, H.; Tada, H. Ultrathin Silicon Oxide film-induced enhancement of charge separation and transport of nanostructured Titanium(IV) Oxide photoelectrode. Chemphyschem 2019, 20, 2054–2059. [Google Scholar] [CrossRef]
- Wang, M.; Sun, L.; Lin, Z.; Cai, J.; Xie, K.; Lin, C. p–n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities. Energy Environ. Sci. 2013, 6, 1211–1220. [Google Scholar] [CrossRef]
- Ji, L.; Spanu, D.; Denisov, N.; Recchia, S.; Schmuki, P.; Altomare, M. A dewetted-dealloyed nanoporous Pt co-catalyst formed on TiO2 nanotube arrays leads to strongly enhanced photocatalytic H2 production. Chem. Asian J. 2019. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Li, X.Z.; Li, F.B. Study of Au/Au3+-TiO2 photocatalysts toward visible photooxidation for water and waste water treatment. Environ. Sci. Technol. 2001, 35, 2381–2387. [Google Scholar] [CrossRef]
- Guo, Y.; He, J.; Wu, S.; Wang, T.; Li, G.; Hu, Y.; Xue, H.; Sun, X.; Tang, J.; Liu, M. Effects of platinum on photo-assisted electrocatalytic activity of fringe-shaped highly ordered mesoporous titanium dioxide film. J. Power Sources 2012, 208, 58–66. [Google Scholar] [CrossRef]
- Meekins, B.H.; Kamat, P.V. Role of water oxidation catalyst IrO2 in shuttling photogenerated holes across TiO2 interface. J. Phys. Chem. Lett. 2011, 2, 2304–2310. [Google Scholar] [CrossRef]
- Kamat, P.V. Manipulation of charge transfer across semiconductor interface. A criterion that cannot be ignored in photocatalyst design. J. Phys. Chem. Lett. 2012, 3, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, W.; Wang, Q.; Guo, Z.; Yuan, W. Spatial separation of Pt and IrO2 cocatalysts on SiC surface for enhanced photocatalysis. Mater. Lett. 2017, 201, 114–117. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Chen, Y.; Wang, C.; Jiang, J.; Dong, J.; Yan, H.; Du, X. Dual enhanced electrochemiluminescence of aminated Au@SiO2/CdS quantum dot superstructures: Electromagnetic field enhancement and chemical enhancement. ACS Appl. Mater. Interfaces 2019, 11, 4488–4499. [Google Scholar] [CrossRef] [PubMed]
- Sui, M.; Zhao, Y.; Ni, Z.; Gu, X. Photoelectrochemical performance and biosensor application for glutathione (GSH) of W-doped BiVO4 thin films. J. Mater. Sci. Mater. Electron 2018, 29, 10109–10116. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, Y.; Zhao, S.; Zhang, P.; Zhang, Y.; Bi, J.; Liu, G.; Yue, Z. Carbon dots based photoelectrochemical sensors for ultrasensitive detection of glutathione and its applications in probing of myocardial infarction. Biosens. Bioelectron. 2018, 99, 251–258. [Google Scholar] [CrossRef]
- Prakasam, H.E.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. A new benchmark for TiO2 nanotube array growth by anodization. J. Phys. Chem. C 2007, 111, 7235–7241. [Google Scholar] [CrossRef]
- Li, W.; Kuang, D.; Gu, P.; Xiang, M. Partially disordered TiO2 nanotube photonic crystal: Randomness characterization and tuning of reflected scattering light. Nanotechnology 2020, 31, 025711. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y. Electrochemically self-doped TiO2 nanotube arrays for supercapacitors. J. Phys. Chem. C 2014, 118, 5626–5636. [Google Scholar] [CrossRef]
- Zhang, R.; Ren, X.; Shi, X.; Xie, F.; Zheng, B.; Guo, X.; Sun, X. Enabling Effective Electrocatalytic N2 Conversion to NH3 by the TiO2 Nanosheets Array under Ambient Conditions. ACS Appl. Mater. Interfaces 2018, 10, 28251–28255. [Google Scholar] [CrossRef]
- Abb, M.J.S.; Weber, T.; Glatthaar, L.; Over, H. Growth of ultrathin single-crystalline IrO2(110) films on a TiO2(110) single crystal. Langmuir 2019, 35, 7720–7726. [Google Scholar] [CrossRef] [PubMed]
- Vovk, E.I.; Kalinkin, A.V.; Smirnov, M.Y.; Klembovskii, I.O.; Bukhtiyarov, V.I. XPS study of stability and reactivity of oxidized Pt nanoparticles supported on TiO2. J. Phys. Chem. C 2017, 121, 17297–17304. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, M.; Yang, Y.; Xu, K.; Luo, X.; Yu, T.; Zhang, W.; Liu, W.; Yuan, C. Highly dispersed Pt nanoparticles on hierarchical titania nanoflowers with {010} facets for gas sensing and photocatalysis. J. Mater. Sci. 2019, 54, 6826–6840. [Google Scholar] [CrossRef]
- Chung, W.-H.; Tsai, D.-S.; Fan, L.-J.; Yang, Y.-W.; Huang, Y.-S. Surface oxides of Ir(111) prepared by gas-phase oxygen atoms. Surf. Sci. 2012, 606, 1965–1971. [Google Scholar] [CrossRef]
- Chakrapani, K.; Sampath, S. The dual role of borohydride depending on reaction temperature: Synthesis of iridium and iridium oxide. Chem. Commun. 2015, 51, 9690–9693. [Google Scholar] [CrossRef]
- Wang, W.N.; An, W.J.; Ramalingam, B.; Mukherjee, S.; Niedzwiedzki, D.M.; Gangopadhyay, S.; Biswas, P. Size and structure matter: Enhanced CO2 photoreduction efficiency by size-resolved ultrafine Pt nanoparticles on TiO2 single crystals. J. Am. Chem. Soc. 2012, 134, 11276–11281. [Google Scholar] [CrossRef]
- Swierk, J.R.; McCool, N.S.; Saunders, T.P.; Barber, G.D.; Strayer, M.E.; Vargas-Barbosa, N.M.; Mallouk, T.E. Photovoltage Effects of Sintered IrO2 Nanoparticle Catalysts in Water-Splitting Dye-Sensitized Photoelectrochemical Cells. J. Phys. Chem. C 2014, 118, 17046–17053. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Xue, Y.; Fang, H.; Wang, W. Facile electrodeposition of environment-friendly Cu2O/ZnO heterojunction for robust photoelectrochemical biosensing. Sens. Actuators B 2014, 191, 619–624. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, S.; Shen, Q.; Jiang, L.P.; Zhu, J.J. Fabrication of glutathione photoelectrochemical biosensor using graphene-CdS nanocomposites. Analyst 2012, 137, 3697–3703. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Gu, Y.; Yan, X.; Bai, Z.; Liu, Y.; Liu, S.; Zhang, X.; Zhang, Z.; Zhang, X.; Zhang, Y. Enhanced photoelectrochemical property of ZnO nanorods array synthesized on reduced graphene oxide for self-powered biosensing application. Biosens. Bioelectron. 2015, 64, 499–504. [Google Scholar] [CrossRef] [PubMed]


| GSH Biosensor | Linear Range (μM) | Detection Limit (μM) | Ref. | |
|---|---|---|---|---|
| Non-enzymatic sensor | Pt-IrO2/TiO2NTs/Ti | 1–10 | 0.8 | This work |
| Cu2O/ZnO | 1–10 and 20–100 | 0.8 | [48] | |
| GR-CdS/ITO | 10–1500 | 3 | [49] | |
| rGO/ZnO | 10–200 | 2.17 | [50] | |
| Porphyrin-Functionalized TiO2-ITO | 50–2400 | 30 | [16] | |
| Enzymatic sensor | IrO2-Hemin-TiO2 nanowire arrays | 0.01–10 | 0.01 | [17] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Zhao, P.; Zhang, S.; Huo, G.; Suo, Z.; Yue, Z.; Zhang, S.; Huang, W.; Zhu, B. Platinum and Iridium Oxide Co-modified TiO2 Nanotubes Array Based Photoelectrochemical Sensors for Glutathione. Nanomaterials 2020, 10, 522. https://doi.org/10.3390/nano10030522
Tian J, Zhao P, Zhang S, Huo G, Suo Z, Yue Z, Zhang S, Huang W, Zhu B. Platinum and Iridium Oxide Co-modified TiO2 Nanotubes Array Based Photoelectrochemical Sensors for Glutathione. Nanomaterials. 2020; 10(3):522. https://doi.org/10.3390/nano10030522
Chicago/Turabian StyleTian, Jing, Peng Zhao, Shasha Zhang, Guona Huo, Zhaochen Suo, Zhao Yue, Shoumin Zhang, Weiping Huang, and Baolin Zhu. 2020. "Platinum and Iridium Oxide Co-modified TiO2 Nanotubes Array Based Photoelectrochemical Sensors for Glutathione" Nanomaterials 10, no. 3: 522. https://doi.org/10.3390/nano10030522
APA StyleTian, J., Zhao, P., Zhang, S., Huo, G., Suo, Z., Yue, Z., Zhang, S., Huang, W., & Zhu, B. (2020). Platinum and Iridium Oxide Co-modified TiO2 Nanotubes Array Based Photoelectrochemical Sensors for Glutathione. Nanomaterials, 10(3), 522. https://doi.org/10.3390/nano10030522




