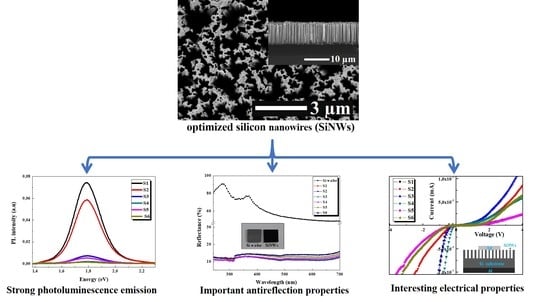
Elucidating the Effect of Etching Time Key-Parameter toward Optically and Electrically-Active Silicon Nanowires
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Samples Preparation
2.3. Characterizations
3. Results and Discussion
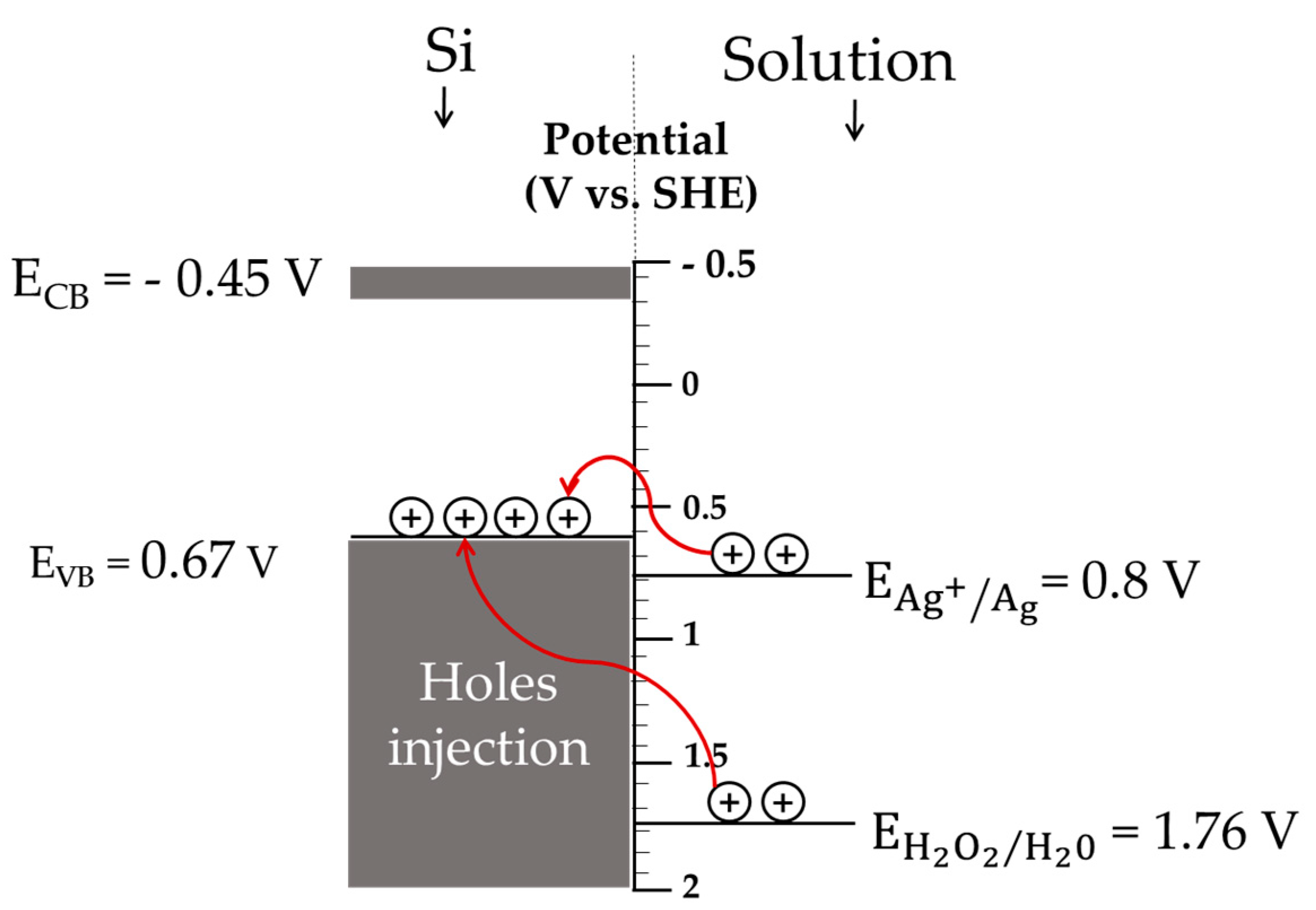
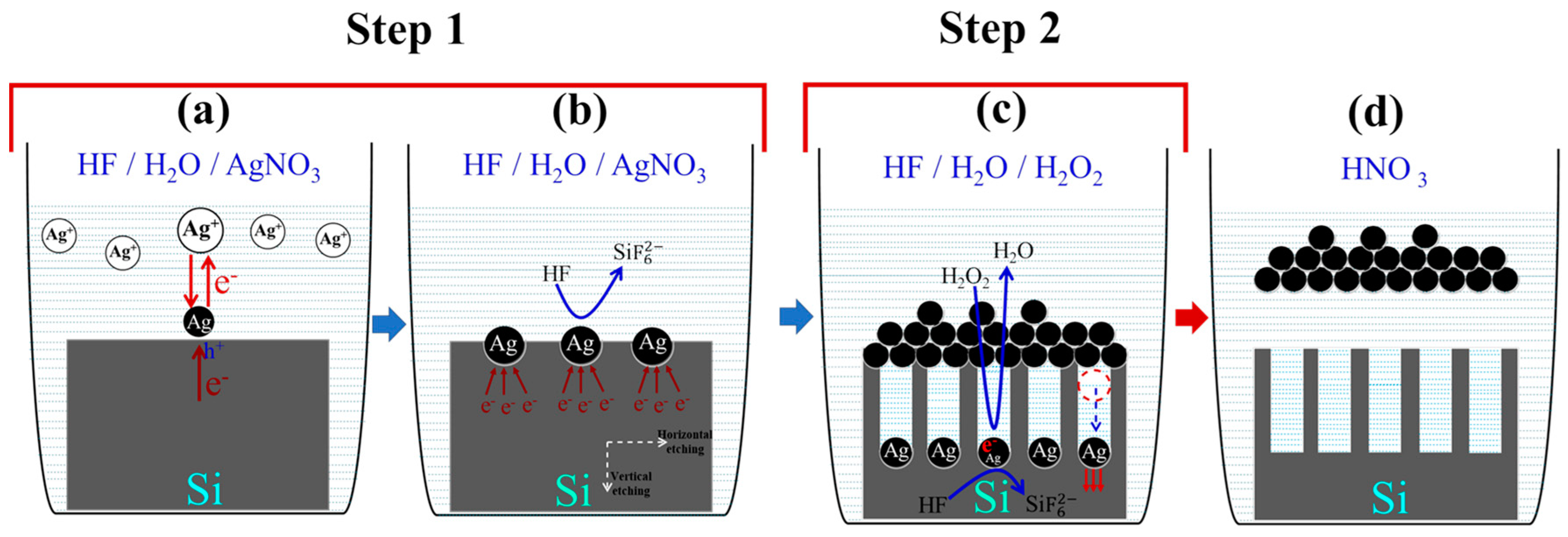
3.1. Detailed Mechanism of SiNWs’ Formation
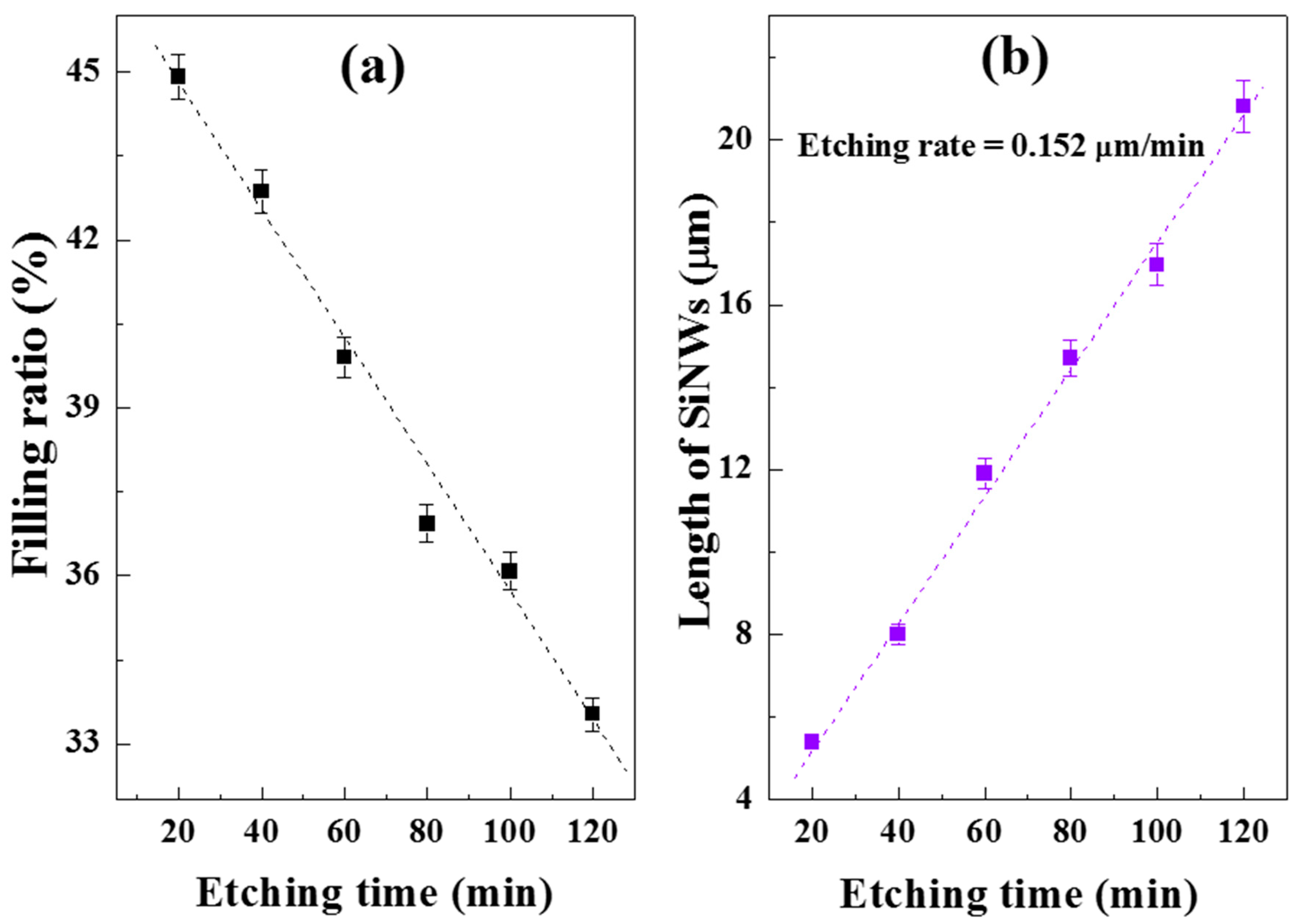
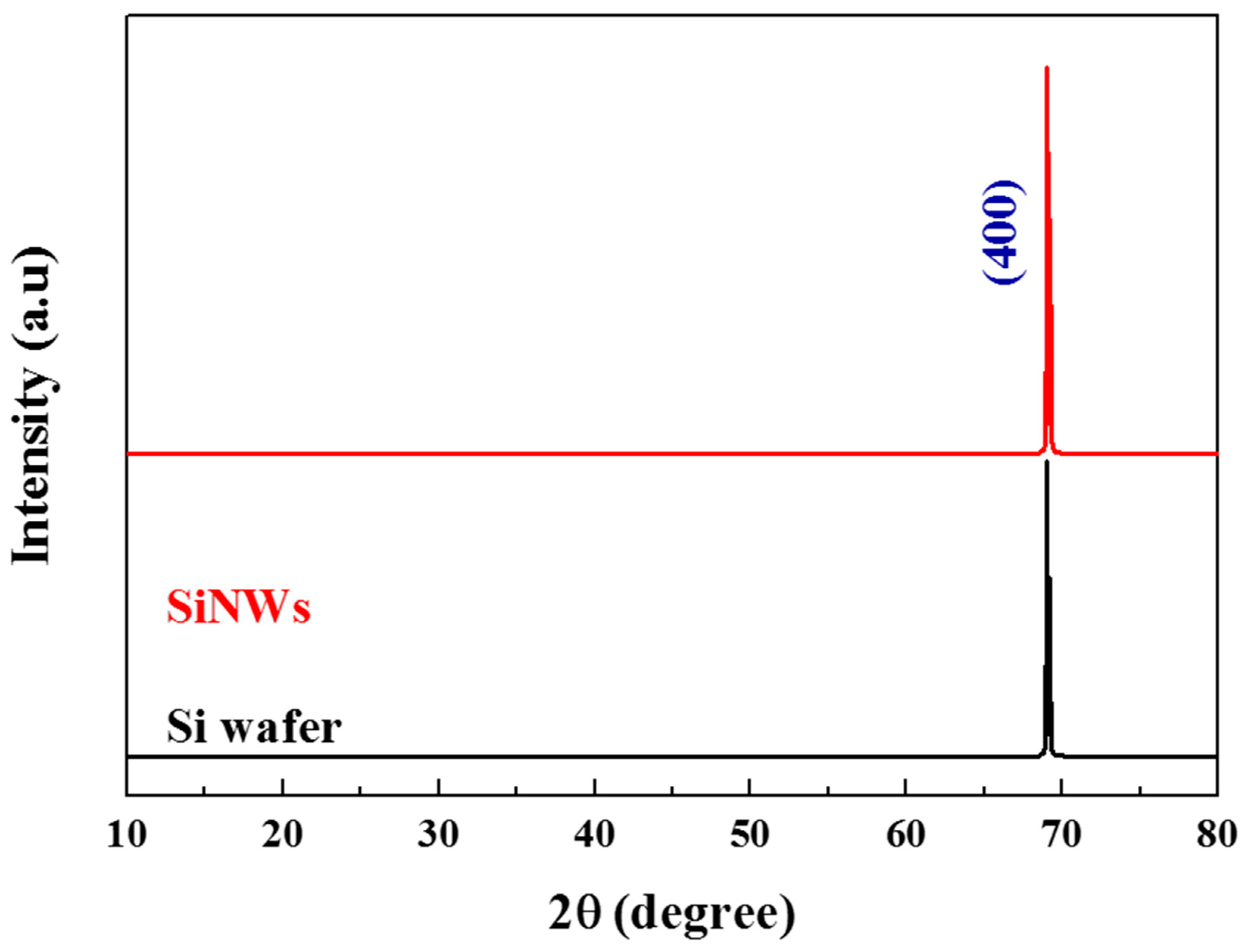
3.2. Etching Time Effect on Morphology of SiNW Arrays
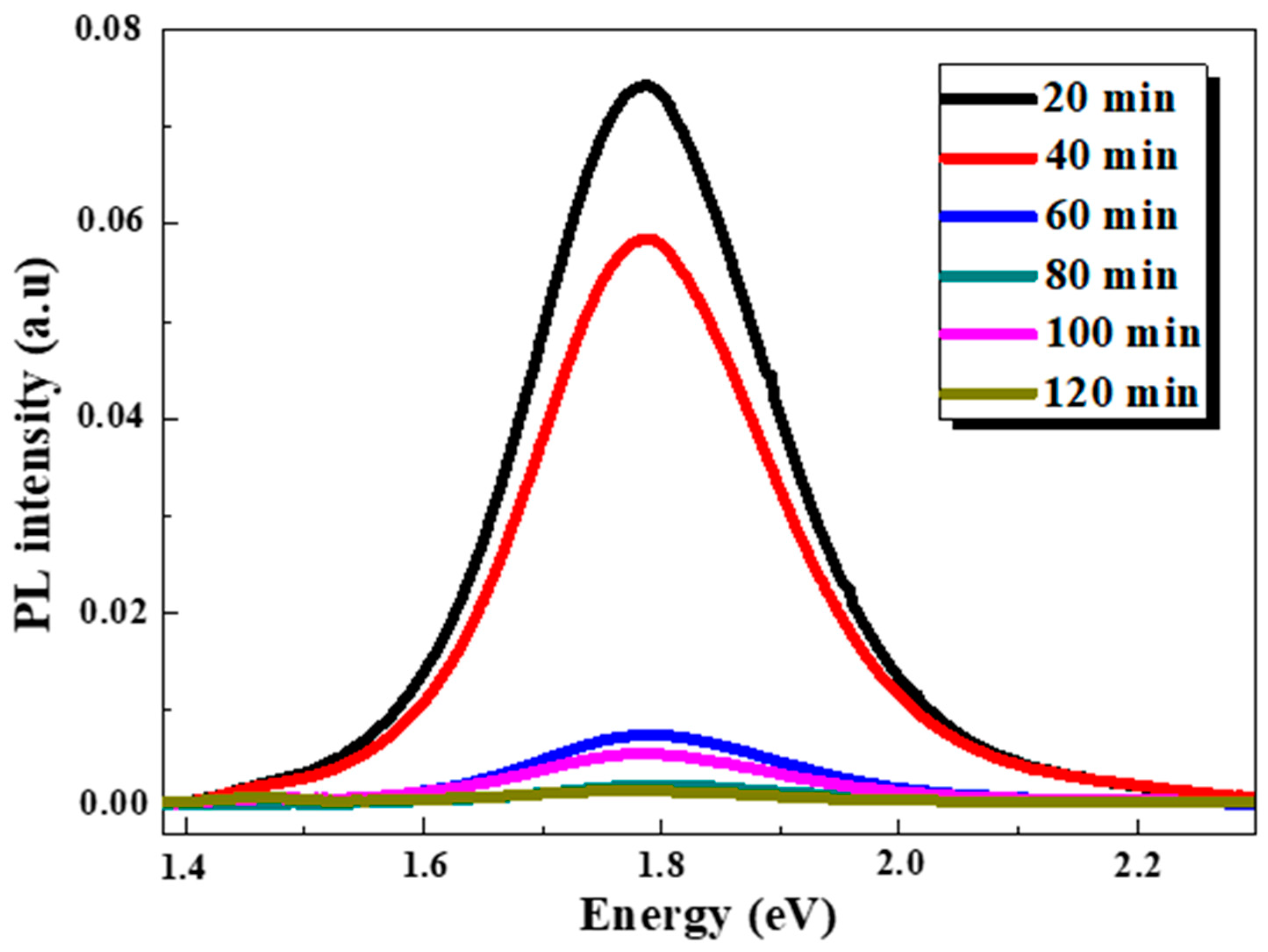
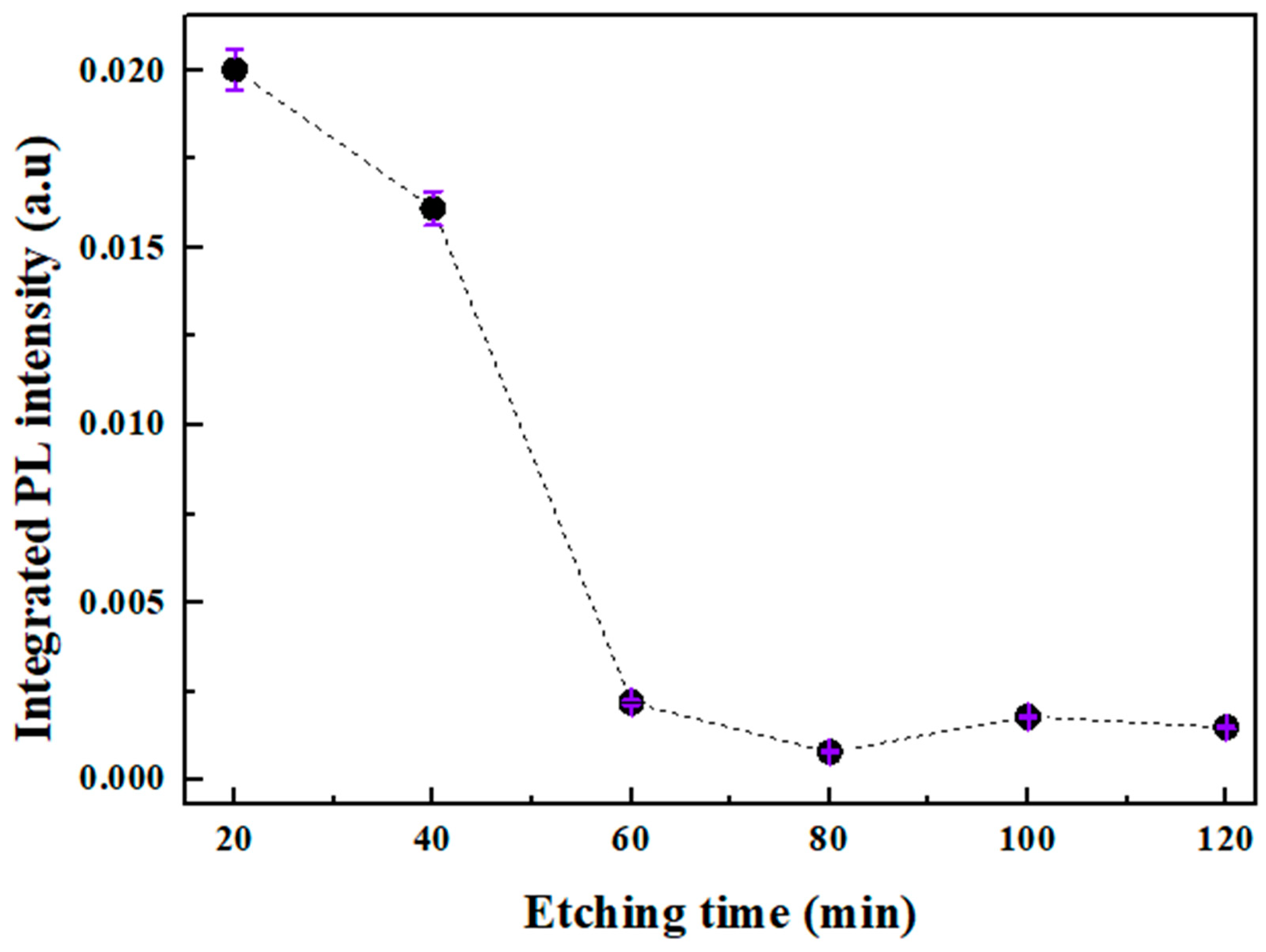
3.3. Photoluminescence Spectroscopy
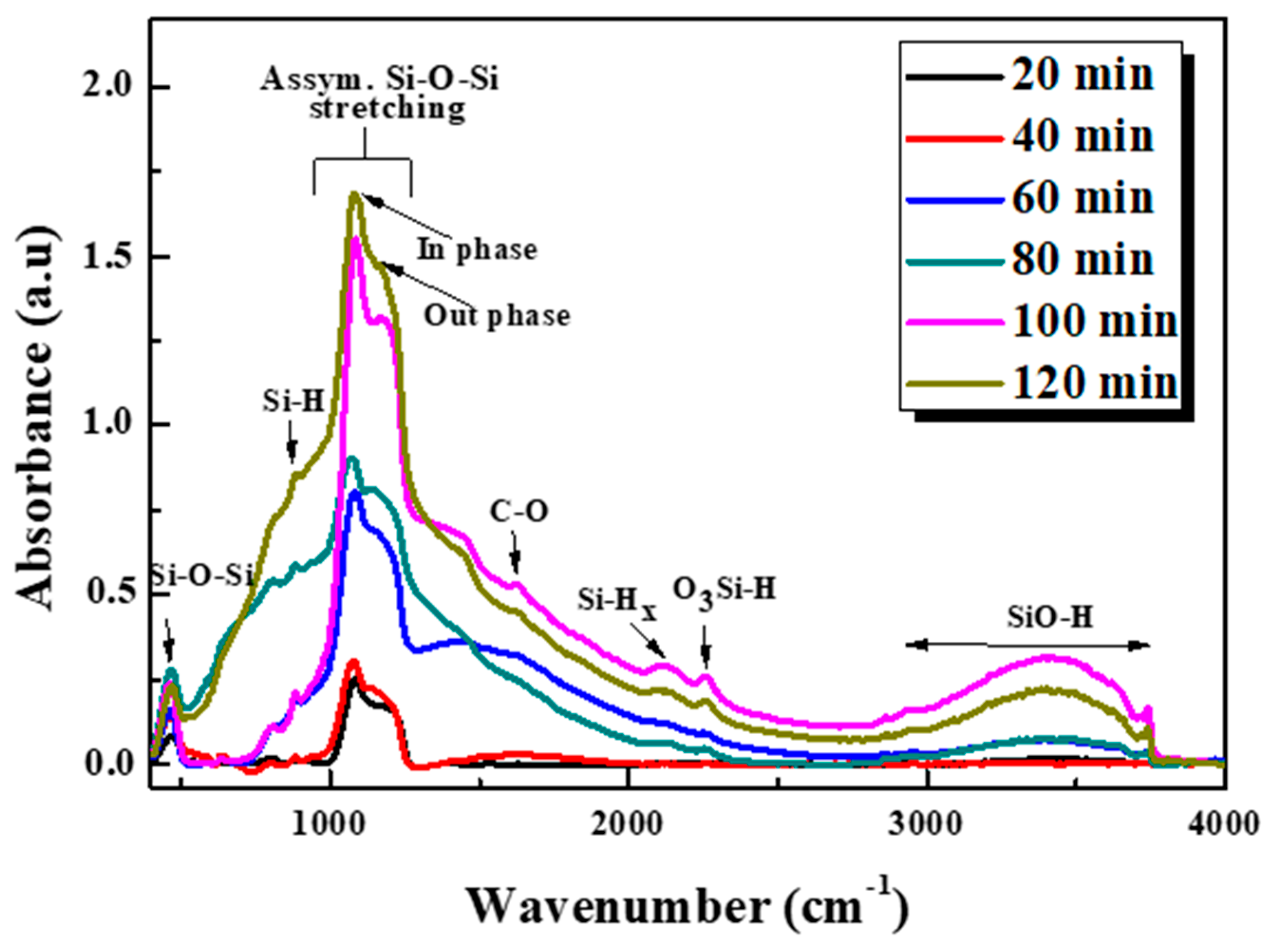
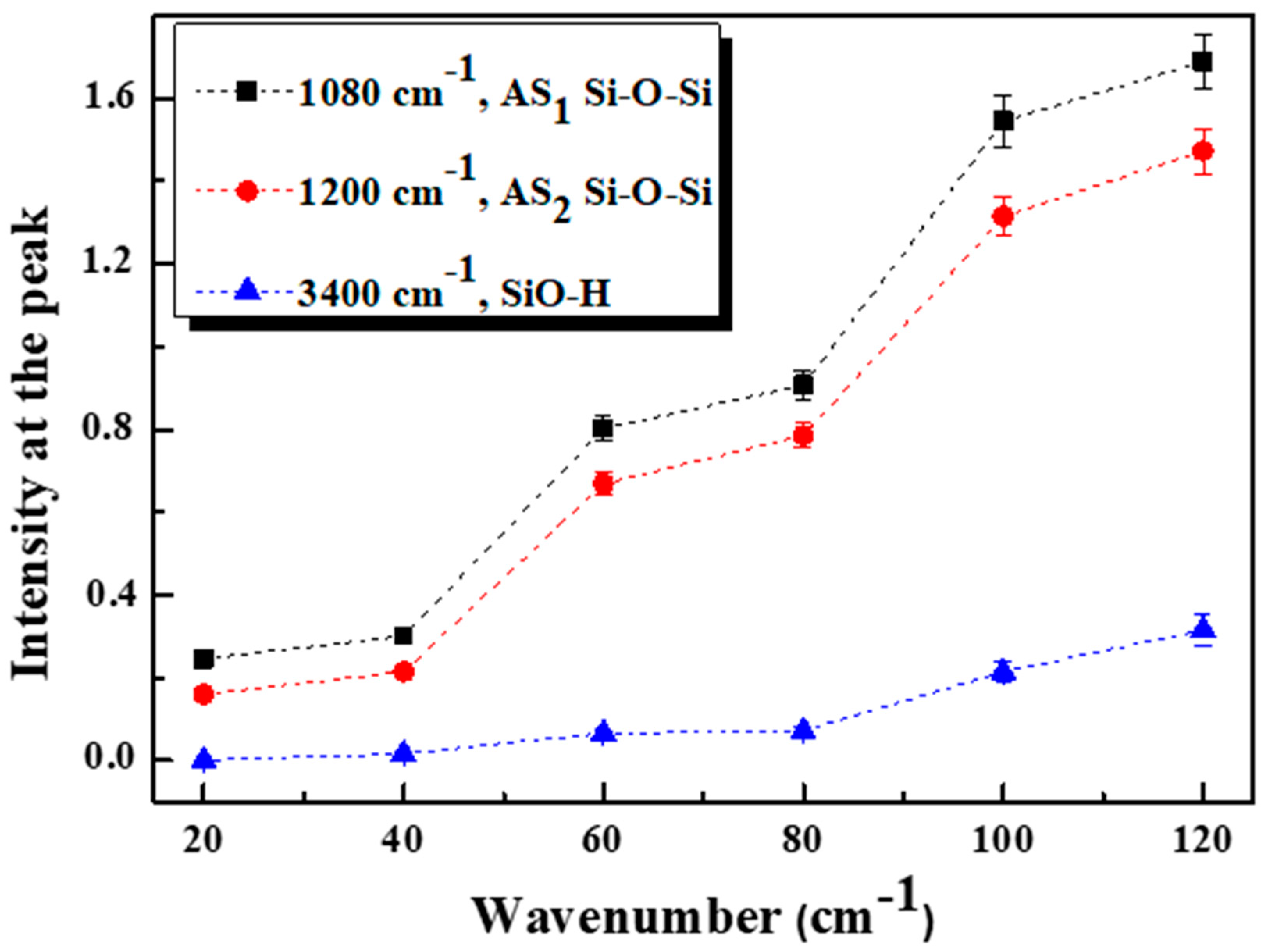
3.4. FTIR Analysis
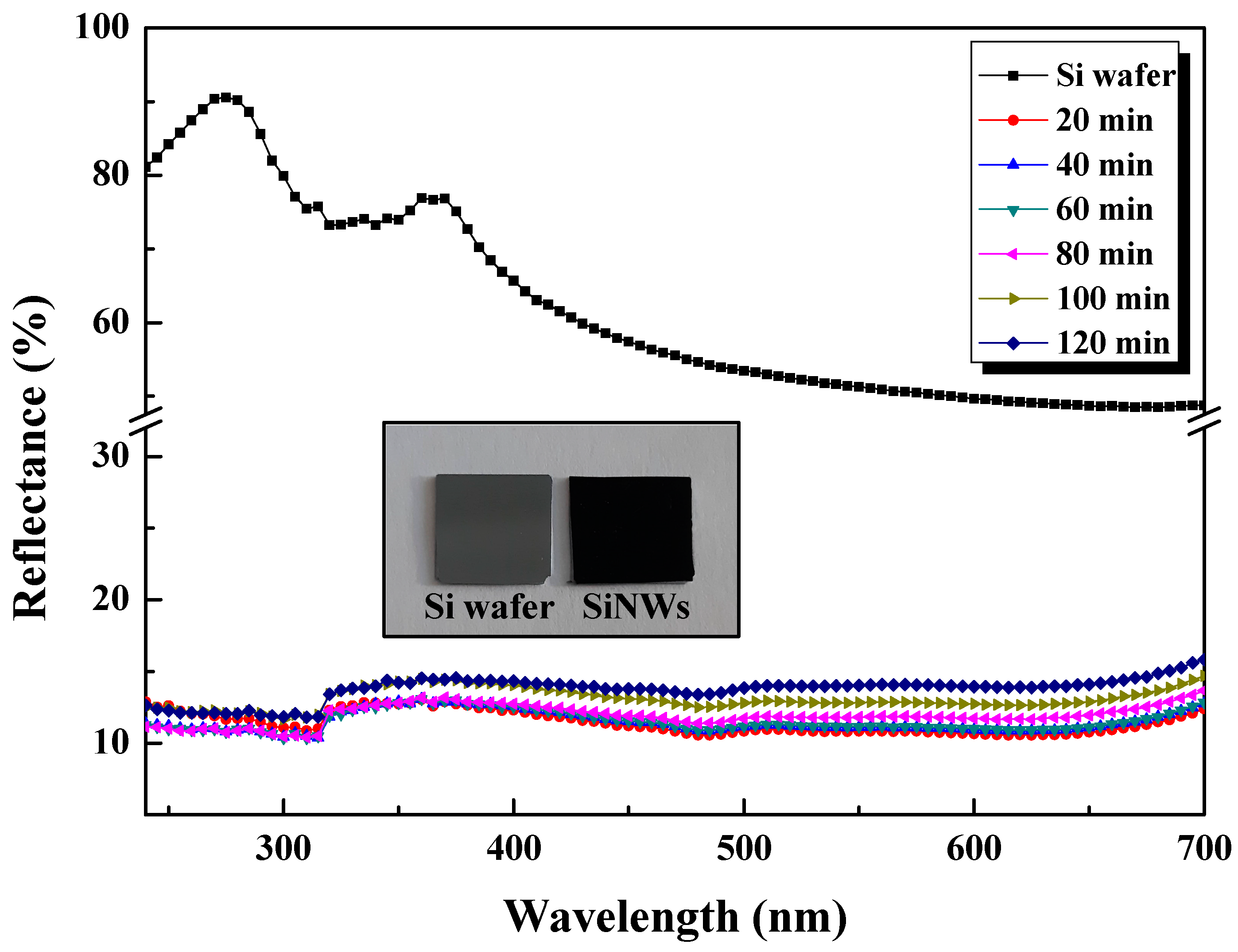
3.5. Reflectance Analysis
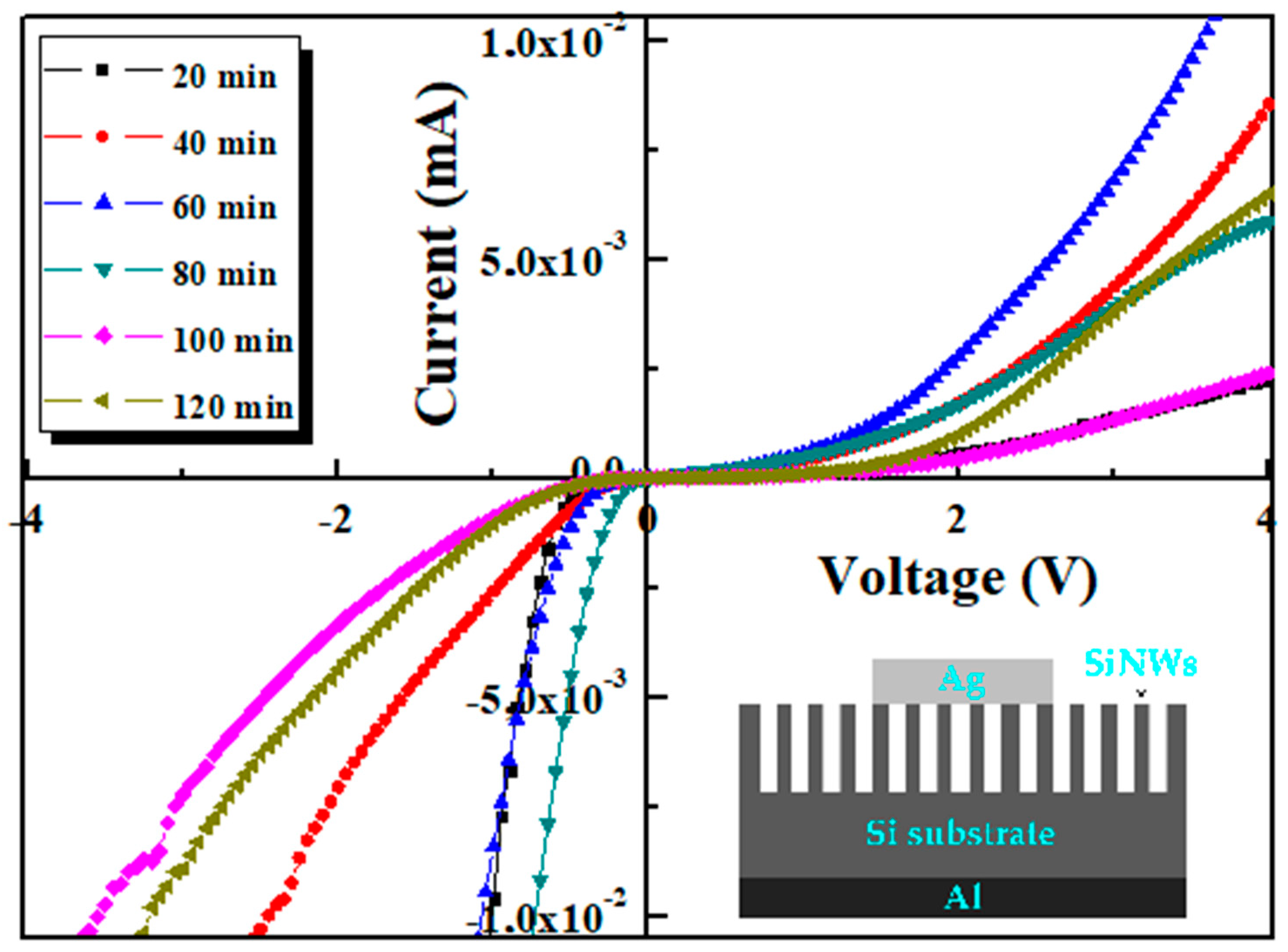
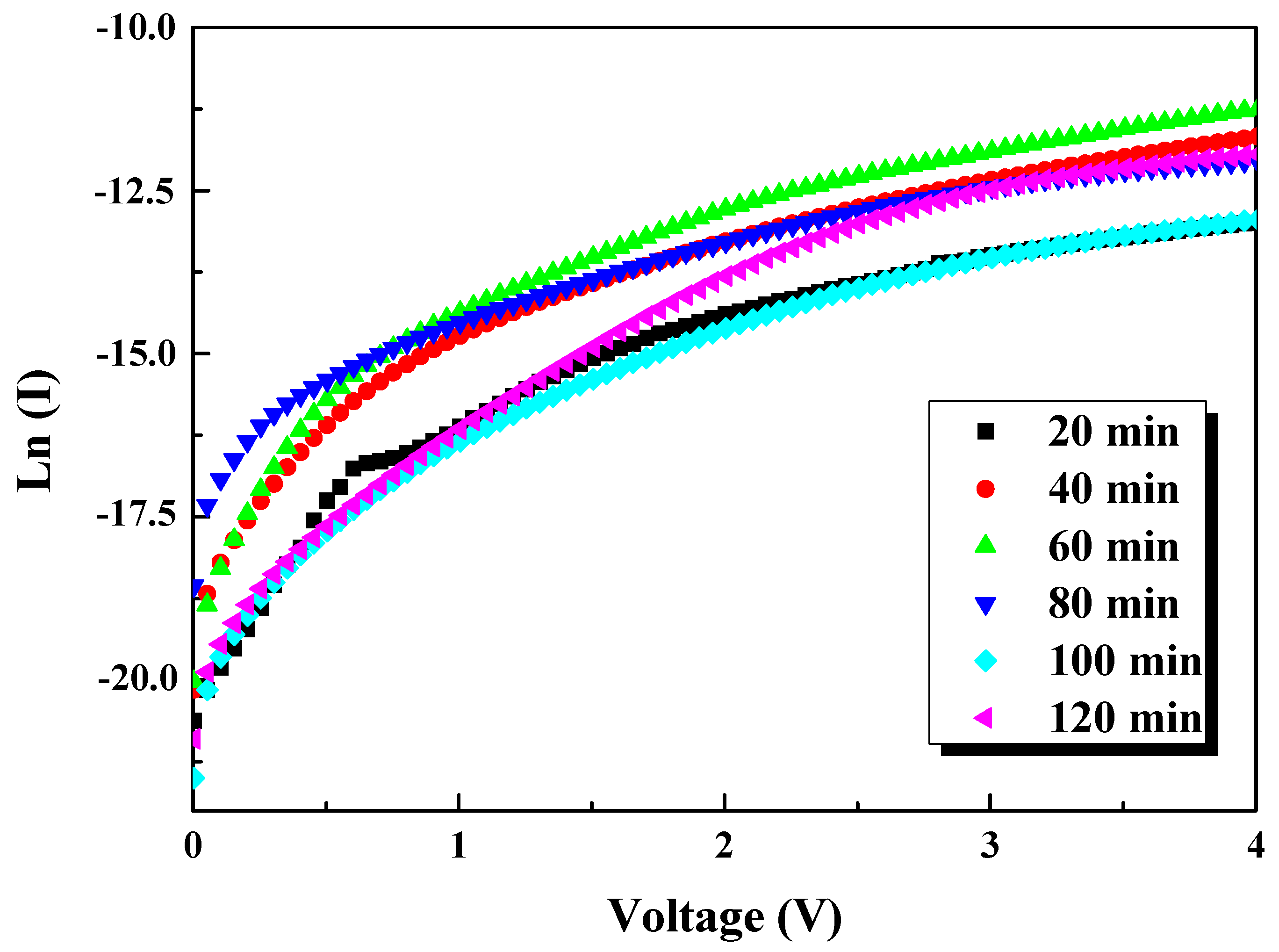
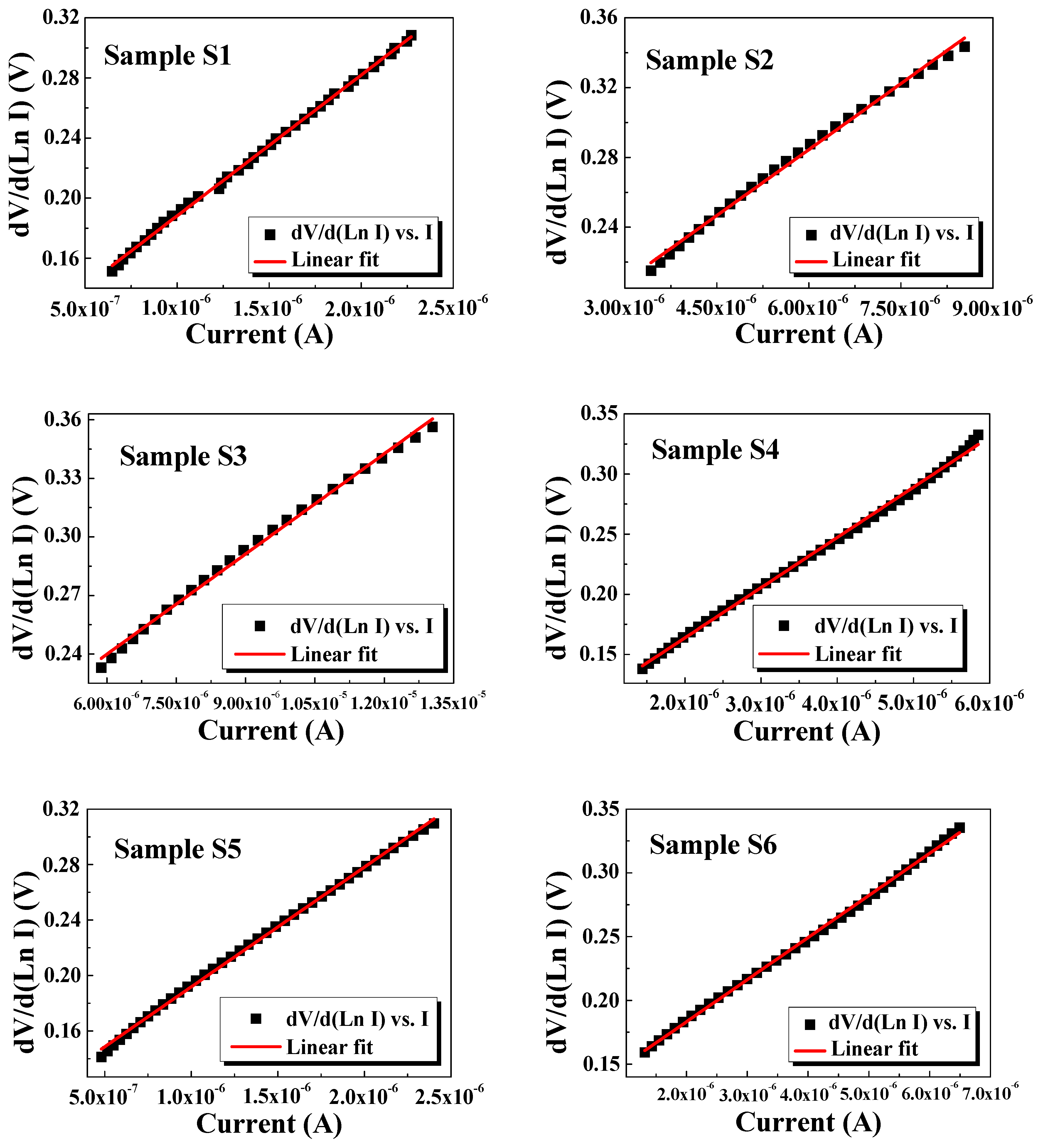
3.6. I–V Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sahoo, M.K.; Kale, P. Integration of silicon nanowires in solar cell structure for efficiency enhancement: A review. J. Mater. 2019, 5, 34–48. [Google Scholar] [CrossRef]
- Cong, L.T.; Ngoc Lam, N.T.; Giang, N.T.; Kien, P.T.; Dung, N.D.; Ha, N.N. N-type silicon nanowires prepared by silver metal-assisted chemical etching: Fabrication and optical properties. Mater. Sci. Semicond. Process. 2019, 90, 198–204. [Google Scholar] [CrossRef]
- Ashrafabadi, S.; Eshghi, H. Single-crystalline Si nanowires fabrication by one-step metal assisted chemical etching: The effect of etching time and resistivity of Si wafer. Superlattices Microstruct. 2018, 120, 517–524. [Google Scholar] [CrossRef]
- Lajvardi, M.; Eshghi, H.; Izadifard, M.; Ghazi, M.E.; Goodarzi, A. Effects of silver and gold catalytic activities on the structural and optical properties of silicon nanowires. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 75, 136–143. [Google Scholar] [CrossRef]
- Amri, C.; Ouertani, R.; Hamdi, A.; Chtourou, R.; Ezzaouia, H. Effect of porous layer engineered with acid vapor etching on optical properties of solid silicon nanowire arrays. Mater. Des. 2016, 111, 394–404. [Google Scholar] [CrossRef]
- Baek, S.-H.; Park, J.-S.; Jeong, Y.-M.; Kim, J.H. Facile synthesis of Ag-coated silicon nanowires as anode materials for high-performance rechargeable lithium battery. J. Alloy. Compd. 2016, 660, 387–391. [Google Scholar] [CrossRef]
- Schwartz, M.; Nguyen, T.C.; Vu, X.T.; Wagner, P.; Thoelen, R.; Ingebrandt, S. Impedimetric Sensing of DNA with Silicon Nanowire Transistors as Alternative Transducer Principle. Phys. Status Solidi A 2018, 215, 1700740. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kang, S.Y.; Choi, S.-W.; Kwon, Y.J.; Choi, M.S.; Bang, J.H.; Kim, S.B.; Kim, H.W. Fabrication and gas sensing properties of vertically aligned Si nanowires. Appl. Surf. Sci. 2018, 427, 215–226. [Google Scholar] [CrossRef]
- Borgne, B.L.; Salaün, A.C.; Pichon, L. Electrical properties of self-aligned gate-allaround polycrystalline silicon nanowires field-effect transistors. Microelectron. Eng. 2016, 150, 32–38. [Google Scholar] [CrossRef]
- Amdouni, S.; Cherifi, Y.; Coffinier, Y.; Addad, A.; Zaïbi, M.A.; Oueslati, M.; Boukherroub, R. Gold nanoparticles coated silicon nanowires for efficient catalytic and photocatalytic applications. Mater. Sci. Semicond. Process. 2018, 75, 206–213. [Google Scholar] [CrossRef]
- Yu, P.; Wu, J.; Liu, S.; Xiong, J.; Jagadish, C.; Wang, Z.M. Design and fabrication of silicon nanowires towards efficient solar cells. Nanotoday 2016, 11, 704–737. [Google Scholar] [CrossRef]
- Latu-Romain, L.; Mouchet, C.; Cayron, C.; Rouviere, E.; Simonato, J.P. Growth parameters and shape specific synthesis of silicon nanowires by the VLS method. J. Nanopart. Res. 2008, 10, 1287–1291. [Google Scholar] [CrossRef]
- Pan, H.; Lim, S.; Poh, C.; Sun, H.; Wu, X.; Feng, Y.; Lin, J. Growth of Si nanowires by thermal evaporation. Nanotechnology 2005, 16, 417–421. [Google Scholar] [CrossRef]
- Fuhrmann, B.; Leipner, H.S.; Hoche, H.-R. Ordered arrays of silicon nanowires produced by nanosphere lithography and molecular beam epitaxy. Nano Lett. 2005, 5, 2524–2527. [Google Scholar] [CrossRef] [PubMed]
- Kokai, F.; Inoue, S.; Hidaka, H.; Uchiyama, K.; Takahashi, Y.; Koshio, A. Catalyst-free growth of amorphous silicon nanowires by laser ablation. Appl. Phys. 2013, 112, 1–7. [Google Scholar] [CrossRef]
- Hamdana, G.; Südkamp, T.; Descoins, M.; Mangelinck, D.; Caccamo, L.; Bertke, M.; Wasisto, H.S.; Bracht, H.; Peiner, E. Towards fabrication of 3D isotopically modulated vertical silicon nanowires in selective areas by nanosphere lithography. Microelectron. Eng. 2017, 179, 74–82. [Google Scholar] [CrossRef]
- Lajvardi, M.; Eshghi, H.; Ghazi, M.E.; Izadifard, M.; Goodarzi, A. Structural and optical properties of silicon nanowires synthesized by Ag-assisted chemical etching. Mater. Sci. Semicond. Process. 2015, 40, 556–563. [Google Scholar] [CrossRef]
- Vinzons, L.U.; Shu, L.; Yip, S.; Wong, C.-Y.; Chan, L.L.H.; Ho, J.C. Unraveling the Morphological Evolution and Etching Kinetics of Porous Silicon Nanowires During Metal-Assisted Chemical Etching. Nanoscale Res. Lett. 2017, 12, 1872. [Google Scholar] [CrossRef]
- Behera, A.K.; Viswanath, R.N.; Lakshmanan, C.; Madapu, K.K.; Kamruddin, M.; Mathews, T. Synthesis, microstructure and visible luminescence properties of vertically aligned lightly doped porous silicon nanowalls. Microporous Mesoporous Mater. 2019, 273, 99–106. [Google Scholar] [CrossRef]
- Ghosh, R.; Pal, A.; Giri, P.K. Quantitative analysis of the phonon confinement effect in arbitrarily shaped Si nanocrystals decorated on Si nanowires and its correlation with the photoluminescence spectrum. J. Raman Spectrosc. 2015, 7, 624–631. [Google Scholar] [CrossRef]
- Leontis, I.; Othonos, A.; Nassiopoulou, A.G. Structure, morphology, and photoluminescence of porous Si nanowires: Effect of different chemical treatments. Nanoscale Res. Lett. 2013, 1, 383. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, Z.; Zhang, M.; Wang, X.; Ji, A.; Yang, F. Electron transport characteristics of silicon nanowires by metal-assisted chemical etching. AIP Adv. 2014, 3, 031307. [Google Scholar] [CrossRef]
- Nafie, N.; Lachiheb, M.; Bouaicha, M. Effect of etching time on morphological, optical, and electronic properties of silicon nanowires. Nanoscale Res. Lett. 2012, 1, 393. [Google Scholar] [CrossRef]
- Hutagalung, S.D.; Fadhali, M.M.; Areshi, R.A.; Tan, F.D. Optical and Electrical Characteristics of Silicon Nanowires Prepared by Electroless Etching. Nanoscale Res. Lett. 2017, 12, 425. [Google Scholar] [CrossRef]
- Hasan, M.; Huq, M.F.; Mahmood, Z.H. A review on electronic and optical properties of silicon nanowire and its different growth techniques. SpringerPlus 2013, 2, 151. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Werner, P.; de Boor, J.; Gösele, U. Metal-Assisted Chemical Etching of Silicon: A Review. Adv. Mater. 2010, 2, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, J.; Mei, L.; Wang, X. Enhanced Photocatalytic Degradation of Rhodamine B by Cu2O Coated Silicon Nanowire Arrays in Presence of H2O2. J. Mater. Sci. Technol. 2014, 11, 1124–1129. [Google Scholar] [CrossRef]
- Lin, L.; Guo, S.; Sun, X.; Feng, J.; Wang, Y. Synthesis and Photoluminescence Properties of Porous Silicon Nanowire Arrays. Nanoscale Res. Lett. 2010, 11, 1822–1828. [Google Scholar] [CrossRef]
- Brahiti, N.; Hadjersi, T.; Amirouche, S.; Menari, H.; ElKechai, O. Photocatalytic degradation of cationic and anionic dyes in water using hydrogen-terminated silicon nanowires as catalyst. J. Hydrog. Energy 2018, 43, 11411–11421. [Google Scholar] [CrossRef]
- Bai, F.; Li, M.; Song, D.; Yu, H.; gosh, B.; Li, Y. One-step synthesis of lightly doped porous silicon nanowires in HF/AgNO3/H2O2 solution at room temperature. J. Solid State Chem. 2012, 96, 596–600. [Google Scholar] [CrossRef]
- Qiu, T.; Wu, X.L.; Mei, Y.F.; Wan, G.J.; Chu, P.K.; Siu, G.G. From Si nanotubes to nanowires: Synthesis, characterization, and self-assembly. J. Cryst. Growth 2005, 277, 143–148. [Google Scholar] [CrossRef]
- Chang, H.-C.; Lai, K.-Y.; Dai, Y.-A.; Wang, H.-H.; Lin, C.-A.; He, J.-H. Nanowire arrays with controlled structure profiles for maximizing optical collection efficiency. Energy Environ. Sci. 2011, 4, 2863. [Google Scholar] [CrossRef]
- Li, S.; Ma, W.; Chen, X.; Xie, K.; Li, Y.; He, X.; Yang, X.; Lei, Y. Structure and antireflection properties of SiNWs arrays form mc-Si wafer through Ag-catalyzed chemical etching. Appl. Surf. Sci. 2016, 369, 232–240. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Kumar, D.; Schmitt, S.W.; Sood, K.N.; Christiansen, S.H.; Singh, P.K. Large area fabrication of vertical silicon nanowire arrays by silver-assisted single-step chemical etching and their formation kinetics. Nanotechnology 2014, 25, 175601. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, B.; Kulakci, M.; Turan, R.; Unalan, H.E. Effect of electroless etching parameters on the growth and reflection properties of silicon nanowires. Nanotechnology 2011, 22, 155606. [Google Scholar] [CrossRef] [PubMed]
- Najar, A.; Slimane, A.B.; Hedhili, M.N.; Anjum, D.; Sougrat, R. Effect of hydrofluoric acid concentration on the evolution of photoluminescence characteristics in porous silicon nanowires prepared by Ag-assisted electroless etching method. J. Appl. Phys. 2012, 112, 33502–33506. [Google Scholar] [CrossRef]
- Vladimir, S.; Voigt, F.; Berger, A.; Bauer, G.; Christiansen, S. Roughness of silicon nanowire sidewalls and room temperature photoluminescence. Phys. Rev. B 2010, 82, 125446. [Google Scholar]
- Chern, W.; Hsu, K.; Chun, I.S.; Azeredo BP, D.; Ahmed, N.; Kim, K.-H.; Zuo, J.-M.; Fang, N.; Ferreira, P.; Li, X. Nonlithographic Patterning and Metal-Assisted Chemical Etching for Manufacturing of Tunable Light-Emitting Silicon Nanowire Arrays. Nano Lett. 2010, 10, 1582–1588. [Google Scholar] [CrossRef]
- Valenta, J.; Bruhn, B.; Linnros, J. Coexistence of 1D and Quasi-0D Photoluminescence from Single Silicon Nanowires, Coexistence of 1D and Quasi-0D. Nano Lett. 2011, 7, 3003–3009. [Google Scholar] [CrossRef]
- Yan, J.-A.; Yang, L.; Chou, M.Y. Size and orientation dependence in the electronic properties of silicon nanowires. Phys. Rev. B 2007, 76, 115318. [Google Scholar] [CrossRef]
- Gonchar, K.A.; Zubairova, A.A.; Schleusener, A.; Osminkina, L.A.; Sivakov, V. Optical Properties of Silicon Nanowires Fabricated by Environment-Friendly Chemistry. Nanoscale Res. Lett. 2016, 1, 357. [Google Scholar] [CrossRef] [PubMed]
- Kulathuran, K.; Mohanraj, K.; Natarajan, B. Structural, optical and electrical characterization of nanostructured porous silicon: Effect of current density. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zamchiy, A.O.; Baranov, E.A.; Khmel, S.Y.; Maximovskiy, E.A.; Gulyaev, D.V.; Zhuravlev, K.S. Deposition time dependence of the morphology and properties of tin-catalyzed silicon oxide nanowires synthesized by the gas-jet electron beam plasma chemical vapor deposition method. Thin Solid Films 2018, 654, 61–68. [Google Scholar] [CrossRef]
- Moumni, B.; Jaballah, A.B. Correlation between oxidant concentrations, morphological aspects and etching kinetics of silicon nanowires during silver-assist electroless etching. Appl. Surf. Sci. 2017, 425, 1–7. [Google Scholar] [CrossRef]
- Chaliyawala, H.A.; Ray, A.; Pati, R.K.; Mukhopadhyay, I. Strong light absorption capability directed by structured profile of vertical Si nanowires. Optical Mater. 2017, 73, 449–458. [Google Scholar] [CrossRef]
- Garzon-Roman, A.; Cuate-Gomez, D.H. Graphene nanoflakes and carbon nanotubes on porous silicon layers by spin coating, for possible applications in optoelectronics. Sens. Actuators A Phys. 2019, 292, 121–128. [Google Scholar] [CrossRef]
- Das, M.; Nath, P.; Sarkar, D. Influence of etching current density on microstructural, optical and electrical properties of porous silicon (PS):n-Si heterostructure. Superlattices Microstruct. 2016, 90, 77–86. [Google Scholar] [CrossRef]
- Al Mortuza, A.; Hafijur, M.; Sinthia, R.; Mou, S.; Islam, M.J.; Ismail, A.B.M. Electrical and optical characteristics of porous silicon impregnated with LaF3 by a novel chemical bath technique. Curr. Appl. Phys. 2012, 12, 565–569. [Google Scholar] [CrossRef]
- Dariani, R.S.; Zabihipour, M. Effect of electrical behavior of ZnO microparticles grown on porous silicon substrate. Appl. Phys. A 2016, 122, 1047. [Google Scholar] [CrossRef]
- Haditale, M.; Zabihipour, A.; Koppelaar, H. A comparison of I-V characteristics of graphene silicon and graphene-porous silicon hybrid structures. Superlattices Microstruct. 2018, 122, 387–393. [Google Scholar] [CrossRef]
- Somvanshi, D.; Jit, S. Analysis of I–V Characteristics of Pd/ZnO Thin Film/n-Si Schottky Diodes with Series Resistance. J. Nanoelectron. Optoelectron. 2014, 9, 1–6. [Google Scholar] [CrossRef]
- Kumar, Y.; Kumr, H.; Rawat, G.; Kumar, C.; Pal, B.N.; Jit, S. Electrical and Optical Characteristics of Pd/ZnO Quantum Dots Based Schottky Photodiode on n-Si. In Proceedings of the 2016 IEEE International Symposium on Nanoelectronic and Information Systems (iNIS), Gwalior, India, 19–21 December 2016. [Google Scholar]

| Constants | Valenta et al. [39] | Yan et al. [40] | Gonchar et al. [41] | Bahera et al. [19] |
|---|---|---|---|---|
| C (eV nm−2) | 2.49 | 4 | 3.73 | 2.4 |
| α | 0.91 | 1.4 | 1.39 | 1.7 |
| d (nm) | 4.25 | 3.59 | 3.45 | 2.12 |
| Ln (I) vs. V (TE model) | Cheung’s Functions | ||||
|---|---|---|---|---|---|
| Etching Time | Is (µA) | η | (eV) | RS (kΩ) | η |
| 20 min | 0.211 | 5.91 | 0.806 | 93.65 | 3.65 |
| 40 min | 0.495 | 5.31 | 0.790 | 32.10 | 3.56 |
| 60 min | 0.695 | 4.38 | 0.771 | 25.06 | 2.60 |
| 80 min | 0.566 | 6.17 | 0.768 | 41.75 | 3.10 |
| 100 min | 0.239 | 6.05 | 0.801 | 86.33 | 4.08 |
| 120 min | 0.224 | 6.41 | 0.804 | 33.01 | 4.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naffeti, M.; Postigo, P.A.; Chtourou, R.; Zaïbi, M.A. Elucidating the Effect of Etching Time Key-Parameter toward Optically and Electrically-Active Silicon Nanowires. Nanomaterials 2020, 10, 404. https://doi.org/10.3390/nano10030404
Naffeti M, Postigo PA, Chtourou R, Zaïbi MA. Elucidating the Effect of Etching Time Key-Parameter toward Optically and Electrically-Active Silicon Nanowires. Nanomaterials. 2020; 10(3):404. https://doi.org/10.3390/nano10030404
Chicago/Turabian StyleNaffeti, Mariem, Pablo Aitor Postigo, Radhouane Chtourou, and Mohamed Ali Zaïbi. 2020. "Elucidating the Effect of Etching Time Key-Parameter toward Optically and Electrically-Active Silicon Nanowires" Nanomaterials 10, no. 3: 404. https://doi.org/10.3390/nano10030404
APA StyleNaffeti, M., Postigo, P. A., Chtourou, R., & Zaïbi, M. A. (2020). Elucidating the Effect of Etching Time Key-Parameter toward Optically and Electrically-Active Silicon Nanowires. Nanomaterials, 10(3), 404. https://doi.org/10.3390/nano10030404