Synthesis and Cytotoxicity Studies on Ru and Rh Nanoparticles as Potential X-Ray Fluorescence Computed Tomography (XFCT) Contrast Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Rh and Ru NPs
2.3. Characterization Methods
2.4. In Vitro Toxicity
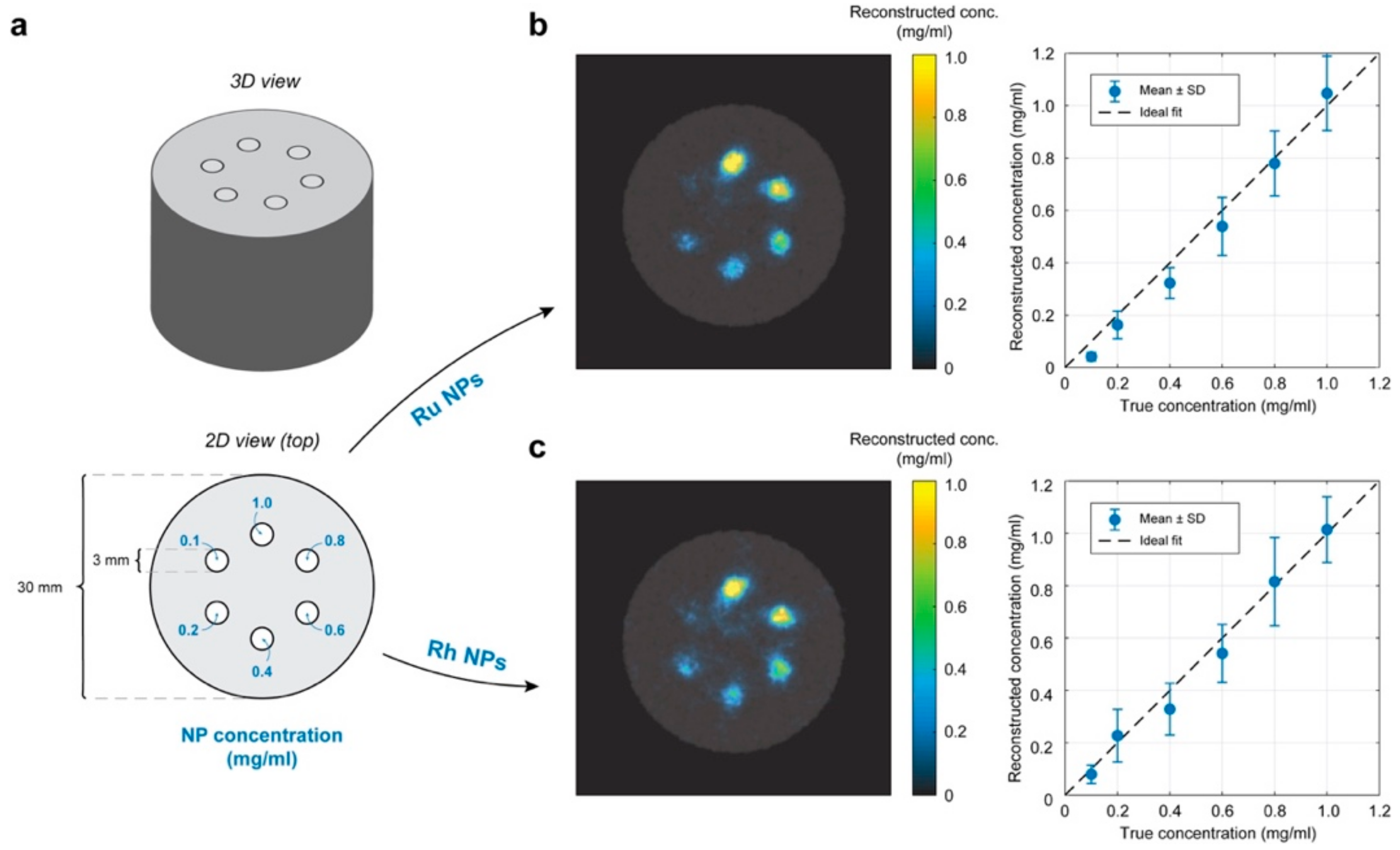
2.5. XFCT Phantom Experiments
3. Results and Discussion
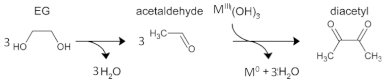
3.1. Mechanism of NP Formation
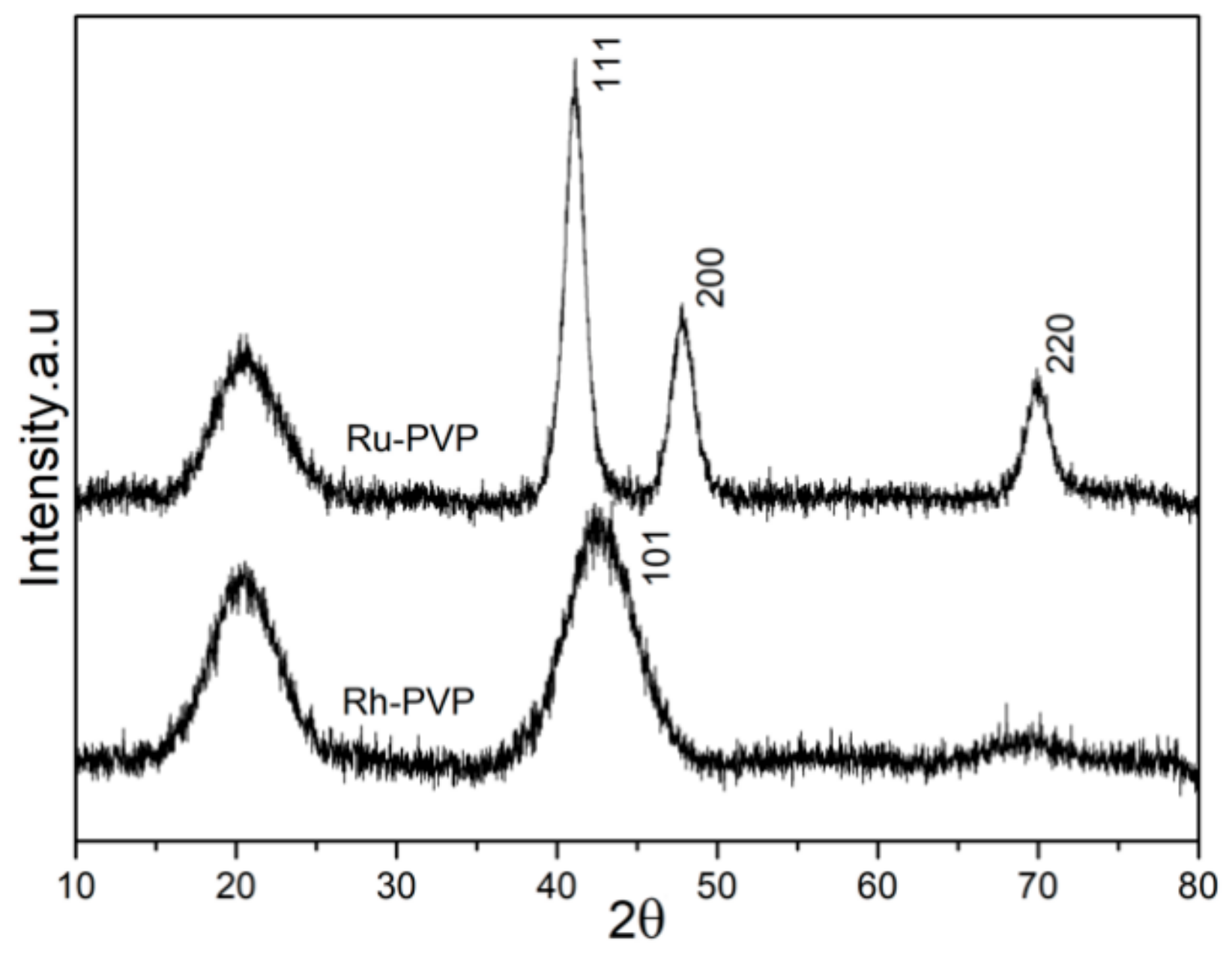
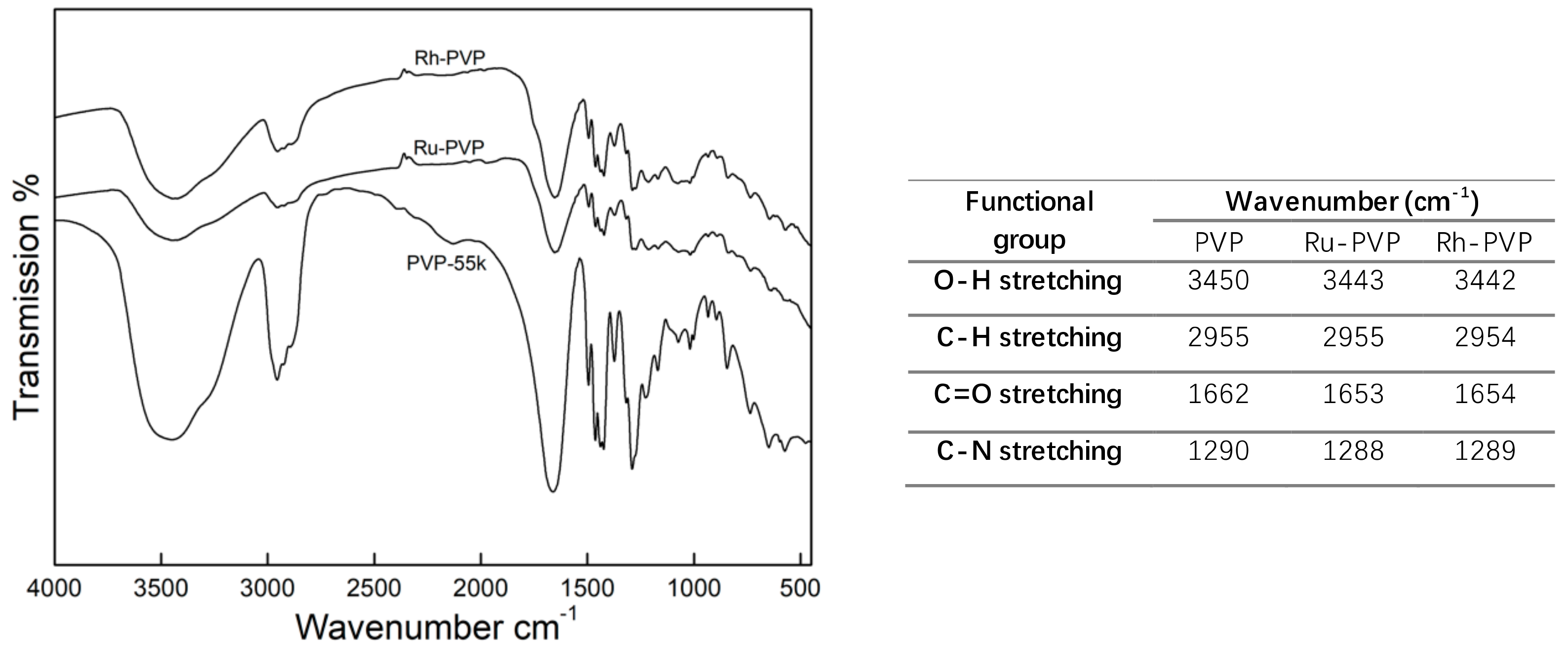
3.2. Characterization of Crystallinity, and Surface Adsorbed Groups
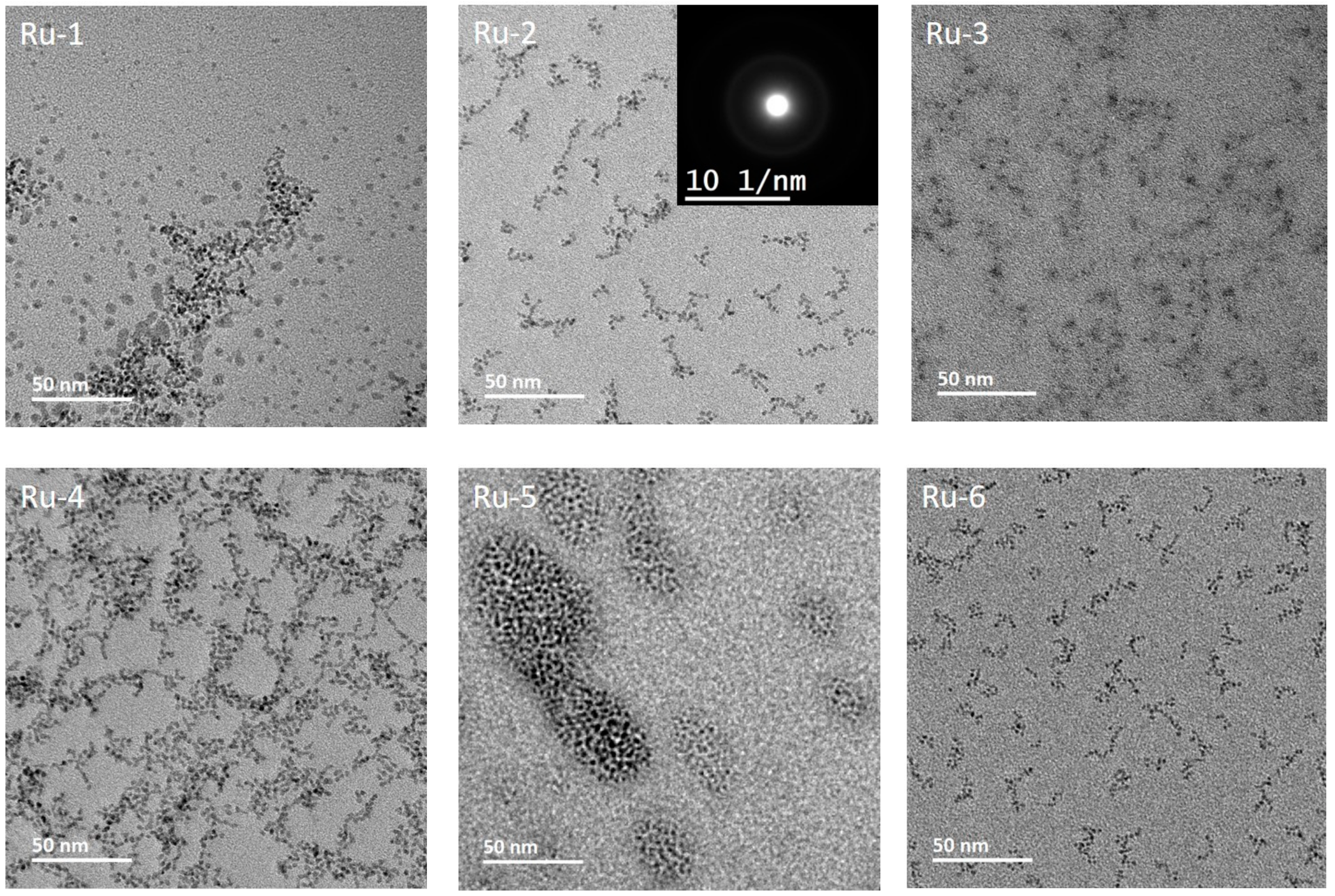
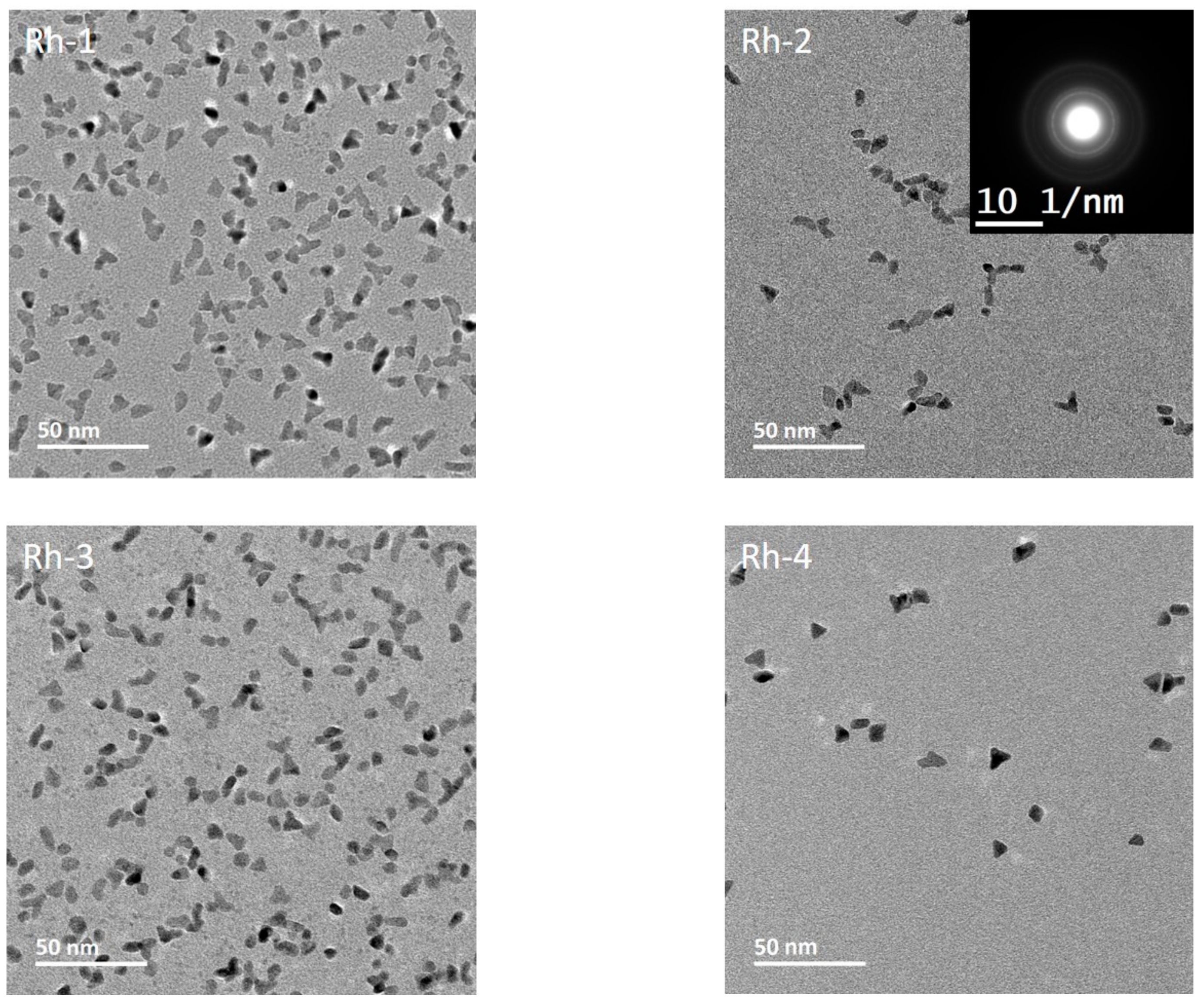
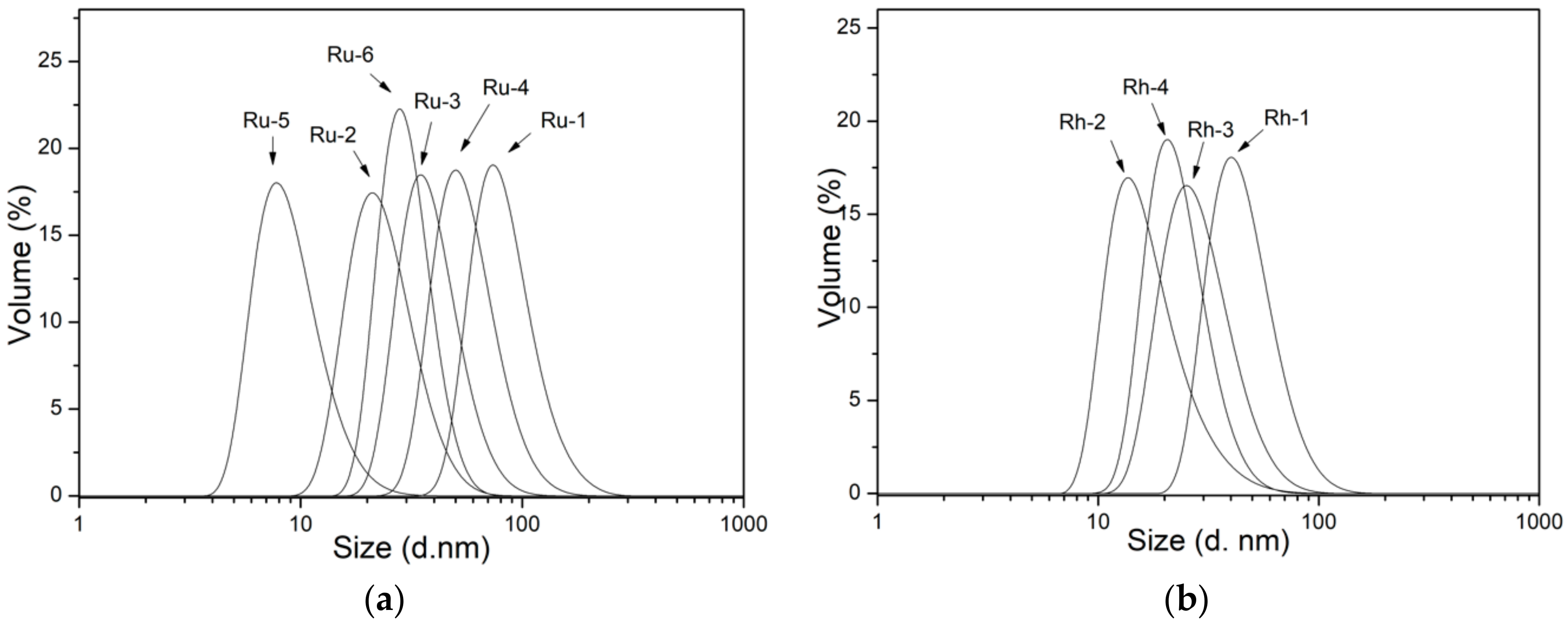
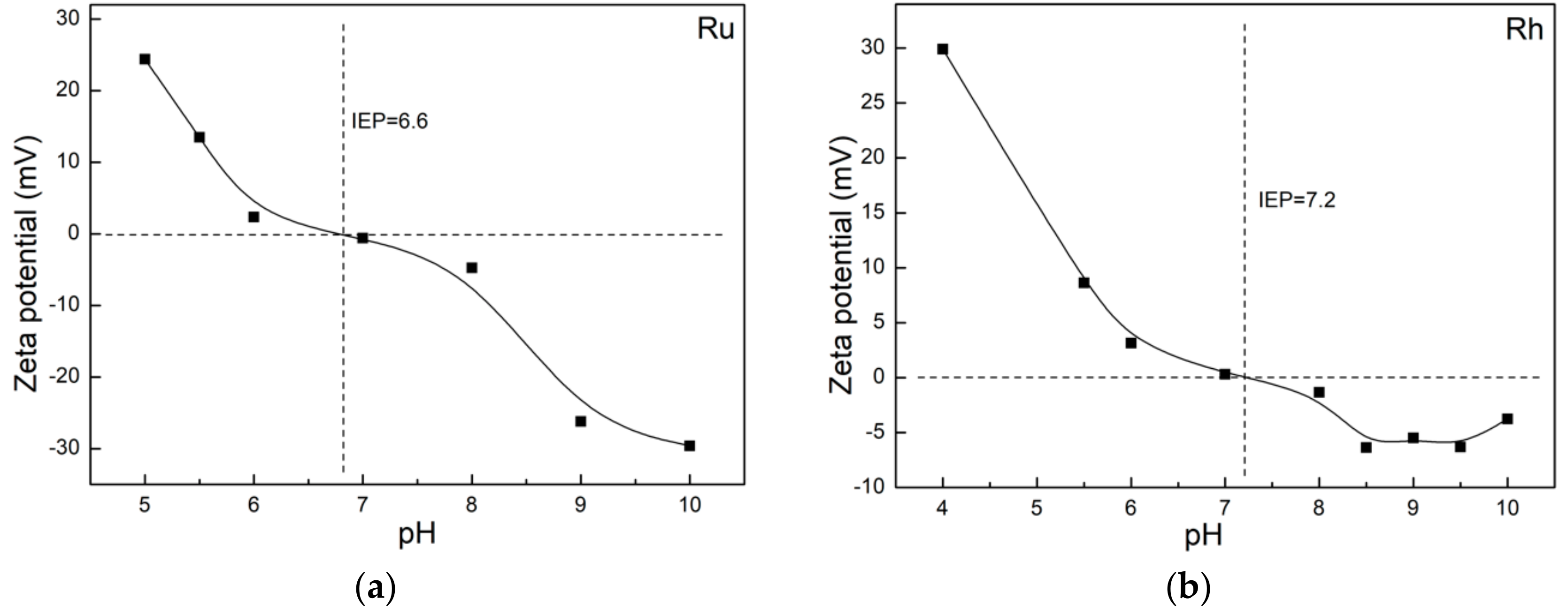
3.3. Morphology, Surface Chemistry and Size Distribution Analysis of NPs
3.4. Cytotoxicity Studies
3.5. XFCT Phantom Demonstrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larsson, J.C.; Vogt, C.; Vågberg, W.; Toprak, M.S.; Dzieran, J.; Henriksson, M.A.; Hertz, H.M. High-spatial-resolution X-Ray fluorescence tomography with spectrally matched nanoparticles. Phys. Med. Biol. 2018, 63, 164001. [Google Scholar] [CrossRef] [PubMed]
- Hertz, H.M.; Larsson, J.C.; Lundström, U.; Larsson, D.H.; Vogt, C. Laboratory X-Ray fluorescence tomography for high-resolution nanoparticle bio-imaging. Opt. Lett. 2014, 39, 2790–2793. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shaker, K.; Larsson, J.C.; Vogt, C.; Hertz, H.M.; Toprak, M.S. A Library of Potential Nanoparticle Contrast Agents for X-Ray Fluorescence Tomography Bioimaging. Contrast Media Mol. Imaging 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Biacchi, A.J.; Schaak, R.E. The Solvent Matters: Kinetic versus Thermodynamic Shape Control in the Polyol Synthesis of Rhodium Nanoparticles. ACS Nano 2011, 5, 8089–8099. [Google Scholar] [CrossRef] [PubMed]
- Linares, E.M.; Formiga, A.; Kubota, L.T.; Galembeck, F.; Thalhammer, S. One-step synthesis of polymer core–shell particles with a carboxylated ruthenium complex: A potential tool for biomedical applications. J. Mater. Chem. B 2013, 1, 2236–2244. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef]
- Garcia, J.P.; Lakshmi, B.A.; Kim, S. Potential anticancer applications of the novel naringin-based ruthenium (II) complex. 3 Biotech 2019, 9, 181. [Google Scholar] [CrossRef]
- Chan, L.; Huang, Y.; Chen, T. Cancer-targeted Tri-block Copolymer Nanoparticles as Payload of Metal Complexes to Achieve Enhanced Cancer Theranosis. J. Mater. Chem. B 2016, 4, 4517–4525. [Google Scholar] [CrossRef]
- Trondl, R.; Heffeter, P.; Kowol, C.R.; Jakupec, M.A.; Berger, W.; Keppler, B.K. NKP-1339, the first ruthenium-based anticancer drug on the edge to clinical application. Chem. Sci. 2014, 5, 2925–2932. [Google Scholar] [CrossRef]
- Kang, S.; Shin, W.; Choi, M.-H.; Ahn, M.; Kim, Y.-K.; Kim, S.; Min, D.-H.; Jang, H. Morphology-Controlled Synthesis of Rhodium Nanoparticles for Cancer Phototherapy. ACS Nano 2018, 12, 6997–7008. [Google Scholar] [CrossRef] [PubMed]
- Poda, A.R.; Kennedy, A.J.; Cuddy, M.F.; Bednar, A.J. Investigations of UV photolysis of PVP-capped silver nanoparticles in the presence and absence of dissolved organic carbon. J. Nanoparticle Res. 2013, 15, 1673. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kang, Y.S.; Lee, W.J.; Jo, B.G.; Jeong, J.H. Synthesis of Cu Nanoparticles Prepared by Using Thermal Decomposition of Cu-oleate Complex. Mol. Cryst. Liq. Cryst. 2006, 445, 231–238. [Google Scholar] [CrossRef]
- Pandey, P.A.; Bell, G.R.; Rourke, J.P.; Sanchez, A.M.; Elkin, M.D.; Hickey, B.J.; Wilson, N.R. Physical Vapor Deposition of Metal Nanoparticles on Chemically Modified Graphene: Observations on Metal-Graphene Interactions. Small 2011, 7, 3202–3210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, F.; Zhuang, J.; Tang, Y.; Wang, D.; Wang, Y.; Dong, A.; Ren, N. Synthesis of silver nanoparticles via electrochemical reduction on compact zeolite film modified electrodes. Chem. Commun. 2002, 23, 2814–2815. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, J.; Zhu, Y.-F.; Che, C.-M. A convenient solvothermal route to ruthenium nanoparticles. New J. Chem. 2000, 24, 739–740. [Google Scholar] [CrossRef]
- Dahal, N.; García, S.; Zhou, J.; Humphrey, S.M. Beneficial Effects of Microwave-Assisted Heating versus Conventional Heating in Noble Metal Nanoparticle Synthesis. ACS Nano 2012, 6, 9433–9446. [Google Scholar] [CrossRef]
- Shaker, K.; Larsson, J.C.; Hertz, H.M. Quantitative predictions in small-animal X-Ray fluorescence tomography. Biomed. Opt. Express 2019, 10, 3773–3788. [Google Scholar] [CrossRef]
- Badal, A.; Badano, A. Accelerating Monte Carlo simulations of photon transport in a voxelized geometry using a massively parallel graphics processing unit. Med Phys. 2009, 36, 4878–4880. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, J.; Wu, C.; Zhang, S.; Deng, Z.; Hu, X.; Chen, M.; Peng, B. Fabrication of silver-decorated sulfonated polystyrene microspheres for surface-enhanced Raman scattering and antibacterial applications. RSC Adv. 2015, 5, 69543–69554. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Skrabalak, S.E.; Polavarapu, L. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Wang, Q.; Ming, M.; Niu, S.; Zhang, Y.; Fan, G.; Hu, J.-S. Scalable Solid-State Synthesis of Highly Dispersed Uncapped Metal (Rh, Ru, Ir) Nanoparticles for Efficient Hydrogen Evolution. Adv. Energy Mater. 2018, 8, 1801698. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Kolli, V.; Karmakar, S.; Sarkar, N. Functionalisation of Polyvinylpyrrolidone on Gold Nanoparticles Enhances Its Anti-Amyloidogenic Propensity towards Hen Egg White Lysozyme. Biomedicines 2017, 5, 19. [Google Scholar]
- Zhang, W. Nanoparticle Aggregation: Principles and Modeling. Curr. Top. Complement 2014, 811, 19–43. [Google Scholar]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles–a new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Germolec, R.R.; Weaver, J.L. Evaluation of nanoparticle immunotoxicity. Nat. Nanotechnol. 2009, 4, 411–414. [Google Scholar] [CrossRef]
- Guidance for Industry: Pyrogen and Endotoxins Testing: Questions and Answers. 2012. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-pyrogen-and-endotoxins-testing-questions-and-answers (accessed on 8 November 2019).
- Steinhäuser, K.G.; Sayre, P.G. Reliability of methods and data for regulatory assessment of nanomaterial risks. NanoImpact 2017, 7, 66–74. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Toprak, M.S.; Fadeel, B. Handbook on the Toxicology of Metals; Academic Press: Cambridge, MA, USA, 2015; Volume 121. [Google Scholar]

| Sample | Precursor (mmol) | T (°C) | Reaction time (h) | PVP (mol) [MW] (kDa) |
|---|---|---|---|---|
| RuCl3·xH2O | ||||
| Ru-1 | 0.2 | 160 | 1.5 | 0.004 [10] |
| Ru-2 | 0.2 | 150 | 1.5 | 0.004 [55] |
| Ru-3 | 0.1 | 150 | 1.5 | 0.004 [55] |
| Ru-4 | 0.2 | 150 | 1.5 | 0.002 [55] |
| Ru-5 | 0.2 | 140 | 1.5 | 0.004 [55] |
| Ru-6 | 0.2 | 150 | 0.5 | 0.004 [55] |
| RhCl3·xH2O | ||||
| Rh-1 | 0.2 | 115 | 1.5 | 0.004 [10] |
| Rh-2 | 0.2 | 115 | 1.5 | 0.004 [55] |
| Rh-3 | 0.2 | 115 | 1.5 | 0.002 [55] |
| Rh-4 | 0.2 | 150 | 1.5 | 0.004 [55] |
| Sample | Particle Size, TEM (nm) | Particle Size (Volume)D-Average (nm)—[PdI] |
|---|---|---|
| Ru-1 | 1.61 | 104.2—[0.108] |
| Ru-2 | 2.51 | 29.86—[0.247] |
| Ru-3 | <1 | 47.86—[0.206] |
| Ru-4 | 2.52 | 69.73—[0.097] |
| Ru-5 | ~1.5 | 12.23—[0.211] |
| Ru-6 | 1.58 | 33.42—[0.226] |
| Rh-1 | 7.02 | 47.80—[0.271] |
| Rh-2 | 6.06 | 25.71—[0.301] |
| Rh-3 | 6.40 | 35.13—[0.214] |
| Rh-4 | 8.74 | 29.09—[0.228] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Shaker, K.; Svenda, M.; Vogt, C.; Hertz, H.M.; Toprak, M.S. Synthesis and Cytotoxicity Studies on Ru and Rh Nanoparticles as Potential X-Ray Fluorescence Computed Tomography (XFCT) Contrast Agents. Nanomaterials 2020, 10, 310. https://doi.org/10.3390/nano10020310
Li Y, Shaker K, Svenda M, Vogt C, Hertz HM, Toprak MS. Synthesis and Cytotoxicity Studies on Ru and Rh Nanoparticles as Potential X-Ray Fluorescence Computed Tomography (XFCT) Contrast Agents. Nanomaterials. 2020; 10(2):310. https://doi.org/10.3390/nano10020310
Chicago/Turabian StyleLi, Yuyang, Kian Shaker, Martin Svenda, Carmen Vogt, Hans M. Hertz, and Muhammet S. Toprak. 2020. "Synthesis and Cytotoxicity Studies on Ru and Rh Nanoparticles as Potential X-Ray Fluorescence Computed Tomography (XFCT) Contrast Agents" Nanomaterials 10, no. 2: 310. https://doi.org/10.3390/nano10020310
APA StyleLi, Y., Shaker, K., Svenda, M., Vogt, C., Hertz, H. M., & Toprak, M. S. (2020). Synthesis and Cytotoxicity Studies on Ru and Rh Nanoparticles as Potential X-Ray Fluorescence Computed Tomography (XFCT) Contrast Agents. Nanomaterials, 10(2), 310. https://doi.org/10.3390/nano10020310







