Almond Shell-Derived, Biochar-Supported, Nano-Zero-Valent Iron Composite for Aqueous Hexavalent Chromium Removal: Performance and Mechanisms
Abstract
1. Introduction
2. Materials and Methods
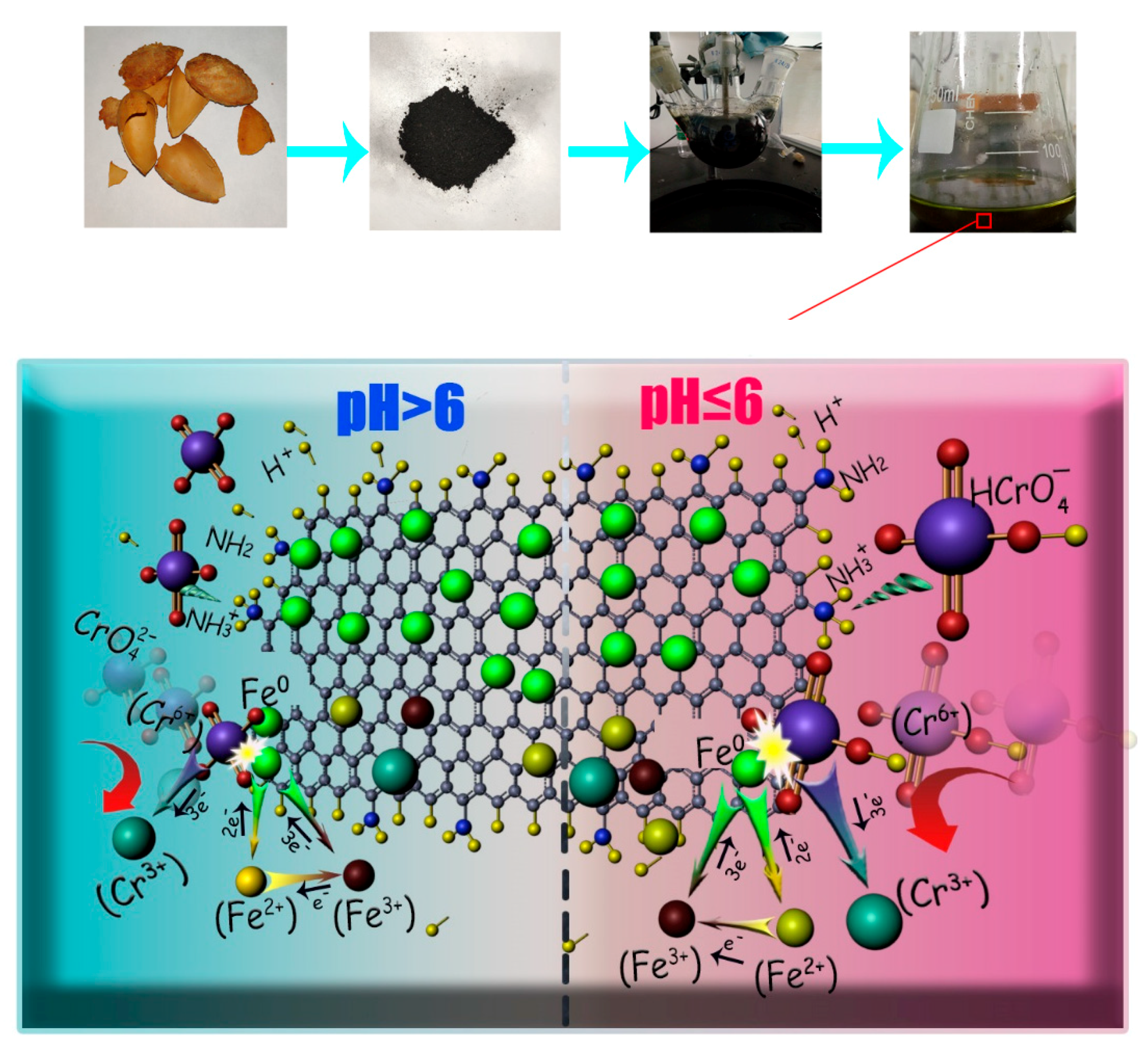
2.1. Material Preparation
2.2. Orthogonal Experimental Design and Statistical Analysis
2.3. Cr(VI) Removal Experiment and Analysis
3. Results and Discussion
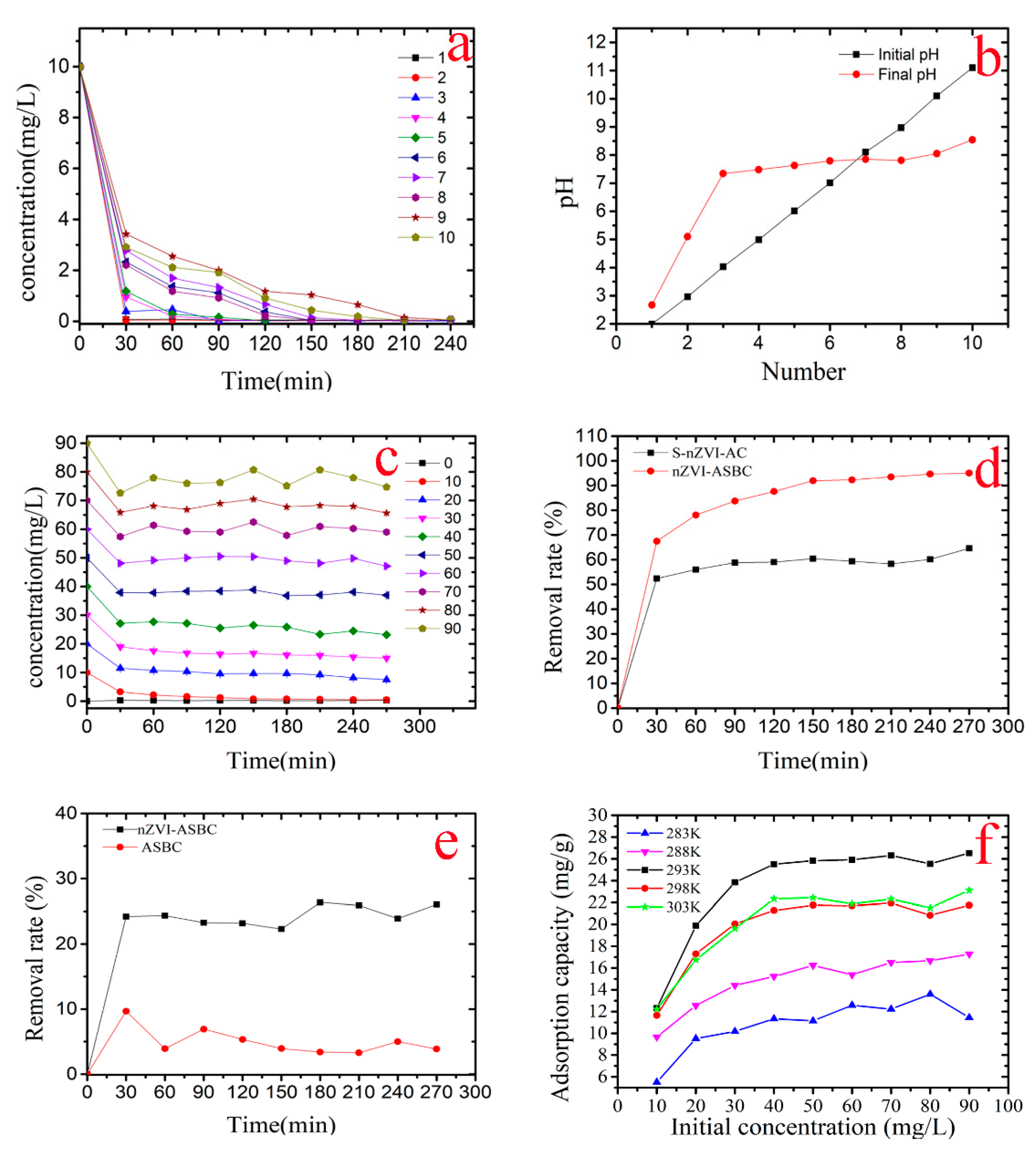
3.1. Orthogonal Experimental Analysis
3.2. Effect of Initial pH
3.3. Effect of the Initial Concentration of Cr(VI)
3.4. Physicochemical Characterization of Cr(VI) Removal by nZVI-ASBC
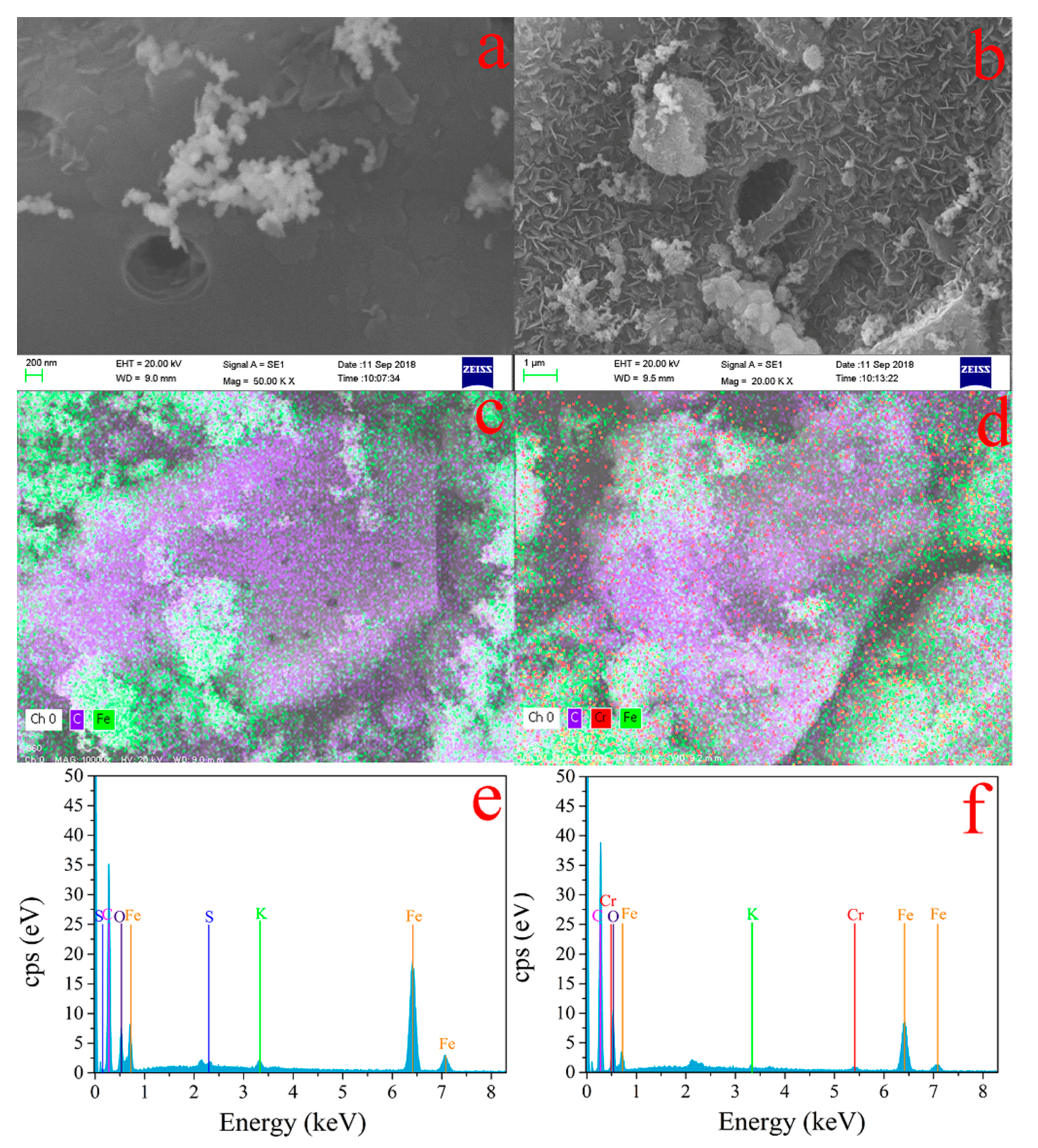
3.4.1. SEM and EDS of Cr(VI)–Fe (0) Reactions
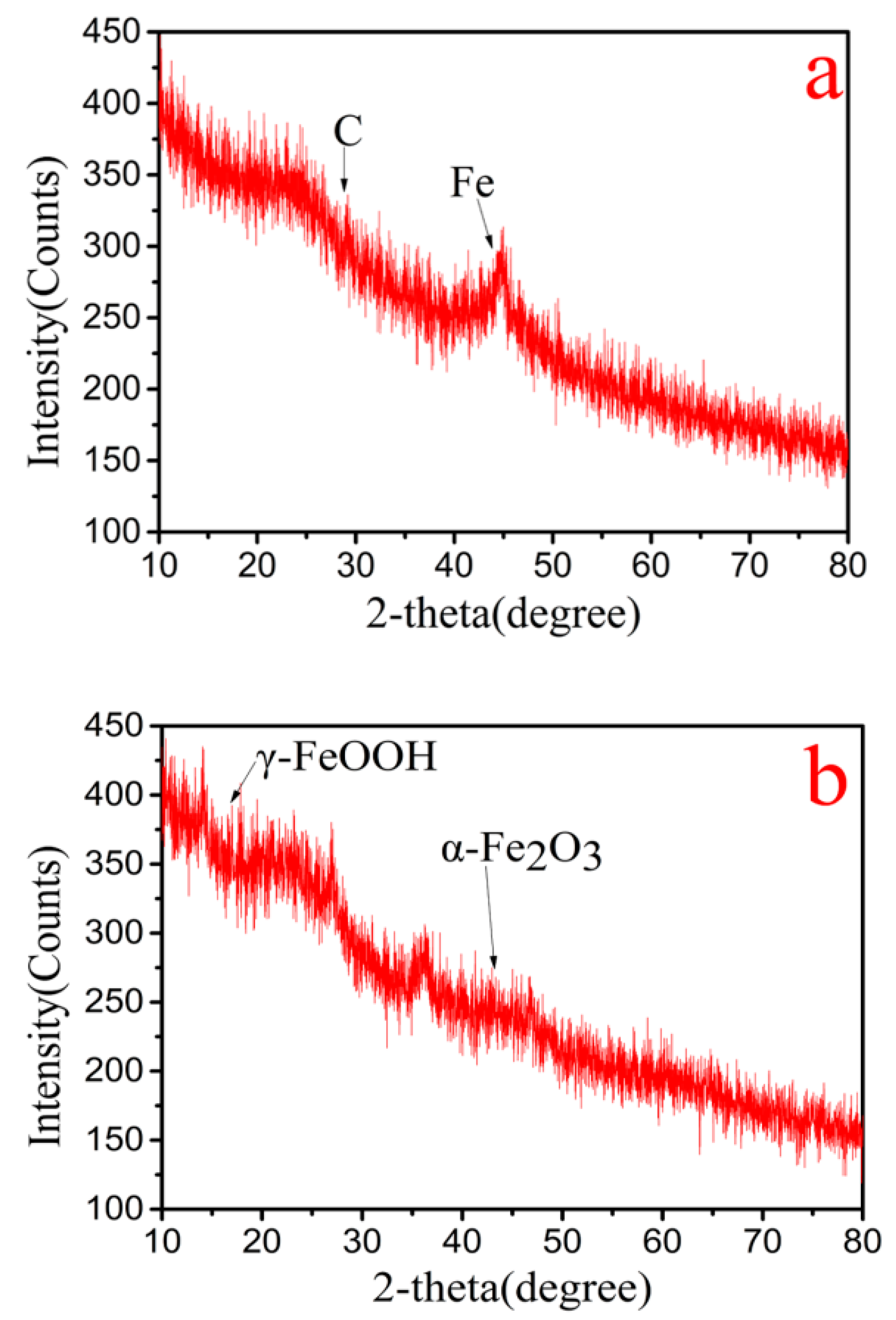
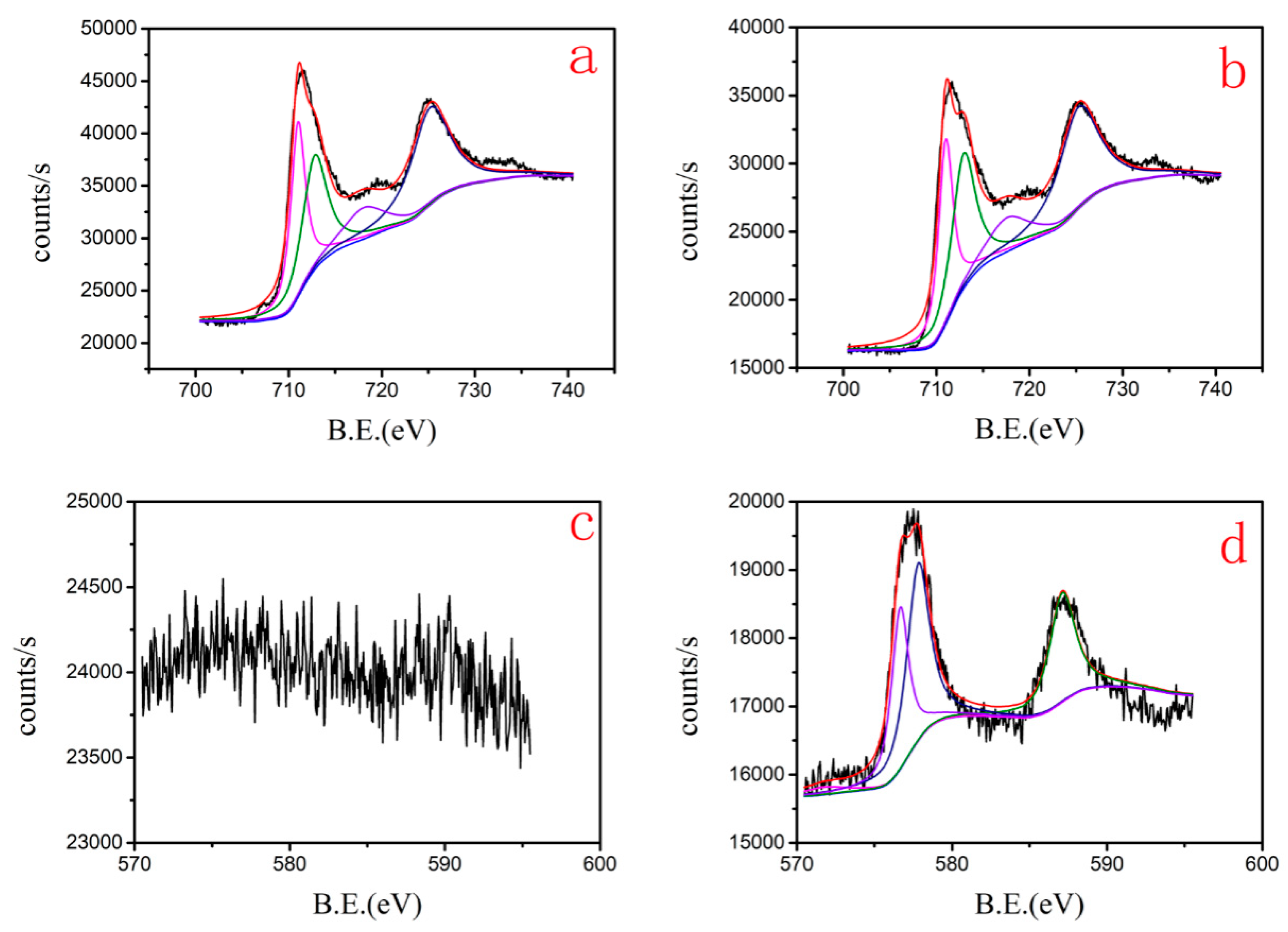
3.4.2. XRD and XPS of Cr(VI)–Fe (0) Reactions
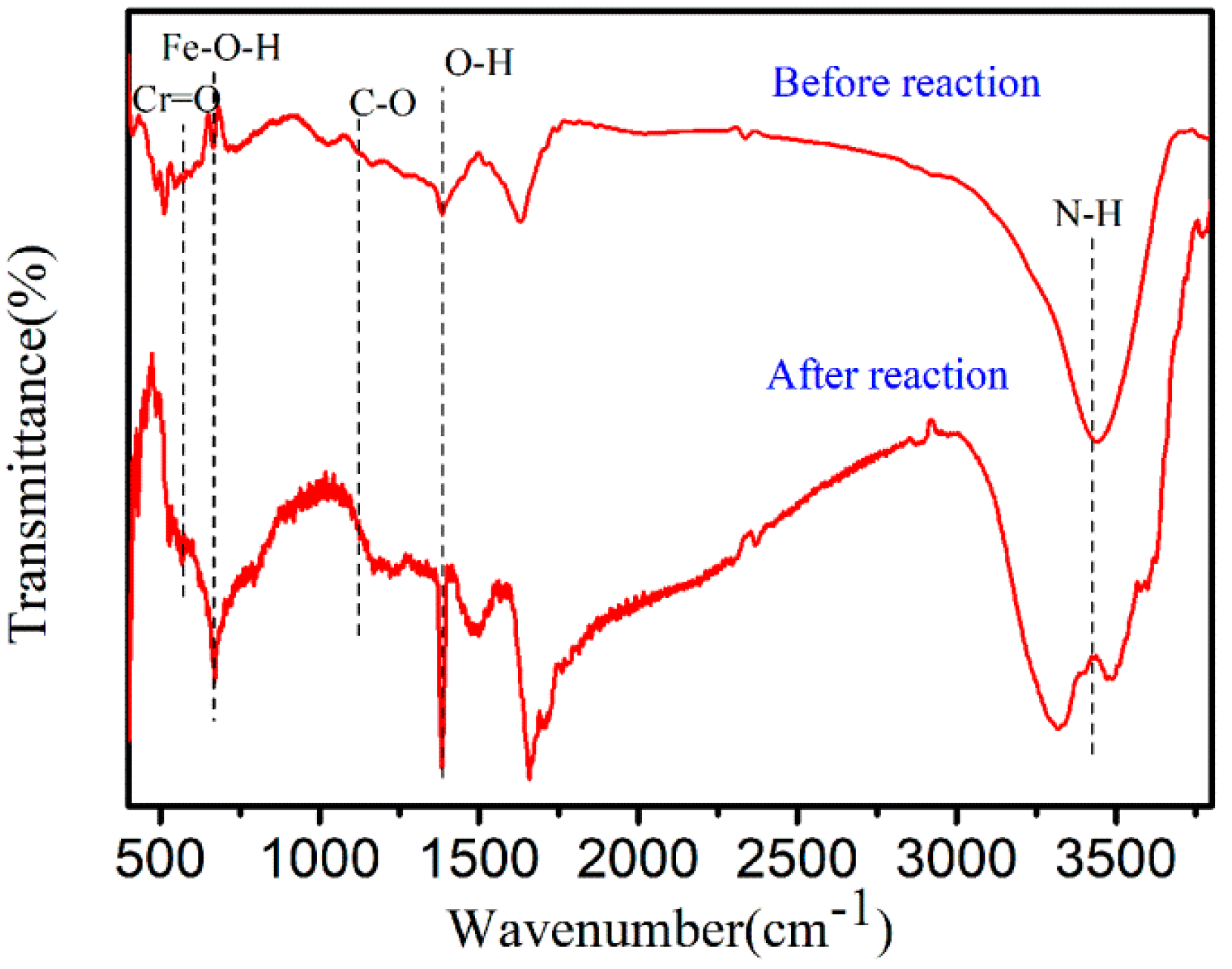
3.4.3. FTIR of Cr(VI)–Fe (0) Reactions
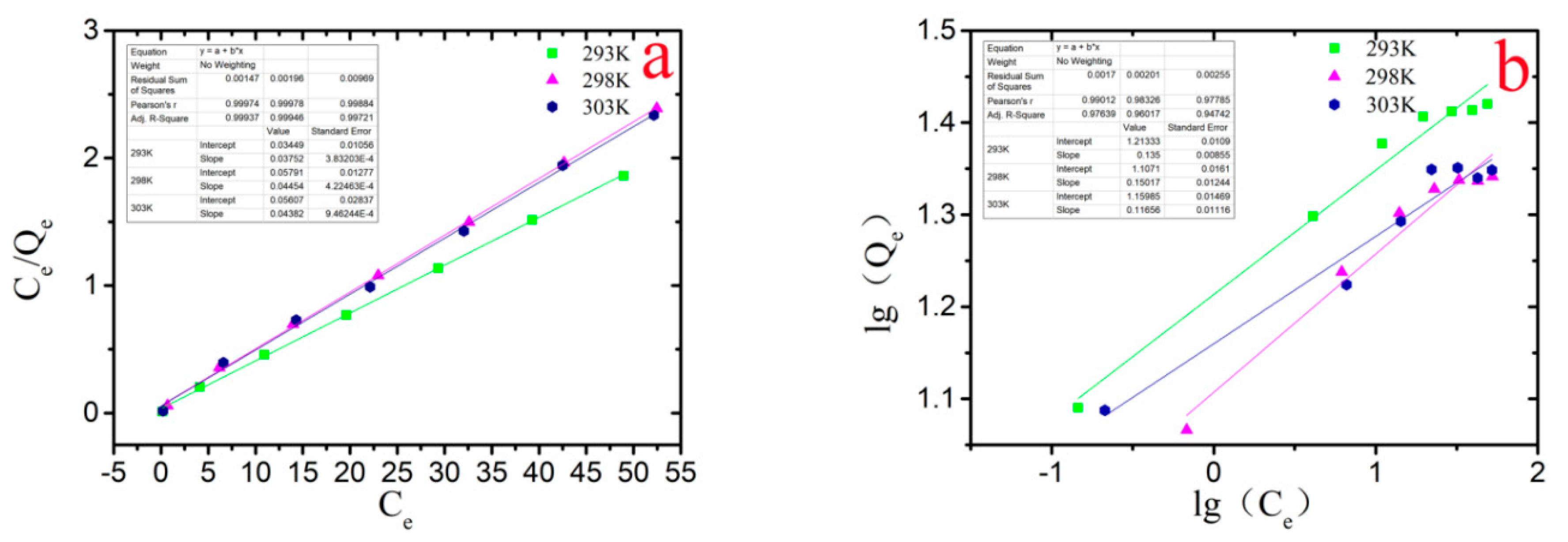
3.5. Adsorption Isotherms and Thermodynamic Study
3.6. Kinetics of Cr(VI) Removal
3.7. Possible Mechanisms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kazemi, M.; Jahanshahi, M.; Peyravi, M. Hexavalent chromium removal by multilayer membrane assisted by photocatalytic couple nanoparticle from both permeate and retentate. J. Hazard. Mater. 2018, 344, 12–22. [Google Scholar] [CrossRef]
- Sharma, A.K.; Devan, R.S.; Arora, M.; Kumar, R.; Ma, Y.; Babu, J.N. Reductive-co-precipitated cellulose immobilized zerovalent iron nanoparticles in ionic liquid/water for Cr(VI) adsorption. Cellulose 2018, 25, 5259–5275. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, W.; Yin, Y.; Chen, Z.; Qiu, R.; Simonnot, M.; Wang, X. Adsorption-reduction removal of Cr(VI) by tobacco petiole pyrolytic biochar: Batch experiment, kinetic and mechanism studies. Bioresour. Technol. 2018, 268, 149–157. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, L.; Qin, Y.; Xu, M.; Jia, X.; Chen, Z. Removal of aqueous Cr(VI) by a magnetic biochar derived from Melia azedarach wood. Bioresour. Technol. 2018, 256, 1–10. [Google Scholar] [CrossRef]
- Yang, P.; Guo, D.; Chen, Z.; Cui, B.; Xiao, B.; Liu, S.; Hu, M. Removal of Cr (VI) from aqueous solution using magnetic biochar synthesized by a single step method. J. Disper. Sci. Technol. 2017, 38, 1665–1674. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Hao, J.; Wang, W. Study of Almond Shell Characteristics. Materials 2018, 11, 1782. [Google Scholar] [CrossRef]
- Simeonidis, K.; Mourdikoudis, S.; Kaprara, E.; Mitrakas, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. Environ. Sci. Water Res. Technol. 2016, 2, 43–70. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, W.; Zhang, M.; Zhang, X.; Zhao, Y. Hydroxyl-functionalized TiO 2 @SiO 2 @Ni/nZVI nanocomposites fabrication, characterization and enhanced simultaneous visible light photocatalytic oxidation and adsorption of arsenite. Chem. Eng. J. 2018, 338, 369–382. [Google Scholar] [CrossRef]
- Jiang, D.; Huang, D.; Lai, C.; Xu, P.; Zeng, G.; Wan, J.; Tang, L.; Dong, H.; Huang, B.; Hu, T. Difunctional chitosan-stabilized Fe/Cu bimetallic nanoparticles for removal of hexavalent chromium wastewater. Sci. Total. Environ. 2018, 644, 1181–1189. [Google Scholar] [CrossRef]
- Dalal, U.; Reddy, S.N. A novel nano zero-valent iron biomaterial for chromium (Cr6+ to Cr3+) reduction. Environ. Sci. Pollut. Res. 2019, 26, 10631–10640. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Wang, Y. Impact of biochar-supported zerovalent iron nanocomposite on the anaerobic digestion of sewage sludge. Environ. Sci. Pollut. Res. 2019, 26, 10292–10305. [Google Scholar] [CrossRef]
- Huang, X.; Ling, L.; Zhang, W. Nanoencapsulation of hexavalent chromium with nanoscale zero-valent iron: High resolution chemical mapping of the passivation layer. J. Environ. Sci. China 2018, 67, 4–13. [Google Scholar] [CrossRef]
- Zhu, N.; Xu, Y.; Dai, L.; Zhang, Y.; Hu, G. Application of sequential extraction analysis to Pb(II) recovery by zerovalent iron-based particles. J. Hazard. Mater. 2018, 351, 138–146. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Ma, J.; Liu, H.; Ning, P. Synthesis and characterization of a new hydrophilic boehmite-PVB/PVDF blended membrane supported nano zero-valent iron for removal of Cr(VI). Sep. Purif. Technol. 2018, 205, 74–83. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, W.; Yan, J.; Han, L.; Chen, Y.; Ouyang, D.; Chen, M. Nanoscale zero-valent iron supported by biochars produced at different temperatures: Synthesis mechanism and effect on Cr(VI) removal. Environ. Pollut. 2017, 223, 153–160. [Google Scholar] [CrossRef]
- Wu, B.; Peng, D.; Hou, S.; Tang, B.; Wang, C.; Xu, H. Dynamic study of Cr(VI) removal performance and mechanism from water using multilayer material coated nanoscale zerovalent iron. Environ. Pollut. 2018, 240, 717–724. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K.S.M. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Huang, T.; Liu, L.; Zhou, L.; Zhang, S. Electrokinetic removal of chromium from chromite ore-processing residue using graphite particle-supported nanoscale zero-valent iron as the three-dimensional electrode. Chem. Eng. J. 2018, 350, 1022–1034. [Google Scholar] [CrossRef]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zeng, G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Han, D.; Huang, W.; Yang, C. New insights into the role of Ni loading on the surface structure and the reactivity of nZVI toward tetrabromo- and tetrachlorobisphenol A. Chem. Eng. J. 2017, 311, 173–182. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Sun, Y.; Cheng, K.; Li, J.; Tsang, D.C.W. Fabrication and characterization of hydrophilic corn stalk biochar-supported nanoscale zero-valent iron composites for efficient metal removal. Bioresour. Technol. 2018, 265, 490–497. [Google Scholar] [CrossRef]
- Su, H.; Fang, Z.; Tsang, P.E.; Fang, J.; Zhao, D. Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil. Environ. Pollut. 2016, 214, 94–100. [Google Scholar] [CrossRef]
- Shang, J.; Zong, M.; Yu, Y.; Kong, X.; Du, Q.; Liao, Q. Removal of chromium (VI) from water using nanoscale zerovalent iron particles supported on herb-residue biochar. J. Environ. Manag. 2017, 197, 331–337. [Google Scholar] [CrossRef]
- Dai, Y.; Hu, Y.; Jiang, B.; Zou, J.; Tian, G.; Fu, H. Carbothermal synthesis of ordered mesoporous carbon-supported nano zero-valent iron with enhanced stability and activity for hexavalent chromium reduction. J. Hazard. Mater. 2016, 309, 249–258. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, Y.; Tang, L.; Yang, G.; Pang, Y.; Zhang, Y.; Zhou, Y.; Li, Z.; Li, M.; Lai, M.; et al. Enhancement of Cd(II) adsorption by polyacrylic acid modified magnetic mesoporous carbon. Chem. Eng. J. 2015, 259, 153–160. [Google Scholar] [CrossRef]
- Yan, J.; Han, L.; Gao, W.; Xue, S.; Chen, M. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol. 2015, 175, 269–274. [Google Scholar] [CrossRef]
- Mohamed, A.; Osman, T.A.; Toprak, M.S.; Muhammed, M.; Yilmaz, E.; Uheida, A. Visible light photocatalytic reduction of Cr(VI) by surface modified CNT/titanium dioxide composites nanofibers. J. Mol. Catal. A Chem. 2016, 424, 45–53. [Google Scholar] [CrossRef]
- Ma, H.; Yang, J.; Gao, X.; Liu, Z.; Liu, X.; Xu, Z. Removal of chromium (VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J. Hazard. Mater. 2019, 369, 550–560. [Google Scholar] [CrossRef]
- Chen, L.; Zuo, L.; Jiang, Z.; Jiang, S.; Liu, K.; Tan, J.; Zhang, L. Mechanisms of shale gas adsorption: Evidence from thermodynamics and kinetics study of methane adsorption on shale. Chem. Eng. J. 2019, 361, 559–570. [Google Scholar] [CrossRef]
- Mortazavian, S.; An, H.; Chun, D.; Moon, J. Activated carbon impregnated by zero-valent iron nanoparticles (AC/nZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: Material characterizations and kinetic studies. Chem. Eng. J. 2018, 353, 781–795. [Google Scholar] [CrossRef]
| Isothermal Adsorption Model | Isothermal Parameter | |||
|---|---|---|---|---|
| Langmuir isotherm model | Temperature (K) | KL (L mol−1) | RL | R2 |
| 293 | 1.0873 | 0.0011 | 0.9994 | |
| 298 | 0.7691 | 0.0016 | 0.9995 | |
| 303 | 0.7815 | 0.0016 | 0.9972 | |
| Freundlich isotherm model | Temperature (K) | KF | R2 | |
| 293 | 16.3418 | 0.9764 | ||
| 298 | 12.7968 | 0.9602 | ||
| 303 | 14.4494 | 0.9474 | ||
| T (K) | △G (KJ·mol−1) | △H (KJ·mol−1) | △S (KJ·mol−1K−1) |
|---|---|---|---|
| 293 | −3.8512 | −47.7670 | −0.1505 |
| 298 | −2.5564 | ||
| 303 | −2.3462 |
| k | R-Square | C0 | |
|---|---|---|---|
| First order | −0.1605 | 0.6662 | 1.2628 |
| Second order | 1.2745 | 0.6092 | 1.2747 |
| k | R-square | qe | |
| Pseudo first order | −0.2062 | 0.6756 | 3.1970 |
| Pseudo second order | 0.1544 | 0.9998 | 24.1546 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, Y.; Ji, B.; Cui, B.; Shi, Y.; Wang, J.; Hu, M.; Luo, S.; Guo, D. Almond Shell-Derived, Biochar-Supported, Nano-Zero-Valent Iron Composite for Aqueous Hexavalent Chromium Removal: Performance and Mechanisms. Nanomaterials 2020, 10, 198. https://doi.org/10.3390/nano10020198
Shu Y, Ji B, Cui B, Shi Y, Wang J, Hu M, Luo S, Guo D. Almond Shell-Derived, Biochar-Supported, Nano-Zero-Valent Iron Composite for Aqueous Hexavalent Chromium Removal: Performance and Mechanisms. Nanomaterials. 2020; 10(2):198. https://doi.org/10.3390/nano10020198
Chicago/Turabian StyleShu, Yaorong, Bin Ji, Baihui Cui, Yuting Shi, Jian Wang, Mian Hu, Siyi Luo, and Dabin Guo. 2020. "Almond Shell-Derived, Biochar-Supported, Nano-Zero-Valent Iron Composite for Aqueous Hexavalent Chromium Removal: Performance and Mechanisms" Nanomaterials 10, no. 2: 198. https://doi.org/10.3390/nano10020198
APA StyleShu, Y., Ji, B., Cui, B., Shi, Y., Wang, J., Hu, M., Luo, S., & Guo, D. (2020). Almond Shell-Derived, Biochar-Supported, Nano-Zero-Valent Iron Composite for Aqueous Hexavalent Chromium Removal: Performance and Mechanisms. Nanomaterials, 10(2), 198. https://doi.org/10.3390/nano10020198






