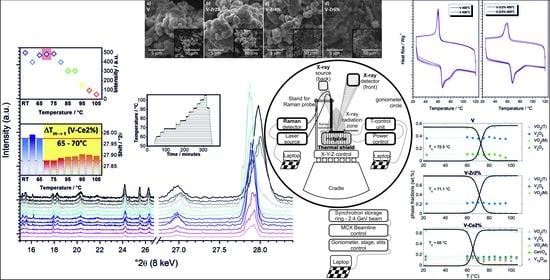
In-Operando Diffraction and Spectroscopic Evaluation of Pure, Zr-, and Ce-Doped Vanadium Dioxide Thermochromic Films Derived via Glycolate Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Powders
2.1.2. Films
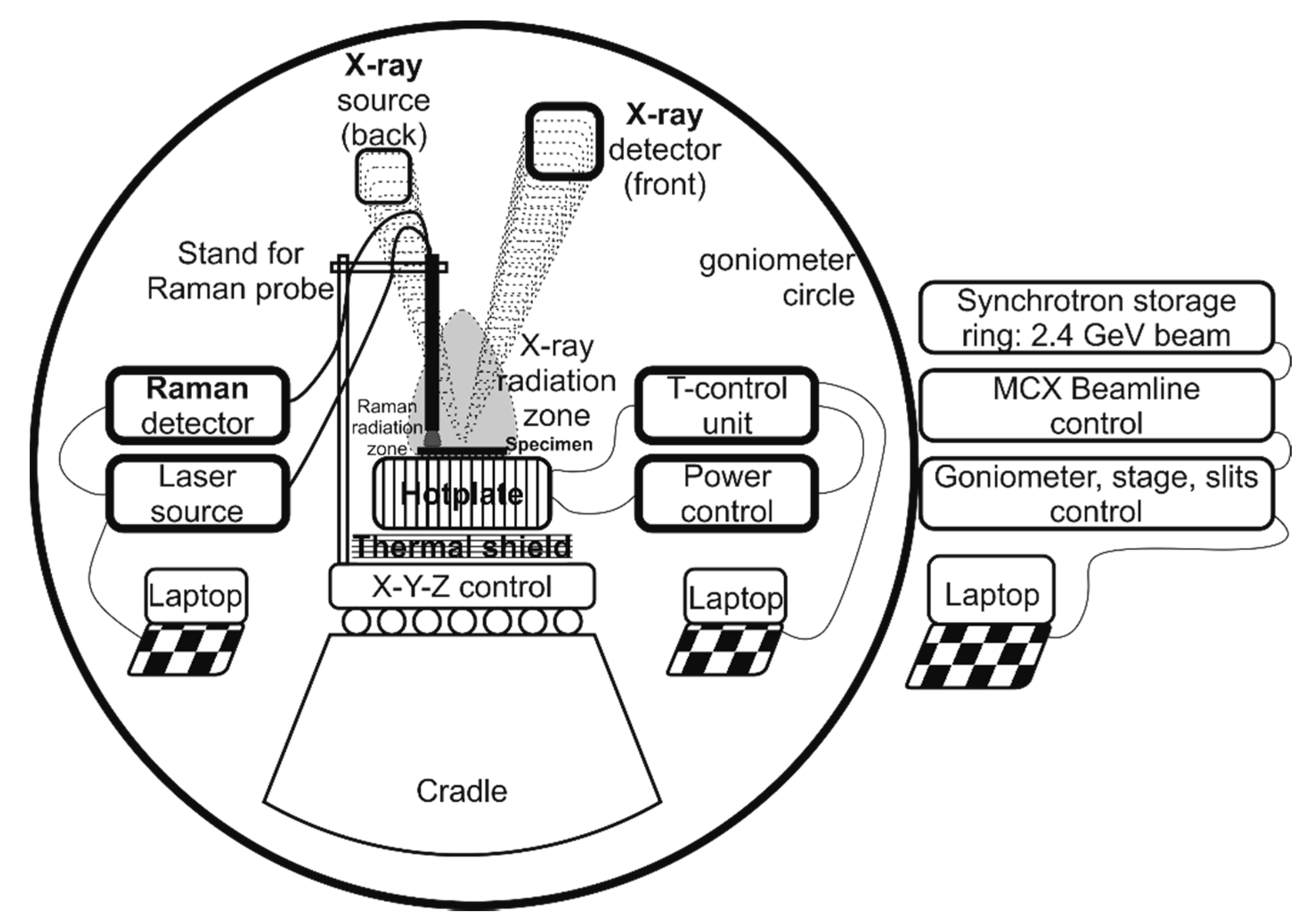
2.2. In-Operando Film Characterisation
2.3. Powder Characterisation
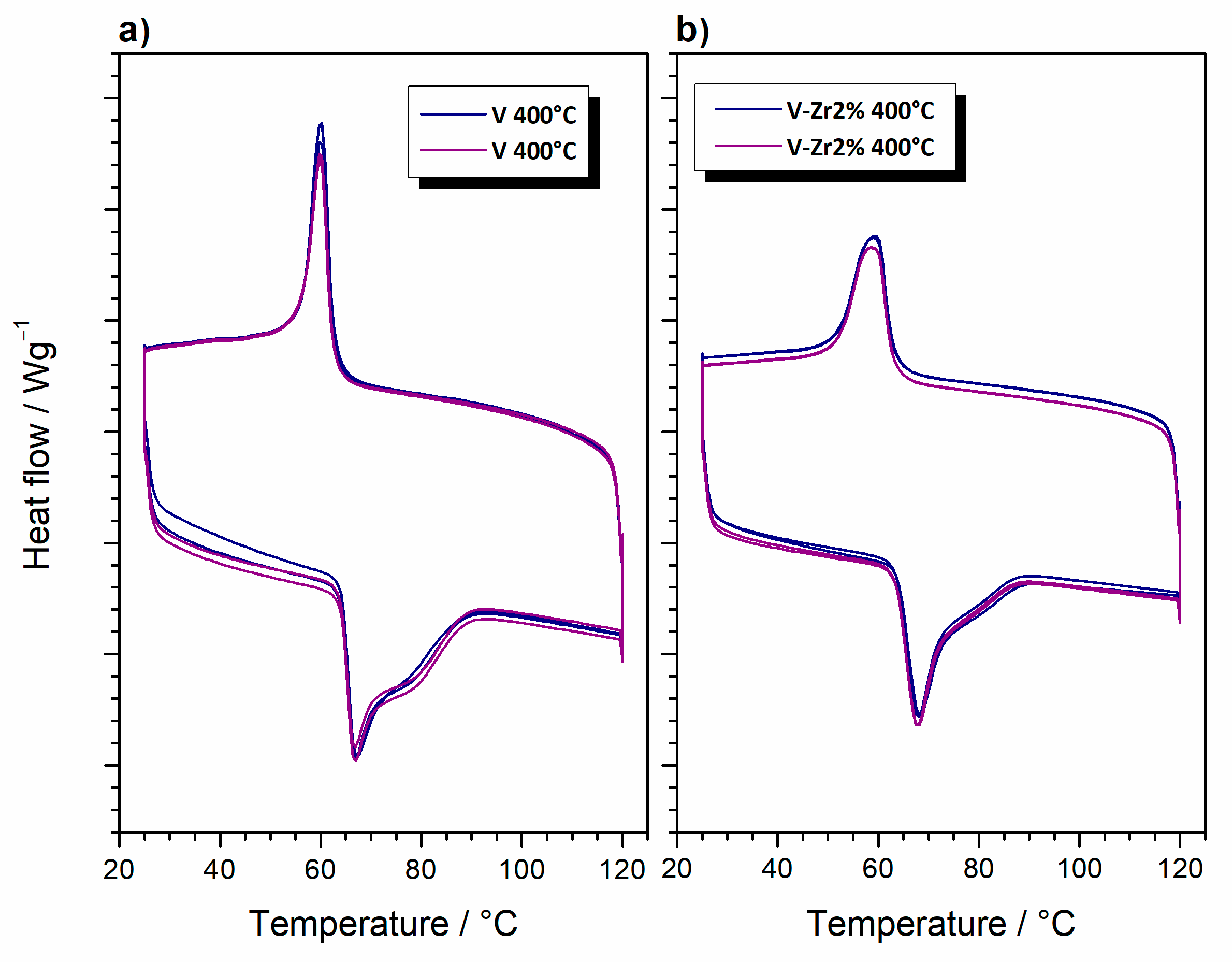
3. Results and Discussion
3.1. In-Operando Characterisation of Films
3.1.1. Setup
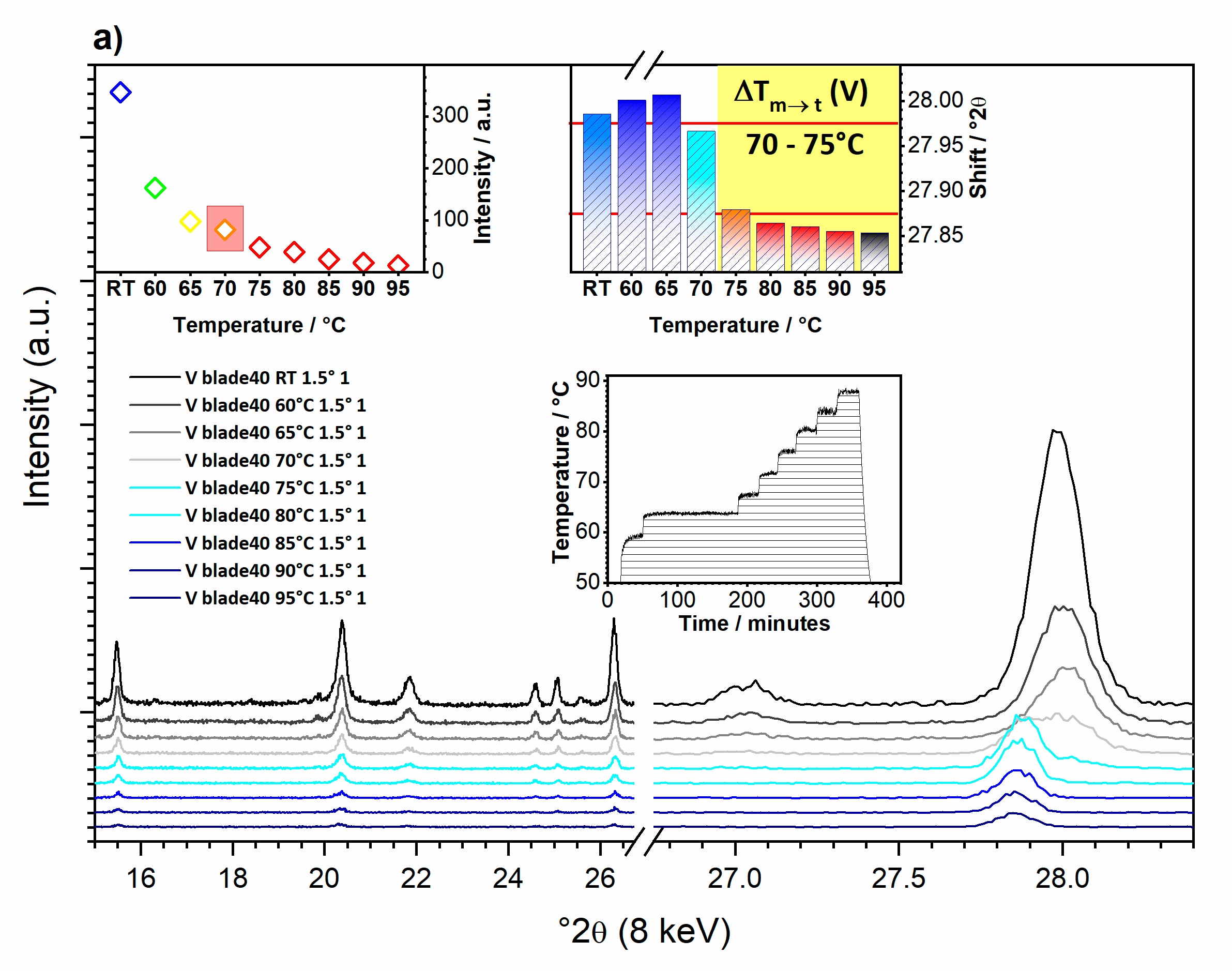
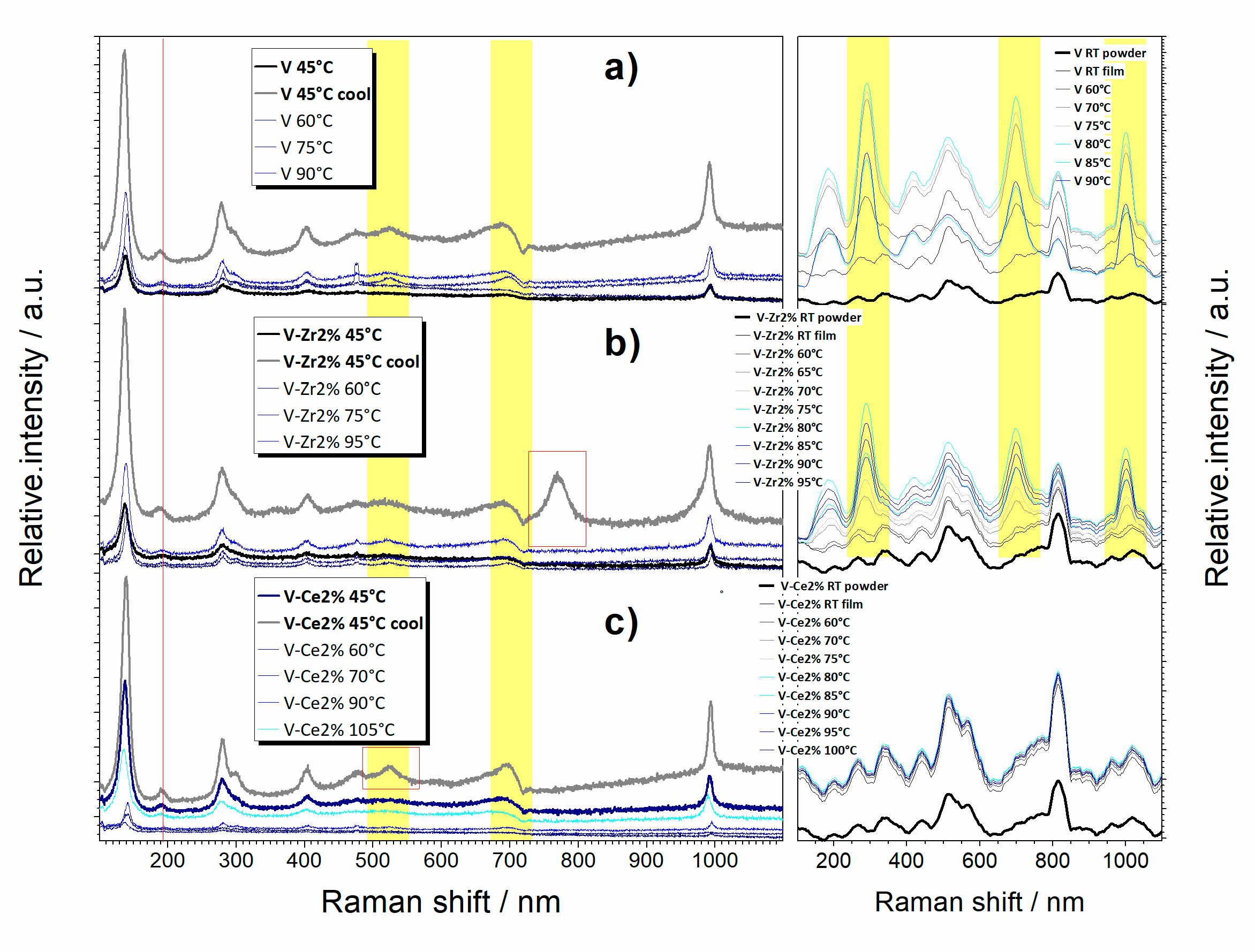
3.1.2. What Can We See at Room Temperature?
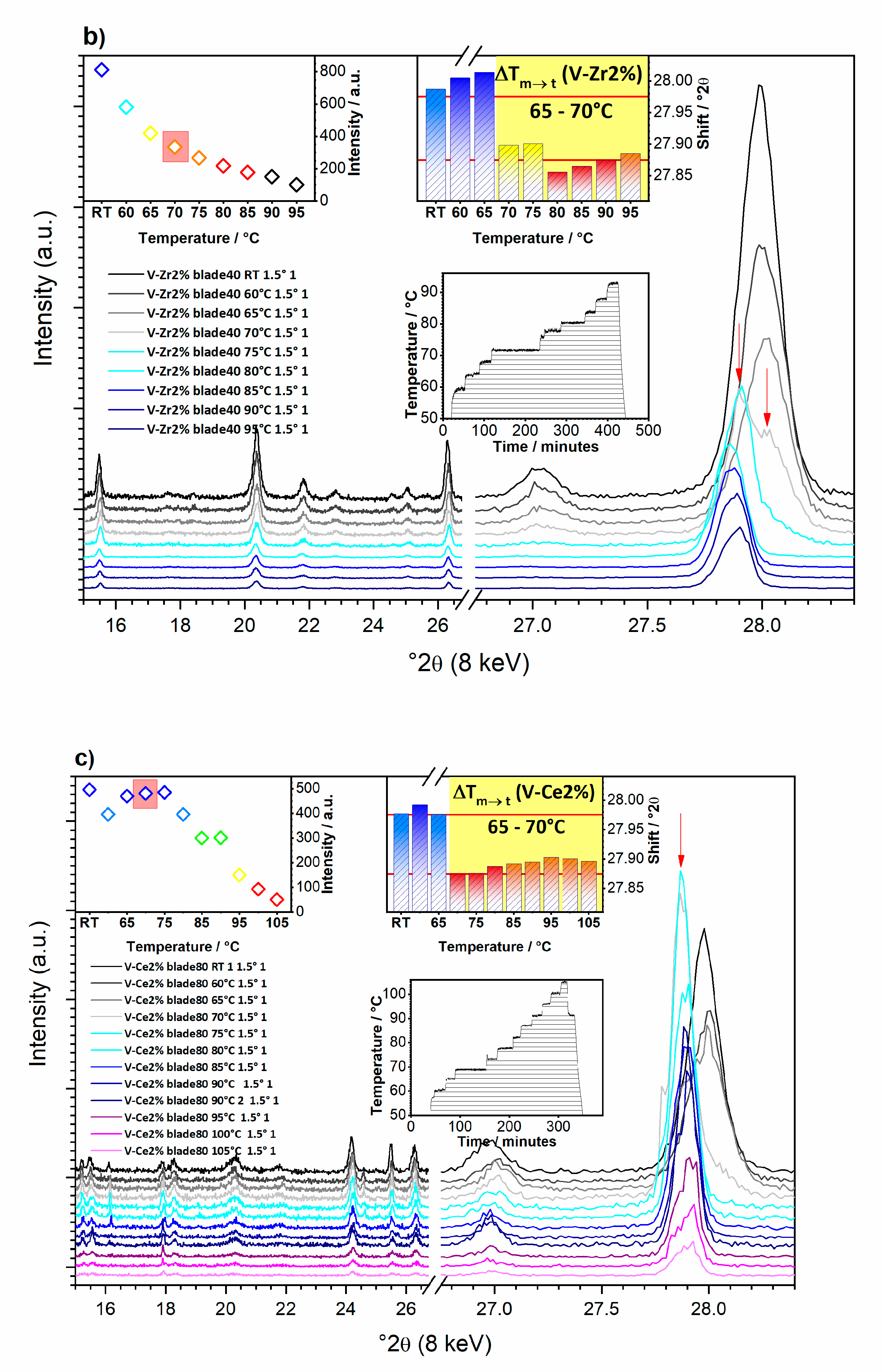
3.1.3. What Can We See with a Temperature Increase?
3.1.4. What Can We Conclude from In-Operando Measurements?
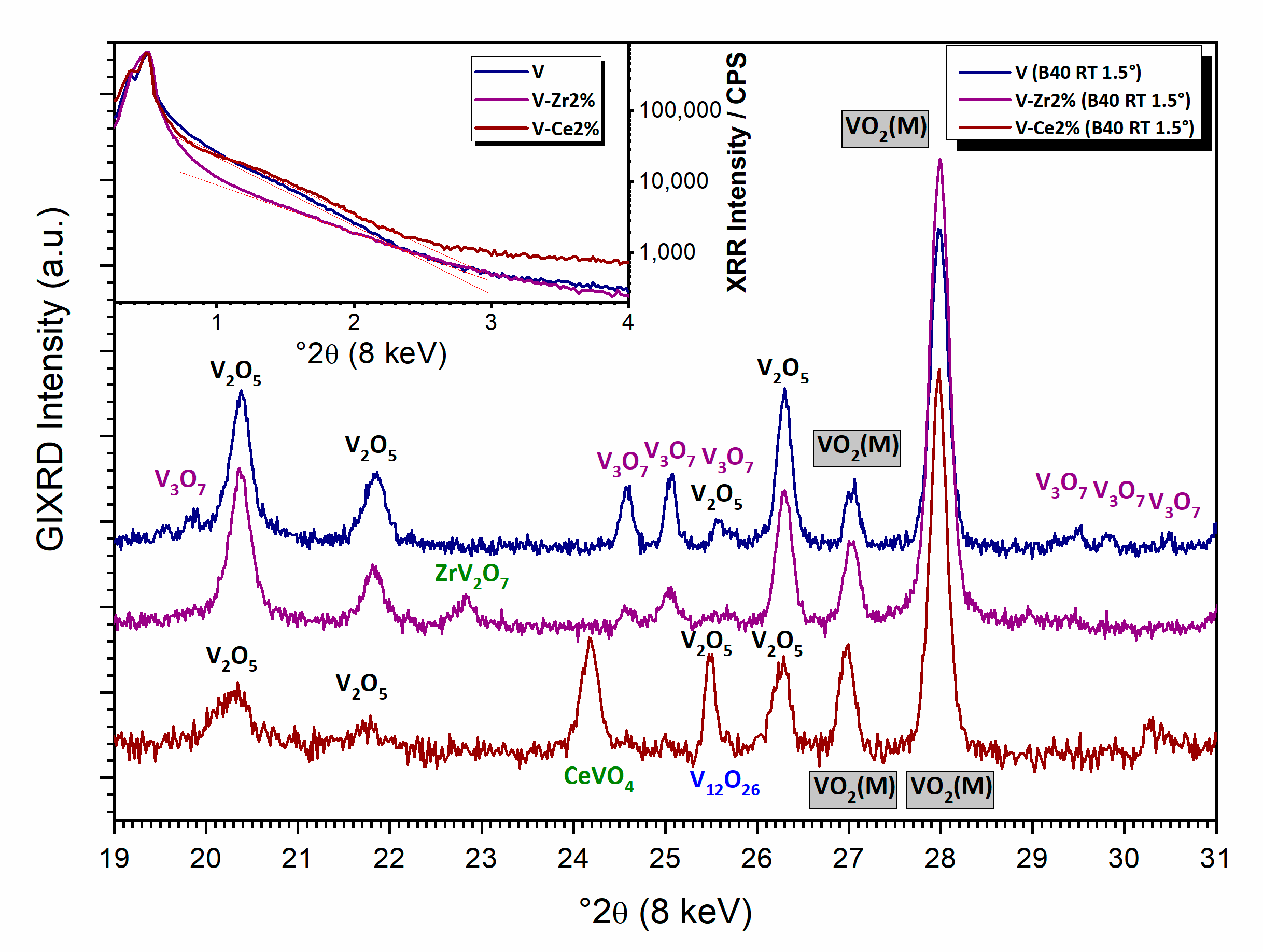
3.2. Characterisation of Bulk Powders
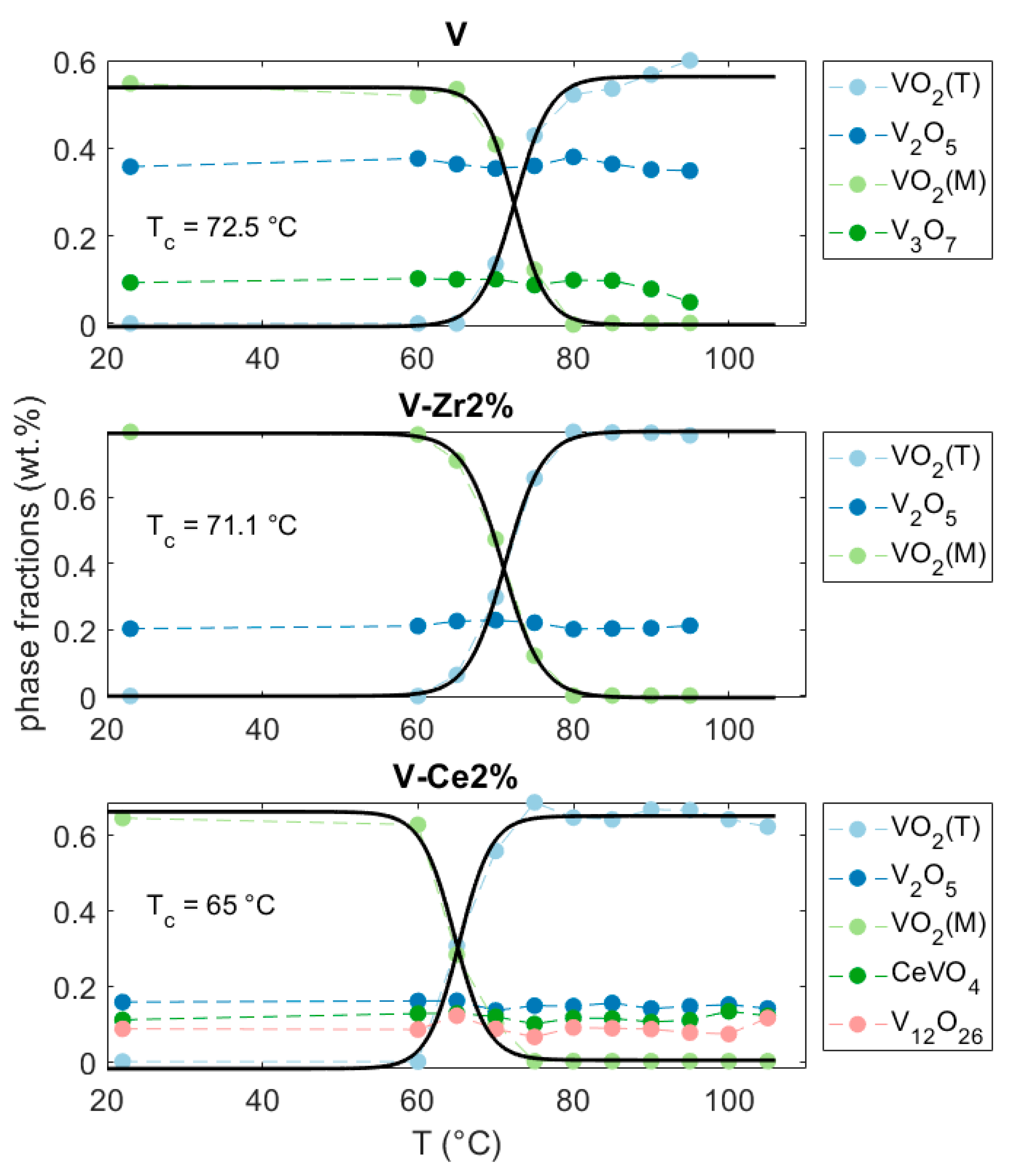
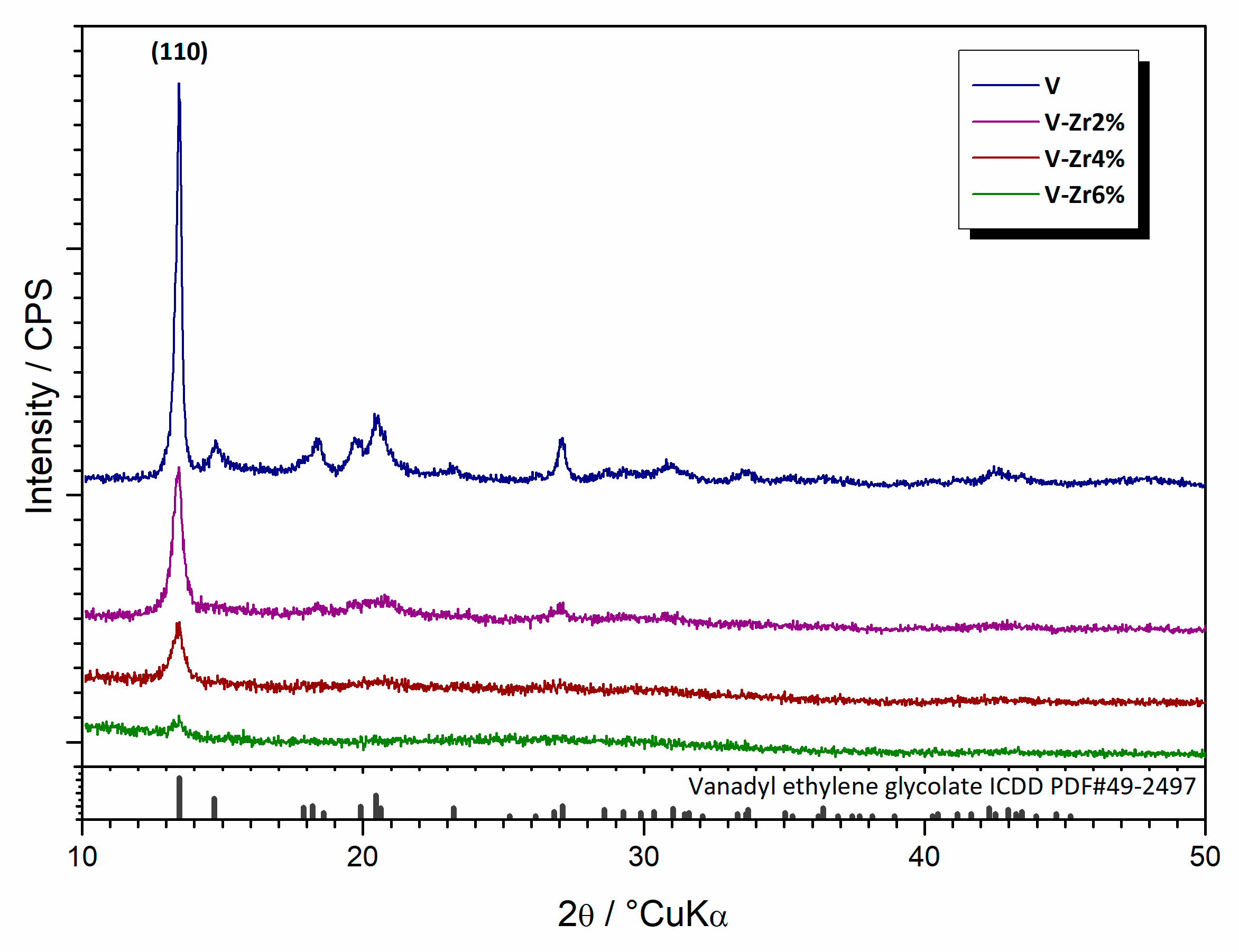
3.2.1. Synthesis and Thermal Evolution
3.2.2. Thermally Treated Powders
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Batista, C.; Ribeiro, R.M.; Teixeira, V. Synthesis and characterization of VO2-based thermochromic thin films for energy-efficient windows. Nanoscale Res. Lett. 2011, 6, 301. [Google Scholar] [CrossRef] [PubMed]
- Alie, D.; Gedvilas, L.; Wang, Z.; Tenent, R.; Engtrakul, C.; Yan, Y.; Shaheen, S.E.; Dillon, A.C.; Ban, C. Direct synthesis of thermochromic VO2 through hydrothermal reaction. J. Solid State Chem. 2014, 212, 237–241. [Google Scholar] [CrossRef]
- Shen, N.; Chen, S.; Chen, Z.; Liu, X.; Cao, C.; Dong, B.; Luo, H.; Liua, J.; Gao, Y. The synthesis and performance of Zr-doped and W–Zr-codoped VO2 nanoparticles and derived flexible foils. J. Mater. Chem. A 2014, 2, 15087–15093. [Google Scholar] [CrossRef]
- Wang, S.; Owusu, K.A.; Mai, L.; Ke, Y.; Zhou, Y.; Hu, P.; Magdassi, S.; Long, Y. Vanadium dioxide for energy conservation and energy storage applications: Synthesis and performance improvement. Appl. Energy 2018, 218, 200–217. [Google Scholar] [CrossRef]
- Wang, S.; Minsu, L.; Lingbing, K.; Long, Y.; Xuchuan, J.; Aibing, Y. Recent progress in VO2 smart coatings: Strategies to improve the thermochromic properties. Prog. Mater. Sci. 2016, 81, 1–54. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J.; Wang, L.; Luo, H.; Gao, Y. Unravelling Mechanism on Reducing Thermal Hysteresis Width of VO2 by Ti Doping: A Joint Experimental and Theoretical Study. J. Phys. Chem. C 2014, 118, 18938–18944. [Google Scholar] [CrossRef]
- Lu, W.; Zhao, G.; Song, B.; Li, J.; Zhang, X.; Han, G. Preparation and thermochromic properties of sol-gel-derived Zr-doped VO2 films. Surf. Coat Technol. 2017, 320, 311–314. [Google Scholar] [CrossRef]
- Cui, Y.; Ke, Y.; Liu, C.; Chen, Z.; Wang, N.; Zhang, L.; Zhou, Y.; Wang, S.; Gao, Y.; Long, Y. Thermochromic VO2 for Energy-Efficient Smart Windows. Joule 2018, 2, 1707–1746. [Google Scholar] [CrossRef]
- Zong, H.; Liu, H.; Yan, L.; Yin, Y.; Bian, L.; Kang, C.; Cao, G.; Li, M. Synchronized improvements of luminous transmittance and solar modulation ability of VO2 films by employing SnO2 buffer layers. Thin Solid Films 2020, 709, 138174. [Google Scholar] [CrossRef]
- Saini, M.; Dehiya, B.S.; Umar, A. VO2(M)@CeO2 core-shell nanospheres for thermochromic smart windows and photocatalytic applications. Ceram. Int. 2020, 46, 986–995. [Google Scholar] [CrossRef]
- Saitzek, S.; Guinneton, F.; Sauques, L.; Aguir, K.; Gavarri, J.-R. Thermochromic CeO2-VO2 bilayers: Role of ceria coating in optical switching properties. Opt. Mater. 2007, 30, 407–415. [Google Scholar] [CrossRef]
- Song, L.; Huang, W.; Zhang, Y.; Li, D.; Shi, Q.; Zheng, S.; Li, N.; Xu, Y. Characteristics of CeOx-VO2 composite thin films synthesized by sol-gel process. J. Mater. Sci. Mater. Electron. 2013, 24, 3496–3503. [Google Scholar] [CrossRef]
- Song, L.; Zhang, Y.; Huang, W.; Shi, Q.; Li, D.; Zhang, Y.; Xu, Y. Preparation and thermochromic properties of Ce-doped VO2 films Mater. Res. Bull. 2013, 48, 2268–2271. [Google Scholar] [CrossRef]
- Guo, B.; Wan, D.; Wang, J.; Zhu, S.; Luo, H.; Gao, Y. Mo-Al co-doped VO2(B) thin films: CVD synthesis, thermal sensitive properties, synchrotron radiation photoelectron and absorption spectroscopy study. J. Alloys Compd. 2018, 745, 247–255. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Z.; Zhang, H.; Niuc, R.; He, Q.; Ji, C.; Wang, J.; Jiang, Y. Enhancement of VO2 thermochromic properties by Si doping. Surf. Coat. Technol. 2015, 276, 248–253. [Google Scholar] [CrossRef]
- Hu, Y.; Shi, Q.; Huang, W.; Zhu, H.; Yue, F.; Xiao, Y.; Liang, S.; Lu, T. Preparation and phase transition properties of Ti-doped VO2 films by sol–gel process. J. Solgel Sci. Technol. 2016, 78, 19–25. [Google Scholar] [CrossRef]
- Kolenatý, D.; Vlček, J.; Bárta, T.; Rezek, J.; Houska, J.; Havaira, S. High-performance thermochromic VO2-based coatings with a low transition temperature deposited on glass by a scalable technique. Sci. Rep. 2020, 10, 11107. [Google Scholar] [CrossRef]
- Houska, J.; Kolenaty, D.; Vlcek, J.; Barta, T.; Rezek, J.; Cerstvy, R. Significant improvement of the performance of ZrO2/V1-xWxO2/ZrO2 thermochromic coatings by utilizing a second-order interference. Sol. Energy Mater. Sol. Cells 2019, 191, 365–371. [Google Scholar] [CrossRef]
- Vlček, J.; Kolenatý, D.; Houška, J.; Kozák, T.; Čerstvý, R. Controlled reactive HiPIMS—Effective technique for low-temperature (300 °C) synthesis of VO2 films with semiconductor-to-metal transition. J. Phys. D Appl. Phys. 2017, 50, 38LT01. [Google Scholar] [CrossRef]
- Houska, J.; Kolenaty, D.; Vlcek, J.; Cerstvy, R. Properties of thermochromic VO2 films prepared by HiPIMS onto unbiased amorphous glass substrates at a low temperature of 300 °C. Thin Solid Films 2018, 660, 463–470. [Google Scholar] [CrossRef]
- Zou, J.; Peng, Y.; Lin, H. A low-temperature synthesis of monoclinic VO2 in an atmosphere of air. J. Mater. Chem. A 2013, 1, 4250–4254. [Google Scholar] [CrossRef]
- Plaisier, J.R.; Nodari, L.; Gigli, L.; Rebollo, S.M.E.P.; Bertoncello, R.; Lausi, A. The X-ray diffraction beamline MCX at Elettra: A case study of non-destructive analysis on stained glass. ACTA IMEKO 2017, 6, 71–75. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Q.; Koughia, C.; Ye, F.; Sanayei, M.; Wen, S.J.; Kasap, S. Characterization of vanadium oxide thin films with different stoichiometry using Raman spectroscopy. Thin Solid Films 2016, 620, 64–69. [Google Scholar] [CrossRef]
- Magg, N.; Immaraporn, B.; Giorgi, J.B.; Schroeder, T.; Bäumer, M.; Döbler, J.; Wu, Z.; Kondratenko, E.; Cherian, M.; Baerns, M.; et al. Vibrational spectra of alumina-and silica-supported vanadia revisited: An experimental and theoretical model catalyst study. J. Catal. 2004, 226, 88–100. [Google Scholar] [CrossRef]
- Uchaker, E.; Zhou, N.; Li, Y.; Cao, G. Polyol-Mediated Solvothermal Synthesis and Electrochemical Performance of Nanostructured V2O5 Hollow Microspheres. J. Phys. Chem. C 2013, 117, 1621–1626. [Google Scholar] [CrossRef]
- Gonçalves, A.; Resende, J.; Marques, A.C.; Pinto, J.V.; Nunes, D.; Marie, A.; Goncalves, R.; Pereira, L.; Martins, R.; Fortunato, E. Smart optically active VO2 nanostructured layers applied in roof-type ceramic tiles for energy efficiency. Sol. Energy Mater. Sol. Cells 2016, 150, 1–9. [Google Scholar] [CrossRef]
- Fu, C.J.; Zhan, Z.W.; Yu, M.; Li, S.M.; Liu, J.H.; Dong, L. Influence of Zr/Si molar ratio on structure, morphology and corrosion resistant of organosilane coatings doped with zirconium (IV) n-propoxide. Int. J. Electrochem. Sci. 2014, 9, 2603–2619. [Google Scholar]
- Krasilnikov, V.N.; Shtin, A.P.; Gyrdasova, O.I.; Baklanova, I.V.; Perelyaeva, L.A. Vanadyl and Titanium Glycolates as Precursors for the Preparation of Oxide Materials in the Form of Elongated Microparticles and Nanoparticles. Nanotechnol. Russ. 2008, 3, 106–111. [Google Scholar]
- Cao, Z.; Xiao, X.; Lu, X.; Zhan, Y.; Cheng, H.; Xu, G. A simple and low-cost combustion method to prepare monoclinic VO2 with superior thermochromic Properties. Sci. Rep. 2016, 6, 39154. [Google Scholar] [CrossRef]
- Basu, R.; Dhara, S. Spectroscopic study of native defects in the semiconductor to metal phase transition in V2O5 nanostructure. J. Appl. Phys. 2018, 123, 161550. [Google Scholar] [CrossRef]
- Ji, H.; Liu, D.; Cheng, H.; Zhang, C.; Yang, L.; Ren, D. Infrared thermochromic properties of monoclinic VO2 nanopowders using a malic acid-assisted hydrothermal method for adaptive camouflage. RSC Adv. 2017, 7, 5189–5194. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Z.; Ji, C.; Feng, H.; Ma, X.; Su, Y.; Zhou, Y.; Wang, J.; Jiang, Y. Influence of infrared optical properties by transformation of the crystal structure in Al-doped vanadium dioxide films. Opt. Mater. Express 2016, 6, 3500–3506. [Google Scholar] [CrossRef]
- Slurca, A.; Orel, B. IR spectroscopy of crystalline V2O5 films in different stages of lithiation. Electrochim. Acta 1999, 44, 3051–3057. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Abeiyan, N. Chemical Synthesis of Vanadium Oxide (V2O5) Nanoparticles Prepared by Sodium Metavanadate. J. Nanomed. Res. 2017, 5, 103. [Google Scholar] [CrossRef]
- Chinchamalatpure, V.R.; Chore, S.M.; Patil, S.S.; Chaudhari, G.N. Synthesis and Electrical Characterization of ZrO2 Thin Films on Si(100). J. Mod. Phys. 2012, 3, 69–73. [Google Scholar] [CrossRef]
- Zhang, Y. VO2(B) conversion to VO2(A) and VO2(M) and their oxidation resistance and optical switching properties. Mater. Sci.-Pol. 2016, 34, 169–176. [Google Scholar] [CrossRef]
- Jin, P.; Tanemura, S. V1-xMoxO2 thermochromic films deposited by reactive magnetron sputtering. Thin Solid Films 1996, 281, 239–242. [Google Scholar] [CrossRef]
- Shelton, D.J.; Coffey, K.R.; Boreman, G.D. Experimental demonstration of tunable phase in a thermochromic infrared-reflectarray metamaterial. Opt. Express 2010, 18, 1330–1335. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Wang, D.; Dang, Y. Effects of Zirconium Ions Doping on the Structural and Thermochromic Properties of VO2 Thin Films. J. Electron. Mater. 2017, 46, 6466–6472. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, L.; Liu, Q.; Chen, S.; Huang, W.; Chen, F.; Liao, G.; Zou, C.; Wu, Z. Decoupling the Lattice Distortion and Charge Doping Effects on the Phase Transition Behavior of VO2 by Titanium (Ti4+) Doping. Sci. Rep. 2015, 5, 9328. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Yao, T.; Long, R.; Sun, Z.; Feng, Y.; Cheng, H.; Yuan, X.; Zhang, W.; Liu, Q.; Wu, C.; et al. Unraveling Metal-insulator Transition Mechanism of VO2 Triggered by Tungsten Doping. Sci. Rep. 2012, 2, 466. [Google Scholar] [CrossRef] [PubMed]
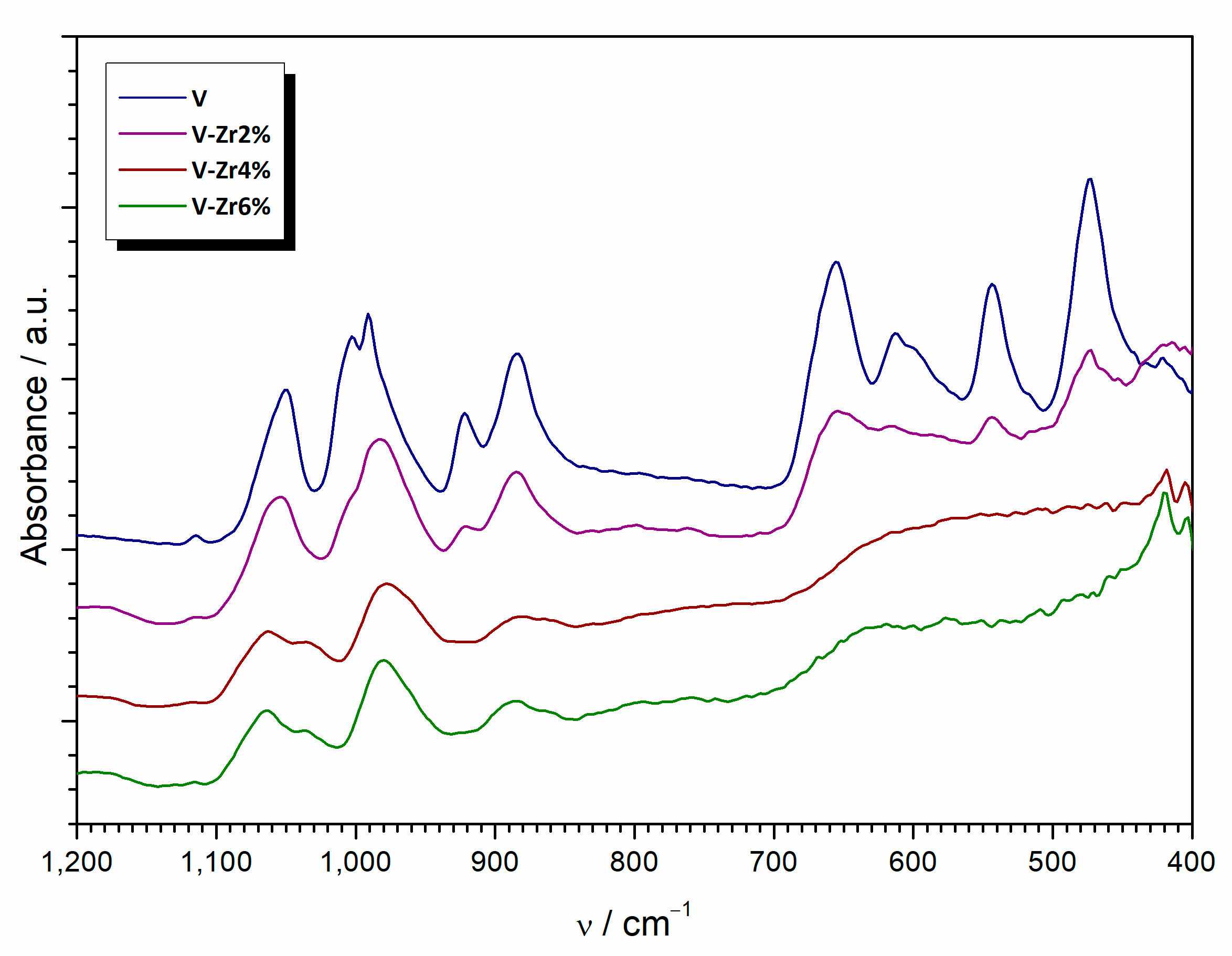


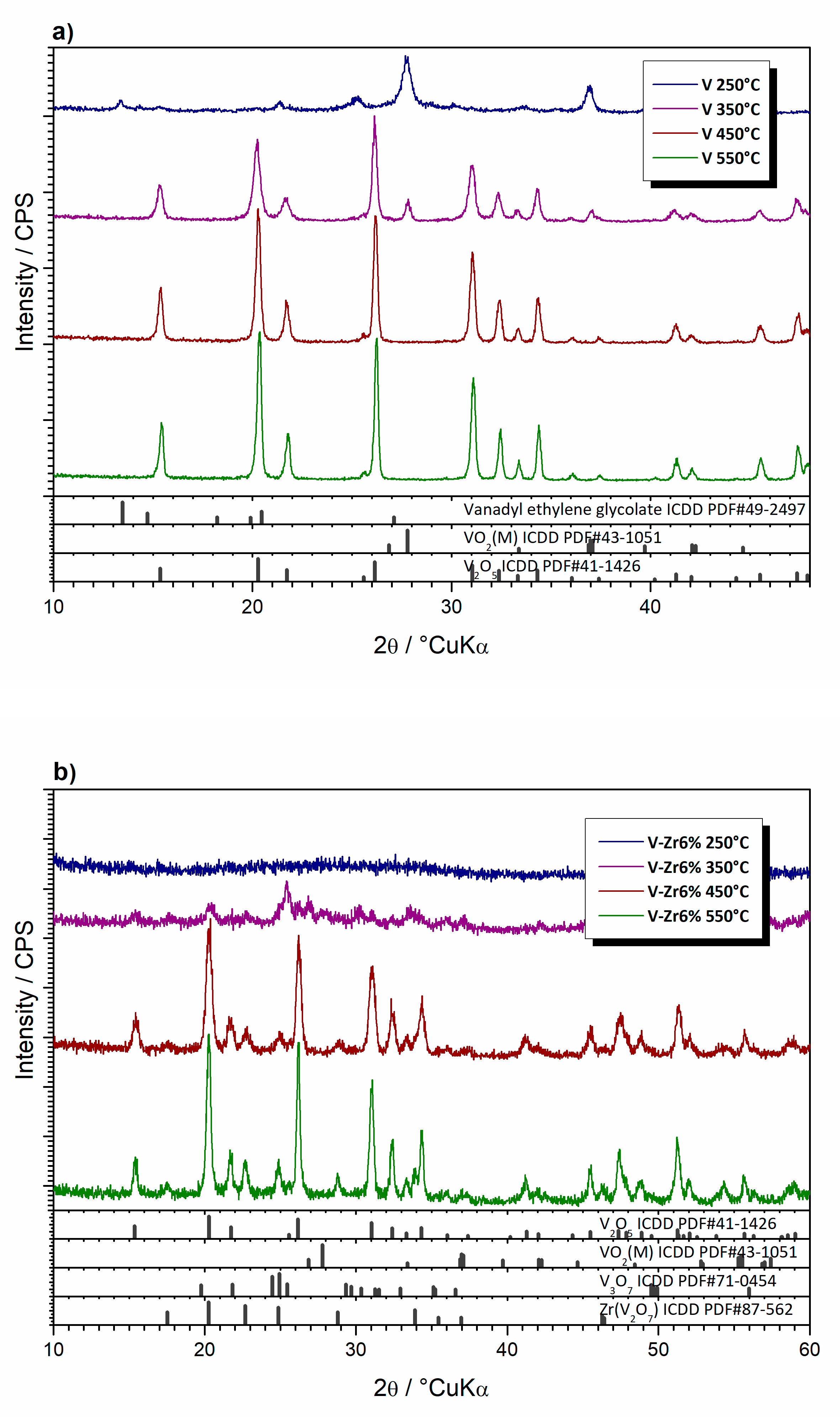
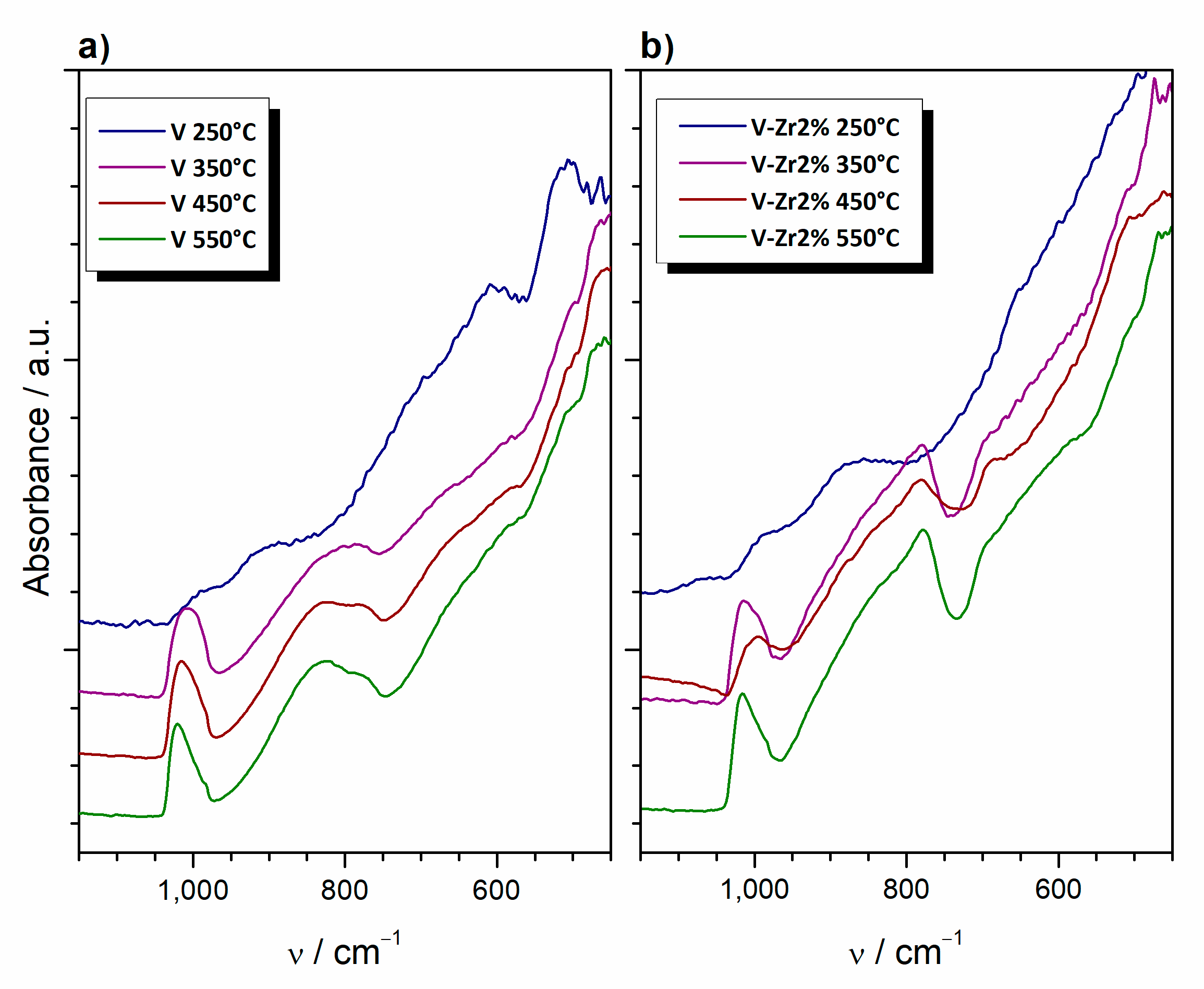
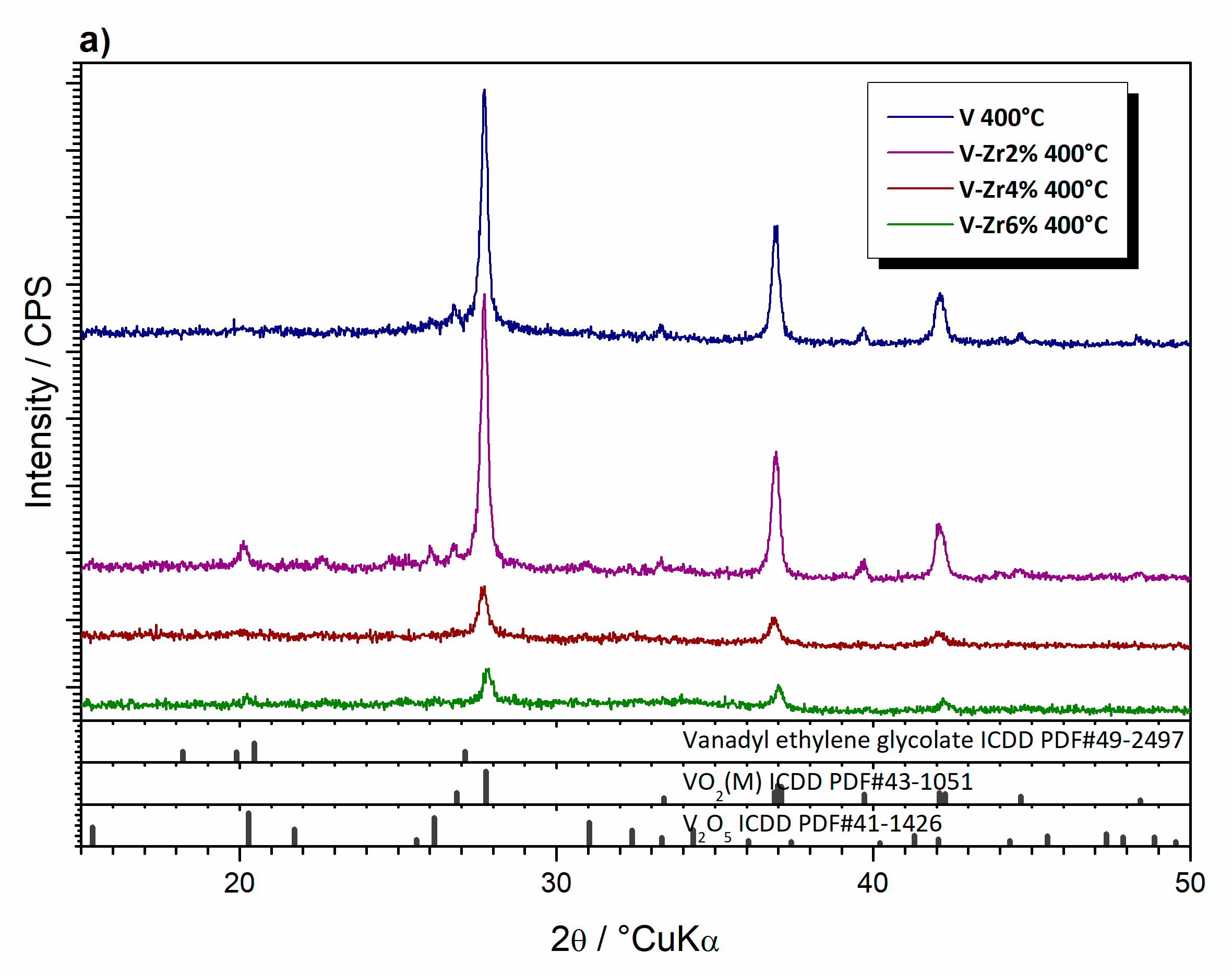


| Sample | Composition | Type | Thermal Treatment (°C, min) |
|---|---|---|---|
| V | VO2 | powder | 150, 60 |
| V-Zr2% | V0.98Zr0.02O2 | powder | 150, 60 |
| V-Zr4% | V0.96Zr0.04O2 | powder | 150, 60 |
| V-Zr6% | V0.94Zr0.06O2 | powder | 150, 60 |
| V | VO2 | film | 100, 360 |
| V-Zr2% | V0.98Zr0.02O2 | film | 100, 360 |
| V-Ce2% | V0.98Ce0.02O2 | film | 100, 360 |
| Sample | Type | Crystallites (nm) | Specific Surface Area (Arbitrary Units) | Film Thickness (μm) | Thermochromic Quality (Arbitrary Units) |
|---|---|---|---|---|---|
| V | powder | 140 | moderate | - | ••••• |
| V-Zr2% | powder | 122 | moderate | - | •••• |
| V-Zr4% | powder | 84 | high | - | •• |
| V-Zr6% | powder | 77 | high | - | • |
| V | film | 50 | - | 24 | ••• |
| V-Zr2% | film | 44 | - | 24 | •••• |
| V-Ce2% | film | 55 | - | 24 | ••••• |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurajica, S.; Mandić, V.; Panžić, I.; Gaboardi, M.; Mužina, K.; Lozančić, A.; Šipušić, J.; Munda, I.K.; Višić, L.; Blagojević, S.L.; et al. In-Operando Diffraction and Spectroscopic Evaluation of Pure, Zr-, and Ce-Doped Vanadium Dioxide Thermochromic Films Derived via Glycolate Synthesis. Nanomaterials 2020, 10, 2537. https://doi.org/10.3390/nano10122537
Kurajica S, Mandić V, Panžić I, Gaboardi M, Mužina K, Lozančić A, Šipušić J, Munda IK, Višić L, Blagojević SL, et al. In-Operando Diffraction and Spectroscopic Evaluation of Pure, Zr-, and Ce-Doped Vanadium Dioxide Thermochromic Films Derived via Glycolate Synthesis. Nanomaterials. 2020; 10(12):2537. https://doi.org/10.3390/nano10122537
Chicago/Turabian StyleKurajica, Stanislav, Vilko Mandić, Ivana Panžić, Mattia Gaboardi, Katarina Mužina, Ana Lozančić, Juraj Šipušić, Ivana Katarina Munda, Lucija Višić, Sanja Lučić Blagojević, and et al. 2020. "In-Operando Diffraction and Spectroscopic Evaluation of Pure, Zr-, and Ce-Doped Vanadium Dioxide Thermochromic Films Derived via Glycolate Synthesis" Nanomaterials 10, no. 12: 2537. https://doi.org/10.3390/nano10122537
APA StyleKurajica, S., Mandić, V., Panžić, I., Gaboardi, M., Mužina, K., Lozančić, A., Šipušić, J., Munda, I. K., Višić, L., Blagojević, S. L., Gigli, L., & Plaisier, J. R. (2020). In-Operando Diffraction and Spectroscopic Evaluation of Pure, Zr-, and Ce-Doped Vanadium Dioxide Thermochromic Films Derived via Glycolate Synthesis. Nanomaterials, 10(12), 2537. https://doi.org/10.3390/nano10122537