Germination and Early Development of Three Spontaneous Plant Species Exposed to Nanoceria (nCeO2) with Different Concentrations and Particle Sizes
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticles Characterization
2.2. Experimental Setup
2.3. Ce concentration in Plant Seedlings
2.4. Internalization of nCeO2 in Plant Tissues
2.5. Data Analysis
3. Results
3.1. Characterization of nCeO2
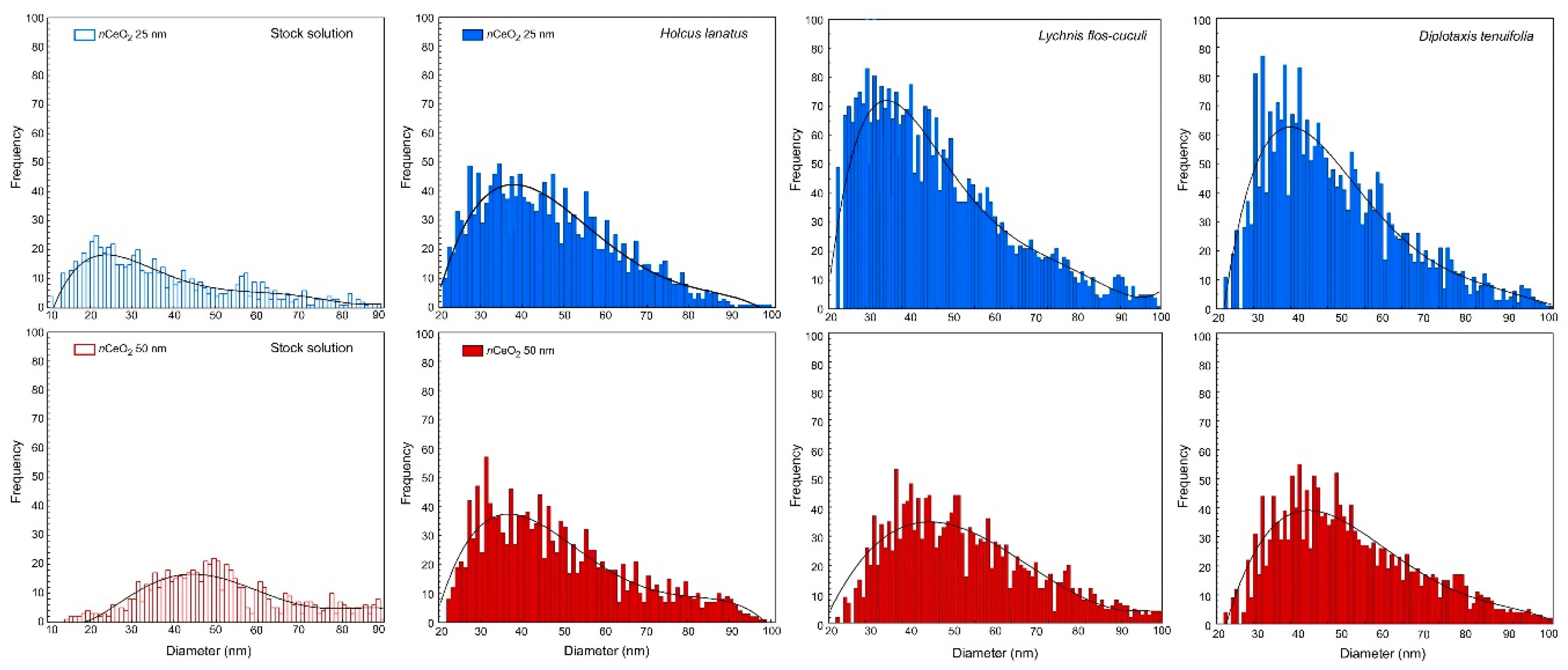
3.2. nCeO2 Plant Internalization
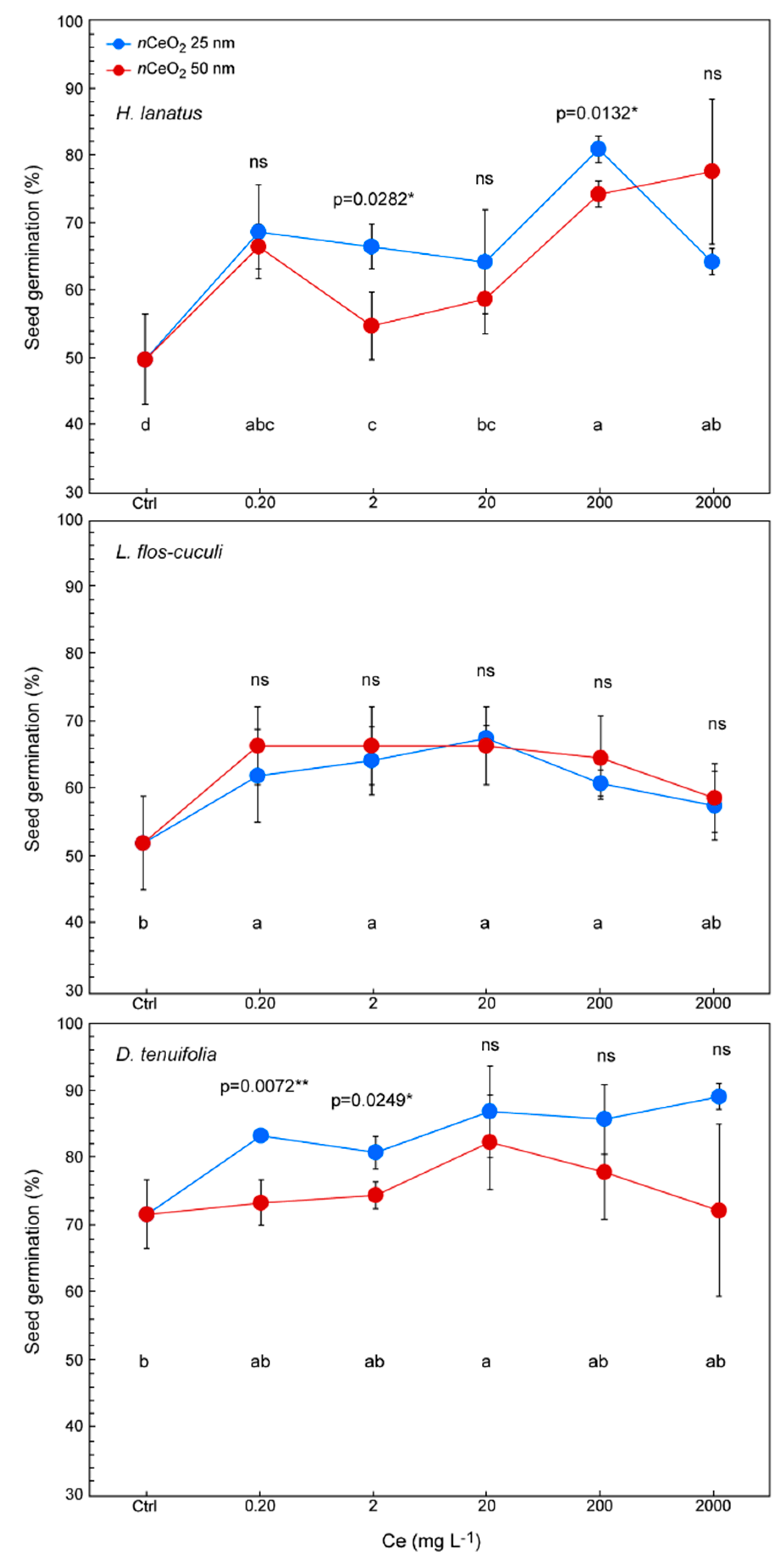
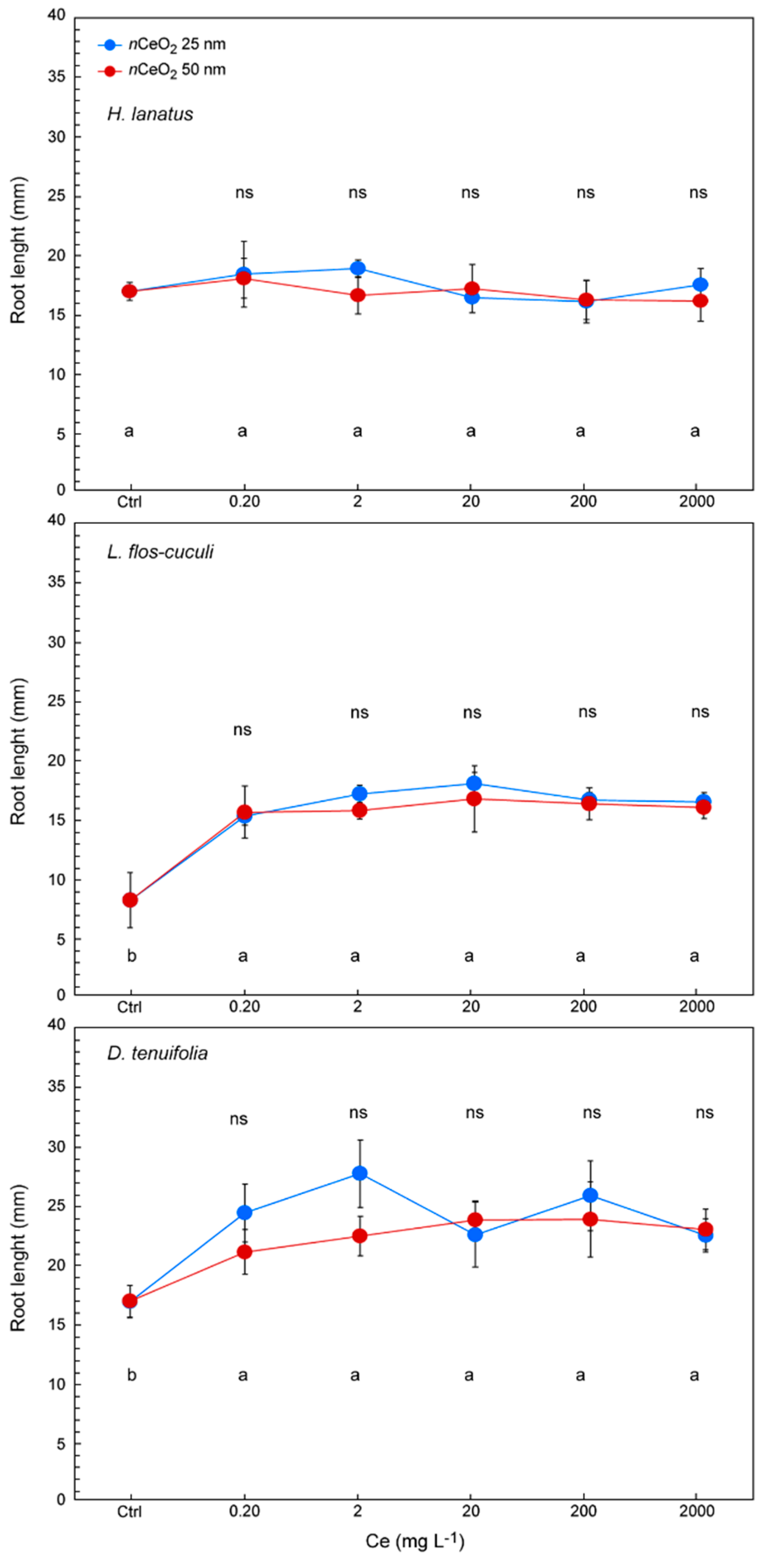
3.3. Seed Germination and Root Length
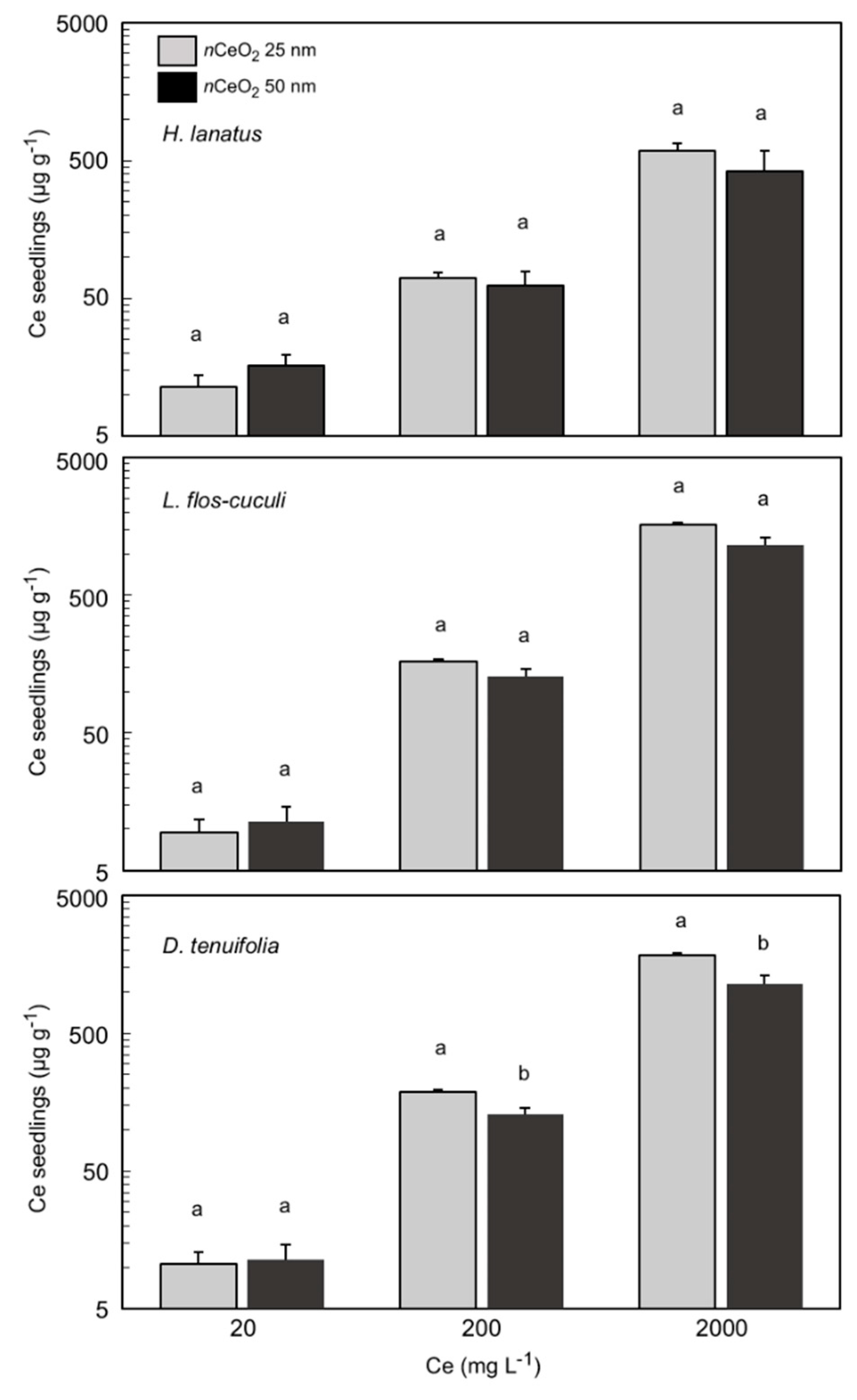
3.4. Ce Concentration in Plant Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bainbridge, W.S.; Roco, M.C. Science and technology convergence: With emphasis for nanotechnology-inspired convergence. J. Nanopart. Res. 2016, 18, 211. [Google Scholar] [CrossRef]
- Mortimer, M.; Holden, P.A. Fate of engineered nanomaterials in natural environments and impacts on ecosystems. In Exposure to Engineered Nanomaterials in the Environment, 1st ed.; Marmiroli, N., White, J., Song, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–103. [Google Scholar] [CrossRef]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Holden, P.A.; Gardea-Torresdey, J.L.; Klaessig, F.; Turco, R.F.; Mortimer, M.; Hund-Rinke, K.; Cohen Hubal, E.A.; Avery, D.; Barceló, D.; Behra, R.; et al. Considerations of environmentally relevant test conditions for improved evaluation of ecological hazards of engineered nanomaterials. Environ. Sci. Technol. 2016, 50, 6124–6145. [Google Scholar] [CrossRef] [PubMed]
- Reddy Pullagurala, V.L.; Adisa, I.O.; Rawat, S.; White, J.C.; Zuverza-Mena, N.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Fate of engineered nanomaterials in agroenvironments and impacts on agroecosystems. In Exposure to Engineered Nanomaterials in the Environment, 1st ed.; Marmiroli, N., White, J., Song, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 105–142. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 5, 16506–16511. [Google Scholar] [CrossRef]
- Priester, J.H.; Ge, Y.; Mielke, R.E.; Horst, A.M.; Moritz, S.C.; Espinosa, K.; Gelb, J.; Walker, S.L.; Nisbet, R.M.; An, Y.J.; et al. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc. Natl. Acad. Sci. USA 2012, 109, 2451–2456. [Google Scholar] [CrossRef]
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernàndez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses. A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef]
- Marchiol, L.; Iafisco, M.; Fellet, G.; Adamiano, A. Nanotechnology support the next agricultural revolution: Perspectives to enhancement of nutrient use efficiency. Adv. Agron. 2020, 161, 27–116. [Google Scholar] [CrossRef]
- Hawthorne, J.; De la Torre Roche, R.; Xing, B.; Newman, L.A.; Ma, X.; Majumdar, S.; Gardea-Torresdey, J.G.; White, J.C. Particle-size dependent accumulation and trophic transfer of cerium oxide through a terrestrial food chain. Environ. Sci. Technol. 2014, 48, 13102–13109. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Xu, Y.; Yin, Y.; Ji, R.; Guo, H. Risk assessment of engineered nanoparticles and other contaminants in terrestrial plants. Curr. Opin. Environ. Sci. Health 2018, 6, 21–28. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Avellan, A.; Bland, G.; Tappero, R.V.; Acerbo, A.S.; Unrine, J.M.; Giraldo, J.P.; Lowry, G.V. Nanoparticle surface charge influences translocation and leaf distribution in vascular plants with contrasting anatomy. Environ. Sci. Nano 2019, 6, 2508–2519. [Google Scholar] [CrossRef]
- Asztemborska, M.; Bembenek, M.; Jakubiak, M.; Stęborowski, R.; Bystrzejewska-Piotrowska, G. The effect of nanoparticles with sorption capacity on the bioaccumulation of divalent ions by aquatic plants. Int. J. Environ. Res. 2018, 12, 245–253. [Google Scholar] [CrossRef]
- Ding, Y.; Bai, X.; Ye, Z.; Ma, L.; Liang, L. Toxicological responses of Fe3O4 nanoparticles on Eichhornia crassipes and associated plant transportation. Sci. Total Environ. 2019, 671, 558–567. [Google Scholar] [CrossRef]
- Movafeghi, A.; Khataee, A.; Abedi, M.; Tarrahi, R.; Dadpour, M.; Vafaei, F. Effects of TiO2 nanoparticles on the aquatic plant Spirodela polyrrhiza: Evaluation of growth parameters, pigment contents and antioxidant enzyme activities. J. Environ. Sci. 2018, 64, 130–138. [Google Scholar] [CrossRef]
- Ekperusia, A.O.; Sikokic, F.D.; Nwachukwud, E.O. Application of common duckweed (Lemna minor) in phytoremediation of chemicals in the environment: State and future perspective. Chemosphere 2019, 223, 285–309. [Google Scholar] [CrossRef]
- Geitner, N.K.; Cooper, J.L.; Avellan, A.; Castellon, B.T.; Perrotta, B.G.; Bossa, N.; Simonin, M.; Anderson, S.M.; Inoue, S.; Hochella, M.F.; et al. Size-based differential transport, uptake, and mass distribution of ceria (CeO2) nanoparticles in wetland mesocosms. Environ. Sci. Technol. 2018, 52, 9768–9776. [Google Scholar] [CrossRef]
- Yin, L.; Colman, B.P.; McGill, B.M.; Wright, J.P.; Bernhardt, E.S. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 2012, 7, e47674. [Google Scholar] [CrossRef]
- Jacob, D.L.; Borchardt, J.D.; Navaratnam, L.; Otte, M.L.; Bezbaruah, A.N. Uptake and translocation of Ti nanoparticles in crops and wetland plants. Int. J. Phytoremediation 2013, 15, 142–153. [Google Scholar] [CrossRef]
- Song, U.; Lee, S. Phytotoxicity and accumulation of zinc oxide nanoparticles on the aquatic plants Hydrilla verticillata and Phragmites australis: Leaf-type-dependent responses. Environ. Sci. Pollut. Res. 2016, 23, 8539–8545. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrowicz-Trzcińska, M.; Bederska-Błaszczyk, M.; Szaniawski, A.; Olchowik, J.; Studnicki, M. The effects of copper and silver nanoparticles on container-grown Scots pine (Pinus sylvestris L.) and Pedunculate oak (Quercus robur L.) seedlings. Forests 2019, 10, 269. [Google Scholar] [CrossRef]
- Dietz, K.J.; Herth, S. Plant nanotoxicology. Trends Plant Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Gottschalk, F.; Hungerbuhler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014, 185, 69–76. [Google Scholar] [CrossRef]
- OECD. List of manufactured nanomaterials and list of endpoints for phase one of the OECD testing programme. In Safety of Manufactured Nanomaterials No. 6; ENV/JM/MONO(2008)13/REV; Organisation for Economic Co-operation and Development: Paris, France, 2008. [Google Scholar]
- Ramirez-Olvera, S.M.; Trejo-Téllez, L.I.; García-Morales, S.; Pérez-Sato, J.R.; Gomez-Merino, F.C. Cerium enhances germination and shoot growth, and alters mineral nutrient concentration in rice. PLoS ONE 2018, 13, e0194691. [Google Scholar] [CrossRef] [PubMed]
- Lizzi, D.; Mattiello, A.; Marchiol, L. Impacts of Cerium Oxide Nanoparticles (nCeO2) on Crop Plants: A Concentric Overview. In Nanomaterials in Plants, Algae and Micro-Organisms. Concepts and Controversies; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 311–322. [Google Scholar] [CrossRef]
- Skiba, E.; Wolf, W.M. Cerium oxide nanoparticles affect heavy metals uptake by Pea in a divergent way than their ionic and bulk counterparts. Water Air Soil Pollut. 2019, 230, 248. [Google Scholar] [CrossRef]
- Adisa, I.O.; Rawat, S.; Reddy Pullagurala, V.L.; Dimkpa, C.O.; Elmer, W.E.; White, J.C.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Nutritional status of tomato (Solanum lycopersicum) fruit grown in Fusarium-infested soil: Impact of cerium oxide nanoparticles. J. Agric. Food Chem. 2020, 68, 1986–1997. [Google Scholar] [CrossRef]
- Thompson, J.D.; Turkington, R. The biology of Canadian weeds—Holcus lanatus L. Can. J. Plant Sci. 1988, 68, 131–147. [Google Scholar] [CrossRef]
- Jalas, J.; Suominen, J. Atlas Florae Europaeae (AFE)—Distribution of vascular plants in Europe 7 (Caryophyllaceae (Silenioideae)); The Committee for Mapping the Flora of Europe and Societas Biologica Fennica Vanamo: Helsinki, Finland, 1998. [Google Scholar]
- Hall, M.K.D.; Jobling, J.J.; Rogers, G.S. Some perspectives on rocket as a vegetable crop: A review. Veg. Crops Res. Bull. 2012, 76, 21–41. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Packer, A.P.; Lariviere, D.; Li, C.; Chen, M.; Fawcett, A.; Nielsen, K.; Mattson, K.; Chatt, A.; Scriver, C.; Erhardt, L.S. Validation of an inductively coupled plasma mass spectrometry (ICP-MS) method for the determination of cerium, strontium and titanium in ceramic materials used in radiological dispersal devices (RDDs). Anal. Chim. Acta 2007, 588, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Lamana, J.; Wojcieszek, J.; Jakubiak, M.; Asztemborska, M.; Szpunar, J. Single particle ICP-MS characterization of platinum nanoparticles uptake and bioaccumulation by Lepidium sativum and Sinapis alba plants. J. Anal. At. Spectrom. 2016, 31, 2321. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, Z.; Rao, P.; Lo, I.M.C. Uptake and toxicity studies of magnetic TiO2-based nanophotocatalyst in Arabidopsis thaliana. Chemosphere 2019, 224, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Weaver, J.W. Aggregation and charge behavior of metallic and nonmetallic nanoparticles in the presence of competing similarly-charged inorganic ions. Environ. Sci. Technol. 2010, 44, 3332–3338. [Google Scholar] [CrossRef]
- Yang, L.; Watts, D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005, 158, 122–132. [Google Scholar] [CrossRef]
- Lin, D.H.; Xing, B.S. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Lin, D.H.; Xing, B.S. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Rico, C.M.; White, J.C. Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments. Environ. Sci. Technol. 2014, 48, 2526–2540. [Google Scholar] [CrossRef]
- López-Moreno, M.L.; De La Rosa, G.; Hernàndez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J. Agric. Food Chem. 2010, 58, 3689–3693. [Google Scholar] [CrossRef]
- Andersen, C.P.; King, G.; Plocher, M.; Storm, M.; Pokhrel, L.R.; Johnson, M.G.; Rygiewicz, P.T. Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ. Toxicol. Chem. 2016, 35, 2223–2229. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Schoenfisch, M.H. Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 10247–10254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, Y.; Zhang, Z.; He, X.; Zhang, J.; Guo, Z.; Tai, R.; Zhao, Y.; Chai, Z. Biotransformation of ceria nanoparticles in cucumber plants. ACS Nano 2012, 6, 9943–9950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, X.; Zhang, H.; Ma, Y.; Zhang, P.; Ding, Y.; Zhao, Y. Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics 2011, 3, 816–822. [Google Scholar] [CrossRef]
- Layet, C.; Auffan, M.; Santaella, C.; Chevassus-Rosset, C.; Montes, M.; Ortet, P.; Barakat, M.; Collin, B.; Legros, S.; Bravin, M.N.; et al. Evidence that soil properties and organic coating drive the phytoavailability of cerium oxide nanoparticles. Environ. Sci. Technol. 2017, 51, 9756–9764. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.; Ma, X.; Zhang, W.; Liu, K. Single particle ICP-MS method development for the determination of plant uptake and accumulation of CeO2 nanoparticles. Anal. Bioanal. Chem. 2016, 408, 5157–5167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, Y.; Liu, S.; Wang, G.; Zhang, J.; He, X.; Zhang, J.; Rui, Y.; Zhang, Z. Phytotoxicity, uptake and transformation of nano-CeO2 in sand cultured romaine lettuce. Environ. Pollut. 2017, 220, 1400–1408. [Google Scholar] [CrossRef]
- Zhang, W.; Dan, Y.; Shi, H.; Ma, X. Elucidating the mechanisms for plant uptake and in-planta speciation of cerium in radish (Raphanus sativus L.) treated with cerium oxide nanoparticles. J. Environ. Chem. Eng. 2017, 5, 572–577. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, P.; Zhang, Z.; He, X.; Zhang, J.; Ding, Y.; Zhang, J.; Zheng, L.; Guo, Z.; Zhang, L.; et al. Where does the transformation of precipitated ceria nanoparticles in hydroponic plants take place? Environ. Sci. Technol. 2015, 9, 10667–10674. [Google Scholar] [CrossRef]
- Bao, D.; Oh, Z.G.; Chen, Z. Characterization of silver nanoparticles internalized by Arabidopsis plants using single particle ICP-MS analysis. Front. Plant Sci. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Corredor, E.; Testillano, P.S.; Coronado, M.J.; Gonzalez-Melendi, P.; Fernandez-Pacheco, R.; Marquina, C.; Ibarra, M.R.; De La Fuente, J.M.; Rubiales, D.; Perez De Luque, A.; et al. Nanoparticle penetration and transport in living pumpkin plants: In situ subcellular identification. BMC Plant Biol. 2009, 9, 45–56. [Google Scholar] [CrossRef]
- Avellan, A.; Schwab, F.; Masion, A.; Chaurand, P.; Borschneck, D.; Vidal, V.; Rose, J.; Santaella, C.; Levard, C. Nanoparticle uptake in plants: Gold nanomaterial localized in roots of Arabidopsis thaliana by X-ray computed nanotomography and hyperspectral imaging. Environ. Sci. Technol. 2017, 51, 8682–8691. [Google Scholar] [CrossRef] [PubMed]
- Geisler–Lee, J.; Wang, Q.; Yao, Y.; Zhang, W.; Geisler, M.; Li, K.; Huang, Y.; Chen, Y.; Kolmakov, A.; Ma, X. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 2013, 7, 323–337. [Google Scholar] [CrossRef]
- Ma, Y.H.; He, X.; Zhang, P.; Zhang, Z.Y.; Guo, Z.; Tai, R.Z.; Xu, Z.J.; Zhang, L.J.; Ding, Y.Y.; Zhao, Y.L.; et al. Phytotoxicity and biotransformation of La2O3 nanoparticles in a terrestrial plant cucumber (Cucumis sativus). Nanotoxicology 2011, 5, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Geiser-Lee, M.J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Lv, J.T.; Zhang, S.Z.; Luo, L.; Zhang, J.; Yang, K.; Christie, P. Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize. Environ. Sci. Nano 2015, 2, 68–77. [Google Scholar] [CrossRef]
- Schymura, S.; Fricke, T.; Hildebrand, H.; Franke, K. Elucidating the role of dissolution in CeO2 nanoparticle plant uptake by smart radiolabeling. Angew. Chem. Int. Ed. 2017, 56, 7411–7414. [Google Scholar] [CrossRef]
- McCully, M. How do real roots work? (Some new views of root structure). Plant Physiol. 1995, 109, 1–6. [Google Scholar] [CrossRef]
- Dan, Y.; Zhang, W.; Xue, R.; Ma, X.; Stephan, C.; Shi, H. Characterization of gold nanoparticle uptake by tomato plants using enzymatic extraction followed by single-particle inductively coupled plasma-mass spectrometry analysis. Environ. Sci. Technol. 2015, 49, 3007–3014. [Google Scholar] [CrossRef]
- López-Moreno, M.L.; de la Rosa, G.; Hernàndez-Viezcas, J.A.; Castillo Michel, H.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ. Sci. Technol. 2010, 44, 7315–7320. [Google Scholar] [CrossRef]
- Mattiello, A.; Pošćić, F.; Musetti, R.; Giordano, C.; Vischi, M.; Filippi, A.; Bertolini, A.; Marchiol, L. Evidences of genotoxicity and phytotoxicity in Hordeum vulgare exposed to CeO2 and TiO2 nanoparticles. Front. Plant Sci. 2015, 6, 1043. [Google Scholar] [CrossRef] [PubMed]
- Shtangeeva, I. Europium and cerium accumulation in wheat and rye seedlings. Water Air Soil Pollut. 2014, 225, 1964–1973. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R.G. Rare earth elements in the soil environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Pošćić, F.; Schat, H.; Marchiol, L. Cerium negatively impacts the nutritional status in rapeseed. Sci. Total Environ. 2017, 593–594, 735–744. [Google Scholar] [CrossRef]
- Zhao, J.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Banerjee, K.; Pramanik, P.; Maity, A.; Joshi, D.C.; Wani, S.H.; Krishnan, P. Methods of using nanomaterials to plant systems and their delivery to plants (Mode of entry, uptake, translocation, accumulation, biotransformation and barriers). In Advances in Phytonanotechnology: From Synthesis to Application; Ghorbanpour, M., Wani, S.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–152. [Google Scholar] [CrossRef]
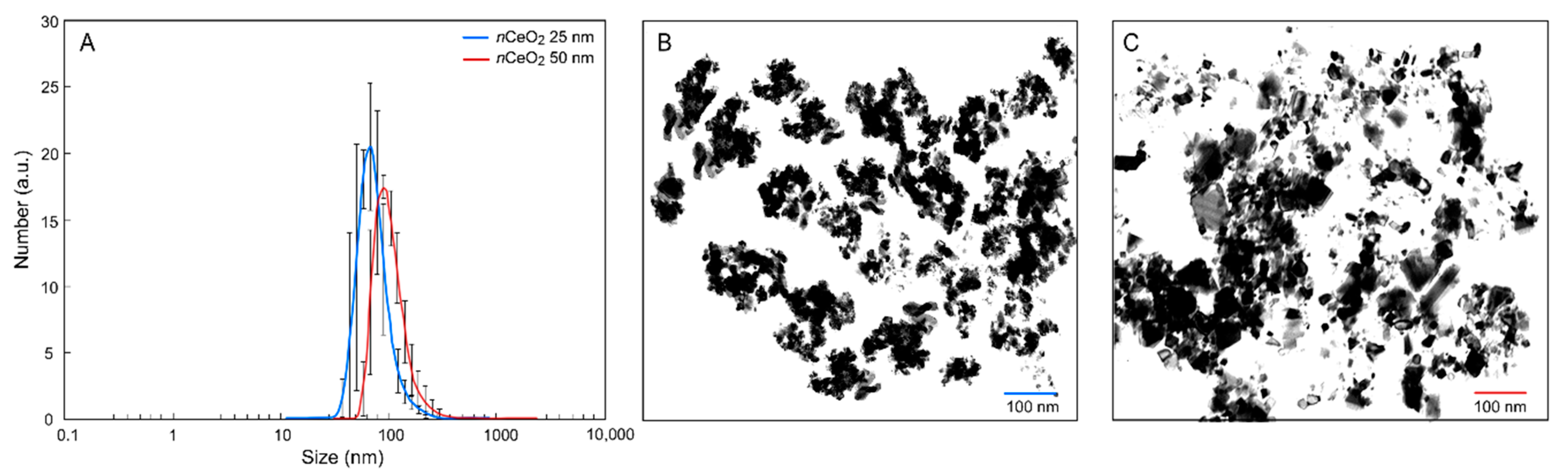
| Material | Z-Average | PDI | ζ-Potential |
|---|---|---|---|
| (nm) | (mV) | ||
| nCeO2 25 nm | 126.7 ± 1.0 | 0.17 ± 0.01 | 39.2 ± 1.1 |
| nCeO2 50 nm | 205.7 ± 1.0 | 0.25 ± 0.02 | 24.1 ± 0.8 |
| Treatment | Species | Most Frequent Size (nm) | Mean Size (nm) | N. of Peaks (n) | Dissolved Ce (ppb) |
|---|---|---|---|---|---|
| nCeO2 25 nm | H. lanatus | 40 | 41 | 1632 | 0.12 |
| D. tenuifolia | 35 | 43 | 2630 | 0.08 | |
| L. flos-cuculi | 31 | 41 | 3079 | 0.03 | |
| nCeO2 50 nm | H. lanatus | 41 | 44 | 1388 | 0.22 |
| D. tenuifolia | 40 | 48 | 1871 | 0.20 | |
| L. flos-cuculi | 41 | 49 | 1842 | 0.21 |
| Effect | % Germination | Root Elongation | Ce Concentration in Seedlings |
|---|---|---|---|
| Species | 0.0000 *** | 0.0000 *** | 0.0000 *** |
| nCeO2 size | 0.0000 *** | 0.0000 *** | 0.0000 *** |
| Ce concentration | 0.0161 * | 0.0000 *** | 0.0000 *** |
| Species × nCeO2 size | 0.0000 *** | 0.0000 *** | 0.0000 *** |
| Species × Ce concentration | 0.0115 * | 0.0000 *** | 0.0000 *** |
| nCeO2 size × Ce concentration | 0.7171 ns | 0.0000 *** | 0.0895 ns |
| Species × nCeO2 size × Ce concentration | 0.0463 * | 0.0000 *** | 0.0848 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizzi, D.; Mattiello, A.; Piani, B.; Fellet, G.; Adamiano, A.; Marchiol, L. Germination and Early Development of Three Spontaneous Plant Species Exposed to Nanoceria (nCeO2) with Different Concentrations and Particle Sizes. Nanomaterials 2020, 10, 2534. https://doi.org/10.3390/nano10122534
Lizzi D, Mattiello A, Piani B, Fellet G, Adamiano A, Marchiol L. Germination and Early Development of Three Spontaneous Plant Species Exposed to Nanoceria (nCeO2) with Different Concentrations and Particle Sizes. Nanomaterials. 2020; 10(12):2534. https://doi.org/10.3390/nano10122534
Chicago/Turabian StyleLizzi, Daniel, Alessandro Mattiello, Barbara Piani, Guido Fellet, Alessio Adamiano, and Luca Marchiol. 2020. "Germination and Early Development of Three Spontaneous Plant Species Exposed to Nanoceria (nCeO2) with Different Concentrations and Particle Sizes" Nanomaterials 10, no. 12: 2534. https://doi.org/10.3390/nano10122534
APA StyleLizzi, D., Mattiello, A., Piani, B., Fellet, G., Adamiano, A., & Marchiol, L. (2020). Germination and Early Development of Three Spontaneous Plant Species Exposed to Nanoceria (nCeO2) with Different Concentrations and Particle Sizes. Nanomaterials, 10(12), 2534. https://doi.org/10.3390/nano10122534