Ultra-Thin SnS2-Pt Nanocatalyst for Efficient Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
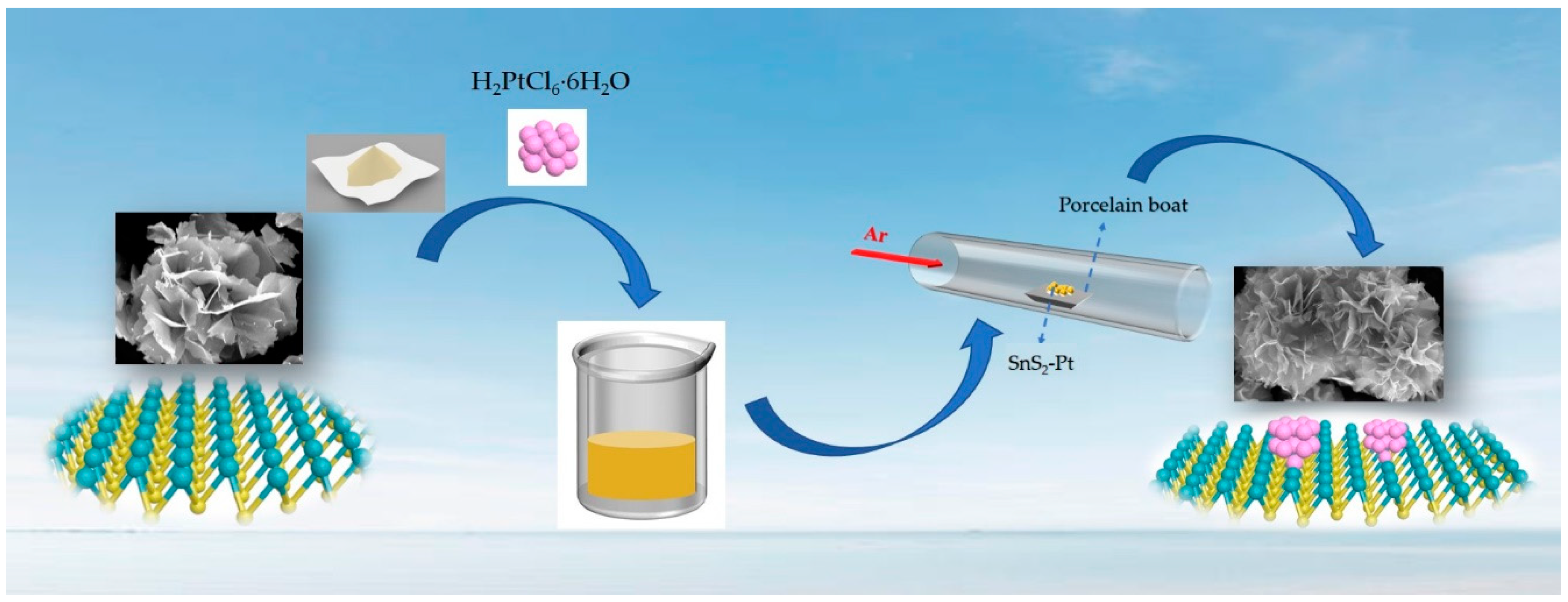
2.2. Synthesis
2.3. Characterization
2.4. Electrochemical Measurements
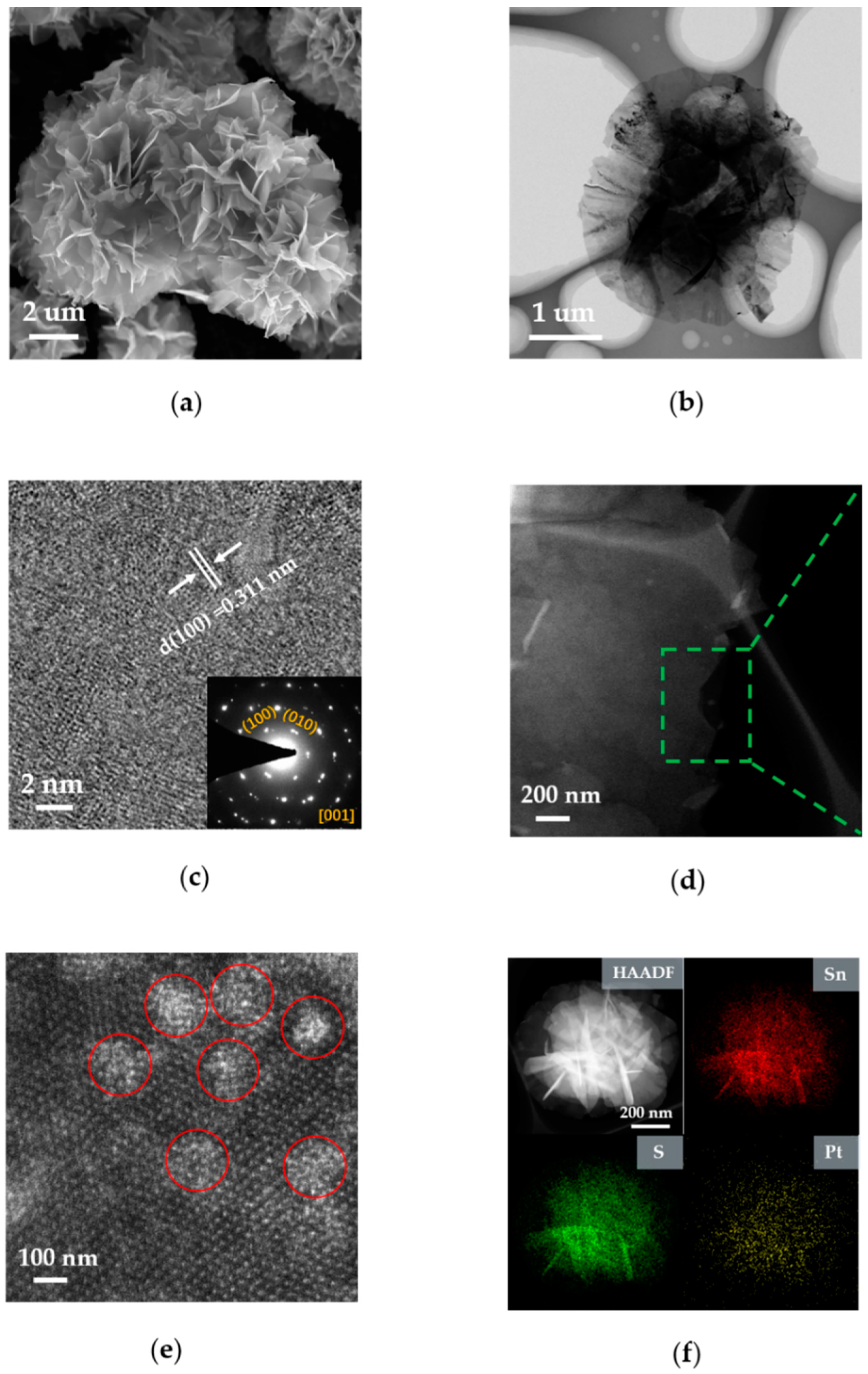
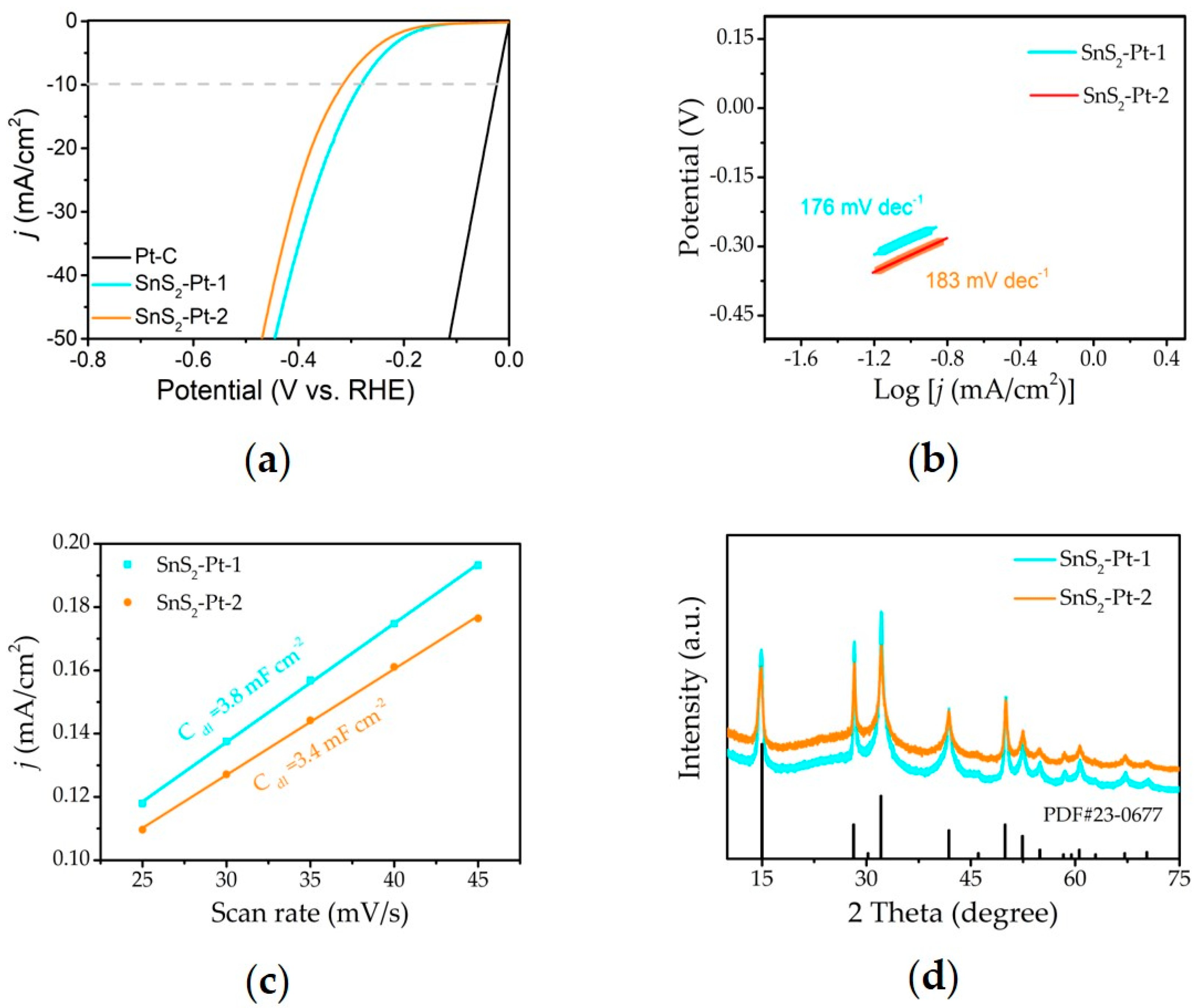
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, H.; Liu, P.; Cheng, C.; Zhu, F.; Hirata, A.; Chen, M. 3D Nanoporous metal phosphides toward high-efficiency electrochemical hydrogen production. Adv. Mater. 2016, 28, 2951–2955. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ren, P.; Deng, D.; Yu, L.; Yang, F.; Bao, X. Highly active and durable non-precious-metal catalysts encapsulated in carbon nanotubes for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 1919–1923. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Tsai, C.; Chan, K.; Benck, J.D.; Nørskov, J.K.; Abild-Pedersen, F.; Jaramillo, T.F. Designing an improved transition metal phosphide catalyst for hydrogen evolution using experimental and theoretical trends. Energy Environ. Sci. 2015, 8, 3022–3029. [Google Scholar] [CrossRef]
- Staszak-Jirkovsky, J.; Malliakas, C.D.; Lopes, P.P.; Danilovic, N.; Kota, S.S.; Chang, K.C.; Genorio, B.; Strmcnik, D.; Stamenkovic, V.R.; Kanatzidis, M.G.; et al. Design of active and stable Co-Mo-Sx chalcogels as pH-universal catalysts for the hydrogen evolution reaction. Nat. Mater. 2016, 15, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2017, 16, 57–69. [Google Scholar] [CrossRef]
- Popczun, E.J.; Read, C.G.; Roske, C.W.; Lewis, N.S.; Schaak, R.E. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 5427–5430. [Google Scholar] [CrossRef]
- Ma, Z.; Tian, H.; Meng, G.; Peng, L.; Chen, Y.; Chen, C.; Chang, Z.; Cui, X.; Wang, L.; Jiang, W.; et al. Size effects of platinum particles@CNT on HER and ORR performance. Sci. China Mater. 2020, in press. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem. Int. Ed. 2015, 54, 52–65. [Google Scholar] [CrossRef]
- Luo, J.; Im, J.H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.G.; Tilley, S.D.; Fan, H.J.; Gratzel, M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef]
- Durst, J.; Siebel, A.; Simon, C.; Hasché, F.; Herranz, J.; Gasteiger, H.A. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014, 7, 2255–2260. [Google Scholar] [CrossRef]
- Balogun, M.S.; Qiu, W.; Yang, H.; Fan, W.; Huang, Y.; Fang, P.; Li, G.; Ji, H.; Tong, Y. A monolithic metal-free electrocatalyst for oxygen evolution reaction and overall water splitting. Energy Environ. Sci. 2016, 9, 3411–3416. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, S.; Wilson, A.R.; Ha, Y.-C.; Wiley, B.J. Optically transparent hydrogen evolution catalysts made from networks of copper–platinum core–shell nanowires. Energy Environ. Sci. 2014, 7, 1461–1467. [Google Scholar] [CrossRef]
- Chen, J.; Yu, D.; Liao, W.; Zheng, M.; Xiao, L.; Zhu, H.; Zhang, M.; Du, M.; Yao, J. WO3-x nanoplates grown on carbon nanofibers for an efficient electrocatalytic hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 18132–18139. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2016, 55, 5277–5281. [Google Scholar] [CrossRef]
- Lu, X.F.; Gu, L.F.; Wang, J.W.; Wu, J.X.; Liao, P.Q.; Li, G.R. Bimetal-organic framework derived CoFe2O4/C porous hybrid nanorod arrays as high-performance electrocatalysts for oxygen evolution reaction. Adv. Mater. 2017, 29, 1604437. [Google Scholar] [CrossRef]
- Lai, F.; Miao, Y.E.; Huang, Y.; Zhang, Y.; Liu, T. Nitrogen-doped carbon nanofiber/molybdenum disulfide nanocomposites derived from bacterial cellulose for high-efficiency electrocatalytic hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 3558–3566. [Google Scholar] [CrossRef]
- Yang, Y.; Fei, H.; Ruan, G.; Li, Y.; Tour, J.M. Vertically aligned WS2 nanosheets for water splitting. Adv. Funct. Mater. 2015, 25, 6199–6204. [Google Scholar] [CrossRef]
- Youn, D.H.; Han, S.; Kim, J.Y.; Kim, J.Y.; Park, H.; Choi, S.H.; Lee, J.S. Highly active and stable hydrogen evolution electrocatalysts based on molybdenum compounds on carbon nanotube-graphene hybrid support. ACS Nano 2014, 8, 5164–5173. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, S.; Li, Y.; Steinmann, S.N.; Yang, W.; Cao, L. Layer-dependent electrocatalysis of MoS2 for hydrogen evolution. Nano Lett. 2014, 14, 553–558. [Google Scholar] [CrossRef]
- Xie, J.; Li, S.; Zhang, X.; Zhang, J.; Wang, R.; Zhang, H.; Pan, B.; Xie, Y. Atomically-thin molybdenum nitride nanosheets with exposed active surface sites for efficient hydrogen evolution. Chem. Sci. 2014, 5, 4615–4620. [Google Scholar] [CrossRef]
- Wan, C.; Regmi, Y.N.; Leonard, B.M. Multiple phases of molybdenum carbide as electrocatalysts for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2014, 53, 6407–6410. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Chen, H.; Wu, Y.; Feng, L.; Liu, Y.; Li, G.; Zou, X. Growth of molybdenum carbide micro-islands on carbon cloth toward binder-free cathodes for efficient hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 16320–16326. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. Int. Ed. 2012, 51, 12703–12706. [Google Scholar] [CrossRef]
- Chia, X.; Pumera, M. Characteristics and performance of two-dimensional materials for electrocatalysis. Nat. Catal. 2018, 1, 909–921. [Google Scholar] [CrossRef]
- Xue, Z.; Su, H.; Yu, Q.; Zhang, B.; Wang, H.; Li, X.; Chen, J. Janus Co/CoP nanoparticles as efficient mott-schottky electrocatalysts for overall water splitting in wide pH range. Adv. Eng. Mater. 2017, 7, 1602355. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, X.; Liu, S.; Chiang, C.L.; Kuai, X.; Peng, C.K.; Lin, Y.C.; Meng, X.; Zhao, J.; Choi, J.; et al. Structural and electronic optimization of MoS2 edges for hydrogen evolution. J. Am. Chem. Soc. 2019, 141, 18578–18584. [Google Scholar] [CrossRef]
- Wu, L.; Longo, A.; Dzade, N.Y.; Sharma, A.; Hendrix, M.; Bol, A.A.; de Leeuw, N.H.; Hensen, E.J.M.; Hofmann, J.P. The origin of high activity of amorphous MoS2 in the hydrogen evolution reaction. Chem. Sustain. Chem. 2019, 12, 4383–4389. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, W.; Ling, X.; Shi, X.; Li, L.; Deng, Y.; An, C.; Han, X. Controlled synthesis of Ni-doped MoS2 hybrid electrode for synergistically enhanced water-splitting process. Chemistry 2019, in press. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, P.; Zhang, J.; Song, W.; Feng, Y.; Gao, D.; Ding, J. Dual-functional N dopants in edges and basal plane of MoS2 nanosheets toward efficient and durable hydrogen evolution. Adv. Energy Mater. 2017, 7, 1602086. [Google Scholar] [CrossRef]
- Nikand, B.; Khalilzadeh, M.A. Liquid phase determination of bisphenol A in food samples using novel nanostructure ionic liquid modified sensor. J. Mol. Liq. 2016, 215, 253–257. [Google Scholar]
- Khalilzadeh, M.A.; Karimi-Maleh, H.; Gupta, V.K. A nanostructure based electrochemical sensor for square wave voltammetric determination of L-Cysteine in the presence of high concentration of folic acid. Electroanalysis 2015, 27, 1766–1773. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Arab, Z. High Sensitive Nanostructure Square Wave Voltammetric sensor for determination of vanillin in food samples. Curr. Anal. Chem. 2017, 13, 81–86. [Google Scholar] [CrossRef]
- Ashjari, M.; Karimi-Maleh, H.; Ahmadpour, F.; Shabani-Nooshabadi, M.; Sadrnia, A.; Khalilzadeh, M.A. Voltammetric analysis of mycophenolate mofetil in pharmaceutical samples via electrochemical nanostructure based sensor modified with ionic liquid and MgO/SWCNTs. J. Taiwan Inst. Chem. E 2017, 80, 989–996. [Google Scholar] [CrossRef]
- Mali, J.M.; Arbuj, S.S.; Ambekar, J.D.; Rane, S.B.; Mulik, U.P.; Amalnerkar, D.P. Hydrothermal synthesis of SnS2 faceted nano sheets and their visible light driven photocatalytic performance. Sci. Adv. Mater. 2013, 5, 1994–1998. [Google Scholar] [CrossRef]
- Tian, H.; Fan, C.; Liu, G.; Zhang, Y.; Wang, M.; Li, E. Hydrothermal synthesis and fast photoresponsive characterization of SnS2 hexagonal nanoflakes. J. Mater. Sci. 2019, 54, 2059–2065. [Google Scholar] [CrossRef]
- Xu, J.; He, J.; Ding, Y.; Luo, J. X-ray imaging of atomic nuclei. Sci. China Mater. 2020, 63, 1788–1796. [Google Scholar] [CrossRef]
- Xu, J.; Lai, S.; Hu, M.; Ge, S.; Xie, R.; Li, F.; Hua, D.; Xu, H.; Zhou, H.; Wu, R. Semimetal 1H-SnS2 enables high-efficiency electroreduction of CO2 to CO. Small Methods 2020, 4, 2000567. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Liu, H.; Sun, J.; Xie, R.; Qiu, Y.; Lü, F.; Liu, Y.; Zhuo, L.; Liu, X.; et al. Amorphous MoOX-Stabilized single platinum atoms with ultrahigh mass activity for acidic hydrogen evolution. Nano Energy 2020, 70, 104529. [Google Scholar] [CrossRef]
- Zhou, M.; Bao, S.; Bard, A.J. Probing Size and Substrate Effects on the Hydrogen Evolution Reaction by Single Isolated Pt Atoms, Atomic Clusters, and Nanoparticles. J. Am. Chem. Soc. 2019, 141, 7327–7332. [Google Scholar] [CrossRef]
- Cheng, X.; Li, Y.; Zheng, L.; Yan, Y.; Zhang, Y.; Chen, G.; Sun, S.; Zhang, J. Highly active, stable oxidized platinum clusters as electrocatalysts for the hydrogen evolution reaction. Energy Environ. Sci. 2017, 10, 2450–2458. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Y.; Xu, X.; Yang, T.; Zhang, D. Preparation of the flower-like MoS2/SnS2 heterojunction as an efficient electrocatalyst for hydrogen evolution reaction. Mol. Catal. 2020, 487, 110890. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Lao, M.; Rui, K.; Zheng, X.; Yu, H.; Ma, J.; Dou, S.; Sun, W. Electrocatalytically inactive SnS2 promotes water adsorption/dissociation on molybdenum dichalcogenides for accelerated alkaline hydrogen evolution. Nano Energy 2019, 64, UNSP 103918. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Pillai, V.V.; Patole, S.P.; Alhassan, S.M. Scalable in situ synthesis of 2D-2D-Type graphene-wrapped SnS2 nanohybrids for enhanced supercapacitor and electrocatalytic applications. ACS Appl. Energy Mater. 2020, 3, 4995–5005. [Google Scholar] [CrossRef]
- Shao, G.; Xue, X.; Wu, B.; Lin, Y.; Ouzounian, M.; Hu, T.S.; Xu, Y.; Liu, X.; Li, S.; Suenaga, K.; et al. Template-assisted synthesis of metallic 1T ’-Sn0.3W0.7S2 nanosheets for hydrogen evolution reaction. Adv. Funct. Mater. 2020, 30, 1906069. [Google Scholar] [CrossRef]
- Kadam, S.R.; Ghosh, S.; Bar-Ziv, R.; Bar-Sadan, M. Structural transformation of SnS2 to SnS by Mo doping produces electro/photocatalyst for hydrogen production. Chem. Eur. J. 2020, 26, 6679–6685. [Google Scholar] [CrossRef]


| Electrode Material | Synthesis Method | Electrolyte | Overpotential at 10 mA/cm2 | Tafel Slope | Reference |
|---|---|---|---|---|---|
| SnS2 SnS2-Pt-3 | Hydrothermal synthesis | 0.5 M H2SO4 | −780 mV −210 mV | 282 mV dec−1 126 mV dec−1 | This work |
| SnS2 MoS2/SnS2 | Hydrothermal method | 0.5 M H2SO4 | −288 mV −580 mV | 76 mV dec−1 50 mV dec−1 | Ref [42] |
| MoSe2 MoSe2/SnS2-2.5 | Hydrothermal method | 1.0 M KOH | −367 mV −285 mV | 149 mV dec−1 109 mV dec−1 | Ref [43] |
| MoS2 MoS2/SnS2-2.5 | Hydrothermal method | 0.5 M H2SO4 | −419 mV −343 mV | 216 mV dec−1 157 mV dec−1 | Ref [43] |
| SnS2 SnS2/G | Solid-state ball-milling approach | 1.0 M KOH | −600 mV −360 mV | 375 mV dec−1 257 mV dec−1 | Ref [44] |
| SnS2 Sn0.3W0.7S2 | Hydrothermal method | 0.5 M H2SO4 | −481 mV −345 mV | 398 mV dec−1 114 mV dec−1 | Ref [45] |
| SnS2 5% Mo-SnS 10% Mo-SnS | Colloidal technique | 0.5 M H2SO4 | −600 mV −486 mV −377 mV | 328 mV dec−1 177 mV dec−1 100 mV dec−1 | Ref [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Xu, J.; Zhang, J.; Li, F.; Fu, J.; Li, C.; An, C. Ultra-Thin SnS2-Pt Nanocatalyst for Efficient Hydrogen Evolution Reaction. Nanomaterials 2020, 10, 2337. https://doi.org/10.3390/nano10122337
Yu Y, Xu J, Zhang J, Li F, Fu J, Li C, An C. Ultra-Thin SnS2-Pt Nanocatalyst for Efficient Hydrogen Evolution Reaction. Nanomaterials. 2020; 10(12):2337. https://doi.org/10.3390/nano10122337
Chicago/Turabian StyleYu, Yanying, Jie Xu, Jianwei Zhang, Fan Li, Jiantao Fu, Chao Li, and Cuihua An. 2020. "Ultra-Thin SnS2-Pt Nanocatalyst for Efficient Hydrogen Evolution Reaction" Nanomaterials 10, no. 12: 2337. https://doi.org/10.3390/nano10122337
APA StyleYu, Y., Xu, J., Zhang, J., Li, F., Fu, J., Li, C., & An, C. (2020). Ultra-Thin SnS2-Pt Nanocatalyst for Efficient Hydrogen Evolution Reaction. Nanomaterials, 10(12), 2337. https://doi.org/10.3390/nano10122337




