Synthesis, Structure and Electrical Resistivity of Carbon Nanotubes Synthesized over Group VIII Metallocenes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qin, L.C. CVD synthesis of carbon nanotubes. J. Mater. Sci. Lett. 1997, 16, 457–459. [Google Scholar] [CrossRef]
- Yellampalli, S. Carbon Nanotubes-Synthesis; Yellampalli, S., Ed.; IntechOpen: London, UK, 2011; ISBN 9789533074979. [Google Scholar]
- Yang, Y.; Hu, Z.; Tian, Y.J.; Lü, Y.N.; Wang, X.Z.; Chen, Y. High-yield production of quasi-aligned carbon nanotubes by catalytic decomposition of benzene. Nanotechnology 2003, 14, 733–737. [Google Scholar] [CrossRef]
- Panic, S.; Bajac, B.; Rakić, S.; Kukovecz, Á.; Kónya, Z.; Srdić, V.; Boskovic, G. Molybdenum anchoring effect in Fe–Mo/MgO catalyst for multiwalled carbon nanotube synthesis. React. Kinet. Mech. Catal. 2017, 122, 775–791. [Google Scholar] [CrossRef]
- Zhu, H.W.; Xu, C.L.; Wu, D.H.; Wei, B.Q.; Vajtai, R.; Ajayan, P.M. Direct synthesis of long single-walled carbon nanotube strands. Science 2002, 296, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Vilatela, J.J.; Windle, A.H. Yarn-like carbon nanotube fibers. Adv. Mater. 2010, 22, 4959–4963. [Google Scholar] [CrossRef]
- Dong, L.; Park, J.G.; Leonhardt, B.E.; Zhang, S.; Liang, R. Continuous Synthesis of Double-Walled Carbon Nanotubes with Water-Assisted Floating Catalyst Chemical Vapor Deposition. Nanomaterials 2020, 10, 365. [Google Scholar] [CrossRef]
- Abdullah, H.B.; Ramli, I.; Ismail, I.; Yusof, N.A. Synthesis and mechanism perspectives of a carbon nanotube aerogel via a floating catalyst chemical vapour deposition method. Bull. Mater. Sci. 2019, 42. [Google Scholar] [CrossRef]
- Tessonnier, J.P.; Su, D.S. Recent progress on the growth mechanism of carbon nanotubes: A review. ChemSusChem 2011, 4, 824–847. [Google Scholar] [CrossRef]
- Mordkovich, V.Z.; Dolgova, E.A.; Karaeva, A.R.; Kharitonov, D.N.; Maslov, I.A.; Kamenev, A.A.; Tretjakov, V.F. Synthesis of carbon nanotubes by catalytic conversion of methane: Competition between active components of catalyst. Carbon 2007. [Google Scholar] [CrossRef]
- Frantsuzov, V.K.; Peshnev, B.V.; Karaeva, A.R.; Estrin, R.I.; Asilova, N.Y.; Lapidus, A.L. Parameters of carbon of fibrous-tubular structure and the related carbonaceous materials. Khimiya Tverd. Topl. 2002, 6, 51–61. [Google Scholar]
- Kenneth, B.K.; Teo Charanjeet, S.; Manish Cha, W.I.M. Catalytic Synthesis of Carbon Nanotubes and Nanofiber. Nanosci. Nanotechnol. 2003, 22, 665–686. [Google Scholar]
- Zhao, T.K.; Zhao, X.; Yan, J.; Du, L.; Li, T.H. Diameter-controlled synthesis of single-walled carbon nanotubes. Adv. Mater. Res. 2013, 652–654, 151–154. [Google Scholar] [CrossRef]
- Li, Y.L.; Kinloch, I.A.; Windle, A.H. Direct Spinning of Carbon Nanotube Fibers from Chemical Vapor Deposition Synthesis. Science 2004, 304, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Reguero, V.; Alemán, B.; Mas, B.; Vilatela, J.J. Controlling carbon nanotube type in macroscopic fibers synthesized by the direct spinning process. Chem. Mater. 2014, 26, 3550–3557. [Google Scholar] [CrossRef]
- Mordkovich, V.Z.; Kazennov, N.V.; Ermolaev, V.S.; Zhukova, E.A.; Karaeva, A.R. Scaled-up process for producing longer carbon nanotubes and carbon cotton by macro-spools. Diam. Relat. Mater. 2018, 83. [Google Scholar] [CrossRef]
- Karaeva, A.R.; Khaskov, M.A.; Mitberg, E.B.; Kulnitskiy, B.A.; Perezhogin, I.; Ivanov, L.A.; Denisov, V.N.; Kirichenko, A.N.; Mordkovich, V.Z. Longer Carbon Nanotubes by Controlled Catalytic Growth in the Presence of Water Vapor. Full Nanotub. Carbon Nanostruct. 2012, 20, 411–418. [Google Scholar] [CrossRef]
- Mayne, M.; Grobert, N.; Terrones, M.; Kamalakaran, R.; Rühle, M.; Kroto, H.W.; Walton, D.R.M. Pyrolytic production of aligned carbon nanotubes from homogeneously dispersed benzene-based aerosols. Chem. Phys. Lett. 2001, 338, 101–107. [Google Scholar] [CrossRef]
- Tapasztó, L.; Kertész, K.; Vértesy, Z.; Horváth, Z.E.; Koós, A.A.; Osváth, Z.; Sárközi, Z.; Darabont, A.; Biró, L.P. Diameter and morphology dependence on experimental conditions of carbon nanotube arrays grown by spray pyrolysis. Carbon 2005, 43, 970–977. [Google Scholar] [CrossRef]
- Stano, K.L.; Koziol, K.; Pick, M.; Motta, M.S.; Moisala, A.; Vilatela, J.J.; Frasier, S.; Windle, A.H. Direct spinning of carbon nanotube fibres from liquid feedstock. Int. J. Mater. Form. 2008, 1, 59–62. [Google Scholar] [CrossRef]
- Reynaud, O.; Nasibulin, A.G.; Anisimov, A.S.; Anoshkin, I.V.; Jiang, H.; Kauppinen, E.I. Aerosol feeding of catalyst precursor for CNT synthesis and highly conductive and transparent film fabrication. Chem. Eng. J. 2014, 255, 134–140. [Google Scholar] [CrossRef]
- Adnan, N.L.; Ismail, I.; Hashim, M. Effect of Ferrocene Concentration on the Carbon Nanotube Cotton Synthesized Via Floating Catalyst CVD Method. Aust. J. Basic Appl. Sci. 2015, 9, 109–113. [Google Scholar]
- Hoecker, C.; Smail, F.; Pick, M.; Weller, L.; Boies, A.M. The Dependence of CNT Aerogel Synthesis on Sulfur-driven Catalyst Nucleation Processes and a Critical Catalyst Particle Mass Concentration. Sci. Rep. 2017, 7, 14519. [Google Scholar] [CrossRef] [PubMed]
- Shandakov, S.D.; Kosobutsky, A.V.; Rybakov, M.S.; Sevostyanov, O.G.; Russakov, D.M.; Lomakin, M.V.; Vershinina, A.I.; Chirkova, I.M. Effect of gaseous and condensate products of ethanol decomposition on aerosol CVD synthesis of single-walled carbon nanotubes. Carbon 2018, 126, 522–531. [Google Scholar] [CrossRef]
- Ambriz-Torres, J.M.; Granados-Martínez, F.G.; Contreras-Navarrete, J.D.J.; Gutiérrez-García, C.J.; García-Ruiz, D.L.; Mondragón-Sánchez, M.D.L.; Hernández-Cristóbal, O.; Arredondo-León, Y.; García, L.; Zamora-Peredo, L.; et al. Carbon nanotubes and carbon nanobeads synthesis by one-pot chemical vapor deposition method: Morphology and crystallinity. Mater. Res. Express 2018, 5, 1–20. [Google Scholar] [CrossRef]
- Weller, L.; Smail, F.R.; Elliott, J.A.; Windle, A.H.; Boies, A.M.; Hochgreb, S. Mapping the parameter space for direct-spun carbon nanotube aerogels. Carbon 2019, 146, 789–812. [Google Scholar] [CrossRef]
- Kumari, R.; Tyagi, P.K.; Puri, N.K. Electron irradiation induced wall-to-wall joining of multiwalled carbon nanotubes. Appl. Surf. Sci. 2018, 453, 153–158. [Google Scholar] [CrossRef]
- Savilov, S.V.; Cherkasov, N.; Egorov, A.V.; Ivanov, A.S.; Shen, Z.; Lunin, V.V. Sulphur-free synthesis of helical carbon nanotubes. Mater. Technol. 2016, 30, 115–120. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Ukhina, A.V.; Dudina, D.V.; Katsui, H.; Goto, T.; Kato, H. Multiwalled carbon nanotube forests grown on the surface of synthetic diamond crystals. Ceram. Int. 2017, 43, 10606–10609. [Google Scholar] [CrossRef]
- Sen, R.; Govindaraj, A.; Rao, C.N.R. Carbon nanotubes by the metallocene route. Chem. Phys. Lett. 1997, 267, 276–280. [Google Scholar] [CrossRef]
- Mayne, M.; Grobert, N.; Terrones, M.; Kamalakaran, R.; Kroto, H.W. Pure and aligned carbon nanotubes produced by the pyrolysis of benzene-based aerosols. AIP Conf. Proc. 2001, 591, 204. [Google Scholar] [CrossRef]
- Asokan, V.; Madsen, D.N.; Kosinski, P.; Myrseth, V. Transformation of carbon black into carbon nano-beads and nanotubes: The effect of catalysts. Xinxing Tan Cailiao New Carbon Mater. 2015, 30, 19–29. [Google Scholar] [CrossRef]
- Moon, S.Y.; Kim, W.S. The Synergistic Effect of a Bimetallic Catalyst for the Synthesis of Carbon Nanotube Aerogels and their Predominant Chirality. Chem. Eur. J. 2019, 25, 13635–13639. [Google Scholar] [CrossRef] [PubMed]
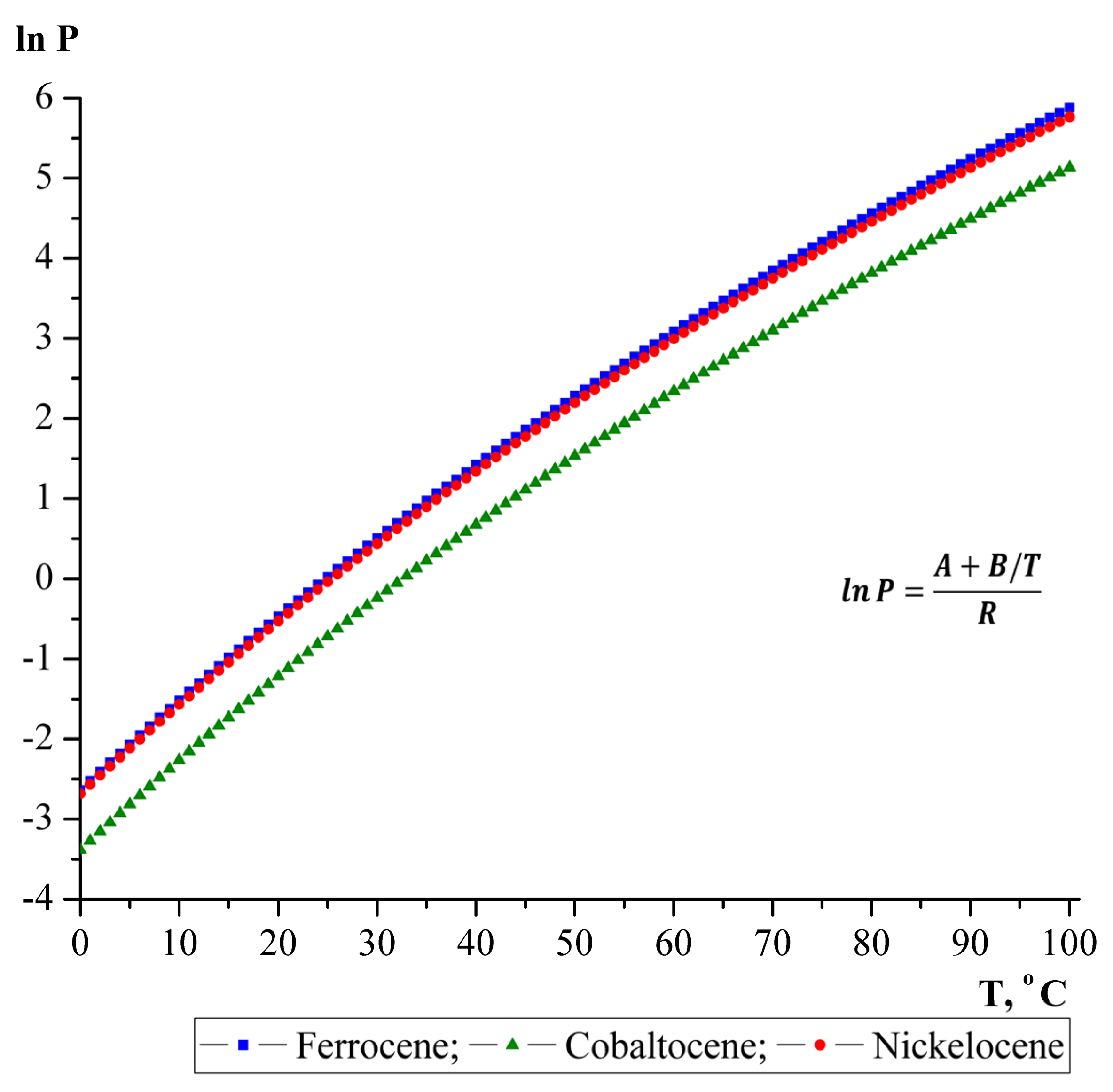
- Torres-Gómez, L.A.; Barreiro-Rodríguez, G.; Méndez-Ruíz, F. Vapour pressures and enthalpies of sublimation of ferrocene, cobaltocene and nickelocene. Thermochim. Acta 1988, 124, 179–183. [Google Scholar] [CrossRef]
- Chiang, W.H.; Sankaran, R.M. The influence of bimetallic catalyst composition on single-walled carbon nanotube yield. Carbon 2012, 50, 1044–1050. [Google Scholar] [CrossRef]
- Shyu, Y.M.; Chau-Nan Hong, F. The effects of pre-treatment and catalyst composition on growth of carbon nanofibers at low temperature. Diam. Relat. Mater. 2001, 10, 1241–1245. [Google Scholar] [CrossRef]
- Chiang, W.H.; Sankaran, R.M. Synergistic effects in bimetallic nanoparticles for low temperature carbon nanotube growth. Adv. Mater. 2008, 20, 4857–4861. [Google Scholar] [CrossRef]
- Hardeman, D.; Esconjauregui, S.; Cartwright, R.; Bhardwaj, S.; D’Arsié, L.; Oakes, D.; Clark, J.; Cepek, C.; Ducati, C.; Robertson, J. The synergistic effect in the Fe-Co bimetallic catalyst system for the growth of carbon nanotube forests. J. Appl. Phys. 2015, 117. [Google Scholar] [CrossRef]
- Kapoor, A.; Singh, N.; Dey, A.B.; Nigam, A.K.; Bajpai, A. 3d transition metals and oxides within carbon nanotubes by co-pyrolysis of metallocene & camphor: High filling efficiency and self-organized structures. Carbon 2018, 132, 733–745. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef]
- Ageeva, E.A.; Zhukova, E.A.; Karaeva, A.R.; Mordkovich, V.Z. Changes in physical properties of super long carbon nanotubes after different methods of purification. Izv. Vyss. Uchebnykh Zaved. Khimiya Khimicheskaya Tekhnol. 2018, 59, 74. [Google Scholar] [CrossRef]
- Rossella, F.; Soldano, C.; Bellani, V.; Tommasini, M. Metal-filled carbon nanotubes as a novel class of photothermal nanomaterials. Adv. Mater. 2012, 24, 2453–2458. [Google Scholar] [CrossRef] [PubMed]
- Rossella, F.; Mozzati, M.C.; Bordonali, L.; Lascialfari, A.; Soldano, C.; Ortolani, L.; Mozzati, M.C.; Bordonali, L.; Lascialfari, A.; Soldano, C.; et al. Nanostructured magnetic metamaterials based on metal-filled carbon nanotubes. Carbon 2015. [Google Scholar] [CrossRef]


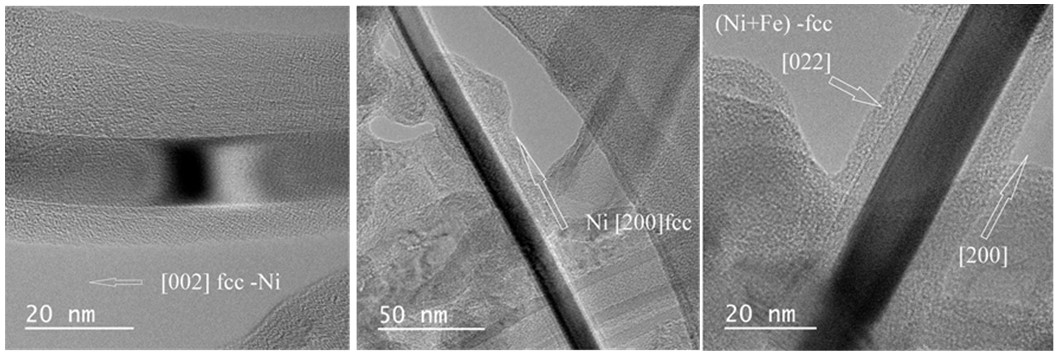
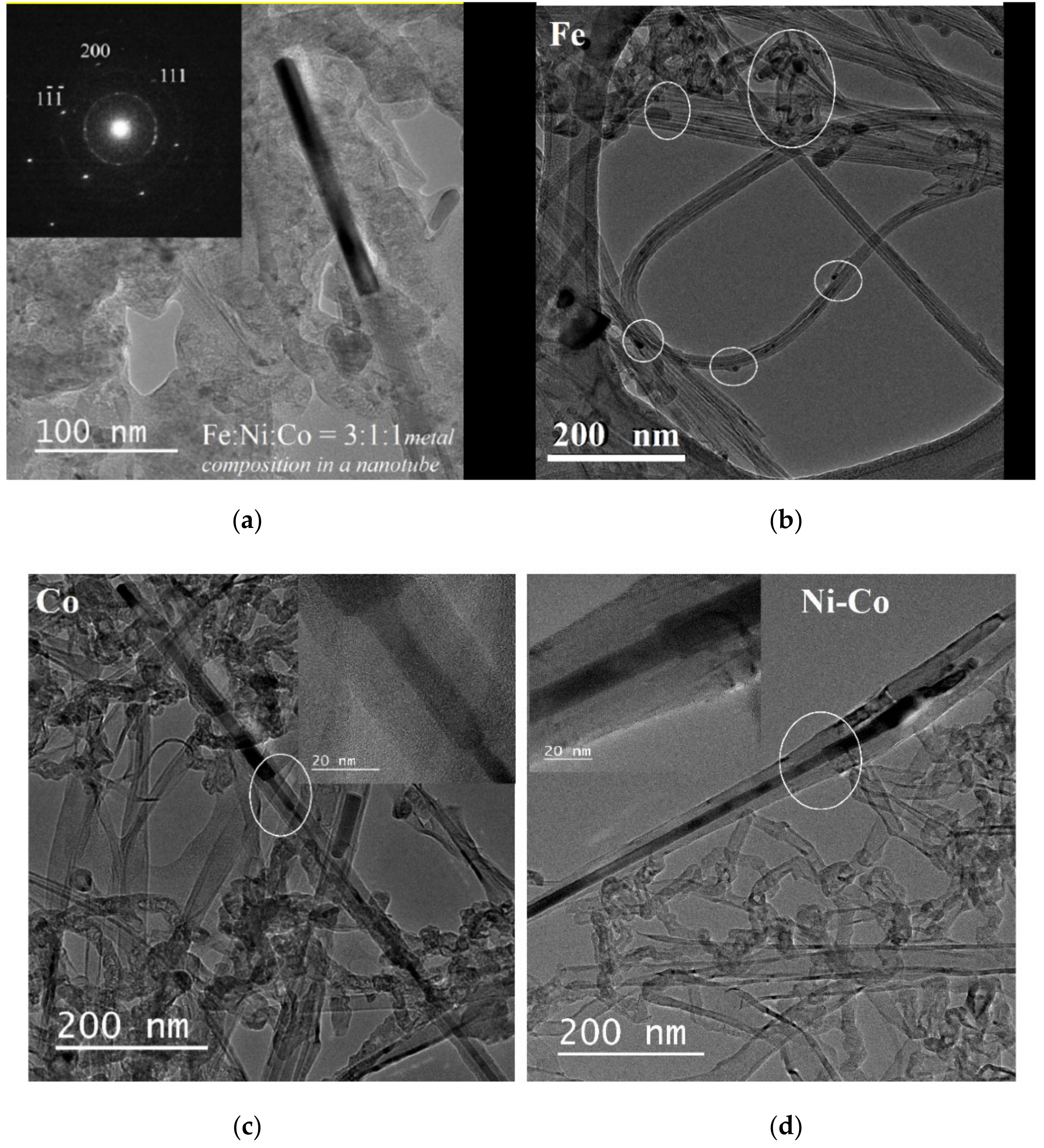
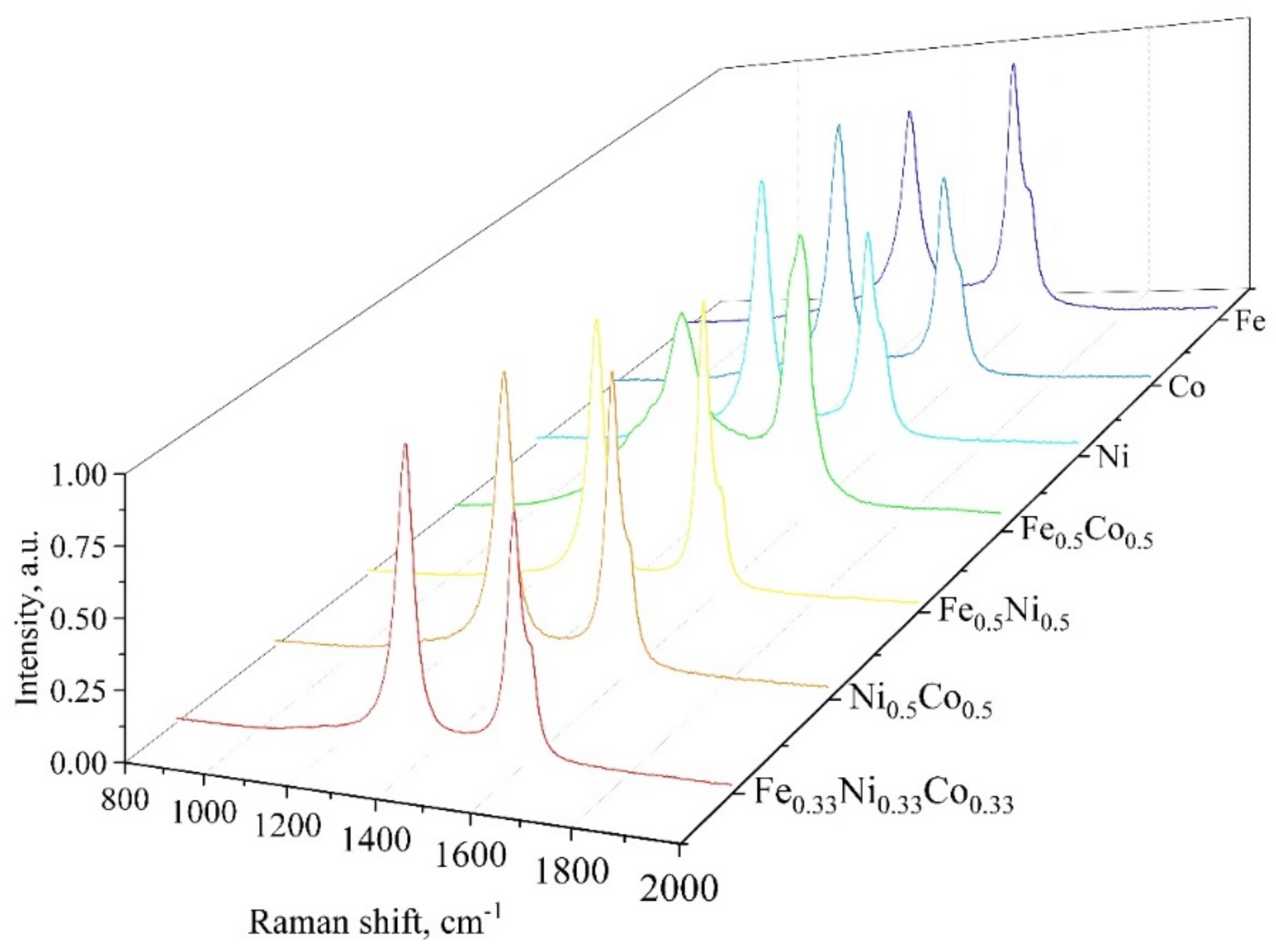
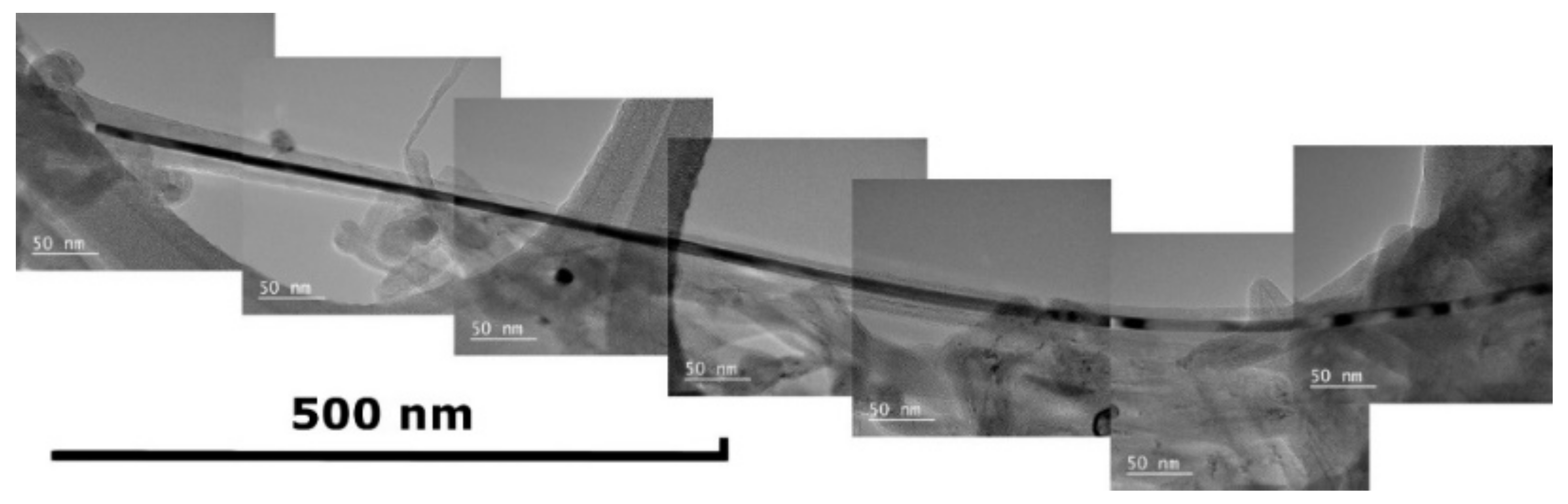
| # | Precursor | Synthesis Conditions | Products | References |
|---|---|---|---|---|
| 1 | Ferrocene | Methane, Thiophene, 1290 °C, H2, plasma spark generator | The outer diameter of bundled CNT is 6–40 nm; length over 100 μm | [23] |
| Cobaltocene | ||||
| Nickelocene | Clusters of curled short CNTs with varying outer diameter | |||
| 2 | Ferrocene | Benzene, 900 °C, Ar:H2 = 3:1;17:3 Flow rate 50, 1000 sccm, quartz boat | MWCNTs, outer diameter 40–90 nm and metal-filled onion-like structures | [30] |
| Cobaltocene | ||||
| Nickelocene | ||||
| 3 | Ferrocene | Benzole, 800–950 °C, Ar, “atomised” | Thick CNTs, outer diameter 60–120 nm and 90–200 nm. Thin CNTs, outer diameter: 10–40 nm and 10–70 nm (more than 4–10 walls). | [31] |
| Ferrocene: Nickelocene (25:75), (65:35) | ||||
| 4 | CB *:ferrocene:nickelocene (9.1:45:45) (9.1:91:0) (12.5:62.5:25) (12.5:25:62.5) (20:40:40) | Toluene, 1000 °C, N2, alumina boat | MWCNT and bulbous structures. Outer diameter: 20–150 nm 50 nm 10–100 nm 10–50 nm 10–30 nm | [32] |
| No | Catalyst Precursor * | Metal | Ratio | Product |
|---|---|---|---|---|
| 1 | Ferrocene | Fe | 100 | “stocking” |
| 2 | Nickelocene | Ni | 100 | threads |
| 3 | Cobaltocene | Co | 100 | “stocking” |
| 4 | Ferrocene/Nickelocene | Fe:Ni | 25:75 | threads |
| 5 | Ferrocene/Nickelocene | Fe:Ni | 50:50 | “stocking” |
| 6 | Ferrocene/Nickelocene | Fe:Ni | 75:25 | “stocking” |
| 7 | Ferrocene/Nickelocene | Fe:Ni | 90:10 | “stocking” |
| 8 | Ferrocene/Cobaltocene | Fe:Co | 25:75 | threads |
| 9 | Ferrocene/Cobaltocene | Fe:Co | 50:50 | “stocking” |
| 10 | Ferrocene/Cobaltocene | Fe:Co | 75:25 | “stocking” |
| 11 | Nickelocene/Cobaltocene | Ni:Co | 50:50 | “stocking” |
| 12 | Ferrocene/Nickelocene/Cobaltocene | Fe:Ni:Co | 33:33:33 | “stocking” |
| No | Active Metal in Catalyst | Ratio | Catalyst Residual Content in CNT, wt% * | Electrical Properties | Description of Nanotubes | |
|---|---|---|---|---|---|---|
| Resistivity, Ω∙m | Conductivity, S/m | |||||
| 1 | Fe | 100 | 5.5 | 3.3 × 10−4 | 3.0 × 103 | straight |
| 2 | Ni | 100 | 6.3 | 6.7 × 10−3 | 1.5 × 102 | straight |
| 3 | Co | 100 | 7.4 | 8.3 × 10−4 | 1.2 × 103 | segmented |
| 4 | Fe:Ni | 25:75 | 2.4 | 8.3 × 10−3 | 1.2 × 102 | straight |
| 5 | Fe:Ni | 50:50 | 3.7 | 4.3 × 10−4 | 2.3 × 103 | straight |
| 6 | Fe:Ni | 75:25 | 5.6 | 1.9 × 10−4 | 5.4 × 103 | straight |
| 7 | Fe:Ni | 90:10 | 2.5 | 1.2 × 10−4 | 8.2 × 103 | straight |
| 8 | Fe:Co | 25:75 | 6.1 | 1.5 × 10−3 | 6.5 × 102 | straight, segmented |
| 9 | Fe:Co | 50:50 | 6.3 | 5.9 × 10−4 | 1.7 × 103 | straight, segmented |
| 10 | Fe:Co | 75:25 | 9.9 | 3.7 × 10−4 | 2.7 × 103 | straight, segmented |
| 11 | Ni:Co | 50:50 | 1.8 | 1.8 × 10−3 | 5.5 × 102 | straight, segmented |
| 12 | Fe:Ni:Co | 33:33:33 | 3.8 | 1.0 × 10−3 | 1.0 × 103 | straight, segmented |
| 13 | Fe | 100 | 0.2 | 1.1 × 10−5 | 9.4 × 104 | straight, purified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaeva, A.R.; Urvanov, S.A.; Kazennov, N.V.; Mitberg, E.B.; Mordkovich, V.Z. Synthesis, Structure and Electrical Resistivity of Carbon Nanotubes Synthesized over Group VIII Metallocenes. Nanomaterials 2020, 10, 2279. https://doi.org/10.3390/nano10112279
Karaeva AR, Urvanov SA, Kazennov NV, Mitberg EB, Mordkovich VZ. Synthesis, Structure and Electrical Resistivity of Carbon Nanotubes Synthesized over Group VIII Metallocenes. Nanomaterials. 2020; 10(11):2279. https://doi.org/10.3390/nano10112279
Chicago/Turabian StyleKaraeva, Aida R., Sergey A. Urvanov, Nikita V. Kazennov, Eduard B. Mitberg, and Vladimir Z. Mordkovich. 2020. "Synthesis, Structure and Electrical Resistivity of Carbon Nanotubes Synthesized over Group VIII Metallocenes" Nanomaterials 10, no. 11: 2279. https://doi.org/10.3390/nano10112279
APA StyleKaraeva, A. R., Urvanov, S. A., Kazennov, N. V., Mitberg, E. B., & Mordkovich, V. Z. (2020). Synthesis, Structure and Electrical Resistivity of Carbon Nanotubes Synthesized over Group VIII Metallocenes. Nanomaterials, 10(11), 2279. https://doi.org/10.3390/nano10112279





