Empirical Modelling of Hydrodynamic Effects on Starch Nanoparticles Precipitation in a Spinning Disc Reactor
Abstract
1. Introduction
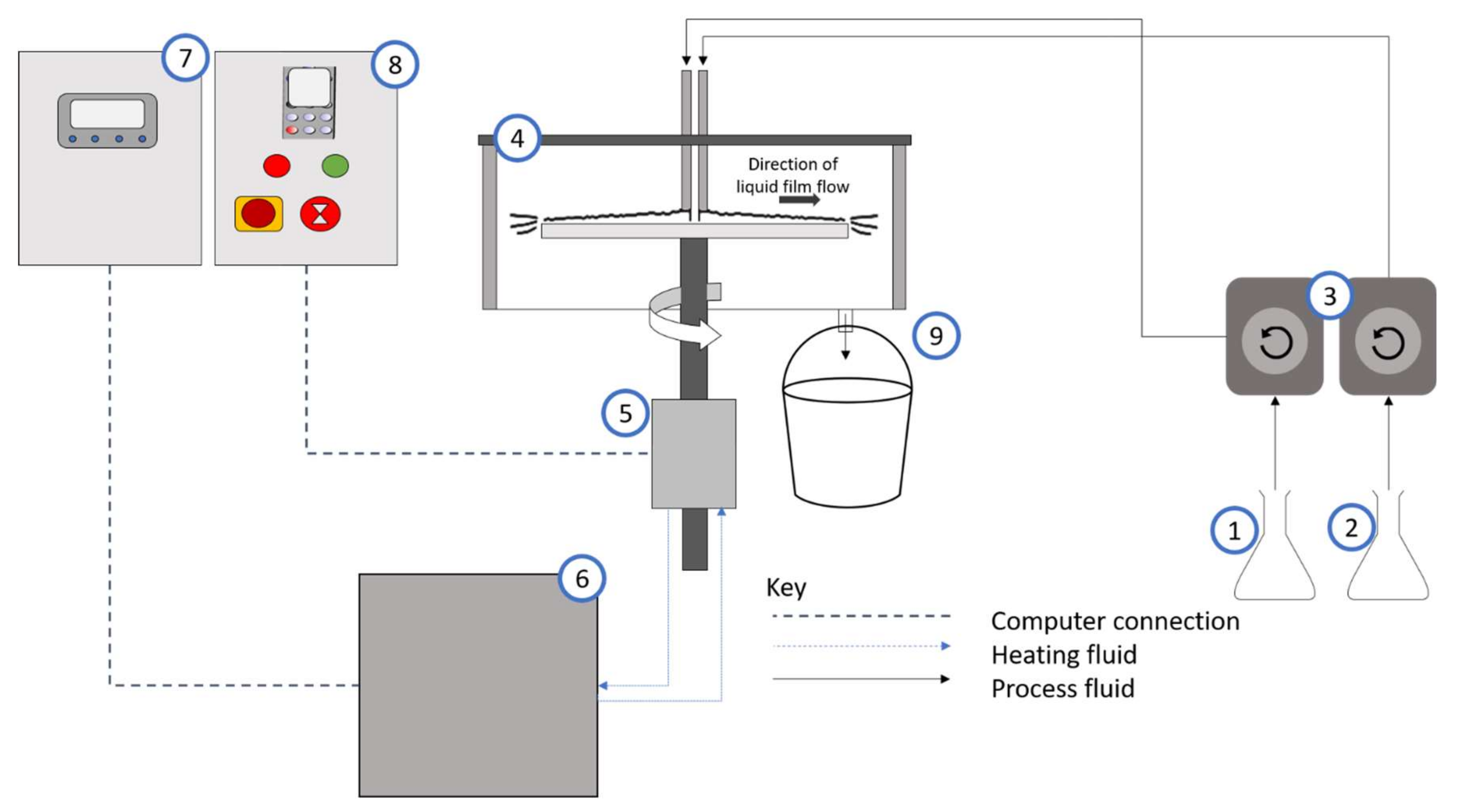
2. Methodology
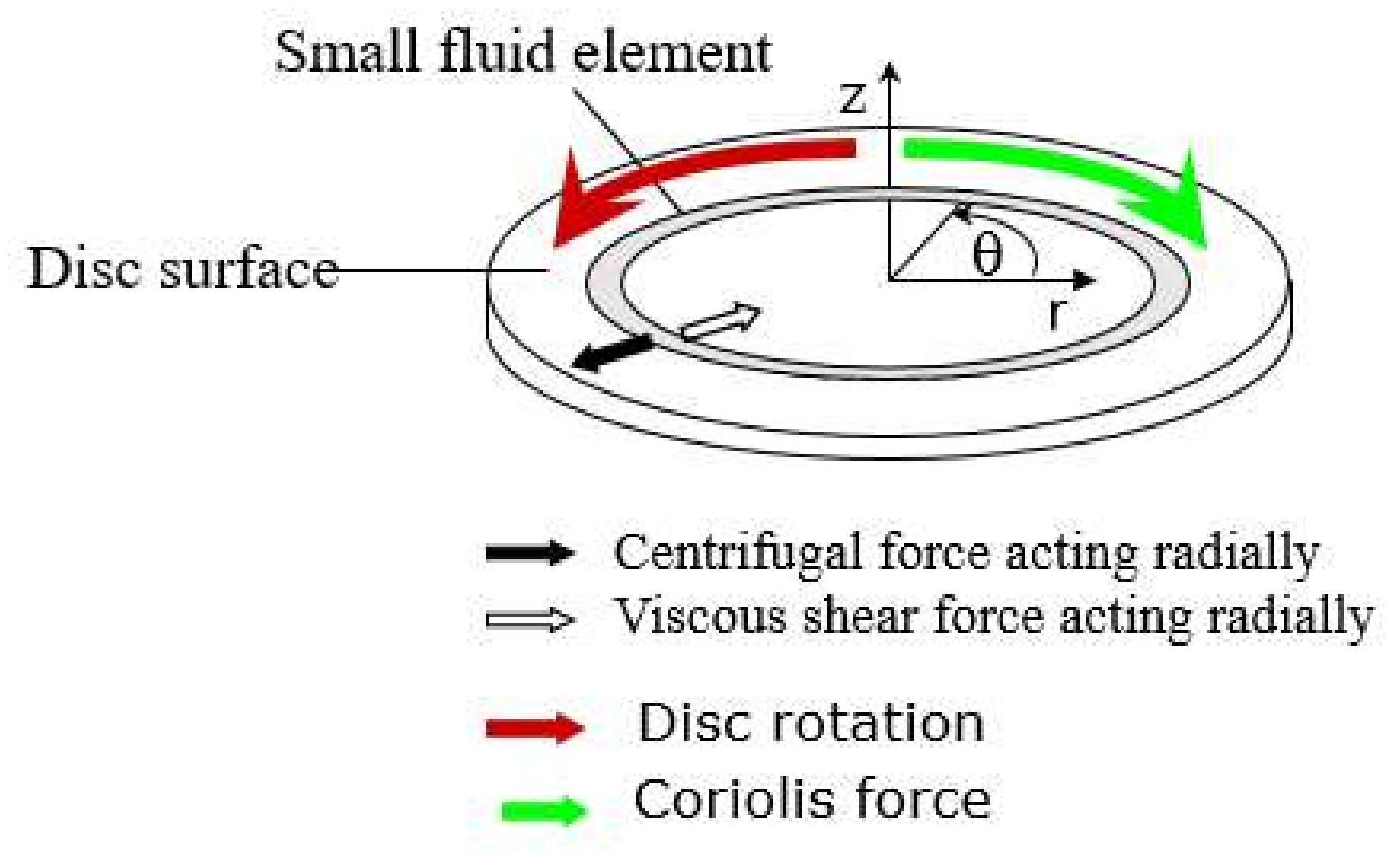
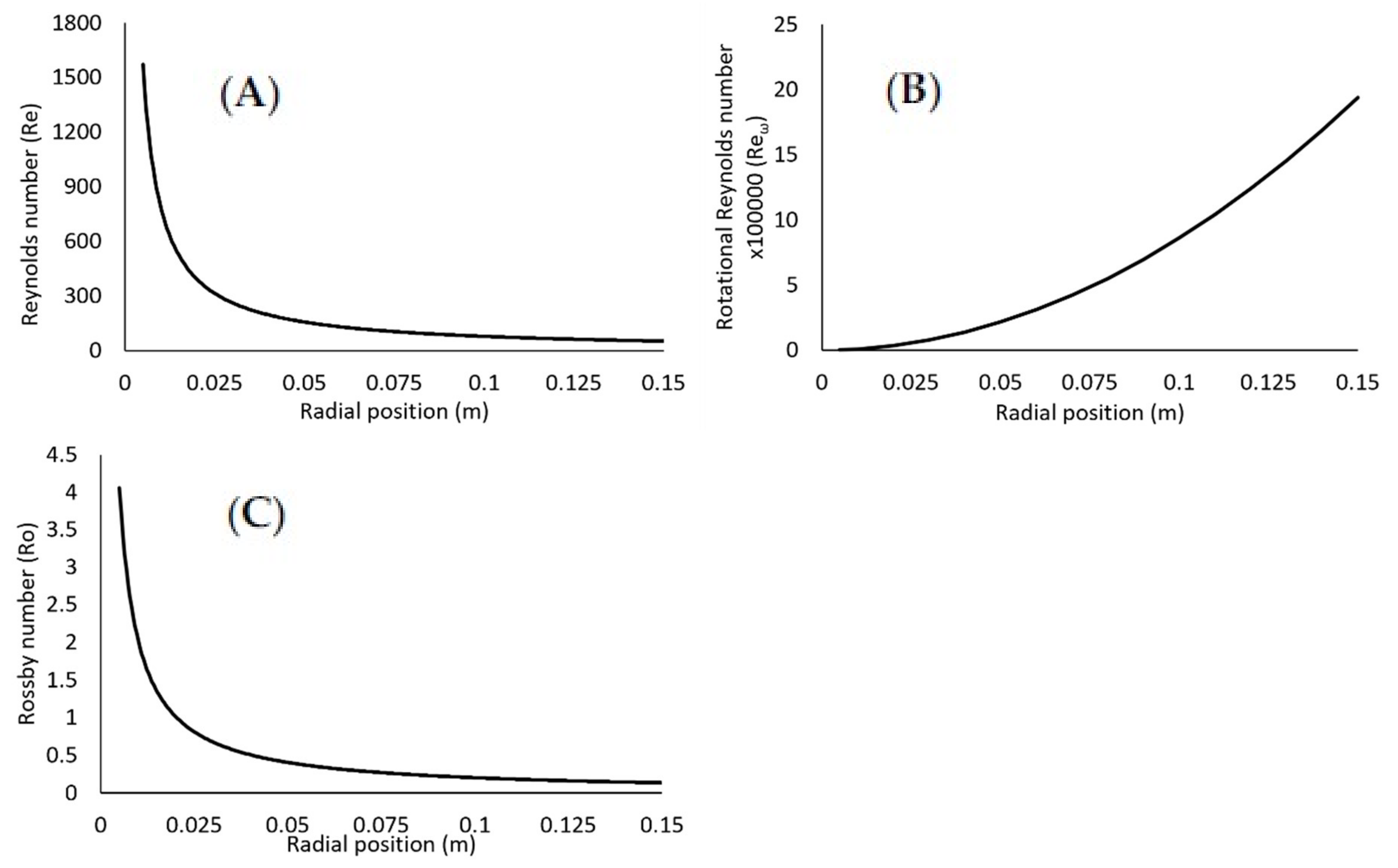
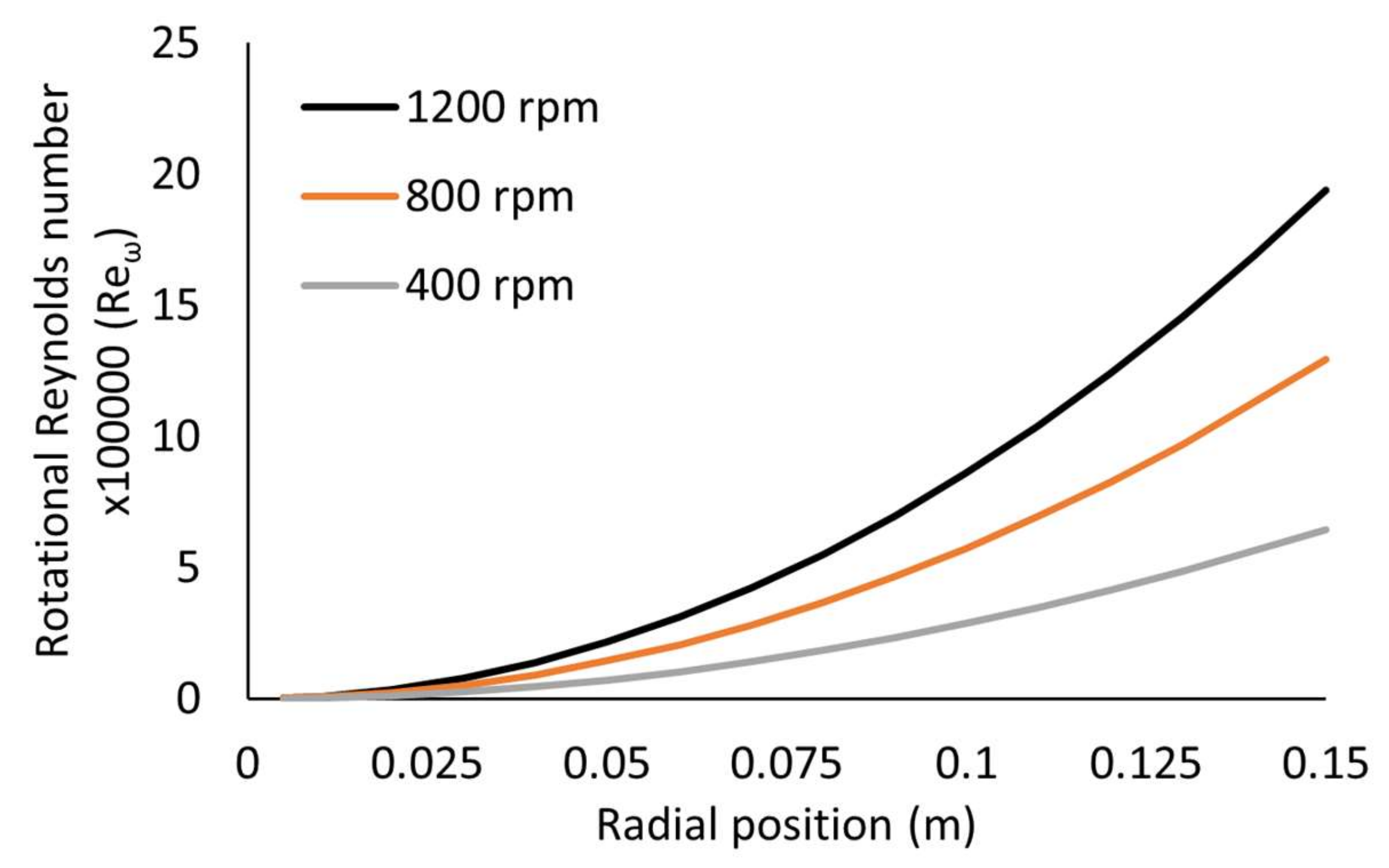
Dimensionless Numbers
- : Smooth laminar flow
- : Small amplitude waves
- : Sinusoidal waves replaced by regular waves
- : Wavy-laminar flow
- : Turbulent flow regime
- < 104: Laminar regime
- 104<105: Flow instabilities increase and flow is in transition to turbulent regime
- 105: Turbulent regime
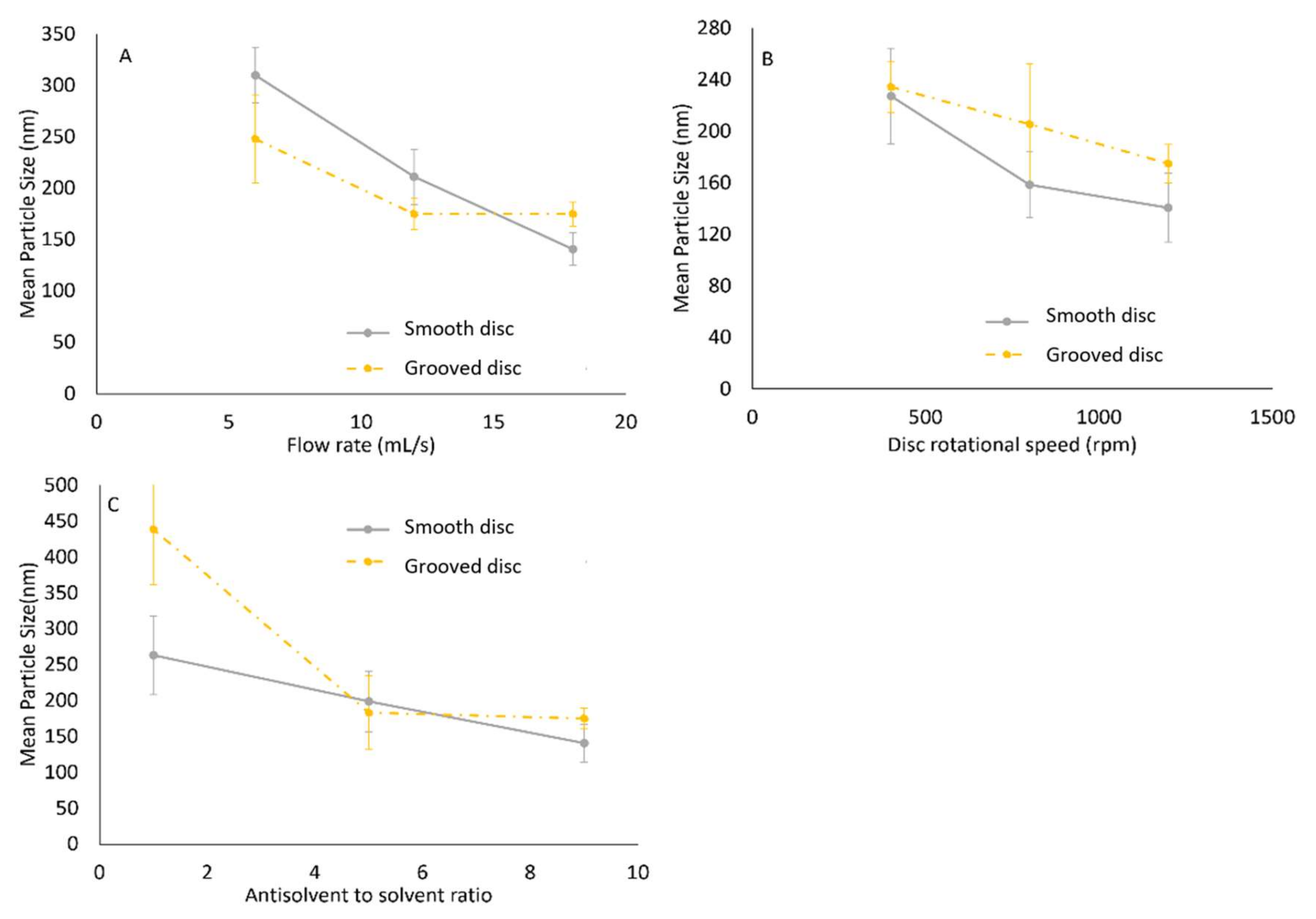
3. Results and Discussion
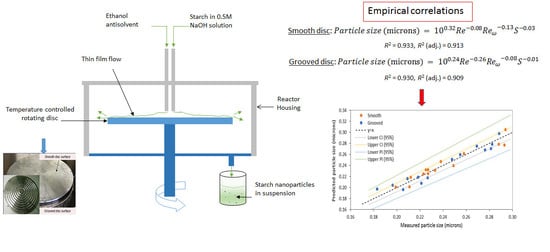
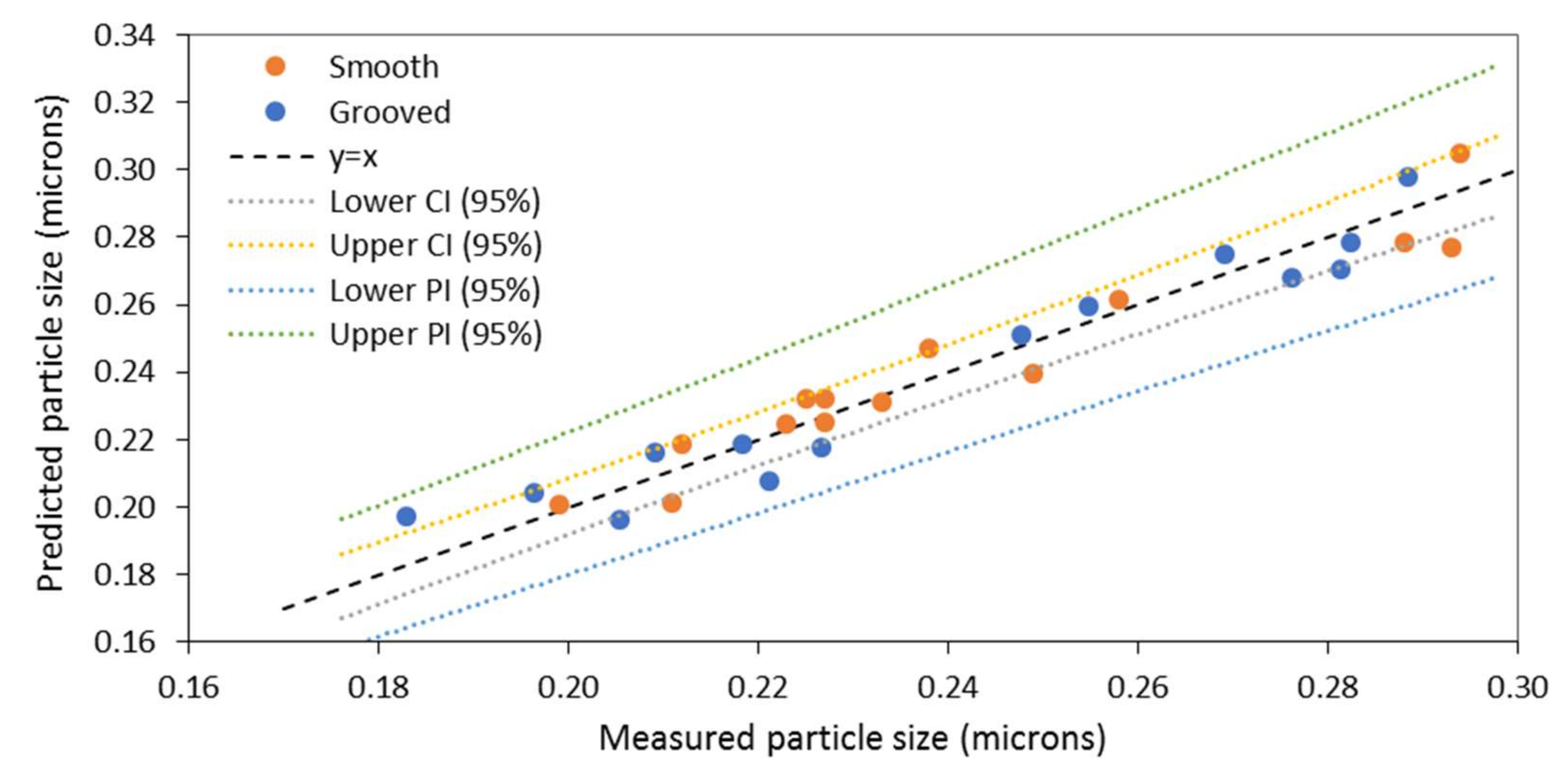
3.1. Model 1: Using Rotational Reynolds Number to Characterise Disc Rotational Speed
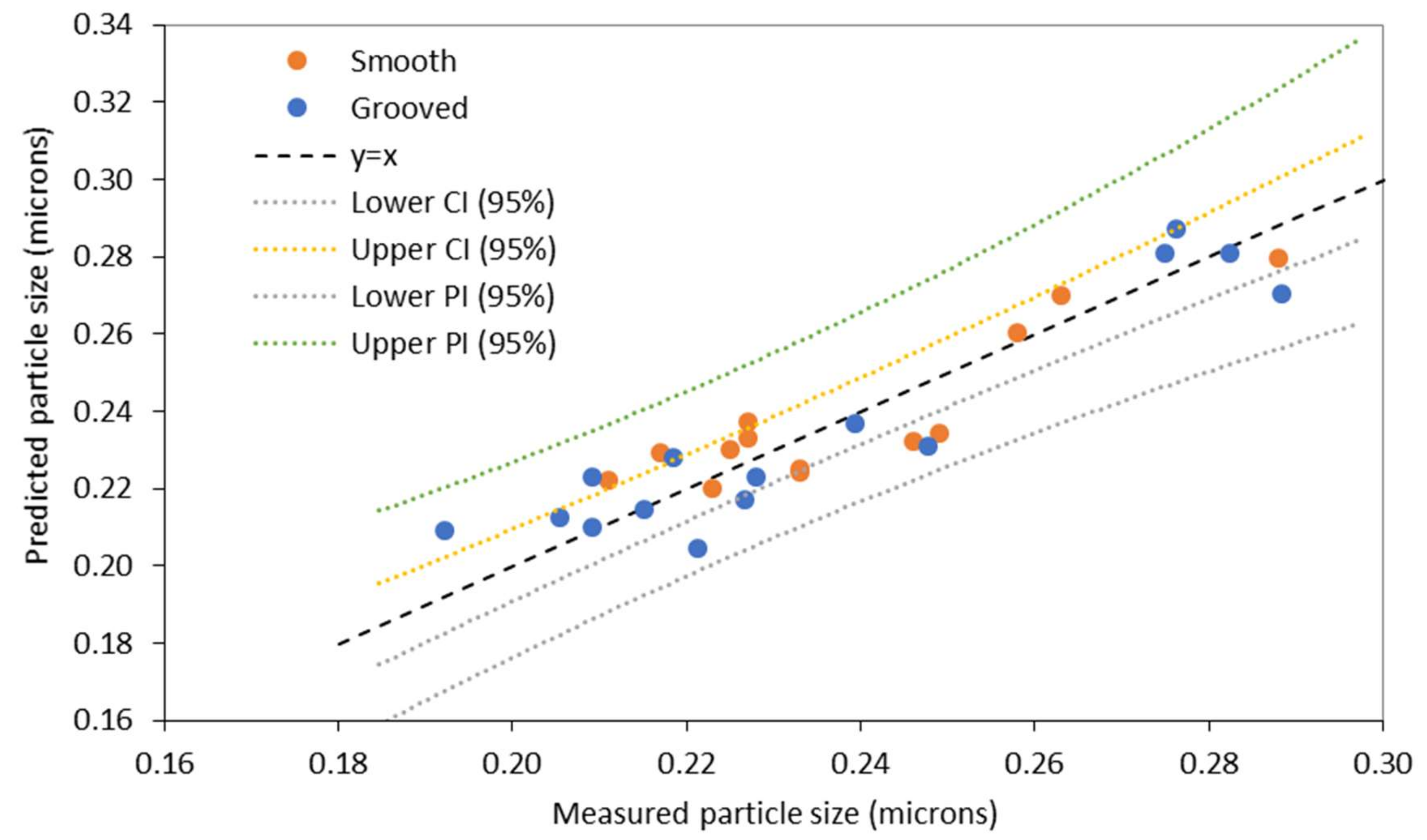
3.2. Model 2: Using Rossby Number to Characterise Disc Rotational Speed
3.3. Evaluation of the Models
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature:
| Solute concentration (g solute/g solvent) | |
| Equilibrium concentration (g solute/g solvent) | |
| Volumetric flow rate (m3 s−1) | |
| Radial position from centre of disc (m) | |
| Mean disc residence time (s) | |
| Film thickness-averaged radial velocity on disc (m s−1) | |
| Inlet velocity (m s−1) | |
| Instantaneous disc radial velocity (m s−1) | |
| Greek symbols | |
| Liquid film thickness (m) | |
| Shear rate (s−1) | |
| ω | Angular velocity (rad s−1) |
| ν | Kinematic viscosity (m2 s−1) |
| Dimensionless numbers | |
| Ekman number, (-) | |
| Reynolds number, (-) | |
| Rotational Reynolds number, (-) | |
| Rossby number, (-) | |
| Supersaturation (-) | |
| Subscripts | |
| i | Inlet |
| o | Outlet |
References
- Rose, J.; Auffan, M.; Proux, O.; Niviere, V.; Bottero, J.Y. Physicochemical properties of nanoparticles in relation with toxicity. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; p. 2085. [Google Scholar] [CrossRef]
- Santander-Ortega, M.J.; Stauner, T.; Loretz, B.; Ortega-Vinuesa, J.L.; Bastos-González, D.; Wenz, G.; Schaefer, U.F.; Lehr, C.M. Nanoparticles made from novel starch derivatives for transdermal drug delivery. J. Control. Release 2010, 141, 85–92. [Google Scholar] [CrossRef]
- Elvira, C.; Mano, J.F.; San Román, J.; Reis, R.L. Starch-based biodegradable hydrogels with potential biomedical applications as drug delivery systems. Biomaterials 2002, 23, 1955–1966. [Google Scholar] [CrossRef]
- Brecher, M.E.; Owen, H.G.; Bandarenko, N. Alternatives to albumin: Starch replacement for plasma exchange. J. Clin. Apher. 1997, 12, 146–153. [Google Scholar] [CrossRef]
- Thielemans, W.; Belgacem, M.N.; Dufresne, A. Starch Nanocrystals with Large Chain Surface Modifications. Langmuir 2006, 22, 4804–4810. [Google Scholar] [CrossRef] [PubMed]
- Angellier, H.; Molina-Boisseau, S.; Dufresne, A. Mechanical Properties of Waxy Maize Starch Nanocrystal Reinforced Natural Rubber. Macromolecules 2005, 38, 9161–9170. [Google Scholar] [CrossRef]
- Alila, S.; Aloulou, F.; Thielemans, W.; Boufi, S. Sorption potential of modified nanocrystals for the removal of aromatic organic pollutant from aqueous solution. Ind. Crop. Prod. 2011, 33, 350–357. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Nain, V. Starch nanoparticles: Their preparation and applications. In Plant Biotechnology: Recent Advancements and Developments; Gahlawat, S.K., Salar, R.K., Siwach, P., Duhan, J.S., Kumar, S., Kaur, P., Eds.; Springer: Singapore, 2017; pp. 213–232. [Google Scholar] [CrossRef]
- Kristo, E.; Biliaderis, C.G. Physical properties of starch nanocrystal-reinforced pullulan films. Carbohydr. Polym. 2007, 68, 146–158. [Google Scholar] [CrossRef]
- Ma, X.; Jian, R.; Chang, P.R.; Yu, J. Fabrication and Characterization of Citric Acid-Modified Starch Nanoparticles/Plasticized-Starch Composites. Biomacromolecules 2008, 9, 3314–3320. [Google Scholar] [CrossRef]
- García, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.I.; Goyanes, S. Physico-Mechanical Properties of Biodegradable Starch Nanocomposites. Macromol. Mater. Eng. 2009, 294, 169–177. [Google Scholar] [CrossRef]
- Bel Haaj, S.; Thielemans, W.; Magnin, A.; Boufi, S. Starch nanocrystals and starch nanoparticles from waxy maize as nanoreinforcement: A comparative study. Carbohydr. Polym. 2016, 143, 310–317. [Google Scholar] [CrossRef]
- Srichuwong, S.; Sunarti, T.C.; Mishima, T.; Isono, N.; Hisamatsu, M. Starches from different botanical sources I: Contribution of amylopectin fine structure to thermal properties and enzyme digestibility. Carbohydr. Polym. 2005, 60, 529–538. [Google Scholar] [CrossRef]
- Thorat, A.A.; Dalvi, S.V. Liquid antisolvent precipitation and stabilization of nanoparticles of poorly water soluble drugs in aqueous suspensions: Recent developments and future perspective. Chem. Eng. J. 2012, 181, 1–34. [Google Scholar] [CrossRef]
- Sun, Q.; Li, G.; Dai, L.; Ji, N.; Xiong, L. Green preparation and characterisation of waxy maize starch nanoparticles through enzymolysis and recrystallisation. Food Chem. 2014, 162, 223–228. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Rafie, M.H.; EL-Sheikh, M.A.; El-Naggar, M.E. Ultra-Fine Characteristics of Starch Nanoparticles Prepared Using Native Starch With and Without Surfactant. J. Inorg. Organomet. Polym. Mater. 2014, 24, 515–524. [Google Scholar] [CrossRef]
- Wu, X.; Chang, Y.; Fu, Y.; Ren, L.; Tong, J.; Zhou, J. Effects of non-solvent and starch solution on formation of starch nanoparticles by nanoprecipitation. Starch 2016, 68, 258–263. [Google Scholar] [CrossRef]
- Chin, S.F.; Pang, S.C.; Tay, S.H. Size controlled synthesis of starch nanoparticles by a simple nanoprecipitation method. Carbohydr. Polym. 2011, 86, 1817–1819. [Google Scholar] [CrossRef]
- LeCorre, D.; Bras, J.; Dufresne, A. Influence of botanic origin and amylose content on the morphology of starch nanocrystals. J. Nanopart. Res. 2011, 13, 7193–7208. [Google Scholar] [CrossRef]
- Chen, G.; Wei, M.; Chen, J.; Huang, J.; Dufresne, A.; Chang, P.R. Simultaneous reinforcing and toughening: New nanocomposites of waterborne polyurethane filled with low loading level of starch nanocrystals. Polymer 2008, 49, 1860–1870. [Google Scholar] [CrossRef]
- Namazi, H.; Dadkhah, A. Surface modification of starch nanocrystals through ring-opening polymerization of ε-caprolactone and investigation of their microstructures. J. Appl. Polym. Sci. 2008, 110, 2405–2412. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, D.J.; Lim, S.T. Fragmentation of Waxy Rice Starch Granules by Enzymatic Hydrolysis. Cereal Chem. 2008, 85, 182–187. [Google Scholar] [CrossRef]
- Lamanna, M.; Morales, N.J.; García, N.L.; Goyanes, S. Development and characterization of starch nanoparticles by gamma radiation: Potential application as starch matrix filler. Carbohydr. Polym. 2013, 97, 90–97. [Google Scholar] [CrossRef]
- Bel Haaj, S.; Magnin, A.; Pétrier, C.; Boufi, S. Starch nanoparticles formation via high power ultrasonication. Carbohydr. Polym. 2013, 92, 1625–1632. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, D.H.; Kim, J.Y. Effect of heat-moisture treatment under mildly acidic condition on fragmentation of waxy maize starch granules into nanoparticles. Food Hydrocoll. 2017, 63, 59–66. [Google Scholar] [CrossRef]
- Park, E.Y.; Kim, M.J.; Cho, M.; Lee, J.H.; Kim, J.Y. Production of starch nanoparticles using normal maize starch via heat-moisture treatment under mildly acidic conditions and homogenization. Carbohydr. Polym. 2016, 151, 274–282. [Google Scholar] [CrossRef]
- Chang, R.; Ji, N.; Li, M.; Qiu, L.; Sun, C.; Bian, X.; Qiu, H.; Xiong, L.; Sun, Q. Green preparation and characterization of starch nanoparticles using a vacuum cold plasma process combined with ultrasonication treatment. Ultrason. Sonochem. 2019, 58, 104660. [Google Scholar] [CrossRef]
- Sana, S.; Boodhoo, K.; Zivkovic, V. Production of starch nanoparticles through solvent–antisolvent precipitation in a spinning disc reactor. Green Process. Synth. 2019, 8, 507. [Google Scholar] [CrossRef]
- O’Grady, D.; Barrett, M.; Casey, E.; Glennon, B. The Effect of Mixing on the Metastable Zone Width and Nucleation Kinetics in the Anti-Solvent Crystallization of Benzoic Acid. Chem. Eng. Res. Des. 2007, 85, 945–952. [Google Scholar] [CrossRef]
- Dong, Y.; Ng, W.K.; Shen, S.; Kim, S.; Tan, R.B.H. Controlled antisolvent precipitation of spironolactone nanoparticles by impingement mixing. Int. J. Pharm. 2011, 410, 175–179. [Google Scholar] [CrossRef]
- Dong, Y.; Ng, W.K.; Hu, J.; Shen, S.; Tan, R.B.H. A continuous and highly effective static mixing process for antisolvent precipitation of nanoparticles of poorly water-soluble drugs. Int. J. Pharm. 2010, 386, 256–261. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.X.; Wang, Q.A.; Chen, J.F.; Yun, J. Controlled Liquid Antisolvent Precipitation of Hydrophobic Pharmaceutical Nanoparticles in a Microchannel Reactor. Ind. Eng. Chem. Res. 2007, 46, 8229–8235. [Google Scholar] [CrossRef]
- Kim, K.J. Crystal Growth in Drowning-Out Crystallization using a T-Mixer. Chem. Eng. Technol. 2006, 29, 1267–1272. [Google Scholar] [CrossRef]
- Wong, S.H.; Ward, M.C.L.; Wharton, C.W. Micro T-mixer as a rapid mixing micromixer. Sens. Actuators B Chem. 2004, 100, 359–379. [Google Scholar] [CrossRef]
- McGlone, T.; Briggs, N.E.B.; Clark, C.A.; Brown, C.J.; Sefcik, J.; Florence, A.J. Oscillatory Flow Reactors (OFRs) for Continuous Manufacturing and Crystallization. Org. Process Res. Dev. 2015, 19, 1186–1202. [Google Scholar] [CrossRef]
- Brown, C.J.; Ni, X. Online evaluation of paracetamol antisolvent crystallization growth rate with video imaging in an oscillatory baffled crystallizer. Cryst. Growth Des. 2011, 11, 719–725. [Google Scholar] [CrossRef]
- Kuang, Y.Y.; Zhang, Z.B.; Xie, M.L.; Wang, J.X.; Le, Y.; Chen, J.F. Large-Scale Preparation of Amorphous Cefixime Nanoparticles by Antisolvent Precipitation in a High-Gravity Rotating Packed Bed. Ind. Eng. Chem. Res. 2015. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Zhang, H.; Shen, Z.; Yun, J.; Chen, J. Facile Preparation of Danazol Nanoparticles by High-Gravity Anti-solvent Precipitation (HGAP) Method. Chin. J. Chem. Eng. 2009, 17, 318–323. [Google Scholar] [CrossRef]
- Valente, I.; Celasco, E.; Marchisio, D.L.; Barresi, A.A. Nanoprecipitation in confined impinging jets mixers: Production, characterization and scale-up of pegylated nanospheres and nanocapsules for pharmaceutical use. Chem. Eng. Sci. 2012, 77, 217–227. [Google Scholar] [CrossRef]
- D’ Intino, A.F.; De Caprariis, B.; Santarelli, M.L.; Verdone, N.; Chianese, A. Best operating conditions to produce hydroxyapatite nanoparticles by means of a spinning disc reactor. Front. Chem. Sci. Eng. 2014, 8, 156–160. [Google Scholar] [CrossRef]
- Dabir, H.; Davarpanah, M.; Ahmadpour, A. Effects of different operating parameters on the particle size of silver chloride nanoparticles prepared in a spinning disk reactor. Appl. Phys. A Mater. Sci. Process. 2015, 120, 105–113. [Google Scholar] [CrossRef]
- Tai, C.Y.; Wang, Y.H.; Liu, H.S. A green process for preparing silver nanoparticles using spinning disk reactor. Aiche J. 2008, 54, 445–452. [Google Scholar] [CrossRef]
- Cafiero, L.M.; Baffi, G.; Chianese, A.; Jachuck, R.J.J. Process Intensification: Precipitation of Barium Sulfate Using a Spinning Disk Reactor. Ind. Eng. Chem. Res. 2002, 41, 5240–5246. [Google Scholar] [CrossRef]
- De Caprariis, B.; Di Rita, M.; Stoller, M.; Verdone, N.; Chianese, A. Reaction-precipitation by a spinning disc reactor: Influence of hydrodynamics on nanoparticles production. Chem. Eng. Sci. 2012, 76, 73–80. [Google Scholar] [CrossRef]
- Dehkordi, A.M.; Vafaeimanesh, A. Synthesis of barium sulfate nanoparticles using a spinning disk reactor: Effects of supersaturation, disk rotation speed, free ion ratio, and disk diameter. Ind. Eng. Chem. Res. 2009, 48, 7574–7580. [Google Scholar] [CrossRef]
- Tai, C.Y.; Tai, C.T.; Chang, M.H.; Liu, H.S. Synthesis of magnesium hydroxide and oxide nanoparticles using a spinning disk reactor. Ind. Eng. Chem. Res. 2007, 46, 5536–5541. [Google Scholar] [CrossRef]
- Mohammadi, S.; Harvey, A.; Boodhoo, K.V.K. Synthesis of TiO2 nanoparticles in a spinning disc reactor. Chem. Eng. J. 2014, 258, 171–184. [Google Scholar] [CrossRef]
- Ahoba-Sam, C.; Boodhoo, K.V.K.; Olsbye, U.; Jens, K.J. Tailoring Cu Nanoparticle Catalyst for Methanol Synthesis Using the Spinning Disk Reactor. Materials 2018, 11, 154. [Google Scholar] [CrossRef]
- Vilardi, G.; Stoller, M.; Di Palma, L.; Boodhoo, K.; Verdone, N. Metallic iron nanoparticles intensified production by spinning disk reactor: Optimization and fluid dynamics modelling. Chem. Eng. Process. Process Intensif. 2019, 146, 107683. [Google Scholar] [CrossRef]
- Chen, X.; Smith, N.M.; Iyer, K.S.; Raston, C.L. Controlling nanomaterial synthesis, chemical reactions and self assembly in dynamic thin films. Chem. Soc. Rev. 2014, 43, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Oxley, P.; Brechtelsbauer, C.; Ricard, F.; Lewis, N.; Ramshaw, C. Evaluation of spinning disk reactor technology for the manufacture of pharmaceuticals. Ind. Eng. Chem. Res. 2000, 39, 2175–2182. [Google Scholar] [CrossRef]
- Khan, W.H.; Rathod, V.K. Process intensification approach for preparation of curcumin nanoparticles via solvent–nonsolvent nanoprecipitation using spinning disc reactor. Chem. Eng. Process. Process Intensif. 2014, 80, 1–10. [Google Scholar] [CrossRef]
- Burns, J.R.; Jachuck, R.J.J. Determination of liquid–solid mass transfer coefficients for a spinning disc reactor using a limiting current technique. Int. J. Heat Mass Transf. 2005, 48, 2540–2547. [Google Scholar] [CrossRef]
- Boodhoo, K.V.K.; Al-Hengari, S.R. Micromixing Characteristics in a Small-Scale Spinning Disk Reactor. Chem. Eng. Technol. 2012, 35, 1229–1237. [Google Scholar] [CrossRef]
- Boodhoo, K. Spinning disc reactor for green processing and synthesis. In Process Intensification for Green Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 59–90. [Google Scholar] [CrossRef]
- Jacobsen, N.C.; Hinrichsen, O. Micromixing Efficiency of a Spinning Disk Reactor. Ind. Eng. Chem. Res. 2012, 51, 11643–11652. [Google Scholar] [CrossRef]
- Mohammadi, S.; Boodhoo, K.V.K. Online conductivity measurement of residence time distribution of thin film flow in the spinning disc reactor. Chem. Eng. J. 2012, 207, 885–894. [Google Scholar] [CrossRef]
- Boodhoo, K.V.K.; Jachuck, R.J. Process intensification: Spinning disk reactor for styrene polymerisation. Appl. Therm. Eng. 2000, 20, 1127–1146. [Google Scholar] [CrossRef]
- Emslie, A.G.; Bonner, F.T.; Peck, L.G. Flow of a Viscous Liquid on a Rotating Disk. J. Appl. Phys. 1958, 29, 858–862. [Google Scholar] [CrossRef]
- Boodhoo, K. Process Intensification: Spinning Disc Reactor for the Polymerisation of Styrene. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 1999. [Google Scholar]
- Shevchuk, I.V. Modelling of Convective Heat and Mass Transfer in Rotating Flows; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–235. [Google Scholar] [CrossRef]
- Ozar, B.; Cetegen, B.M.; Faghri, A. Experiments on the flow of a thin liquid film over a horizontal stationary and rotating disk surface. Exp. Fluids 2003, 34, 556–565. [Google Scholar] [CrossRef]
- Saw, C.B.; Anderson, G.K.; Howarth, C.R.; Porter, J.E. Application of the spinning disc reactor as an ozone contractor. In Proceedings of the Industrial Waste Conference, Purdue University, West Lafayette, IN, USA, 14–16 May 1985; pp. 813–823. [Google Scholar]
- Khan, J.R. Heat Transfer on a Rotating Surface with and Without Phase Change. Ph.D. Thesis, Newcastle University, Newcastle Upon Tyne, UK, 1986. [Google Scholar]
- Mohammadi, S. Nano-TiO₂ Precipitation in SDRs: Experimental and Modelling Studies. Ph.D. Thesis, Newcastle University, Newcastle Upon Tyne, UK, 2014. [Google Scholar]
- Basu, S.; Cetegen, B.M. Analysis of hydrodynamics and heat transfer in a thin liquid film flowing over a rotating disk by the integral method. J. Heat Transf. 2006, 128, 217–225. [Google Scholar] [CrossRef][Green Version]
- Leneweit, G.; Roesner, K.G.; Koehler, R. Surface instabilities of thin liquid film flow on a rotating disk. Exp. Fluids 1999, 26, 75–85. [Google Scholar] [CrossRef]
- Scheichl, B.; Kluwick, A. Laminar spread of a circular liquid jet impinging axially on a rotating disc. J. Fluid Mech. 2019, 864, 449–489. [Google Scholar] [CrossRef]
- Rauscher, J.W.; Kelly, R.E.; Cole, J.D. An Asymptotic Solution for the Laminar Flow of a Thin Film on a Rotating Disk. J. Appl. Mech. 1973, 40, 43–47. [Google Scholar] [CrossRef]
- Prieling, D. Computational Investigation of Liquid Film Flow on Rotating Disks. Master’s Thesis, Graz University of Technology, Graz, Austria, 2013. [Google Scholar]
- Allen, M.P. The problem of multicollinearity. In Understanding Regression Analysis; Springer: Boston, MA, USA, 1997; pp. 176–180. [Google Scholar]

| Technique | Starch Nanoparticle Production Process | Size | Ref. |
|---|---|---|---|
| Hydrolysis (top-down) | Acid hydrolysis | 20–90 nm (SEM) 40–70 nm (TEM) 70–100 nm (TEM) | [19] [20] [21] |
| Physical methods (top-down) | Enzymatic hydrolysis | 500 nm (DLS) | [22] |
| Gamma radiation | 20–30 nm (DLS) | [23] | |
| Ultrasonication | 30–100 nm (SEM) | [24] | |
| High pressure homogenisation | <50 nm (TEM) | [25,26] | |
| Cold plasma and ultrasound | 300–1000 nm (DLS) | [27] | |
| Precipitation (bottom-up) | Solvent–antisolvent precipitation | 50–100 nm (SEM) 150 nm (SEM) 130–400 nm (DLS) 140–450 nm (DLS) | [10] [18] [16] [28] |
| Factor | Low | Centre | High |
|---|---|---|---|
| Disc rotational speed (rpm) | 400 | 800 | 1200 |
| Total flow rate (mL/s) | 6 | 12 | 18 |
| Antisolvent to solvent ratio (vol/vol basis) | 1:1 | 5:1 | 9:1 |
| Model | Standard Error |
|---|---|
| Model 1 ) Grooved disc | 0.020 |
| Model 1 ) Smooth disc | 0.016 |
| Model 2 Grooved disc | 0.023 |
| Model 2 Smooth disc | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sana, S.; Zivkovic, V.; Boodhoo, K. Empirical Modelling of Hydrodynamic Effects on Starch Nanoparticles Precipitation in a Spinning Disc Reactor. Nanomaterials 2020, 10, 2202. https://doi.org/10.3390/nano10112202
Sana S, Zivkovic V, Boodhoo K. Empirical Modelling of Hydrodynamic Effects on Starch Nanoparticles Precipitation in a Spinning Disc Reactor. Nanomaterials. 2020; 10(11):2202. https://doi.org/10.3390/nano10112202
Chicago/Turabian StyleSana, Sahr, Vladimir Zivkovic, and Kamelia Boodhoo. 2020. "Empirical Modelling of Hydrodynamic Effects on Starch Nanoparticles Precipitation in a Spinning Disc Reactor" Nanomaterials 10, no. 11: 2202. https://doi.org/10.3390/nano10112202
APA StyleSana, S., Zivkovic, V., & Boodhoo, K. (2020). Empirical Modelling of Hydrodynamic Effects on Starch Nanoparticles Precipitation in a Spinning Disc Reactor. Nanomaterials, 10(11), 2202. https://doi.org/10.3390/nano10112202