Biotransformation of Food-Grade and Nanometric TiO2 in the Oral–Gastro–Intestinal Tract: Driving Forces and Effect on the Toxicity toward Intestinal Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanomaterials
2.1.1. Specific Surface Area
2.1.2. AF4/DLS Analysis
2.2. Treatment of TiO2 Samples with the Simulated Human Digestive System (SHDS)
2.2.1. Preparation of Fluids
2.2.2. Treatment with Single Simulated Digestive Fluids
2.2.3. Simulated Digestion Cascade
2.2.4. Reversibility of the Biotransformation
2.3. Dynamic Light Scattering Analysis
2.4. Electrophoretic Light Scattering Analysis
2.5. Geometrical Diameter Distribution Measured by Flow Particle Imaging Analyzer
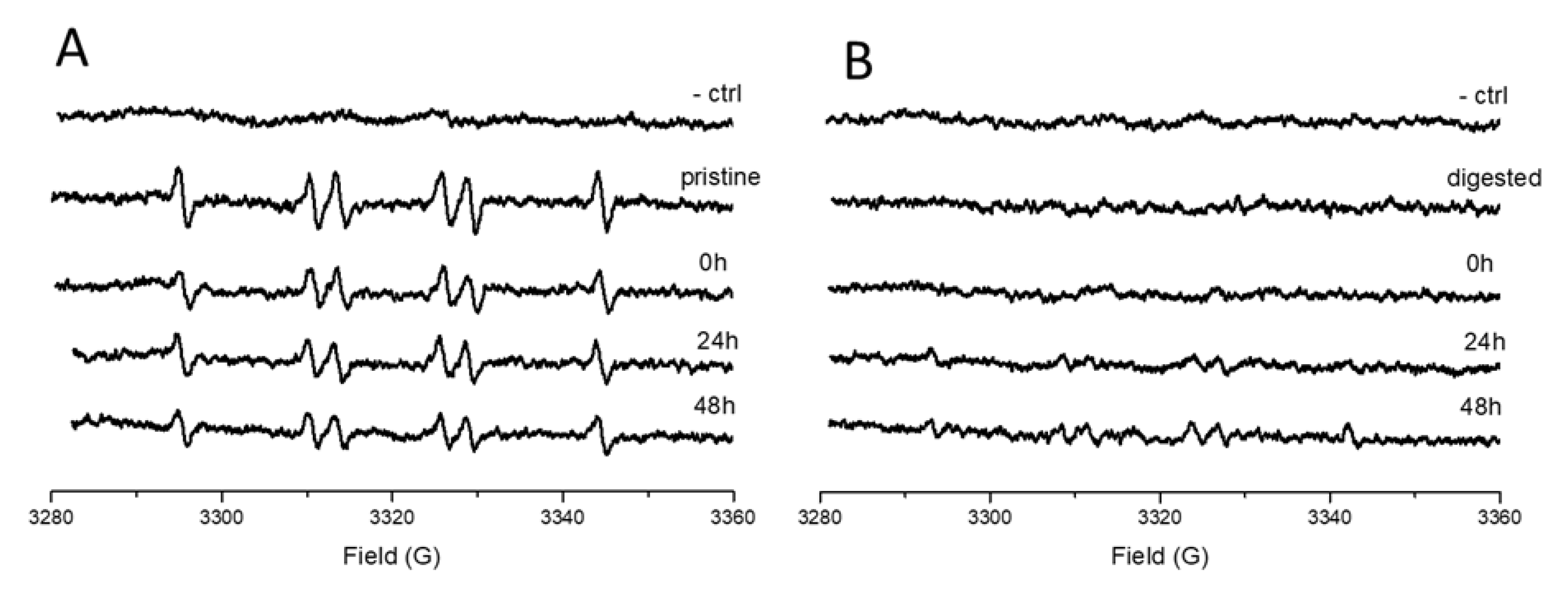
2.6. Surface Reactivity
2.7. Cellular Experiments
2.7.1. Preparation the Suspensions in Cell Media
2.7.2. Cell Culture
2.7.3. WST-1 Assay
2.7.4. DHR123 Assay
2.7.5. 53BP1 Immunostaining and Foci Count
2.7.6. Statistical Analysis
3. Results
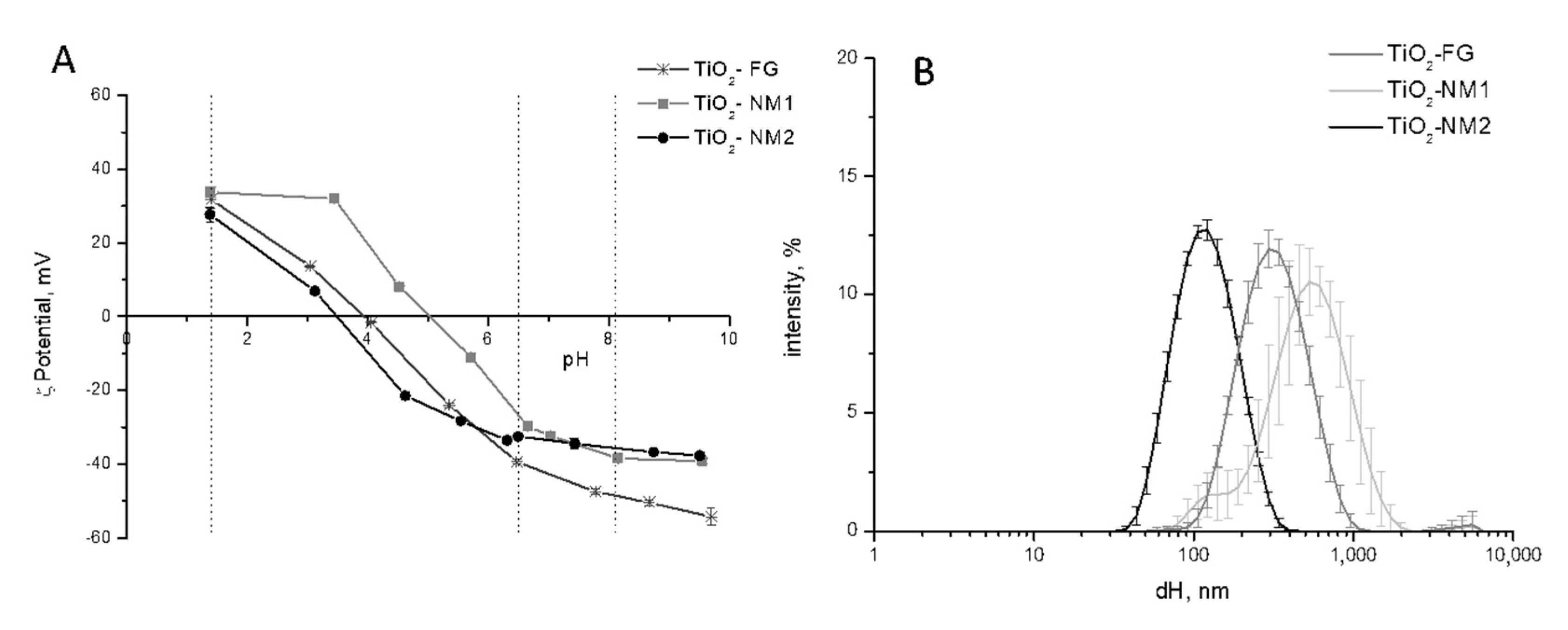
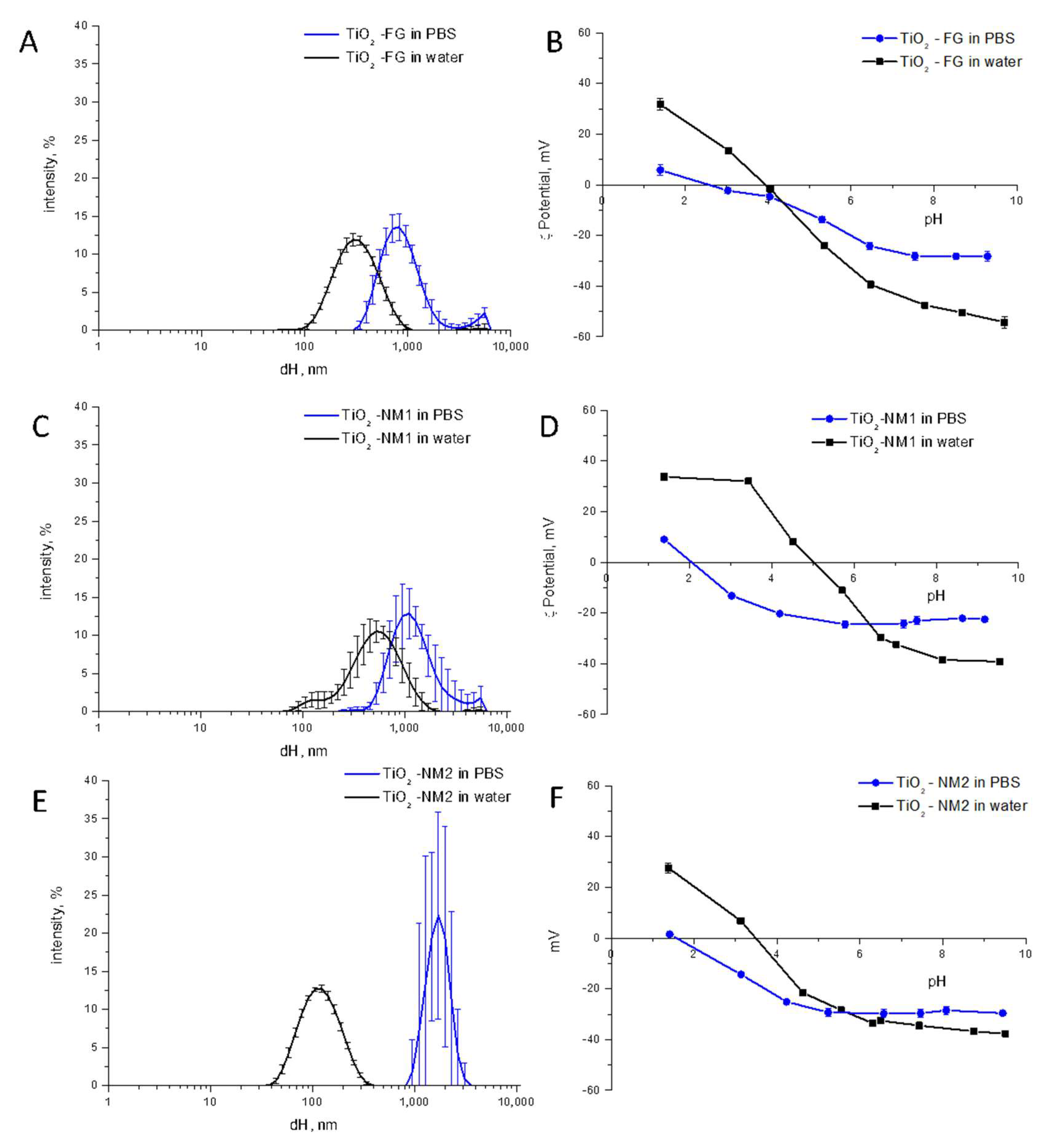
3.1. Size Distribution and ζ-Potential of the TiO2 Samples in Water
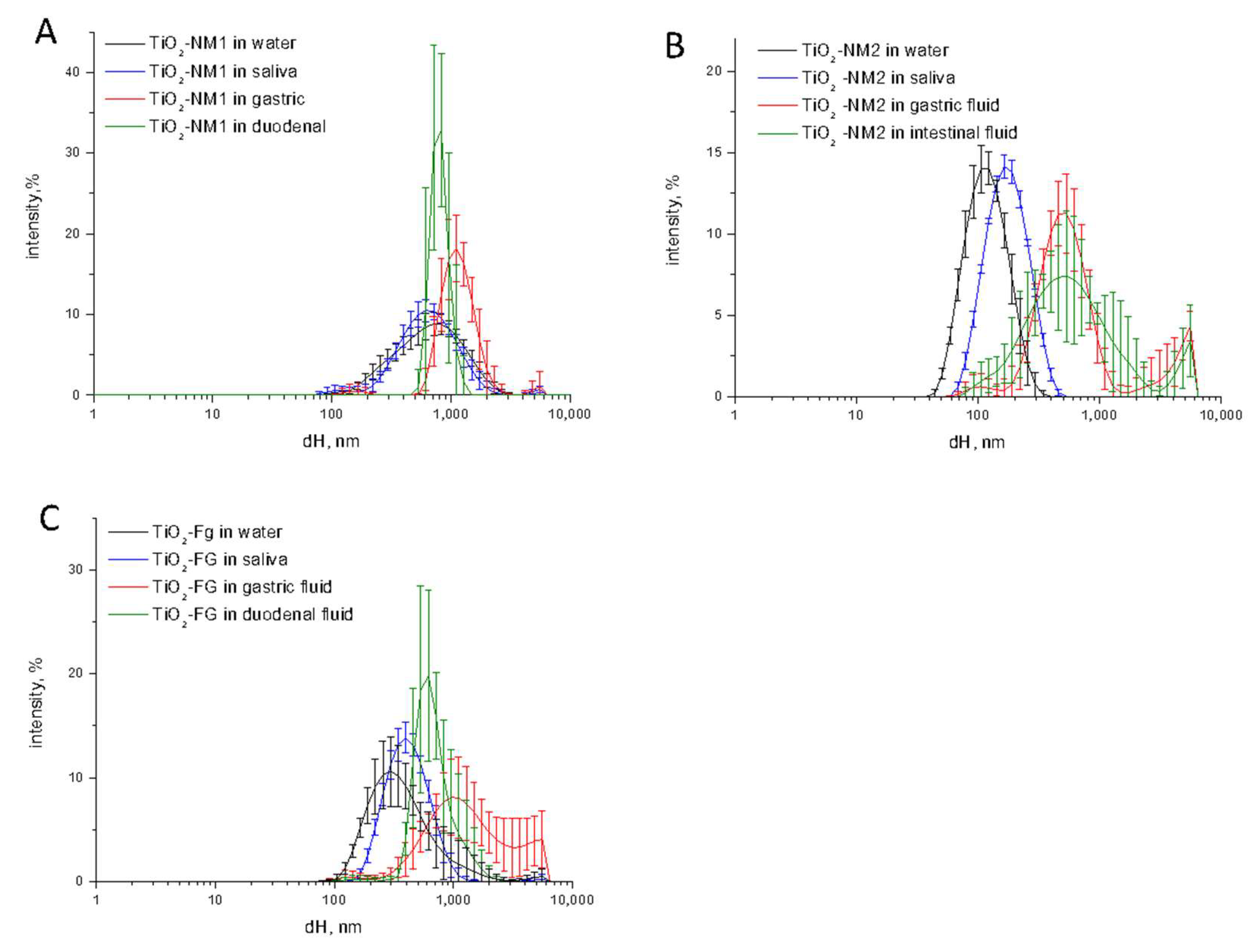
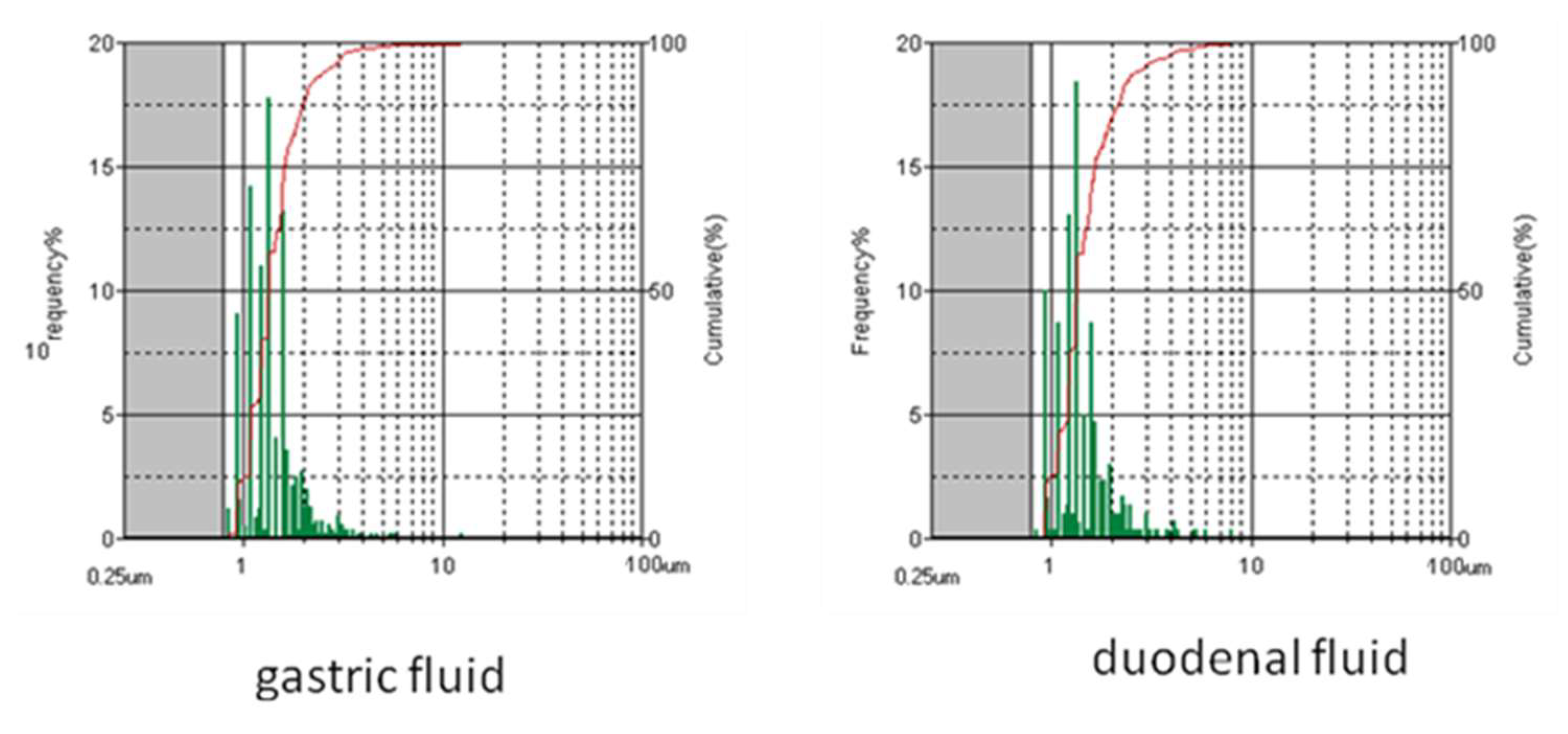
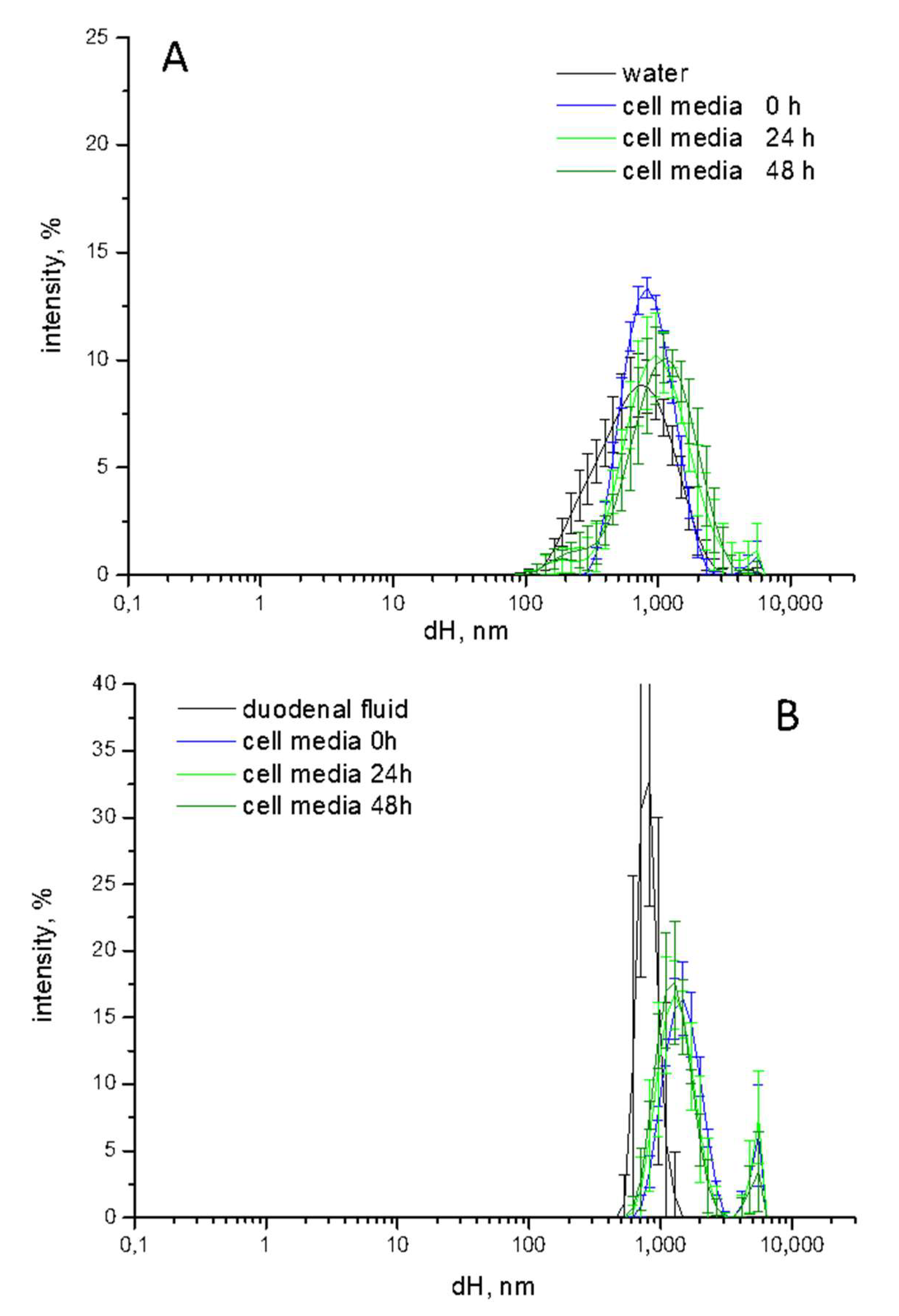
3.2. Size Distribution Changes during the Simulated Digestion Cascade
3.3. Irreversibility of Size Distribution Changes
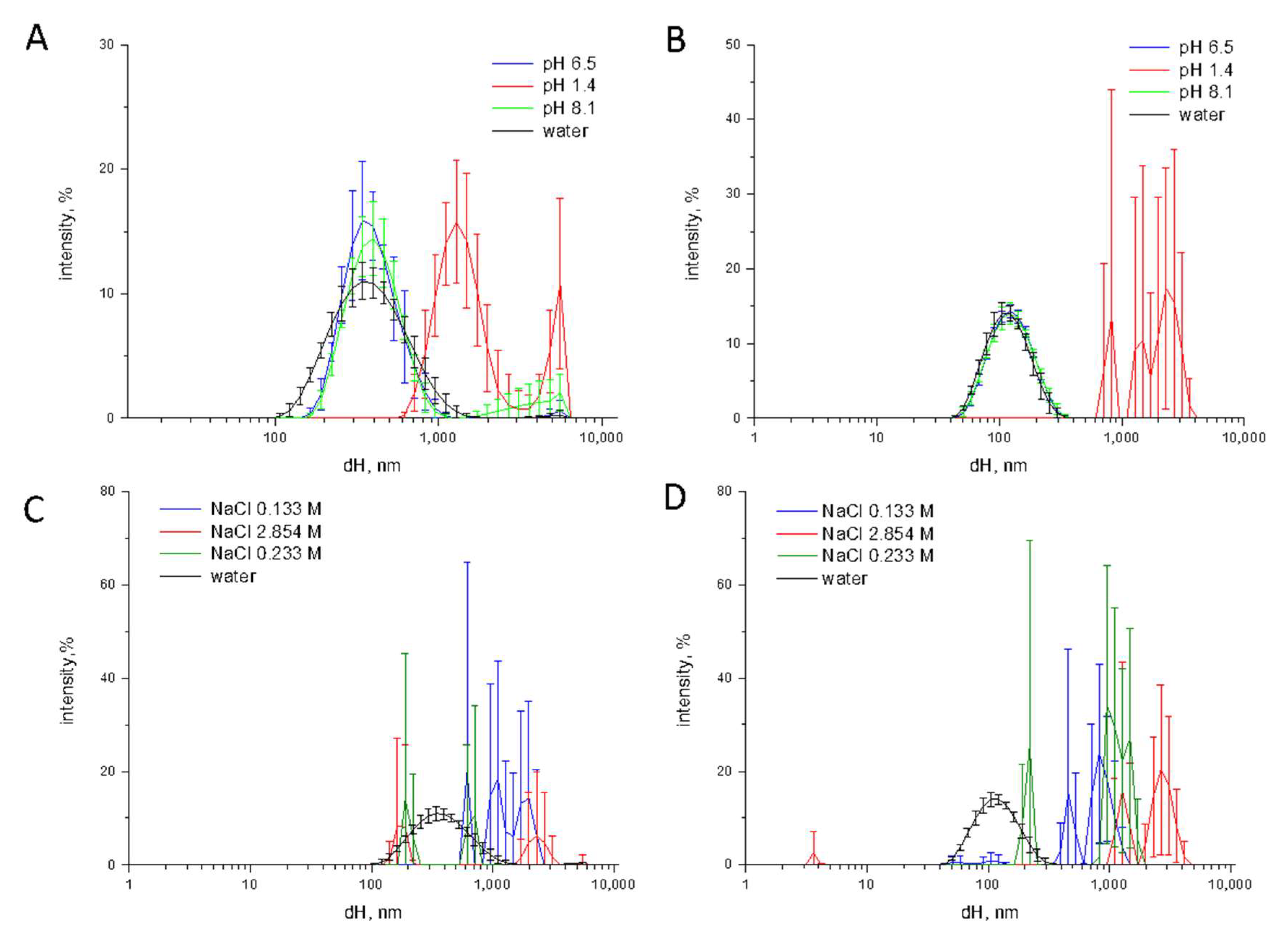
3.4. Effect of the pH, Ionic Strength, and Phosphate Ions on Aggregation/Agglomeration
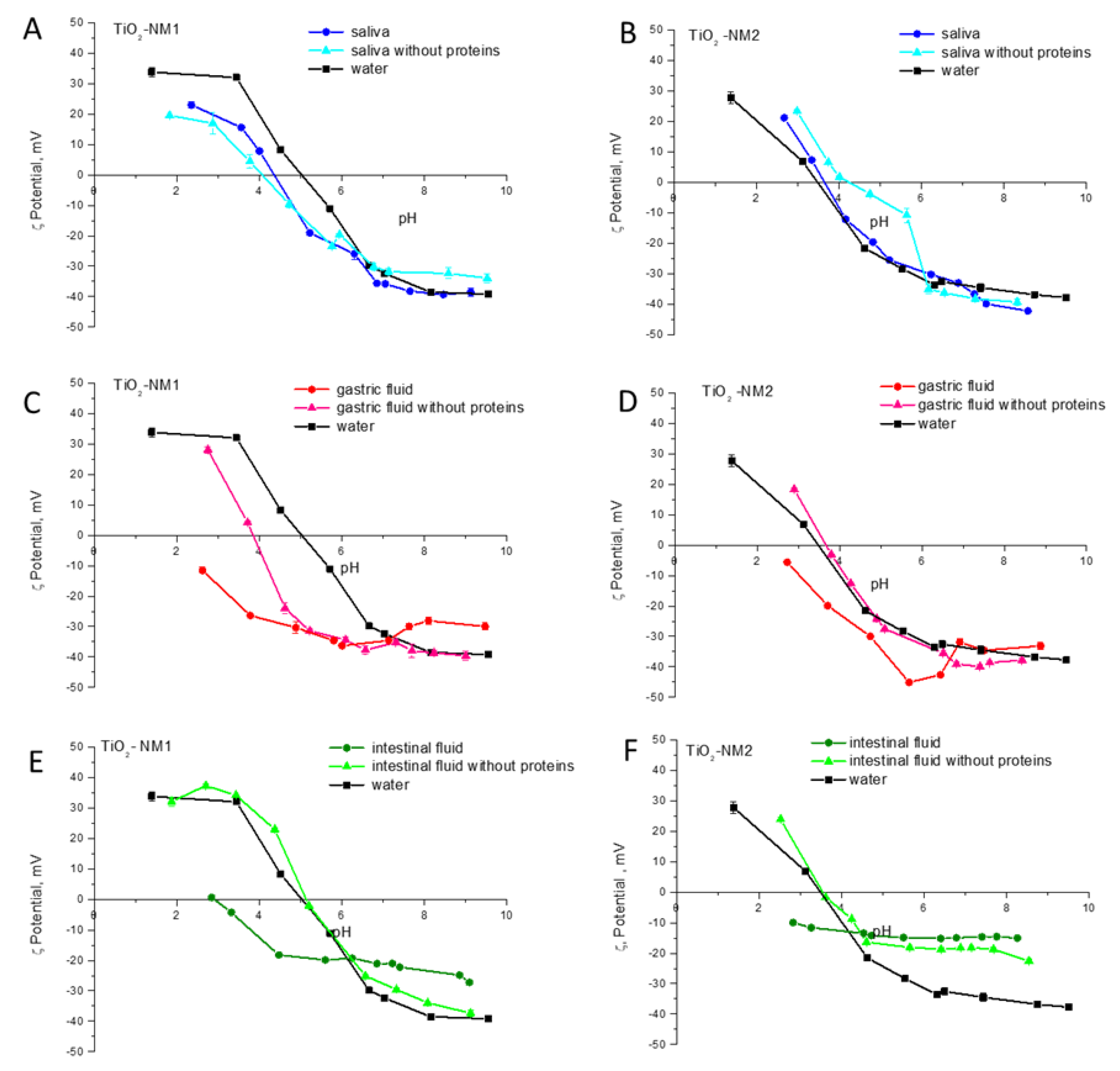
3.5. Bio-Corona Formation
3.6. Characterization of TiO2-NM1 in Cell Media
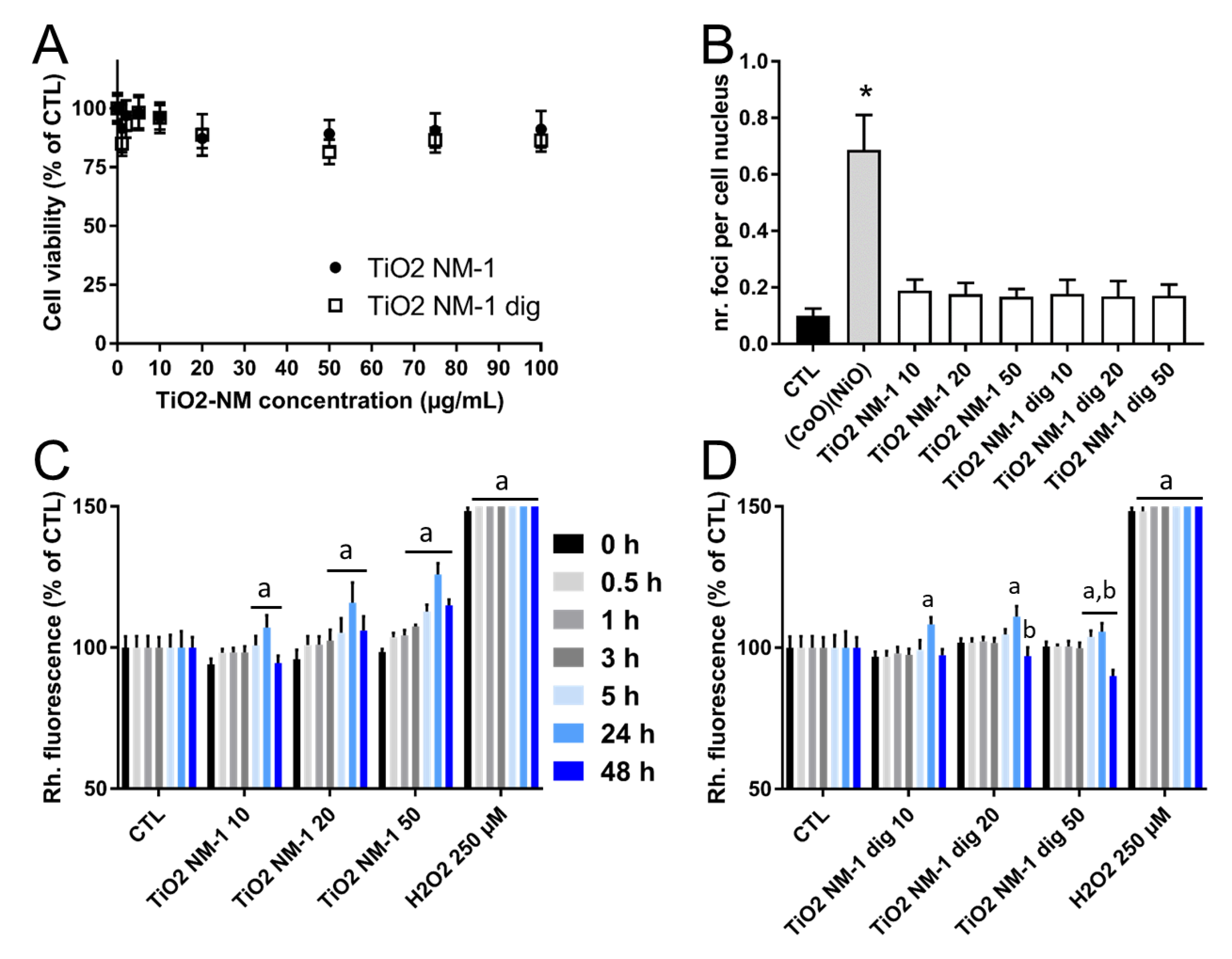
3.7. Cytotoxicity, Genotoxicity and Oxidative Stress on HCT116 Cells Exposed to Pristine or Treated TiO2-NM1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; Is it safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.J.B.; Van Bemmel, G.; Herrera-Rivera, Z.; Helsper, H.P.; Marvin, H.J.P.; Weigel, S.; Tromp, P.C.; Oomen, A.G.; Bouwmeester, H. Characterization of titanium dioxide nanoparticles in food product: Analytical methods to define nanoparticles. J. Agric. Food Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016, 14, 4545. [Google Scholar] [CrossRef]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.-H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef]
- Bachler, G.; von Goetz, N.; Hungerbuhler, K. Using physiologically based pharmacokinetic modeling for dietary risk assessment of titanium dioxide nanoparticles. Nanotoxicology 2015, 9, 373–380. [Google Scholar] [CrossRef]
- Otmar, G.; Ponti, J.; Senaldi, C.; Bianchi, I.; Mehn, D.; Barrero, J.; Gilliland, D.; Matissek, R.; Anklam, E. Characterisation of food grade titania with respect to nanoparticle content in pristine additives and in their related food products. Food Addit. Contam. Part A 2020, 37, 239–253. [Google Scholar] [CrossRef]
- Yang, Y.; Doudrick, K.; Bi, X.; Hristovsk, K.; Herckes, P.; Westerhoff, P.; Kaegi, R. Characterisation of food grade titanium dioxide the presence of nanosize particles. Environ. Sci. Technol. 2014, 48, 6391–6400. [Google Scholar] [CrossRef]
- Pele, L.; Thoree, V.; Bruggraber, S.F.A.; Koller, D.; Thompson, R.P.H.; Lomer, M.C.; Powell, J.J. Pharmaceutical/food grade titanium dioxide particles are absorbed into the bloodstream of human volunteers. Part. Fiber Toxicol. 2015, 12. [Google Scholar] [CrossRef]
- Jones, K.; Morton, J.; Smith, I.; Jurkschat, K.; Harding, A.H.; Evans, G. Human in vivo and in vitro studies on gastrointestinal absorption of titanium dioxide nanoparticles. Toxicol. Lett. 2015, 233, 95–101. [Google Scholar] [CrossRef]
- Koeneman, B.A.; Zhang, Y.; Westerhoff, P.; Chen, Y.; Crittenden, J.C.; Capco, D.G. Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol. Toxicol. 2010, 26, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Brun, E.; Barreau, F.; Veronesi, G.; Fayard, B.; Sorieul, S.; Chanéac, C.; Carrière, M. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part. Fibre Toxicol. 2014, 11, 13. [Google Scholar] [CrossRef]
- Warheit, D.B.; Brown, S.C.; Donner, E.M. Acute and subchronic oral toxicity studies in rats with nanoscale and pigment grade titanium dioxide particles. Food Chem. Toxicol. 2015, 84, 208–224. [Google Scholar] [CrossRef]
- Urrutia-Ortega, I.M.; Garduño-Balderas, L.G.; Delgado-Buenrostro, N.L.; Freyre-Fonseca, V.; Flores-Flores, J.O.; González-Robles, A.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Rodríguez-Sosa, M.; León-Cabrera, S.; et al. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food Chem. Toxicol. 2016, 93, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.A.; Moron, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 Inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373–40386. [Google Scholar] [CrossRef]
- Blevins, L.K.; Crawford, R.B.; Bach, A.; Rizzo, M.D.; Zhou, J.; Henriquez, J.E.; Olive Khan, D.M.I.; Sermet, S.; Arnold, L.L.; Pennington, K.L.; et al. Evaluation of immunologic and intestinal effects in rats administered an E 171-containing diet, a food grade titanium dioxide (TiO2). Food Chem. Toxicol. 2019, 133, 110793. [Google Scholar] [CrossRef]
- Sohal, I.S.; O’Fallon, K.S.; Gaines, P.; Demokritou, P.; Bello, D. Ingested engineered nanomaterials: State of science in nanotoxicity testing and future research needs. Part. Fibre Toxicol. 2018, 15, 29. [Google Scholar] [CrossRef]
- Dorier, M.; Beal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology 2017, 11, 751–761. [Google Scholar] [CrossRef]
- Dorier, M.; Béal, D.; Tisseyre, C.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Rabilloud, T.; Carriere, M. The food additive E171 and titanium dioxide nanoparticles indirectly alter the homeostasis of human intestinal epithelial cells in vitro. Environ. Sci. Nano 2019, 6, 1549–1561. [Google Scholar] [CrossRef]
- Cho, W.S.; Kang, B.C.; Lee, J.K.; Jeong, J.; Che, J.H.; Seok, S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013, 10, 9. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Bergamaschi, E.; Campagna, M.; Campagnolo, L.; De Palma, G.; Iavicoli, S.; Leso, V.; Magrini, A.; Miragoli, M.; Pedata, P.; et al. The unrecognized occupational relevance of the interaction between engineered nanomaterials and the gastro-intestinal tract: A consensus paper from a multidisciplinary working group. Part. Fibre Toxicol. 2017, 14. [Google Scholar] [CrossRef]
- Sohal, I.S.; Cho, Y.K.; O’Fallon, K.S.; Gaines, P.; Demokritou, P.; Bello, D. Dissolution behavior and biodurability of ingested engineered nanomaterials in the gastrointestinal environment. ACS Nano 2018, 12, 115–8128. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Cheng, B.; Yang, Y.X.; Cao, A.; Liu, J.H.; Du, L.J.; Liu, Y.; Zhao, Y.; Wang, H. Characterization and preliminary toxicity assay of nanotitanium dioxide additive in sugar coated chewing gum. Small 2012, 9, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Liu, J.; Feng, X.; Wei, L.; Shao, L. A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res. Lett. 2015, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Ault, A.P.; Stark, D.I.; Axson, J.L.; Keeney, J.N.; Maynard, A.D.; Berginc, I.L.; Philbert, M.A. Protein corona induce modification on silver nanoparticles aggregation in simulated gastric fluid. Environ. Sci. Nano 2016, 3, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Radziwill-Bienkowska, J.M.; Talbot, P.; Kamphuis, J.B.J.; Robert, V.; Cartier, C.; Fourquaux, I.; Lentzen, E.; Audinot, J.-N.; Jamme, F.; Réfrégiers, M.; et al. Toxicity of Food-Grade TiO2 to Commensal Intestinal and Transient Food-Borne Bacteria: New Insights Using Nano-SIMS and Synchrotron UV Fluorescence Imaging. Front. Microbiol. 2018, 9, 794. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, T.; DeLoid, G.M.; Gaffrey, M.J.; Weitz, K.K.; Thrall, B.D.; Qian, W.-J.; Demokritou, P. Evaluation of the cytotoxic and cellular proteome impacts of food-grade TiO2 (E171) using simulated gastrointestinal digestions and a tri-culture small intestinal epithelial model. NanoImpact 2020, 17, 100202. [Google Scholar] [CrossRef]
- Rasmussen, K.; Mech, A.; Mast, J.; De Temmerman, P.-J.; Verleysen, E.; Waegeneers, N.; Van Steen, F.; Pizzolon, J.C.; De Temmerman, L.; Van Doren, E.; et al. Titanium Dioxide, NM-100, NM-101, NM-102, NM-103, NM-104, NM-105: Characterisation and Physico-Chemical Properties; JRC science and Policy Reports; European Commission: Luxembourg, 2014. [Google Scholar]
- McClements, D.J.; Li, Y. Review of in vitro digestion models for rapid screening of emulsion-based systems. Food Funct. 2010, 1, 32–59. [Google Scholar] [CrossRef]
- Versantvoort, C.H.M.; Oomen, A.G.; Van de Kamp, E.; Rompelberg, C.J.M.; Sips, A.J. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef]
- Marucco, A.; Carella, E.; Fenoglio, I. A comparative study on the efficacy of different probes to predict the photo-activity of nano-titanium dioxide toward biomolecules. RSC Adv. 2015, 5, 89559–89568. [Google Scholar] [CrossRef]
- EU FP7 Project Nanogenotox. 2011. Available online: http://www.nanogenotox.eu/files/PDF/Deliverables/nanogenotox%20deliverable%203_wp4_%20dispersion%20protocol.pdf (accessed on 15 July 2014).
- Marucco, A.; Aldieri, E.; Leinardi, R.; Bergamaschi, E.; Riganti, C.; Fenoglio, I. Applicability and Limitations in the Characterization of Poly-Dispersed Engineered Nanomaterials in Cell Media by Dynamic Light Scattering (DLS). Materials 2019, 12, 3833. [Google Scholar] [CrossRef] [PubMed]
- Marucco, A.; Catalano, F.; Fenoglio, I.; Turci, F.; Martra, G.; Fubini, B. Possible chemical source of discrepancy between in vitro and in vivo tests in nanotoxicology caused by strong adsorption of buffer components. Chem. Res. Toxicol. 2015, 28, 87–91. [Google Scholar] [CrossRef]
- Marucco, A.; Pellegrino, F.; Oliaro-Bosso, S.; Maurino, V.; Martra, G.; Fenoglio, I. Indoor illumination: A possible pitfall in toxicological assessment of photo-active nanomaterials. J. Photochem. Photob. A Chem. 2018, 350, 23–31. [Google Scholar] [CrossRef]
- McClements, D.J.; DeLoid, G.; Pyrgiotakis, G.; Shatkin, J.A.; Xiao, H.; Demokritou, P. The role of the food matrix and gastrointestinal tract in the assessment of biological properties of ingested engineered nanomaterials (iENMs): State of the science and knowledge gaps. NanoImpact 2016, 3–4, 47–57. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical−Chemical Aspects of Protein Corona: Relevance to in Vitro and in Vivo Biological Impacts of Nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, I.; Fubini, B.; Ghibaudi, E.; Turci, F. Multiple aspects of the interaction of biomacromolecules with inorganic surfaces. Adv. Drug Deliver. Rev. 2011, 63, 1186–1209. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.G.; Allegri, M.; Chiu, M.; Costa, L.; Blosi, M.; Ortelli, S.; Bussolati, O.; Bergamaschi, E. Lipopolysaccharide Adsorbed to the Bio-Corona of TiO2 Nanoparticles Powerfully Activates Selected Pro-inflammatory Transduction Pathways. Front. Immun. 2017, 8, 866. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Xu, X.; Tang, W.; Xiong, L.; Sun, Q. Formation of Protein Corona on Nanoparticles with Digestive Enzymes in Simulated Gastrointestinal Fluids. J. Agric. Food Chem. 2019, 67, 2296–2306. [Google Scholar] [CrossRef]
- Lieleg, O.; Vladescu, I.; Ribbeck, K. Characterization of particle translocation through mucin hydrogels. Biophys. J. 2010, 98, 1782–1789. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. Nanomed. 2014, 9, 51–63. [Google Scholar] [CrossRef]
- Gerloff, K.; Fenoglio, I.; Carella, E.; Kolling, J.; Albrecht, C.; Boots, A.; Förster, I.; Schins, R. Distinctive toxicity of TiO2 rutile/anatase mixed phases nanoparticles on Caco-2 cells. Chem. Res. Toxicol. 2012, 25, 646–655. [Google Scholar] [CrossRef]

| TiO2-FG | TiO2-NM1 c | TiO2–NM2 d | |
|---|---|---|---|
| Specific surface area (m2/g) | 9 ± 0.45 a | 316.07 ± 15.80 | 35–65 |
| Primary particle size (nm) | 10–350 b | 5–6 | 70 (mean) |
| Aggregate size (nm) | - | 10–170 | - |
| Crystalline phase | Anatase (>99%) | Anatase (98.1%) | Anatase (85%); Rutile (15%) |
| Impurities | - | Al, Na, P, S | <0.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marucco, A.; Prono, M.; Beal, D.; Alasonati, E.; Fisicaro, P.; Bergamaschi, E.; Carriere, M.; Fenoglio, I. Biotransformation of Food-Grade and Nanometric TiO2 in the Oral–Gastro–Intestinal Tract: Driving Forces and Effect on the Toxicity toward Intestinal Epithelial Cells. Nanomaterials 2020, 10, 2132. https://doi.org/10.3390/nano10112132
Marucco A, Prono M, Beal D, Alasonati E, Fisicaro P, Bergamaschi E, Carriere M, Fenoglio I. Biotransformation of Food-Grade and Nanometric TiO2 in the Oral–Gastro–Intestinal Tract: Driving Forces and Effect on the Toxicity toward Intestinal Epithelial Cells. Nanomaterials. 2020; 10(11):2132. https://doi.org/10.3390/nano10112132
Chicago/Turabian StyleMarucco, Arianna, Marion Prono, David Beal, Enrica Alasonati, Paola Fisicaro, Enrico Bergamaschi, Marie Carriere, and Ivana Fenoglio. 2020. "Biotransformation of Food-Grade and Nanometric TiO2 in the Oral–Gastro–Intestinal Tract: Driving Forces and Effect on the Toxicity toward Intestinal Epithelial Cells" Nanomaterials 10, no. 11: 2132. https://doi.org/10.3390/nano10112132
APA StyleMarucco, A., Prono, M., Beal, D., Alasonati, E., Fisicaro, P., Bergamaschi, E., Carriere, M., & Fenoglio, I. (2020). Biotransformation of Food-Grade and Nanometric TiO2 in the Oral–Gastro–Intestinal Tract: Driving Forces and Effect on the Toxicity toward Intestinal Epithelial Cells. Nanomaterials, 10(11), 2132. https://doi.org/10.3390/nano10112132






