In Situ Synthesis of Alumina Nanoparticles in a Binary Carbonate Salt Eutectic for Enhancing Heat Capacity
Abstract
1. Introduction
2. Method
3. Results and Discussion
3.1. Thermogravimetric Analysis (TGA)
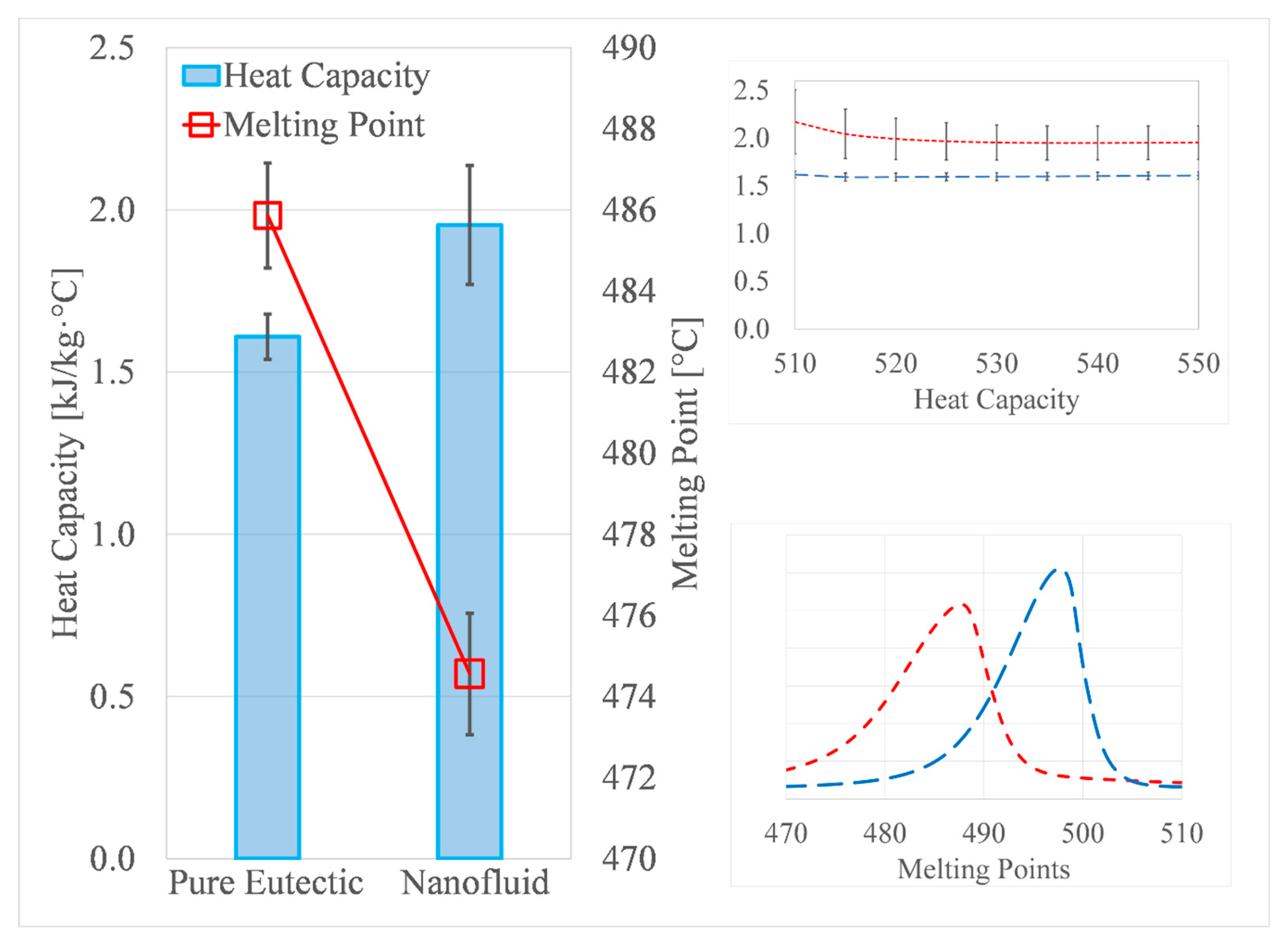
3.2. DSC Measurements
3.3. Material Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Wu, Z.; Li, B.; Sundén, B. A review on molten-salt-based and ionic-liquid-based nanofluids for medium-to-high temperature heat transfer. J. Therm. Anal. Calorim. 2018, 136, 1037–1051. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Mujumdar, A.S. Heat transfer characteristics of nanofluids: A review. Int. J. Therm. Sci. 2007, 46, 1–19. [Google Scholar] [CrossRef]
- Patel, H.E.; Das, S.K.; Sundararajan, T.; Nair, A.S.; George, B.; Pradeep, T. Thermal conductivities of naked and monolayer protected metal nanoparticle based nanofluids: Manifestation of anomalous enhancement and chemical effects. Appl. Phys. Lett. 2003, 83, 2931–2933. [Google Scholar] [CrossRef]
- Choi, S.; Zhang, Z.G.; Yu, W.; Lockwood, F.E.; Grulke, E.A. Anomalous thermal conductivity enhancement in nanotube suspensions. Appl. Phys. Lett. 2001, 79, 2252–2254. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Choi, S.U.S. Thermal Conductivity of Nanoparticle—Fluid Mixture. J. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]
- Dudda, B.; Shin, D. Effect of nanoparticle dispersion on specific heat capacity of a binary nitrate salt eutectic for concentrated solar power applications. Int. J. Therm. Sci. 2013, 69, 37–42. [Google Scholar] [CrossRef]
- Tiznobaik, H.; Shin, D. Enhanced specific heat capacity of high-temperature molten salt-based nanofluids. Int. J. Heat Mass Transf. 2013, 57, 542–548. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Enhancement of specific heat capacity of high-temperature silica-nanofluids synthesized in alkali chloride salt eutectics for solar thermal-energy storage applications. Int. J. Heat Mass Transf. 2011, 54, 1064–1070. [Google Scholar] [CrossRef]
- Wang, L.; Tan, Z.; Meng, S.; Liang, D.; Li, G. Enhancement of Molar Heat Capacity of Nanostructured Al2O3. J. Nano Part. Res. 2001, 3, 483–487. [Google Scholar] [CrossRef]
- Xue, L.; Keblinski, P.; Phillpot, S.; Choi, S.-S.; Eastman, J. Effect of liquid layering at the liquid–solid interface on thermal transport. Int. J. Heat Mass Transf. 2004, 47, 4277–4284. [Google Scholar] [CrossRef]
- Keblinski, P.; Phillpot, S.; Choi, S.; Eastman, J. Mechanisms of heat flow in suspensions of nano-sized particles (nanofluids). Int. J. Heat Mass Transf. 2002, 45, 855–863. [Google Scholar] [CrossRef]
- Shin, D.; Tiznobaik, H.; Banerjee, D. Specific heat mechanism of molten salt nanofluids. Appl. Phys. Lett. 2014, 104, 121914. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Enhanced Specific Heat Capacity of Nanomaterials Synthesized by Dispersing Silica Nanoparticles in Eutectic Mixtures. J. Heat Transf. 2013, 135, 032801. [Google Scholar] [CrossRef]
- Ho, M.X.; Pan, C. Optimal concentration of alumina nanoparticles in molten Hitec salt to maximize its specific heat capacity. Int. J. Heat Mass Transf. 2014, 70, 174–184. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-T.; Zhang, L.-D.; Wang, X.; Ma, C.-F. Experimental study on thermophysical properties of molten salt nanofluids prepared by high-temperature melting. Sol. Energy Mater. Sol. Cells 2019, 191, 209–217. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-T.; Zhang, L.-D.; Wang, X.; Ma, C.-F. Experimental study on the specific heat and stability of molten salt nanofluids prepared by high-temperature melting. Sol. Energy Mater. Sol. Cells 2018, 176, 42–48. [Google Scholar] [CrossRef]
- Lasfargues, M.; Geng, Q.; Cao, H.; Ding, Y. Mechanical Dispersion of Nanoparticles and Its Effect on the Specific Heat Capacity of Impure Binary Nitrate Salt Mixtures. Nanomaterials 2015, 5, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Lasfargues, M.; Bell, A.; Ding, Y. In situ production of titanium dioxide nanoparticles in molten salt phase for thermal energy storage and heat-transfer fluid applications. J. Nano Part. Res. 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Lasfargues, M.; Stead, G.; Amjad, M.; Ding, Y.; Wen, D. In Situ Production of Copper Oxide Nanoparticles in a Binary Molten Salt for Concentrated Solar Power Plant Applications. Materials 2017, 10, 537. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, X.; Li, Y.; Yu, G.; Xu, K.; Li, G. Effect of in-situ synthesized nano-MgO on thermal properties of NaNO3-KNO3. Sol. Energy 2018, 160, 208–215. [Google Scholar] [CrossRef]
- Luo, Y.; Du, X.; Awad, A.; Wen, D. Thermal energy storage enhancement of a binary molten salt via in-situ produced nanoparticles. Int. J. Heat Mass Transf. 2017, 104, 658–664. [Google Scholar] [CrossRef]
- Janz, G.J. Molten Salts Handbook; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lee, S.; Kim, M.; Hwang, M.; Kim, K.; Jeon, C.; Song, J. Thermal stability and viscosity behaviors of hot molten carbonate mixtures. Exp. Therm. Fluid Sci. 2013, 49, 94–104. [Google Scholar] [CrossRef]
- Venables, H.J.; Wells, J.I. Powder Mixing. Drug Dev. Ind. Pharm. 2001, 27, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Dehsari, H.S.; Heidari, M.; Ribeiro, A.H.; Tremel, W.; Jakob, G.; Donadio, D.; Potestio, R.; Asadi, K. Combined Experimental and Theoretical Investigation of Heating Rate on Growth of Iron Oxide Nanoparticles. Chem. Mater. 2017, 29, 9648–9656. [Google Scholar] [CrossRef]
- Guardia, P.; Pérez-Juste, J.; Labarta, A.; Batlle, X.; Liz-Marzán, L.M. Heating rate influence on the synthesis of iron oxide nanoparticles: The case of decanoic acid. Chem. Commun. 2010, 46, 6108–6110. [Google Scholar] [CrossRef]
- Chen, M.; Liu, A.J.P.; Sun, S. One-Step Synthesis of FePt Nanoparticles with Tunable Size. J. Am. Chem. Soc. 2004, 126, 8394–8395. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Wang, Y.; Wang, Z.L. Preparation of Monodispersed Fe−Mo Nanoparticles as the Catalyst for CVD Synthesis of Carbon Nanotubes. Chem. Mater. 2001, 13, 1008–1014. [Google Scholar] [CrossRef]
- Shevchenko, E.V.; Talapin, D.V.; Schnablegger, H.; Kornowski, A.; Festin, Ö.; Svedlindh, P.; Haase, M.; Weller, H. Study of nucleation and growth in the organometallic synthesis of magnetic alloy nanocrystals: The role of nucleation rate in size control of CoPt3 nanocrystals. J. Am. Chem. Soc. 2003, 125, 9090–9101. [Google Scholar] [CrossRef]
- Hong, W.; Zhao, F.; Liu, J.; Tian, D.; Luo, Z. A Novel Preparation Method of Nanometer CuO Powder. Chin. J. Explos. Propellants 2000, 3, 7–8. [Google Scholar]
- Saita, S.; Maenosono, S. Formation Mechanism of FePt Nanoparticles Synthesized via Pyrolysis of Iron (III) Ethoxide and Platinum (II) Acetylacetonate. Chem. Mater. 2005, 17, 6624–6634. [Google Scholar] [CrossRef]
- Rizvi, S.M.M.; El Far, B.; Nayfeh, Y.; Shin, D. Investigation of time–temperature dependency of heat capacity enhancement in molten salt nanofluids. RSC Adv. 2020, 10, 22972–22982. [Google Scholar] [CrossRef]
- Active Standard ASTM E1269. Standard Test Method for Determining Specific Heat Capacity by Differential Scanning Calorimetry; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Grosu, Y.; Nithiyanantham, U.; González-Fernández, L.; Faik, A. Preparation and characterization of nanofluids based on molten salts with enhanced thermophysical properties for thermal energy storage at concentrate solar power. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2019; p. 200021. [Google Scholar] [CrossRef]
- Song, W.; Lu, Y.; Wu, Y.; Ma, C. Effect of SiO2 nanoparticles on specific heat capacity of low-melting-point eutectic quaternary nitrate salt. Sol. Energy Mater. Sol. Cells 2018, 179, 66–71. [Google Scholar] [CrossRef]
- Sang, L.; Liu, T. The enhanced specific heat capacity of ternary carbonates nanofluids with different nanoparticles. Sol. Energy Mater. Sol. Cells 2017, 169, 297–303. [Google Scholar] [CrossRef]
- Rizvi, S.M.M.; Shin, D. Mechanism of heat capacity enhancement in molten salt nanofluids. Int. J. Heat Mass Transf. 2020, 161, 120260. [Google Scholar] [CrossRef]
- El-Shereafy, E.; Abousekkina, M.M.; Mashaly, A.; El-Ashry, M. Mechanism of thermal decomposition and γ-pyrolysis of aluminum nitrate nonahydrate [AI(NO3)3 9H2O]. J. Radioanal. Nucl. Chem. 1998, 237, 183–186. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Wen, S.-B.; Wenglin, L.; Lin, C.-P. Nano α-Al2O3 Powder Preparation by Calcining an Emulsion Precursor. J. Am. Ceram. Soc. 2007, 90, 1723–1727. [Google Scholar] [CrossRef]
- Oskam, G. Metal oxide nanoparticles: Synthesis, characterization and application. J. Sol-Gel Sci. Technol. 2006, 37, 161–164. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Enhanced Specific Heat of Silica Nanofluid. J. Heat Transf. 2010, 133, 024501. [Google Scholar] [CrossRef]
- Ercole, D.; Manca, O.; Vafai, K. An investigation of thermal characteristics of eutectic molten salt-based nanofluids. Int. Commun. Heat Mass Transf. 2017, 87, 98–104. [Google Scholar] [CrossRef]
- Araújo, A.A.S.; Bezerra, M.D.S.; Storpirtis, S.; Matos, J.D.R. Determination of the melting temperature, heat of fusion, and purity analysis of different samples of zidovudine (AZT) using DSC. Braz. J. Pharm. Sci. 2010, 46, 37–43. [Google Scholar] [CrossRef]
- Höhne, G.; Cammenga, H.; Eysel, W.; Gmelin, E.; Hemminger, W. The temperature calibration of scanning calorimeters. Thermochim. Acta 1990, 160, 1–12. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M. Effect of nanoparticles on heat capacity of nanofluids based on molten salts as PCM for thermal energy storage. Nanoscale Res. Lett. 2013, 8, 448. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Miliozzi, A.; Crescenzi, T.; Torre, L.; Kenny, J.M. A New Phase Change Material Based on Potassium Nitrate with Silica and Alumina Nanoparticles for Thermal Energy Storage. Nanoscale Res. Lett. 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gavarrell, P.G.; Fereres, S. An Experimental Study of the Effect of SiO2 Nanoparticles on the Phase Change Characteristics of KNO3-NaNO3 Mixtures for Thermal Energy Storage. In Proceedings of the ASME 2015 International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 13–19 November 2015. [Google Scholar] [CrossRef]
- Olivares, R.I.; Wright, S.; Chen, C. The Thermal Stability of Molten Lithium–Sodium–Potassium Carbonate and the Influence of Additives on the Melting Point. J. Sol. Energy Eng. 2012, 134, 041002. [Google Scholar] [CrossRef]
- Pfleger, N.; Bauer, T.; Martin, C.; Eck, M.; Wörner, A. Thermal energy storage—Overview and specific insight into nitrate salts for sensible and latent heat storage. Beilstein J. Nanotechnol. 2015, 6, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-X.; Zhou, L.-P.; Peng, X.-F. Surface and Size Effects on the Specific Heat Capacity of Nanoparticles. Int. J. Thermophys. 2006, 27, 139–151. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Ma, H.; Yang, M. Molecular dynamics simulation of effect of liquid layering around the nanoparticle on the enhanced thermal conductivity of nanofluids. J. Nano Part. Res. 2009, 12, 811–821. [Google Scholar] [CrossRef]
- Mondragón, R.; Juliá, J.E.; Cabedo, L.; Navarrete, N. On the relationship between the specific heat enhancement of salt-based nanofluids and the ionic exchange capacity of nanoparticles. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Specific heat of nanofluids synthesized by dispersing alumina nanoparticles in alkali salt eutectic. Int. J. Heat Mass Transf. 2014, 74, 210–214. [Google Scholar] [CrossRef]
- Tiznobaik, H.; Banerjee, D.; Shin, D. Effect of formation of “long range” secondary dendritic nanostructures in molten salt nanofluids on the values of specific heat capacity. Int. J. Heat Mass Transf. 2015, 91, 342–346. [Google Scholar] [CrossRef]
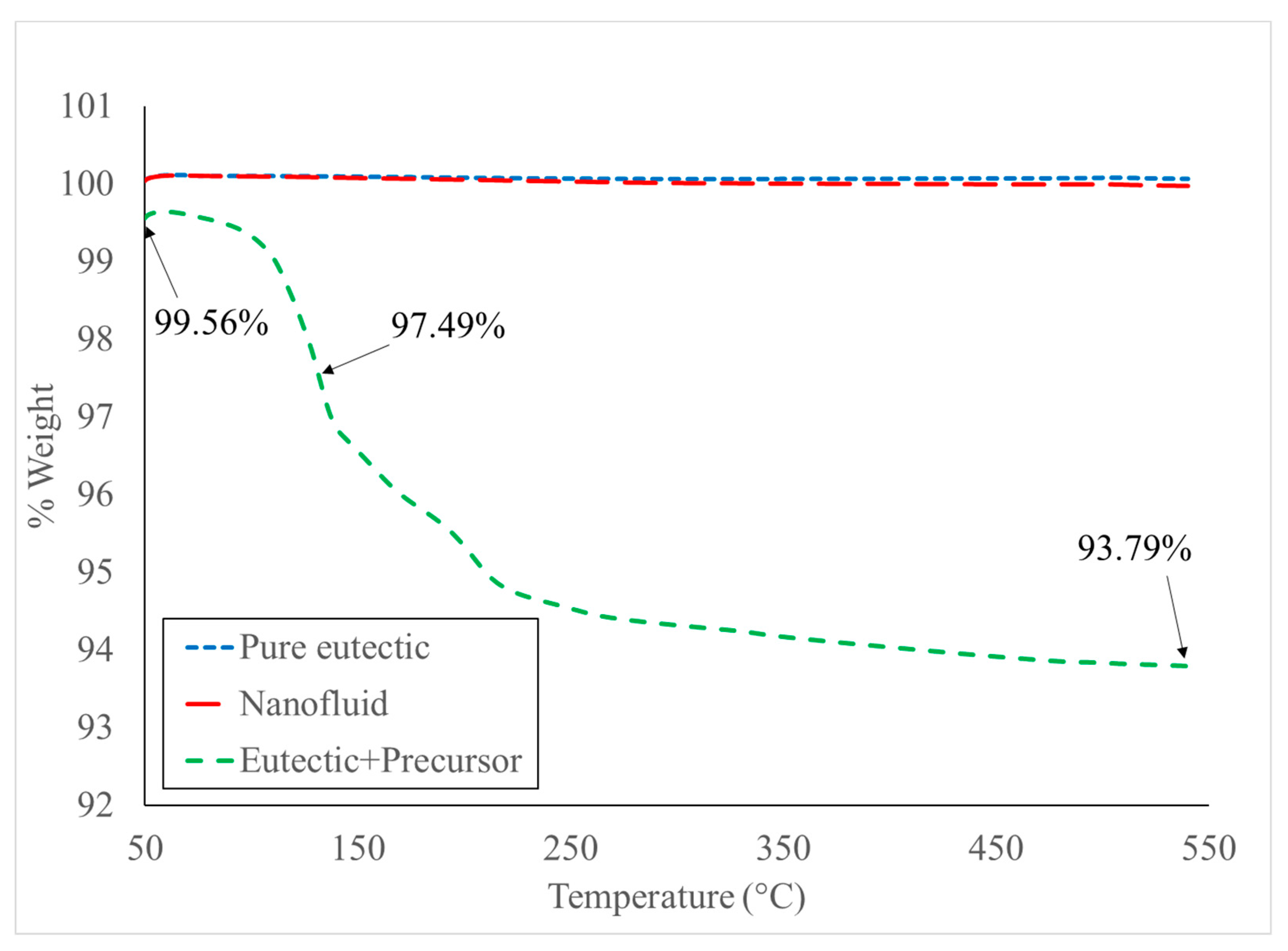
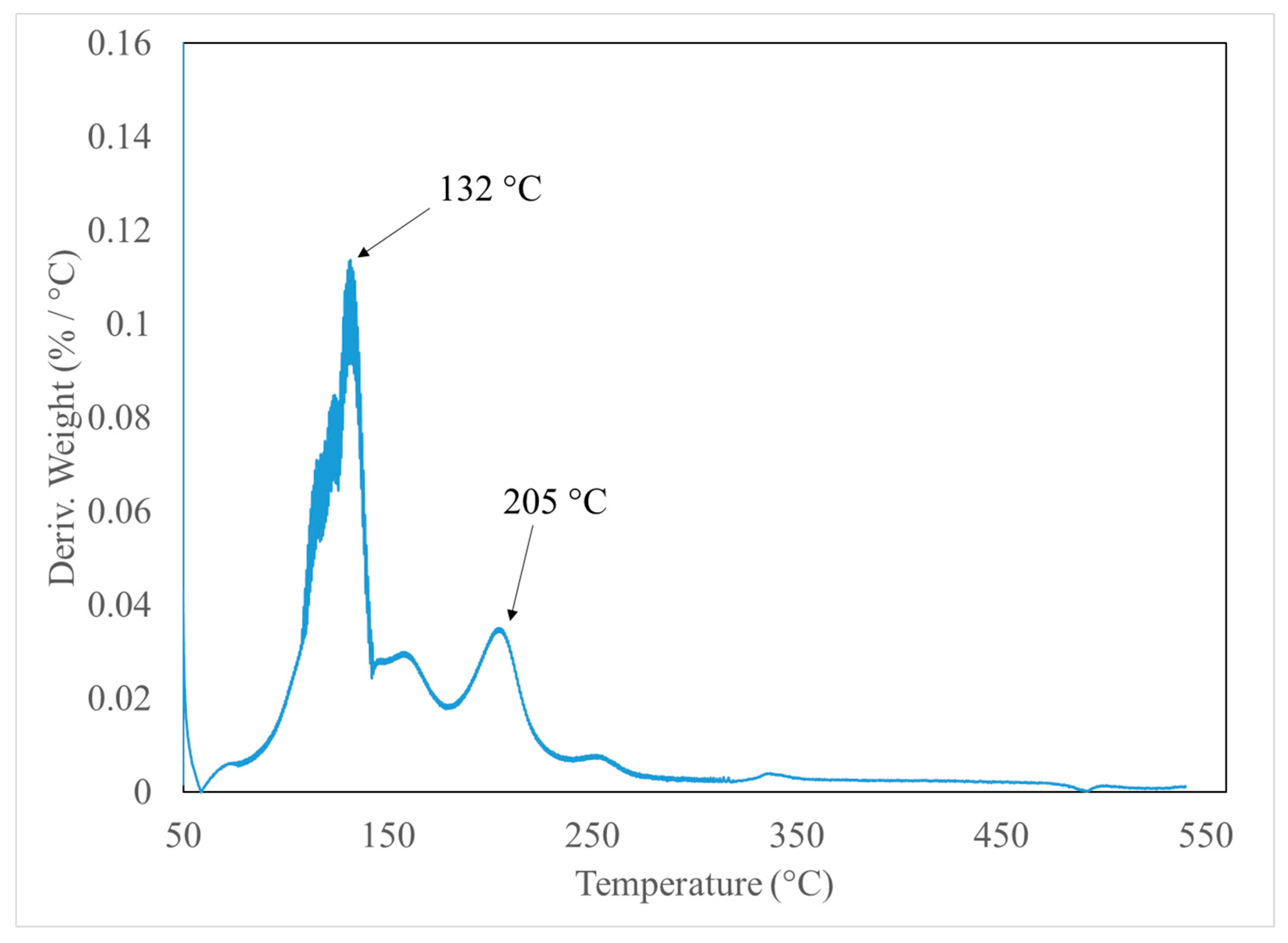
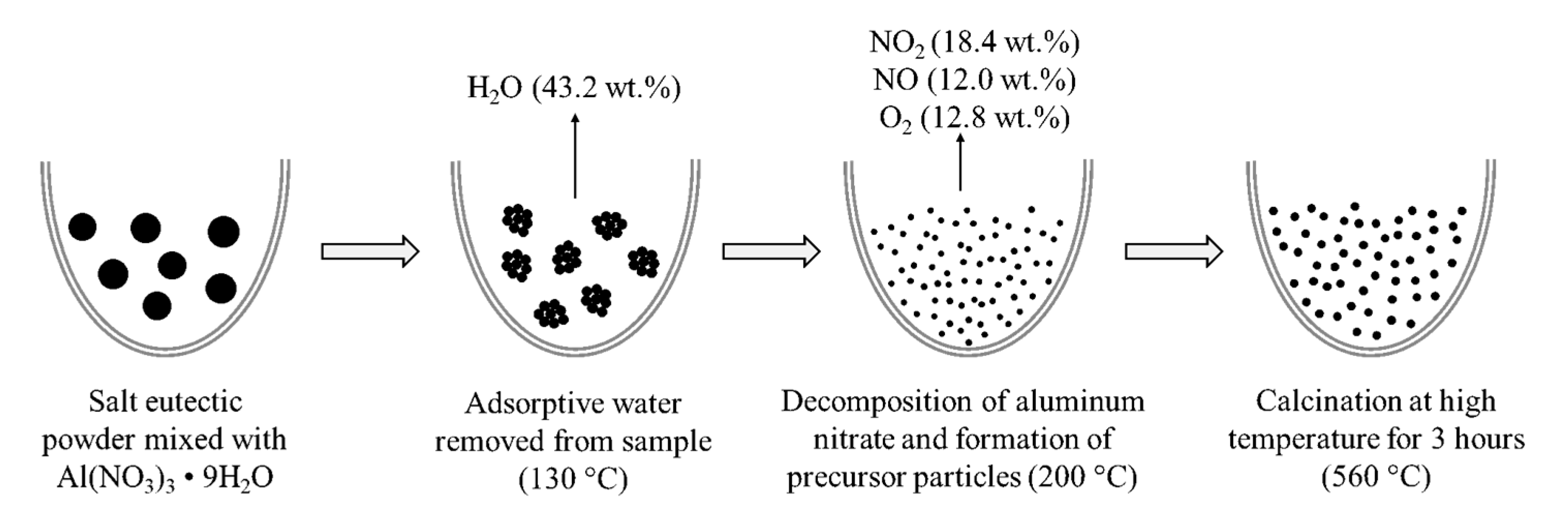
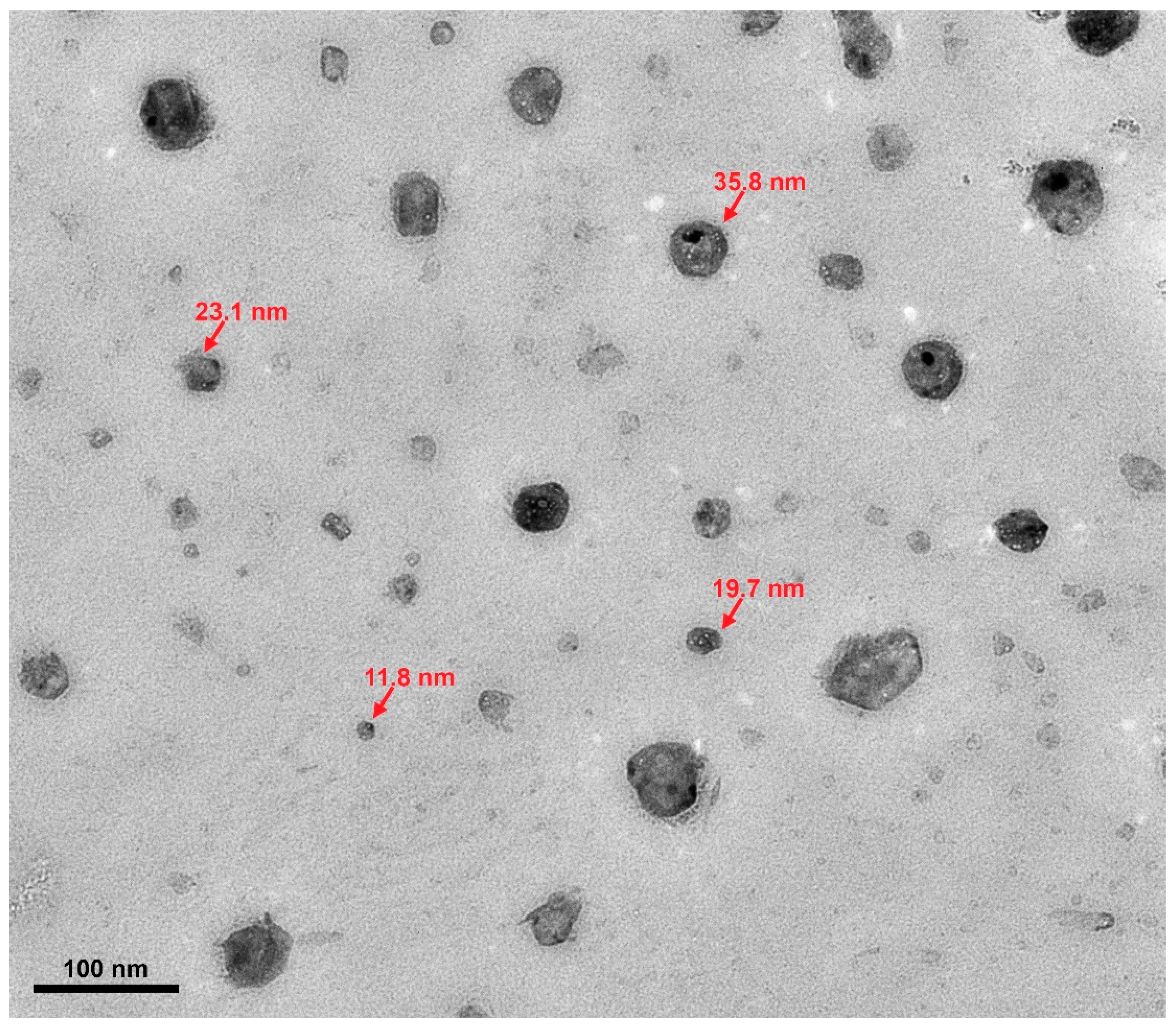
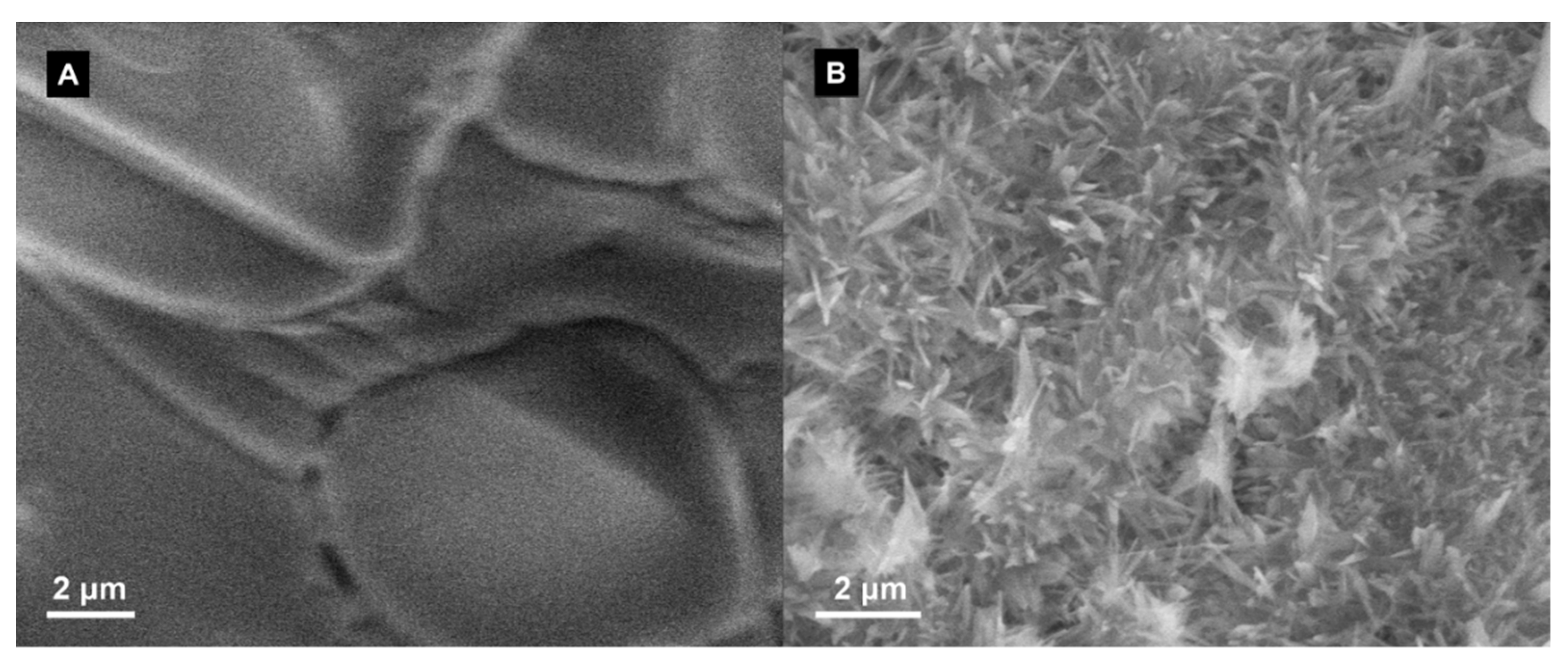

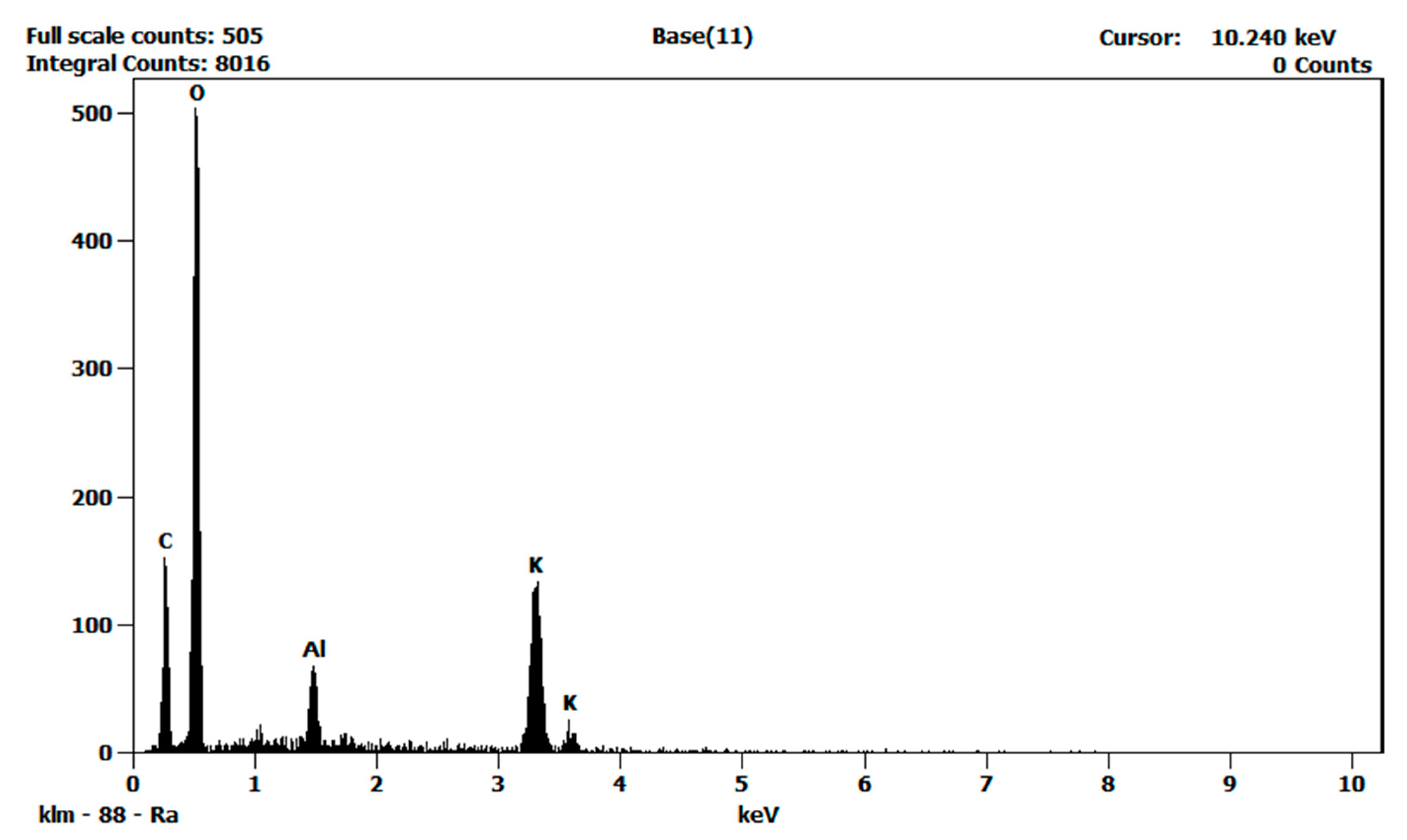
| Reaction | Temperature | %Weight | ||
|---|---|---|---|---|
| Ref. [39] | Measured | Theoretical | Measured | |
| 130 °C | 132 °C | 97.01% | 97.49% (132 °C) | |
| 200 °C | 205 °C | 94.02% | 95.17% (205 °C) 93.79% (540 °C) | |
| Samples | Heat Capacity (STDEV) (kJ/kg·°C) | Melting Point (STDEV) (°C) |
|---|---|---|
| 1 | 1.63 (0.09) | 486 (0.02) |
| 2 | 1.57 (0.06) | 484 (0.60) |
| 3 | 1.62 (0.06) | 485 (0.96) |
| Average | 1.61 | 485 |
| Standard deviation | 0.065 | 1.22 |
| Measurement uncertainty (%) | 3.31 | 0.21 |
| Samples | Heat Capacity (STDEV) (kJ/kg·°C) | Melting Point (STDEV) (°C) |
|---|---|---|
| 1 | 2.29 (0) | 476 (0.9) |
| 2 | 1.9 (0.02) | 475 (0.01) |
| 3 | 1.68 (0.08) | 474 (0.12) |
| 4 | 2.05 (0.09) | 474 (0.1) |
| 5 | 1.78 (0.09) | 473 (0.05) |
| 6 | 1.94 (0.11) | 473 (0.22) |
| 7 | 1.87 (0.14) | 473 (0.23) |
| 8 | 1.84 (0.11) | 473 (0.23) |
| 9 | 1.81 (0.05) | 471 (2.3) |
| 10 | 2.14 (0.02) | 475 (0.1) |
| 11 | 2.09 (0.14) | 476 (0.02) |
| 12 | 2.06 (0.02) | 475 (0.07) |
| Average | 1.95 | 474 |
| Standard deviation | 0.18 | 1.48 |
| Measurement uncertainty (%) | 3.18 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayfeh, Y.; Rizvi, S.M.M.; El Far, B.; Shin, D. In Situ Synthesis of Alumina Nanoparticles in a Binary Carbonate Salt Eutectic for Enhancing Heat Capacity. Nanomaterials 2020, 10, 2131. https://doi.org/10.3390/nano10112131
Nayfeh Y, Rizvi SMM, El Far B, Shin D. In Situ Synthesis of Alumina Nanoparticles in a Binary Carbonate Salt Eutectic for Enhancing Heat Capacity. Nanomaterials. 2020; 10(11):2131. https://doi.org/10.3390/nano10112131
Chicago/Turabian StyleNayfeh, Yousof, Syed Muhammad Mujtaba Rizvi, Baha El Far, and Donghyun Shin. 2020. "In Situ Synthesis of Alumina Nanoparticles in a Binary Carbonate Salt Eutectic for Enhancing Heat Capacity" Nanomaterials 10, no. 11: 2131. https://doi.org/10.3390/nano10112131
APA StyleNayfeh, Y., Rizvi, S. M. M., El Far, B., & Shin, D. (2020). In Situ Synthesis of Alumina Nanoparticles in a Binary Carbonate Salt Eutectic for Enhancing Heat Capacity. Nanomaterials, 10(11), 2131. https://doi.org/10.3390/nano10112131




