Experimental Analysis and Characterization of High-Purity Aluminum Nanoparticles (Al-NPs) by Electromagnetic Levitation Gas Condensation (ELGC)
Abstract
:1. Introduction
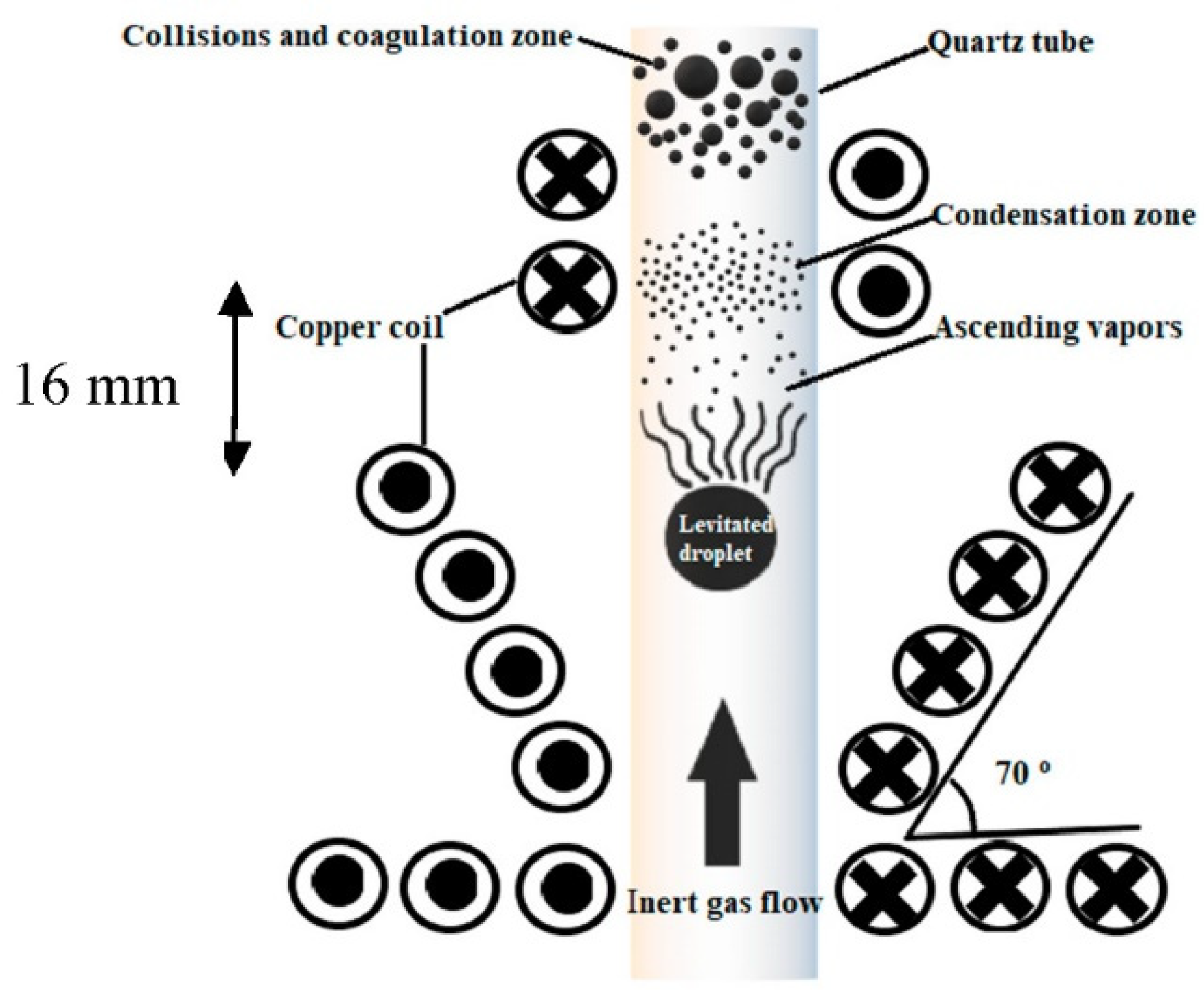
2. Methodology
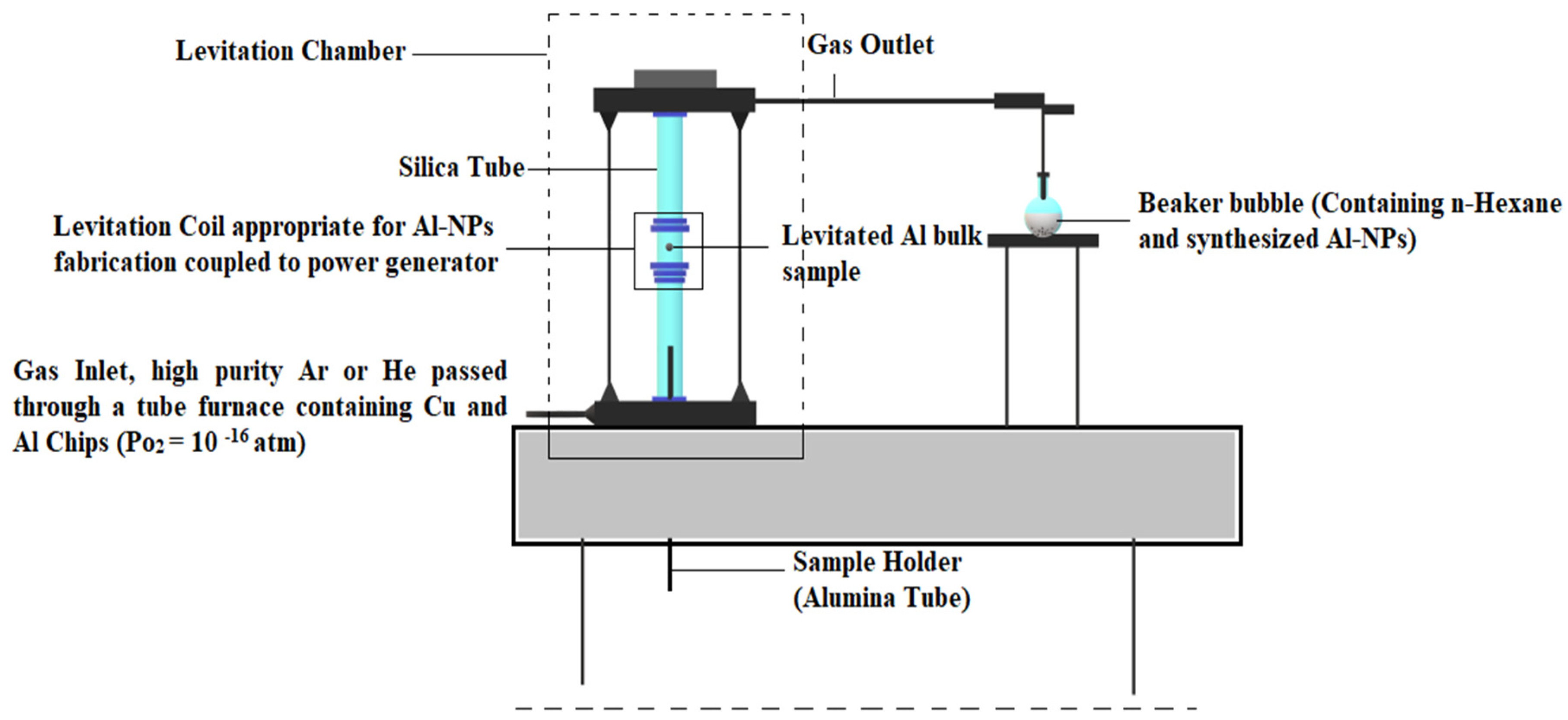
3. Experimental Setup
4. Identification and Characterization of High-Purity NPs
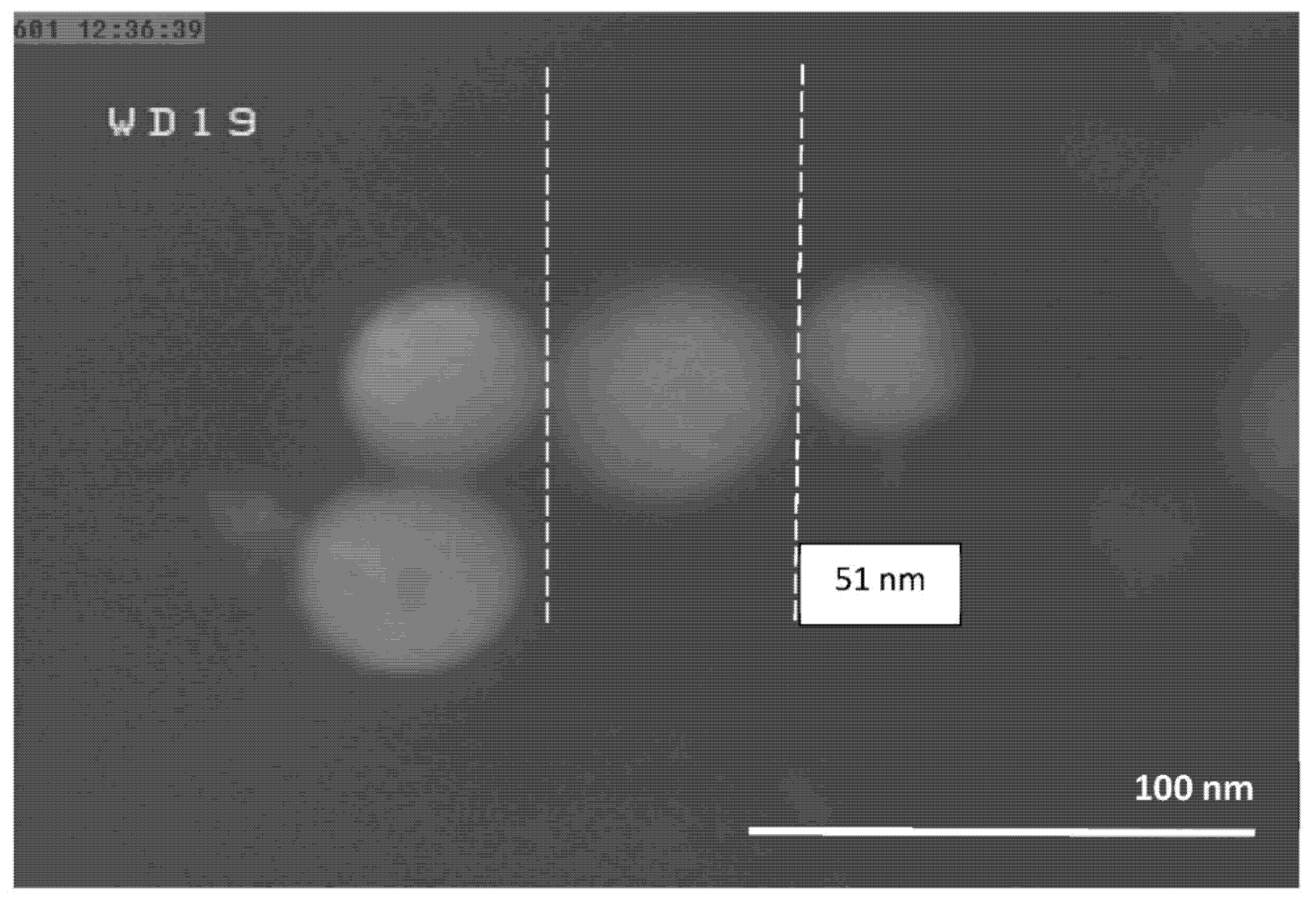
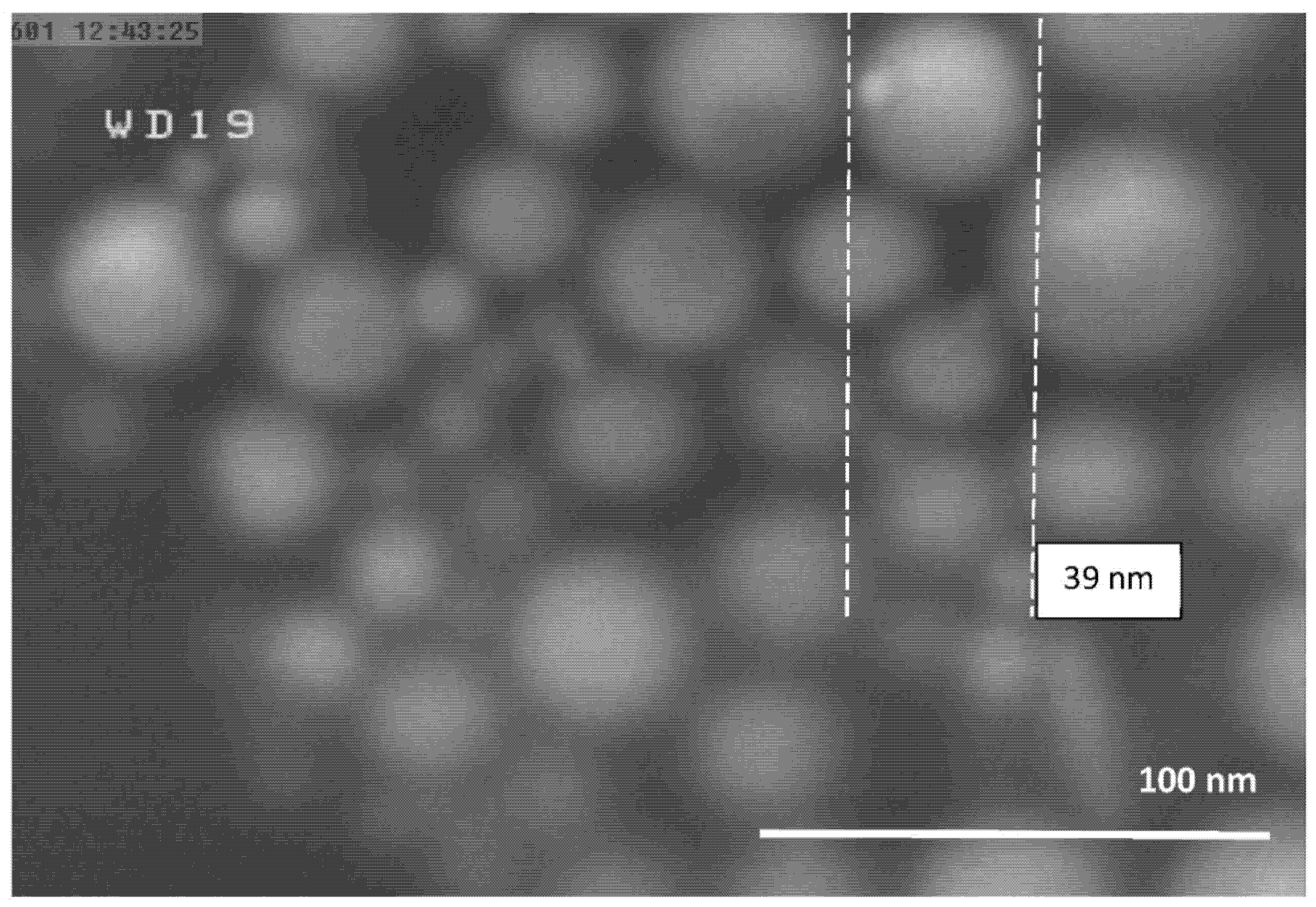
4.1. Morphology of the Al-NPs
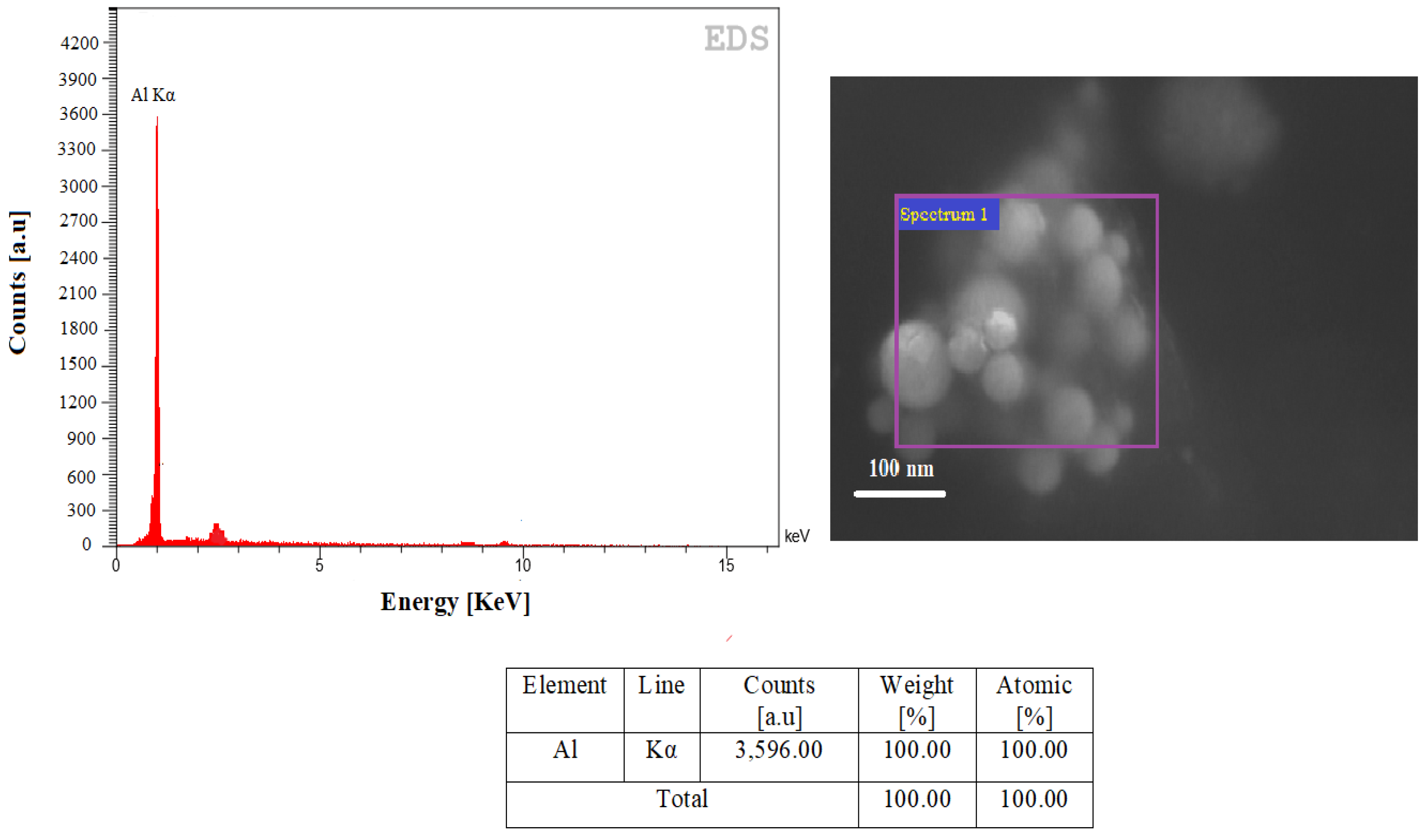
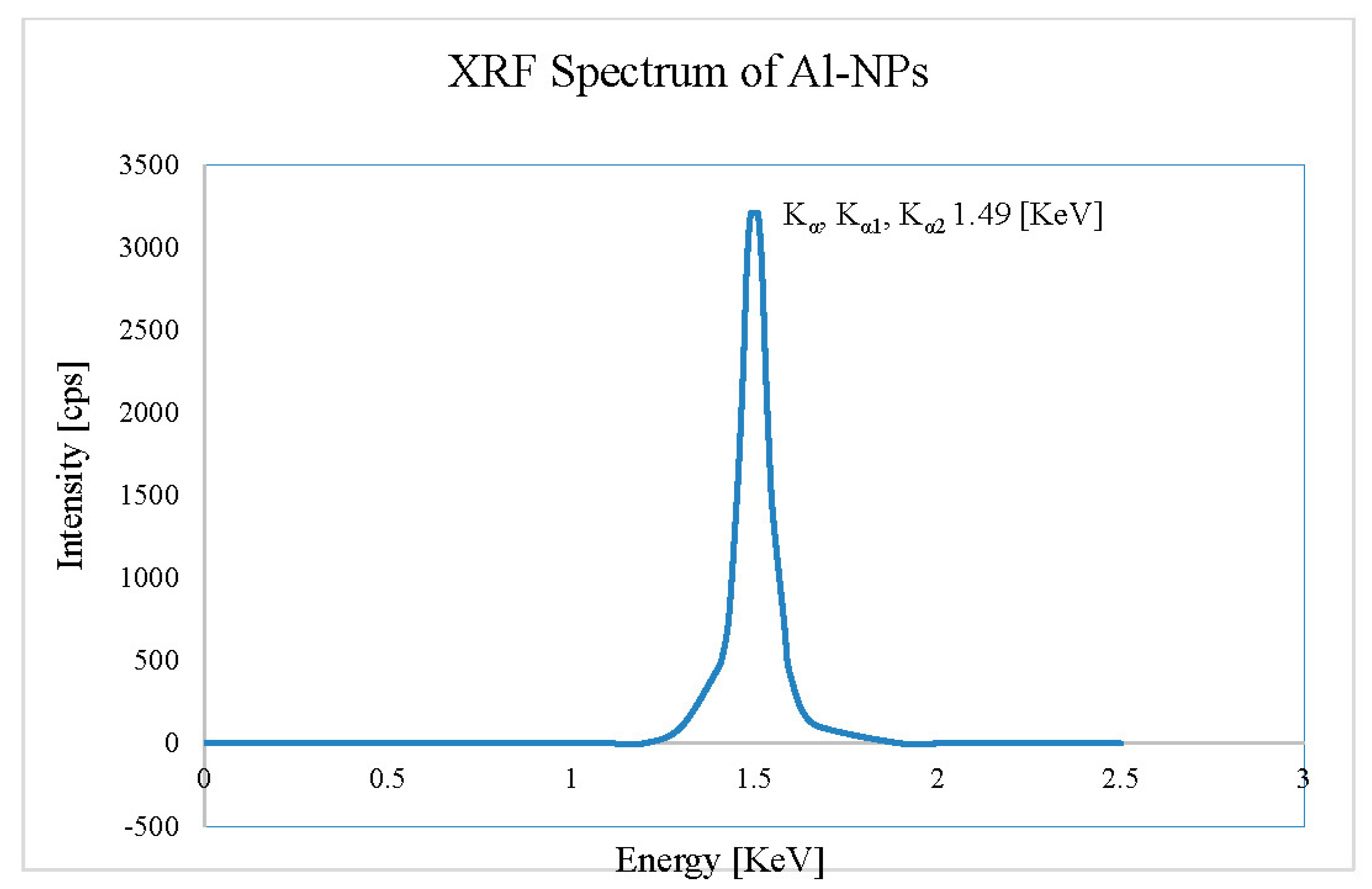
4.2. EDS Analysis
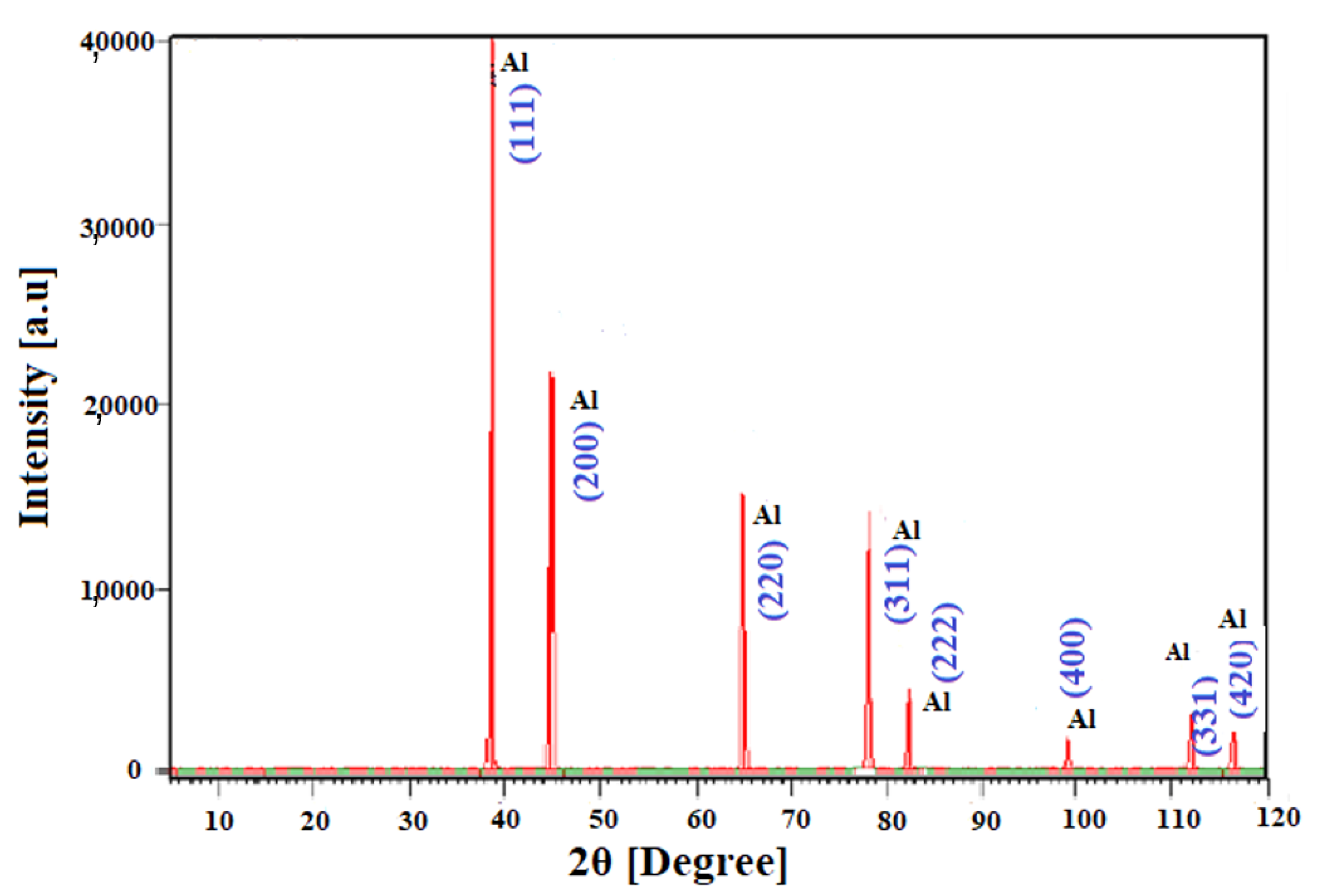
4.3. Phase and Purity of the Al-NPs
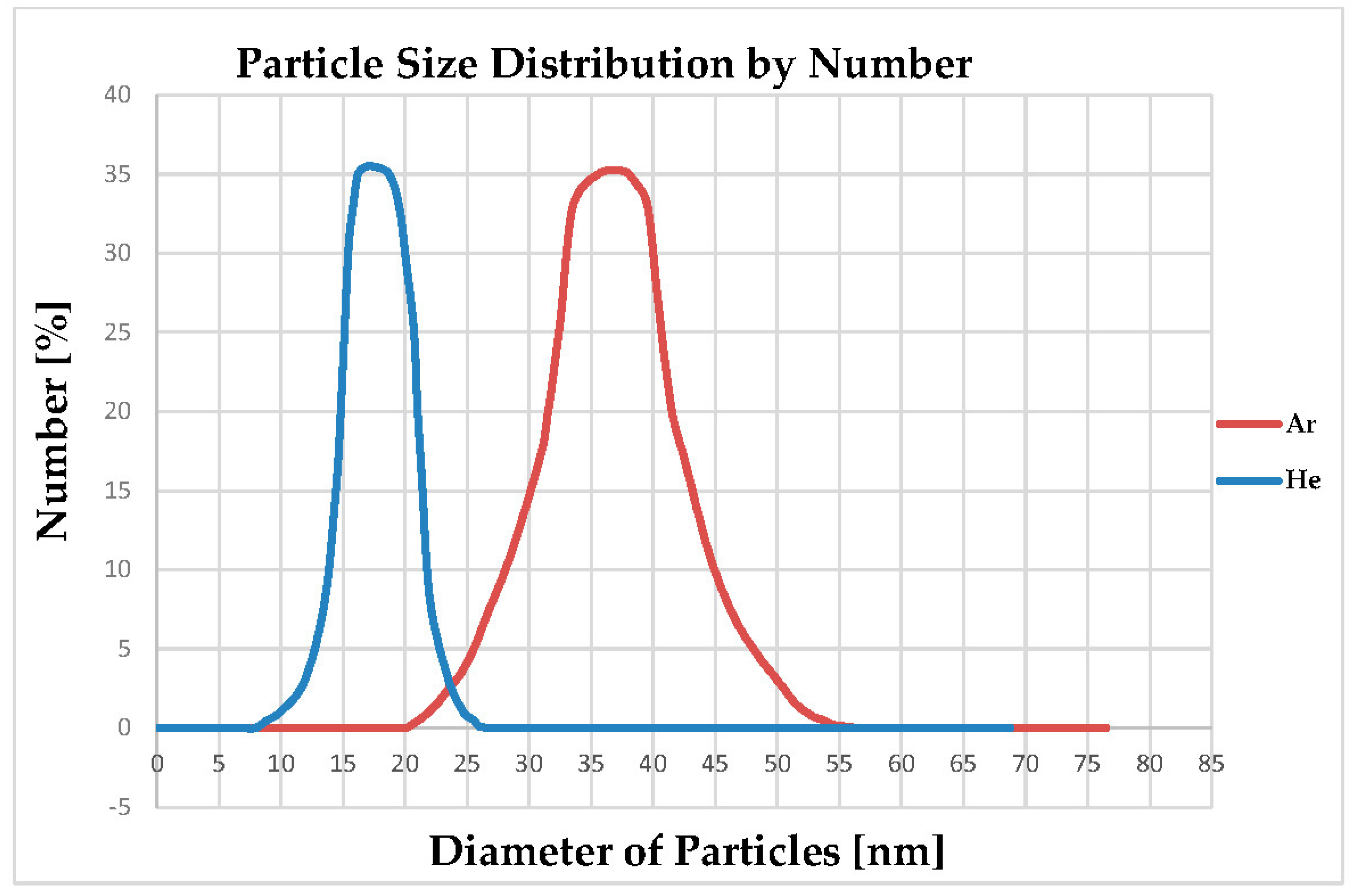
4.4. Particle Size Distribution of Produced Al-NPs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chun, B.-H.; Kang, H.U.; Kim, S. Effect of alumina nanoparticles in the fluid on heat transfer in double-pipe heat exchanger system. Korean J. Chem. Eng. 2008, 25, 966–971. [Google Scholar] [CrossRef]
- Soltani, N.; Sadrnezhaad, S.K.; Bahrami, A. Manufacturing Wear-Resistant 10Ce-TZP/Al2O3Nanoparticle Aluminum Composite by Powder Metallurgy Processing. Mater. Manuf. Process. 2014, 29, 1237–1244. [Google Scholar] [CrossRef]
- Wagner, A.J.; Bleckmann, C.A.; Murdock, R.C.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Cellular Interaction of Different Forms of Aluminum Nanoparticles in Rat Alveolar Macrophages. J. Phys. Chem. B 2007, 111, 7353–7359. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, P.; Panahi, Y.; Ebrahimi-Kalan, A.; Akbarzadeh, A.; Davaran, S.; Nasibova, A.N.; Khalilov, R.; Kavetskyy, T. Biomedical applications of aluminium oxide nanoparticles. Micro Nano Lett. 2018, 13, 1227–1231. [Google Scholar] [CrossRef]
- Baladi, A.; Mamoory, R.S. Investigation of different liquid media and ablation times on pulsed laser ablation synthesis of aluminum nanoparticles. Appl. Surf. Sci. 2010, 256, 7559–7564. [Google Scholar] [CrossRef]
- Ghorbani, H.R. A Review of Methods for Synthesis of Al Nanoparticles. Orient. J. Chem. 2014, 30, 1941–1949. [Google Scholar] [CrossRef] [Green Version]
- Baldé, C.P.; Hereijgers, B.P.C.; Bitter, J.H.; Bitter, J.H.; de Jong, K.P. Facilitated hydrogen storage in NaAlH4 supported on carbon nanofibers. Angew. Chem. 2006, 118, 3581–3583. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Del-Valle, M.D.P.; Cruz-Manjarrez, H.D.J.; Rodriguez, A. Simple Fabrication and Characterization of an Aluminum Nanoparticle Monolayer with Well-Defined Plasmonic Resonances in the Far Ultraviolet. Metals 2018, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Altuwirqi, R.M.; Baatiyah, B.; Nugali, E.; Hashim, Z.; Al-Jawhari, H. Synthesis and Characterization of Aluminum Nanoparticles Prepared in Vinegar Using a Pulsed Laser Ablation Technique. J. Nanomater. 2020, 2020, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Jiang, A.; Wang, F.; Xia, D.; Li, M.; Qiang, L.; Zhu, Z.; Wang, P.; Fan, R.; Lin, K.; Fan, R. Aluminum nanoparticles manufactured using a ball-milling method with ammonium chloride as a grinding aid: Achieving energy release at low temperature. New J. Chem. 2019, 43, 1851–1856. [Google Scholar] [CrossRef]
- Gazanfari, M.; Karimzadeh, M.; Ghorbani, S.; Sadeghi, M.R.; Azizi, G.; Karimi, H.; Fattahi, N.; Karimzadeh, Z. Synthesis of aluminium nanoparticles by arc evaporation of an aluminium cathode surface. Bull. Mater. Sci. 2014, 37, 871–876. [Google Scholar] [CrossRef]
- Li, Z.; Quan, R.W.; Jacobsen, E.N. Mechanism of the (Diimine)copper-Catalyzed Asymmetric Aziridination of Alkenes. Nitrene Transfer via Ligand-Accelerated Catalysis. J. Am. Chem. Soc. 1995, 117, 5889–5890. [Google Scholar] [CrossRef]
- Ferrara, J.D.; Claire, A.D.; Youngs, T.; Youngs, W.J. Synthesis and characterization of a silver (I) triflate sandwich complex of 1, 2: 5, 6: 9, 10-tribenzocyclododeca-1, 5, 9-triene-3, 7, 11-triyne. The first example of a 12-membered macrocycle sandwich complex. J. Am. Chem. Soc. 1988, 110, 647–649. [Google Scholar] [CrossRef]
- Ghanta, S.R.; Muralidharan, K.; Muralidharan, K. Chemical synthesis of aluminum nanoparticles. J. Nanopart. Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Kermanpur, A.; Dadfar, M.R.; Rizi, B.N.; Eshraghi, M. Synthesis of aluminum nanoparticles by electromagnetic levitational gas condensation method. J. Nanosci. Nanotechnol. 2010, 10, 6251–6255. [Google Scholar] [CrossRef] [PubMed]
- Anggraeni, S.D.; Kurniawan, F. Synthesis of Aluminium Nanoparticles Using Electrochemical Method. J. Phys. Conf. Ser. 2020, 1445, 012013. [Google Scholar] [CrossRef]
- Piao, R.; Yang, S.; Ma, L.; Wang, T. Vacuum Electromagnetic Levitation Melting of Ti–Al Based Alloy Prepared by Aluminothermic Reduction of Acid Soluble Ti Bearing Slag. Met. Mater. Int. 2019, 26, 130–142. [Google Scholar] [CrossRef]
- Sassonker, I.; Kuperman, A. Electro-mechanical modeling of electromagnetic levitation melting system driven by a series resonant inverter with experimental validation. Energy Convers. Manag. 2020, 208, 112578. [Google Scholar] [CrossRef]
- Sassonker, I.; Elkayam, M.S.; Shvartsas, M.; Darhovsky, Y.; Shoihet, A.; Kuperman, A. Small-signal modeling and active damping of resonant electromagnetic levitation melting system with experimental verification. Energy Convers. Manag. 2020, 215, 112906. [Google Scholar] [CrossRef]
- Kermanpur, A.; Rizi, B.N.; Vaghayenegar, M.; Yazdabadi, H.G. Bulk synthesis of monodisperse Fe nanoparticles by electromagnetic levitational gas condensation method. Mater. Lett. 2009, 63, 575–577. [Google Scholar] [CrossRef]
- Vaghayenegar, M.; Kermanpur, A.; Abbasi, M.; Yazdabadi, H.G. Effects of process parameters on synthesis of Zn ultrafine/nanoparticles by electromagnetic levitational gas condensation. Adv. Powder Technol. 2010, 21, 556–563. [Google Scholar] [CrossRef]
- Malekzadeh, M.; Halali, M. Production of silver nanoparticles by electromagnetic levitation gas condensation. Chem. Eng. J. 2011, 168, 441–445. [Google Scholar] [CrossRef]
- Malekzadeh, M.; Halali, M. Method of Producing High Purity Silver Nanoparticles. U.S. Patent 8480784B2, 9 July 2013. [Google Scholar]
- VahidMohammadi, A.; Halali, M. Synthesis and characterization of pure metallic titanium nanoparticles by an electromagnetic levitation melting gas condensation method. RSC Adv. 2014, 4, 7104–7108. [Google Scholar] [CrossRef]
- Dokko, W.; Bautista, R.G. The high temperature heat content and heat capacity of liquid cerium-copper alloys by levitation calorimetry. Met. Mater. Trans. A 1980, 11, 511–518. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, G.; Yang, Y.; McLean, A. Numerical Investigation of the Position and Asymmetric Deformation of a Molten Droplet in the Electromagnetic Levitation System. Met. Mater. Trans. A 2019, 51, 247–257. [Google Scholar] [CrossRef]
- Wegner, K.; Walker, B.; Tsantilis, S.; Pratsinis, S.E. Design of metal nanoparticle synthesis by vapor flow condensation. Chem. Eng. Sci. 2002, 57, 1753–1762. [Google Scholar] [CrossRef]
- Ferrara, J.D.; Youngs, C.T.; Youngs, W.J. Synthesis and characterization of a copper (I) triflate complex of 1, 2: 5, 6: 9, 10-tribenzocyclododeca-1, 5, 9-triene-3, 7, 11-triyne. Organometallics 1987, 6, 676–678. [Google Scholar] [CrossRef]
- Chen, T.Y.; Omnès, L.; Vaisserman, J.; Doppelt, P. Observation of specific intermolecular interactions in the X-ray crystal structure of Cu(I) (1,1,1,3,5,5,5-hepta-fluoropentane-2,4-dionato) (vinyltrimethylsilane). Inorg. Chim. Acta 2004, 357, 1299–1302. [Google Scholar] [CrossRef]
- Yavorovsky, N.; Domashenko, V.; Balukhtin, P. Production of ultradispersed powders with electric explosion method. In Proceedings of the KORUS 2000, 4th Korea-Russia International Symposium On Science and Technology, Ulsan, Korea, 27 June–1 July 2000. [Google Scholar]
- Gromov, A.; Ilyin, A.; Förter-Barth, U.; Teipel, U. Characterization of Aluminum Powders: II. Aluminum Nanopowders Passivated by Non-Inert Coatings. Propellants Explos. Pyrotech. 2006, 31, 401–409. [Google Scholar] [CrossRef]
- Lin, T.-C.; Cao, C.; Sokoluk, M.; Jiang, L.; Wang, X.; Schoenung, J.M.; Lavernia, E.J.; Li, X. Aluminum with dispersed nanoparticles by laser additive manufacturing. Nat. Commun. 2019, 10, 4124. [Google Scholar] [CrossRef] [Green Version]
- Mahendiran, C.; Ganesan, R.; Gedanken, A. Sonoelectrochemical Synthesis of Metallic Aluminum Nanoparticles. Eur. J. Inorg. Chem. 2009, 2009, 2050–2053. [Google Scholar] [CrossRef]
- Bramston-Cook, R.; Neumann, H. Operating Parameters for the Thermal Conductivity Detector in Varian 3800 and 3900 Gas Chromatographs, Long Beach, CA, USA. 2007. Available online: http://lotusinstruments.com/wp/wp-content (accessed on 21 August 2020).
- Pauzon, C.; Forêt, P.; Hryha, E.; Arunprasad, T.; Nyborg, L. Argon-helium mixtures as Laser-Powder Bed Fusion atmospheres: Towards increased build rate of Ti-6Al-4V. J. Mater. Process. Technol. 2020, 279, 116555. [Google Scholar] [CrossRef]

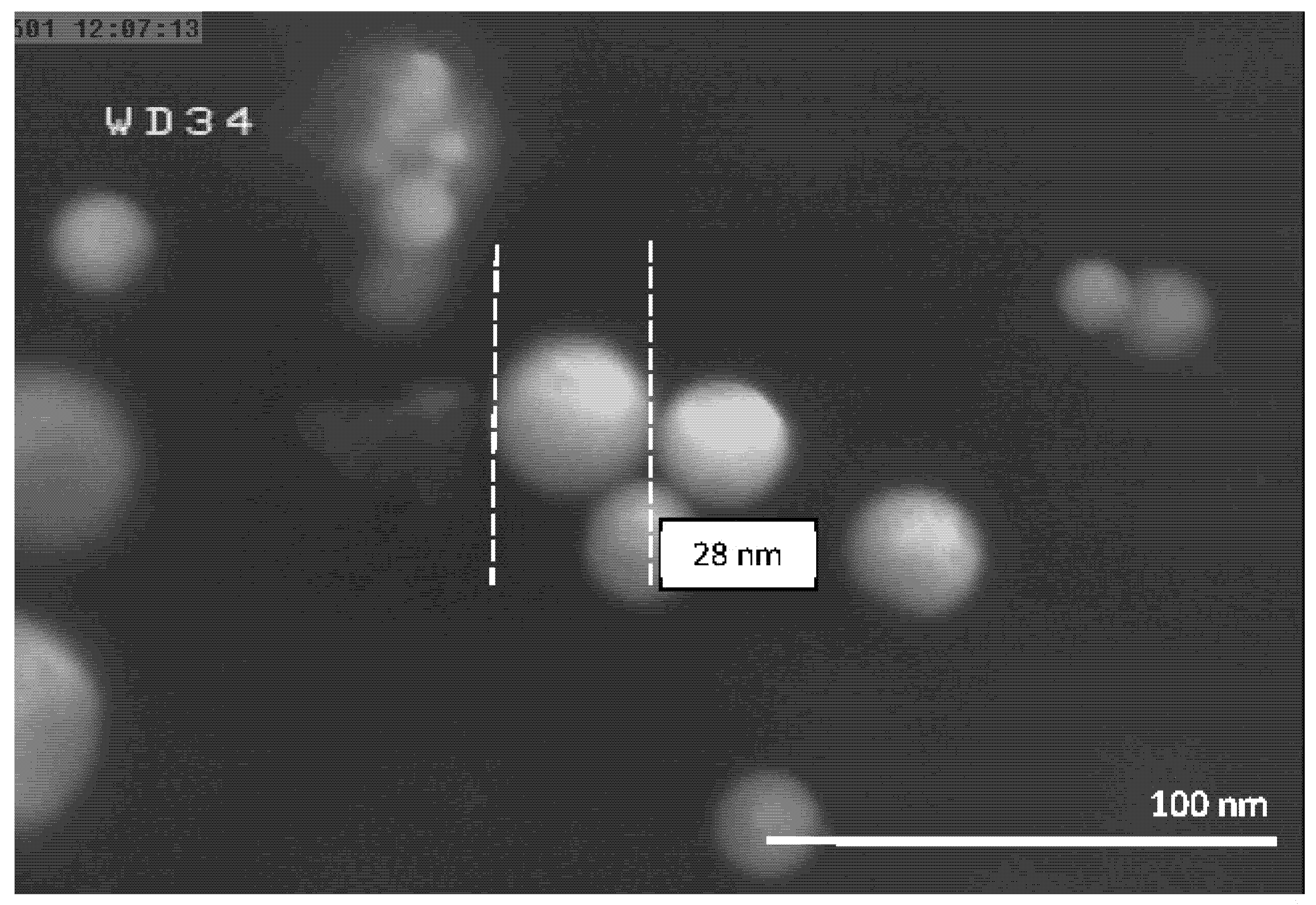
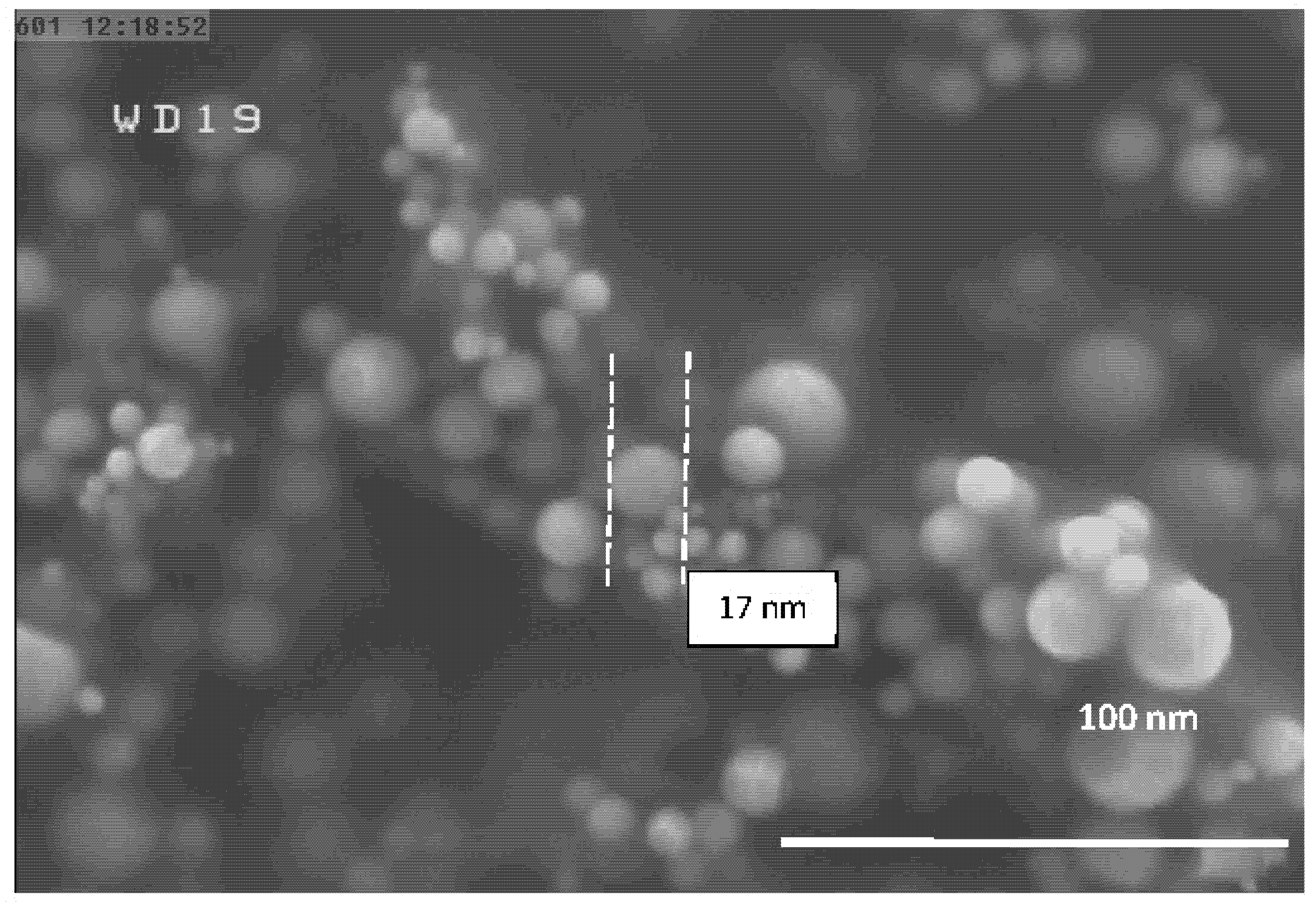
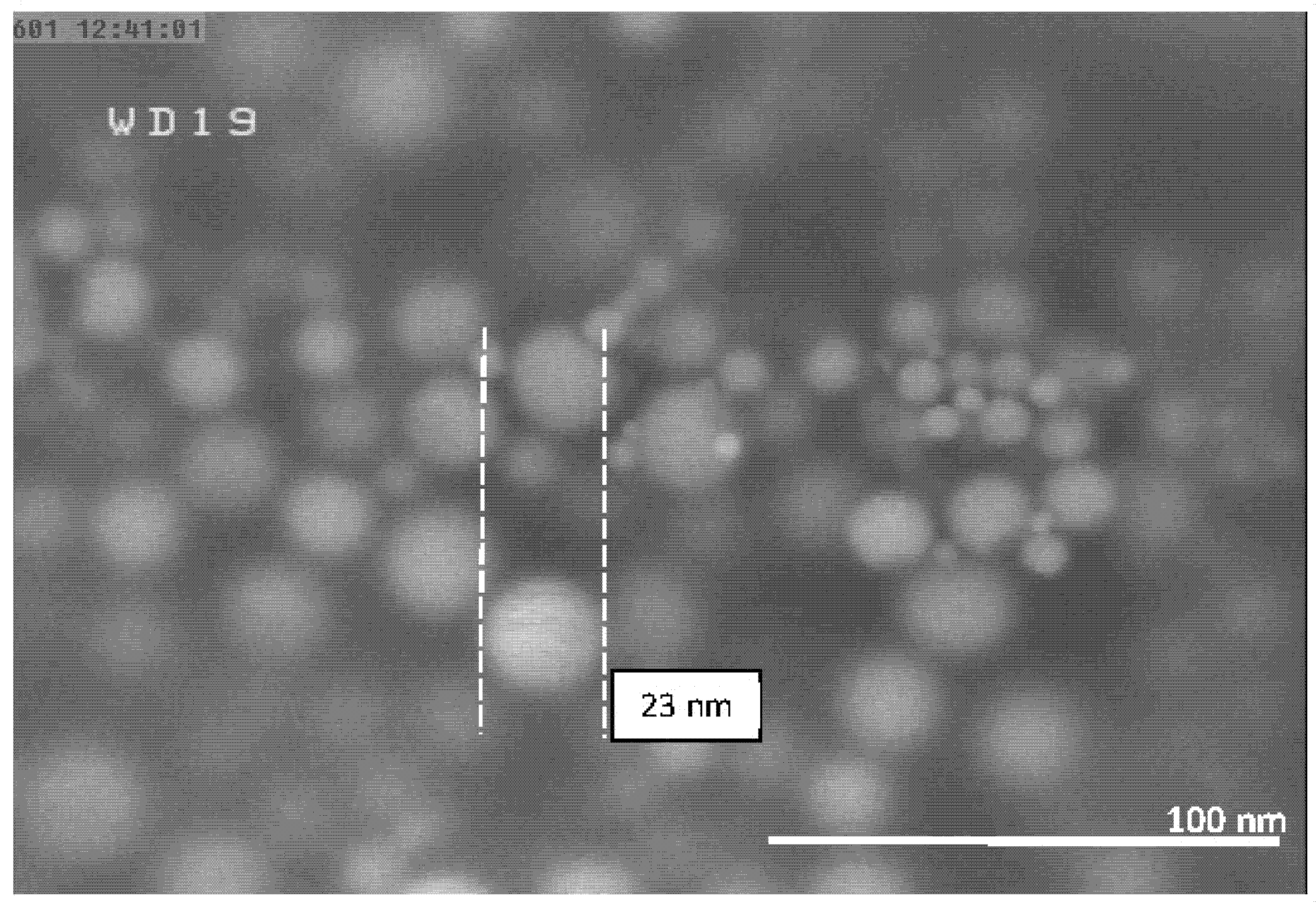
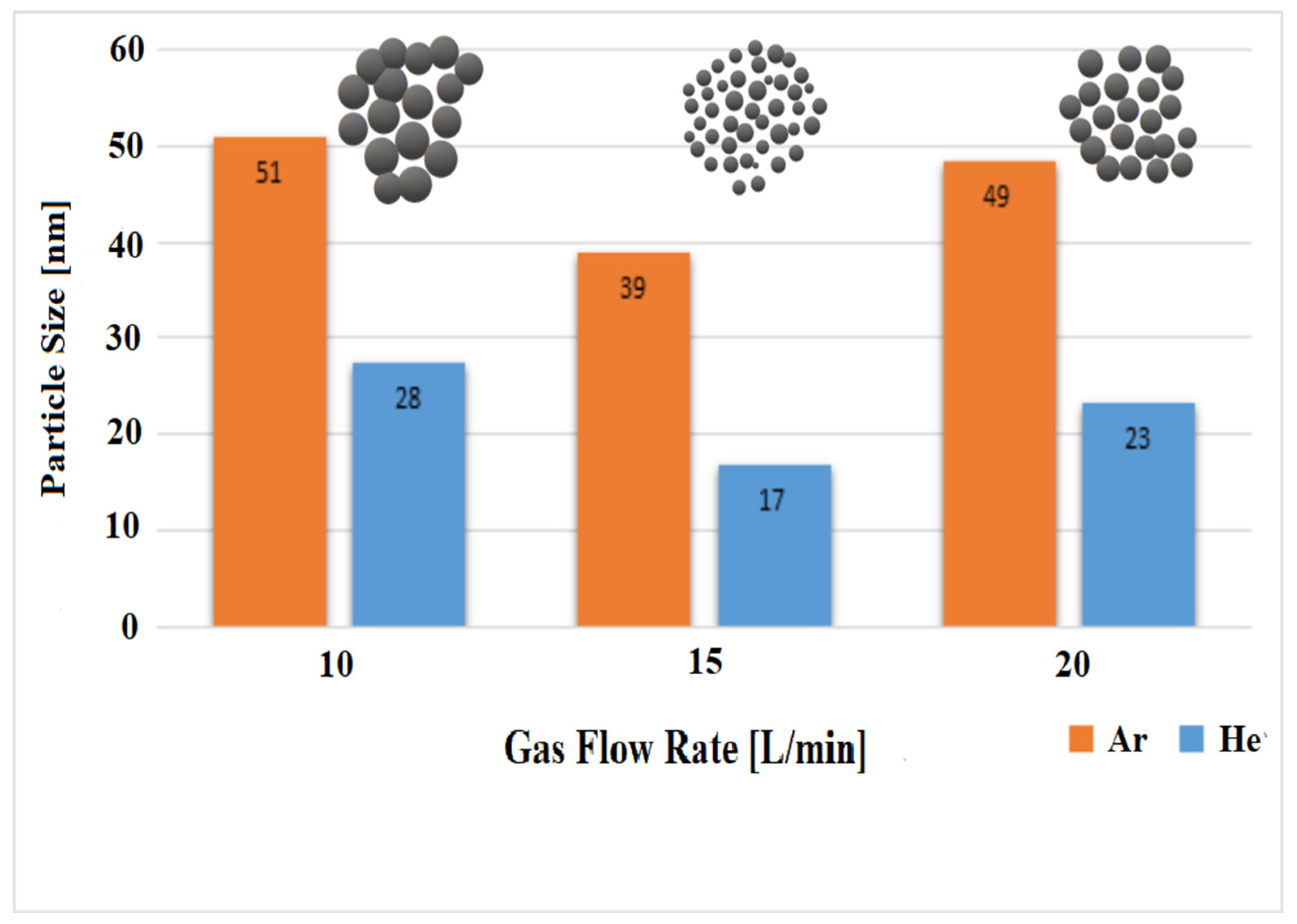
| Experiment Number | Ar Flow Rate (L/min) | Temperature of Al Droplet (K) | Particle Size (nm) |
|---|---|---|---|
| 1 | 10 | 1683 ± 10 | 51 |
| 2 | 15 | 1683 ± 10 | 39 |
| 3 | 20 | 1683 ± 10 | 49 |
| Experiment Number | He Flow Rate (L/min) | Temperature of Al Droplet (K) | Particle Size (nm) |
| 4 | 10 | 1683 ± 10 | 28 |
| 5 | 15 | 1683 ± 10 | 17 |
| 6 | 20 | 1683 ± 10 | 23 |
| 2θ (Degree) | Intensity (a.u) | d-Spacing (A°) | Plane | ||
|---|---|---|---|---|---|
| 38.5624 | 40,404.19 | 2.33472 | 1 | 1 | 1 |
| 44.7953 | 21,916.62 | 2.02328 | 2 | 0 | 0 |
| 65.1216 | 16,239.63 | 2.43245 | 2 | 2 | 0 |
| 78.2060 | 14,216.02 | 1.22232 | 3 | 1 | 1 |
| 82.4127 | 4451.56 | 1.17026 | 2 | 2 | 2 |
| 99.0613 | 1887.77 | 1.0346 | 4 | 0 | 0 |
| 111.9845 | 3151.97 | 0.93000 | 3 | 3 | 1 |
| 116.5297 | 2654.67 | 0.90571 | 4 | 2 | 0 |
| Component | Symbol | Concentration Range Mass (%) |
|---|---|---|
| Aluminum | Al | 99.976 |
| Magnesium | Mg | 0.009 |
| Manganese | Mn | 0.009 |
| Iron | Fe | 0.003 |
| Silicon | Si | 0.001 |
| Copper | Cu | 0.001 |
| Researcher(s) | Content of Al (wt.%) |
|---|---|
| Gromov et al. [31] | 90 |
| Gazanfari et al. [11] | 92.5 |
| Lin et al. [32] | 99.0 |
| Mahendiran et al. [33] | 99.0 |
| Ar Atmosphere | Diameter (nm) | Intensity (%) | Standard Deviation (nm) | PDI |
|---|---|---|---|---|
| Peak | 38 | 94.2 | 29 | 0.567 |
| He Atmosphere | Diameter (nm) | Intensity (%) | Standard Deviation (nm) | PDI |
| Peak | 17 | 93.7 | 11 | 0.442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabouni Tabari, R.; Halali, M.; A. Javadi, A.; Khanjanpour, M.H. Experimental Analysis and Characterization of High-Purity Aluminum Nanoparticles (Al-NPs) by Electromagnetic Levitation Gas Condensation (ELGC). Nanomaterials 2020, 10, 2084. https://doi.org/10.3390/nano10102084
Sabouni Tabari R, Halali M, A. Javadi A, Khanjanpour MH. Experimental Analysis and Characterization of High-Purity Aluminum Nanoparticles (Al-NPs) by Electromagnetic Levitation Gas Condensation (ELGC). Nanomaterials. 2020; 10(10):2084. https://doi.org/10.3390/nano10102084
Chicago/Turabian StyleSabouni Tabari, Rana, Mohammad Halali, Akbar A. Javadi, and Mohammad Hassan Khanjanpour. 2020. "Experimental Analysis and Characterization of High-Purity Aluminum Nanoparticles (Al-NPs) by Electromagnetic Levitation Gas Condensation (ELGC)" Nanomaterials 10, no. 10: 2084. https://doi.org/10.3390/nano10102084




