Graphene Derivatives in Biopolymer-Based Composites for Food Packaging Applications
Abstract
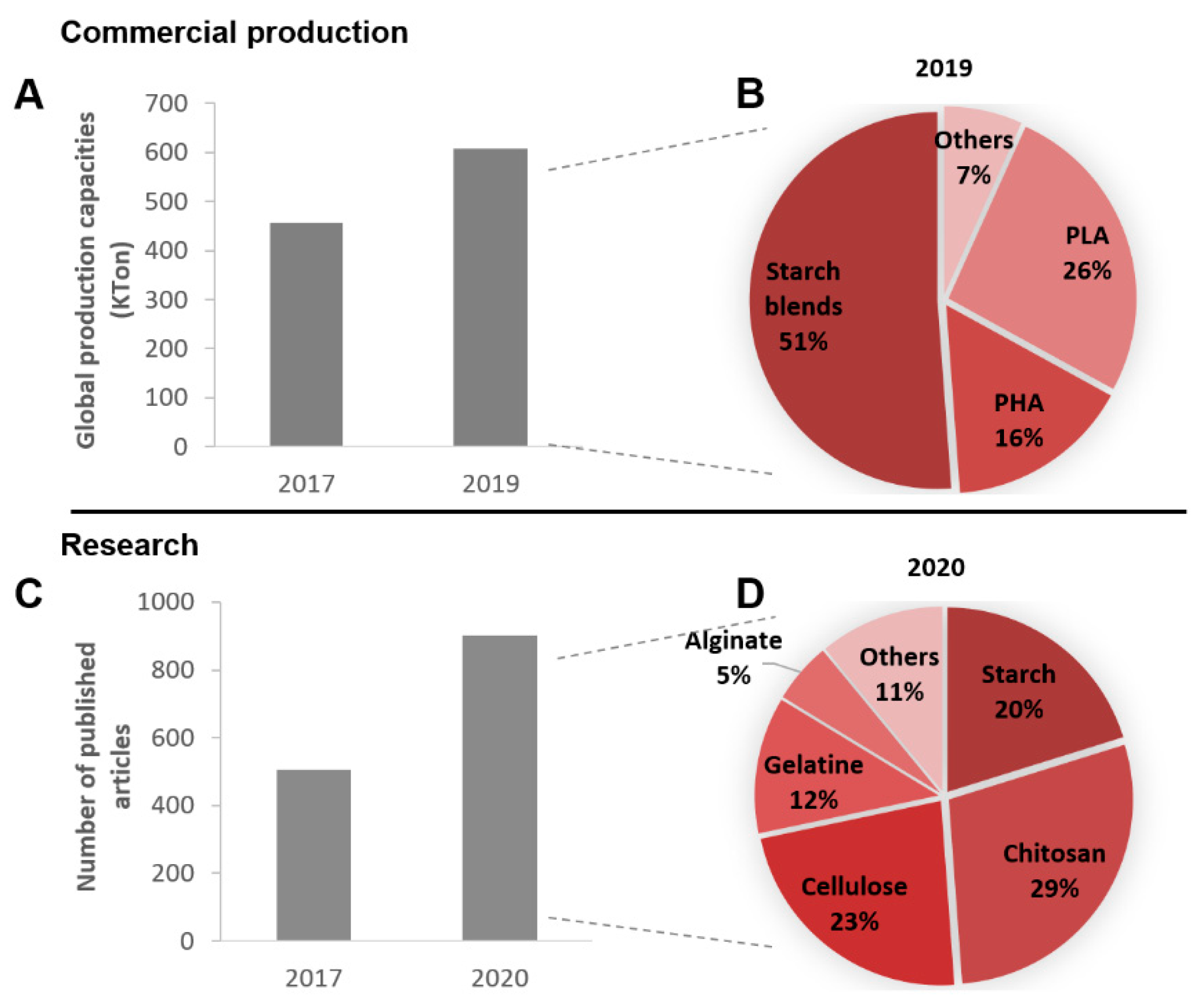
:1. Introduction
2. Biopolymers as Food Packaging Raw Materials
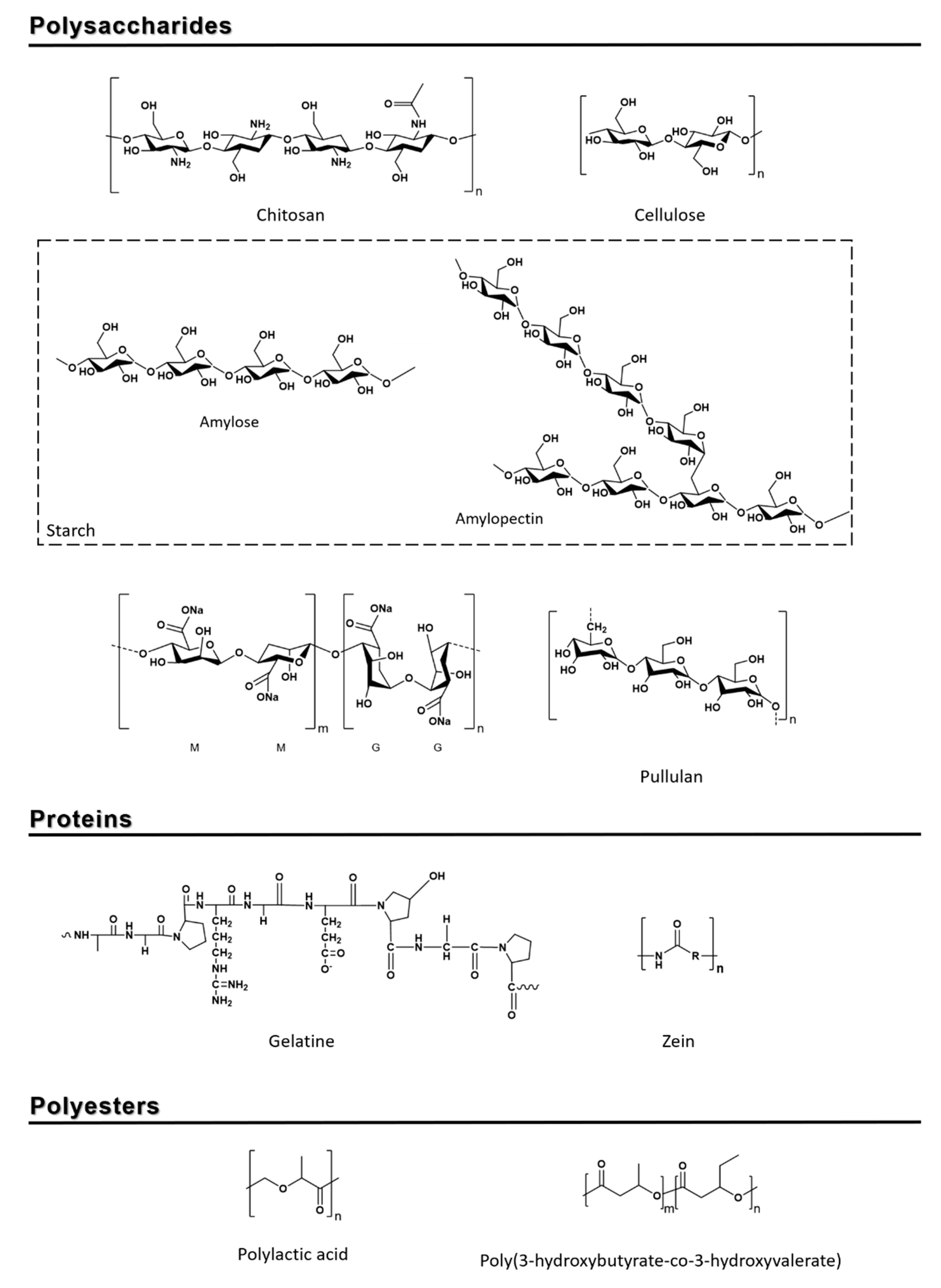
2.1. Polysaccharides
2.1.1. Starch
2.1.2. Cellulose
2.1.3. Chitosan
2.1.4. Alginate
2.1.5. Pullulans
2.2. Proteins
2.2.1. Gelatin
2.2.2. Zein
2.3. Polyesters
2.3.1. Polylactic Acid
2.3.2. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)
3. Graphene Derivatives-Based Biocomposites as Food Packaging Materials
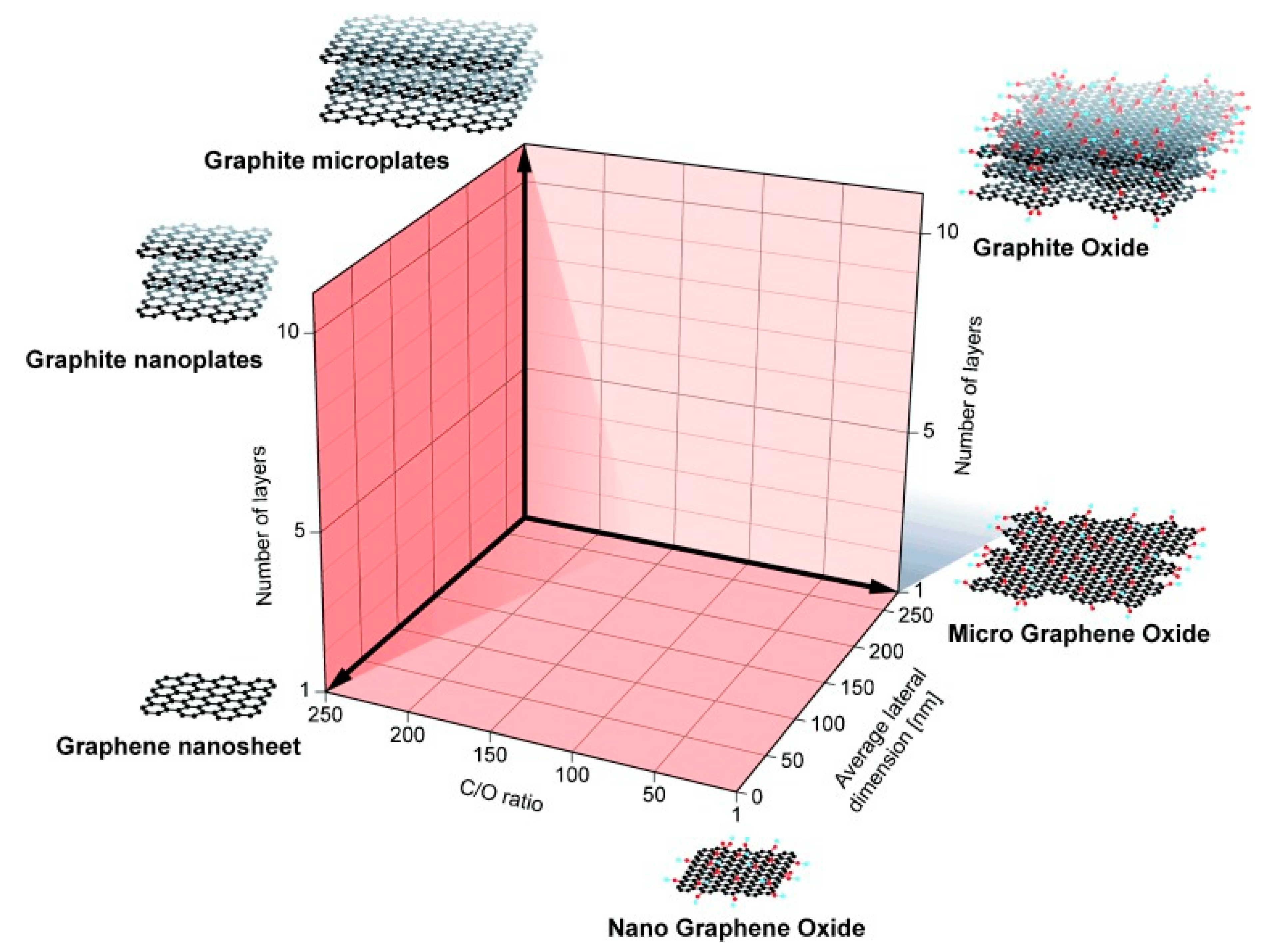
3.1. Graphene Derivatives
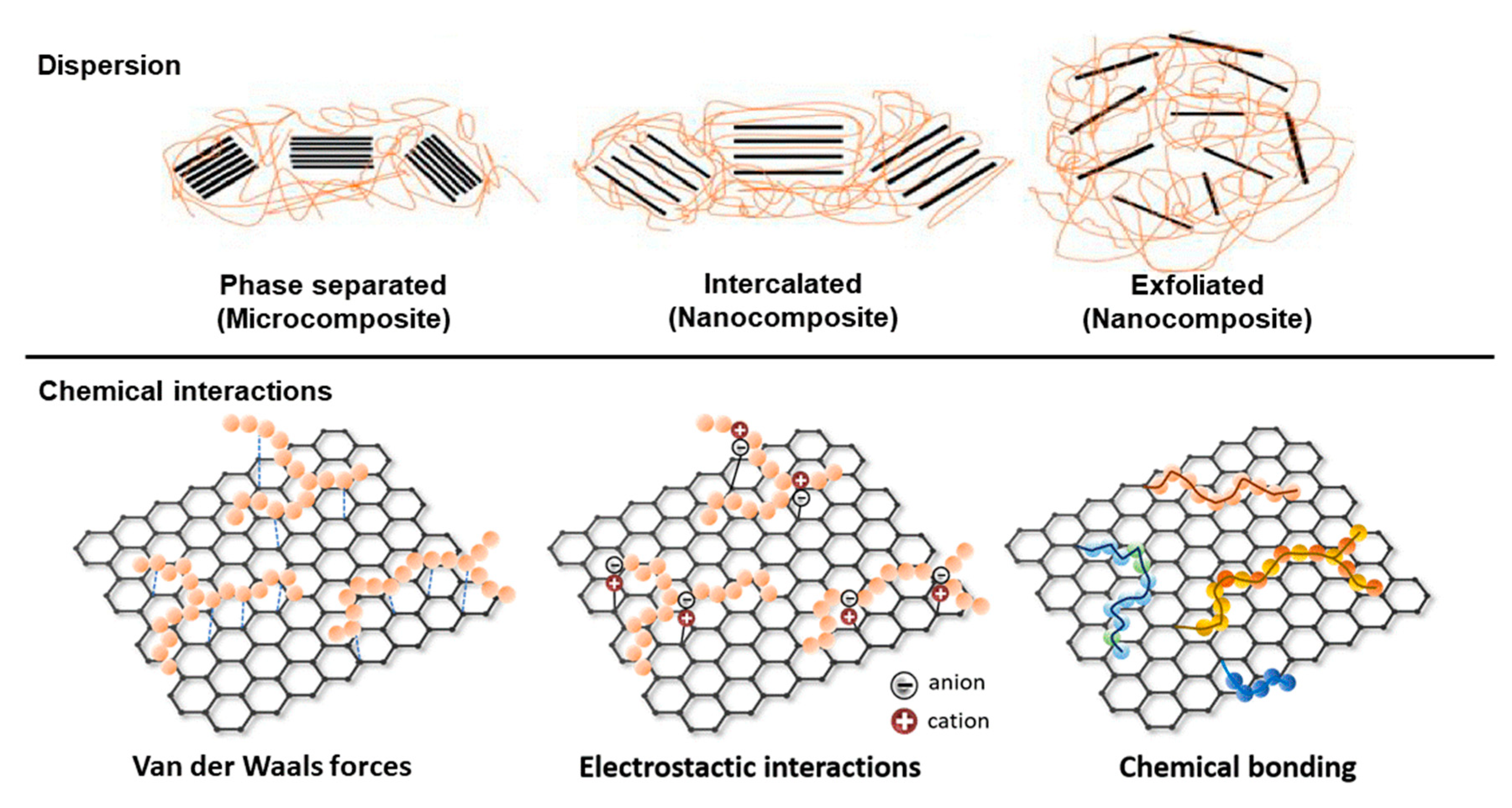
3.2. Properties of Biopolymer-Based Composites with Graphene Derivatives
3.2.1. Mechanical and Thermal Stability Properties
3.2.2. Barrier Properties
3.2.3. Surface Hydrophobicity Properties
3.2.4. Biodegradability
3.2.5. Active Properties
3.2.6. Clay–Graphene Bionanocomposites
3.3. Emerging Application for Biopolymer-Based with Graphene Derivatives
| Biopolymer | Graphene Derivative | σ (S m−1) | Applications | Ref. |
|---|---|---|---|---|
| PLA | 4.3 wt% MWCNT | 59.30 | EMI shielding | [172] |
| 15 wt% GNP | 0.35 | - | [227] | |
| 3 wt% MWCNT | 6.42 | EMI shielding | [175] | |
| 2 wt% MWCNT | 19.70 | EMI shielding | [174] | |
| 5 wt% SWCNT | 1010 | Organic devices | [228] | |
| 15 wt% GNP | 0.36 | Electronics | [229] | |
| PLA/Starch PLA/PBAT Cellulose derivatives | 5 wt% rGO | 0.001 | Packaging | [190] |
| 40 wt% GNP | 338 | Electronic devices | [219] | |
| 5 wt% rGO | 15,200 | Electronic devices | [230] | |
| 4.5 wt% MWCNT | 10 | Electrochemical devices | [201] | |
| 10 wt% MWCNT | 37.6 | Electronics | [231] | |
| 9 wt% graphene | 2.4 | Diverse | [223] | |
| 9 wt% rGO | 1.4 | Diverse | [223] | |
| 50 wt% rGO (AC) | 0.83 | Electronic devices | [225] | |
| 50 wt% rGO (HI) | 22.22 | Electronic devices | [225] | |
| 50 wt% rGO (TR) | 23.42 | Electronic devices | [225] | |
| Cellulose/SPI CS | 0.25 wt% *MWCNT | 0.82 | - | [218] |
| 50 wt% rGO | 0.7 | Food packaging | [170] | |
| 50 wt% rGO-Fe3-xO4 | 0.016 | Biomedical | [12] | |
| 55 wt% GNP/5 wt% MWCNT | 2900 | Bioelectrocatalysis | [221] | |
| 2.5 wt% rGO | 0.08 | Biomedical | [224] |
4. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- UN Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals (accessed on 20 September 2020).
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Shapi’i, R.A.; Othman, S.H.; Nordin, N.; Kadir Basha, R.; Nazli Naim, M. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 1–6. [Google Scholar] [CrossRef]
- Institute for Bioplastics and Biocomposites European Bioplastics. Available online: https://www.european-bioplastics.org/market/ (accessed on 19 August 2020).
- Report Linker. Available online: https://www.reportlinker.com/market-report/Packaging/6286/Packaging?gclid=CjwKCAjwnK36BRBVEiwAsMT8WNhZAbfnSMF48UwrjnqHM4QD24JfVUjqiN5HmTXzuMhLAC0s-LLqkBoCYzYQAvD_BwE&fbclid=IwAR120hj1aHAXE4k4qhK_o032LoO6XCLeqMx-vhe3mSBXIbnNx-A-pc-dyQU (accessed on 6 September 2020).
- RameshKumar, S.; Shaiju, P.; O’Connor, K.E.; P, R.B. Bio-based and biodegradable polymers - State-of-the-art, challenges and emerging trends. Curr. Opin. Green Sustain. Chem. 2020, 21, 75–81. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.S.; Lee, S.-Y.; Mahajan, R.L.; Kim, Y.-T. Exploration of hybrid nanocarbon composite with polylactic acid for packaging applications. Int. J. Biol. Macromol. 2020, 144, 135–142. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Preparation and characterization of environmentally safe and highly biodegradable microbial polyhydroxybutyrate (PHB) based graphene nanocomposites for potential food packaging applications. Int. J. Biol. Macromol. 2020, 154, 866–877. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Structure and properties of thermomechanically processed chitosan/carboxymethyl cellulose/graphene oxide polyelectrolyte complexed bionanocomposites. Int. J. Biol. Macromol. 2020, 158, 420–429. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Z.; Owens, A.C.E.; Kulaots, I.; Chen, Y.; Kane, A.B.; Hurt, R.H. Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology. Nanoscale 2014, 6, 11744–11755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, C.; Coimbra, M.A.; Ferreira, P. Tailoring Functional Chitosan-Based Composites for Food Applications. Chem. Rec. 2018, 18, 1138–1149. [Google Scholar] [CrossRef]
- Barra, A.; Alves, Z.; Ferreira, N.M.; Martins, M.A.; Oliveira, H.; Ferreira, L.P.; Cruz, M.M.; de Deus Carvalho, M.; Neumayer, S.M.; Rodriguez, B.J.; et al. Biocompatible chitosan-based composites with properties suitable for hyperthermia therapy. J. Mater. Chem. B 2020, 8, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef] [PubMed]
- Salit, M.S. Tropical Natural Fibre Composites—Chapter 3 Biopolymer. In Tropical Natural Fibre Composites; Engineering Materials; Springer: Singapore, 2014; p. 41. ISBN 978-981-287-154-1. [Google Scholar]
- Niranjana Prabhu, T.; Prashantha, K. A review on present status and future challenges of starch based polymer films and their composites in food packaging applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Tan, I.; Torley, P.J.; Halley, P.J. Combined rheological and optical investigation of maize, barley and wheat starch gelatinisation. Carbohydr. Polym. 2008, 72, 272–286. [Google Scholar] [CrossRef]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent Advances in Biopolymers and Biopolymer-Based Nanocomposites for Food Packaging Materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef]
- Bagheri, R. Effect of processing on the melt degradation of starch-filled polypropylene. Polym. Int. 1999, 48, 1257–1263. [Google Scholar] [CrossRef]
- Thunwall, M.; Kuthanova, V.; Boldizar, A.; Rigdahl, M. Film blowing of thermoplastic starch. Carbohydr. Polym. 2008, 71, 583–590. [Google Scholar] [CrossRef]
- Tabi, T.; Kovacs, J.G. Examination of injection moulded thermoplastic maize starch. Express Polym. Lett. 2007, 1, 804–809. [Google Scholar] [CrossRef]
- Rodríguez-Castellanos, W.; Martínez-Bustos, F.; Rodrigue, D.; Trujillo-Barragán, M. Extrusion blow molding of a starch–gelatin polymer matrix reinforced with cellulose. Eur. Polym. J. 2015, 73, 335–343. [Google Scholar] [CrossRef]
- Gumul, D.; Krystyjan, M.; Buksa, K.; Ziobro, R.; Zięba, T. The influence of oxidation, extrusion and oxidation/extrusion on physico-chemical properties of potato starch. Starch Stärke 2014, 66, 190–198. [Google Scholar] [CrossRef]
- Sapper, M.; Talens, P.; Chiralt, A. Improving Functional Properties of Cassava Starch-Based Films by Incorporating Xanthan, Gellan, or Pullulan Gums. Int. J. Polym. Sci. 2019, 2019, 5367164. [Google Scholar] [CrossRef] [Green Version]
- Ribba, L.; Garcia, N.L.; D’Accorso, N.; Goyanes, S. Disadvantages of Starch-Based Materials, Feasible Alternatives in Order to Overcome These Limitations. In Starch-Based Materials in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 37–76. ISBN 9780128122570. [Google Scholar]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Grifffin, G.J.L. Biodegradable Fillers in Thermoplastics. In Advances in Chemistry; Deanin, R.D., Schott, N.R., Eds.; American Chemical Society: Washington, DC, USA, 1974; pp. 159–170. [Google Scholar]
- Tian, H.; Xu, G. Processing and Characterization of Glycerol-Plasticized Soy Protein Plastics Reinforced with Citric Acid-Modified Starch Nanoparticles. J. Polym. Environ. 2011, 19, 582–588. [Google Scholar] [CrossRef]
- Hochschule Hannover–University of Applied Sciences and Arts IfBB–Institute for Bioplastics and Biocomposites. Available online: https://www.ifbb-hannover.de/en/facts-and-statistics.html (accessed on 12 May 2020).
- Spierling, S.; Knüpffer, E.; Behnsen, H.; Mudersbach, M.; Krieg, H.; Springer, S.; Albrecht, S.; Herrmann, C.; Endres, H.J. Bio-based plastics-A review of environmental, social and economic impact assessments. J. Clean. Prod. 2018, 185, 476–491. [Google Scholar] [CrossRef]
- Reis, M.O.; Olivato, J.B.; Bilck, A.P.; Zanela, J.; Grossmann, M.V.E.; Yamashita, F. Biodegradable trays of thermoplastic starch/poly (lactic acid) coated with beeswax. Ind. Crops Prod. 2018, 112, 481–487. [Google Scholar] [CrossRef]
- Chollakup, R.; Pongburoos, S.; Boonsong, W.; Khanoonkon, N.; Kongsin, K.; Sothornvit, R.; Sukyai, P.; Sukatta, U.; Harnkarnsujarit, N. Antioxidant and antibacterial activities of cassava starch and whey protein blend films containing rambutan peel extract and cinnamon oil for active packaging. LWT 2020, 130, 109573. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of Agricultural and Industrial Waste in the Field of Nanocellulose and Recent Industrial Developments of Wood Based Nanocellulose: A Review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828. [Google Scholar] [CrossRef]
- Lindman, B.; Medronho, B.; Alves, L.; Costa, C.; Edlund, H.; Norgren, M. The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys. Chem. Chem. Phys. 2017, 19, 23704–23718. [Google Scholar] [CrossRef] [Green Version]
- Mallick, P.K. Thermoplastics and thermoplastic–matrix composites for lightweight automotive structures. In Materials, Design and Manufacturing for Lightweight Vehicles; Elsevier: Amsterdam, The Netherlands, 2010; pp. 174–207. [Google Scholar]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J.M. Multifunctional bionanocomposite films of poly(lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, T.; Song, Y.; Qian, F.; Tuo, Y.; Mu, G. Mechanical properties of whey protein concentrate based film improved by the coexistence of nanocrystalline cellulose and transglutaminase. Int. J. Biol. Macromol. 2019, 126, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Tang, Y.; Zhao, R.; Zhou, Y.; Wang, Z. Preparation of nanofibrillated cellulose and application in reinforced PLA/starch nanocomposite film. J. Polym. Environ. 2019, 27, 728–738. [Google Scholar] [CrossRef]
- Bergamonti, L.; Potenza, M.; Haghighi Poshtiri, A.; Lorenzi, A.; Sanangelantoni, A.M.; Lazzarini, L.; Lottici, P.P.; Graiff, C. Ag-functionalized nanocrystalline cellulose for paper preservation and strengthening. Carbohydr. Polym. 2020, 231, 115773. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhang, M.; Feng, J.; Zhang, S.; Wang, X. High Oxygen Barrier Property of Poly(propylene carbonate)/Polyethylene Glycol Nanocomposites with Low Loading of Cellulose Nanocrytals. ACS Sustain. Chem. Eng. 2017, 5, 11246–11254. [Google Scholar] [CrossRef]
- Dreyfuss-Deseigne, R. Nanocellulose Films in Art Conservation. J. Pap. Conserv. 2017, 18, 18–29. [Google Scholar] [CrossRef]
- Alavi, M. Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications. e-Polymers 2019, 19, 103–119. [Google Scholar] [CrossRef]
- Hedenqvist, M.S.; Plackett, D. V Properties of plasticized composite films prepared from nanofibrillated cellulose and birch wood xylan. Cellulose 2015, 19, 2015–2031. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Vartiainen, J.; Peresin, M.S.; Lahtinen, P.; Lagaron, J.M. Improving the water resistance of nanocellulose-based films with polyhydroxyalkanoates processed by the electrospinning coating technique. Cellulose 2018, 25, 1291–1307. [Google Scholar]
- Dehnad, D.; Mirzaei, H.; Emam-Djomeh, Z.; Jafari, S.M.; Dadashi, S. Thermal and antimicrobial properties of chitosan-nanocellulose films for extending shelf life of ground meat. Carbohydr. Polym. 2014, 109, 148–154. [Google Scholar]
- Moreirinha, C.; Vilela, C.; Silva, N.H.C.S.; Pinto, R.R.J.; Almeida, A.; Rocha, M.A.M.; Coelho, E.; Coimbra, M.A.; Silvestre, A.A.J.; Freire, C.S.R. Antioxidant and antimicrobial films based on brewers spent grain arabinoxylans, nanocellulose and feruloylated compounds for active packaging. Food Hydrocoll. 2020, 108, 105836. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, Y.; Cha, R.; Yang, J.; Jiang, X. Water-soluble nanocrystalline cellulose films with highly transparent and oxygen barrier properties. Nanoscale 2016, 8, 973–978. [Google Scholar] [CrossRef]
- Missio, A.L.; Mattos, B.D.; Ferreira, D.d.F.; Magalhães, W.L.E.; Bertuol, D.A.; Gatto, D.A.; Petutschnigg, A.; Tondi, G. Nanocellulose-tannin films: From trees to sustainable active packaging. J. Clean. Prod. 2018, 184, 143–151. [Google Scholar] [CrossRef]
- Tyagi, P.; Lucia, L.A.; Hubbe, M.A.; Pal, L. Nanocellulose-based multilayer barrier coatings for gas, oil, and grease resistance. Carbohydr. Polym. 2019, 206, 281–288. [Google Scholar] [CrossRef]
- Pourmoazzen, Z.; Sadeghifar, H.; Chen, J.; Yang, G.; Zhang, K.; Lucia, L. The morphology, self-assembly, and host-guest properties of cellulose nanocrystals surface grafted with cholesterol. Carbohydr. Polym. 2020, 233, 115840. [Google Scholar] [CrossRef]
- Bian, H.; Gao, Y.; Wang, R.; Liu, Z.; Wu, W.; Dai, H. Contribution of lignin to the surface structure and physical performance of cellulose nanofibrils film. Cellulose 2018, 25, 1309–1318. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Francis Suh, J.-K.; Matthew, H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef]
- Mano, J.F. Viscoelastic Properties of Chitosan with Different Hydration Degrees as Studied by Dynamic Mechanical Analysis. Macromol. Biosci. 2008, 8, 69–76. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Zhan, X.; Xie, W.; Yang, X.; Cui, S.W.; Xia, W. Development and properties of new kojic acid and chitosan composite biodegradable films for active packaging materials. Int. J. Biol. Macromol. 2020, 144, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, Y.; Duan, S.; Li, C.; Hu, B.; Liu, A.; Wu, D.; Cui, H.; Lin, L.; He, J.; et al. Preparation and characterization of chitosan films with three kinds of molecular weight for food packaging. Int. J. Biol. Macromol. 2020, 155, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Prata, A.S.; Grosso, C.R.F. Production of microparticles with gelatin and chitosan. Carbohydr. Polym. 2015, 116, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Botelho da Silva, S.; Krolicka, M.; van den Broek, L.A.M.; Frissen, A.E.; Boeriu, C.G. Water-soluble chitosan derivatives and pH-responsive hydrogels by selective C-6 oxidation mediated by TEMPO-laccase redox system. Carbohydr. Polym. 2018, 186, 299–309. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan—Present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Braz, E.M.A.; Silva, S.C.C.C.; Sousa Brito, C.A.R.; Brito, L.M.; Barreto, H.M.; Carvalho, F.A.A.; Santos, L.S.; Lobo, A.O.; Osajima, J.A.; Sousa, K.S.; et al. Spectroscopic, thermal characterizations and bacteria inhibition of chemically modified chitosan with phthalic anhydride. Mater. Chem. Phys. 2020, 240, 122053. [Google Scholar] [CrossRef]
- Prabaharan, M.; Mano, J.F. Stimuli-Responsive Hydrogels Based on Polysaccharides Incorporated with Thermo-Responsive Polymers as Novel Biomaterials. Macromol. Biosci. 2006, 6, 991–1008. [Google Scholar] [CrossRef]
- Castillejo, M.; Rebollar, E.; Oujja, M.; Sanz, M.; Selimis, A.; Sigletou, M.; Psycharakis, S.; Ranella, A.; Fotakis, C. Fabrication of porous biopolymer substrates for cell growth by UV laser: The role of pulse duration. Appl. Surf. Sci. 2012, 258, 8919–8927. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.W.; Mirhosseini, H.; Taip, F.S.; Ling, T.C.; Tan, C.P. Comparative study on the physicochemical properties of κ-carrageenan extracted from Kappaphycus alvarezii (doty) doty ex Silva in Tawau, Sabah, Malaysia and commercial κ-carrageenans. Food Hydrocoll. 2013, 30, 581–588. [Google Scholar] [CrossRef]
- Pu, S.; Li, J.; Sun, L.; Zhong, L.; Ma, Q. An in vitro comparison of the antioxidant activities of chitosan and green synthesized gold nanoparticles. Carbohydr. Polym. 2019, 211, 161–172. [Google Scholar] [CrossRef]
- Darmadji, P.; Izumimoto, M. Effect of chitosan in meat preservation. Meat Sci. 1994, 38, 243–254. [Google Scholar] [CrossRef]
- Ouattara, B.; Simard, R.E.; Piette, G.; Begin, A.; Holley, R.A. Diffusion of Acetic and Propionic Acids from Chitosan-based Antimicrobial Packaging Films. J. Food Sci. 2000, 65, 768–773. [Google Scholar] [CrossRef]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef]
- Lim, H.N.; Huang, N.M.; Loo, C.H. Facile preparation of graphene-based chitosan films: Enhanced thermal, mechanical and antibacterial properties. J. Non. Cryst. Solids 2012, 358, 525–530. [Google Scholar] [CrossRef]
- Carson, L.; Kelly-Brown, C.; Stewart, M.; Oki, A.; Regisford, G.; Luo, Z.; Bakhmutov, V.I. Synthesis and characterization of chitosan–carbon nanotube composites. Mater. Lett. 2009, 63, 617–620. [Google Scholar] [CrossRef] [Green Version]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Marcos, B.; Aymerich, T.; Monfort, J.M.; Garriga, M. Use of antimicrobial biodegradable packaging to control Listeria monocytogenes during storage of cooked ham. Int. J. Food Microbiol. 2007, 120, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T. Effect of alginate coating on physicochemical and sensory qualities of button mushrooms (Agaricus bisporus) under a high oxygen modified atmosphere. Postharvest Biol. Technol. 2013, 76, 91–97. [Google Scholar] [CrossRef]
- Rhim, J.W.; Wu, Y.; Weller, C.L.; Schnepf, M. Physical Characteristics of a Composite Film of Soy Protein Isolate and Propyleneglycol Alginate. J. Food Sci. 1999, 64, 149–152. [Google Scholar] [CrossRef]
- Gu, C.H.; Wang, J.J.; Yu, Y.; Sun, H.; Shuai, N.; Wei, B. Biodegradable multilayer barrier films based on alginate/polyethyleneimine and biaxially oriented poly(lactic acid). Carbohydr. Polym. 2013, 92, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Fayaz, A.; Balaji, K.; Girilal, M.; Kalaichelvan, P.T.; Venkatesan, R. Mycobased Synthesis of Silver Nanoparticles and Their Incorporation into Sodium Alginate Films for Vegetable and Fruit Preservation. J. Agric. Food Chem. 2009, 57, 6246–6252. [Google Scholar] [CrossRef] [PubMed]
- Cutter, C.N.; Siragusa, G.R. Reduction of Brochothrix thermosphacta on beef surfaces following immobilization of nisin in calcium alginate gels. Lett. Appl. Microbiol. 1996, 23, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshi, R.; Kim, H.J.; Rhim, J.W. Effect of sulfur nanoparticles on properties of alginate-based films for active food packaging applications. Food Hydrocoll. 2021, 110, 106155. [Google Scholar] [CrossRef]
- Pawar, S.G.; Pathade, G.R.; Rale, V.B. Pullulan production from cane molasses by Aureobasidium mausonii strain NCIM 1226. Anal. Chem. 2007, 6, 4–8. [Google Scholar]
- Wu, S.; Chen, H.; Jin, Z.; Tong, Q. Effect of two-stage temperature on pullulan production by Aureobasidium pullulans. World J. Microbiol. Biotechnol. 2010, 26, 737–741. [Google Scholar] [CrossRef]
- Ma, Z.-C.; Liu, N.-N.; Chi, Z.; Liu, G.-L.; Chi, Z.-M. Genetic Modification of the Marine-Isolated Yeast Aureobasidium melanogenum P16 for Efficient Pullulan Production from Inulin. Mar. Biotechnol. 2015, 17, 511–522. [Google Scholar] [CrossRef]
- Yurlova, N.A.; De Hoog, G.S. A new variety of Aureobasidium pullulans characterized by exopolysaccharide structure, nutritional physiology and molecular features. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 1997, 72, 141–147. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Han, K.; Cai, Y.; Ma, M.; Tong, Q.; Sheng, L. Effect of nano-TiO2 on the physical, mechanical and optical properties of pullulan film. Carbohydr. Polym. 2019, 218, 95–102. [Google Scholar] [CrossRef]
- Sheng, L.; Su, P.; Han, K.; Chen, J.; Cao, A.; Zhang, Z.; Jin, Y.; Ma, M. Synthesis and structural characterization of lysozyme–pullulan conjugates obtained by the Maillard reaction. Food Hydrocoll. 2017, 71, 1–7. [Google Scholar] [CrossRef]
- Niu, B.; Shao, P.; Chen, H.; Sun, P. Structural and physiochemical characterization of novel hydrophobic packaging films based on pullulan derivatives for fruits preservation. Carbohydr. Polym. 2019, 208, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Saeaeh, K.; Thummarungsan, N.; Paradee, N.; Choeichom, P.; Phasuksom, K.; Lerdwijitjarud, W.; Sirivat, A. Soft and highly responsive multi-walled carbon nanotube/pullulan hydrogel composites as electroactive materials. Eur. Polym. J. 2019, 120, 109231. [Google Scholar] [CrossRef]
- Brown, M.L. A Comparative Study of the Effects of Non-starch Polysaccharide Gums on Physical Properties of Single-screw Extruded Aquafeed. J. Food Process. Technol. 2015, 6, 457. [Google Scholar] [CrossRef]
- Sarraf, A.G.; Tissot, H.; Tissot, P.; Alfonso, D.; Gurny, R.; Doelker, E. Influence of hot-melt extrusion and compression molding on polymer structure organization, investigated by differential scanning calorimetry. J. Appl. Polym. Sci. 2001, 81, 3124–3132. [Google Scholar] [CrossRef]
- Ganduri, V.S.R. Evaluation of pullulan-based edible active coating methods on Rastali and Chakkarakeli bananas and their shelf-life extension parameters studies. J. Food Process. Preserv. 2020, 44, e14378. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, C.; Liu, X.; Zhang, N.; Xu, T.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Improvement of storage quality of strawberries by pullulan coatings incorporated with cinnamon essential oil nanoemulsion. LWT 2020, 122, 109054. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.; Zhao, H. Application of Pullulan and Chitosan Multilayer Coatings in Fresh Papayas. Coatings 2019, 9, 745. [Google Scholar] [CrossRef] [Green Version]
- Treviño-Garza, M.Z.; García, S.; Heredia, N.; Alanís-Guzmán, M.G.; Arévalo-Niño, K. Layer-by-layer edible coatings based on mucilages, pullulan and chitosan and its effect on quality and preservation of fresh-cut pineapple (Ananas comosus). Postharvest Biol. Technol. 2017, 128, 63–75. [Google Scholar] [CrossRef]
- Shao, P.; Niu, B.; Chen, H.; Sun, P. Fabrication and characterization of tea polyphenols loaded pullulan-CMC electrospun nanofiber for fruit preservation. Int. J. Biol. Macromol. 2018, 107, 1908–1914. [Google Scholar] [CrossRef]
- Qian, Y.F.; Zheng, L.J.; Song, R.Y.; Du, B. Electrospinning of Pullulan Nanofibers for Food Package Materials. Adv. Mater. Res. 2013, 821–822, 1321–1325. [Google Scholar] [CrossRef]
- Son, T.-W.; Lee, G.-M.; Lee, D.-W.; Lee, J.-H.; Lim, H.-S. Preparation and Characterization of Electrospun Pullulan Webs. Polym. Korea 2012, 36, 196–201. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, F.; Li, Y.; Shoemaker, C.F.; Xia, W. Preparation and characterization of pullulan-chitosan and pullulan-carboxymethyl chitosan blended films. Food Hydrocoll. 2013, 30, 82–91. [Google Scholar] [CrossRef]
- Kanmani, P.; Lim, S.T. Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem. 2013, 141, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.J.B.; Almeida, A.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Trindade, T. Antifungal activity of transparent nanocomposite thin films of pullulan and silver against Aspergillus niger. Colloids Surf. B Biointerfaces 2013, 103, 143–148. [Google Scholar] [CrossRef]
- Kristo, E.; Biliaderis, C.G. Physical properties of starch nanocrystal-reinforced pullulan films. Carbohydr. Polym. 2007, 68, 146–158. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, D.; Kanmani, P. Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity. Carbohydr. Polym. 2019, 224, 115159. [Google Scholar] [CrossRef]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Papon, P.; Leblond, J.; Meijer, P.H. (Eds.) Gelation and Transitions in Biopolymers. In The Physics of Phase Transitions; Springer: Berlin/Heidelberg, Germany, 2006; pp. 189–213. [Google Scholar]
- Shankar, S.; Jaiswal, L.; Rhim, J.-W. Gelatin-Based Nanocomposite Films. In Antimicrobial Food Packaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 339–348. [Google Scholar]
- Ortiz-Zarama, M.A.; Jiménez-Aparicio, A.R.; Solorza-Feria, J. Obtainment and partial characterization of biodegradable gelatin films with tannic acid, bentonite and glycerol. J. Sci. Food Agric. 2016, 96, 3424–3431. [Google Scholar] [CrossRef]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; Garrigós, M.d.C.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef] [Green Version]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Mechanical and Physical Properties of Cassava Starch-Gelatin Composite Films. Int. J. Polym. Mater. 2012, 61, 778–792. [Google Scholar] [CrossRef]
- Malherbi, N.M.; Schmitz, A.C.; Grando, R.C.; Bilck, A.P.; Yamashita, F.; Tormen, L.; Fakhouri, F.M.; Velasco, J.I.; Bertan, L.C. Corn starch and gelatin-based films added with guabiroba pulp for application in food packaging. Food Packag. Shelf Life 2019, 19, 140–146. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, F.; Xu, W.; Han, X. Enhanced antibacterial performance of gelatin/chitosan film containing capsaicin loaded MOFs for food packaging. Appl. Surf. Sci. 2020, 510, 145418. [Google Scholar] [CrossRef]
- Halder, D.; Mitra, A.; Bag, S.; Raychaudhuri, U.; Chakraborty, R. Study on Gelatin-Silver Nanoparticle Composite Towards the Development of Bio-Based Antimicrobial Film. J. Nanosci. Nanotechnol. 2011, 11, 10374–10378. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Juszczak, L.; Kawecka, A.; Bytesnikova, Z.; Milosavljevic, V.; Makarewicz, M. Development of furcellaran-gelatin films with Se-AgNPs as an active packaging system for extension of mini kiwi shelf life. Food Packag. Shelf Life 2019, 21, 100339. [Google Scholar] [CrossRef]
- Samsi, M.S.; Kamari, A.; Din, S.M.; Lazar, G. Synthesis, characterization and application of gelatin–carboxymethyl cellulose blend films for preservation of cherry tomatoes and grapes. J. Food Sci. Technol. 2019, 56, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Kingwascharapong, P.; Arisa, K.; Karnjanapratum, S.; Tanaka, F.; Tanaka, F. Effect of gelatin-based coating containing frog skin oil on the quality of persimmon and its characteristics. Sci. Hortic. Amst. 2020, 260, 108864. [Google Scholar] [CrossRef]
- Pellá, M.C.G.; Silva, O.A.; Pellá, M.G.; Beneton, A.G.; Caetano, J.; Simões, M.R.; Dragunski, D.C. Effect of gelatin and casein additions on starch edible biodegradable films for fruit surface coating. Food Chem. 2020, 309, 125764. [Google Scholar] [CrossRef]
- Sagis, L.M.C. Microencapsulation and Microspheres for Food Applications; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780128003503. [Google Scholar]
- Fabra, M.J.; Lopez-Rubio, A.; Lagaron, J.M. High barrier polyhydroxyalcanoate food packaging film by means of nanostructured electrospun interlayers of zein. Food Hydrocoll. 2013, 32, 106–114. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Ahammed, S.; Liu, F.; Khin, M.N.; Yokoyama, W.H.; Zhong, F. Improvement of the water resistance and ductility of gelatin film by zein. Food Hydrocoll. 2020, 105, 105804. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.W. Plasticizers for Zein: Their Effect on Tensile Properties and Water Absorption of Zein Films. Cereal Chem. J. 2004, 81, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Lai, H.-M.; Padua, G.W. Water Vapor Barrier Properties of Zein Films Plasticized with Oleic Acid. Cereal Chem. J. 1998, 75, 194–199. [Google Scholar] [CrossRef]
- Doğan Atik, İ.; Özen, B.; Tıhmınlıoğlu, F. Water vapour barrier performance of corn-zein coated polypropylene (PP) packaging films. J. Therm. Anal. Calorim. 2008, 94, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Zhan, F.; Yan, X.; Sheng, F.; Li, B. Facile in situ synthesis of silver nanoparticles on tannic acid/zein electrospun membranes and their antibacterial, catalytic and antioxidant activities. Food Chem. 2020, 330, 127172. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Y.; Jin, N.; Li, J.; Dong, S.; Li, S.; Zhang, Z.; Chen, Y. Zein films with porous polylactic acid coatings via cold plasma pre-treatment. Ind. Crops Prod. 2020, 150, 112382. [Google Scholar] [CrossRef]
- Park, H.-Y.; Kim, S.-J.; Kim, K.M.; You, Y.-S.; Kim, S.Y.; Han, J. Development of Antioxidant Packaging Material by Applying Corn-Zein to LLDPE Film in Combination with Phenolic Compounds. J. Food Sci. 2012, 77, E273–E279. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Alves de Oliveira, R.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Suryanegara, L.; Nakagaito, A.N.; Yano, H. The effect of crystallization of PLA on the thermal and mechanical properties of microfibrillated cellulose-reinforced PLA composites. Compos. Sci. Technol. 2009, 69, 1187–1192. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-hindi, R. Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne Pathogens: A review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef] [PubMed]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Mohsen, A.H.; Ali, N.A. Mechanical, Color and Barrier, Properties of Biodegradable Nanocomposites Polylactic Acid/Nanoclay. J. Bioremediation Biodegrad. 2018, 9, 455. [Google Scholar] [CrossRef]
- Gross, R.A. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [Green Version]
- Corneillie, S.; Smet, M. PLA architectures: The role of branching. Polym. Chem. 2015, 6, 850–867. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, M.; Pircheraghi, G.; Bagheri, R.; Altstädt, V. Poly(lactic acid)/coplasticized thermoplastic starch blend: Effect of plasticizer migration on rheological and mechanical properties. Polym. Adv. Technol. 2019, 30, 839–851. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S.M. Mechanical, dynamic, and thermomechanical properties of coir/pineapple leaf fiber reinforced polylactic acid hybrid biocomposites. Polym. Compos. 2019, 40, 2000–2011. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Briso, A.; Serrano-Aroca, Á. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Yu, H.; Wang, Y.; Zhou, Y.; Zhang, H.; Yao, J.; Abdalkarim, S.Y.H.; Tam, K.C. Natural Biodegradable Poly(3-hydroxybutyrate- co -3-hydroxyvalerate) Nanocomposites with Multifunctional Cellulose Nanocrystals/Graphene Oxide Hybrids for High-Performance Food Packaging. J. Agric. Food Chem. 2019, 67, 10954–10967. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. ZnO-Reinforced Poly(3-hydroxybutyrate- co -3-hydroxyvalerate) Bionanocomposites with Antimicrobial Function for Food Packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Fu, J.; Ma, X. Improvement in thermal, mechanical, and barrier properties of biocomposite of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)/modified nano-SiO2. Polym. Compos. 2019, 41, 381–390. [Google Scholar] [CrossRef]
- Chen, G.X.; Hao, G.J.; Guo, T.Y.; Song, M.D.; Zhang, B.H. Structure and mechanical properties of poly (3-hydroxybutyrate- co-3-hydroxyvalerate) (PHBV)/clay nanocomposites. J. Mater. Sci. Lett. 2002, 21, 1587–1589. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Ma, X.; Li, J. Synthesis of a novel biocomposite of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) reinforced with acetylated cellulose nanocrystals. Cellulose 2019, 26, 8729–8743. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Lyn, F.H.; Peng, T.C.; Ruzniza, M.Z.; Hanani, Z.A.N. Effect of oxidation degrees of graphene oxide (GO) on the structure and physical properties of chitosan/GO composite films. Food Packag. Shelf Life 2019, 21, 100373. [Google Scholar] [CrossRef]
- Purkait, T.; Singh, G.; Singh, M.; Kumar, D.; Dey, R.S. Large area few-layer graphene with scalable preparation from waste biomass for high-performance supercapacitor. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.Z.; Yang, Q.; Kuang, W.J.; Stebunov, Y.V.; Xiong, W.Q.; Yu, J.; Nair, R.R.; Katsnelson, M.I.; Yuan, S.J.; Grigorieva, I.V.; et al. Limits on gas impermeability of graphene. Nature 2020, 579, 229–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassei, J.K.; Kaner, R.B. Graphene, a promising transparent conductor. Mater. Today 2010, 13, 52–59. [Google Scholar] [CrossRef]
- Shokrieh, M.M.; Esmkhani, M.; Shahverdi, H.R.; Vahedi, F. Effect of graphene nanosheets (GNS) and graphite nanoplatelets (GNP) on the mechanical properties of epoxy nanocomposites. Sci. Adv. Mater. 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Grácio, J. Surface Modification of Graphene Nanosheets with Gold Nanoparticles: The Role of Oxygen Moieties at Graphene Surface on Gold Nucleation and Growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Compton, O.C.; Jain, B.; Dikin, D.A.; Abouimrane, A.; Amine, K.; Nguyen, S.T. Chemically active reduced graphene oxide with tunable C/O ratios. ACS Nano 2011, 5, 4380–4391. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Wu, H.; Yang, H.; Zhang, J.; Guo, S. Reducing Graphene Oxide via Hydroxylamine: A Simple and Efficient Route to Graphene. J. Phys. Chem. C 2011, 115, 11957–11961. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials. Angew. Chemie Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Sun, H.; Li, H.; Peng, H. Developing polymer composite materials: Carbon nanotubes or graphene? Adv. Mater. 2013, 25, 5153–5176. [Google Scholar] [CrossRef]
- Wang, P.; Tang, S.; Sheng, F.; Cai, J.; Fei, P.; Nawaz, A.; Walayat, N.; Bakhsh, A.; Xiong, H. Crystallization, thermal stability, barrier property, and aging resistance application of multi-functionalized graphene oxide/poly (lactide)/starch nanocomposites. Int. J. Biol. Macromol. 2019, 132, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Fabrication of polylactic acid/carbon nanotubes/chitosan composite fibers by electrospinning for strawberry preservation. Int. J. Biol. Macromol. 2019, 121, 1329–1336. [Google Scholar] [CrossRef]
- Gouvêa, R.F.; Del Aguils, E.M.; Paschoalin, V.M.F.; Andrade, C.T. Extruded hybrids based on poly (3-hydroxybutyrate- co -3-hydroxyvalerate) and reduced graphene oxide composite for active food packaging. Food Packag. Shelf Life 2018, 16, 77–85. [Google Scholar] [CrossRef]
- Barra, A.; Ferreira, N.M.; Martins, M.A.; Lazar, O.; Pantazi, A.; Jderu, A.A.; Neumayer, S.M.; Rodriguez, B.J.; Enăchescu, M.; Ferreira, P.; et al. Eco-friendly preparation of electrically conductive chitosan-reduced graphene oxide flexible bionanocomposites for food packaging and biological applications. Compos. Sci. Technol. 2019, 173, 53–60. [Google Scholar] [CrossRef]
- Afshar, S.; Banisadi, H. Investigation the effect of graphene oxide and gelatin/starch weight ratio on the properties of starch/gelatin/GO nanocomposite films: The RSM study. Int. J. Biol. Macromol. 2017, 109, 1019–1028. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, Z.; Fu, S.; Meng, L.; Liu, Y.; Chen, F.; Wang, K.; Fu, Q. Fabrication of PLA/CNC/CNT conductive composites for high electromagnetic interference shielding based on Pickering emulsions method. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105558. [Google Scholar] [CrossRef]
- Montes, S.; Etxeberria, A.; Mocholi, V.; Rekondo, A.; Grande, H.; Labidi, J. Effect of combining cellulose nanocrystals and graphene nanoplatelets on the properties of poly (lactic acid) based films. Polym. Lett. 2018, 12, 543–555. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Cui, C.; Ren, P.; Yan, D.; Li, Z. Enhanced Mechanical Performance of Segregated Carbon Nanotube / Poly (lactic acid) Composite for E fficient Electromagnetic Interference Shielding. Ind. Eng. Chem. Res. 2019, 58, 4454–4461. [Google Scholar] [CrossRef]
- Ren, F.; Li, Z.; Xu, L.; Sun, Z.; Ren, P.; Yan, D.; Li, Z. Large-scale preparation of segregated PLA/carbon nanotube composite with high efficient electromagnetic interference shielding and favourable mechanical properties. Compos. Part B Eng. 2018, 155, 405–413. [Google Scholar] [CrossRef]
- Husna, N.; Che, M.; Mustapha, M.; Sallih, N.; Eng, K.; Ahmad, R. Progress in Organic Coatings Graphene-based polymer nanocomposites as barrier coatings for corrosion protection. Prog. Org. Coatings 2019, 135, 82–99. [Google Scholar] [CrossRef]
- Sharma, B.; Malik, P.; Jain, P. Biopolymer reinforced nanocomposites: A comprehensive review. Mater. Today Commun. 2018, 16, 353–363. [Google Scholar] [CrossRef]
- Terzioglu, P.; Altin, Y.; Kalemtas, A.; Celik Bedeloglu, A. Graphene oxide and zinc oxide decorated chitosan nanocomposite biofilms for packaging applications. J. Polym. Eng. 2020, 40, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Weng, S.X.; Youse, N.; Tufenkji, N. Self-Assembly of Ultralarge Graphene Oxide Nanosheets and Alginate into Layered Nanocomposites for Robust Packaging Materials. ACS Appl. Nano Mater. 2019, 2, 1431–1444. [Google Scholar] [CrossRef]
- Xie, D.; Liu, Q.; Xu, D.; Wu, X. Graphene oxide–polyoctahedral silsesquioxane–chitosan composite films with improved mechanical and water-vapor-transport properties. J. Appl. Polym. Sci. 2019, 47748, 1–9. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, Y.; Chen, G.; Cai, M. Enhanced mechanical and hydrophobic properties of composite cassava starch films with stearic acid modified MCC (microcrystalline cellulose)/NCC (nanocellulose) as strength agent. Int. J. Biol. Macromol. 2020, 142, 846–854. [Google Scholar] [CrossRef]
- Mallakpour, S.; Rashidimoghadam, S. Application of ultrasonic irradiation as a benign method for production of glycerol plasticized-starch/ascorbic acid functionalized MWCNTs nanocomposites: Investigation of methylene blue adsorption and electrical properties. Ultrason. Sonochem. 2018, 40, 419–432. [Google Scholar] [CrossRef]
- Mergen, O.B.; Arda, E.; Evingur, G.A. Electrical, optical and mechanical properties of chitosan biocomposites. J. Compos. Mater. 2019, 54, 846–854. [Google Scholar] [CrossRef]
- Jia, S.; Wang, Z.; Chen, L. A feasible strategy to constructing hybrid conductive networks in PLA—Based composites modified by CNT-d-RGO particles and PEG for mechanical and electrical properties. Polym. Adv. Technol. 2019, 31, 699–712. [Google Scholar] [CrossRef]
- Idumah, C.I.; Hassan, A.; Ihuoma, D.E. Recently emerging trends in polymer nanocomposites packaging materials. Polym. Technol. Mater. 2019, 58, 1054–1109. [Google Scholar] [CrossRef]
- Xie, X.; Li, L.; Ye, S.; Zhang, Q.; Chen, X.; Huang, X. Photocatalytic degradation of ethylene by TiO2 nanotubes/ reduced graphene oxide prepared by gamma irradiation. Radiat. Phys. Chem. 2019, 165, 108371. [Google Scholar] [CrossRef]
- Pal, N.; Dubey, P.; Gopinath, P.; Pal, K. Combined effect of cellulose nanocrystal and reduced graphene oxide into poly-lactic acid matrix nanocomposite as a scaffold and its anti-bacterial activity. Int. J. Biol. Macromol. 2017, 95, 94–105. [Google Scholar] [CrossRef]
- Shen, X.J.; Yang, S.; Shen, J.X.; Ma, J.L.; Wu, Y.Q.; Zeng, X.L.; Fu, S.Y. Improved mechanical and antibacterial properties of silver-graphene oxide hybrid/polylactid acid composites by in-situ polymerization. Ind. Crops Prod. 2019, 130, 571–579. [Google Scholar] [CrossRef]
- Bher, A.; Unalan, I.U.; Auras, R.; Rubino, M.; Schvezov, C.E. Graphene modifies the biodegradation of poly(lactic acid)-thermoplastic cassava starch reactive blend films. Polym. Degrad. Stab. 2019, 164, 187–197. [Google Scholar] [CrossRef]
- Ferreira, W.H.; Dahmouche, K.; Andrade, C.T. Dispersion of reduced graphene oxide within thermoplastic starch / poly (lactic acid) blends investigated by small-angle X-ray scattering. Carbohydr. Polym. 2019, 208, 124–132. [Google Scholar] [CrossRef]
- De Carvalho, A.P.A.; Conte Junior, C.A. Green strategies for active food packagings: A systematic review on active properties of graphene-based nanomaterials and biodegradable polymers. Trends Food Sci. Technol. 2020, 103, 130–143. [Google Scholar] [CrossRef]
- Cui, R.; Jiang, K.; Yuan, M.; Cao, J.; Li, L.; Tang, Z.; Qin, Y. Antimicrobial film based on polylactic acid and carbon nanotube for controlled cinnamaldehyde release. J. Mater. Res. Technol. 2020, 9, 10130–10138. [Google Scholar] [CrossRef]
- Xie, J.; Huang, L.; Wang, R.; Ye, S.; Song, X. Novel visible light-responsive graphene oxide/Bi2WO6/starch composite membrane for efficient degradation of ethylene. Carbohydr. Polym. 2020, 246, 116640. [Google Scholar] [CrossRef] [PubMed]
- Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Bionanocomposites: A new concept of ecological, bioinspired, and functional hybrid materials. Adv. Mater. 2007, 19, 1309–1319. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Ariga, K.; Lvov, Y.M. Bio-inorganic Hybrid Nanomaterials: Strategies, Synthesis, Characterization and Applications; Wiley-VCH: Weinheim, Germany, 2008; ISBN 978-3-527-31718-9. [Google Scholar]
- Fernandes, F.M.; Darder, M.; Ruiz, A.I.; Aranda, P.; Ruiz-Hitzky, E. Gelatine-based bio-nanocomposites. In Nanocomposites with Biodegradable Polymers: Synthesis, Properties, and Future Perspectives; Mittal, V., Ed.; Oxford University Press: New York, NY, USA, 2011; p. 209. [Google Scholar]
- Alcântara, A.C.S.; Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Zein-fibrous clays biohybrid materials. Eur. J. Inorg. Chem. 2012, 5216–5224. [Google Scholar] [CrossRef]
- Aranda, P.; Darder, M.; Fernandes, F.M. Clay Mineral–Polymer Nanocomposites. In Handbook of Clay Science. Part A: Fundamentals; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands; Oxford, UK, 2013; p. 721. [Google Scholar]
- Ruiz-Hitzky, E.; Darder, M.; Fernandes, F.M.; Wicklein, B.; Alcântara, A.C.S.; Aranda, P. Fibrous clays based bionanocomposites. Prog. Polym. Sci. 2013, 38, 1392–1414. [Google Scholar] [CrossRef] [Green Version]
- Alcântara, A.C.S.; Darder, M.; Aranda, P.; Ayral, A.; Ruiz-Hitzky, E. Bionanocomposites based on polysaccharides and fibrous clays for packaging applications. J. Appl. Polym. Sci. 2016, 133, 42362. [Google Scholar] [CrossRef]
- González, M.M.; Darder, M.; Aranda, P.; Akkari, M.; Huttel, Y.; Mayoral, A.; Bettini, J.; Ruiz-hitzky, E. Functional Hybrid Nanopaper by Assembling Nanofibers of Cellulose and Sepiolite. Adv. Funct. Mater. 2018, 28, 1–13. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Ruiz-García, C.; Fernandes, F.M.; Ruiz-Hitzky, E. The Meeting Point of Carbonaceous Materials and Clays: Toward a New Generation of Functional Composites. Adv. Funct. Mater. 2017, 28, 1704323. [Google Scholar] [CrossRef]
- Ruiz-García, C.; Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Toward a green way for the chemical production of supported graphenes using porous solids. J. Mater. Chem. A 2017, 2, 2009–2017. [Google Scholar] [CrossRef]
- Bandyopadhyaya, R.; Nativ-Roth, E.; Regev, O.; Yerushalmi-Rozen, R. Stabilization of Individual Carbon Nanotubes in Aqueous Solutions. Nano Lett. 2002, 2, 25–28. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L.; Sun, J. Production of aqueous colloidal dispersions of carbon nanotubes. J. Colloid Interface Sci. 2003, 260, 89–94. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion of carbon nanotubes: Mixing, sonication, stabilization, and composite properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, R.M.F.; Abreu, B.; Claro, B.; Buzaglo, M.; Regev, O.; Furó, I.; Marques, E.F. Dispersing Carbon Nanotubes with Ionic Surfactants under Controlled Conditions: Comparisons and Insight. Langmuir 2015, 31, 10955–10965. [Google Scholar] [CrossRef] [PubMed]
- Kharisov, B.I.; Kharissova, O.V.; Dimas, A.V. The dispersion, solubilization and stabilization in “solution” of single-walled carbon nanotubes. RSC Adv. 2016, 6, 68760–68787. [Google Scholar] [CrossRef]
- Fernandes, F.M.; Ruiz-Hitzky, E. Assembling nanotubes and nanofibres: Cooperativeness in sepiolite-carbon nanotube materials. Carbon N. Y. 2014, 72, 296–303. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Sobral, M.M.C.; Gómez-Avilés, A.; Nunes, C.; Ruiz-García, C.; Ferreira, P.; Aranda, P. Clay-Graphene Nanoplatelets Functional Conducting Composites. Adv. Funct. Mater. 2016, 26, 7394–7405. [Google Scholar] [CrossRef]
- Pillet, F.; Formosa-Dague, C.; Baaziz, H.; Dague, E.; Rols, M.P. Cell wall as a target for bacteria inactivation by pulsed electric fields. Sci. Rep. 2016, 6, 19778. [Google Scholar] [CrossRef]
- Sanchez-Vega, R.; Elez-Martinez, P.; Martin-Belloso, O. Influence of high-intensity pulsed electric field processing parameters on antioxidant compounds of broccoli juice. Innov. Food Sci. Emerg. Technol. 2015, 29, 70–77. [Google Scholar] [CrossRef]
- Quintão-Teixeira, L.J.; Soliva-Fortuny, R.; Mota Ramos, A.; Martín-Belloso, O. Kinetics of peroxidase inactivation in carrot juice treated with pulsed electric fields. J. Food Sci. 2013, 78, E222–E228. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wang, J. Designs of pulsed electric fields treatment chambers for liquid foods pasteurization process: A review. J. Food Eng. 2009, 95, 227–239. [Google Scholar] [CrossRef]
- Roodenburg, B.; Morren, J.; Berg, H.E.; de Haan, S.W.H. Metal release in a stainless steel pulsed electric field (PEF) system Part II. The treatment of orange juice; related to legislation and treatment chamber lifetime. Innov. Food Sci. Emerg. Technol. 2005, 6, 337–345. [Google Scholar] [CrossRef]
- Roodenburg, B.; de Haan, S.W.H.; van Boxtel, L.B.J.; Hatt, V.; Wouters, P.C.; Coronel, P.; Ferreira, J.A. Conductive plastic film electrodes for Pulsed Electric Field (PEF) treatment-A proof of principle. Innov. Food Sci. Emerg. Technol. 2010, 11, 274–282. [Google Scholar] [CrossRef]
- Roodenburg, B.; De Haan, S.W.H.; Ferreira, J.A.; Coronel, P.; Wouters, P.C.; Hatt, V. Toward 6 log10 pulsed electric field inactivation with conductive plastic packaging material. J. Food Process Eng. 2013, 36, 77–86. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.; Kang, H.; Zhang, W.; Zhang, S.; Li, J. Mussel byssus-inspired engineering of synergistic nanointerfacial interactions as sacrificial bonds into carbon nanotube-reinforced soy protein/nanofibrillated cellulose nanocomposites: Versatile mechanical enhancement. Appl. Surf. Sci. 2018, 434, 1086–1100. [Google Scholar] [CrossRef]
- Guo, Y.; Zuo, X.; Xue, Y.; Tang, J.; Gouzman, M.; Fang, Y.; Zhou, Y.; Wang, L.; Yu, Y.; Rafailovich, M.H. Engineering thermally and electrically conductive biodegradable polymer nanocomposites. Compos. Part B Eng. 2020, 189, 107905. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, H. Alginate/pectin aerogel microspheres for controlled release of proanthocyanidins. Int. J. Biol. Macromol. 2019, 136, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Lo Dico, G.; Wicklein, B.; Lisuzzo, L.; Lazzara, G.; Aranda, P.; Ruiz-Hitzky, E. Multicomponent bionanocomposites based on clay nanoarchitectures for electrochemical devices. Beilstein J. Nanotechnol. 2019, 10, 1303–1315. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Narita, A.; Müllen, K. Precision synthesis versus bulk-scale fabrication of graphenes. Nat. Rev. Chem. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Phiri, J.; Johansson, L.; Gane, P.; Maloney, T.C. Co-exfoliation and fabrication of graphene based microfibrillated cellulose composites–mechanical and thermal stability and functional conductive properties. Nano Res. 2018, 10, 9569–9582. [Google Scholar] [CrossRef] [Green Version]
- Cobos, M.; González, B.; Fernández, M.J.; Fernández, M.D. International Journal of Biological Macromolecules Study on the effect of graphene and glycerol plasticizer on the properties of chitosan-graphene nanocomposites via in situ green chemical reduction of graphene oxide. Int. J. Biol. Macromol. 2018, 114, 599–613. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Zhang, L.; Du, C.; Fang, T.; Hu, J. Direct Reduction of Graphene Oxide/Nanofibrillated Cellulose Composite Film and its Electrical Conductivity Research. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with carbon nanotubes and graphene: An outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashi, S.; Gupta, R.K.; Baum, T.; Kao, N.; Bhattacharya, S.N. Phase transition and anomalous rheological behaviour of polylactide/graphene nanocomposites. Compos. Part B Eng. 2018, 135, 25–34. [Google Scholar] [CrossRef]
- Fryń, P.; Bogdanowicz, K.A.; Górska, N.; Rysz, J.; Krysiak, P.; Marzec, M.; Marzec, M.; Iwan, A.; Januszko, A. Hybrid materials based on L,D-poly(lactic acid) and Single-Walled Carbon Nanotubes as flexible substrate for organic devices. Polymers 2018, 10, 1271. [Google Scholar] [CrossRef] [Green Version]
- Kashi, S.; Gupta, R.K.; Kao, N.; Hadigheh, S.A.; Bhattacharya, S.N. Influence of graphene nanoplatelet incorporation and dispersion state on thermal, mechanical and electrical properties of biodegradable matrices. J. Mater. Sci. Technol. 2018, 34, 1026–1034. [Google Scholar] [CrossRef]
- Dhar, P.; Pratto, B.; Jose, A.; Cruz, G.; Bankar, S. Valorization of sugarcane straw to produce highly conductive bacterial cellulose/graphene nanocomposite fi lms through in situ fermentation: Kinetic analysis and property evaluation. J. Clean. Prod. 2019, 238, 117859. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Hubbe, M.A.; Pal, L. Highly conductive carbon nanotubes and flexible cellulose nano fibers composite membranes with semi-interpenetrating networks structure. Carbohydr. Polym. 2019, 222, 115013. [Google Scholar] [CrossRef]
- Siripongpreda, T.; Siralertmukul, K.; Rodthongkum, N. Colorimetric sensor and LDI-MS detection of biogenic amines in food spoilage based on porous PLA and graphene oxide. Food Chem. 2020, 329, 127165. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Akkari, M.; Khaorapapong, N.; Ogawa, M. Photoactive nanoarchitectures based on clays incorporating TiO2 and ZnO nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1140–1156. [Google Scholar] [CrossRef] [Green Version]
- Lizundia, E.; Ruiz-Rubio, L.; Vilas, J.L.; León, L.M. Poly(L-lactide)/ZnO nanocomposites as efficient UV-shielding coatings for packaging applications. J. Appl. Polym. Sci. 2016, 133, 42426. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E. Molecular access to intracrystalline tunnels of sepiolite. J. Mater. Chem. 2001, 11, 86–91. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P. Novel architectures in porous materials based on clays. J. Sol Gel Sci. Technol. 2014, 70, 307–316. [Google Scholar] [CrossRef]
- Aranda, P.; Ruiz-Hitzky, E. Immobilization of Nanoparticles on Fibrous Clay Surfaces: Towards Promising Nanoplatforms for Advanced Functional Applications. Chem. Rec. 2018, 18, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety Assessment of Graphene-Based Materials: Focus on Human Health and the Environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.; Rodrigues, A.F.; Jasim, D.A.; Vacchi, I.A.; Ménard-Moyon, C.; Bianco, A.; Bussy, C.; Kostarelos, K. Nose-to-Brain Translocation and Cerebral Biodegradation of Thin Graphene Oxide Nanosheets. Cell Rep. Phys. Sci. 2020, 1, 100176. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Newman, L.; Jasim, D.; Mukherjee, S.P.; Wang, J.; Vacchi, I.A.; Ménard-Moyon, C.; Bianco, A.; Fadeel, B.; Kostarelos, K.; et al. Size-Dependent Pulmonary Impact of Thin Graphene Oxide Sheets in Mice: Toward Safe-by-Design. Adv. Sci. 2020, 7, 1–17. [Google Scholar] [CrossRef]
| Polymer | Nanomaterial | Preparation Method | Nanomaterial Dispersion Strategies | Main Mechanical Effects | Ref. |
|---|---|---|---|---|---|
| PHBV | 0.5 to 0.7 wt% GO, 1:0.5 wt% non-grafted GO/CNC, 1 wt% grafted GO-CNC | Solvent casting | Physical blending (stirring); chemical grafting | Covalently grafted GO-CNC achieved the highest YM, TS, and EB values, which were up to 138%, 170%, and 52% higher than neat polymer. | [149] |
| CS | 0.5 wt% GO with different degrees of oxidation | Solvent casting | Ultrasonic dispersion | By increasing of oxidation degree of GO, the TS and YM increase and the EB decreases. | [152] |
| 0.25 wt% GO and 3 wt% ball-milled maleamic acid–isobutyl polyoctahedral silsesquioxanes (MAIPS) | Solvent casting | Physical blending | Synergistic reinforcements were found on the composite with GO and MAIPS: highest YM and TS (e.g., 50% and 38% higher, respectively, than neat polymer). | [186] | |
| 5 wt% GNP and 5 wt% ZnO | Solvent casting | Ultrasonic dispersion | The simultaneous incorporation of GNP and ZnO lead to highest values of YM and TS, and to a slight decrease of EB. | [178] | |
| GO (0, 25, 40, 45, 48, or 50 wt%, in relation to CS weight) | Solvent casting | Ultrasonic dispersion | CS/GO showed higher TS (improvements of 70% to 110%), YM (improvements of 500%), and lower EB (decay of 90%) when compared with chitosan films. No significative differences were found in CS-based composites with 40 to 50 wt% of GO. | [170] | |
| 0 to 30 wt% GNP or MWCNT | Solvent casting | Ultrasonic dispersion | At the same ratios, CS/GNP and CS/MWCNT exhibited similar TS and YM values. The highest values of TS were achieved by incorporation of 15 wt% of GNP or MWCNT, which represented improvements of 49% and 64% when compared to those of neat polymer. In turn, the highest values of YM were achieved by incorporation of 30 wt% of GNP or MWCNT, which represented improvements of 109% and 115% when compared to those of neat polymer. | [183] | |
| Starch | 3, 6, and 9 wt% MWCNT grafted with ascorbic acid (AA-MWCNT) | Solvent casting | Ultrasonic dispersion | The YM and TS were reduced and the EB was increased by enhancing the AA-MWCNT loading in the composite. | [182] |
| PLA | 1 wt% GNP and CNC (ratio 50/50) | Hot pressing | Melt blending with the Triton X-100 surfactant | Improvements on YM, TS, and EB were achieved by simultaneous incorporation of both nanofillers. | [173] |
| 0.5 wt% GO and 1 wt% CNC | Solvent casting | Physical blending | Increase of PLA/CNC/rGO nanocomposite TS up to 23%. | [187] | |
| 0.05 to 2 wt% GO-Ag hybrids | Solvent casting or direct mechanical melt blending | Physical blending or melt blending | Higher flexural strength was achieved when higher amounts of GO-Ag hybrids were added and when physical blending and solvent casting subsequent methods were applied. | [188] | |
| 2 wt% CNT | Solvent casting | Ultrasonic dispersion | The TS and EB have an enhancement of 52% and 36%, respectively, in comparison with PLA films. | [174] | |
| 0.5, 1.0, 2.0, and 3.0 wt% MWCNT | Injection molding | Mechanical blending | Increments of 32.70% and 67.17% were obtained for the TS and EB with the inclusion of 3 wt% of MWCNT. | [175] | |
| PLA/CNCs | 0.9 to 8.3 wt% CNT | Compression molding | Pickering emulsions | The mechanical performance of the sample was maintained a high level (tensile strength: 45.52 MPa, Young’s modulus: 3152 MPa) after the incorporation of 4.3 wt% CNT. | [172] |
| Alginate | 0 to 25 wt% GO | Solvent casting | Physical blending | The inclusion of >2 wt% GO content into alginate-based composites demonstrated remarkable improvements in YM. The maximum upgrade achieved was of 230% in comparison with pure alginate (15 wt% GO). The evolution of the TS suggested the inclusion of defects in the microstructure as GO increased. | [179] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barra, A.; Santos, J.D.C.; Silva, M.R.F.; Nunes, C.; Ruiz-Hitzky, E.; Gonçalves, I.; Yildirim, S.; Ferreira, P.; Marques, P.A.A.P. Graphene Derivatives in Biopolymer-Based Composites for Food Packaging Applications. Nanomaterials 2020, 10, 2077. https://doi.org/10.3390/nano10102077
Barra A, Santos JDC, Silva MRF, Nunes C, Ruiz-Hitzky E, Gonçalves I, Yildirim S, Ferreira P, Marques PAAP. Graphene Derivatives in Biopolymer-Based Composites for Food Packaging Applications. Nanomaterials. 2020; 10(10):2077. https://doi.org/10.3390/nano10102077
Chicago/Turabian StyleBarra, Ana, Jéssica D. C. Santos, Mariana R. F. Silva, Cláudia Nunes, Eduardo Ruiz-Hitzky, Idalina Gonçalves, Selçuk Yildirim, Paula Ferreira, and Paula A. A. P. Marques. 2020. "Graphene Derivatives in Biopolymer-Based Composites for Food Packaging Applications" Nanomaterials 10, no. 10: 2077. https://doi.org/10.3390/nano10102077
APA StyleBarra, A., Santos, J. D. C., Silva, M. R. F., Nunes, C., Ruiz-Hitzky, E., Gonçalves, I., Yildirim, S., Ferreira, P., & Marques, P. A. A. P. (2020). Graphene Derivatives in Biopolymer-Based Composites for Food Packaging Applications. Nanomaterials, 10(10), 2077. https://doi.org/10.3390/nano10102077











