Transport Properties of Electro-Sprayed Polytetrafluoroethylene Fibrous Layer Filled with Aerogels/Phase Change Materials
Abstract
:1. Introduction
2. Methodology
2.1. Materials
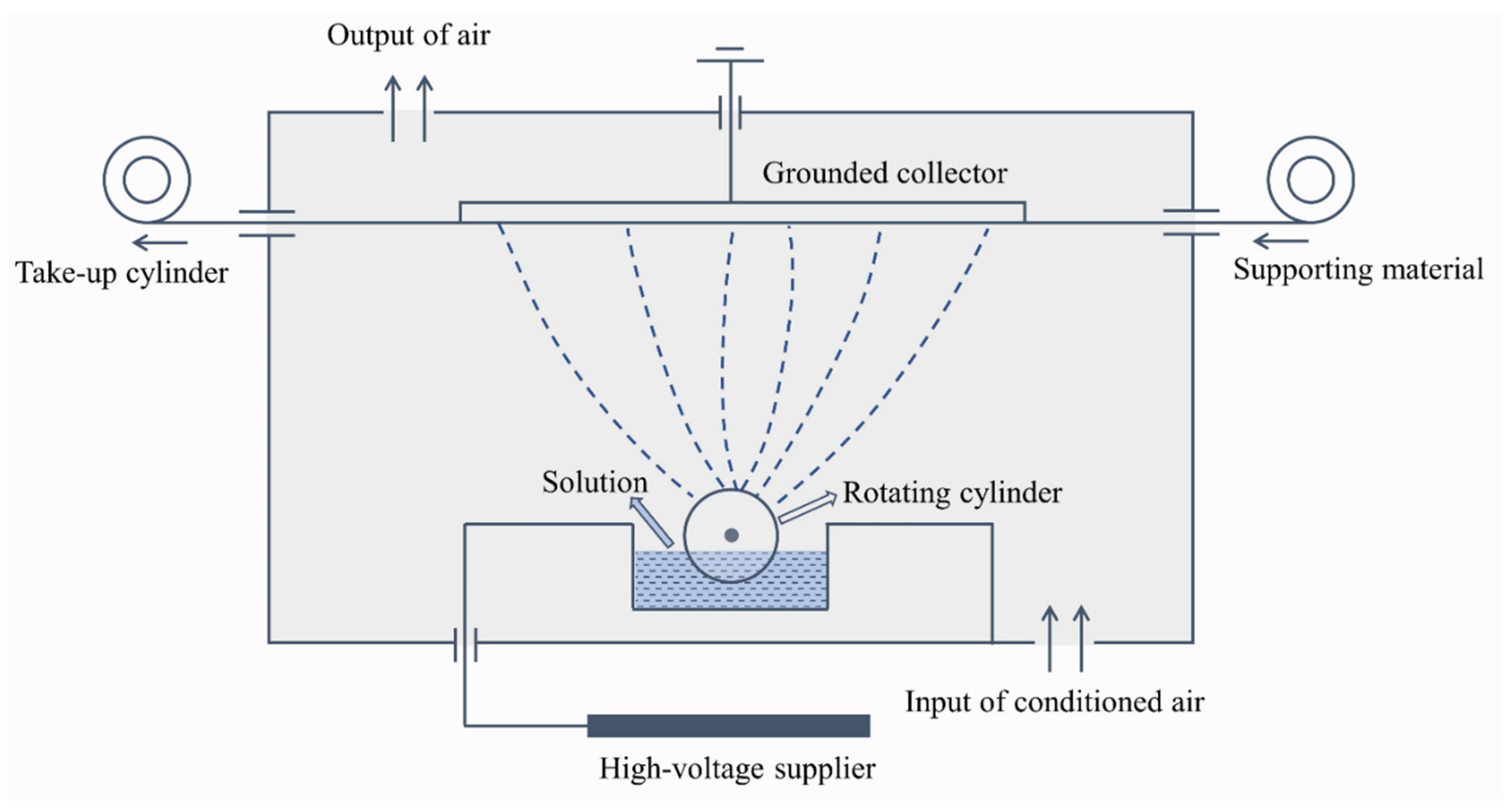
2.2. Fabrication of Electro-Sprayed PTFE Embedded with Aerogel/PCM
3. Characterization
3.1. SEM-EDX
3.2. Particle Size Distribution
3.3. Thickness and Areal Density of the Materials
3.4. Air Permeability
3.5. Thermal Properties from Alambeta
3.6. Water Vapor Permeability
4. Results and Discussion
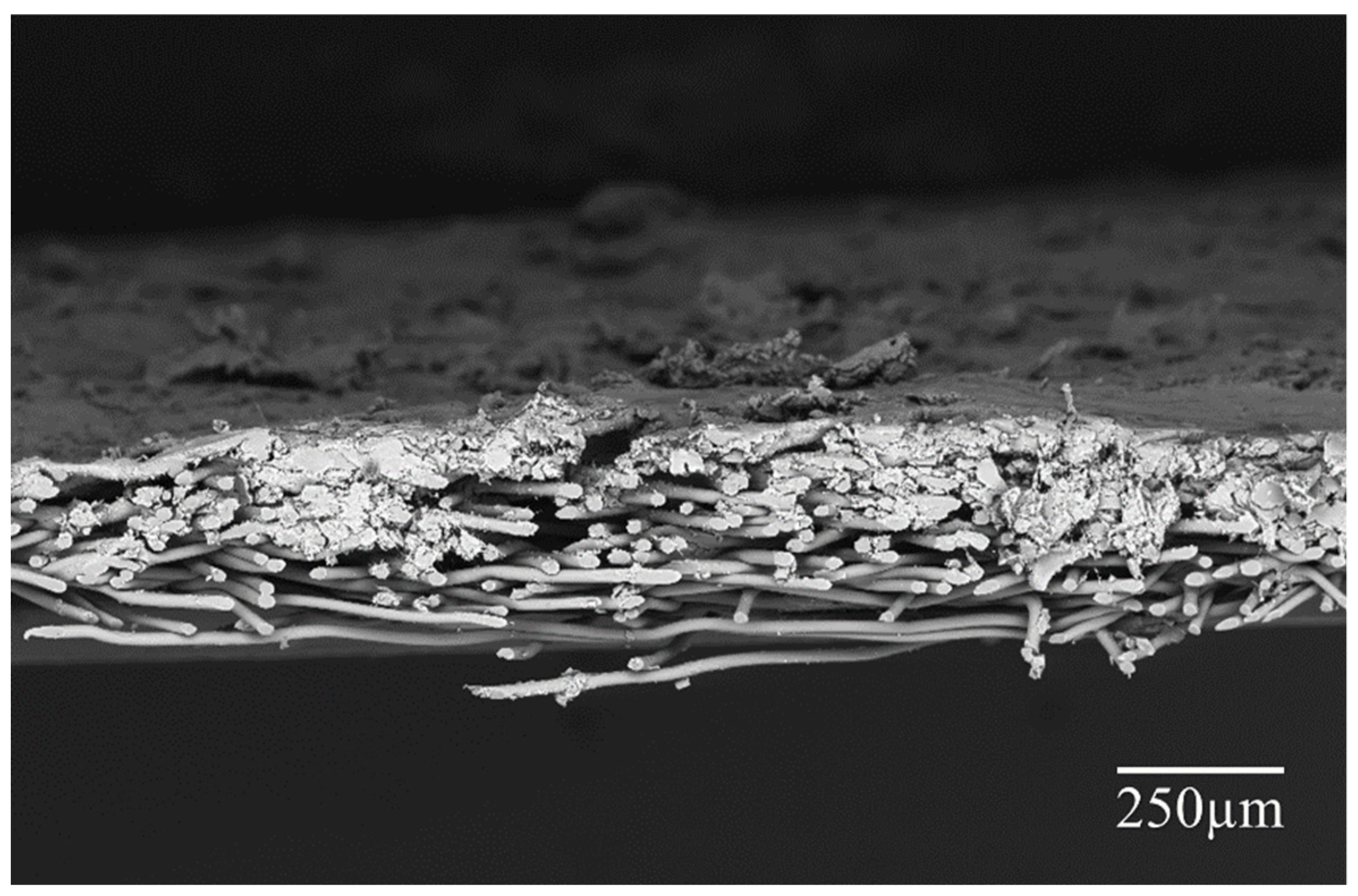
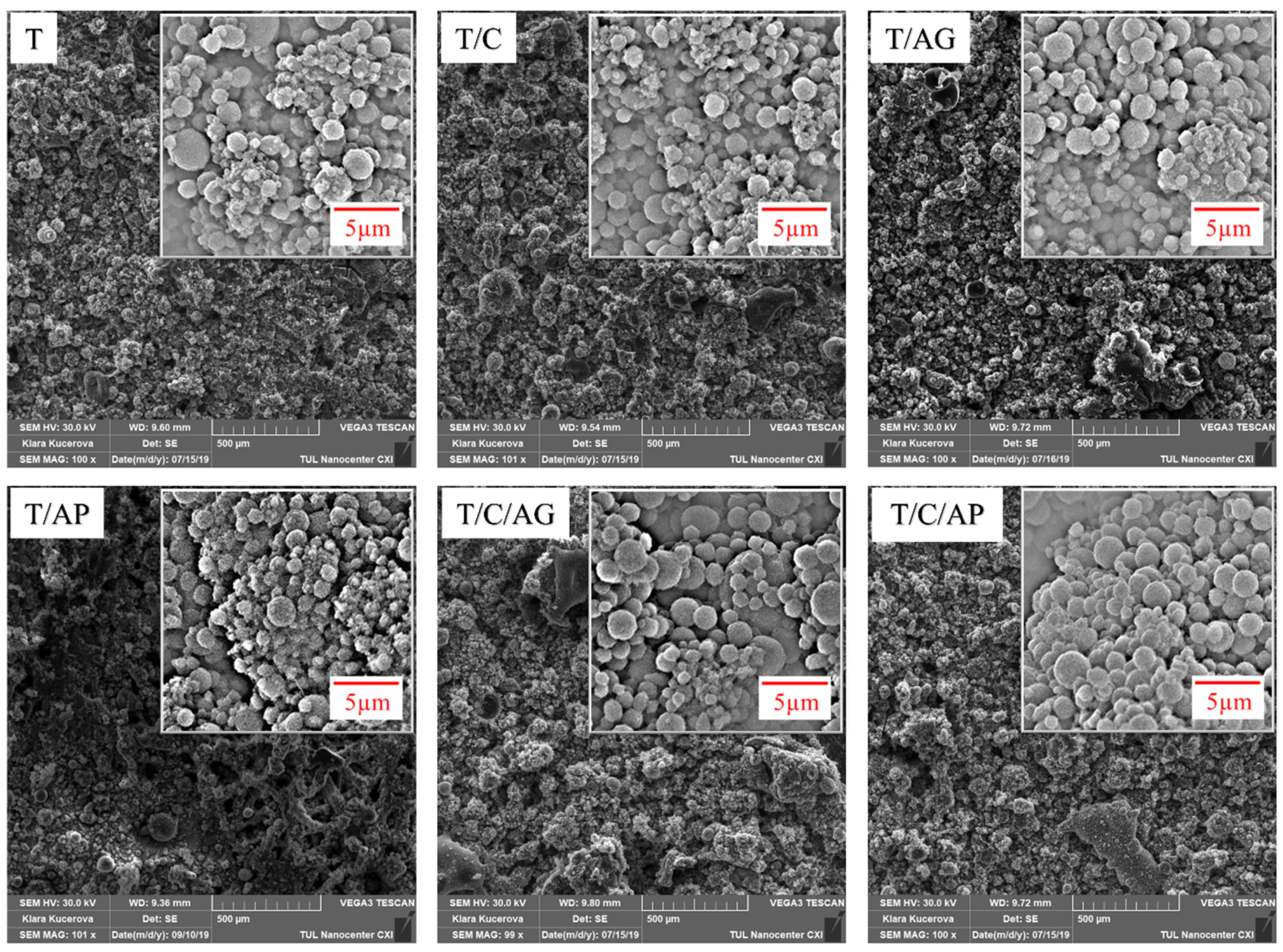
4.1. SEM Analysis of the Electro-Sprayed Materials
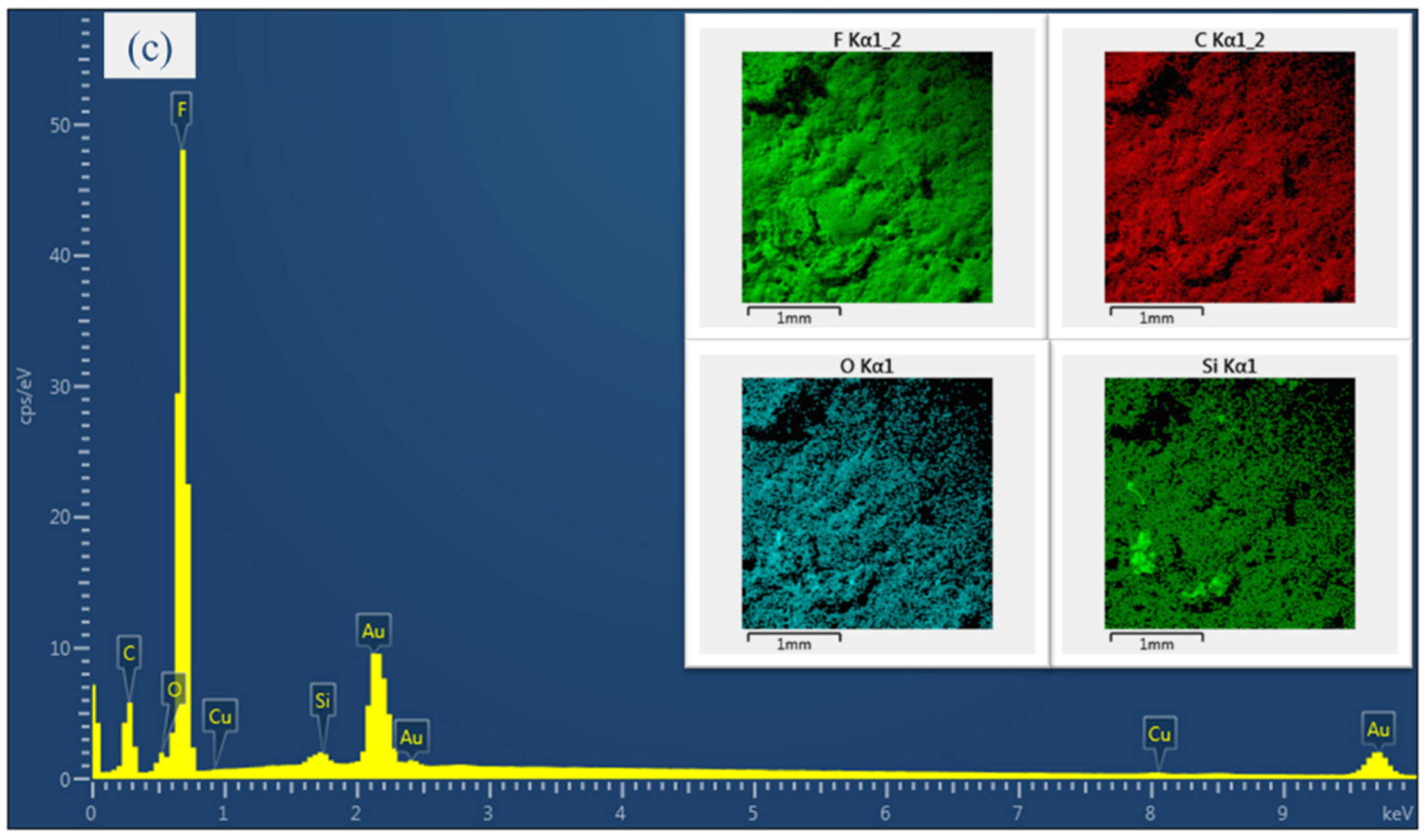
4.2. EDX Analysis of the Electro-Sprayed Materials
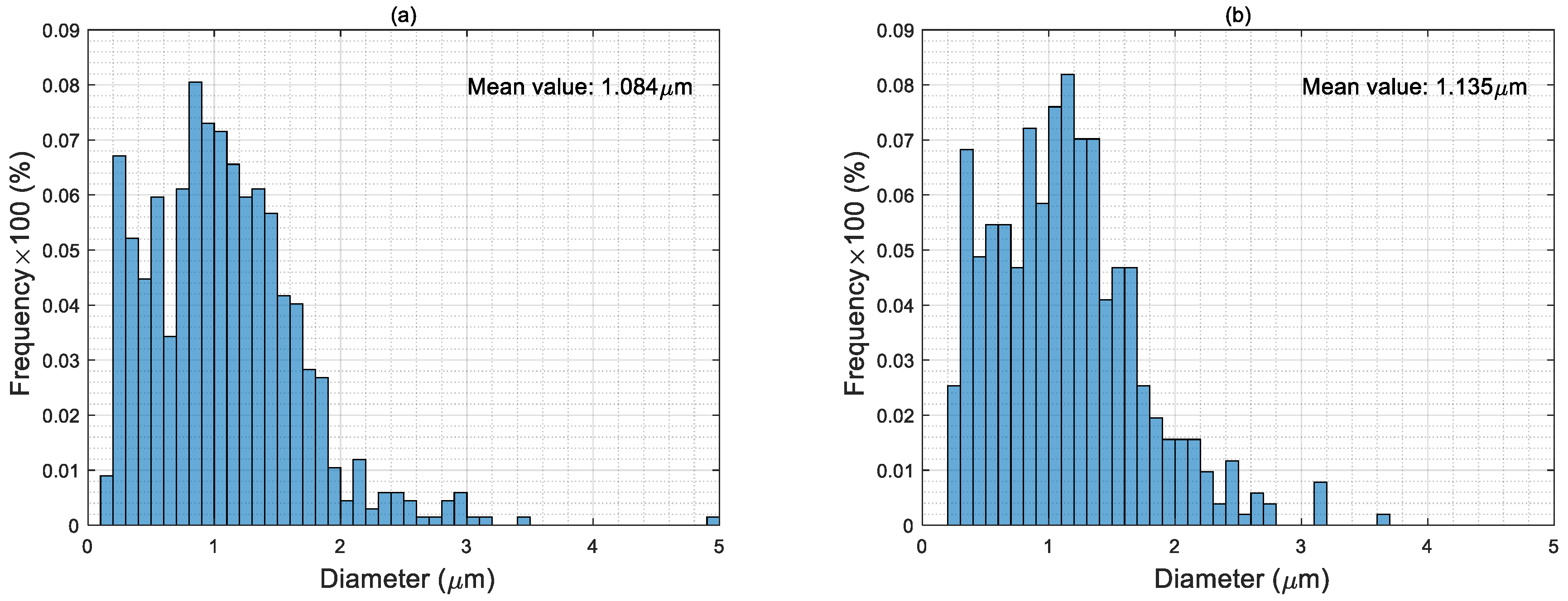
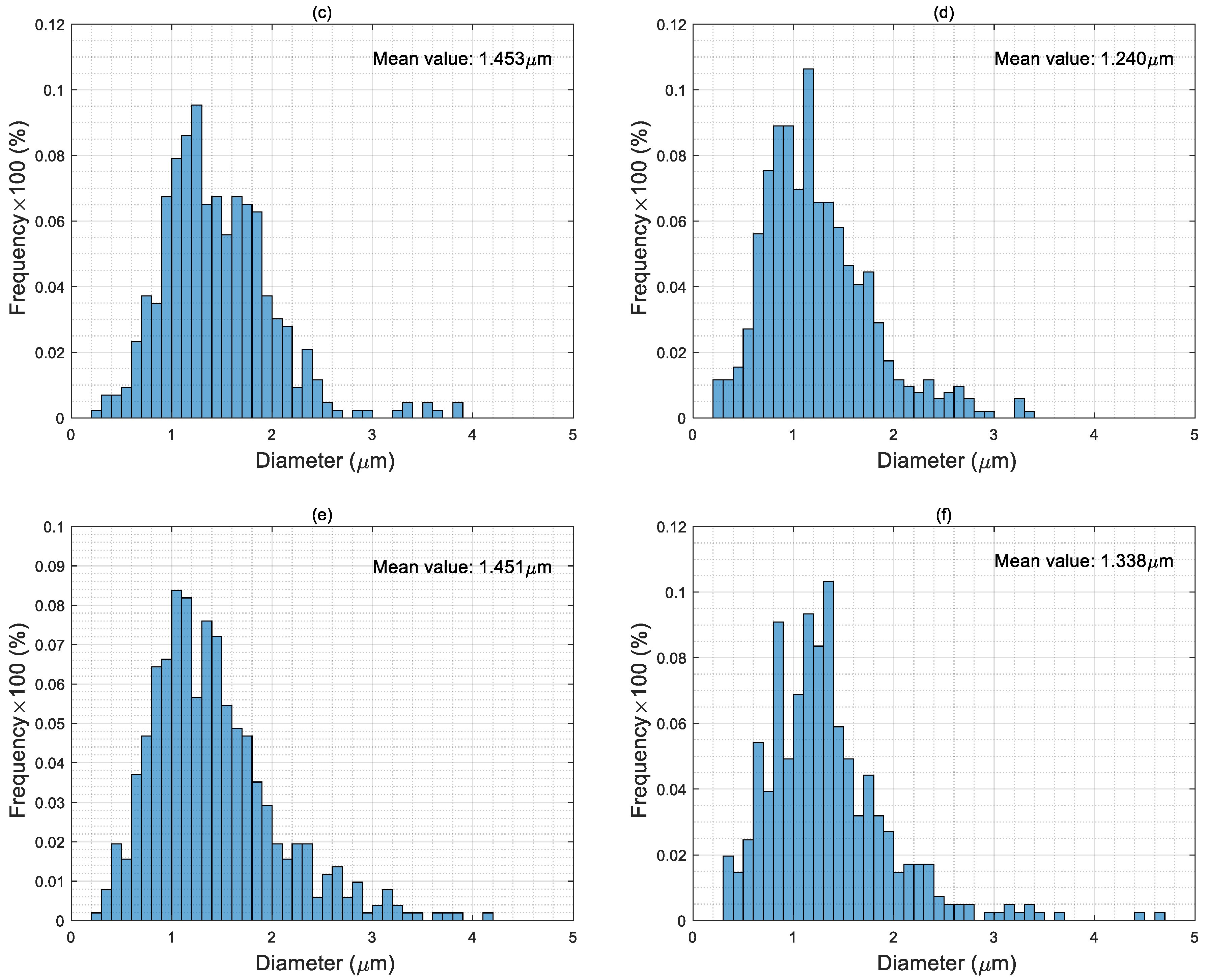
4.3. Particle Size Distribution of Electro-Sprayed Materials
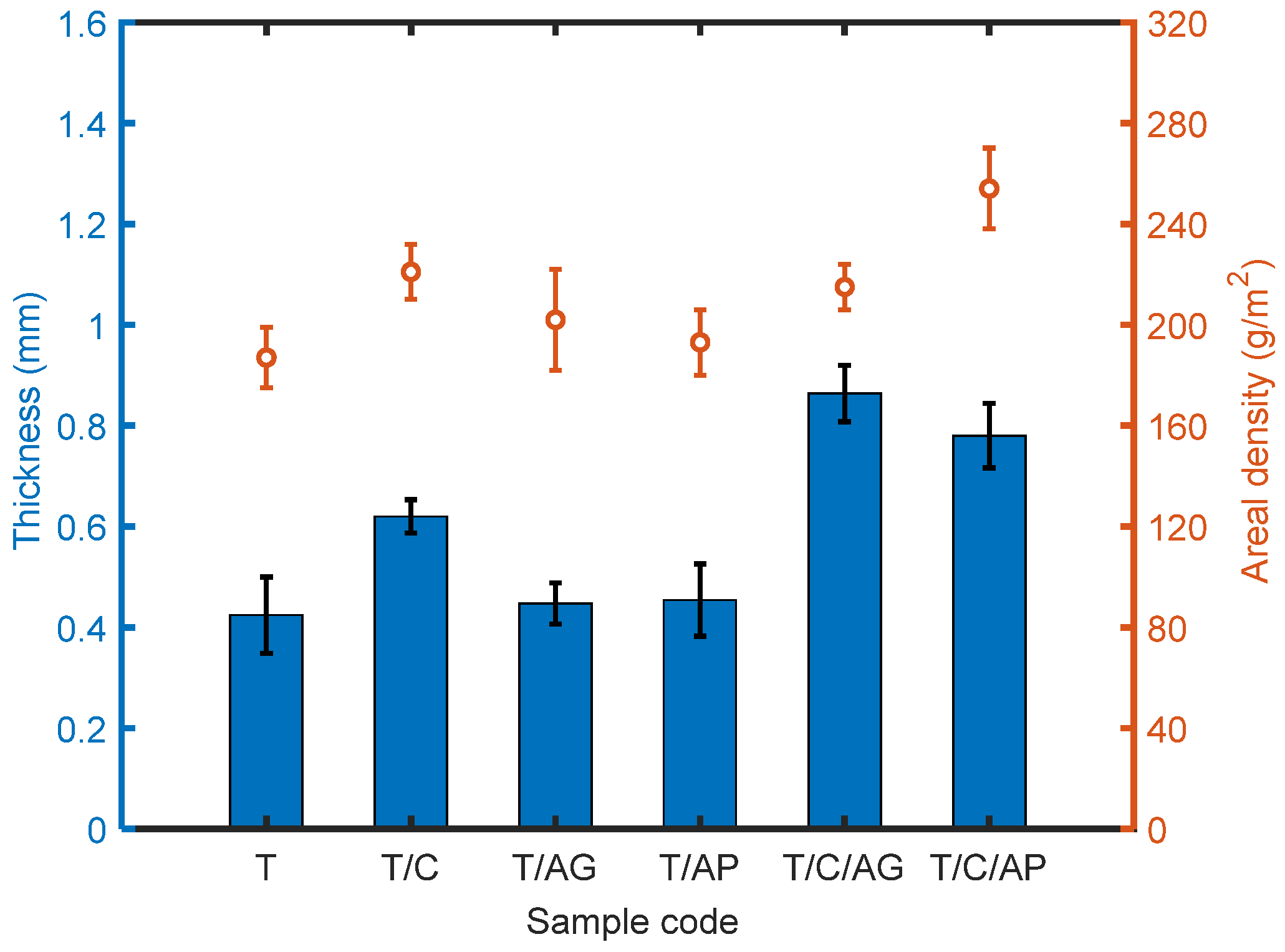
4.4. Effect of Aerogels and PCMs on Thickness and Areal Density of the Materials
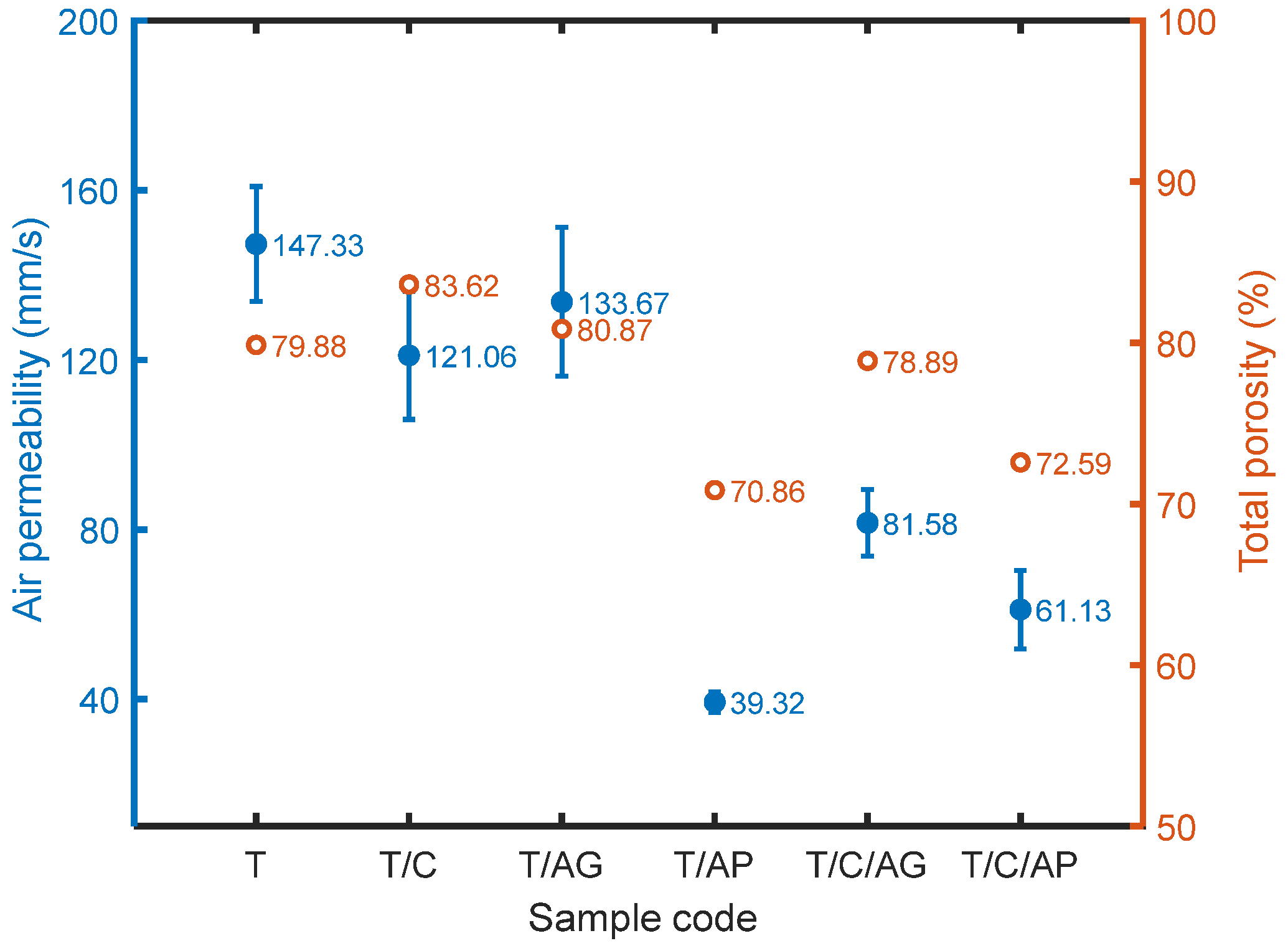
4.5. Effect of Aerogels and PCMs on Air Permeability
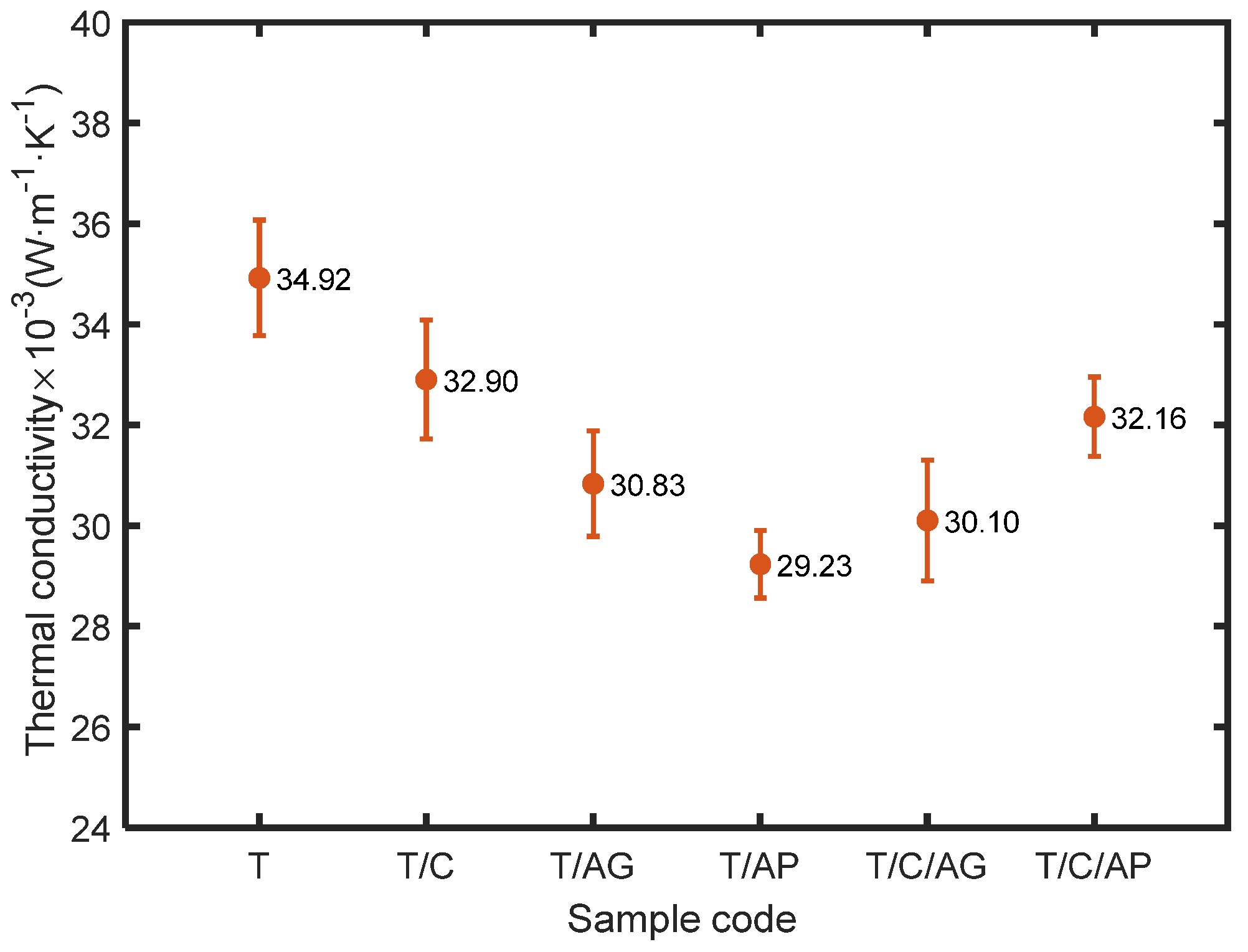
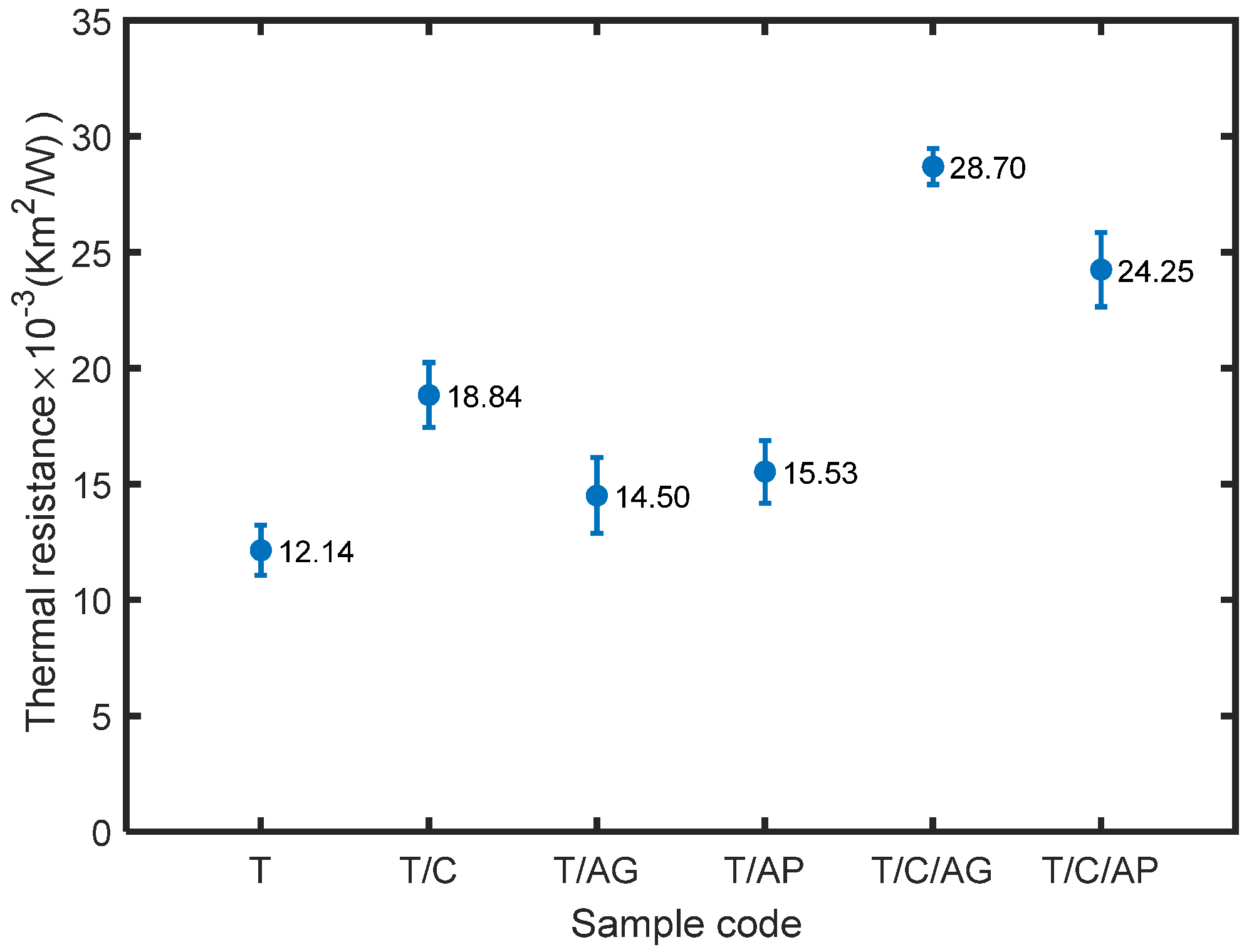
4.6. Effect of Aerogels and PCMs on Thermal Comfort Properties
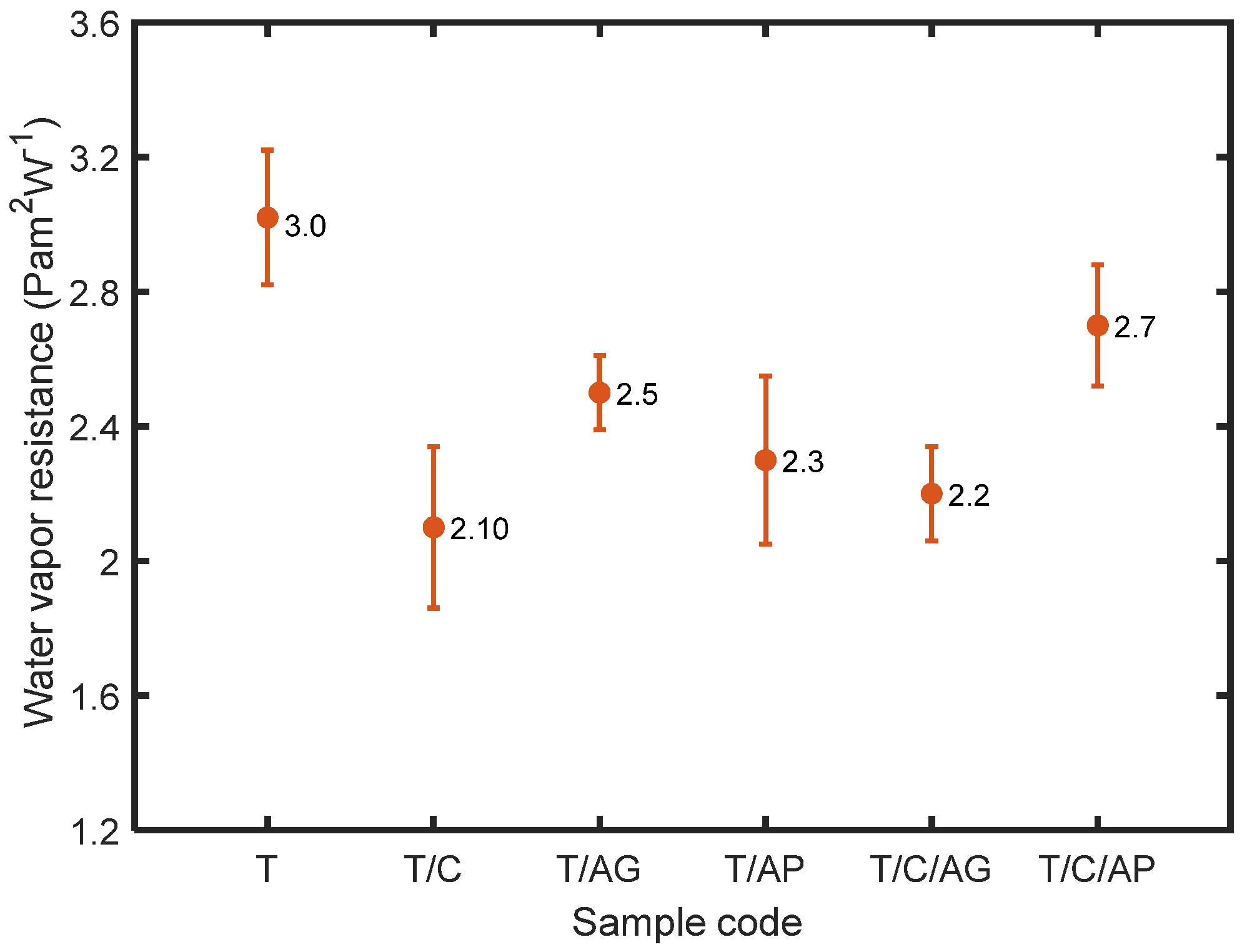
4.7. Effect of Aerogels and PCMs on Water Vapor Transmission
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and perspectives in PTFE membrane: Preparation, modification, and applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Brown, N.A.; Zhu, Y.; German, G.K.; Yong, X.; Chiarot, P.R. Electrospray deposit structure of nanoparticle suspensions. J. Electrost. 2017, 90, 67–73. [Google Scholar] [CrossRef]
- Vail, J.R.; Krick, B.A.; Marchman, K.R.; Sawyer, W.G. Polytetrafluoroethylene (PTFE) fiber reinforced polyetheretherketone composites. Wear 2011, 270, 737–741. [Google Scholar] [CrossRef]
- Huang, S.; Chen, T.; Chen, H. Study on the composites of two sized silica filled in PTFE. J. Reinf. Plast. Compos. 2006, 25, 1053–1058. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lin, H.C.; Lee, Y.D. The effects of filler content and size on the properties of PTFE/SiO2 composites. J. Polym. Res. 2003, 10, 247–258. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 243–4265. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Shen, Y.; Yuan, Q.; Wang, C.; Shi, L. Preparation and characterization of silica aerogel/polytetrafluoroethylene composites. Mater. Res. Express 2019, 6, 115021. [Google Scholar] [CrossRef]
- Rosace, G.; Guido, E.; Colleoni, C.; Barigozzi, G. Influence of textile structure and silica-based finishing on thermal insulation properties of cotton fabrics. Int. J. Polym. Sci. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Shaid, A.; Fergusson, M.; Wang, L. Thermo-physiological comfort analysis of aerogel nanoparticle incorporated fabric for fire fighter’s protective clothing. Chem. Mater. Eng. 2014, 2, 37–43. [Google Scholar]
- Xiong, X.; Venkataraman, M.; Jašíková, D.; Yang, T.; Mishra, R.; Militký, J.; Petrů, M. An experimental evaluation of convective heat transfer in multi-layered fibrous materials composed by different middle layer structures. J. Ind. Text. 2019. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, T.; Mishra, R.; Kanai, H.; Militky, J. Thermal and compression characteristics of aerogel-encapsulated textiles. J. Ind. Text. 2017, 7, 1998–2013. [Google Scholar] [CrossRef]
- Venkataraman, M.; Mishra, R.; Militky, J.; Xiong, X.; Marek, J.; Yao, J.; Zhu, G. Electrospun nanofibrous membranes embedded with aerogel for advanced thermal and transport properties. Polym. Adv. Technol. 2018, 29, 2583–2592. [Google Scholar] [CrossRef]
- Shaid, A.; Wang, L.; Padhye, R. The thermal protection and comfort properties of aerogel and PCM-coated fabric for firefighter garment. J. Ind. Text. 2016, 45, 611–625. [Google Scholar] [CrossRef]
- Cardoso, I.; Gomes, J.R. The application of microcapsules of PCM in flame resistant nonwoven materials. Int. J. Cloth. Sci. Technol. 2009, 21, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Feng, Q.; Liu, R.; Yu, B.; Zhou, Y. Enhancing the thermal protective performance of firefighters’ protective fabrics by incorporating phase change materials. Fibres Text. East. Eur. 2015, 23, 68–73. [Google Scholar]
- Hu, Y.; Huang, D.; Qi, Z.; He, S.; Yang, H.; Zhang, H. Modeling thermal insulation of firefighting protective clothing embedded with phase change material. Heat Mass Transf. 2013, 49, 567–573. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Q.; Liu, H.; Zhang, C.; You, Y.; Li, N.; Xiao, C. Preparation, characterization, and applications of electrospun ultrafine fibrous PTFE porous membranes. J. Membr. Sci. 2017, 523, 317–326. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, Y.; Gao, S.; Zhang, M.; Xiao, C. Novel Ultrafine Fibrous Poly(tetrafluoroethylene)Hollow Fiber Membrane Fabricated by Electrospinning. Polymers 2018, 10, 464. [Google Scholar] [CrossRef] [Green Version]
- Aderikha, V.N.; Shapovalov, V.A. Tribological behavior of polytetrafluoroethylene-silica composites. J. Frict. Wear 2011, 32, 124–132. [Google Scholar] [CrossRef]
- Basu, B.J.; Kumar, V.D. Fabrication of superhydrophobic nanocomposite coatings using polytetrafluoroethylene and silica nanoparticles. Int. Sch. Res. Not. 2011. [Google Scholar] [CrossRef] [Green Version]
- Burkarter, E.; Saul, C.K.; Thomazi, F.; Cruz, N.C.; Zanata, S.M.; Roman, L.S.; Schreiner, W.H. Superhydrophobic electrosprayed PTFE: A non-contaminating surface. J. Phys. D Appl. Phys. 2007, 40, 7778–7781. [Google Scholar] [CrossRef]
- Burkarter, E.; Saul, C.K.; Thomazi, F.; Cruz, N.C.; Roman, L.S.; Schreiner, W.H. Superhydrophobic electrosprayed PTFE. Surf. Coat. Technol. 2007, 202, 194–198. [Google Scholar] [CrossRef]
- Venkataraman, M.; Yang, K.; Xiong, X.; Militky, J.; Kremenakova, D.; Zhu, G.; Yao, J.; Wang, Y.; Zhang, G. Preparation of electrosprayed, microporous particle filled Layers. Polymers 2020, 12, 1352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cai, C.; Wang, Y.; Liu, G.; Zhou, L.; Yao, J.; Militky, J.; Marek, J.; Venkataraman, M.; Zhu, G. Preparation and evaluation of thermo-regulating bamboo fabric treated by microencapsulated phase change materials. Text. Res. J. 2019, 89, 3387–3393. [Google Scholar] [CrossRef]
- Costa, L.M.M.; Bretas, R.E.S.; Gregorio, R. Effect of solution concentration on the electrospray/electrospinning transition and on the crystalline phase of PVDF. Mater. Sci. Appl. 2010, 1, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Dolezal, I.; Hes, L.; Bal, K. A non-destructive single plate method for measurement of thermal resistance of polymer sheets and fabrics. Int. J. Occup. Saf. Ergon. 2019, 25, 562–567. [Google Scholar] [CrossRef]
- Lenggoro, I.W.; Xia, B.; Okuyama, K. Sizing of colloidal nanoparticles by electrospray and differential mobility analyzer methods. Langmuir 2002, 18, 4584–4591. [Google Scholar] [CrossRef]
- Gomez, A.; Tang, K. Charge and fission of droplets in electrostatic sprays. Phys. Fluids 1994, 6, 404–414. [Google Scholar] [CrossRef]
- Gibson, P.W. Factors influencing steady-state heat and water vapor transfer measurements for clothing materials. Text. Res. J. 1993, 63, 749–764. [Google Scholar] [CrossRef]
- Swarbrick, J.; Amann, A.H.; Lindstrom, R.E. Factors affecting water vapor transmission through free polymer films. J. Pharm. Sci. 1972, 61, 1645–1647. [Google Scholar] [CrossRef]

| Properties | Aerogel Powder |
|---|---|
| Majority particle size range | 2~40 μm |
| Pore diameter | ~20 nm |
| Porosity | 90~95% |
| Surface area | 600~800 m2/g |
| Particle density | 120~150 kg/m3 |
| Thermal conductivity | 0.012 W m−1 K−1 at 25 °C |
| Sample Code | Component | Spinning Liquids Used for Electro-Spraying | |
|---|---|---|---|
| Basic Solution | Fillers | ||
| T | PTFE | TeflonTM PTFE 30 aqueous dispersion | None |
| T/C | PTFE + PCM | PCM (3 g/L) | |
| T/AG | PTFE + aerogel granule | Aerogel granule (3 g/L) | |
| T/AP | PTFE + aerogel powder | Aerogel powder (3 g/L) | |
| T/C/AG | PTFE + PCM +aerogel granule | PCM (3 g/L) + Aerogel granule (3 g/L) | |
| T/C/AP | PTFE + PCM + aerogel powder | PCM (3 g/L) + Aerogel powder (3 g/L) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Venkataraman, M.; Yang, T.; Kucerova, K.; Militký, J.; Yang, K.; Zhu, G.; Yao, J. Transport Properties of Electro-Sprayed Polytetrafluoroethylene Fibrous Layer Filled with Aerogels/Phase Change Materials. Nanomaterials 2020, 10, 2042. https://doi.org/10.3390/nano10102042
Xiong X, Venkataraman M, Yang T, Kucerova K, Militký J, Yang K, Zhu G, Yao J. Transport Properties of Electro-Sprayed Polytetrafluoroethylene Fibrous Layer Filled with Aerogels/Phase Change Materials. Nanomaterials. 2020; 10(10):2042. https://doi.org/10.3390/nano10102042
Chicago/Turabian StyleXiong, Xiaoman, Mohanapriya Venkataraman, Tao Yang, Klara Kucerova, Jiří Militký, Kai Yang, Guocheng Zhu, and Juming Yao. 2020. "Transport Properties of Electro-Sprayed Polytetrafluoroethylene Fibrous Layer Filled with Aerogels/Phase Change Materials" Nanomaterials 10, no. 10: 2042. https://doi.org/10.3390/nano10102042
APA StyleXiong, X., Venkataraman, M., Yang, T., Kucerova, K., Militký, J., Yang, K., Zhu, G., & Yao, J. (2020). Transport Properties of Electro-Sprayed Polytetrafluoroethylene Fibrous Layer Filled with Aerogels/Phase Change Materials. Nanomaterials, 10(10), 2042. https://doi.org/10.3390/nano10102042









