Recent Advances in the Development of Nano-Sculpted Films by Magnetron Sputtering for Energy-Related Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Characterization Techniques
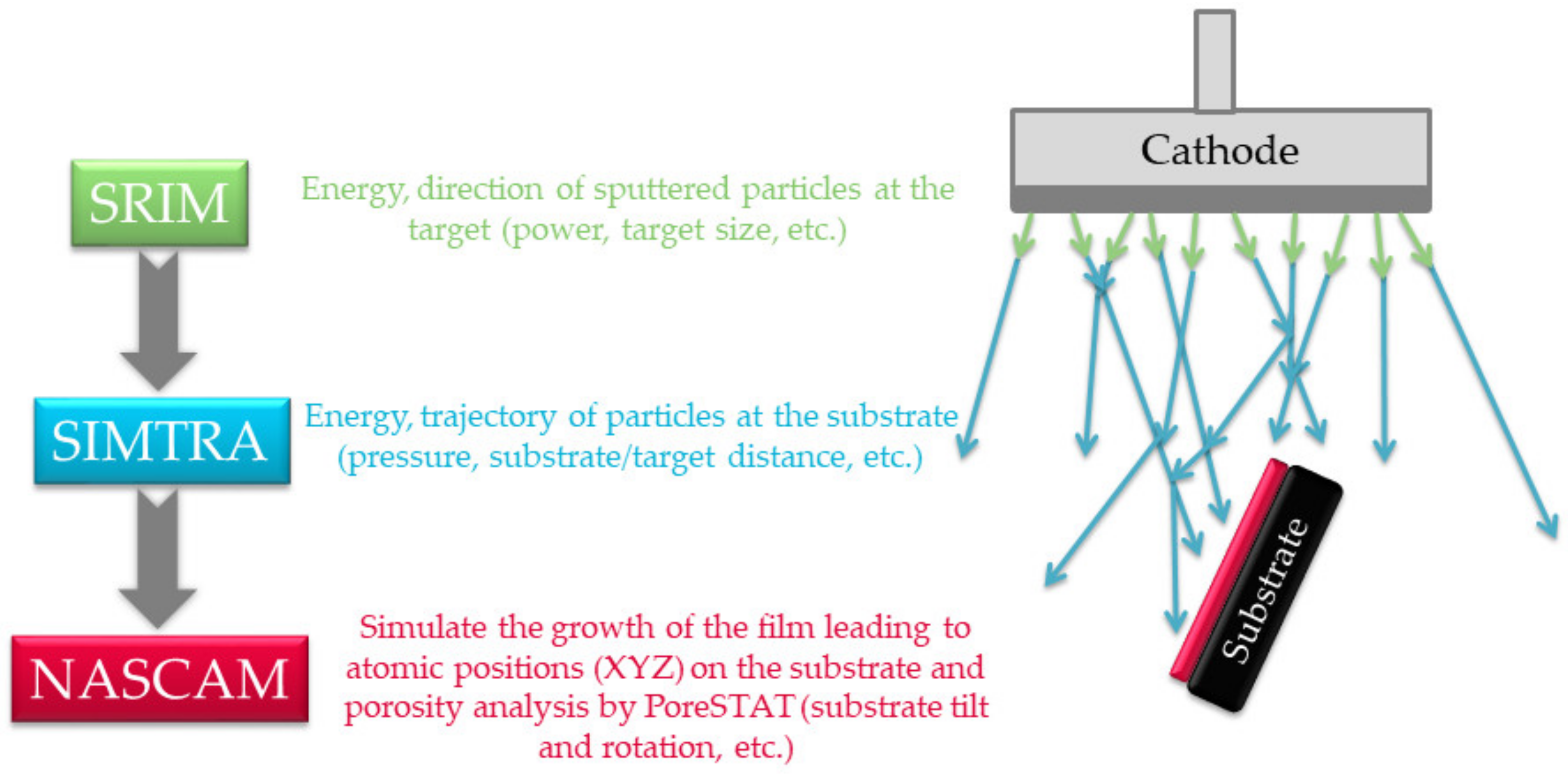
2.3. Simulation Protocol
3. Results and Discussion
3.1. Growth Mechanisms of Metallic Films
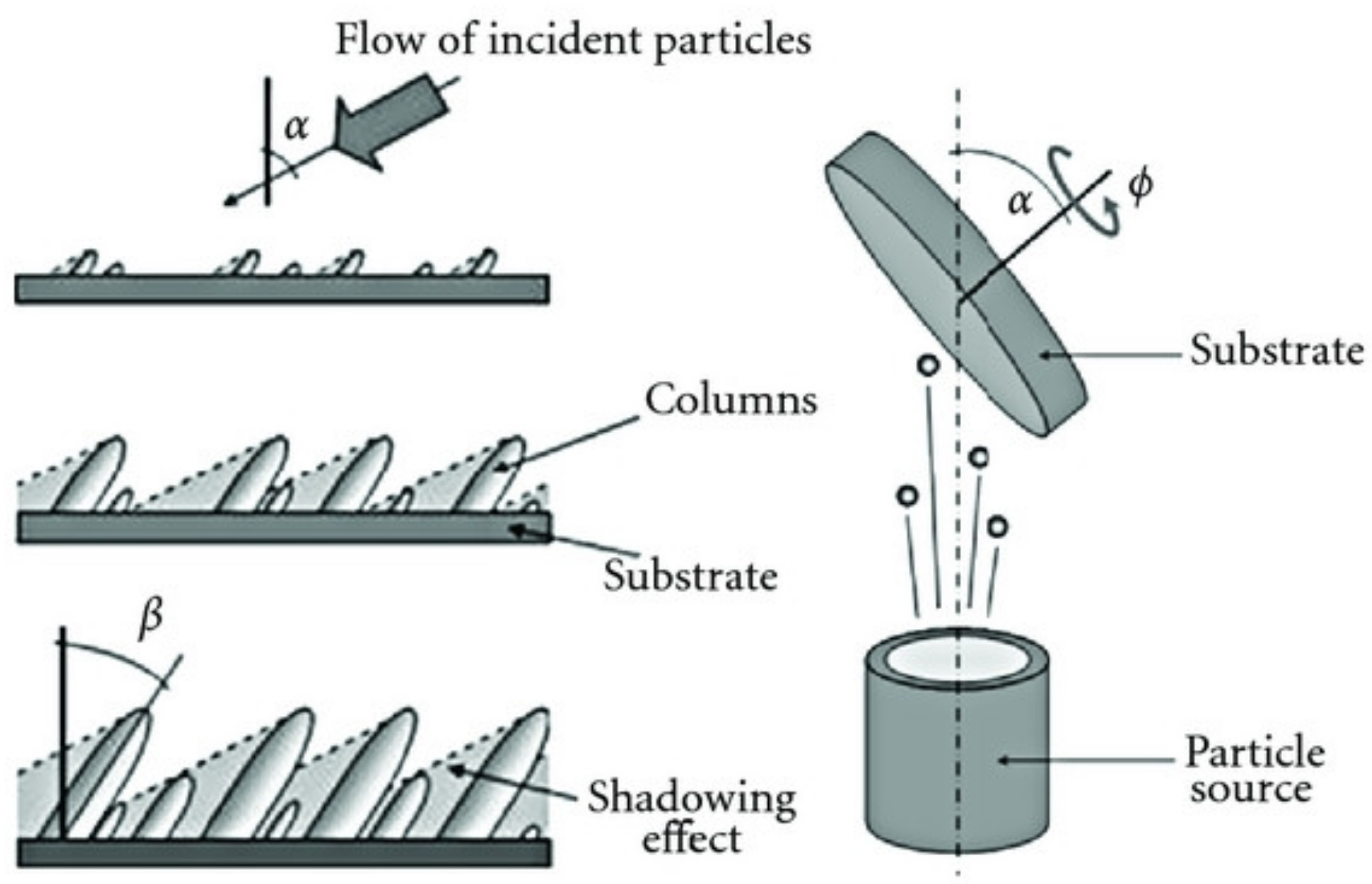
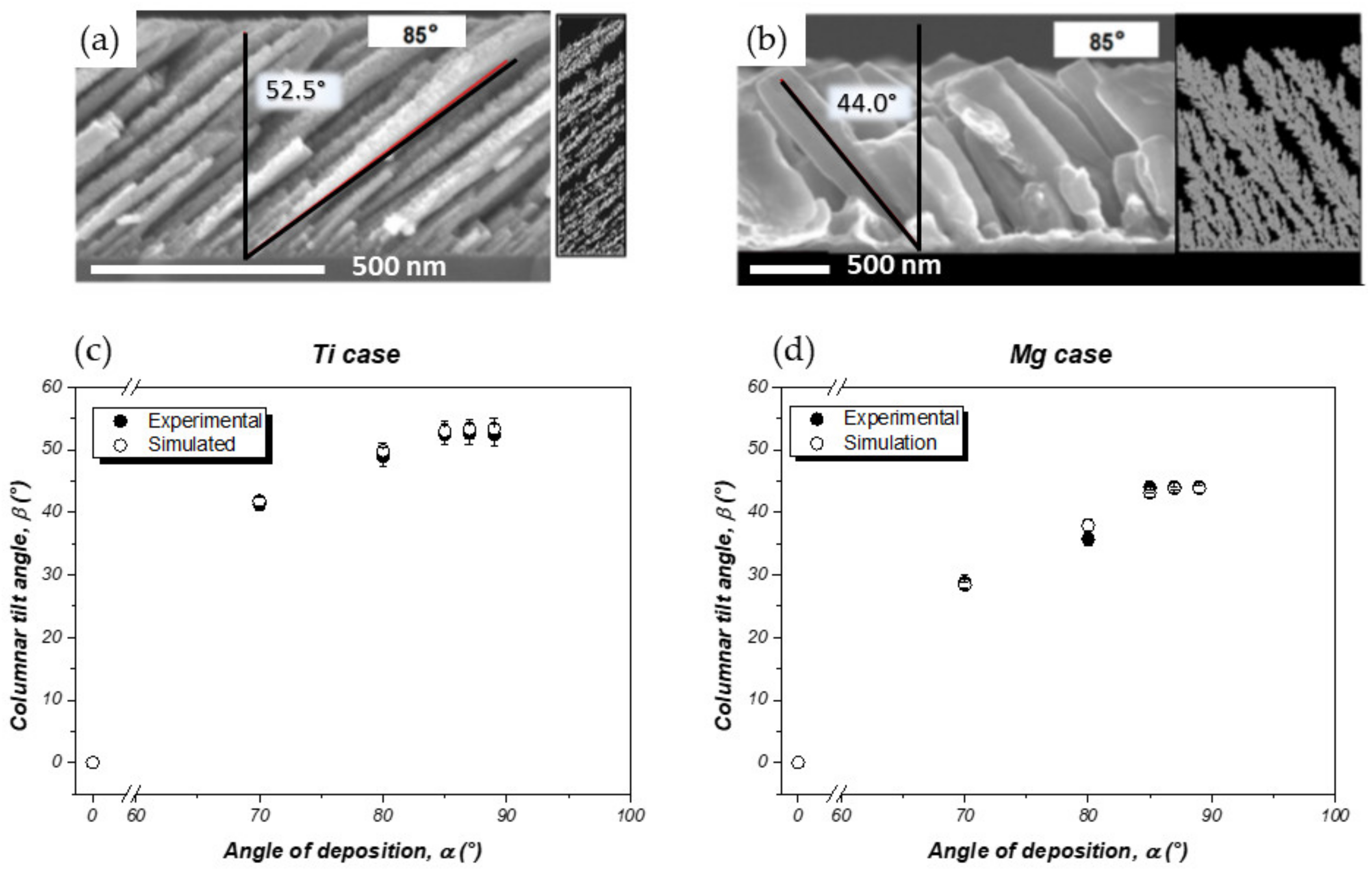
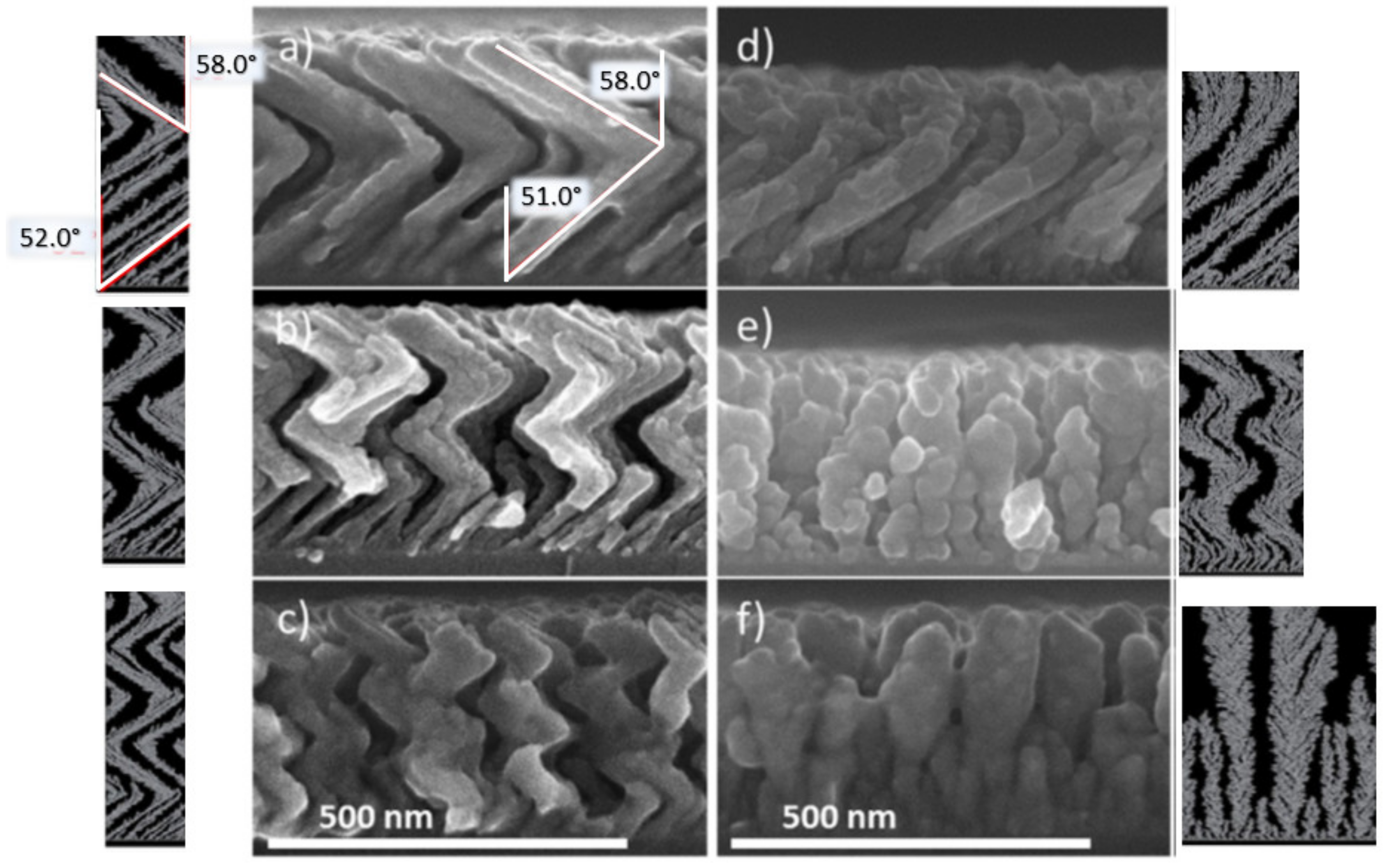
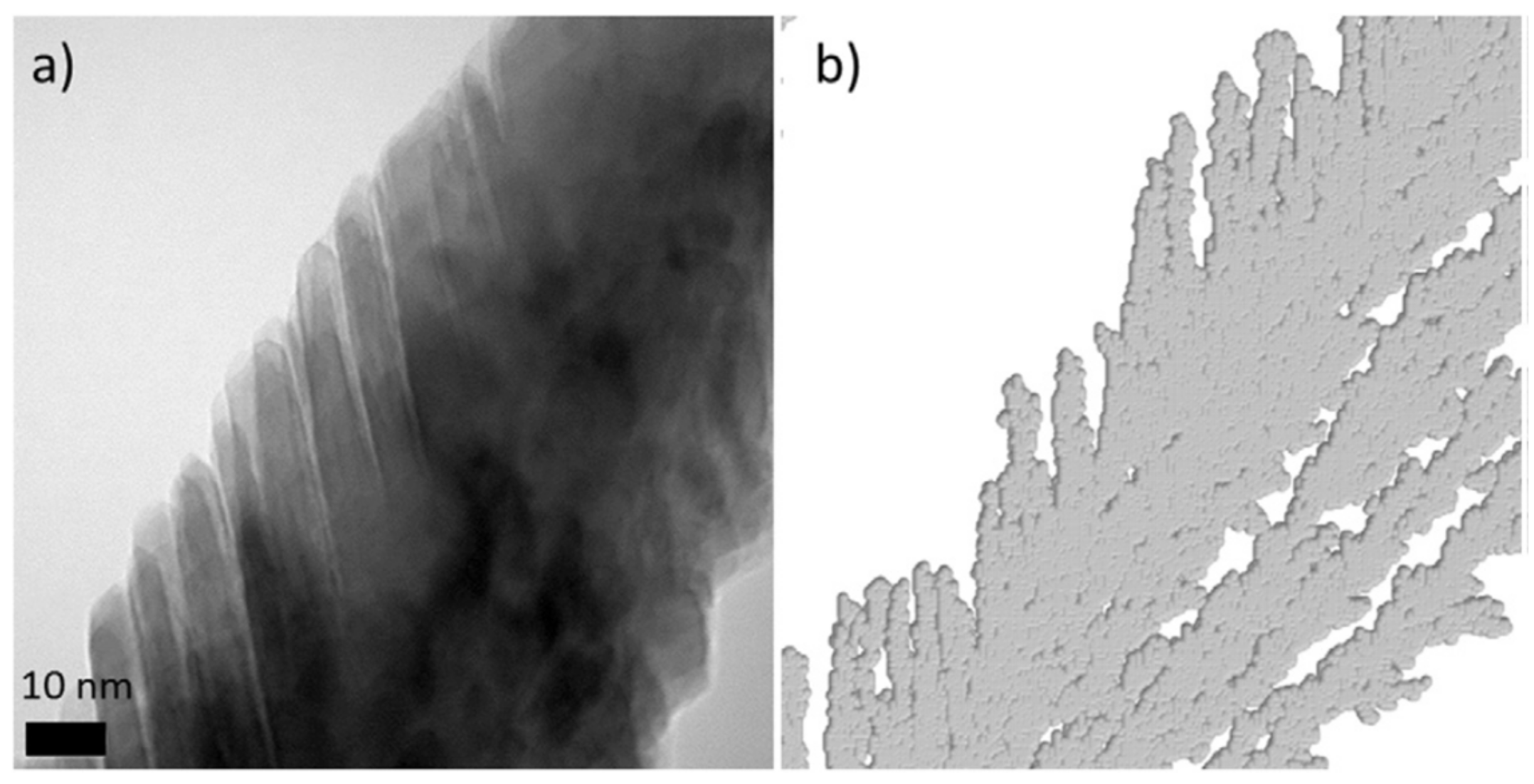
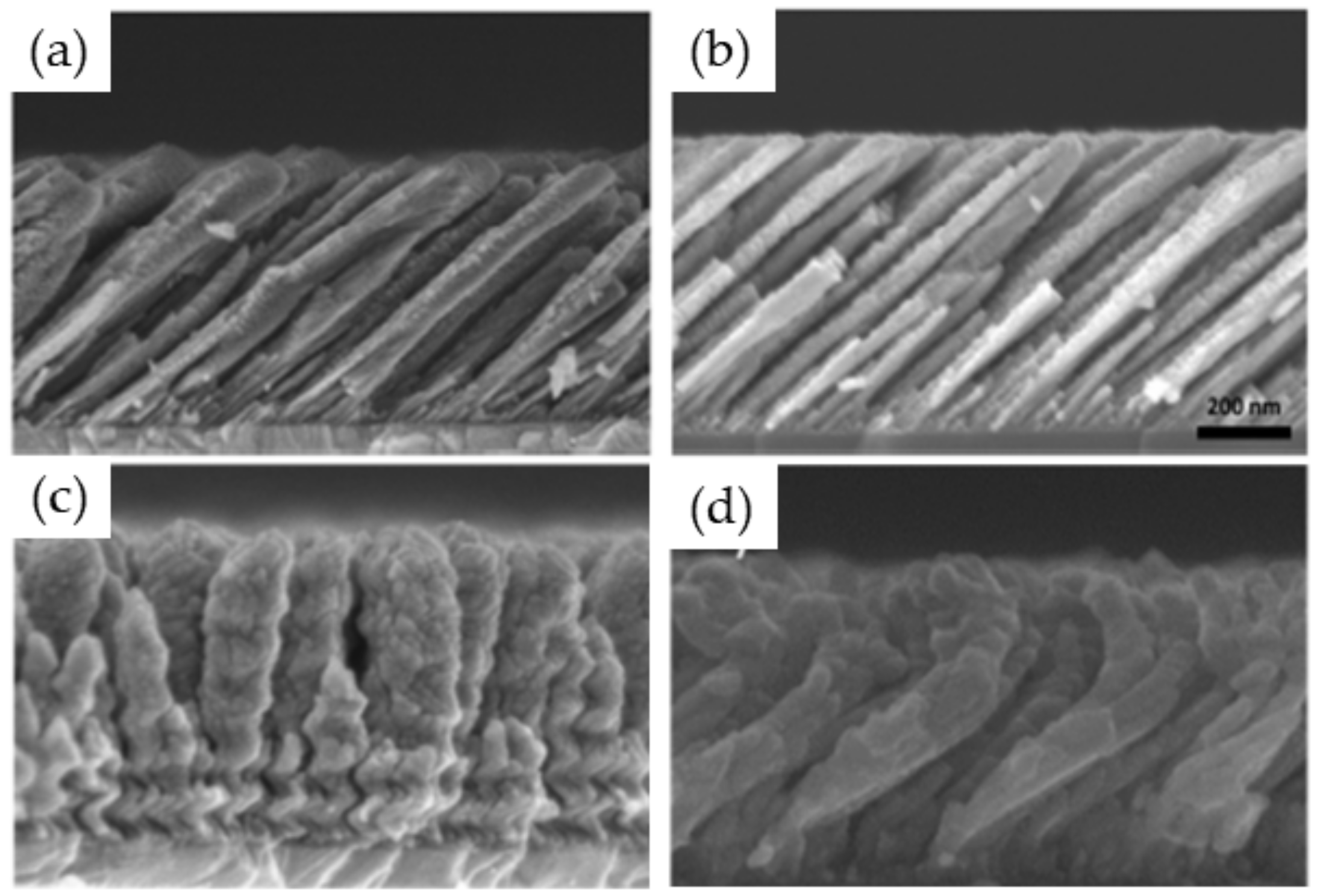
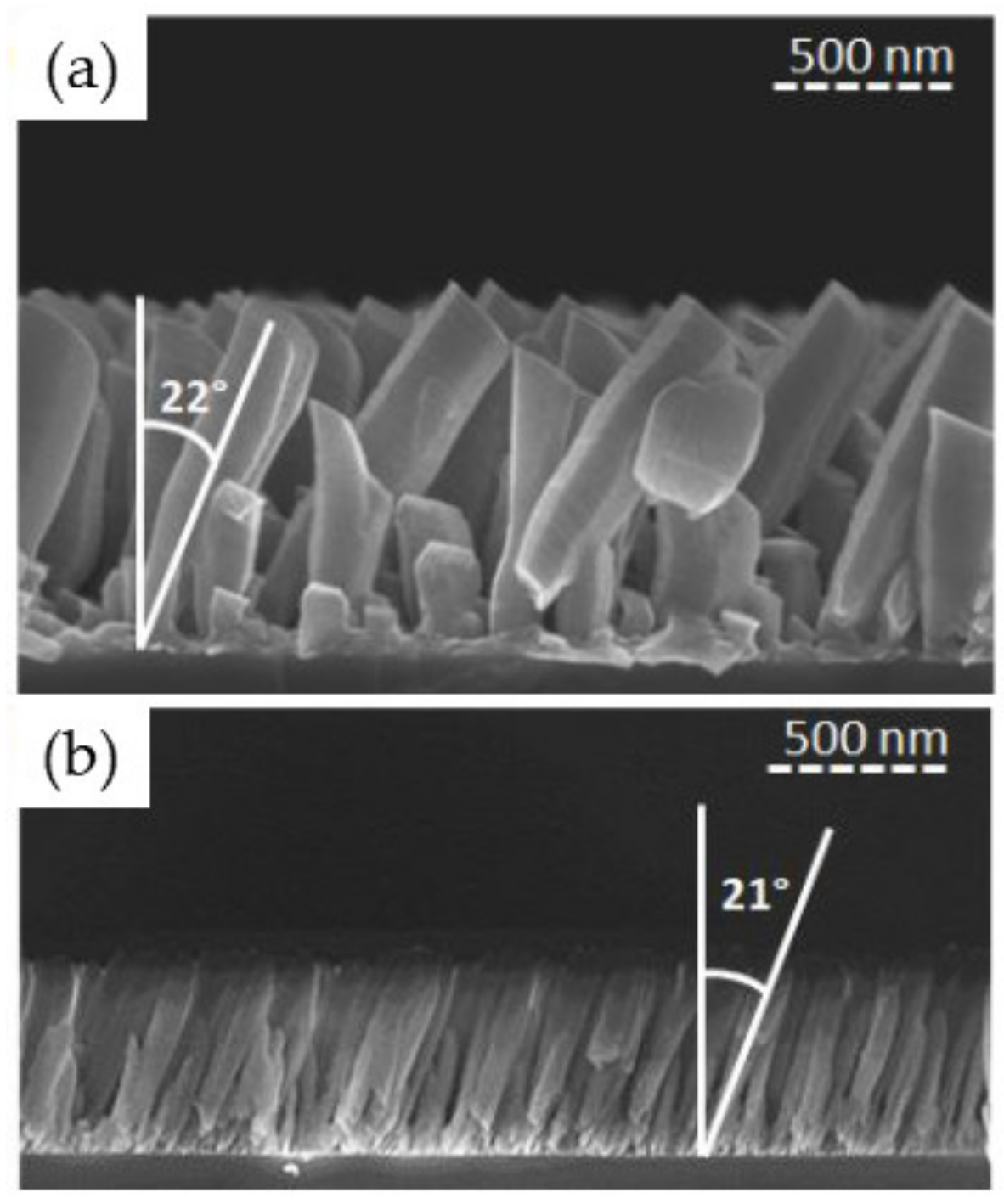
3.1.1. Effect of the Angle of Deposition
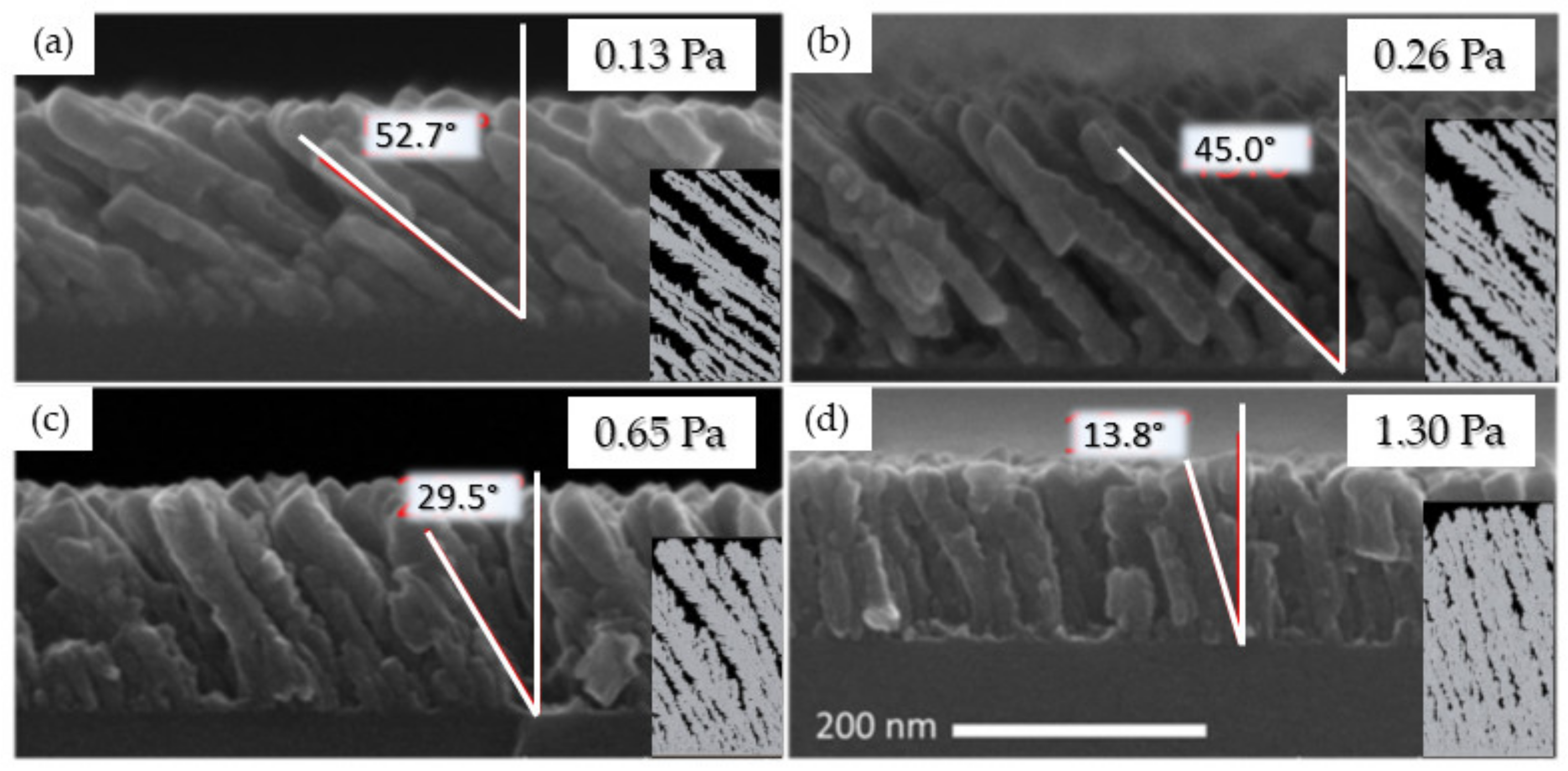
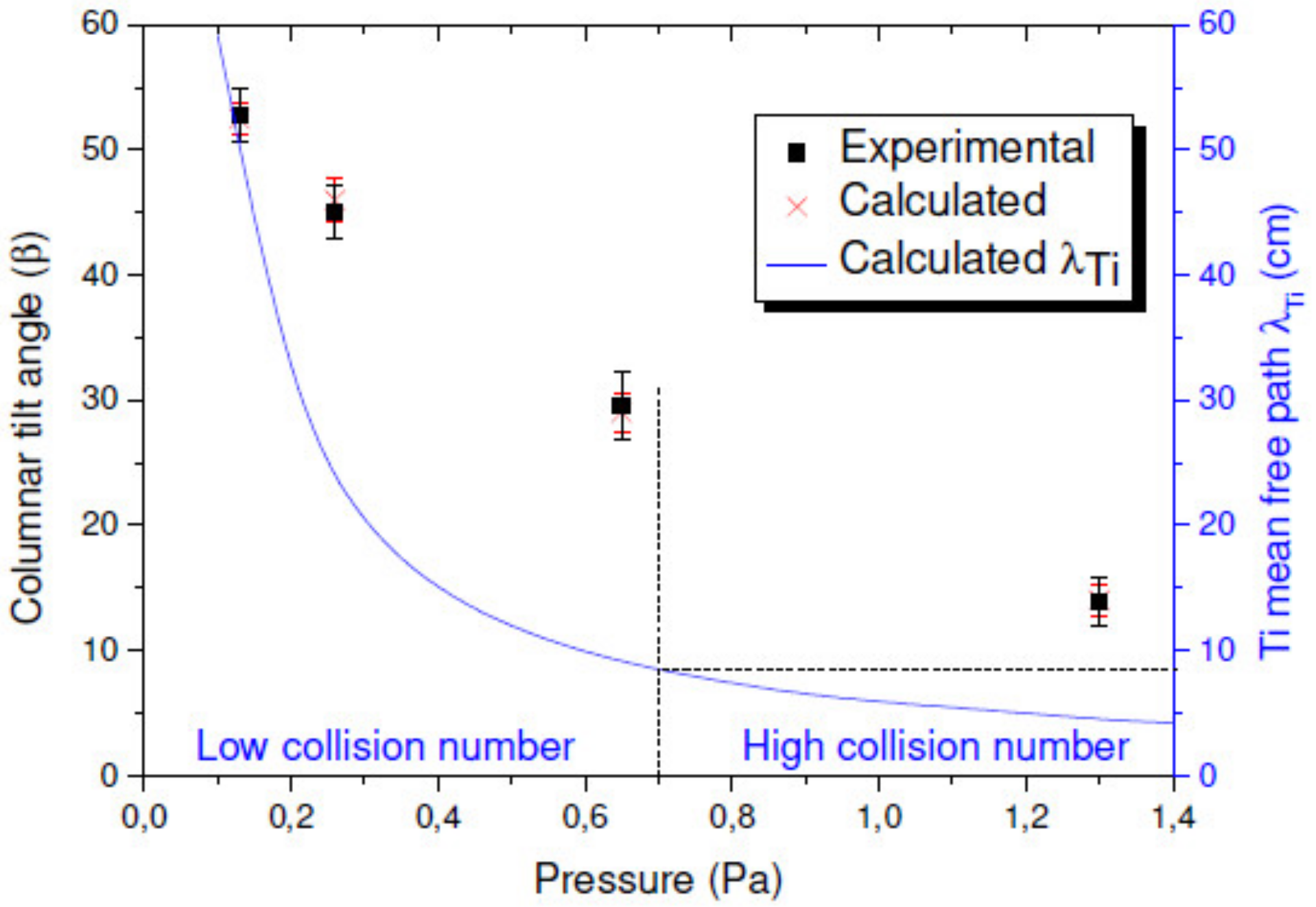
3.1.2. Effect of the Deposition Pressure
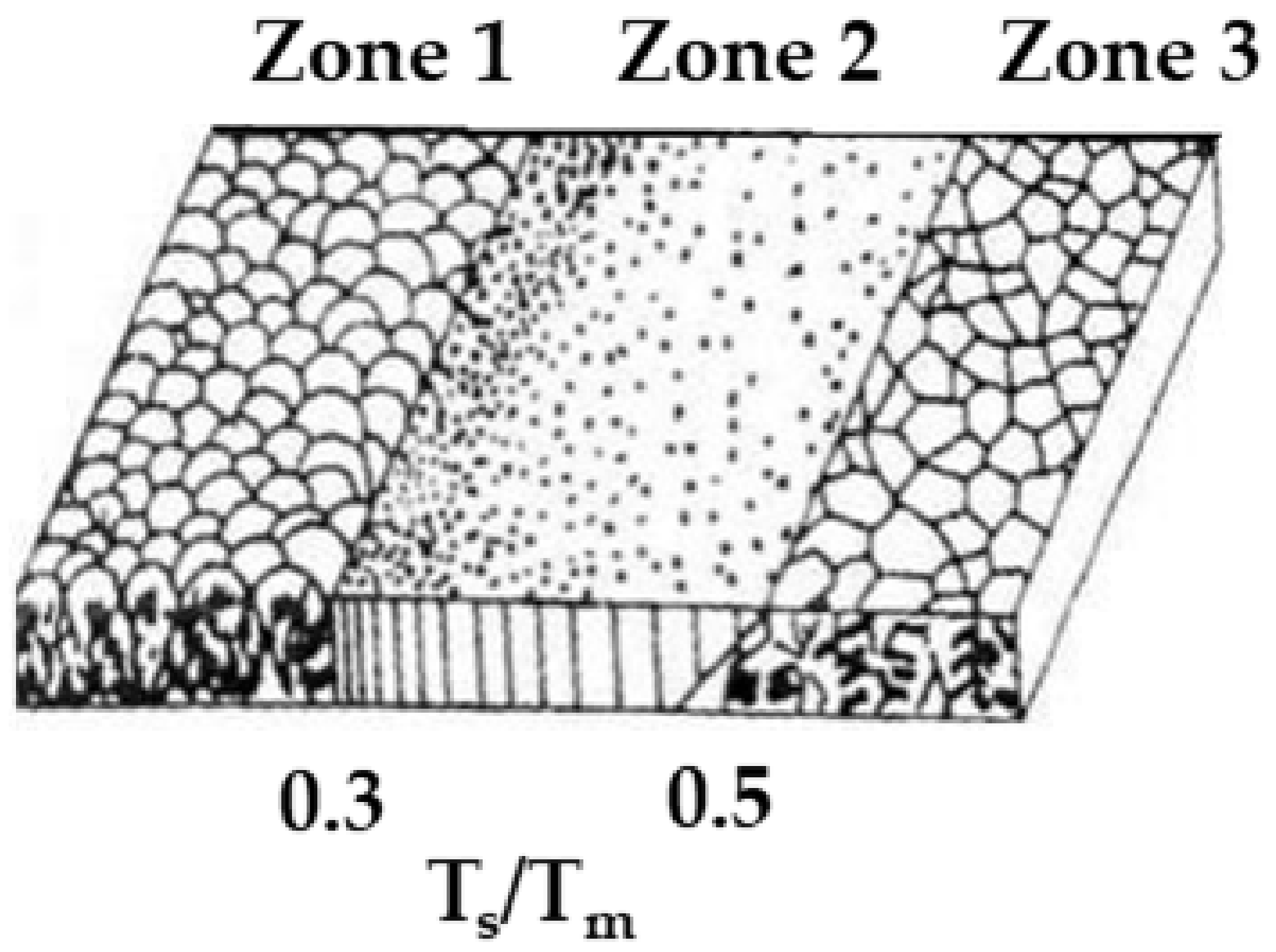
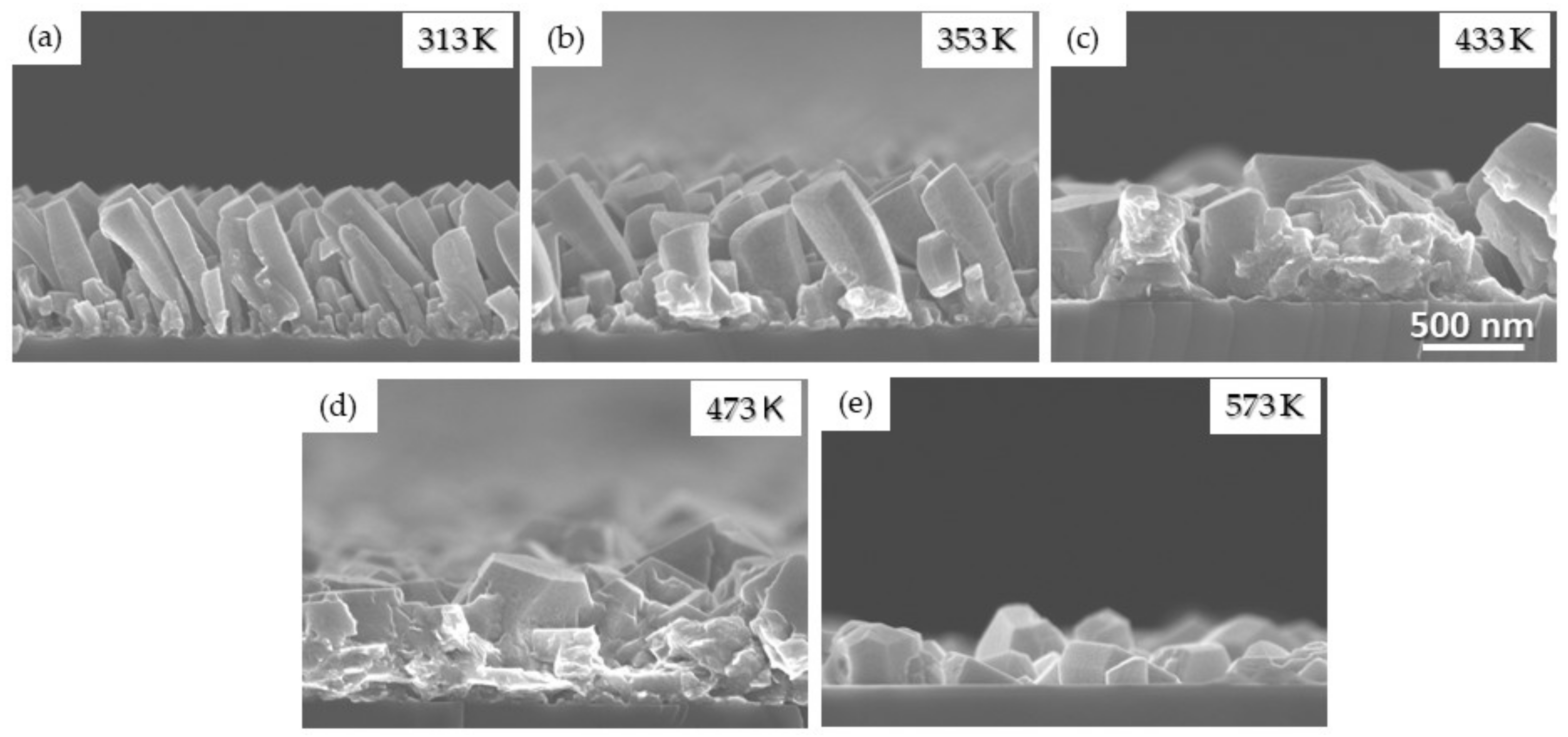
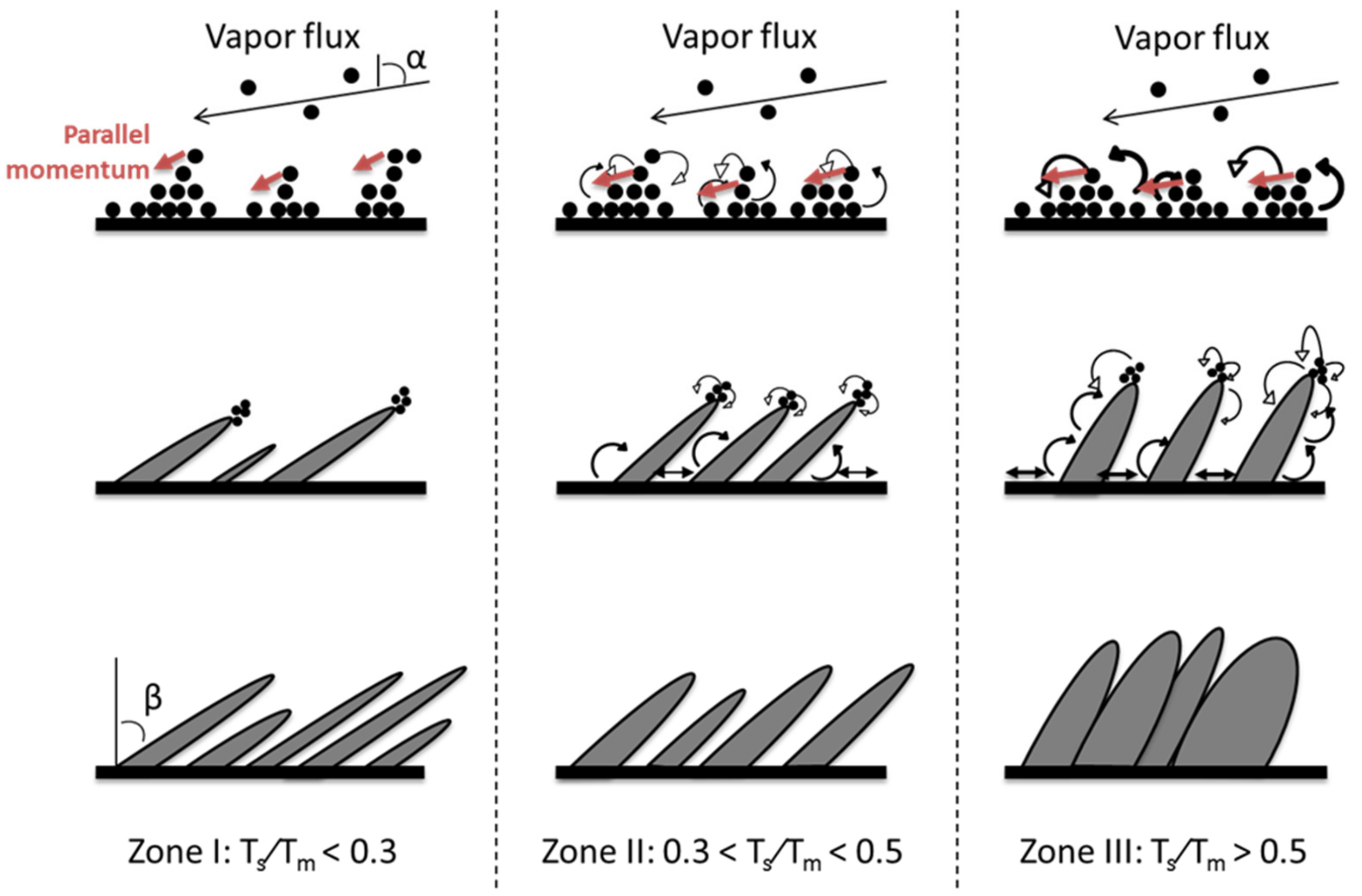
3.1.3. Effect of the Substrate Temperature
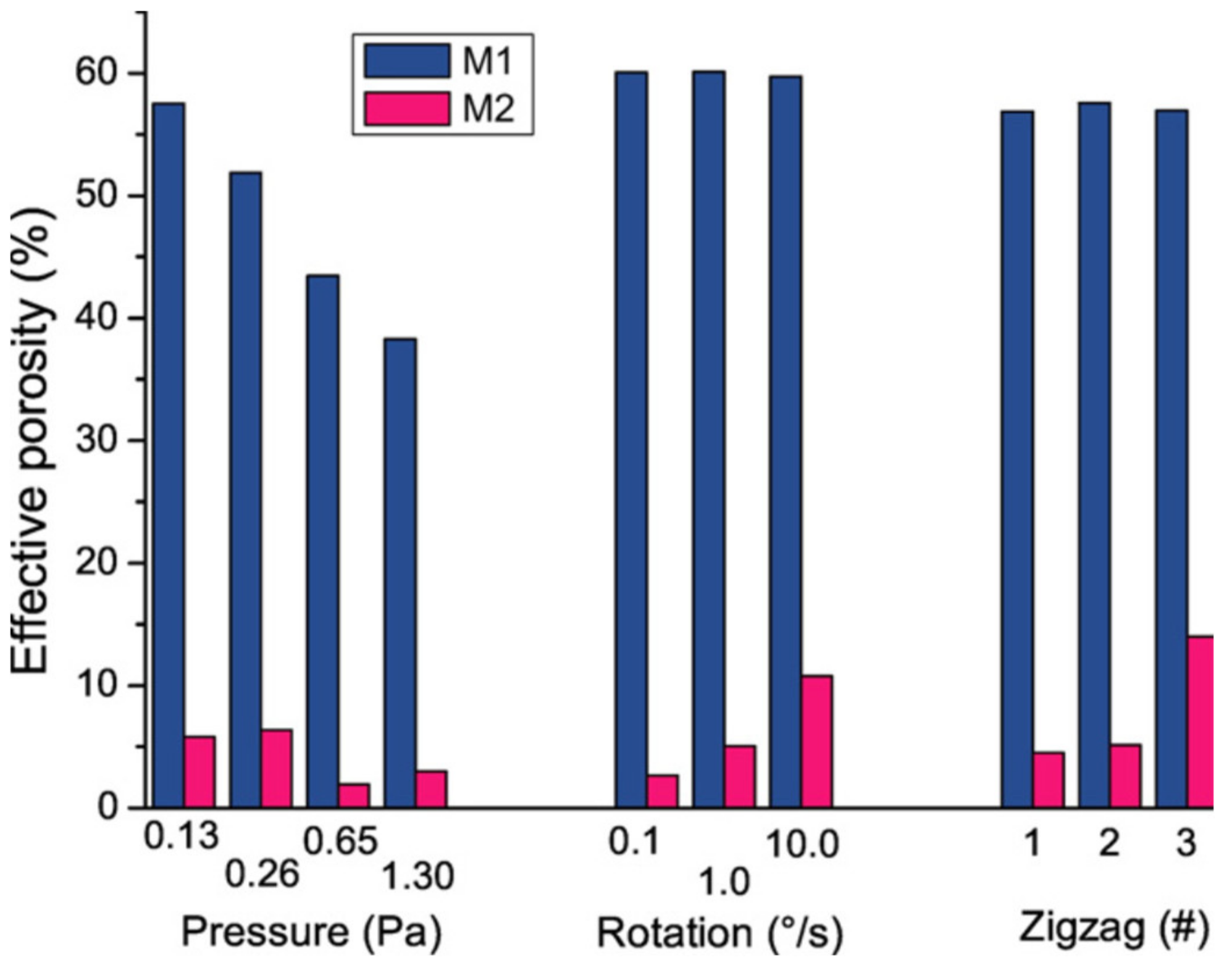
3.1.4. Effect of the Substrate Rotation
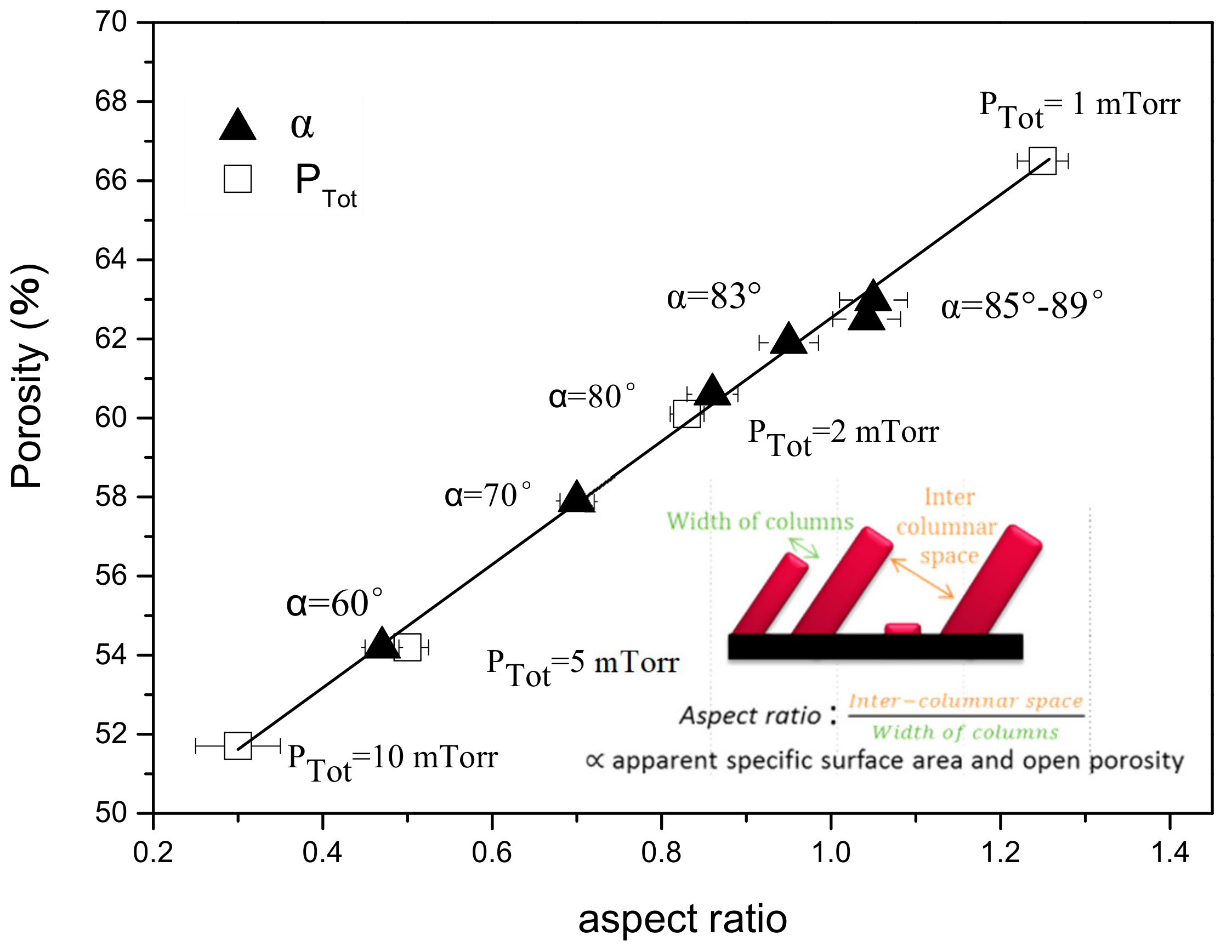
3.1.5. Evolution of the Porosity
3.2. Nano-Sculpted Oxide Films
3.3. Growth Model for GLAD Deposited Thin Films
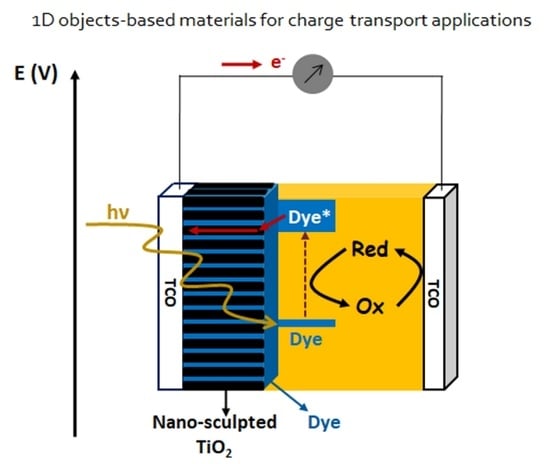
3.4. Nano-Sculpted TiO2 Films for Dye-Sensitized Solar Cell Applications
3.4.1. Context
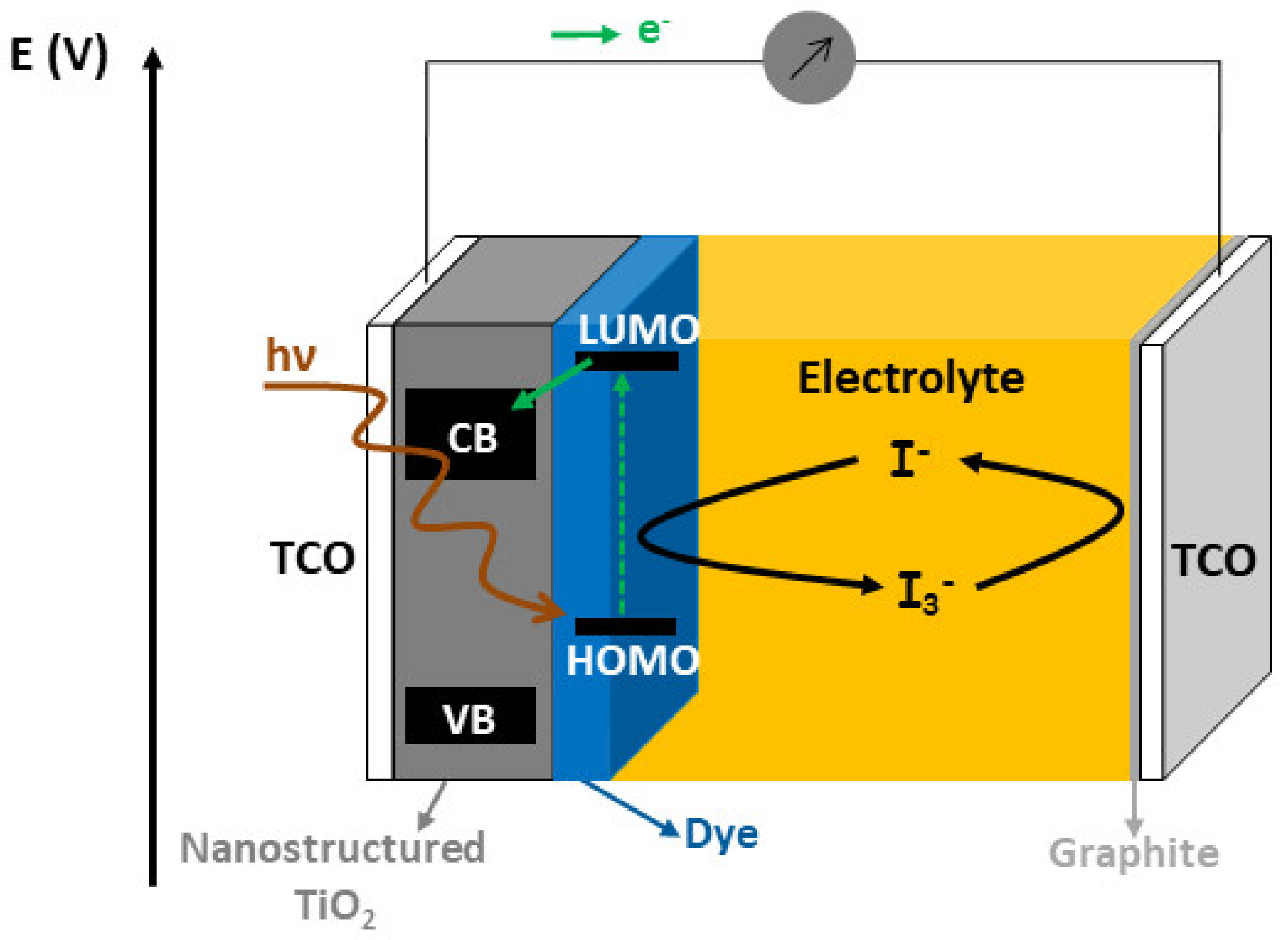
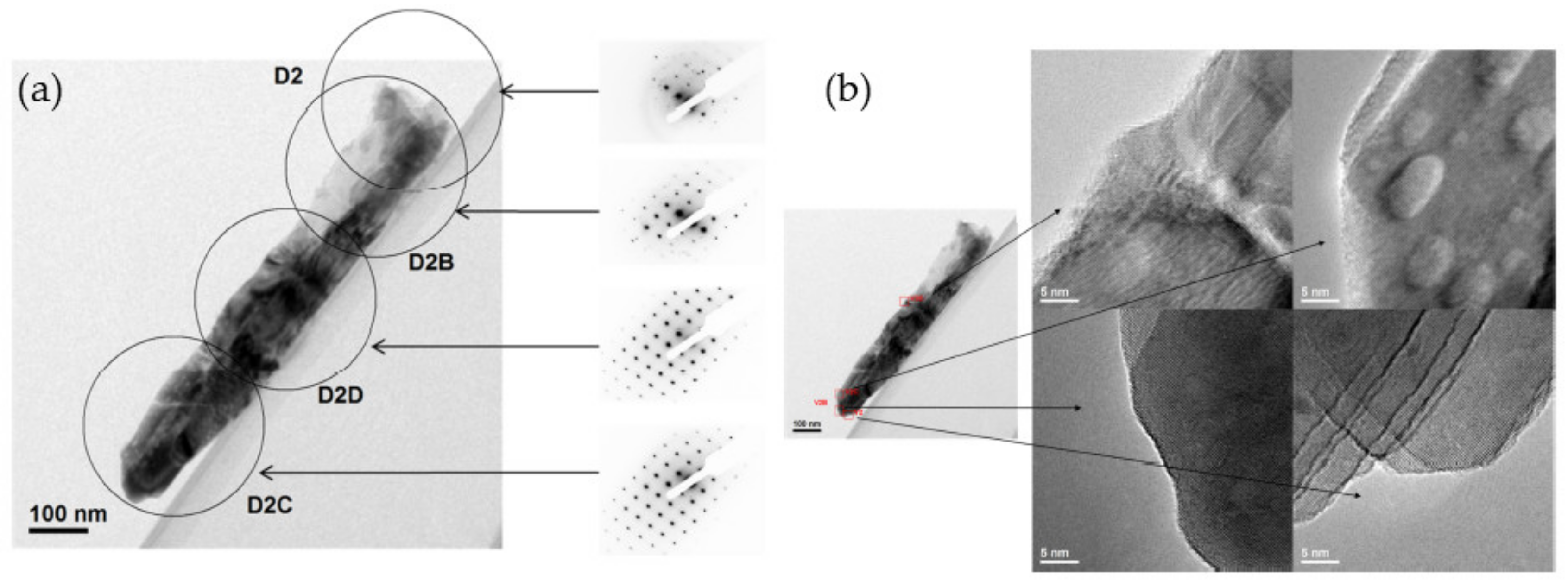
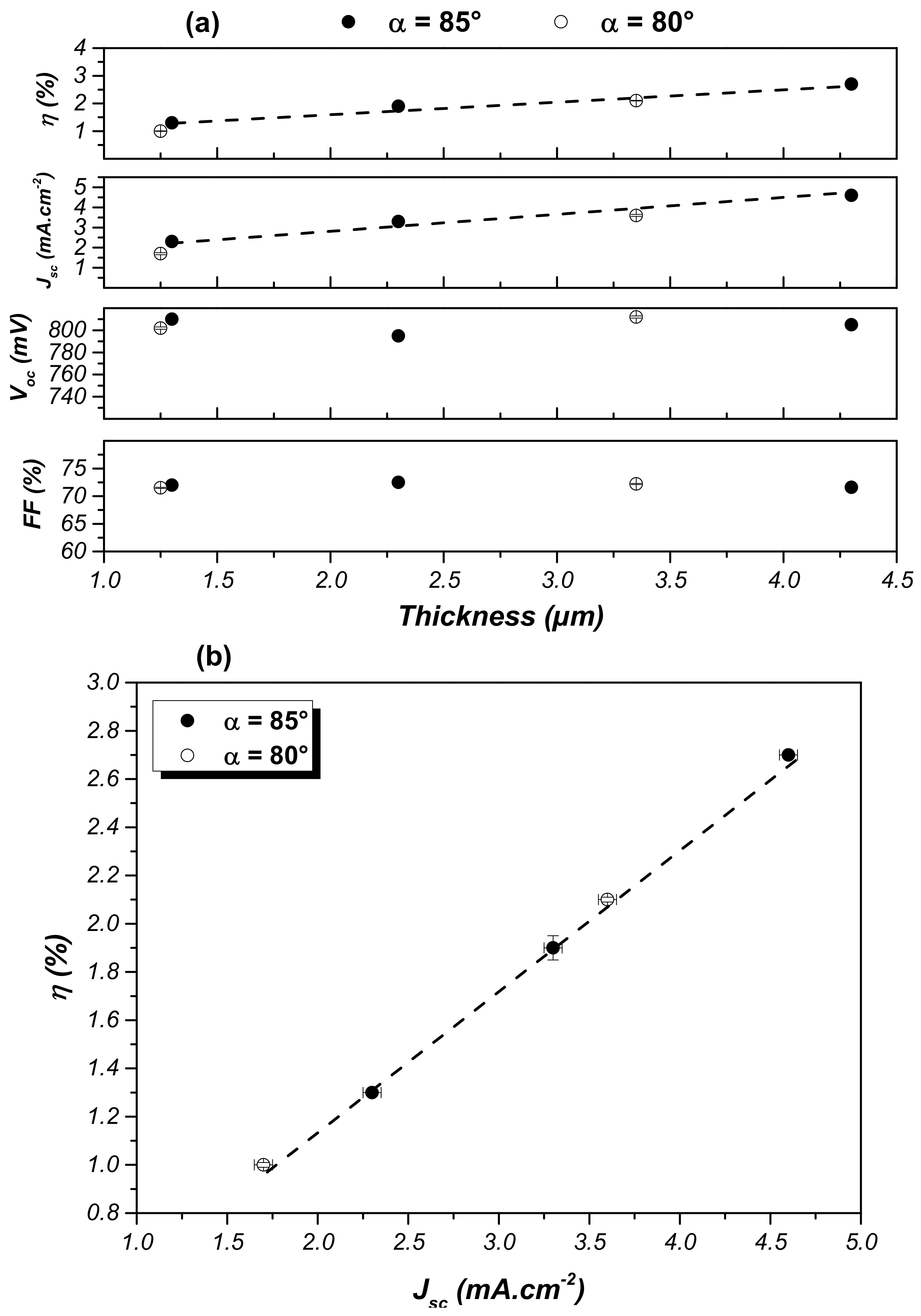
3.4.2. Nano-Sculpted TiO2 Thin Films as Photoanode Material
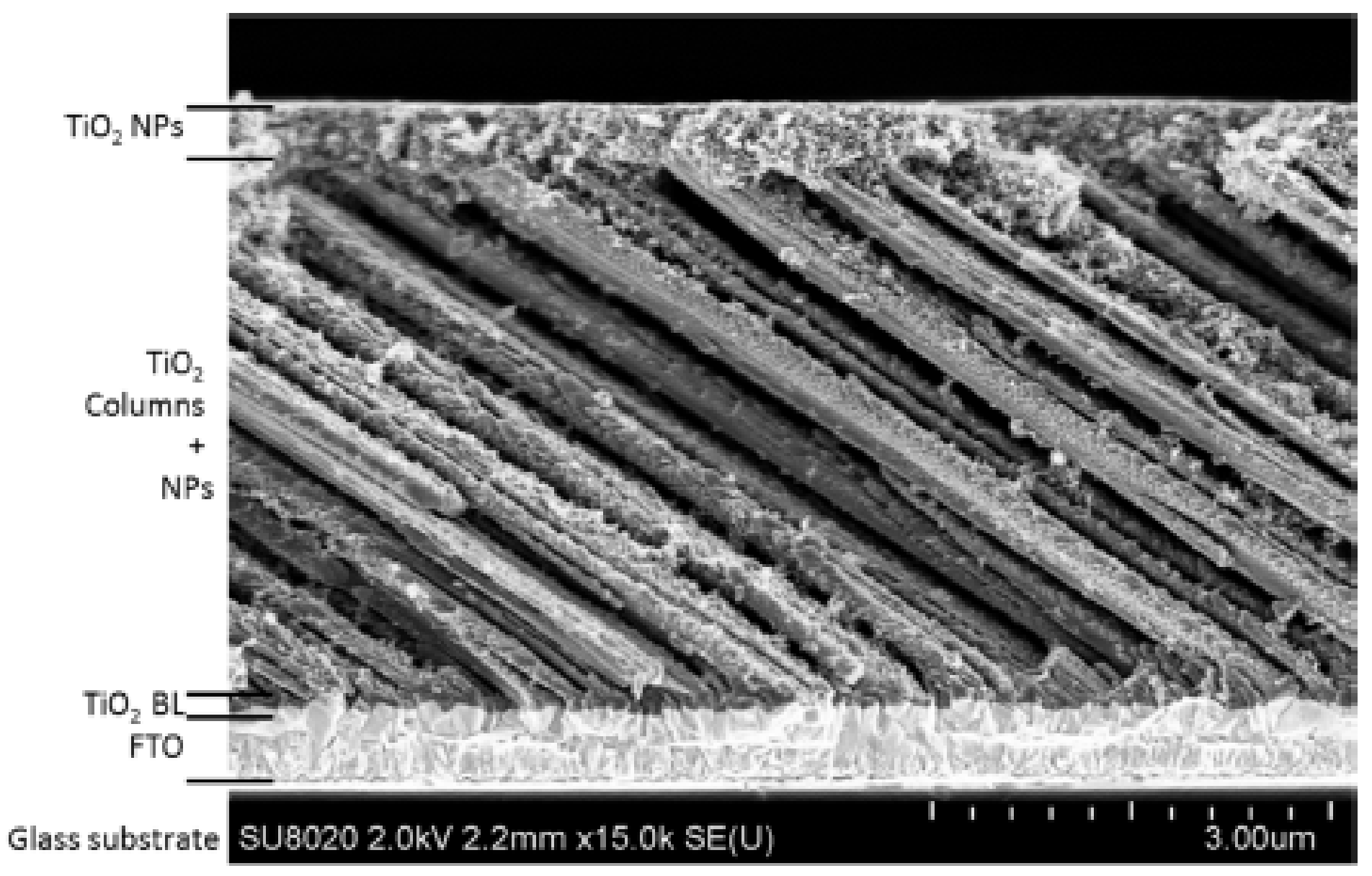
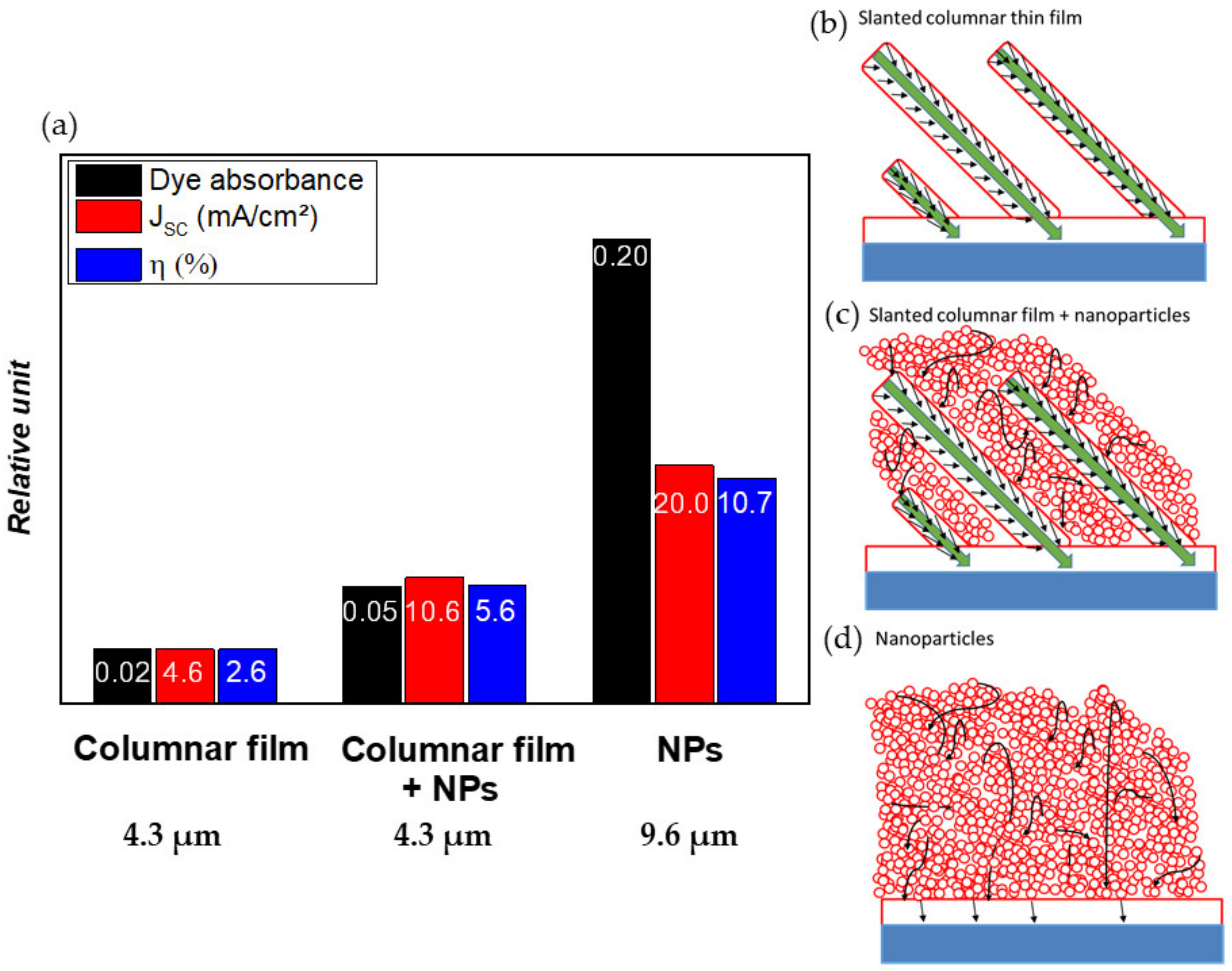
3.4.3. A Nano-Sculpted TiO2/TiO2 Nanoparticles Hybrid Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hawkeye, M.M.; Taschuk, M.T.; Brett, M.J. Glancing Angle Deposition of Thin Films; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Forro, L.; Chauvet, O.; Emin, D.; Zuppiroli, L. High mobility n-type charge carriers in large single crystals of anatase (TiO2). J. Appl. Phys. 1994, 75, 633–635. [Google Scholar] [CrossRef]
- Agrell, H.G.; Boschloo, G.; Hagfeldt, A. Conductivity Studies of Nanostructured TiO2 Films Permeated with Electrolyte. J. Phys. Chem. B 2004, 108, 12388–12396. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of Highly-Ordered TiO2 Nanotube Arrays in Dye-Sensitized Solar Cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef]
- Du, S.; Koenigsmann, C.; Sun, S. One-dimensional nanostructures for PEM fuel cell applications. In Hydrogen and Fuel Cells Primers; Pollet, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Xie, Z.; Henry, B.; Kirov, K.; Smith, H.; Barkhouse, A.; Grovenor, C.; Assender, H.; Briggs, G.; Webster, G.; Burn, P.L.; et al. Study of the effect of changing the microstructure of titania layers on composite solar cell performance. Thin Solid Films 2006, 511, 523–528. [Google Scholar] [CrossRef]
- Govardhan Reddy, K.; Deepak, T.G.; Anjusree, G.S.; Thomas, S.; Vadukumpully, S.; Subramanian, K.R.V.; Nair, S.V.; Nair, A.S. On Global Energy Scenario, Dye-sensitized Solar Cells and the Promise of Nanotechnology Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 1166–1169. [Google Scholar]
- Dervaux, J.; Cormier, P.-A.; Konstantinidis, S.; Di Ciuccio, R.; Coulembier, O.; Dubois, P.; Snyders, R. Deposition of porous titanium oxide thin films as anode material for dye sensitized solar cells. Vacuum 2015, 114, 213–220. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Feng, H.-L.; Rao, H.-S.; Xu, Y.-F.; Kuang, D.-B.; Su, C.-Y. Maximizing omnidirectional light harvesting in metal oxide hyperbranched array architectures. Nat. Commun. 2014, 5, 3968. [Google Scholar] [CrossRef] [Green Version]
- Krumpmann, A. Anodized TiO2 nanotubes as a photoelectrode material for solid-state dye-sensitized solar cells. PhD℉Thesis, University of Mons, Mons, Belgium, 2018. [Google Scholar]
- Colgan, M.; Djurfors, B.; Ivey, D.; Brett, M. Effects of annealing on titanium dioxide structured films. Thin Solid Films 2004, 466, 92–966. [Google Scholar] [CrossRef]
- Sander, M.S.; Côté, M.J.; Gu, W.; Kile, B.M.; Tripp, C.P. Template-Assisted Fabrication of Dense, Aligned Arrays of Titania Nanotubes with Well-Controlled Dimensions on Substrates. Adv. Mater. 2004, 16, 2052–2057. [Google Scholar] [CrossRef]
- Shin, H.; Jeong, D.-K.; Lee, J.; Sung, M.M.; Kim, J. Formation of TiO2 and ZrO2 Nanotubes Using Atomic Layer Deposition with Ultraprecise Control of the Wall Thickness. Adv. Mater. 2004, 16, 1197–1200. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh-Attar, A.; Ghamsari, M.S.; Hajiesmaeilbaigi, F.; Mirdamadi, S.; Katagiri, K.; Koumoto, K. Sol–gel template synthesis and characterization of aligned anatase-TiO2 nanorod arrays with different diameter. Mater. Chem. Phys. 2009, 113, 856–860. [Google Scholar] [CrossRef]
- Feng, X.; Zhu, K.; Frank, A.J.; Grimes, C.A.; Mallouk, T.E. Rapid Charge Transport in Dye-Sensitized Solar Cells Made from Vertically Aligned Single-Crystal Rutile TiO2 Nanowires. Angew. Chem. Int. Ed. 2012, 51, 2727–2730. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Grill, A. Cold Plasma Materials Fabrication; Institute of Electrical and Electronics Engineers (IEEE), Wiley: Piscataway, NJ, USA, 1994. [Google Scholar]
- Ershov, A.; Pekker, L. Model of d.c. magnetron reactive sputtering in Ar-O2 gas mixtures. Thin Solid Films 1996, 289, 140–146. [Google Scholar] [CrossRef]
- Safi, I. Recent aspects concerning DC reactive magnetron sputtering of thin films: A review. Surf. Coatings Technol. 2000, 127, 203–218. [Google Scholar] [CrossRef]
- Cormier, P.-A.; Balhamri, A.; Thomann, A.-L.; Dussart, R.; Semmar, N.; Lecas, T.; Snyders, R.; Konstantinidis, S. Titanium oxide thin film growth by magnetron sputtering: Total energy flux and its relationship with the phase constitution. Surf. Coat. Technol. 2014, 254, 291–297. [Google Scholar] [CrossRef]
- Bräuer, G.; Szyszka, B.; Vergöhl, M.; Bandorf, R. Magnetron sputtering – Milestones of 30 years. Vacuum 2010, 84, 1354–1359. [Google Scholar] [CrossRef]
- Young, N.O.; Kowal, J. Optically Active Fluorite Films. Nature, 1959; 183, 104–105. [Google Scholar]
- Michalcik, Z.; Horakova, M.; Spatenka, P.; Klementová, Š.; Zlámal, M.; Martin, N. Photocatalytic Activity of Nanostructured Titanium Dioxide Thin Films. Int. J. Photoenergy 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Robbie, K.; Brett, M.J. Sculptured thin films and glancing angle deposition: Growth mechanics and applications. J. Vac. Sci. Technol. A 1997, 15, 1460–1465. [Google Scholar] [CrossRef]
- Cormier, P.-A.; Thomann, A.-L.; Dolique, V.; Balhamri, A.; Dussart, R.; Semmar, N.; Lecas, T.; Brault, P.; Snyders, R.; Konstantinidis, S. IR emission from the target during plasma magnetron sputter deposition. Thin Solid Films 2013, 545, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Sit, J.C.; Vick, D.; Robbie, K.; Brett, M.J. Thin Film Microstructure Control Using Glancing Angle Deposition by Sputtering. J. Mater. Res. 1999, 14, 1197–1199. [Google Scholar] [CrossRef]
- García-Martín, J.M.; Alvarez, R.; Romero-Gomez, P.; Cebollada, A.; Palmero, A. Tilt angle control of nanocolumns grown by glancing angle sputtering at variable argon pressures. Appl. Phys. Lett. 2010, 97, 173103. [Google Scholar] [CrossRef] [Green Version]
- Anders, A. A structure zone diagram including plasma-based deposition and ion etching. Thin Solid Films 2010, 518, 4087–4090. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Liang, H.; Li, W.; Panepinto, A.; Thiry, D.; Chen, M.; Snyders, R. Experimental evaluation of the role of oxygen on the growth of MgOx nano-sculpted thin films synthesized by reactive magnetron sputtering combined with glancing angle deposition. Thin Solid Films. submitted.
- Liang, H.; Geng, X.; Li, W.; Panepinto, A.; Thiry, D.; Chen, M.; Snyders, R. Experimental and Modeling Study of the Fabrication of Mg Nano-Sculpted Films by Magnetron Sputtering Combined with Glancing Angle Deposition. Coatings 2019, 9, 361. [Google Scholar] [CrossRef] [Green Version]
- Ganciu, M.; Konstantinidis, S.; Paint, Y.; Dauchot, J.P.; Hecq, M.; De Poucques, L.; Vašina, P.; Meško, M.; Imbert, J.C.; Bretagne, J.; et al. Preionised pulsed magnetron discharges for ionised physical vapour deposition. J. Optoelectron. Adv. Mater. 2005, 7, 2481–2484. [Google Scholar]
- Panepinto, A.; Michiels, M.; Dürrschnabel, M.T.; Molina-Luna, L.; Bittencourt, C.; Cormier, P.A.; Snyders, R. Synthesis of Anatase (Core)/Rutile (Shell) Nanostructured TiO2 Thin Films by Magnetron Sputtering Methods for Dye-Sensitized Solar Cell Applications. ACS Appl. Energy Mater. 2020, 3, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Cormier, P.-A.; Dervaux, J.; Szuwarski, N.; Pellegrin, Y.; Odobel, F.; Gautron, E.; Boujtita, M.; Snyders, R.; Boujita, M. Single Crystalline-like and Nanostructured TiO2Photoanodes for Dye Sensitized Solar Cells Synthesized by Reactive Magnetron Sputtering at Glancing Angle. J. Phys. Chem. C 2018, 122, 20661–20668. [Google Scholar] [CrossRef]
- Bortz, A.; Kalos, M.; Lebowitz, J. A new algorithm for Monte Carlo simulation of Ising spin systems. J. Comput. Phys. 1975, 17, 10–18. [Google Scholar] [CrossRef]
- Claassens, C.H.; Hoffman, M.J.H.; Terblans, J.; Swart, H.C. Kinetic Monte Carlo Simulation of the Growth of Various Nanostructures through Atomic and Cluster Deposition: Application to Gold Nanostructure Growth on Graphite. J. Phys. Conf. Ser. 2006, 29, 185–189. [Google Scholar] [CrossRef]
- Meakin, P.; Krug, J. Three-dimensional ballistic deposition at oblique incidence. Phys. Rev. A 1992, 46, 3390–3399. [Google Scholar] [CrossRef] [PubMed]
- Smy, T.; Vick, D.; Brett, M.J.; Dew, S.K.; Wu, A.T.; Sit, J.C.; Harris, K.D. Three-dimensional simulation of film microstructure produced by glancing angle deposition. J. Vac. Sci. Technol. A 2000, 18, 2507. [Google Scholar] [CrossRef]
- Lucas, S.; Moskovkin, P. Simulation at high temperature of atomic deposition, islands coalescence, Ostwald and inverse Ostwald ripening with a general simple kinetic Monte Carlo code. Thin Solid Films 2010, 518, 5355–5361. [Google Scholar] [CrossRef]
- NASCAM (NAnoSCAle Modeling). Available online: https://www.unamur.be/sciences/physique/ur/larn/logiciels/nascam (accessed on 5 May 2020).
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM—The stopping and range of ions in matter. Nucl. Instrum. Methods Phys. Res. B 2010, 268, 1818–1823. [Google Scholar] [CrossRef] [Green Version]
- Godinho, V.; Moskovkin, P.; Álvarez, R.; Caballero-Hernández, J.; Schierholz, R.; Bera, B.; Demarche, J.; Palmero, A.; Fernández, A.; Lucas, S. On the formation of the porous structure in nanostructured a-Si coatings deposited by dc magnetron sputtering at oblique angles. Nanotechnology 2014, 25, 355705. [Google Scholar] [CrossRef] [Green Version]
- Thornton, J.A. Influence of apparatus geometry and deposition conditions on the structure and topography of thick sputtered coatings. J. Vac. Sci. Technol. 1974, 11, 666–670. [Google Scholar] [CrossRef]
- Hussla, I.; Enke, K.; Grunwald, H.; Lorenz, G.; Stoll, H. In situ silicon-wafer temperature measurements during RF argon-ion plasma etching via fluoroptic thermometry. J. Phys. D Appl. Phys. 1987, 20, 889–896. [Google Scholar] [CrossRef]
- Kersten, H.; Deutsch, H.; Steffen, H.; Kroesen, G.; Hippler, R. The energy balance at substrate surfaces during plasma processing. Vacuum 2001, 63, 385–431. [Google Scholar] [CrossRef]
- Kersten, H.; Rohde, D.; Steffen, H.; Deutsch, H.; Hippler, R.; Swinkels, G.; Kroesen, G. On the determination of energy fluxes at plasma–surface processes. Appl. Phys. A 2001, 72, 531–540. [Google Scholar] [CrossRef]
- Movchan, B.A.; Demchishin, A.V. Structure and Properties of Thick Condensates of Nickel, Titanium, Tungsten, Aluminium Oxides, and Zirconium Dioxide in Vacuum. Phys. Metal. Metallog. 2014, 28, 653–663. [Google Scholar]
- Jain, I.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Dervaux, J.; Cormier, P.-A.; Moskovkin, P.; Douheret, O.; Konstantinidis, S.; Lazzaroni, R.; Lucas, S.; Snyders, R. Synthesis of nanostructured Ti thin films by combining glancing angle deposition and magnetron sputtering: A joint experimental and modeling study. Thin Solid Films 2017, 636, 644–657. [Google Scholar] [CrossRef]
- Abelmann, L.; Lodder, C. Oblique evaporation and surface diffusion. Thin Solid Films 1997, 305, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Rohlf, J.W. Modern physics from [alpha] to Z0. Wiley: Hoboken, NJ, USA, 1994. [Google Scholar]
- Blocher, J.M.; Campbell, I.E. Vapor Pressure of Titanium. J. Am. Chem. Soc. 1949, 71, 4040–4042. [Google Scholar] [CrossRef]
- Hawkeye, M.M.; Brett, M.J. Glancing angle deposition: Fabrication, properties, and applications of micro- and nanostructured thin films. J. Vac. Sci. Technol. A 2007, 25, 1317. [Google Scholar] [CrossRef]
- Dick, B.; Brett, M.J.; Smy, T. Controlled growth of periodic pillars by glancing angle deposition. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2003, 21, 23. [Google Scholar] [CrossRef]
- Henderson, M.A. A surface perspective on self-diffusion in rutile TiO2. Surf. Sci. 1999, 419, 174–187. [Google Scholar] [CrossRef]
- Yao, K.-S.; Chen, Y.-C.; Chao, C.-H.; Wang, W.-F.; Lien, S.-Y.; Shih, H.C.; Chen, T.-L.; Weng, K.-W. Electrical enhancement of DMFC by Pt–M/C catalyst-assisted PVD. Thin Solid Films 2010, 518, 7225–7228. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Grätzel, M. Conversion of sunlight to electric power by nanocrystalline dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2004, 164, 3–14. [Google Scholar]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Wang, W.; Gurzadyan, G.G.; Li, J.; Li, X.; An, J.; Yu, Z.; Wang, H.; Cai, B.; et al. 13.6% Efficient Organic Dye-Sensitized Solar Cells by Minimizing Energy Losses of the Excited State. ACS Energy Lett. 2019, 4, 943–951. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)-Based Redox Electrolyte Exceed 12 Percent Efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Astani, N.A.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maçaira, J.; Andrade, L.; Mendes, A. Review on nanostructured photoelectrodes for next generation dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2013, 27, 334–349. [Google Scholar] [CrossRef]
- Mardare, D.; Tasca, M.; Delibas, M.; Rusu, G. On the structural properties and optical transmittance of TiO2 r.f. sputtered thin films. Appl. Surf. Sci. 2000, 156, 200–206. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Dervaux, J. Synthesis of nanostructured TiO2 thin films by reactive magnetron sputtering in glancing angle configuration for dye-sensitized solar cell applications. PhD Thesis, University of Mons, Mons, Belgium, 2017. [Google Scholar]
- Kang, M.G.; Ryu, K.S.; Chang, S.H.; Park, N.G.; Hong, J.S.; Kim, K.J. Dependence of TiO2 Film Thickness on Photocurrent-Voltage Characteristics of Dye-Sensitized Solar Cells. Bull. Korean Chem. Soc. 2004, 25, 742–744. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panepinto, A.; Snyders, R. Recent Advances in the Development of Nano-Sculpted Films by Magnetron Sputtering for Energy-Related Applications. Nanomaterials 2020, 10, 2039. https://doi.org/10.3390/nano10102039
Panepinto A, Snyders R. Recent Advances in the Development of Nano-Sculpted Films by Magnetron Sputtering for Energy-Related Applications. Nanomaterials. 2020; 10(10):2039. https://doi.org/10.3390/nano10102039
Chicago/Turabian StylePanepinto, Adriano, and Rony Snyders. 2020. "Recent Advances in the Development of Nano-Sculpted Films by Magnetron Sputtering for Energy-Related Applications" Nanomaterials 10, no. 10: 2039. https://doi.org/10.3390/nano10102039
APA StylePanepinto, A., & Snyders, R. (2020). Recent Advances in the Development of Nano-Sculpted Films by Magnetron Sputtering for Energy-Related Applications. Nanomaterials, 10(10), 2039. https://doi.org/10.3390/nano10102039