Mesoporous Carbons from Polysaccharides and Their Use in Li-O2 Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment Used
2.2. Determination of Real Amylose Content in Commercially Available Starches
2.3. Preparation of Mesoporous Carbons from a Modified Starbon® Based Process
2.3.1. Modified Starbon® Based Process
2.3.2. Modified Starbon® Based Process with a Previous Treatment of the Starches with the IL
2.4. Preparation of Mesoporous Carbons with the “Low-Cost” Process
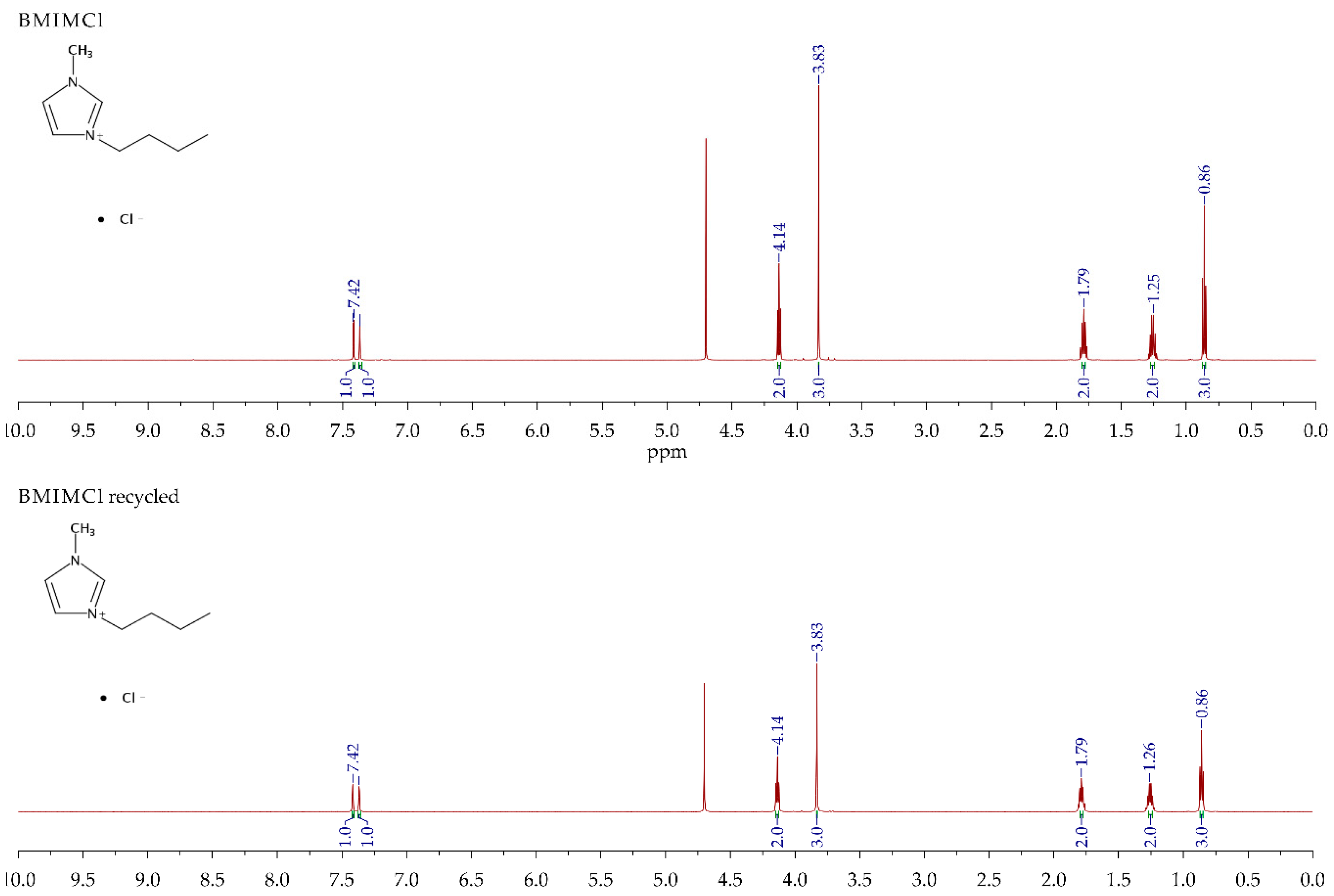
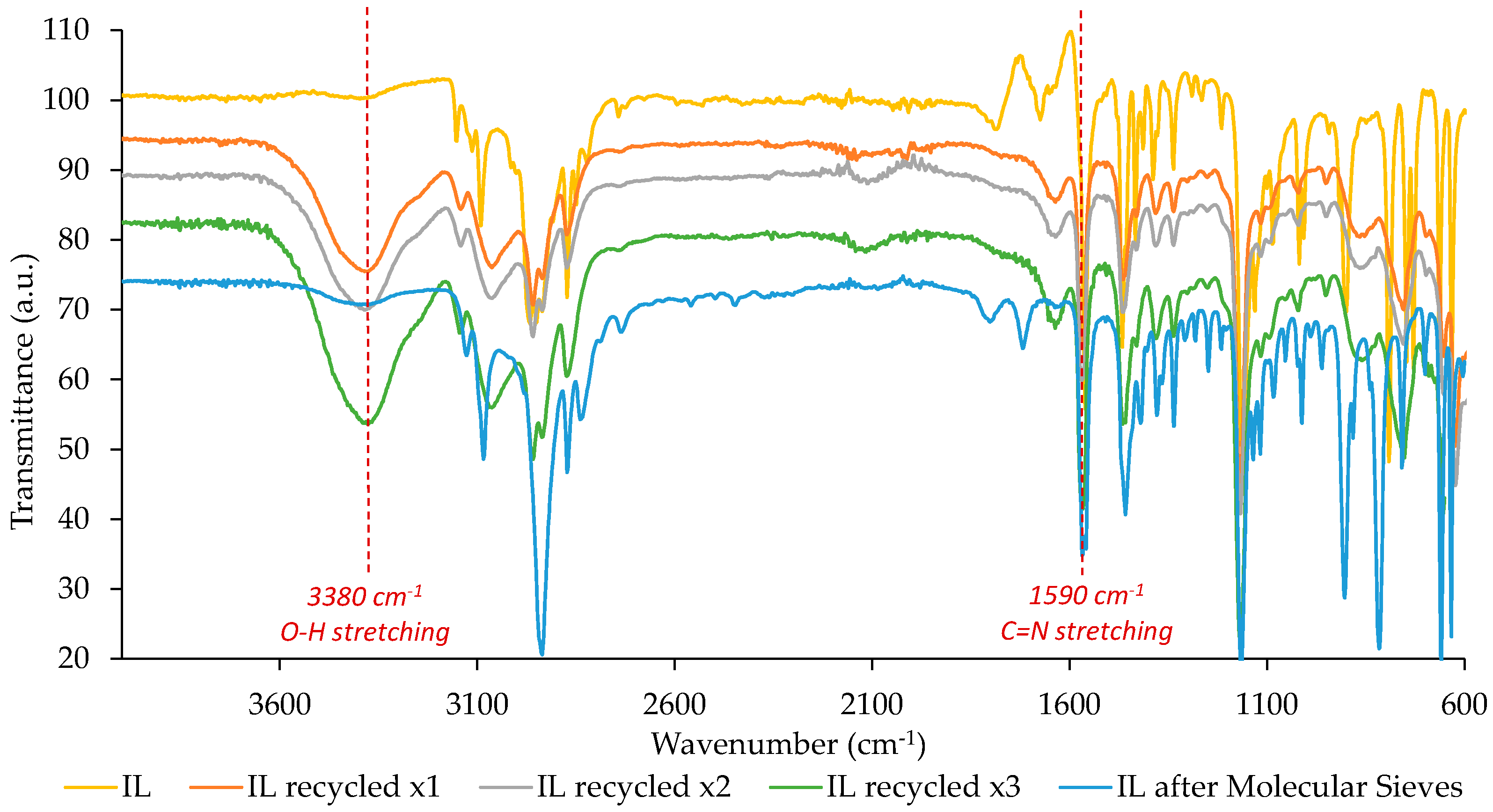
2.5. IL Recyclability
2.6. Preparation of N-Doped Mesoporous Carbon from Chitosan
2.7. Cell Assembly and Electrochemical Characterization
3. Results
3.1. Determination of Real Amylose Content in Commercially Available Starches
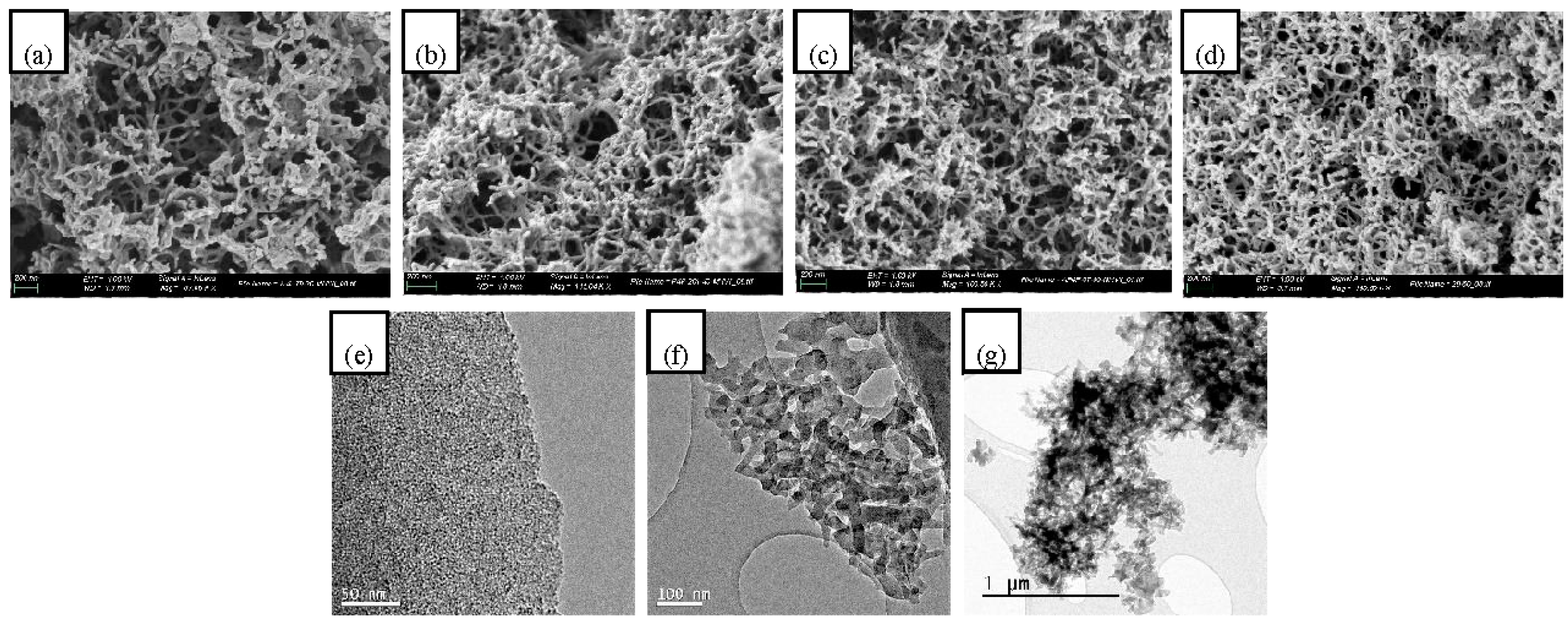
3.2. Preparation of Mesoporous Carbons from a Modified Starbon® Based Process
3.2.1. Modified Starbon® Based Process with a Previous Treatment of the Starches with the IL
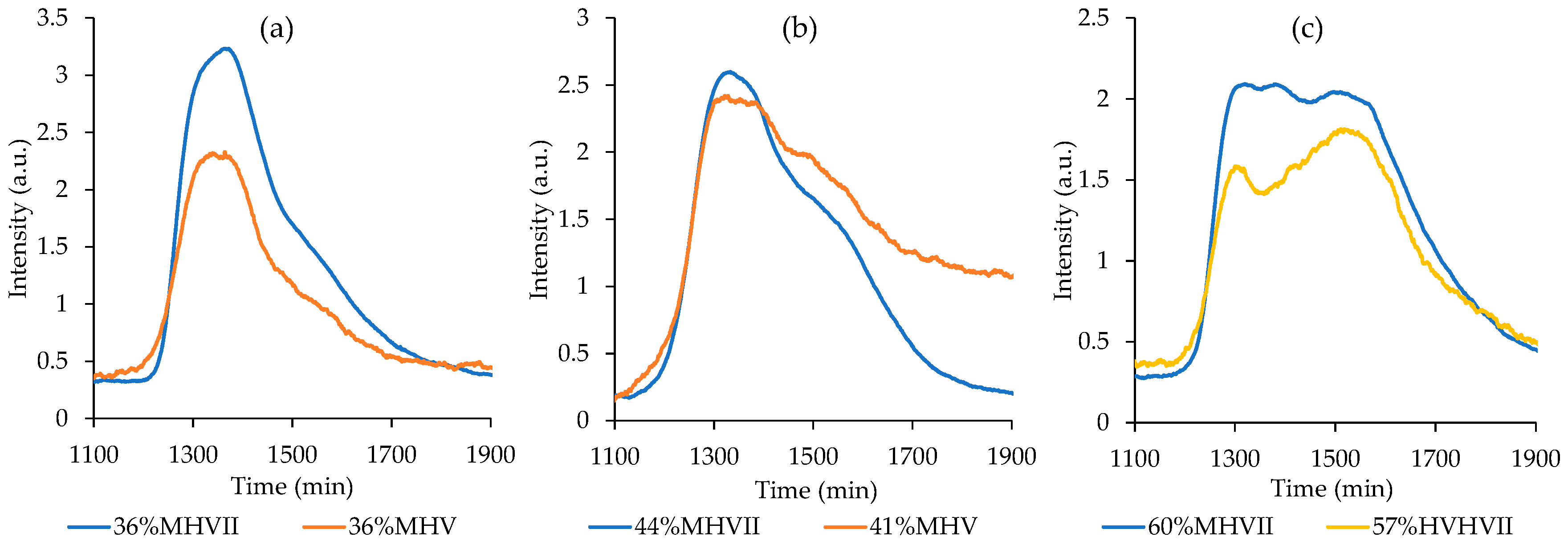
Influence of Starch Mixture Concentration in Carbon Mesoporosity
3.2.2. Modified Starbon® Based Process
3.3. Preparation of Mesoporous Carbons with a “Low-Cost” Process
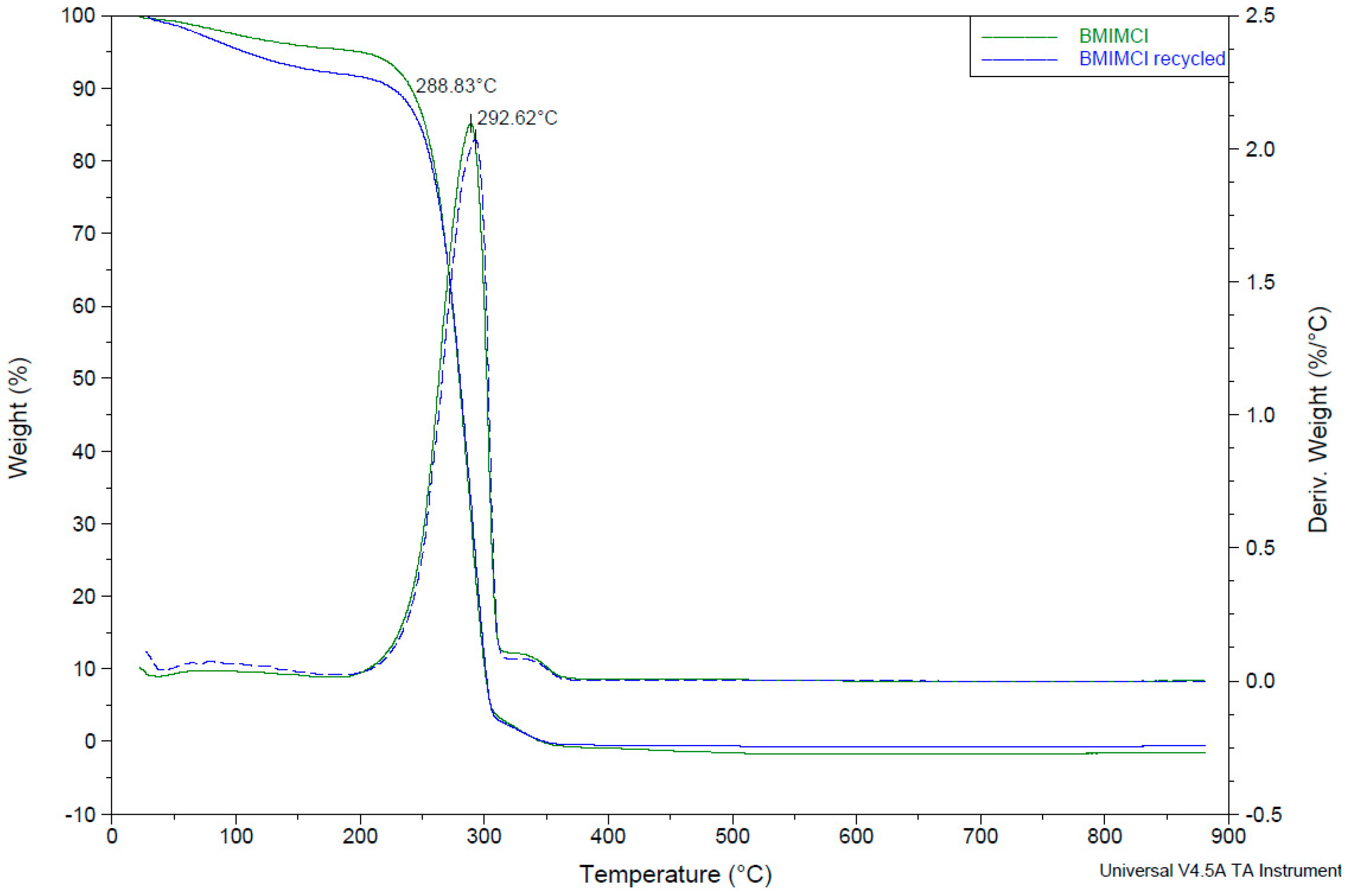
3.4. Recyclability of the IL
3.5. Preparation of Mesoporous Carbon to be Used in Li-O2 Battery Cells Studies
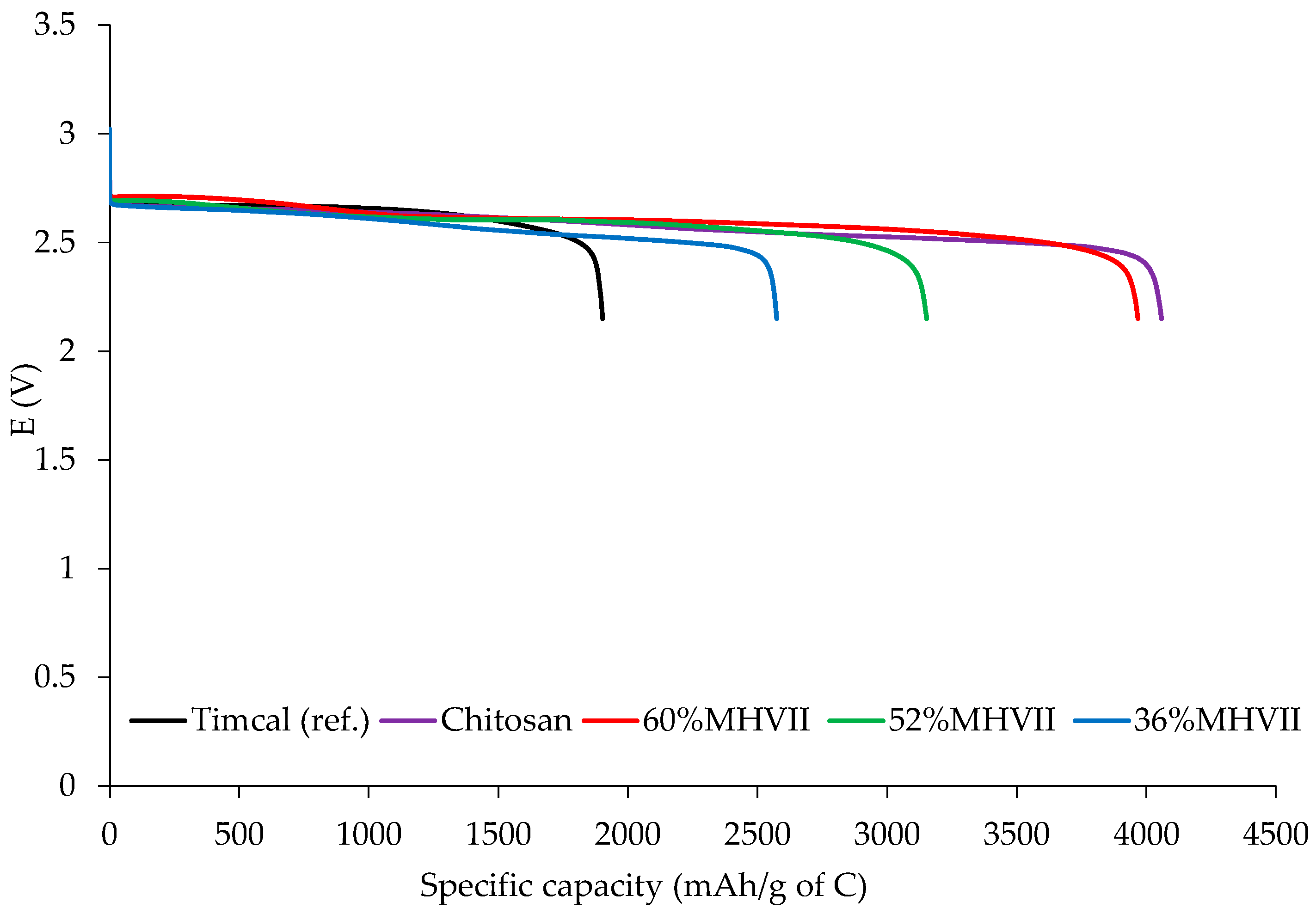
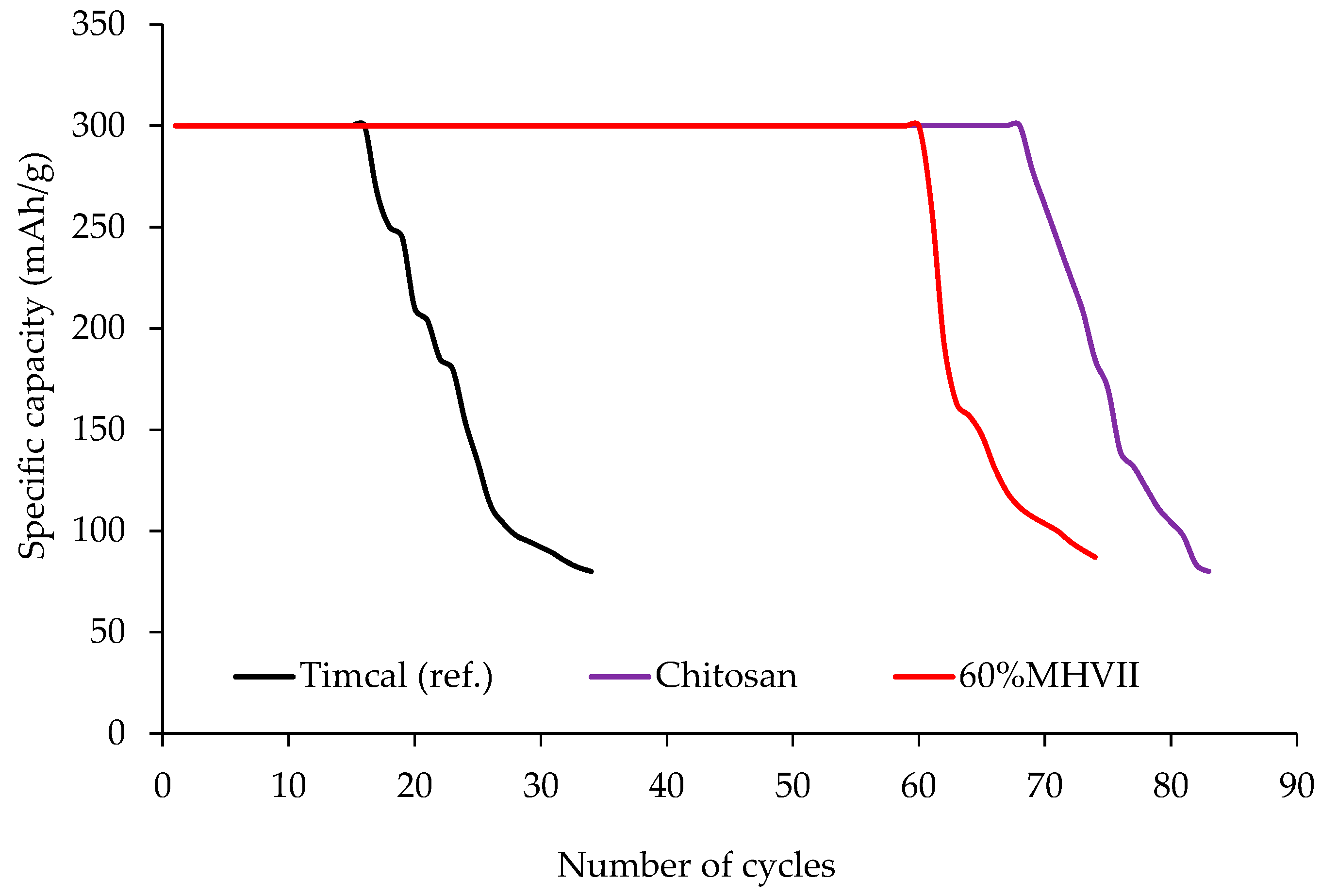
3.6. Application of Mesoporous Carbon as Cathodic Material in Li-O2 Battery Cells
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Budarin, V.; Clark, J.H.; Deswarte, F.E.I.; Hardy, J.J.E.; Hunt, A.J.; Kerton, F.M. Delicious Not Siliceous: Expanded Carbohydrates as Renewable Separation Media for Column Chromatography. Chem. Commun. 2005, 23, 2903–2905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, J.H.; Budarin, V.; Deswarte, F.E.I.; Hardy, J.J.E.; Kerton, F.M.; Hunt, A.J.; Luque, R.; Macquarrie, D.J.; Milkowski, K.; Rodriguez, A.; et al. Green Chemistry and the Biorefinery: A Partnership for a Sustainable Future. Green Chem. 2006, 8, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Budarin, V.L.; Clark, J.H.; Luque, R.; Macquarrie, D.J. Versatile Mesoporous Carbonaceous Materials for Acid Catalysis. Chem. Commun. 2007, 6, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-L.; Chen, L.-L.; Xin, S.; Yin, Y.-X.; Guo, Y.-G.; Kong, Q.-S.; Xia, Y.-Z. Preparation and Li Storage Properties of Hierarchical Porous Carbon Fibers Derived from Alginic Acid. ChemSusChem 2010, 3, 703–707. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Hu, H.; Liu, S.; Cai, Y.; Dong, H.; Zheng, M.; Xiao, Y.; Liu, Y. Ultrahigh-Surface-Area Hierarchical Porous Carbon from Chitosan: Acetic Acid Mediated Efficient Synthesis and Its Application in Superior Supercapacitors. J. Mater. Chem. A 2017, 5, 24775–24781. [Google Scholar] [CrossRef]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Masson, E.; Celzard, A. The Use of Tannin to Prepare Carbon Gels. Part I: Carbon Aerogels. Carbon 2011, 49, 2773–2784. [Google Scholar] [CrossRef]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Celzard, A. The Use of Tannin to Prepare Carbon Gels. Part II. Carbon Cryogels. Carbon 2011, 49, 2785–2794. [Google Scholar] [CrossRef]
- Nita, C.; Bensafia, M.; Vaulot, C.; Delmotte, L.; Matei Ghimbeu, C. Insights on the Synthesis Mechanism of Green Phenolic Resin Derived Porous Carbons via a Salt-Soft Templating Approach. Carbon 2016, 109, 227–238. [Google Scholar] [CrossRef]
- Sopronyi, M.; Sima, F.; Vaulot, C.; Delmotte, L.; Bahouka, A.; Matei Ghimbeu, C. Direct Synthesis of Graphitic Mesoporous Carbon from Green Phenolic Resins Exposed to Subsequent UV and IR Laser Irradiations. Sci. Rep. 2016, 6, 39617. [Google Scholar] [CrossRef]
- Bianco, A.; Chen, Y.; Frackowiak, E.; Holzinger, M.; Koratkar, N.; Meunier, V.; Mikhailovsky, S.; Strano, M.; Tascon, J.M.D.; Terrones, M. Carbon Science Perspective in 2020: Current Research and Future Challenges. Carbon 2020, 161, 373–391. [Google Scholar] [CrossRef]
- White, R.J. Porous Carbon Materials from Sustainable Precursors; Green Chemistry Series; The Royal Society of Chemistry: London, UK, 2015; p. 433. [Google Scholar] [CrossRef]
- White, R.J.; Brun, N.; Budarin, V.L.; Clark, J.H.; Titirici, M.-M. Always Look on the “Light” Side of Life: Sustainable Carbon Aerogels. ChemSusChem 2014, 7, 670–689. [Google Scholar] [CrossRef] [PubMed]
- Antonietti, M.; Lopez-Salas, N.; Primo, A. Adjusting the Structure and Electronic Properties of Carbons for Metal-Free Carbocatalysis of Organic Transformations. Adv. Mater. 2019, 31, 1805719. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, G.A.; Fuertes, A.B.; Sevilla, M.; Titirici, M.-M. Efficient Metal-Free N-Doped Mesoporous Carbon Catalysts for ORR by a Template-Free Approach. Carbon 2016, 106, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Correa, C.; Kruse, A. Biobased Functional Carbon Materials: Production, Characterization, and Applications-A Review. Materials 2018, 11, 1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Nicolae, S.A.; Qiao, M.; Preuss, K.; Szilágyi, P.A.; Moores, A.; Titirici, M. Homogenous Meets Heterogenous and Electro-Catalysis: Iron-Nitrogen Molecular Complexes within Carbon Materials for Catalytic Applications. ChemCatChem 2019, 11, 3602–3625. [Google Scholar] [CrossRef]
- Roberts, A.D.; Li, X.; Zhang, H. Porous Carbon Spheres and Monoliths: Morphology Control, Pore Size Tuning and Their Applications as Li-Ion Battery Anode Materials. Chem. Soc. Rev. 2014, 43, 4341–4356. [Google Scholar] [CrossRef]
- Raj, K.A.; Panda, M.R.; Dutta, D.P.; Mitra, S. Bio-Derived Mesoporous Disordered Carbon: An Excellent Anode in Sodium-Ion Battery and Full-Cell Lab Prototype. Carbon 2019, 143, 402–412. [Google Scholar] [CrossRef]
- Zeng, L.; Pan, F.; Li, W.; Jiang, Y.; Zhong, X.; Yu, Y. Free-Standing Porous Carbon Nanofibers–Sulfur Composite for Flexible Li–S Battery Cathode. Nanoscale 2014, 6, 9579–9587. [Google Scholar] [CrossRef]
- Kunanusont, N.; Shimoyama, Y. Porous Carbon Electrode for Li-Air Battery Fabricated from Solvent Expansion during Supercritical Drying. J. Supercrit. Fluids 2018, 133, 77–85. [Google Scholar] [CrossRef]
- Budarin, V.; Clark, J.H.; Luque, R.; Macquarrie, D.J.; Milkowski, K.; White, R.J. Carbonaceous Materials. U.S. 8790548B2, 29 July 2014. [Google Scholar]
- Mallik, A.K.; Shahruzzaman; Zaman, A.; Biswas, S.; Ahmed, T.; Sakib, N.; Haque, P.; Rahman, M.M. Fabrication of Polysaccharide-Based Materials Using Ionic Liquids and Scope for Biomedical Use. In Functional Polysaccharides for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–171. [Google Scholar] [CrossRef]
- Rogers, R.D. CHEMISTRY: Ionic Liquids--Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellulose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Shitta-Bey, G.O.; Mirzaeian, M.; Hall, P.J. The Electrochemical Performance of Phenol-Formaldehyde Based Activated Carbon Electrodes for Lithium/Oxygen Batteries. J. Electrochem. Soc. 2012, 159, A315–A320. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Hall, P.J. Characterizing Capacity Loss of Lithium Oxygen Batteries by Impedance Spectroscopy. J. Power Sources 2010, 195, 6817–6824. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.; Zhou, Z. Lithium-Air Batteries: Challenges Coexist with Opportunities. APL Mater. 2019, 7, 040701. [Google Scholar] [CrossRef]
- McGrance, S.J.; Cornell, H.J.; Rix, C.J. A Simple and Rapid Colorimetric Method for the Determination of Amylose in Starch Products. Starch-Stärke 1998, 50, 158–163. [Google Scholar] [CrossRef]
- Hoover, R.; Ratnayake, W.S. Determination of Total Amylose Content of Starch. Curr. Protoc. Food Anal. Chem. 2001, 1, E2.3.1–E2.3.5. [Google Scholar] [CrossRef]
- Jayawardena, B.; Pandithavidana, D.R.; Sameera, W. Polysaccharides in Solution: Experimental and Computational Studies. In Solubility of Polysaccharides; Xu, Z., Ed.; InTech: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Ren, Y.; Wang, R.; Zhang, P.; Ding, M.; Li, C.; Zhao, D.; Qian, Z.; Zhang, Z.; Zhang, L.; et al. Atomically Dispersed Cobalt Catalyst Anchored on Nitrogen-Doped Carbon Nanosheets for Lithium-Oxygen Batteries. Nat. Commun. 2020, 11, 1576. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, J.; Li, X.; Liu, J.; Geng, D.; Yang, J.; Li, R.; Sun, X. Nitrogen-Doped Carbon Nanotubes as Cathode for Lithium-Air Batteries. Electrochem. Commun. 2011, 13, 668–672. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhang, Y.; Wang, B.; Peng, H. The Recent Progress of Nitrogen-Doped Carbon Nanomaterials for Electrochemical Batteries. J. Mater. Chem. A 2018, 6, 12932–12944. [Google Scholar] [CrossRef]
- Zahoor, A.; Christy, M.; Hwang, Y.J.; Choi, J.M.; Nahm, K.S. Mesoporous Nitrogen Doped Carbon as Cathode Materials for High Capacity Lithium-Air Batteries. Meet. Abstr. 2012, MA2012-02, 1141. [Google Scholar] [CrossRef]
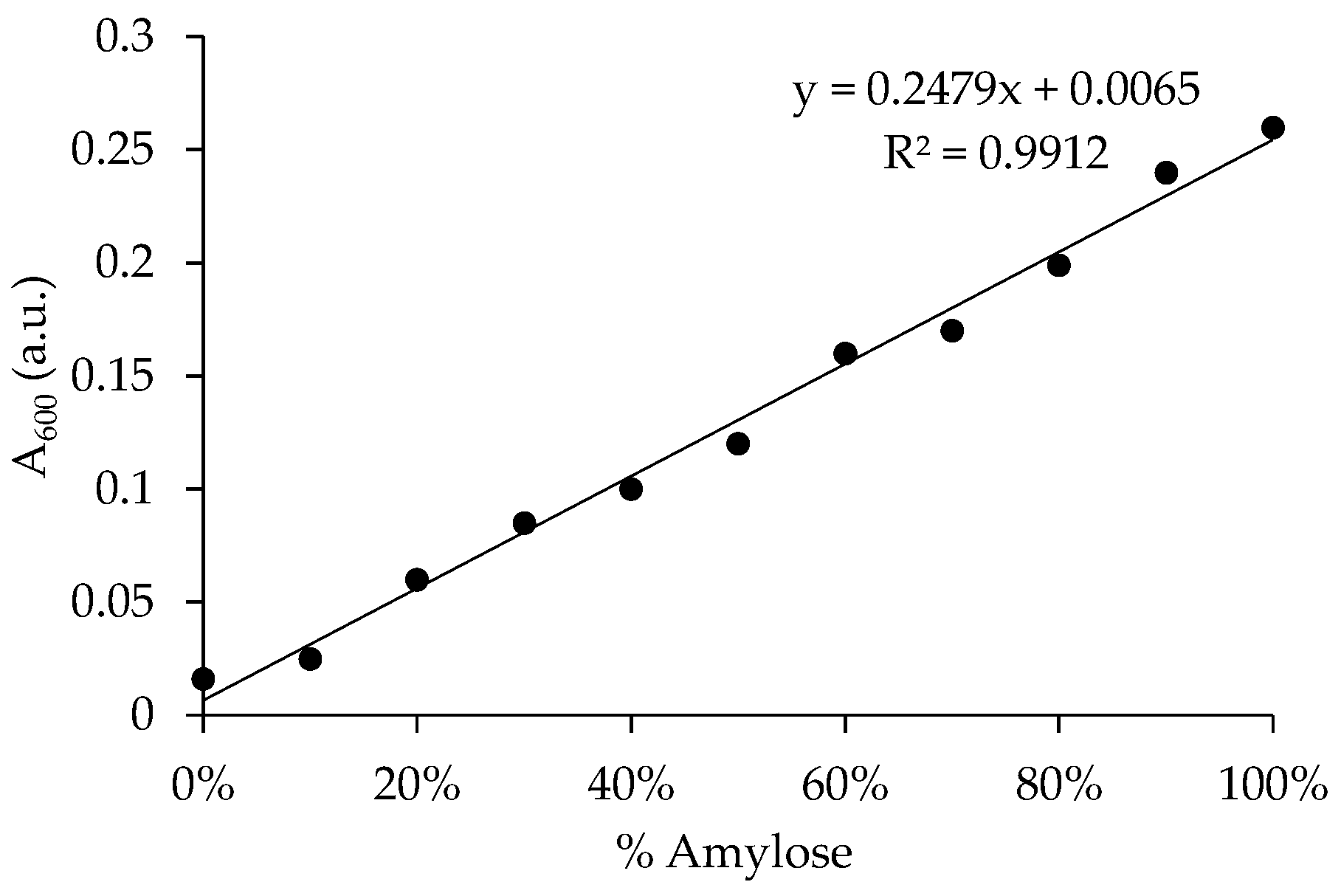


| Starch | wt % Real Amylose |
|---|---|
| Maize | 34% |
| Hylon V | 47% |
| Hylon VII | 70% |
| Precursor | Weight Ratio | wt% Amylose | S.A.BET (m2/g) | Vtotal (cm3/g) | % Mesoporosity | BJH Pore Diameter (nm) |
|---|---|---|---|---|---|---|
| Maize | 1 | 34% | 415 | 0.27 | 52% | 11 |
| HV | 1 | 47% | 458 | 0.54 | 70% | 17 |
| HVII | 1 | 70% | 547 | 0.80 | 77% | 20 |
| 36% MHVII | 1:0.07 | 36% | 424 | 0.29 | 53% | 11 |
| 44% MHVII | 1:0.39 | 44% | 370 | 0.34 | 98% | 16 |
| 52% MHVII | 1:1 | 52% | 484 | 0.50 | 74% | 25 |
| 60% MHVII | 1:2.55 | 60% | 536 | 0.87 | 80% | 22 |
| 36% MHV | 1:0.15 | 36% | 470 | 0.39 | 61% | 15 |
| 41% MHV | 1:1.30 | 41% | 475 | 0.55 | 75% | 23 |
| 57% HVHVII | 1:0.77 | 57% | 458 | 0.46 | 72% | 15 |
| Precursor | wt% Starch Mixture in IL | S.A.BET (m2/g) | Vtotal (cm3/g) | % Mesoporosity | BJH Pore Diameter (nm) |
|---|---|---|---|---|---|
| 60% MHVII 5 | 5 | 449 | 0.52 | 69 | 17 |
| 60% MHVII 10 | 10 | 448 | 0.42 | 60 | 16 |
| 60% MHVII 15 | 15 | 459 | 0.57 | 70 | 21 |
| 60% MHVII 20 | 20 | 536 | 0.87 | 80 | 22 |
| 60% MHVII 25 | 25 | 475 | 0.78 | 79 | 24 |
| Precursor | Weight Ratio | wt% Amylose | S.A.BET (m2/g) | Vtotal (cm3/g) | % Mesoporosity | BJH Pore Diameter (nm) |
|---|---|---|---|---|---|---|
| 60%MHVII no IL | 1:2.55 | 60% | 398 | 0.25 | 31 | 10 |
| 70%HVII no IL | 1 | 70% | 395 | 0.21 | 13 | 6 |
| Precursor | wt% Starch Mixture in IL | S.A.BET (m2/g) | Vtotal (cm3/g) | % Mesoporosity | BJH Pore Diameter (nm) |
|---|---|---|---|---|---|
| 60% MHVII-LC 5 | 5 | 359 | 0.51 | 77 | 14 |
| 60% MHVII-LC 10 | 10 | 410 | 0.37 | 51 | 17 |
| 60% MHVII-LC 15 | 15 | 441 | 0.56 | 70 | 19 |
| 60% MHVII-LC 20 | 20 | 335 | 0.33 | 59 | 14 |
| 60% MHVII-LC 25 | 25 | 412 | 0.38 | 55 | 13 |
| Precursor | S.A.BET (m2/g) | Vtotal (cm3/g) | % Mesoporosity | BJH Pore Diameter (nm) |
|---|---|---|---|---|
| 60% MHVII | 536 | 0.87 | 80 | 22 |
| 60% MHVII-IL recycled x1 | 490 | 0.59 | 67 | 23 |
| 60% MHVII-IL recycled x2 | 486 | 0.49 | 63 | 14 |
| 60% MHVII-IL recycled x3 | 417 | 0.52 | 70 | 23 |
| 60% MHVII-IL recycled + MS | 469 | 0.82 | 81 | 23 |
| Precursor | S.A.BET (m2/g) | Vtotal (cm3/g) | % Mesoporosity | BJH Pore Diameter (nm) | wt% N | ID/IG |
|---|---|---|---|---|---|---|
| Chitosan 1000 | 217 | 0.45 | 96 | 14 | 5.50 | 1.00 |
| 60% MHVII 1000 | 554 | 0.71 | 71 | 21 | - | 1.02 |
| 52% MHVII 1000 | 541 | 0.64 | 69 | 17 | - | 1.00 |
| 36% MHVII 1000 | 529 | 0.54 | 61 | 17 | - | 1.03 |
| Timcal (ref.) | 67 | 0.19 | 100 | 14 | - | 1.01 |
| Precursor | Specific Discharge Capacity (mAh/g of C) | Total Cycles | Active Material (mg) |
|---|---|---|---|
| Timcal (ref.) | 1997 | 14 | 3.14 |
| Chitosan 1000 | 4057 | 69 | 3.36 |
| 60% MHVII 1000 | 3978 | 60 | 3.80 |
| 52% MHVII 1000 | 3157 | - | 3.82 |
| 36% MHVII 1000 | 2570 | - | 4.12 |
| Precursor | Method | S.A.BET (m2/g) | Vtotal (cm3/g) | % Mesoporosity | BJH Pore Diameter (nm) |
|---|---|---|---|---|---|
| 60% MHVII | IL + Starbon® | 536 | 0.87 | 80 | 22 |
| IL | 335 | 0.33 | 59 | 14 | |
| Starbon® | 398 | 0.25 | 31 | 10 | |
| 70% HVII | IL + Starbon® | 547 | 0.80 | 77 | 20 |
| IL | 511 | 0.44 | 59 | 16 | |
| Starbon® | 395 | 0.21 | 13 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uriburu-Gray, M.; Pinar-Serrano, A.; Cavus, G.; Knipping, E.; Aucher, C.; Conesa-Cabeza, A.; Satti, A.; Amantia, D.; Martínez-Crespiera, S. Mesoporous Carbons from Polysaccharides and Their Use in Li-O2 Batteries. Nanomaterials 2020, 10, 2036. https://doi.org/10.3390/nano10102036
Uriburu-Gray M, Pinar-Serrano A, Cavus G, Knipping E, Aucher C, Conesa-Cabeza A, Satti A, Amantia D, Martínez-Crespiera S. Mesoporous Carbons from Polysaccharides and Their Use in Li-O2 Batteries. Nanomaterials. 2020; 10(10):2036. https://doi.org/10.3390/nano10102036
Chicago/Turabian StyleUriburu-Gray, María, Aránzazu Pinar-Serrano, Gokhan Cavus, Etienne Knipping, Christophe Aucher, Aleix Conesa-Cabeza, Amro Satti, David Amantia, and Sandra Martínez-Crespiera. 2020. "Mesoporous Carbons from Polysaccharides and Their Use in Li-O2 Batteries" Nanomaterials 10, no. 10: 2036. https://doi.org/10.3390/nano10102036






