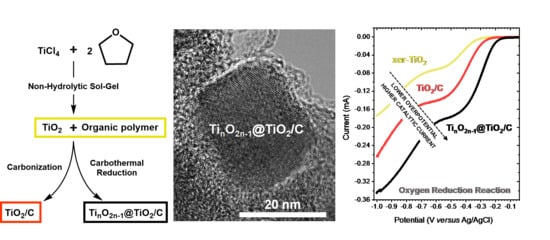
TinO2n−1 Suboxide Phases in TiO2/C Nanocomposites Engineered by Non-hydrolytic Sol–Gel with Enhanced Electrocatalytic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Characterization
2.3. Electrochemical Properties
3. Results
3.1. Synthesis and Characterization
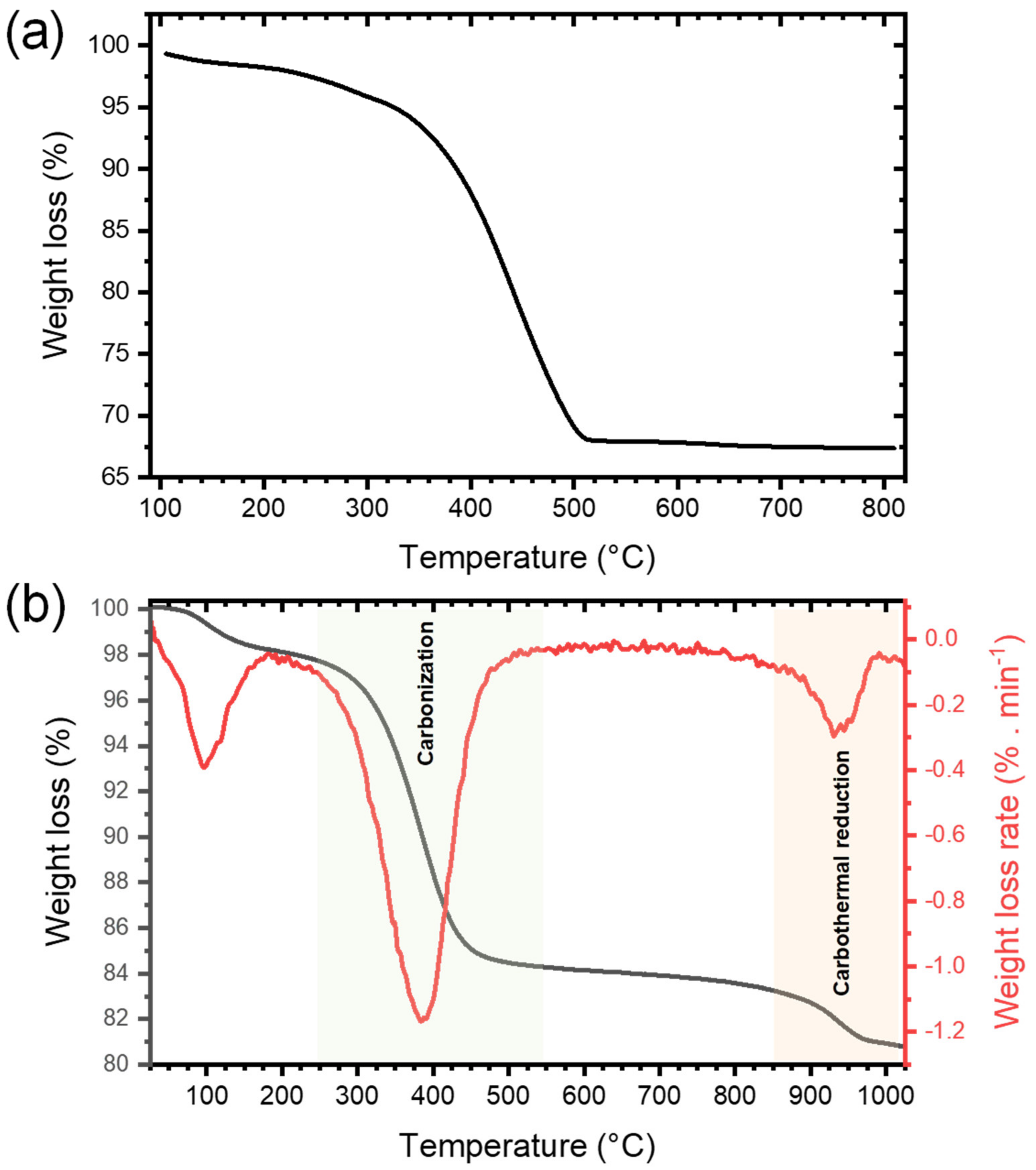
3.1.1. NHSG Ether Route and Carbothermal Reduction: Mechanistic Insights
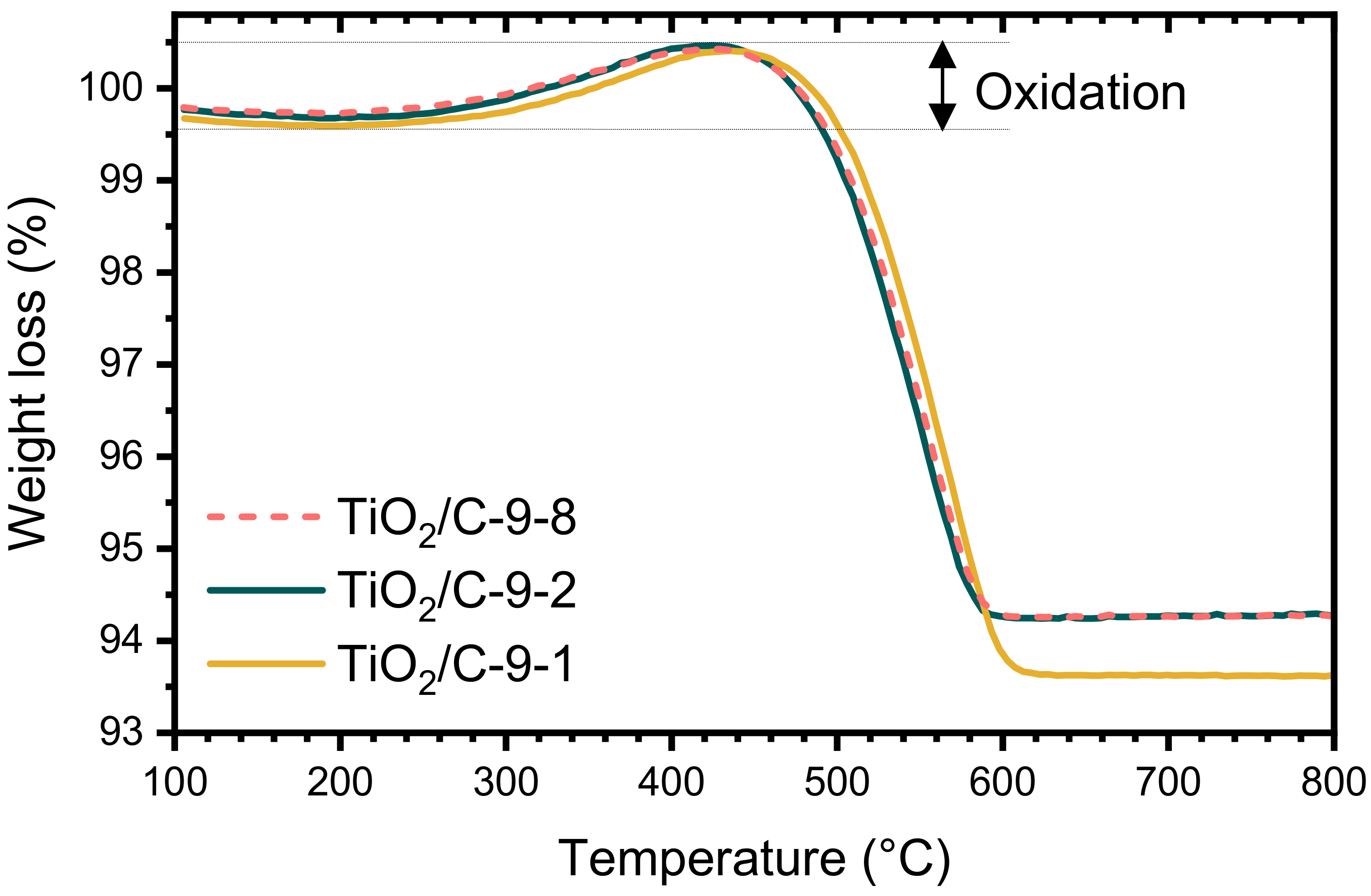
3.1.2. TiO2 and TiO2/C Nanocomposites: Characterization
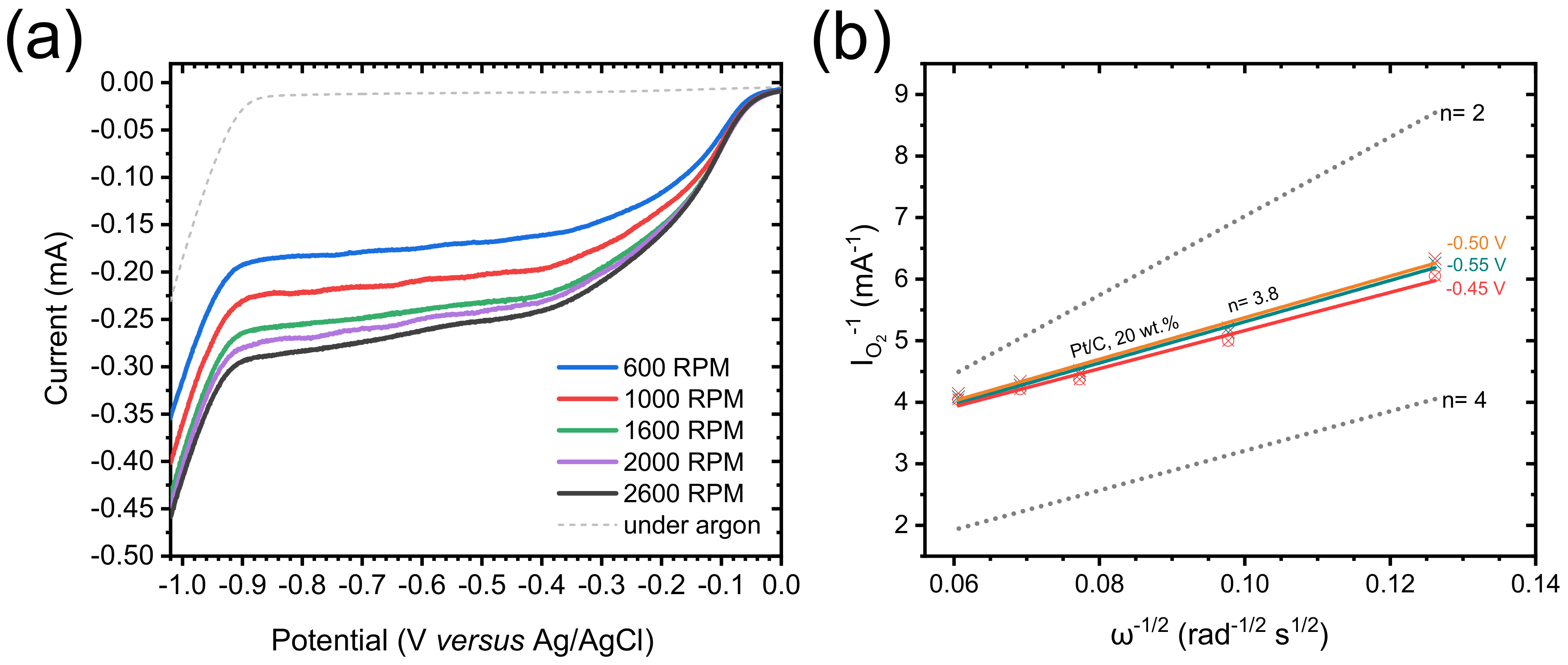
3.2. Electrochemical Properties Towards the Oxygen Reduction Reaction (ORR)
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Harada, S.; Tanaka, K.; Inui, H. Thermoelectric properties and crystallographic shear structures in titanium oxides of the Magneli phases. J. Appl. Phys. 2010, 108, 083703. [Google Scholar] [CrossRef]
- Padilha, A.C.M.; Osorio-Guillen, J.M.; Rocha, A.R.; Dalpian, G.M. TinO2n−1 Magneli phases studied using density functional theory. Phys. Rev. B 2014, 90, 035213. [Google Scholar] [CrossRef]
- Padilha, A.C.M.; Raebiger, H.; Rocha, A.R.; Dalpian, G.M. Charge storage in oxygen deficient phases of TiO2: Defect Physics without defects. Sci. Rep. 2016, 6, 28871. [Google Scholar] [CrossRef] [PubMed]
- Szot, K.; Rogala, M.; Speier, W.; Klusek, Z.; Besmehn, A.; Waser, R. TiO2-a prototypical memristive material. Nanotechnology 2011, 22, 254001. [Google Scholar] [CrossRef]
- Anderson, J.S.; Tilley, R.J.D. Crystallographic Shear in Oxygen-Deficient Rutile: An Electron Microscope Study. J. Solid State Chem. 1970, 2, 472–482. [Google Scholar] [CrossRef]
- Chen, X.B.; Liu, L.; Huang, F.Q. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef]
- Cho, E.; Han, S.; Ahn, H.S.; Lee, K.R.; Kim, S.K.; Hwang, C.S. First-principles study of point defects in rutile TiO2-x. Phys. Rev. B 2006, 73, 193202. [Google Scholar] [CrossRef]
- Andersson, S.; Collen, B.; Kuylenstierna, U.; Magneli, A. Phase Analysis Studies on the Titanium-Oxygen System. Acta Chem. Scand. 1957, 11, 1641–1652. [Google Scholar] [CrossRef]
- Andersson, S.; Collen, B.; Kruuse, G.; Kuylenstierna, U.; Magneli, A.; Pestmalis, H.; Asbrink, S. Identification of Titanium Oxides by X-Ray Powder Patterns. Acta Chem. Scand. 1957, 11, 1653–1657. [Google Scholar] [CrossRef]
- Asbrink, S.; Magneli, A. Crystal Structure Studies on Trititanium Pentoxide, Ti3O5. Acta Crystallogr. 1959, 12, 575–581. [Google Scholar] [CrossRef]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A review of its properties and conflicting trends. Chem. Eng. J. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Bartholomew, R.F.; Frankl, D.R. Electrical Properties of Some Titanium Oxides. Phys. Rev. 1969, 187, 828–833. [Google Scholar] [CrossRef]
- Han, W.Q.; Zhang, Y. Magneli phases TinO2n−1 nanowires: Formation, optical, and transport properties. Appl. Phys. Lett. 2008, 92, 203117. [Google Scholar] [CrossRef]
- Niu, M.; Tan, H.Q.; Cheng, D.J.; Sun, Z.C.; Cao, D.P. Bandgap engineering of Magnei phase TinO2n−1: Electron-hole self-compensation. J. Chem. Phys. 2015, 143, 054701. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.F.; Xu, Z.F.; Ren, L.; Liu, C.; Zhuang, J.C.; Hu, Z.P.; Xu, X.; Chen, J.; Wang, J.O.; Hao, W.C.; et al. Activating Titania for Efficient Electrocatalysis by Vacancy Engineering. ACS Catal. 2018, 8, 4288–4293. [Google Scholar] [CrossRef]
- Oturan, N.; Ganiyu, S.O.; Raffy, S.; Oturan, M.A. Sub-stoichiometric titanium oxide as a new anode material for electro-Fenton process: Application to electrocatalytic destruction of antibiotic amoxicillin. Appl. Catal. B-Environ. 2017, 217, 214–223. [Google Scholar] [CrossRef]
- Pei, D.N.; Gong, L.; Zhang, A.Y.; Zhang, X.; Chen, J.J.; Mu, Y.; Yu, H.Q. Defective titanium dioxide single crystals exposed by high-energy {001} facets for efficient oxygen reduction. Nat. Commun. 2015, 6, 8696. [Google Scholar] [CrossRef]
- Yao, C.H.; Li, F.; Li, X.; Xia, D.G. Fiber-like nanostructured Ti4O7 used as durable fuel cell catalyst support in oxygen reduction catalysis. J. Mater. Chem. 2012, 22, 16560–16565. [Google Scholar] [CrossRef]
- Massazza, D.; Parra, R.; Busalmen, J.P.; Romeo, H.E. New ceramic electrodes allow reaching the target current density in bioelectrochemical systems. Energ. Environ. Sci. 2015, 8, 2707–2712. [Google Scholar] [CrossRef]
- Domaschke, M.; Zhou, X.M.; Wergen, L.; Romeis, S.; Miehlich, M.E.; Meyer, K.; Peukert, W.; Schmuki, P. Magneli-Phases in Anatase Strongly Promote Cocatalyst-Free Photocatalytic Hydrogen Evolution. ACS Catal. 2019, 9, 3627–3632. [Google Scholar] [CrossRef]
- Hamad, H.; Bailon-Garcia, E.; Maldonado-Hodar, F.J.; Perez-Cadenas, A.F.; Carrasco-Marin, F.; Morales-Torres, S. Synthesis of TixOy nanocrystals in mild synthesis conditions for the degradation of pollutants under solar light. Appl. Catal. B-Environ. 2019, 241, 385–392. [Google Scholar] [CrossRef]
- An, X.Q.; Hu, C.Z.; Liu, H.J.; Qu, J.H. Oxygen vacancy mediated construction of anatase/brookite heterophase junctions for high-efficiency photocatalytic hydrogen evolution. J. Mater. Chem. A 2017, 5, 24989–24994. [Google Scholar] [CrossRef]
- Negi, S.S. Enhanced light harvesting and charge separation over wormhole mesoporous TiO2−x nanocrystallites towards efficient hydrogen generation. Sustain. Energ. Fuels 2019, 3, 1191–1200. [Google Scholar] [CrossRef]
- Tao, X.Y.; Wang, J.G.; Ying, Z.G.; Cai, Q.X.; Zheng, G.Y.; Gan, Y.P.; Huang, H.; Xia, Y.; Liang, C.; Zhang, W.K.; et al. Strong Sulfur Binding with Conducting Magneli-Phase TinO2n−1 Nanomaterials for Improving Lithium-Sulfur Batteries. Nano Lett. 2014, 14, 5288–5294. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Rodriguez, E.F.; Best, A.S.; Hollenkamp, A.F.; Chen, D.H.; Caruso, R.A. Chemical Bonding and Physical Trapping of Sulfur in Mesoporous Magneli Ti4O7 Microspheres for High- Performance Li-S Battery. Adv. Energy Mater. 2017, 7, 1601616. [Google Scholar] [CrossRef]
- Kitada, A.; Hasegawa, G.; Kobayashi, Y.; Kanamori, K.; Nakanishi, K.; Kageyama, H. Selective Preparation of Macroporous Monoliths of Conductive Titanium Oxides TinO2n−1 (n = 2, 3, 4, 6). J. Am. Chem. Soc. 2012, 134, 10894–10898. [Google Scholar] [CrossRef]
- Azor-Lafarga, A.; Ruiz-Gonzalez, L.; Parras, M.; Portehault, D.; Sanchez, C.; Gonzalez-Calbet, J.M. Modified Synthesis Strategies for the Stabilization of low n TinO2n-1 Magneli Phases. Chem. Rec. 2018, 18, 1105–1113. [Google Scholar] [CrossRef]
- Portehault, D.; Maneeratana, V.; Candolfi, C.; Deschler, N.; Veremchuk, I.; Grin, Y.; Sanchez, C.; Antonietti, M. Facile General Route toward Tunable Magneli Nanostructures and Their Use As Thermoelectric Metal Oxide/Carbon Nanocomposites. ACS Nano 2011, 5, 9052–9061. [Google Scholar] [CrossRef]
- Huang, S.S.; Lin, Y.H.; Chuang, W.; Shao, P.S.; Chuang, C.H.; Lee, J.F.; Lu, M.L.; Weng, Y.T.; Wu, N.L. Synthesis of High-Performance Titanium Sub-Oxides for Electrochemical Applications Using Combination of Sol-Gel and Vacuum-Carbothermic Processes. ACS Sustain. Chem. Eng. 2018, 6, 3162–3168. [Google Scholar] [CrossRef]
- Tominaka, S.; Tsujimoto, Y.; Matsushita, Y.; Yamaura, K. Synthesis of Nanostructured Reduced Titanium Oxide: Crystal Structure Transformation Maintaining Nanomorphology. Angew. Chem. Int. Edit. 2011, 50, 7418–7421. [Google Scholar] [CrossRef]
- Kitada, A.; Hasegawa, G.; Kobayashi, Y.; Miyazaki, K.; Abe, T.; Kanamori, K.; Nakanishi, K.; Kageyama, H. Hierarchically Porous Monoliths of Oxygen-deficient Anatase TiO2−x with Electronic Conductivity. RSC Adv. 2013, 3, 26475. [Google Scholar] [CrossRef]
- Mei, S.L.; Jafta, C.J.; Lauermann, I.; Ran, Q.; Kargell, M.; Ballauff, M.; Lu, Y. Porous Ti4O7 Particles with Interconnected-Pore Structure as a High-Efficiency Polysulfide Mediator for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2017, 27, 1701176. [Google Scholar] [CrossRef]
- Mutin, P.H.; Vioux, A. Recent advances in the synthesis of inorganic materials via non-hydrolytic condensation and related low-temperature routes. J. Mater. Chem. A 2013, 1, 11504–11512. [Google Scholar] [CrossRef]
- Niederberger, M.; Garnweitner, G. Organic reaction pathways in the nonaqueous synthesis of metal oxide nanoparticles. Chem. Eur. J. 2006, 12, 7282–7302. [Google Scholar] [CrossRef] [PubMed]
- Debecker, D.P.; Hulea, V.; Mutin, P.H. Mesoporous mixed oxide catalysts via non-hydrolytic sol-gel: A review. Appl. Catal. A Gen. 2013, 451, 192–206. [Google Scholar] [CrossRef]
- Pinna, N.; Niederberger, M. Surfactant-free nonaqueous synthesis of metal oxide nanostructures. Angew. Chem. Int. Edit. 2008, 47, 5292–5304. [Google Scholar] [CrossRef]
- Deshmukh, R.; Niederberger, M. Mechanistic Aspects in the Formation, Growth and Surface Functionalization of Metal Oxide Nanoparticles in Organic Solvents. Chem. Eur. J. 2017, 23, 8542–8570. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, M.; Garnweitner, G.; Pinna, N.; Neri, G. Non-aqueous routes to crystalline metal oxide nanoparticles: Formation mechanisms and applications. Prog. Solid State Chem. 2005, 33, 59–70. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous sol-gel routes to metal oxide nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef]
- Hay, J.N.; Raval, H.M. Synthesis of organic-inorganic hybrids via the non-hydrolytic sol-gel process. Chem. Mater. 2001, 13, 3396–3403. [Google Scholar] [CrossRef]
- Wang, Y.H.; Alauzun, J.G.; Mutin, P.H. Water-Stable, Nonsiliceous Hybrid Materials with Tunable Porosity and Functionality: Bridged Titania-Bisphosphonates. Chem. Mater. 2020, 32, 2910–2918. [Google Scholar] [CrossRef]
- Escamilla-Perez, A.M.; Louvain, N.; Boury, B.; Brun, N.; Mutin, P.H. Ethers as Oxygen Donor and Carbon Source in Non-hydrolytic Sol-Gel: One-Pot, Atom-Economic Synthesis of Mesoporous TiO2-Carbon Nanocomposites. Chem. Eur. J. 2018, 24, 4982–4990. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Russo, P.A.; Goubard-Bretesche, N.; Patane, S.; Santangelo, S.; Zhang, R.; Pinna, N. Exploiting the Condensation Reactions of Acetophenone to Engineer Carbon-Encapsulated Nb2O5 Nanocrystals for High-Performance Li and Na Energy Storage Systems. Adv. Energy Mater. 2019, 9, 1902813. [Google Scholar] [CrossRef]
- Masa, J.; Batchelor-McAuley, C.; Schuhmann, W.; Compton, R.G. Koutecky-Levich analysis applied to nanoparticle modified rotating disk electrodes: Electrocatalysis or misinterpretation? Nano Res. 2014, 7, 71–78. [Google Scholar] [CrossRef]
- Escamilla-Perez, A.M.; Louvain, N.; Kaschowitz, M.; Freunberger, S.; Fontaine, O.; Boury, B.; Brun, N.; Mutin, P.H. Lithium insertion properties of mesoporous nanocrystalline TiO2 and TiO2-V2O5 microspheres prepared by non-hydrolytic sol-gel. J. Sol-Gel Sci. Technol. 2016, 79, 270–278. [Google Scholar] [CrossRef]
- Nolan, N.T.; Seery, M.K.; Pillai, S.C. Spectroscopic Investigation of the Anatase-to-Rutile Transformation of Sol-Gel-Synthesized TiO2 Photocatalysts. J. Phys. Chem. C 2009, 113, 16151–16157. [Google Scholar] [CrossRef]
- Tsumura, T.; Kojitani, N.; Izumi, I.; Iwashita, N.; Toyoda, M.; Inagaki, M. Carbon coating of anatase-type TiO2 and photoactivity. J. Mater. Chem. 2002, 12, 1391–1396. [Google Scholar] [CrossRef]
- Escamilla-Pérez, A.M. Non-hydrolytic sol-gel synthesis of TiO2-based electrode materials for Li-ion batteries. Ph.D. Thesis, University Montpellier, Montpellier, France, September 2018. [Google Scholar]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Dai, L.M.; Xue, Y.H.; Qu, L.T.; Choi, H.J.; Baek, J.B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Shao, M.H.; Chang, Q.W.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Ge, X.M.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z.L. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Li, Y.G.; Dai, H.J. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, N.; Mukerjee, S. Alkaline Anion-Exchange Membrane Fuel Cells: Challenges in Electrocatalysis and Interfacial Charge Transfer. Chem. Rev. 2019, 119, 11945–11979. [Google Scholar] [CrossRef] [PubMed]
- Brun, N.; Wohlgemuth, S.A.; Osiceanu, P.; Titirici, M.M. Original design of nitrogen-doped carbon aerogels from sustainable precursors: Application as metal-free oxygen reduction catalysts. Green Chem. 2013, 15, 2514–2524. [Google Scholar] [CrossRef]
- Pham-Truong, T.N.; Petenzi, T.; Ranjan, C.; Randriamahazaka, H.; Ghilane, J. Microwave assisted synthesis of carbon dots in ionic liquid as metal free catalyst for highly selective production of hydrogen peroxide. Carbon 2018, 130, 544–552. [Google Scholar] [CrossRef]
- Appel, A.M.; Helm, M.L. Determining the Overpotential for a Molecular Electrocatalyst. ACS Catal. 2014, 4, 630–633. [Google Scholar] [CrossRef]
- Jha, A.; Yoon, S.J. Formation of titanium carbonitride phases via the reduction of TiO2 with carbon in the presence of nitrogen. J. Mater. Sci. 1999, 34, 307–322. [Google Scholar] [CrossRef]
- Kim, S.; De Bruyn, M.; Alauzun, J.G.; Louvain, N.; Brun, N.; Macquarrie, D.J.; Stievano, L.; Mutin, P.H.; Monconduit, L.; Boury, B. Dehydration of Alginic Acid Cryogel by TiCl4 vapor: Direct Access to Mesoporous TiO2@C Nanocomposites and Their Performance in Lithium-Ion Batteries. Chemsuschem 2019, 12, 2660–2670. [Google Scholar] [CrossRef]


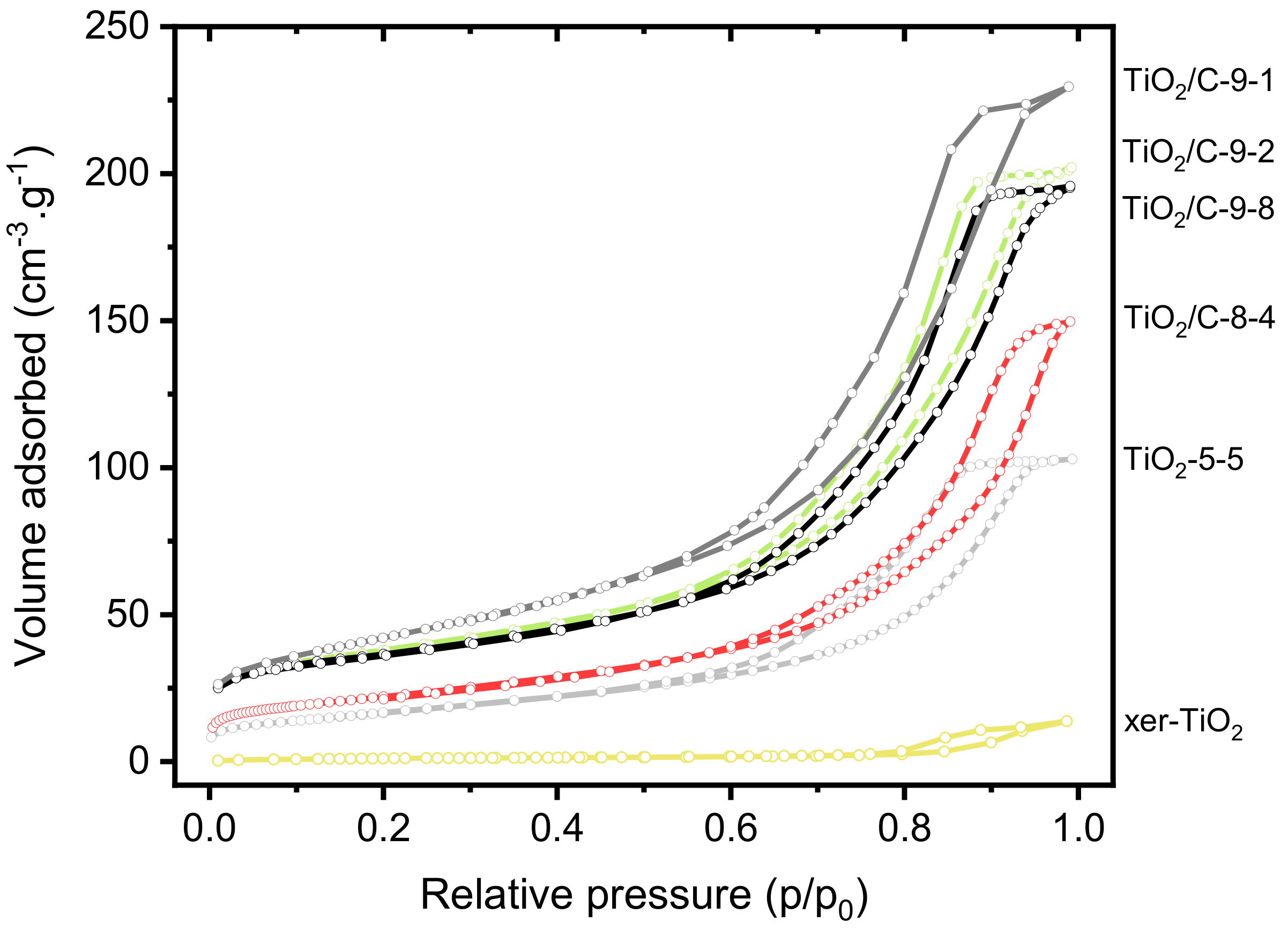


| Sample | Organic (wt%) † | C (wt%) ‡ | H (wt%) ‡ | SSABET (m2 g−1) # | V0.99 (cm3 g−1) | TiO2 Phase(s) | TinO2n−1 Phases |
|---|---|---|---|---|---|---|---|
| xer-TiO2 | 31 ± 3 | 20 ± 2 | 2.4 ± 0.2 | 4 | 0.02 | Anatase (A) | None |
| TiO2-5-5 | n/a | n/a | n/a | 60 | 0.16 | A | None |
| TiO2/C-8-4 | 4.0 ± 0.4 | 5.0 ± 0.1 | 0.1 | 79 | 0.23 | A/Rutile (R) | None |
| TiO2/C-9-1 | 6.8 ± 0.6 | 6.3 ± 0.1 | < 0.1 | 150 | 0.35 | A | Ti3O5/Ti6O11 |
| TiO2/C-9-2 | 6.2 ± 0.5 | n/a | n/a | 130 | 0.31 | A | Ti3O5/Ti6O11 |
| TiO2/C-9-8 | 6.2 ± 0.5 | 6.3 ± 0.1 | < 0.1 | 124 | 0.30 | A | Ti3O5/Ti6O11 |
| Sample | Onset potential (V) a | Ecat/2 (V) b | Icat (mA mg−1) c | Ik (mA mg−1) d | nelectrons e |
|---|---|---|---|---|---|
| xer-TiO2 | −0.20 | −0.43 | 1.27 | n/a | n/a |
| TiO2-5-5 | −0.20 | −0.40 | 1.44 | n/a | n/a |
| TiO2/C-8-4 | −0.16 | −0.39 | 2.80 | n/a | n/a |
| TiO2/C-9-1 | −0.09 | −0.31 | 3.32 | 5.1 | 2.1 ± 0.2 |
| TiO2/C-9-8 | −0.09 | −0.30 | 3.78 | 5.3 | 3.0 ± 0.2 |
| Pt/C, 20 wt.% | −0.01 | −0.13 | 4.80 | 10.0 | 3.8 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, S.; Berthelot, R.; Boury, B.; Mutin, P.H.; Brun, N. TinO2n−1 Suboxide Phases in TiO2/C Nanocomposites Engineered by Non-hydrolytic Sol–Gel with Enhanced Electrocatalytic Properties. Nanomaterials 2020, 10, 1789. https://doi.org/10.3390/nano10091789
Zou S, Berthelot R, Boury B, Mutin PH, Brun N. TinO2n−1 Suboxide Phases in TiO2/C Nanocomposites Engineered by Non-hydrolytic Sol–Gel with Enhanced Electrocatalytic Properties. Nanomaterials. 2020; 10(9):1789. https://doi.org/10.3390/nano10091789
Chicago/Turabian StyleZou, Shuxian, Romain Berthelot, Bruno Boury, Pierre Hubert Mutin, and Nicolas Brun. 2020. "TinO2n−1 Suboxide Phases in TiO2/C Nanocomposites Engineered by Non-hydrolytic Sol–Gel with Enhanced Electrocatalytic Properties" Nanomaterials 10, no. 9: 1789. https://doi.org/10.3390/nano10091789
APA StyleZou, S., Berthelot, R., Boury, B., Mutin, P. H., & Brun, N. (2020). TinO2n−1 Suboxide Phases in TiO2/C Nanocomposites Engineered by Non-hydrolytic Sol–Gel with Enhanced Electrocatalytic Properties. Nanomaterials, 10(9), 1789. https://doi.org/10.3390/nano10091789