Carbon Nanotube Sheet-Synthesis and Applications
Abstract
1. Introduction
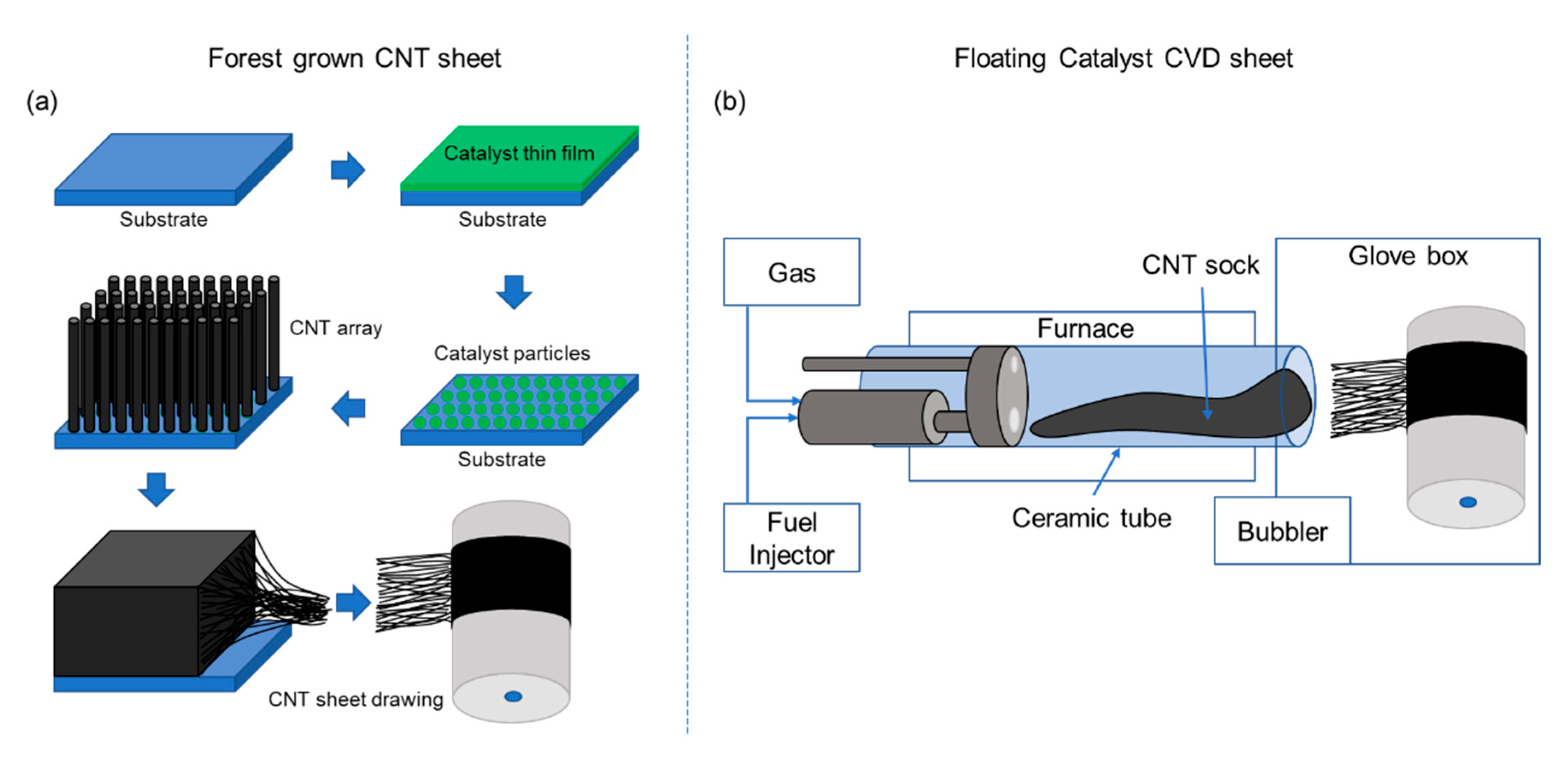
2. Manufacturing CNT Sheet Using the Floating Catalyst Method
2.1. Carbon Nanotube Materials Synthesis
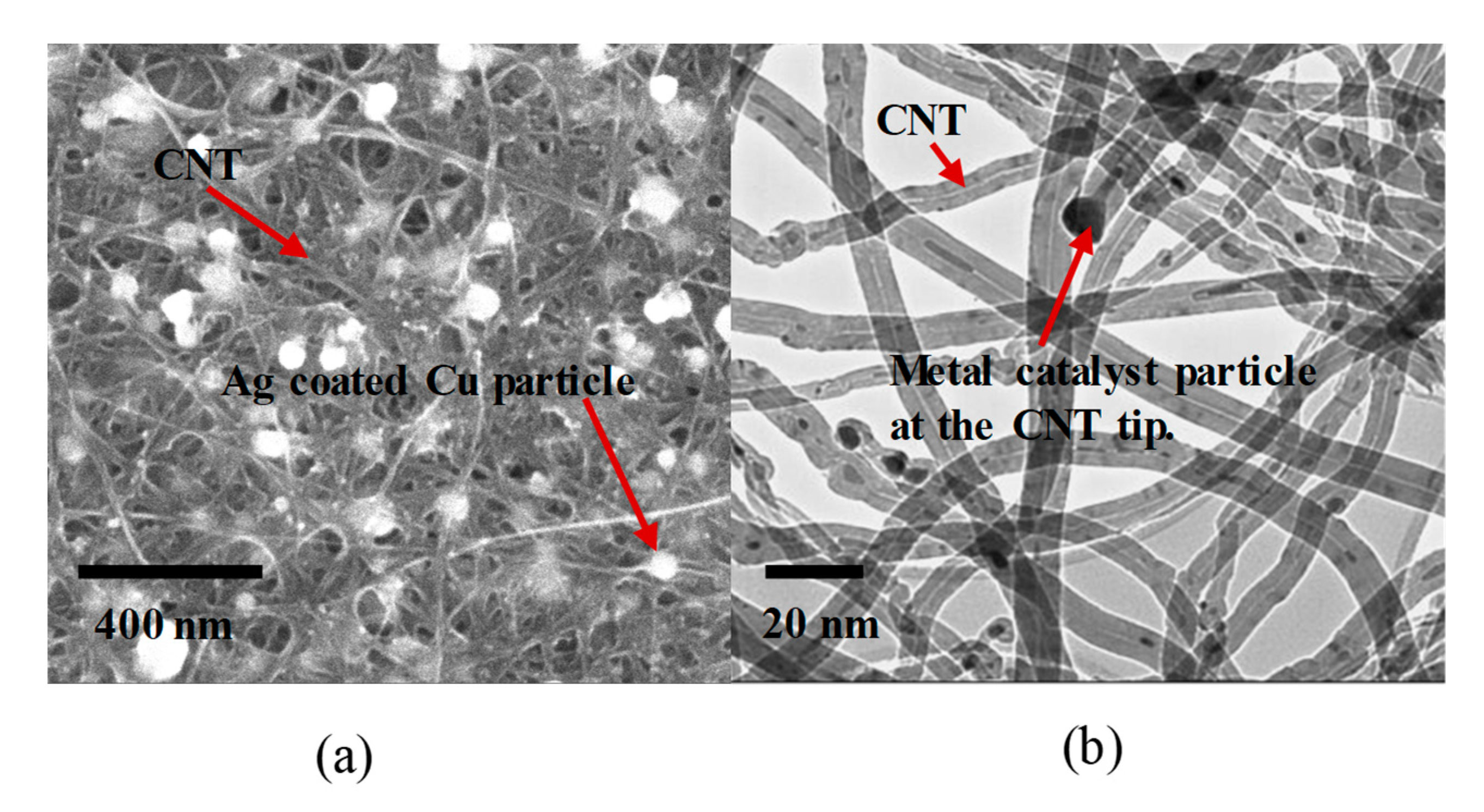
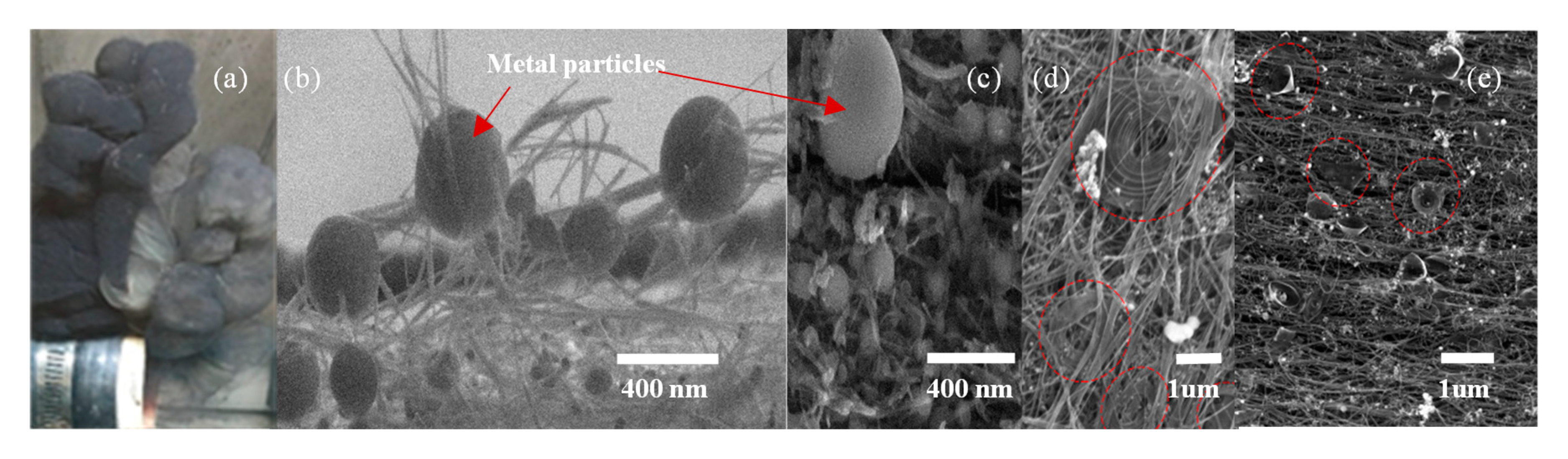
2.2. Integration of Nanoparticles in CNT Sheet
2.3. Materials and Methods
3. Characterization of CNT Material
3.1. CNT Sheet Characterization Using Microscopy
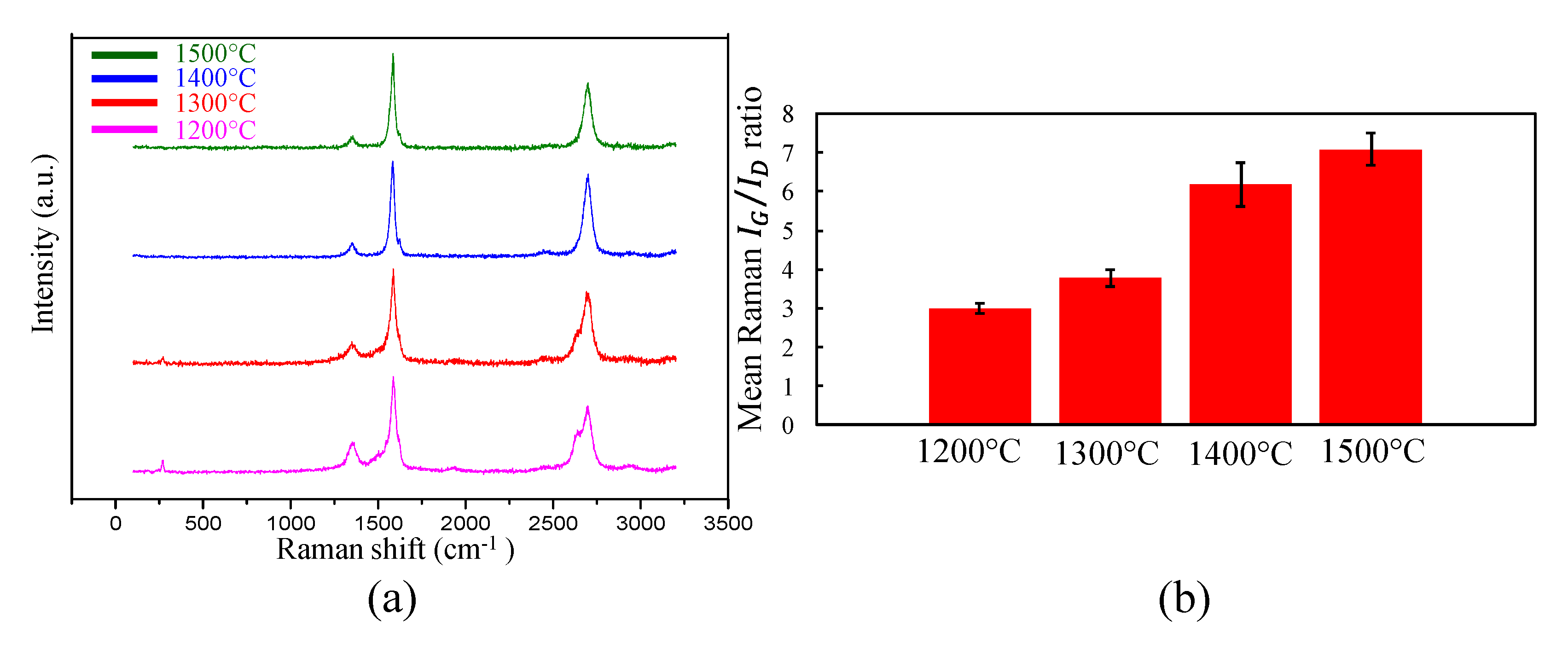
3.2. Raman Spectroscopy
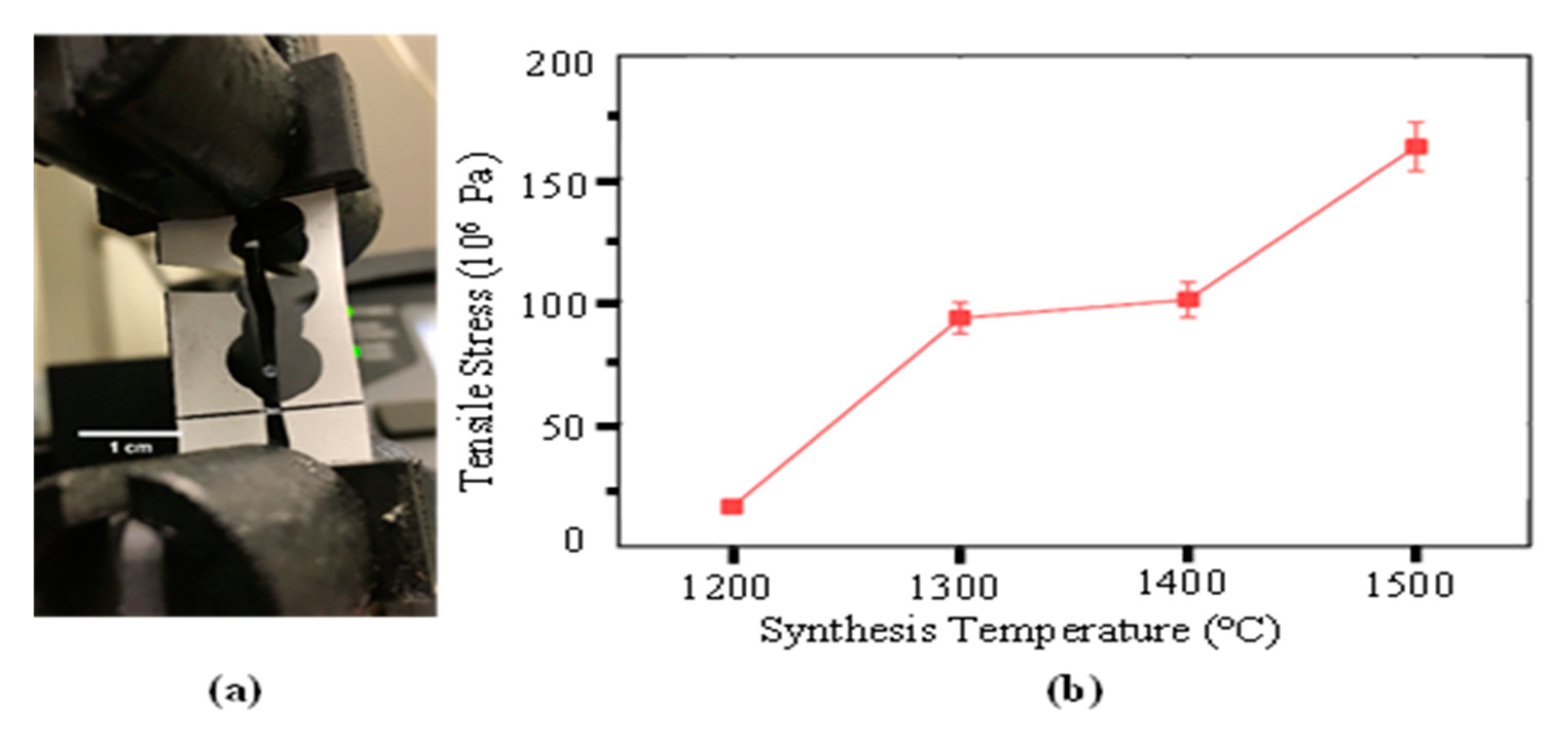
3.3. Tensile Strength Testing
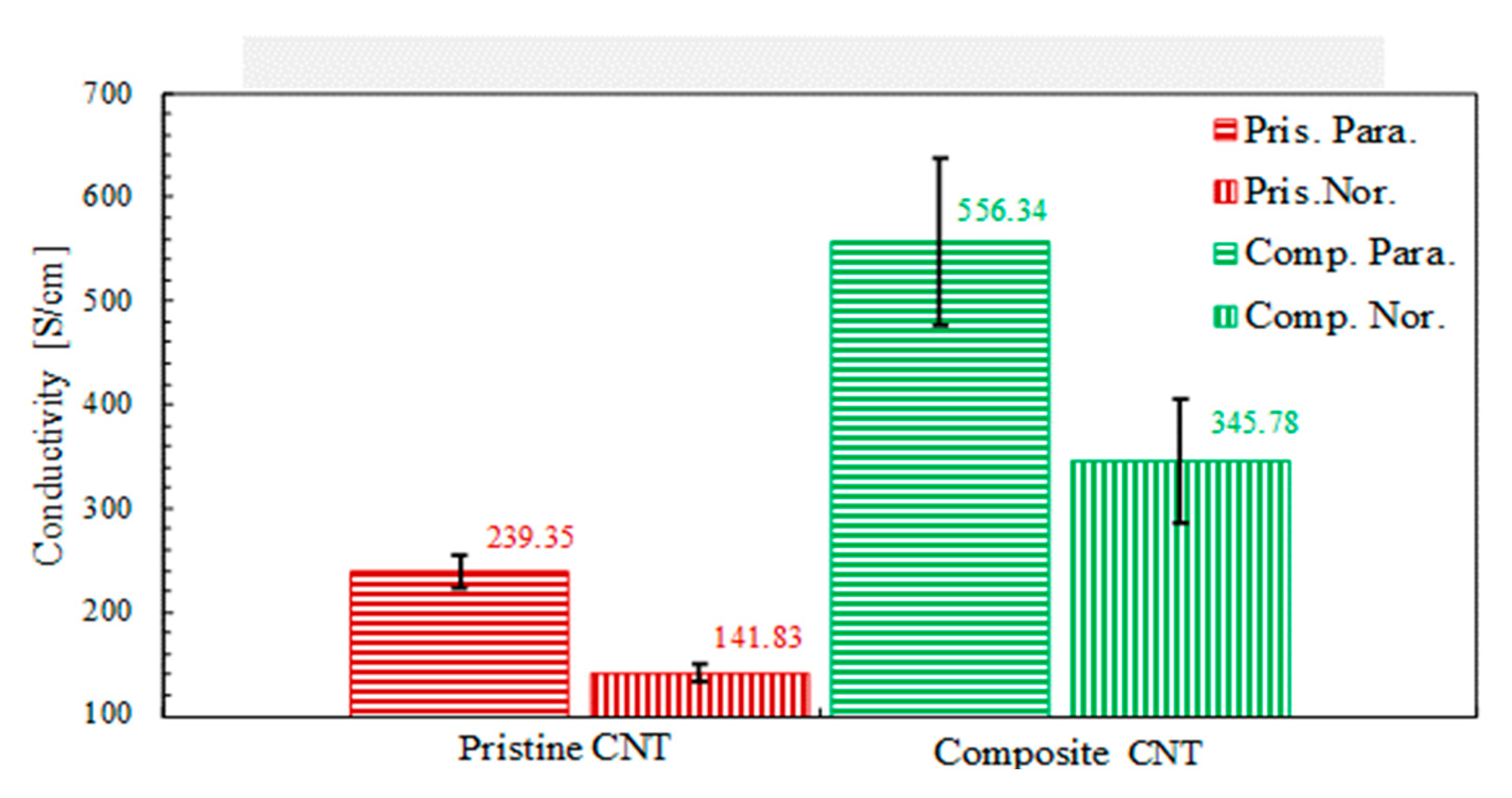
3.4. Electrical Conductivity
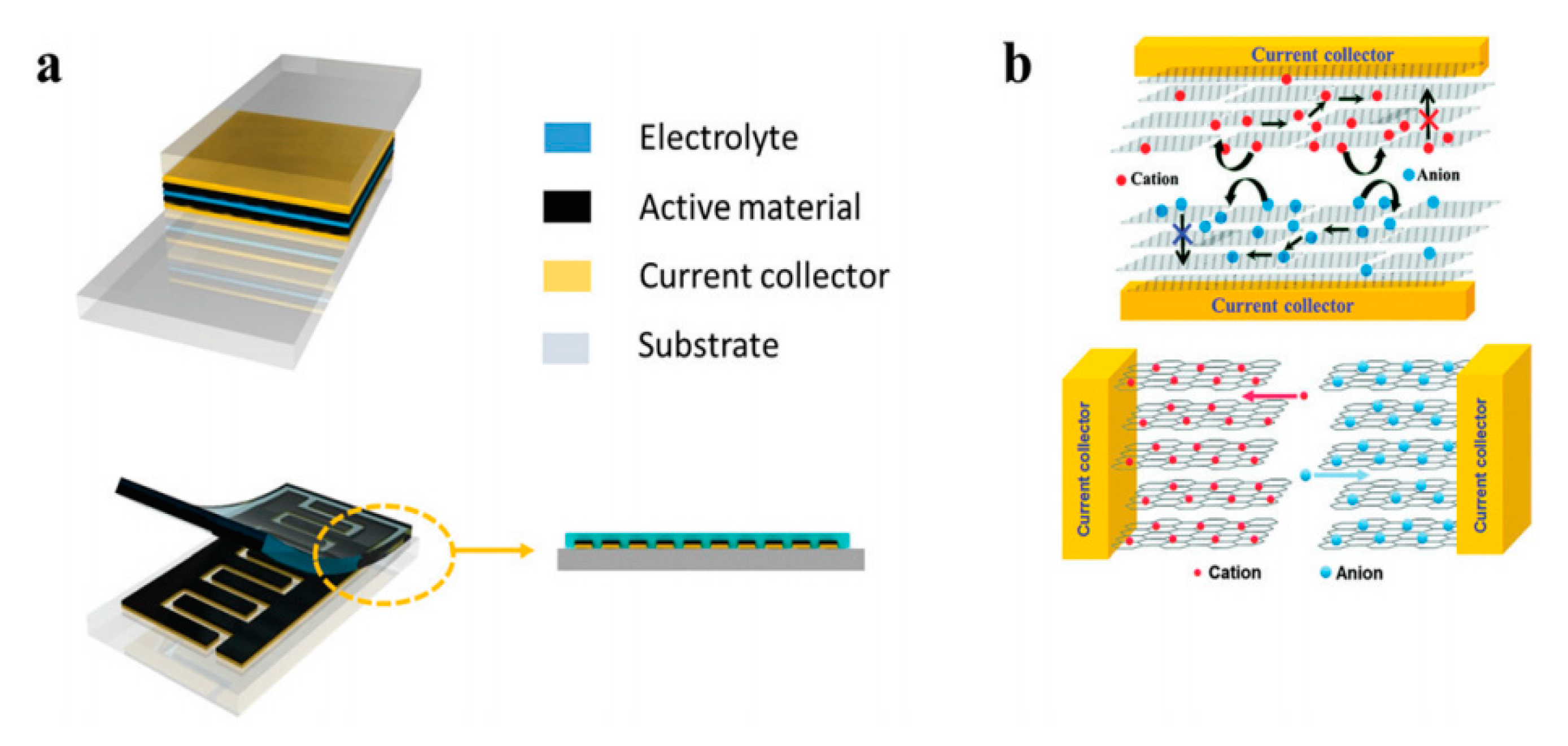
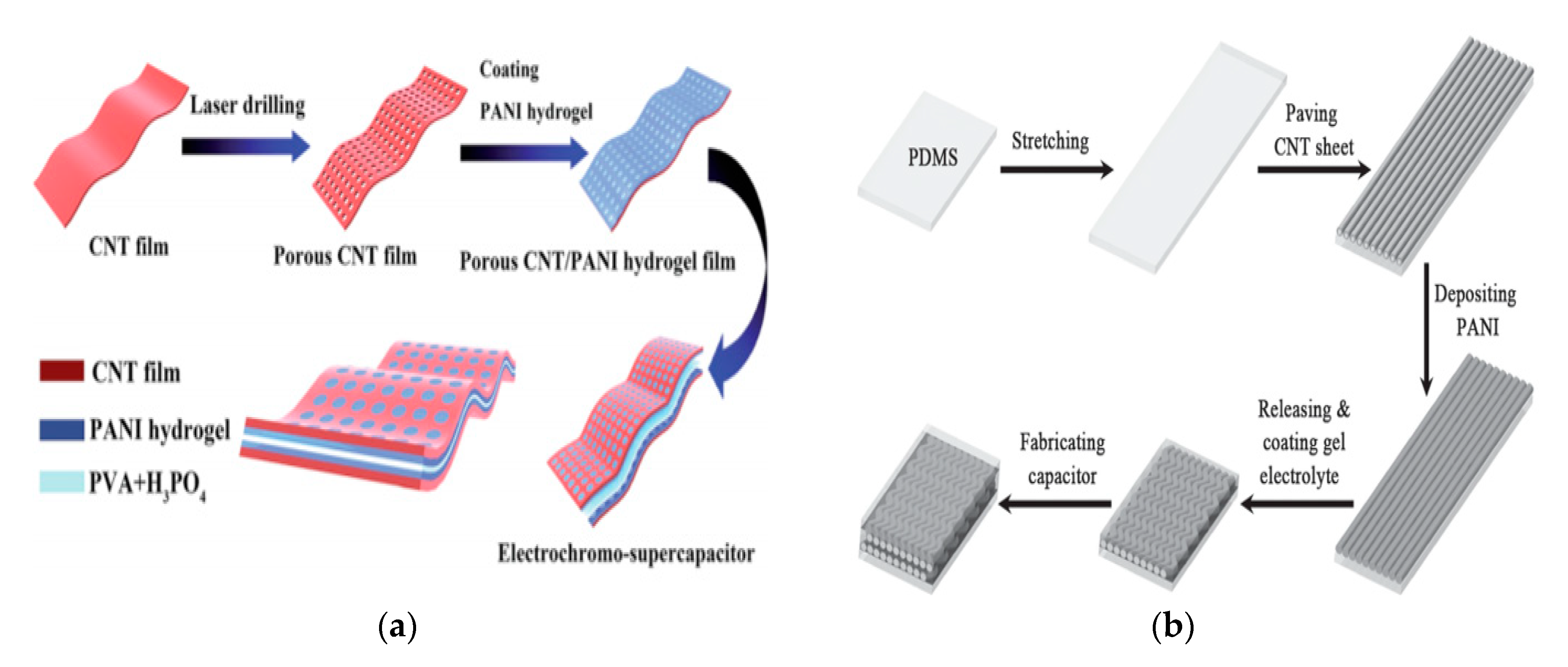
4. CNT for Energy Storage Applications
5. CNT Filtering Applications
6. Safety of CNT Sheet
7. Scaling up Manufacturing
8. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vashist, S.K.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.; Sheu, F.-S. Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol. Adv. 2011, 29, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wei, F. High Efficiency Particulate Air Filters Based on Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 643–666. [Google Scholar]
- Yang, S.; Zhu, Z.; Wei, F.; Yang, X. Carbon nanotubes/activated carbon fiber based air filter media for simultaneous removal of particulate matter and ozone. Build. Environ. 2017, 125, 60–66. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Wang, Y.; Zhang, Q. Roles of carbon nanotubes in novel energy storage devices. Carbon 2017, 122, 462–474. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nat. Cell Biol. 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Lim, S.H.; Luo, Z.; Shen, Z.; Lin, J. Plasma-Assisted Synthesis of Carbon Nanotubes. Nanoscale Res. Lett. 2010, 5, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, H.; Iijima, S. Single-wall carbon nanotubes synthesized by laser ablation in a nitrogen atmosphere. Appl. Phys. Lett. 1998, 73, 3827–3829. [Google Scholar] [CrossRef]
- Bronikowski, M.J. CVD growth of carbon nanotube bundle arrays. Carbon 2006, 44, 2822–2832. [Google Scholar] [CrossRef]
- Gspann, T.S.; Smail, F.R.; Windle, A.H. Spinning of carbon nanotube fibres using the floating catalyst high temperature route: Purity issues and the critical role of sulphur. Faraday Discuss. 2014, 173, 47–65. [Google Scholar] [CrossRef]
- Hou, G.; Su, R.; Wang, A.; Ng, V.; Li, W.; Song, Y.; Zhang, L.; Sundaram, M.; Shanov, V.; Mast, D.; et al. The effect of a convection vortex on sock formation in the floating catalyst method for carbon nanotube synthesis. Carbon 2016, 102, 513–519. [Google Scholar] [CrossRef]
- Hoecker, C.; Smail, F.R.; Pick, M.; Weller, L.; Boies, A. The Dependence of CNT Aerogel Synthesis on Sulfur-driven Catalyst Nucleation Processes and a Critical Catalyst Particle Mass Concentration. Sci. Rep. 2017, 7, 14519. [Google Scholar] [CrossRef]
- Hoecker, C.; Smail, F.R.; Bajada, M.A.; Pick, M.; Boies, A.; Höcker, C. Catalyst nanoparticle growth dynamics and their influence on product morphology in a CVD process for continuous carbon nanotube synthesis. Carbon 2016, 96, 116–124. [Google Scholar] [CrossRef]
- Eatemandi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Zheng, B.; Liu, J. A scalable CVD method for the synthesis of single-walled carbon nanotubes with high catalyst productivity. Chem. Phys. Lett. 2000, 322, 321–326. [Google Scholar] [CrossRef]
- Ci, L.; Wei, J.; Wei, B.; Liang, J.; Xu, C.; Wu, D. Carbon nanofibers and single-walled carbon nanotubes prepared by the floating catalyst method. Carbon 2001, 39, 329–335. [Google Scholar] [CrossRef]
- Cheng, H.-M.; Li, F.; Su, G.; Pan, H.Y.; He, L.; Sun, X.; Dresselhaus, M.S. Large-scale and low-cost synthesis of single-walled carbon nanotubes by the catalytic pyrolysis of hydrocarbons. Appl. Phys. Lett. 1998, 72, 3282–3284. [Google Scholar] [CrossRef]
- Jakubinek, M.B.; Johnson, M.B.; White, M.A.; Jayasinghe, C.; Li, G.; Cho, W.; Schulz, M.J.; Shanov, R.W.A.V. Thermal and electrical conductivity of array-spun multi-walled carbon nanotube yarns. Carbon 2012, 50, 244–248. [Google Scholar] [CrossRef]
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.K.; Bengio, E.A.; Ter Waarbeek, R.F.; De Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, Light, Multifunctional Fibers of Carbon Nanotubes with Ultrahigh Conductivity. Science 2013, 339, 182–186. [Google Scholar] [CrossRef]
- Boncel, S.; Sundaram, R.; Windle, A.H.; Koziol, K. Enhancement of the Mechanical Properties of Directly Spun CNT Fibers by Chemical Treatment. ACS Nano 2011, 5, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Moisala, A.; Li, Q.; Kinloch, I.; Windle, A. Thermal and electrical conductivity of single- and multi-walled carbon nanotube-epoxy composites. Compos. Sci. Technol. 2006, 66, 1285–1288. [Google Scholar] [CrossRef]
- Hone, J.; Llaguno, M.; Biercuk, M.; Johnson, A.; Batlogg, B.; Benes, Z.; Fischer, J. Thermal properties of carbon nanotubes and nanotube-based materials. Appl. Phys. A 2002, 74, 339–343. [Google Scholar] [CrossRef]
- Sullivan, J.; Schulz, M.; Vemaganti, K.; Bhattacharya, A.; Jetter, B.J.; Shanov, V.; Alvarez, N.; Kim, J. Carbon Nanotube Fabric Cooling System for Firefighters and First Responders: Modeling and Simulation. J. Fiber Bioeng. Inform. 2015, 8, 1–12. [Google Scholar] [CrossRef]
- Janas, D.; Rdest, M.; Koziol, K. Flame-retardant carbon nanotube films. Appl. Surf. Sci. 2017, 411, 177–181. [Google Scholar] [CrossRef]
- Schulz, M.J.; Chitranshi, M.; Chauhan, D.; Kubley, A.; Pujari, A.; Xu, C.; Chen, D.; Chaudhary, S.; Hou, G.; Bell, G.; et al. Pioneering carbon nanotube textile engineering & fashion technology. J. Text. Eng. Fash. Technol. 2019, 5. [Google Scholar] [CrossRef]
- Hu, L.; Pasta, M.; La Mantia, F.; Cui, L.; Jeong, S.; DeShazer, H.D.; Choi, J.W.; Han, S.M.; Cui, Y. Stretchable, Porous, and Conductive Energy Textiles. Nano Lett. 2010, 10, 708–714. [Google Scholar] [CrossRef]
- Ashley, K.; Mark, S.; Daniel, R.C.; Motahareh, S.; Vesselin, S.; Sung, Y.K.; Vianessa, N.; Devika, C.; Megha, C.; Anuptha, P. Carbon Nanotube Hybrid Fabric to Filter and Deactivate Virus. U.S. Patent 63046680, 1 July 2020. [Google Scholar]
- Weissker, U.; Hampel, S.; Leonhardt, A.; Buchner, B.K. Carbon Nanotubes Filled with Ferromagnetic Materials. Materials 2010, 3, 4387–4427. [Google Scholar] [CrossRef]
- Dujardin, E.; Ebbesen, T.W.; Hiura, H.; Tanigaki, K. Capillarity and Wetting of Carbon Nanotubes. Science 1994, 265, 1850–1852. [Google Scholar] [CrossRef]
- Ebbesen, T.W. Wetting, filling and decorating carbon nanotubes. J. Phys. Chem. Solids 1996, 57, 951–955. [Google Scholar] [CrossRef]
- Singh, M.; Ohji, T.; Asthana, R.; Mathur, S. Ceramic Integration and Joining Technologies. Ceramic Integr. Join. Technol. 2011. [Google Scholar] [CrossRef]
- Mattevi, C.; Kim, H.; Chhowalla, M. A review of chemical vapour deposition of graphene on copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- He, M.; Magnin, Y.; Amara, H.; Jiang, H.; Cui, H.; Fossard, F.; Castan, A.; Kauppinen, E.I.; Loiseau, A.; Bichara, C. Linking growth mode to lengths of single-walled carbon nanotubes. Carbon 2017, 113, 231–236. [Google Scholar] [CrossRef]
- Aizawa, T.; Souda, R.; Otani, S.; Ishizawa, Y.; Oshima, C. Anomalous bond of monolayer graphite on transition-metal carbide surfaces. Phys. Rev. Lett. 1990, 64, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Planeix, J.M.; Coustel, N.; Coq, B.; Brotons, V.; Kumbhar, P.S.; Dutartre, R.; Geneste, P.; Bernier, P.; Ajayan, P.M. Application of carbon nanotubes as supports in heterogeneous catalysis. J. Am. Chem. Soc. 1994, 116, 7935–7936. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nat. Cell Biol. 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Chauhan, D.; Hou, G.; Ng, V.; Chaudhary, S.; Paine, M.; Moinuddin, K.; Rabiee, M.; Cahay, M.; Lalley, N.; Shanov, R.W.A.V.; et al. Multifunctional smart composites with integrated carbon nanotube yarn and sheet. In Proceedings of the A Tribute Conference Honoring Daniel Inman, Portland, OR, USA, 10 April 2017; SPIE-International Society for Optics and Photonics: Bellingham, WA, USA; Volume 10172, p. 1017205. [Google Scholar]
- Hsieh, Y.-Y.; Zhang, L.; DeArmond, D.; Kanakaraaj, S.N.; Adusei, P.K.; Alvarez, N.T.; Fang, Y.; Daum, J.; Shanov, R.W.A.V. Integrated graphene-sulfur cathode and separator with plasma enhancement for Li-S batteries. Carbon 2018, 139, 1093–1103. [Google Scholar] [CrossRef]
- Kanakaraaj, S.N.; Hsieh, Y.-Y.; Adusei, P.K.; Homan, B.; Fang, Y.; Zhang, G.; Mishra, S.; Gbordzoe, S.; Shanov, R.W.A.V. Nitrogen-doped CNT on CNT hybrid fiber as a current collector for high-performance Li-ion capacitors. Carbon 2019, 149, 407–418. [Google Scholar] [CrossRef]
- Chauhan, D.; Chen, R.; Xu, C.; Mast, D.; Kleismit, R.; Shanov, V.; Kubley, A.; Hou, G.; Chitranshi, M.; Pujari, A.; et al. Carbon Nanotube Hybrid Fabric and Tape. In Nanotube Superfiber Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 239–261. [Google Scholar]
- McConnell, C.; Kanakaraj, S.N.; Dugre, J.; Malik, R.; Zhang, G.; Haase, M.R.; Hsieh, Y.-Y.; Fang, Y.; Mast, D.; Shanov, R.W.A.V. Hydrogen Sensors Based on Flexible Carbon Nanotube-Palladium Composite Sheets Integrated with Ripstop Fabric. ACS Omega 2019, 5, 487–497. [Google Scholar] [CrossRef]
- Schulz, M.J.; Chauhan, D.; Xu, C.; Chen, D.; Kubley, A.; Brandewie, B.; Hou, G.; Li, W.; Ng, V.; Rabiee, M.; et al. Introduction to Carbon Nanotube Hybrid Textiles. J. Text. Sci. Fash. Technol. 2019, 1, 1–7. [Google Scholar] [CrossRef]
- Adusei, P.K.; Gbordzoe, S.; Kanakaraj, S.N.; Hsieh, Y.-Y.; Alvarez, N.T.; Fang, Y.; Johnson, K.; McConnell, C.; Shanov, R.W.A.V. Fabrication and study of supercapacitor electrodes based on oxygen plasma functionalized carbon nanotube fibers. J. Energy Chem. 2020, 40, 120–131. [Google Scholar] [CrossRef]
- Elliott, J.A.; Gspann, T.H.; Terrones, J.E.; Windle, A.L. The measurement of properties in carbon nanotube yarns. WSEAS Trans. Appl. Theor. Mech. 2017, 12, 33–40. [Google Scholar]
- Chauhan, D. Manufacturing and Applications of Carbon Nanotube Sheet and Thread. Master’s Thesis, University of Cincinnati, Cincinnati, OH, USA, 2018. [Google Scholar]
- Tatarnikov, V.M. Use of probes for measuring the electrical conductance of anisotropic plates. Meas. Tech. 1970, 13, 877–881. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Wang, Y.; Cheng, T.; Yao, L.-Q.; Li, X.; Lai, W.-Y.; Huang, W. Printed supercapacitors: Materials, printing and applications. Chem. Soc. Rev. 2019, 3229–3326. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, G.Z. Supercapatteries as High-Performance Electrochemical Energy Storage Devices. Electrochem. Energy Rev. 2020, 3, 271–285. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y. Chemical Oxidative Polymerization of Polyaniline: A Practical Approach for Preparation of Smart Conductive Textiles. J. Chem. Educ. 2016, 93, 1606–1611. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, W.; Yang, Z.; Zhang, Y.; Zhang, H.; Zhang, H.; Guo, H.; Zhang, X.; Li, Q. Smart and flexible supercapacitor based on a porous carbon nanotube film and polyaniline hydrogel. RSC Adv. 2016, 6, 24946–24951. [Google Scholar] [CrossRef]
- Chen, X.; Lin, H.; Chen, P.; Guan, G.; Deng, J.; Peng, H. Smart, Stretchable Supercapacitors. Adv. Mater. 2014, 26, 4444–4449. [Google Scholar] [CrossRef]
- Adusei, P.K.; Kanakaraj, S.N.; Gbordzoe, S.; Johnson, K.; DeArmond, D.; Hsieh, Y.-Y.; Fang, Y.; Mishra, S.; Phan, N.; Alvarez, N.T.; et al. A scalable nano-engineering method to synthesize 3D-graphene-carbon nanotube hybrid fibers for supercapacitor applications. Electrochim. Acta 2019, 312, 411–423. [Google Scholar] [CrossRef]
- Baker, R.T.K. Nucleation and growth of carbon deposits from the nickel catalyzed decomposition of acetylene. J. Catal. 1972, 26, 51–62. [Google Scholar] [CrossRef]
- Kukovitsky, E.; L’Vov, S.; Sainov, N.; Shustov, V.; Chernozatonskii, L. Correlation between metal catalyst particle size and carbon nanotube growth. Chem. Phys. Lett. 2002, 355, 497–503. [Google Scholar] [CrossRef]
- Hinds, B.J.; Chopra, N.; Rantell, T.; Andrews, R.; Gavalas, V.; Bachas, L.G. Aligned Multiwalled Carbon Nanotube Membranes. Science 2004, 303, 62–65. [Google Scholar] [CrossRef]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast Mass Transport Through Sub-2-Nanometer Carbon Nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef]
- Yu, M.; Funke, H.H.; Falconer, J.L.; Noble, R.D. High Density, Vertically-Aligned Carbon Nanotube Membranes. Nano Lett. 2009, 9, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Chopra, N.; Hinds, B.J. Effect of Tip Functionalization on Transport through Vertically Oriented Carbon Nanotube Membranes. J. Am. Chem. Soc. 2005, 127, 9062–9070. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Tan, X.; Chen, C.; Wang, X. Removal of Pb(II) from aqueous solution by oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2008, 154, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Elsehly, E.M.I.; Chechenin, N.G.; Makunin, A.V.; Vorobyeva, E.A.; Motaweh, H.A. Oxidized Carbon Nanotubes Filters for Iron Removal from Aqueous Solutions. Int. J. New Technol. Sci. Eng. 2015, 2, 14–18. [Google Scholar]
- Elsehly, E.; Chechenin, N.G.; Makunin, A.V.; Motaweh, H.A.; Leksina, E.G. Functionalized carbon nanotubes based filters for chromium removal from aqueous solutions. Water Sci. Technol. 2017, 75, 1564–1571. [Google Scholar] [CrossRef]
- Savage, N.; Diallo, M.S. Nanomaterials and Water Purification: Opportunities and Challenges. J. Nanoparticle Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Casavant, M.J.; Walters, D.A.; Schmidt, J.J.; Smalley, R.E. Neat macroscopic membranes of aligned carbon nanotubes. J. Appl. Phys. 2003, 93, 2153. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, O.N.; Talapatra, S.; Vajtai, R.; Ajayan, P.M. Carbon nanotube filters. Nat. Mater. 2004, 3, 610–614. [Google Scholar] [CrossRef]
- Corry, B. Designing Carbon Nanotube Membranes for Efficient Water Desalination. J. Phys. Chem. B 2008, 112, 1427–1434. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, D.-G. Development of CNT-metal-filters by direct growth of carbon nanotubes. Curr. Appl. Phys. 2006, 6, e182–e186. [Google Scholar] [CrossRef]
- Yang, H.Y.; Han, Z.J.; Yu, S.F.; Pey, K.L.; Ostrikov, K.; Karnik, R. Carbon nanotube membranes with ultrahigh specific adsorption capacity for water desalination and purification. Nat. Commun. 2013, 4, 2220. [Google Scholar] [CrossRef] [PubMed]
- Shawky, H.A.; Chae, S.-R.; Lin, S.; Wiesner, M.R. Synthesis and characterization of a carbon nanotube/polymer nanocomposite membrane for water treatment. Desalination 2011, 272, 46–50. [Google Scholar] [CrossRef]
- Majeed, S.; Fierro, D.; Buhr, K.; Wind, J.; Du, B.; Boschetti-De-Fierro, A.; Abetz, V. Multi-walled carbon nanotubes (MWCNTs) mixed polyacrylonitrile (PAN) ultrafiltration membranes. J. Membr. Sci. 2012, 101–109. [Google Scholar] [CrossRef]
- Roy, S.; Ntim, S.A.; Mitra, S.; Sirkar, K.K. Facile fabrication of superior nanofiltration membranes from interfacially polymerized CNT-polymer composites. J. Membr. Sci. 2011, 375, 81–87. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Jeong, S.; Pham, T.T.N.; Kandasamy, J.; Vigneswaran, S. Effect of granular activated carbon filter on the subsequent flocculation in seawater treatment. Desalination 2014, 354, 9–16. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Li, M.-S. Adsorption of selected volatile organic vapors on multiwall carbon nanotubes. J. Hazard. Mater. 2008, 154, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pui, D.Y.H.; Wang, J. Removal of airborne nanoparticles by membrane coated filters. Sci. Total. Environ. 2011, 409, 4868–4874. [Google Scholar] [CrossRef]
- Hung, C.-H.; Leung, W.W.-F. Filtration of nano-aerosol using nanofiber filter under low Peclet number and transitional flow regime. Sep. Purif. Technol. 2011, 79, 34–42. [Google Scholar] [CrossRef]
- Leung, W.W.-F.; Hung, C.-H. Skin effect in nanofiber filtration of submicron aerosols. Sep. Purif. Technol. 2012, 92, 174–180. [Google Scholar] [CrossRef]
- Yildiz, O.; Bradford, P.D. Aligned carbon nanotube sheet high efficiency particulate air filters. Carbon 2013, 64, 295–304. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yang, L. Dual functional nisin-multi-walled carbon nanotubes coated filters for bacterial capture and inactivation. J. Biol. Eng. 2015, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yao, M. Effects of single-walled carbon nanotube filter on culturability and diversity of environmental bioaerosols. J. Aerosol Sci. 2011, 42, 387–396. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Schnoor, M.H.; Rahaman, S.; Schiffman, J.D.; Elimelech, M. Electrochemical Multiwalled Carbon Nanotube Filter for Viral and Bacterial Removal and Inactivation. Environ. Sci. Technol. 2011, 45, 3672–3679. [Google Scholar] [CrossRef]
- Ihsanullah; Laoui, T.; Al-Amer, A.M.; Khalil, A.B.; Abbas, A.; Khraisheh, M.; Atieh, M.A. Novel anti-microbial membrane for desalination pretreatment: A silver nanoparticle-doped carbon nanotube membrane. Desalination 2015, 376, 82–93. [Google Scholar] [CrossRef]
- Zou, Z.; Yao, M. Airflow resistance and bio-filtering performance of carbon nanotube filters and current facepiece respirators. J. Aerosol Sci. 2015, 79, 61–71. [Google Scholar] [CrossRef]
- Kubley, A.; Chauhan, D.; Kanakaraj, S.N.; Shanov, V.; Xu, C.; Chen, R.; Ng, V.; Bell, G.; Verma, P.; Hou, X.; et al. Smart Textiles and Wearable Technology Innovation with Carbon Nanotube Technology. In Nanotube Superfiber Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–311. [Google Scholar]
- Nanocomp Nanotechnology Safety Information. Available online: http://www.miralon.com/health-safety (accessed on 7 September 2020).
- Windle, Q.A. Flow Nanotechnology Safety Information. Available online: https://www.q-flo.com/advanced-technology/ (accessed on 7 September 2020).
- Schulz, M.J.; Hou, G.; Ng, V.; Rabiee, M.; Cahay, M.; Chaudhary, S.; Lindley, D.; Chauhan, D.; Paine, M.; Vijayakumar, D.; et al. Science to Commercialization of Carbon Nanotube Sheet and Yarn. WSEAS Trans. Appl. Theor. Mechan. 2017, 12, 41–50. [Google Scholar]
- Schulz, M.; Hou, G.; Ng, V. Methods of Manufacturing Carbon Nanotube (CNT) Hybrid Sheet and Yarn by Gas Phase Assembly, and CNT-Hybrid Materials. U.S. Patent PCT/US2018/019427, 23 February 2018. [Google Scholar]
- Schulz, M.J.; Kanakaraj, S.; Mast, D.; Shanov, V.; Chauhan, D.; Hou, G.; Ng, V.; Xu, C.; Chen, R.D.; Kubley, A.; et al. Carbon Nanotube Hybrid Material Fabric, Composite Fabric, and Personal Protective Apparel and Equipment. U.S. Patent Application 16/629,714, 27 August 2020. [Google Scholar]
- Available online: https://www.boronite.com/ (accessed on 7 September 2020).
- Available online: https://www.veelotech.com/ (accessed on 7 September 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitranshi, M.; Pujari, A.; Ng, V.; Chen, D.; Chauhan, D.; Hudepohl, R.; Saleminik, M.; Kim, S.Y.; Kubley, A.; Shanov, V.; et al. Carbon Nanotube Sheet-Synthesis and Applications. Nanomaterials 2020, 10, 2023. https://doi.org/10.3390/nano10102023
Chitranshi M, Pujari A, Ng V, Chen D, Chauhan D, Hudepohl R, Saleminik M, Kim SY, Kubley A, Shanov V, et al. Carbon Nanotube Sheet-Synthesis and Applications. Nanomaterials. 2020; 10(10):2023. https://doi.org/10.3390/nano10102023
Chicago/Turabian StyleChitranshi, Megha, Anuptha Pujari, Vianessa Ng, Daniel Chen, Devika Chauhan, Ronald Hudepohl, Motahareh Saleminik, Sung Yong Kim, Ashley Kubley, Vesselin Shanov, and et al. 2020. "Carbon Nanotube Sheet-Synthesis and Applications" Nanomaterials 10, no. 10: 2023. https://doi.org/10.3390/nano10102023
APA StyleChitranshi, M., Pujari, A., Ng, V., Chen, D., Chauhan, D., Hudepohl, R., Saleminik, M., Kim, S. Y., Kubley, A., Shanov, V., & Schulz, M. (2020). Carbon Nanotube Sheet-Synthesis and Applications. Nanomaterials, 10(10), 2023. https://doi.org/10.3390/nano10102023




