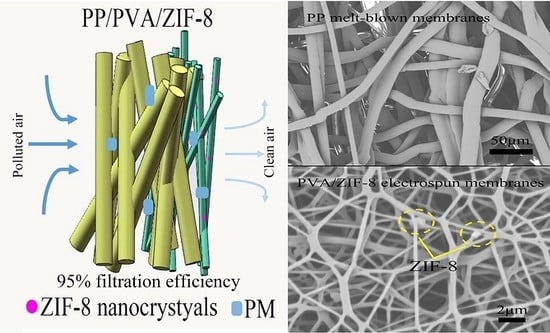
Polypropylene/Polyvinyl Alcohol/Metal-Organic Framework-Based Melt-Blown Electrospun Composite Membranes for Highly Efficient Filtration of PM2.5
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of Polypropylene (PP) Melt-Blown Membranes
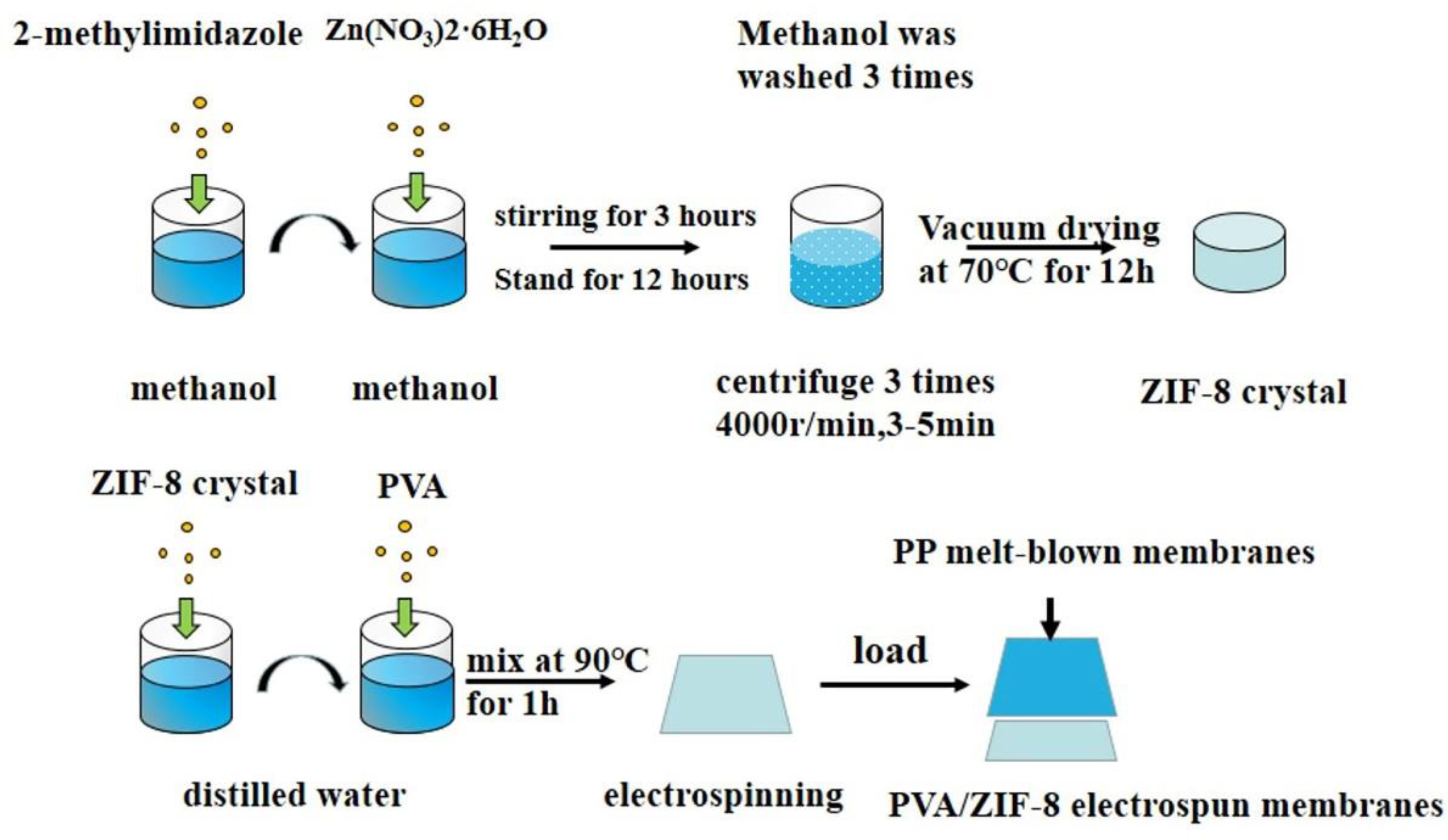
2.3. Synthesis of Zeolite Imidazole Frameworks-8 (ZIF-8)
2.4. Preparation of PP/Polyvinyl Alcohol (PVA)/ZIF-8 Melt-Blown Electrospun Composite Membranes
2.5. Measurements
2.6. Characterization
3. Results and Discussion
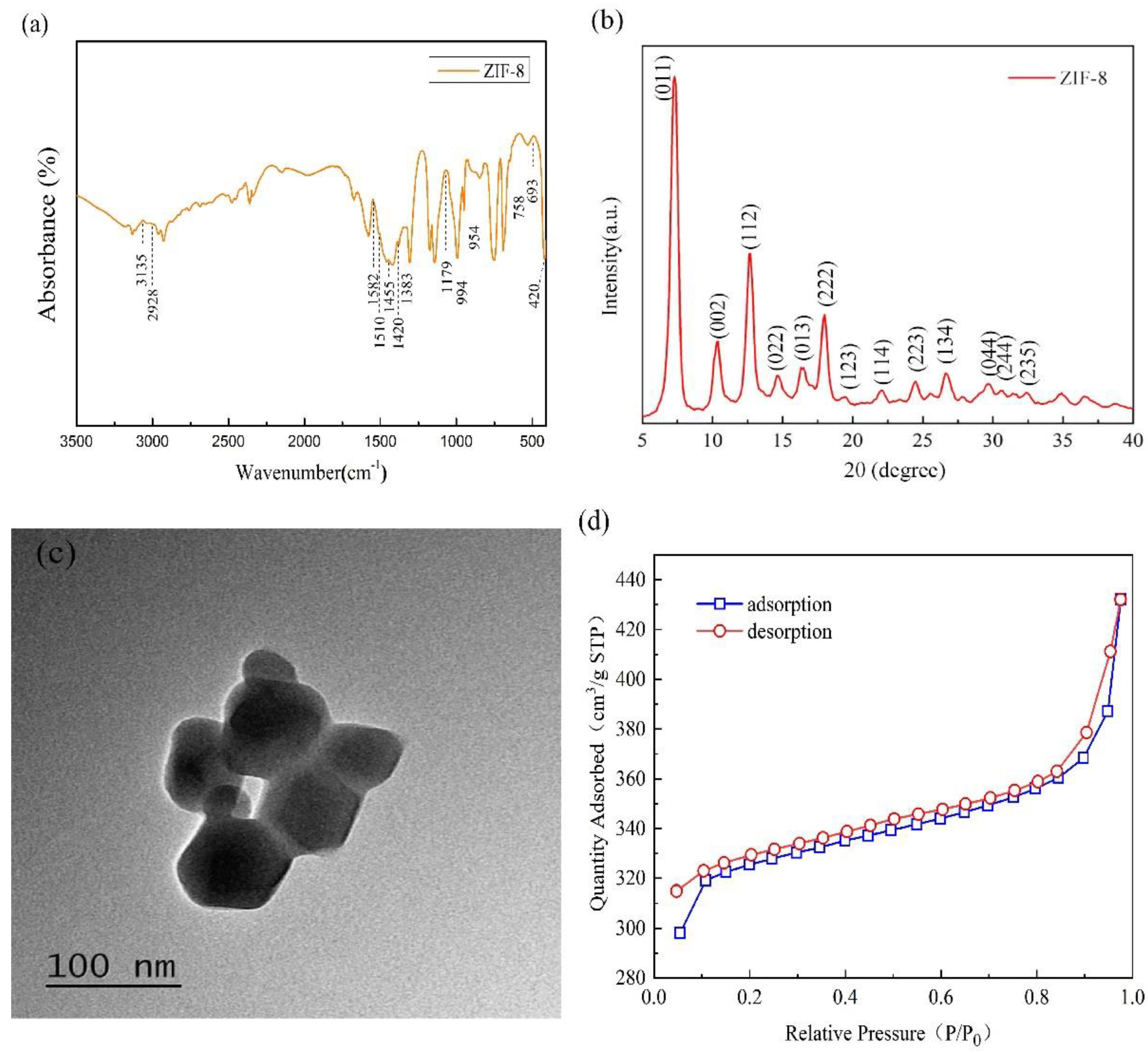
3.1. Property and Characteristics of Synthesized ZIF-8
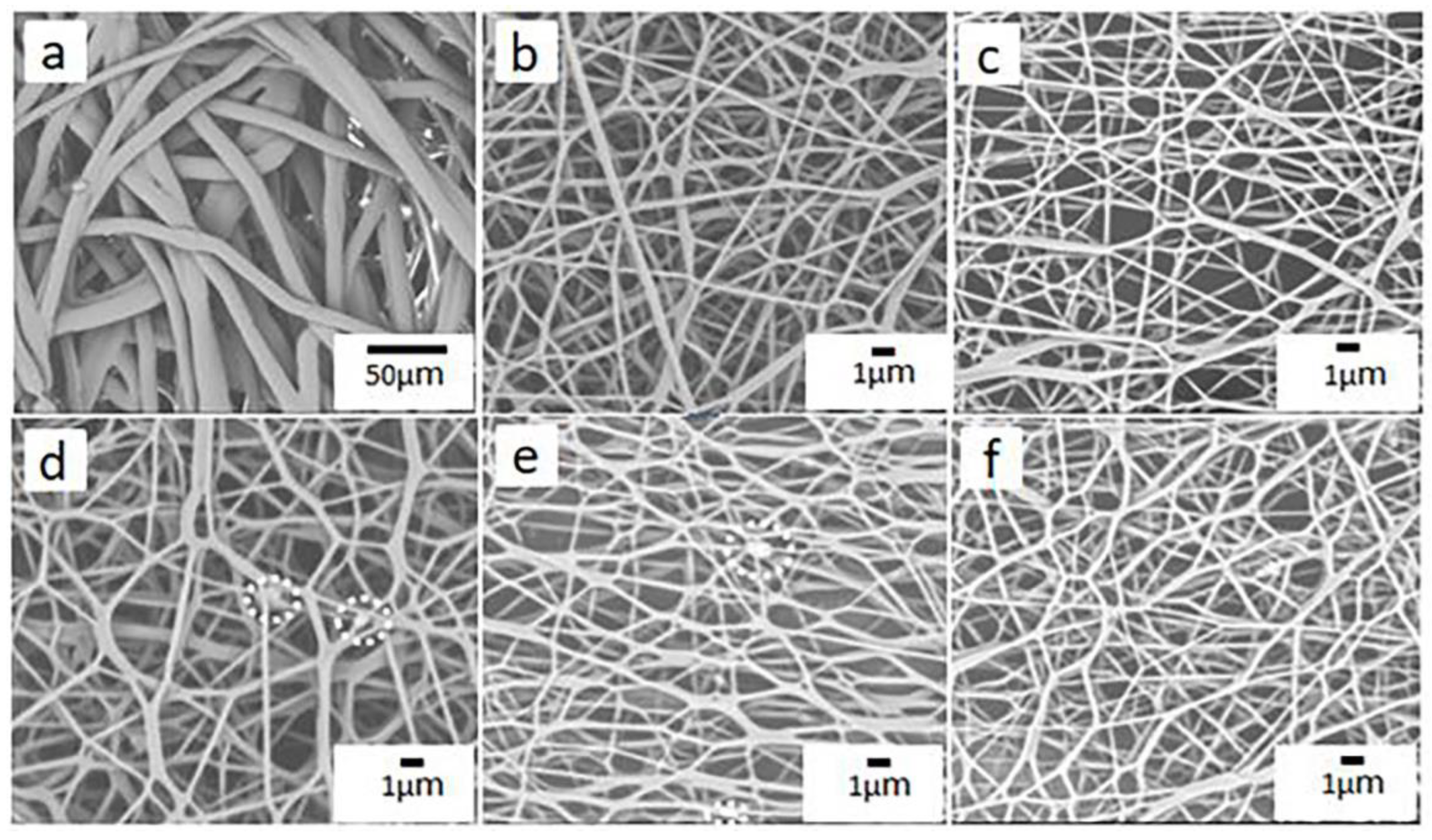
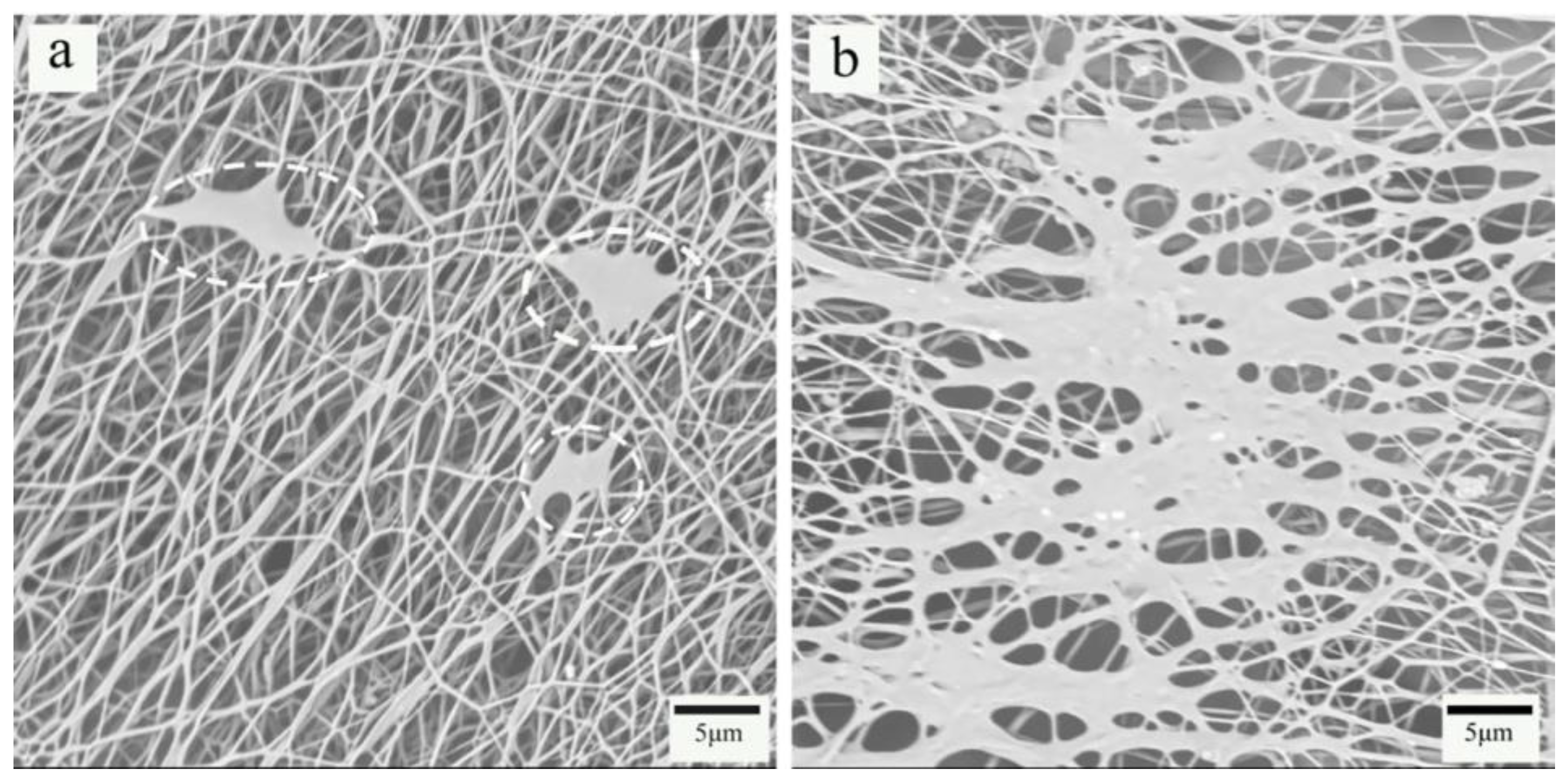
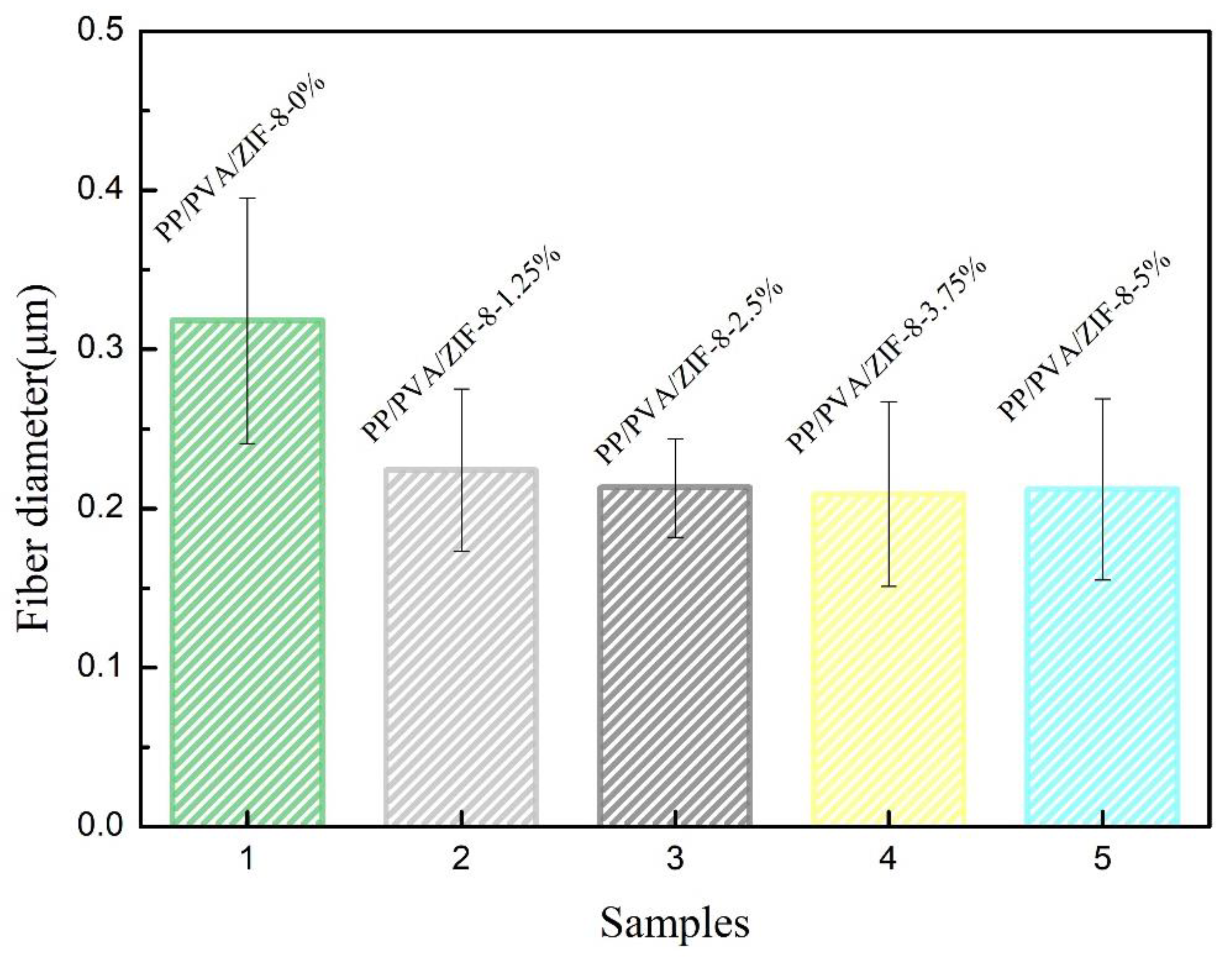
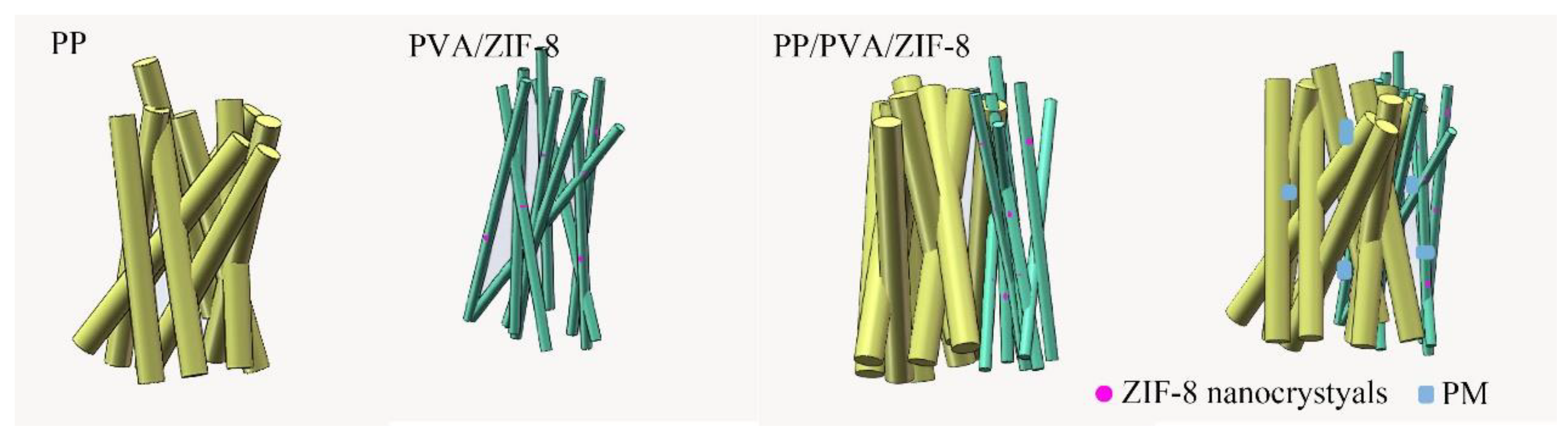
3.2. Surface Morphology of PP Melt-Blown Membrane and PP/PVA/ZIF-8 Melt-Blown Electrospun Composite Membranes
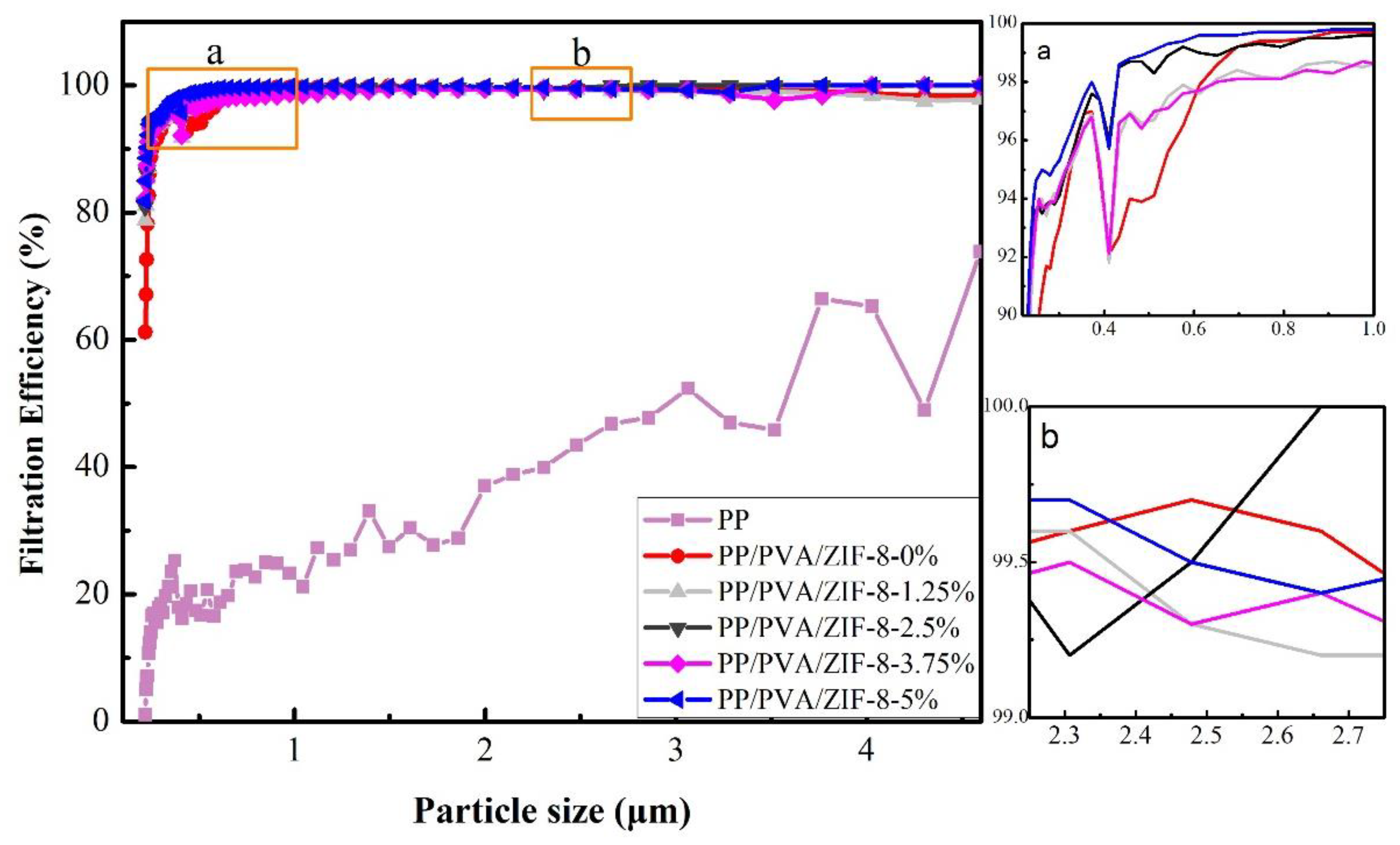
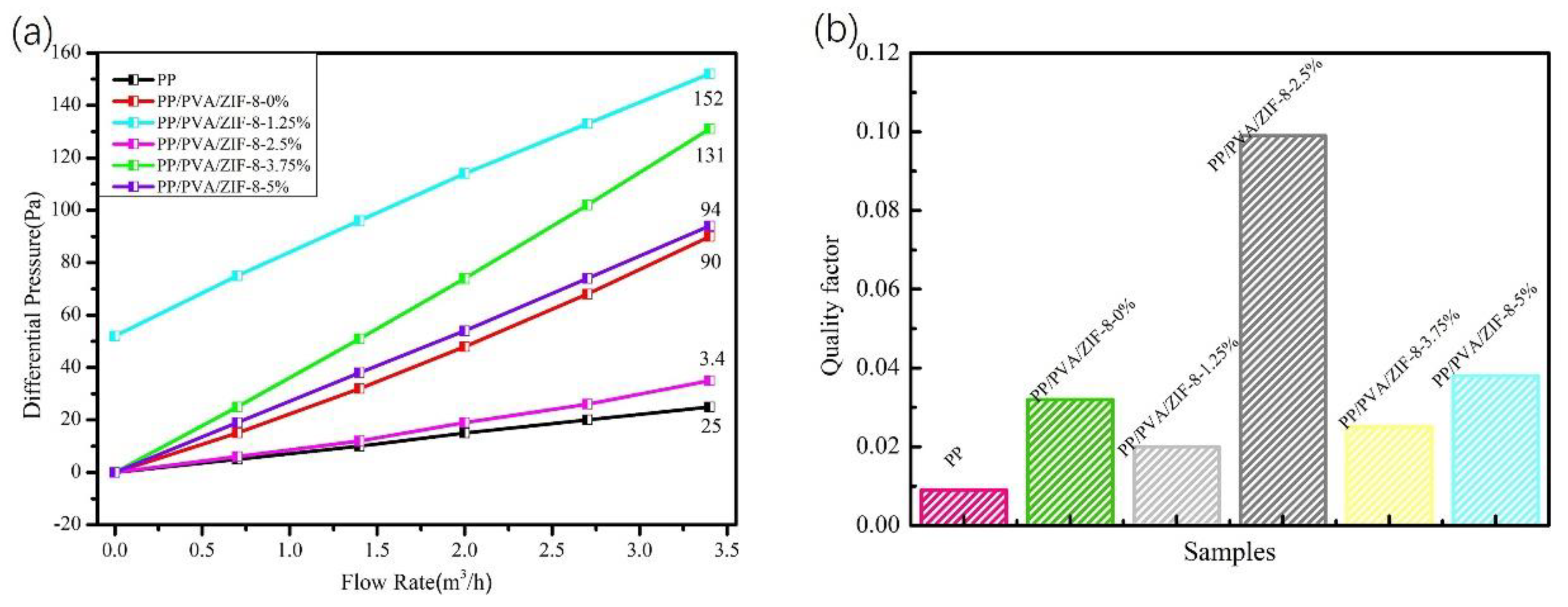
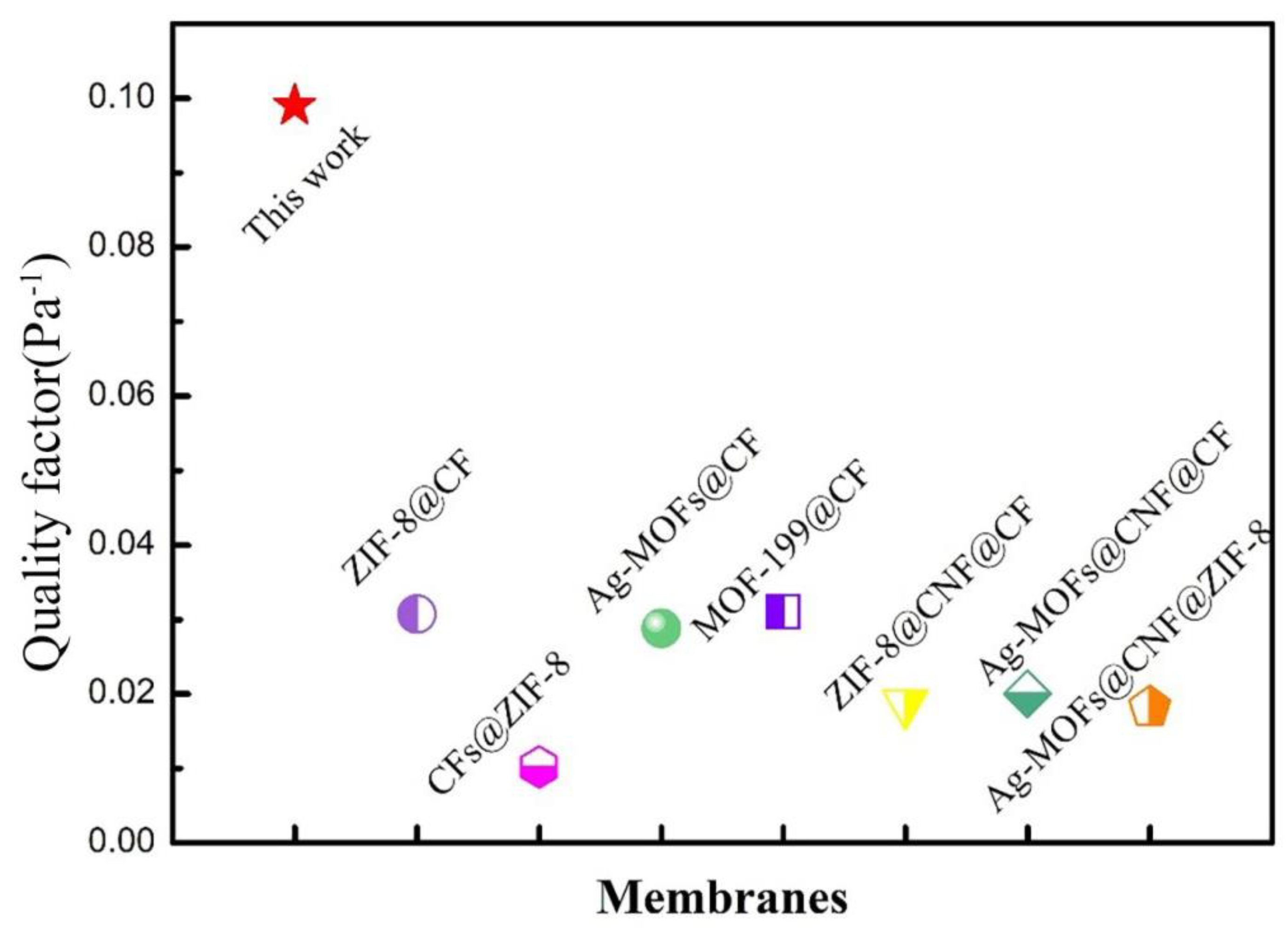
3.3. Filtration Performance of PP Melt-Blown Membranes and PP/PVA/ZIF-8 Melt-Blown Electrospun Composite Membranes
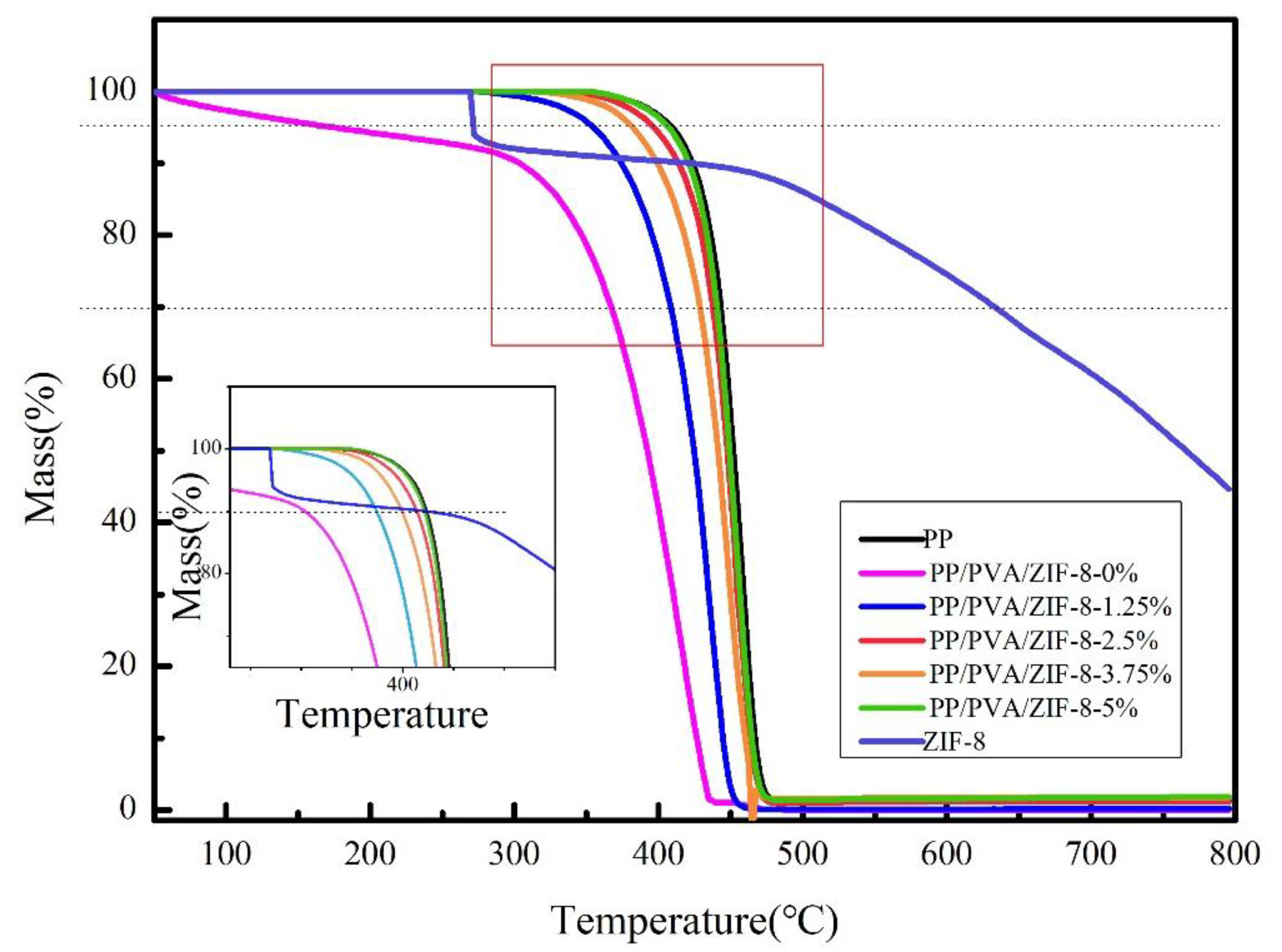
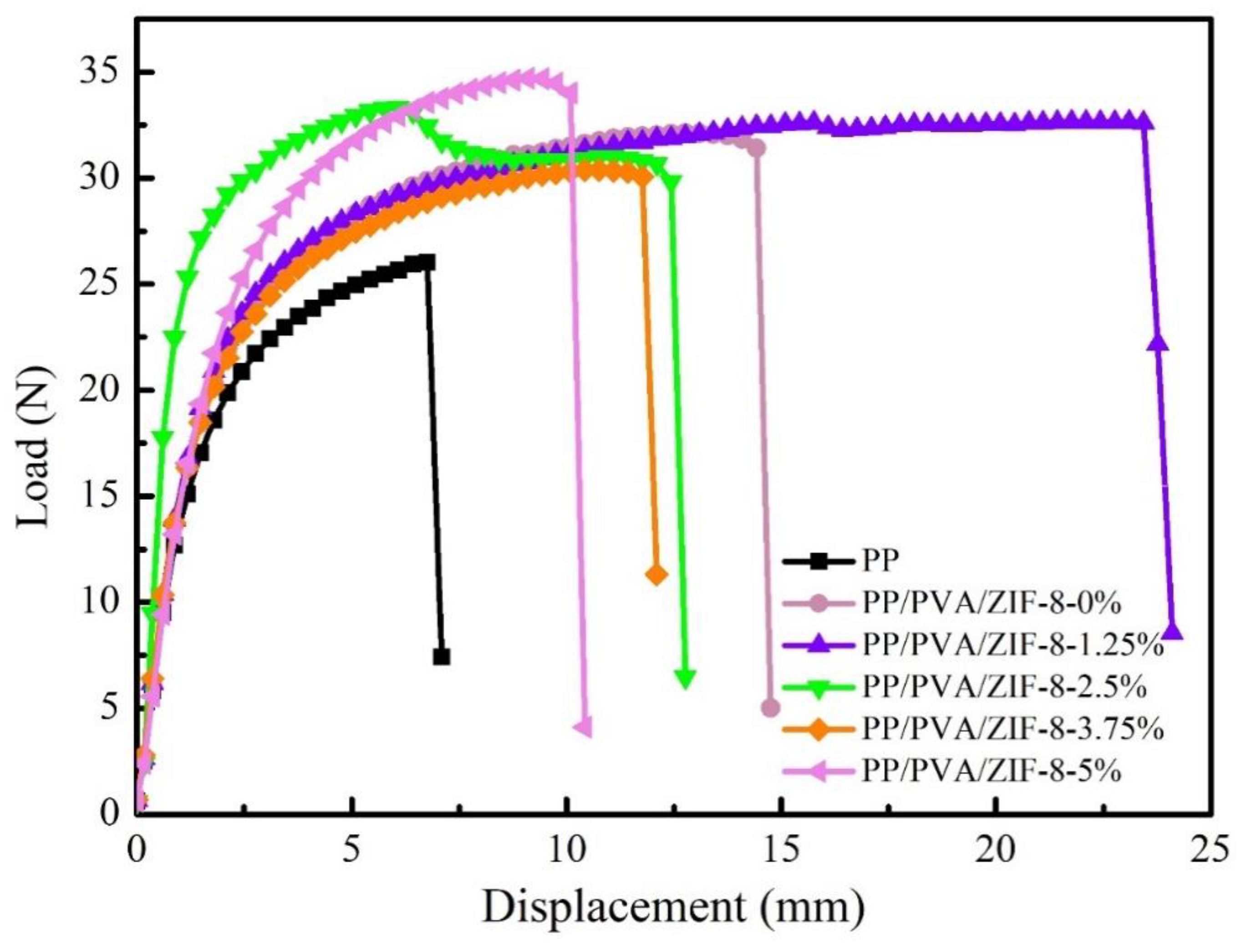
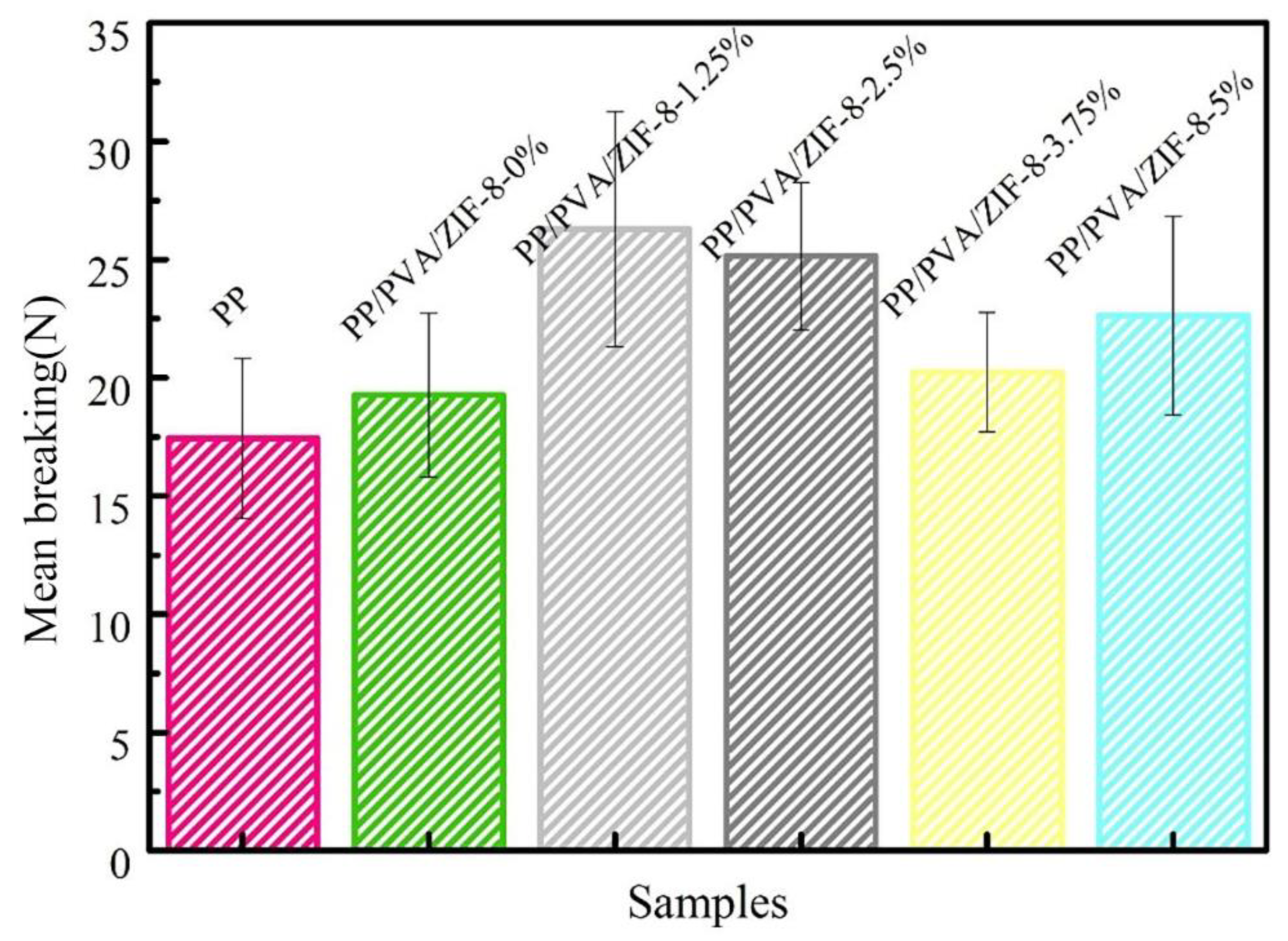
3.4. Thermogravimetric Analyzer (TGA) and Mechanical Properties of PP Melt-Blown Membranes and PP/PVA/ZIF-8 Melt-Blown Electrospun Composite Membranes
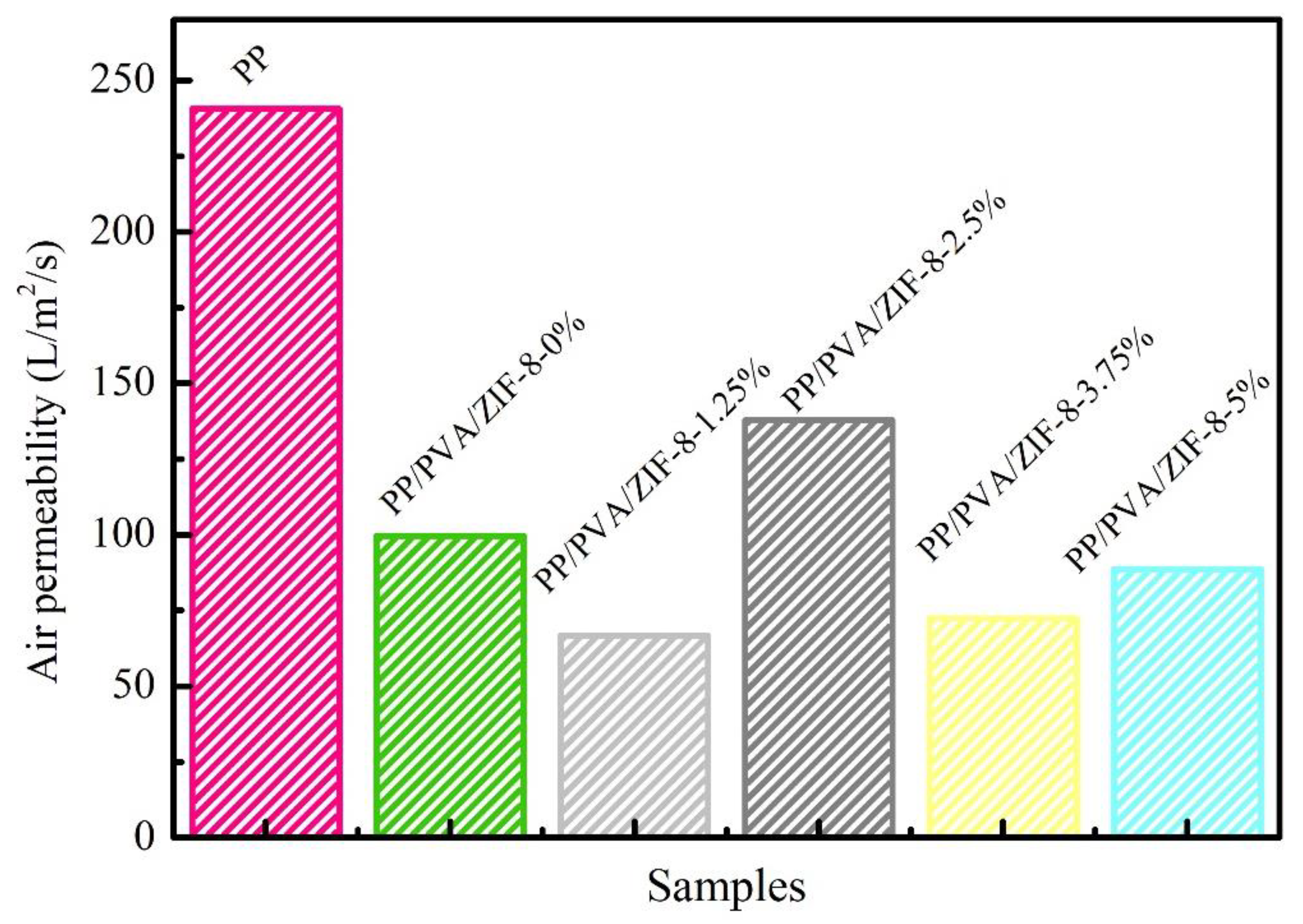
3.5. Air Permeability of PP Melt-Blown Membranes and PP/PVA/ZIF-8 Melt-Blown Electrospun Composite Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.Y.; Jia, Y.; Hou, L.A. Synthesis of zeolitic imidazolate framework-8 on polyester fiber for PM2.5 removal. RSC Adv. 2018, 8, 31471–31477. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.F.; Zhang, S.H.; Cao, S.J.; Li, S.Q.; Chen, F.; Yuan, S.; Xu, C.; Zhou, J.W.; Feng, X.; Ma, X.J.; et al. Roll-to-Roll Production of Metal-Organic Framework Coatings for Particulate Matter Removal. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Tang, N.; Cao, L.T.; Yin, X.; Yu, J.Y.; Ding, B. Highly Integrated Polysulfone/Polyacrylonitrile/Polyamide-6 Air Filter for Multilevel Physical Sieving Airborne Particles. ACS Appl. Mater. Interfaces 2016, 8, 29062–29072. [Google Scholar] [CrossRef]
- Khalid, B.; Bai, X.P.; Wei, H.H.; Huang, Y.; Wu, H.; Cui, Y. Direct Blow-Spinning of Nanofibers on a Window Screen for Highly Efficient PM2.5 Removal. Nano Lett. 2017, 17, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Chen, S.C.; Chang, D.Q.; Xie, X.F.; Sun, J.; Pui, D.Y.H. Filtration efficiency and loading characteristics of PM2.5 through composite filter media consisting of commercial HVAC electret media and nanofiber layer. Sep. Purif. Technol. 2018, 198, 137–145. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yuan, S.; Feng, X.; Li, H.W.; Zhou, J.W.; Wang, B. Preparation of Nanofibrous Metal-Organic Framework Filters for Efficient Air Pollution Control. J. Am. Chem. Soc. 2016, 138, 5785–5788. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Niu, Z.C.; Wang, S.; Chen, J.S.; Zhang, F.W.; Chen, X.Q.; He, C.; Lin, L.F.; Yin, L.Q.; Xu, L.L. Source contributions to carbonaceous species in PM2.5 and their uncertainty analysis at typical urban, peri-urban and background sites in southeast China. Environ. Pollut. 2013, 181, 107–114. [Google Scholar] [CrossRef]
- Li, T.T.; Cen, X.X.; Ren, H.T.; Wu, L.W.; Peng, H.K.; Wang, W.; Gao, B.; Lou, C.W.; Lin, J.H. Zeolitic Imidazolate Framework-8/Polypropylene-Polycarbonate Barklike Meltblown Fibrous Membranes by a Facile in Situ Growth Method for Efficient PM2.5 Capture. ACS Appl. Mater. Interfaces 2020, 12, 8730–8739. [Google Scholar] [CrossRef]
- Yang, K.; Yu, Z.H.; Yu, C.C.; Chen, H.B.; Pan, F. An Electrically Renewable Air Filter with Integrated 3D Nanowire Networks. Adv. Mater. Technol. US 2019, 4, 1900101. [Google Scholar] [CrossRef]
- Zhang, H.F.; Liu, J.X.; Zhang, X.; Huang, C.; Jin, X.Y. Design of electret polypropylene melt blown air filtration material containing nucleating agent for effective PM2.5 capture. RSC Adv. 2018, 8, 7932–7941. [Google Scholar] [CrossRef] [Green Version]
- Tien, C.Y.; Chen, J.P.; Li, S.H.; Li, Z.Y.; Zheng, Y.M.; Peng, A.S.; Zhou, F.; Tsai, C.J.; Chen, S.C. Experimental and theoretical analysis of loading characteristics of different electret media with various properties toward the design of ideal depth filtration for nanoparticles and fine particles. Sep. Purif. Technol. 2020, 233, 116002. [Google Scholar] [CrossRef]
- Xie, S.; Han, W.L.; Jiang, G.J.; Chen, C. Turbulent air flow field in slot-die melt blowing for manufacturing microfibrous nonwoven materials. J. Mater. Sci. 2018, 53, 6991–7003. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Pararneswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Rutledge, G.C.; Fridrikh, S.V. Formation of fibers by electrospinning. Adv. Drug. Deliver. Rev. 2007, 59, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.C.; He, J.X.; Cui, S.Z.; Gao, W.D. Fabrication of nanofibers by a modified air-jet electrospinning method. Iran. Polym. J. 2013, 23, 13–25. [Google Scholar] [CrossRef]
- Li, T.T.; Yan, M.X.; Zhong, Y.Q.; Ren, H.T.; Lou, C.W.; Huang, S.Y.; Lin, J.H. Processing and characterizations of rotary linear needleless electrospun polyvinyl alcohol(PVA)/Chitosan(CS)/Graphene(Gr) nanofibrous membranes. J. Mater. Res. Technol. 2019, 8, 5124–5132. [Google Scholar] [CrossRef]
- Castro-Munoz, R.; Agrawal, K.V.; Coronas, J. Ultrathin permselective membranes: The latent way for efficient gas separation. RSC Adv. 2020, 10, 12653–12670. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, S.W.; Joshi, M.K.; Kim, C.S. Fabrication and characterization of silver nanoparticle-incorporated bilayer electrospun–melt-blown micro/nanofibrous membrane. Int. J. Polym. Mater. Polym. 2017, 66, 514–520. [Google Scholar] [CrossRef]
- Ji, H.; Lee, S.; Park, J.; Kim, T.; Choi, S.; Oh, M. Improvement in Crystallinity and Porosity of Poorly Crystalline Metal-Organic Frameworks (MOFs) through Their Induced Growth on a Well-Crystalline MOF Template. Inorg. Chem. 2018, 57, 9048–9054. [Google Scholar] [CrossRef]
- Li, Z.J.; Ju, Y.; Yu, B.W.; Wu, X.L.; Lu, H.J.; Li, Y.X.; Zhou, J.; Guo, X.F.; Zhang, Z.H.; Lin, J.; et al. Modulated synthesis and isoreticular expansion of Th-MOFs with record high pore volume and surface area for iodine adsorption. Chem. Commun. 2020, 56, 6715–6718. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.M.; Zhou, W.; Chen, B.L. Porous Metal-Organic Frameworks for Gas Storage and Separation: What, How, and Why? J. Phys. Chem. Lett. 2014, 5, 3468–3479. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kim, H.; Choi, J.; Yip, A.C.K. Thermal stability of ZIF-8 under oxidative and inert environments: A practical perspective on using ZIF-8 as a catalyst support. Chem. Eng. J. 2015, 278, 293–300. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.Q.; Du, X.Y.; Shen, Y.Y.; Ma, Z.W. Effect of Lanthanum Doping on the Microstructure, Thermal Stability, and CO2 Adsorption Property of ZIF-8. Adv. Mater. Sci. Eng. 2019, 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.M.; Wu, J.T.; Wang, C.L.; Liu, J.G. Electrospun Polyimide/Metal-Organic Framework Nanofibrous Membrane with Superior Thermal Stability for Efficient PM2.5 Capture. ACS Appl. Mater. Interfaces 2019, 11, 11904–11909. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.S.; Zhang, M.Y.; Nie, J.Y.; Tan, J.J.; Yang, B.; Song, S.X. Design of double-component metal-organic framework air filters with PM2.5 capture, gas adsorption and antibacterial capacities. Carbohyd. Polym. 2019, 203, 415–422. [Google Scholar] [CrossRef]
- Su, Z.P.; Zhang, M.Y.; Lu, Z.Q.; Song, S.X.; Zhao, Y.S.; Hao, Y. Functionalization of cellulose fiber by in situ growth of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals for preparing a cellulose-based air filter with gas adsorption ability. Cellulose 2018, 25, 1997–2008. [Google Scholar] [CrossRef]
- Wang, C.H.; Zheng, T.; Luo, R.; Liu, C.; Zhang, M.; Li, J.S.; Sun, X.Y.; Shen, J.Y.; Han, W.Q.; Wang, L.J. In Situ Growth of ZIF-8 on PAN Fibrous Filters for Highly Efficient U(VI) Removal. ACS Appl. Mater. Interfaces 2018, 10, 24164–24171. [Google Scholar] [CrossRef]
- Fausey, C.L.; Zucker, I.; Lee, D.E.; Shaulsky, E.; Zimmerman, J.B.; Elimelech, M. Tunable Molybdenum Disulfide-Enabled Fiber Mats for High-Efficiency Removal of Mercury from Water. ACS Appl. Mater. Interfaces 2020, 12, 18446–18456. [Google Scholar] [CrossRef]
- Castro-Munoz, R.; Boczkaj, G.; Gontarek, E.; Cassano, A.; Fila, V. Membrane technologies assisting plant-based and agro-food by-products processing: A comprehensive review. Trends. Food. Sci. Tech. 2020, 95, 219–232. [Google Scholar] [CrossRef]
- Li, C.X.; Kuang, S.Y.; Chen, Y.H.; Wang, Z.L.; Li, C.J.; Zhu, G. In Situ Active Poling of Nanofiber Networks for Gigantically Enhanced Particulate Filtration. ACS Appl. Mater. Interfaces 2018, 10, 24332–24338. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.N.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.F.; Chen, R.Z.; Wang, K.; Wang, H.T. Direct synthesis of zeolitic imidazolate framework-8/chitosan composites in chitosan hydrogels. Micropor. Mesopor. Mater. 2013, 165, 200–204. [Google Scholar] [CrossRef]
- He, M.; Yao, J.F.; Liu, Q.; Wang, K.; Chen, F.Y.; Wang, H.T. Facile synthesis of zeolitic imidazolate framework-8 from a concentrated aqueous solution. Micropor. Mesopor. Mater. 2014, 184, 55–60. [Google Scholar] [CrossRef]
- Pan, Y.C.; Liu, Y.Y.; Zeng, G.F.; Zhao, L.; Lai, Z.P. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Jian, M.P.; Liu, B.; Zhang, G.S.; Liu, R.P.; Zhang, X.W. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloid. Surf. A 2015, 465, 67–76. [Google Scholar] [CrossRef]
- Li, T.T.; Cen, X.X.; Ren, H.T.; Sun, F.; Lin, Q.; Lou, C.W.; Lin, J.H. One-Step Bark-Like Imitated Polypropylene (PP)/Polycarbonate (PC) Nanofibrous Meltblown Membrane for Efficient Particulate Matter Removal. Polymers 2019, 11, 1307. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.D.; Khosravi, A.; O’Connor, L.; Tagaban, A.Q.; Wilson, L.; Houck, B.; Liu, Q.L.; Lind, M.L. Effect of ZIF-71 Particle Size on Free-Standing ZIF-71/PDMS Composite Membrane Performances for Ethanol and 1-Butanol Removal from Water through Pervaporation. Ind. Eng. Chem. Res. 2017, 56, 9167–9176. [Google Scholar] [CrossRef]
- Maze, B.; Vahedi Tafreshi, H.V.; Wang, Q.; Pourdeyhimi, B. A simulation of un-steady-state filtration via nanofiber media at reduced operating pressures. J. Aerosol. Sci. 2007, 38, 550–571. [Google Scholar] [CrossRef]
- Liang, Z.Y.; Deng, Z.L.; Qin, X.H.; Wei, L. Theoretical analysis and of three dimensional free surface of electrospinning. J. King Saud. Univ. Sci. 2019, 31, 460–463. [Google Scholar] [CrossRef]
- Dai, X.; Li, X.; Wang, X.L. Morphology controlled porous poly(lactic acid)/zeolitic imidazolate framework-8 fibrous membranes with superior PM2.5 capture capacity. Chem. Eng. J. 2018, 338, 82–91. [Google Scholar] [CrossRef]
Publisher′s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Screw 1 (°C) | Screw 2 (°C) | Screw 3 (°C) | Pipeline (°C) | Metering Pump (°C) | Die (°C) | Hot Air Duct Temperature (°C) |
|---|---|---|---|---|---|---|
| 180 | 280 | 310 | 310 | 200 | 193 | 210 |
| Screw Pressure (Mpa) | Metering Pump Flow (r·min−1) | Air Pressure (MPa) | Collector Speed (cm·min−1) | Distance/cm |
|---|---|---|---|---|
| 0.1–1 | 7.6 | 0.03 | 44 | 20 |
| Pump-Flow (mL/h) | Pump-Area (mm2) | Drum Diameter (mm) | Winding Speed (mm/s) | Round Trip Distance (mm) |
|---|---|---|---|---|
| 0.8 | 187.62 | 19.11 | 150.00 | 120.00 |
| Moving Speed (mm/s) | Reduction Ratio | Voltage (kV) | Current (mA) | |
| 30.00 | 1.00 | 24.00 | 0.01 |
| Sample | Ingredient |
|---|---|
| PP/PVA | PVA(10%)ZIF-8(0%) |
| PP/PVA/ZIF-8-1.25% | PVA(10%)ZIF-8(1.25%) |
| PP/PVA/ZIF-8-2.5% | PVA(10%)ZIF-8(2.5%) |
| PP/PVA/ZIF-83.75% | PVA(10%)ZIF-8(3.75%) |
| PP/PVA/ZIF-8-5% | PVA(10%)ZIF-8(5%) |
| Sample | PM (μm) | Average Filtration Efficiency (%) | Pressure Drop Resistance (Pa) | Quality Factor |
|---|---|---|---|---|
| PP | 0.218–2.478 | 21.0 | 24 | 0.009 |
| 0.218–4.595 | 26.5 | 0.013 | ||
| PP/PVA | 0.218–2.478 | 93.8 | 88 | 0.032 |
| 0.218–4.595 | 94.6 | 0.033 | ||
| PP/PVA/ZIF-8-1.25% | 0.218–2.478 | 95.6 | 152 | 0.020 |
| 0.218–4.595 | 96.1 | 0.021 | ||
| PP/PVA/ZIF-8-2.5% | 0.218–2.478 | 96.5 | 34 | 0.099 |
| 0.218–4.595 | 97.1 | 0.103 | ||
| PP/PVA/ZIF-8-3.75% | 0.218–2.478 | 95.9 | 130 | 0.025 |
| 0.218–4.595 | 96.4 | 0.026 | ||
| PP/PVA/ZIF-8-5% | 0.218–2.478 | 97.1 | 94 | 0.038 |
| 0.218–4.595 | 97.4 | 0.039 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.-T.; Fan, Y.; Cen, X.; Wang, Y.; Shiu, B.-C.; Ren, H.-T.; Peng, H.-K.; Jiang, Q.; Lou, C.-W.; Lin, J.-H. Polypropylene/Polyvinyl Alcohol/Metal-Organic Framework-Based Melt-Blown Electrospun Composite Membranes for Highly Efficient Filtration of PM2.5. Nanomaterials 2020, 10, 2025. https://doi.org/10.3390/nano10102025
Li T-T, Fan Y, Cen X, Wang Y, Shiu B-C, Ren H-T, Peng H-K, Jiang Q, Lou C-W, Lin J-H. Polypropylene/Polyvinyl Alcohol/Metal-Organic Framework-Based Melt-Blown Electrospun Composite Membranes for Highly Efficient Filtration of PM2.5. Nanomaterials. 2020; 10(10):2025. https://doi.org/10.3390/nano10102025
Chicago/Turabian StyleLi, Ting-Ting, Yujia Fan, Xixi Cen, Yi Wang, Bing-Chiuan Shiu, Hai-Tao Ren, Hao-Kai Peng, Qian Jiang, Ching-Wen Lou, and Jia-Horng Lin. 2020. "Polypropylene/Polyvinyl Alcohol/Metal-Organic Framework-Based Melt-Blown Electrospun Composite Membranes for Highly Efficient Filtration of PM2.5" Nanomaterials 10, no. 10: 2025. https://doi.org/10.3390/nano10102025
APA StyleLi, T.-T., Fan, Y., Cen, X., Wang, Y., Shiu, B.-C., Ren, H.-T., Peng, H.-K., Jiang, Q., Lou, C.-W., & Lin, J.-H. (2020). Polypropylene/Polyvinyl Alcohol/Metal-Organic Framework-Based Melt-Blown Electrospun Composite Membranes for Highly Efficient Filtration of PM2.5. Nanomaterials, 10(10), 2025. https://doi.org/10.3390/nano10102025