Highly Porous Free-Standing rGO/SnO2 Pseudocapacitive Cathodes for High-Rate and Long-Cycling Al-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Single Components
2.2. Ice-Templating and Annealing
2.3. Microstructural Characterization
2.4. Mechanical Characterization
2.5. Electrochemical Characterization
3. Results and Discussion
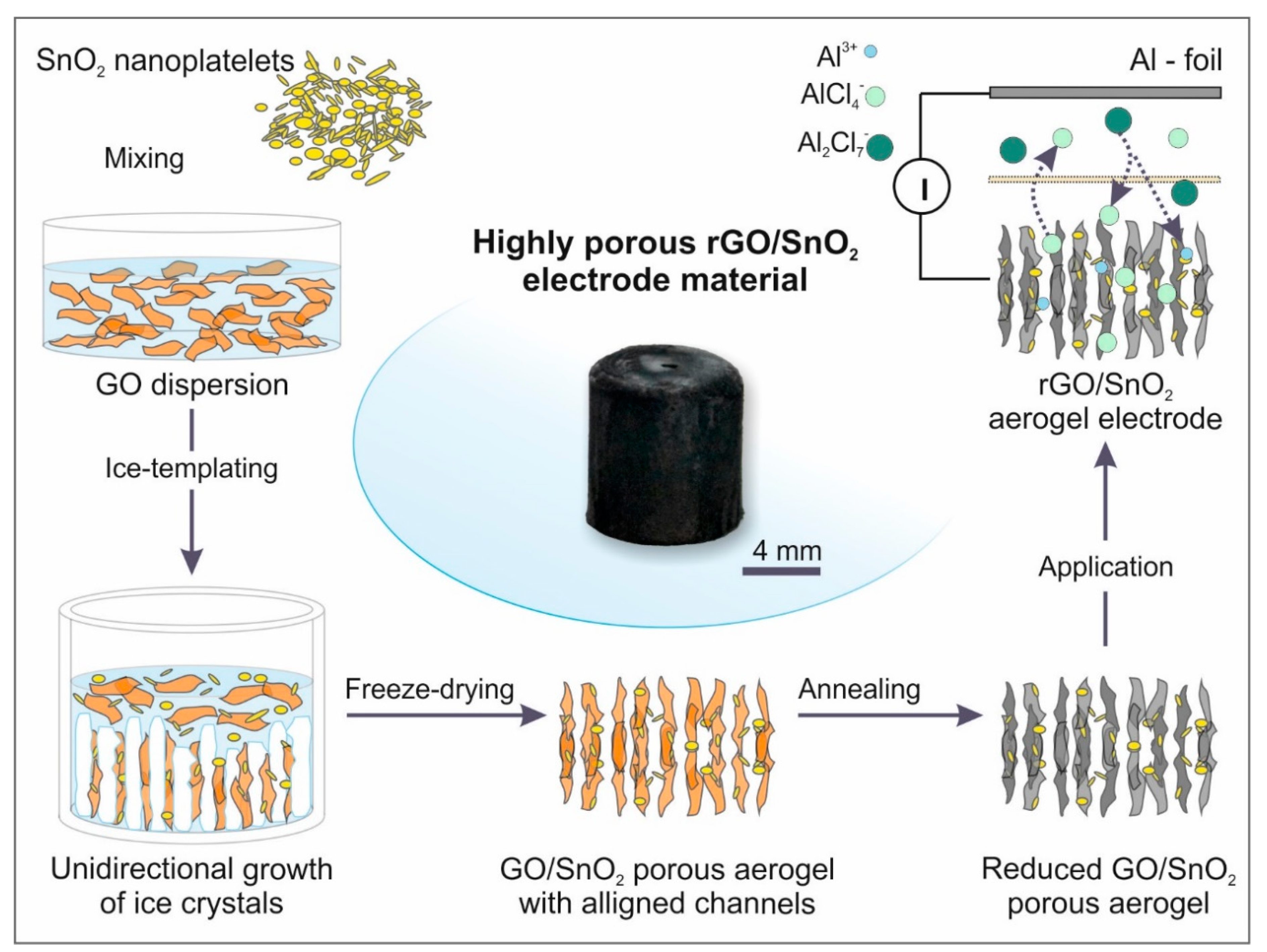
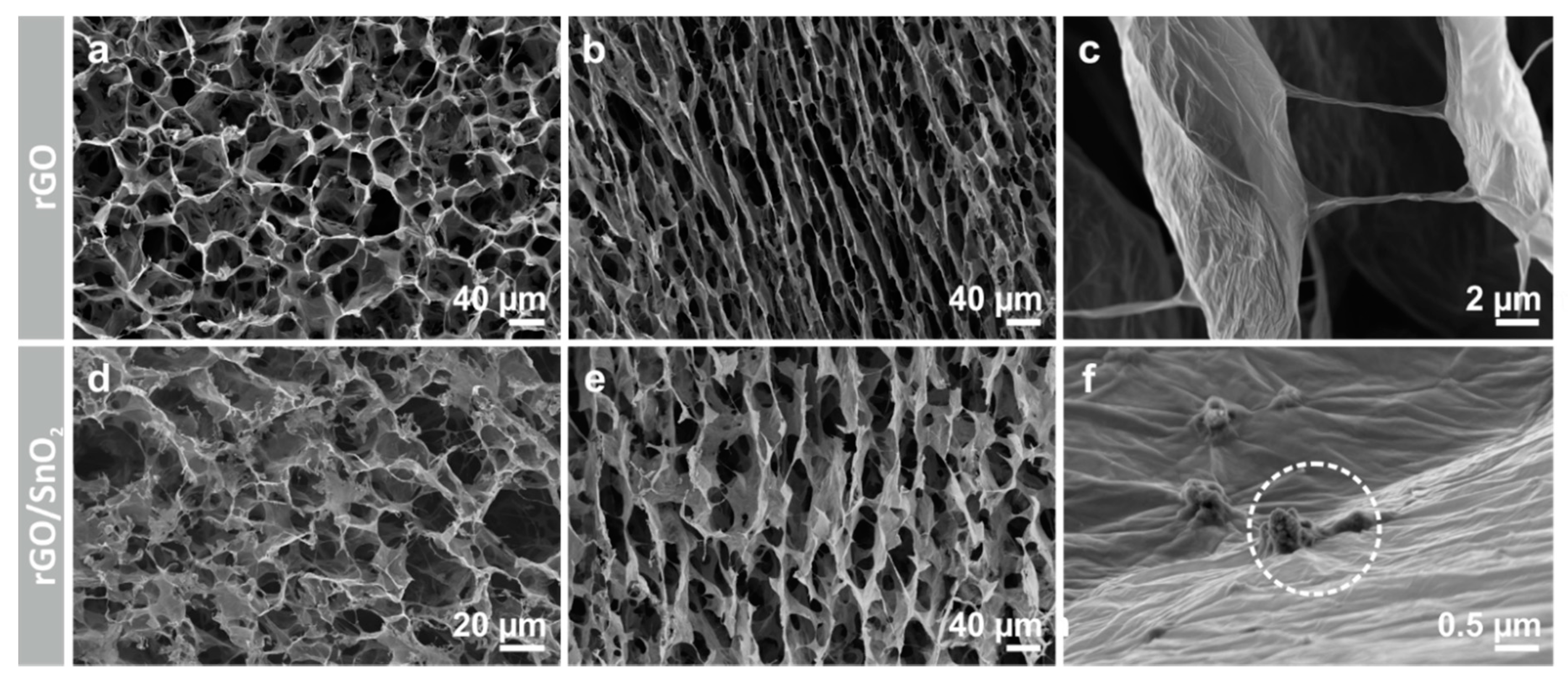
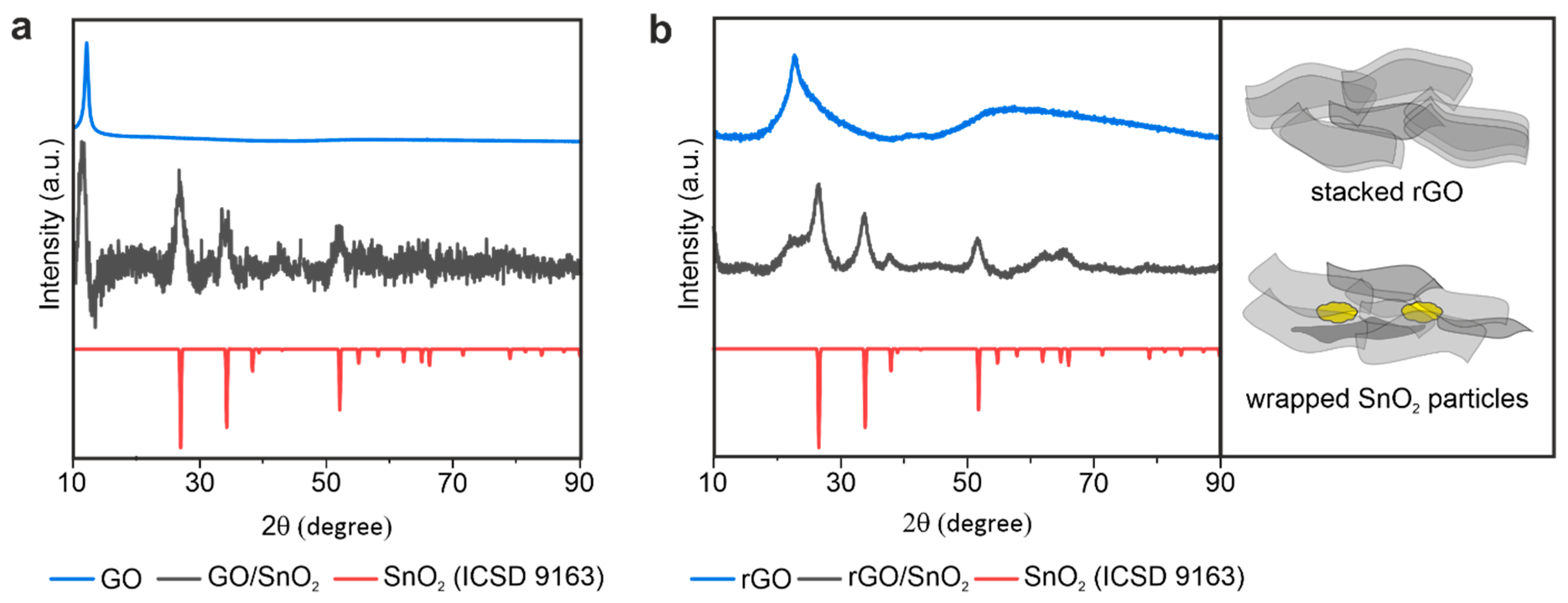
3.1. Fabrication of the Porous, Binder-Free rGO/SnO2 Aerogels
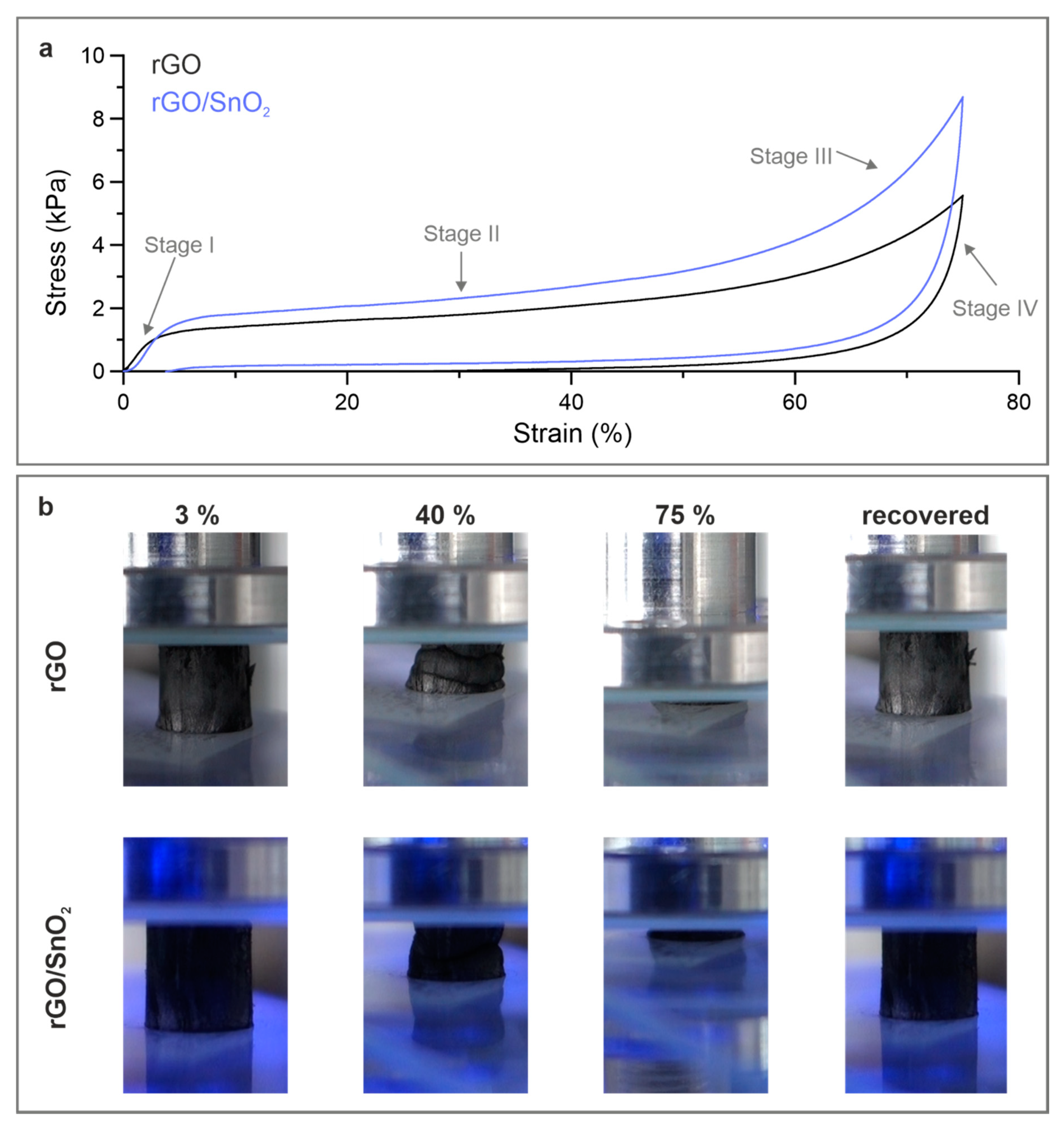
3.2. Mechanical Performance of the rGO/SnO2 Aerogels
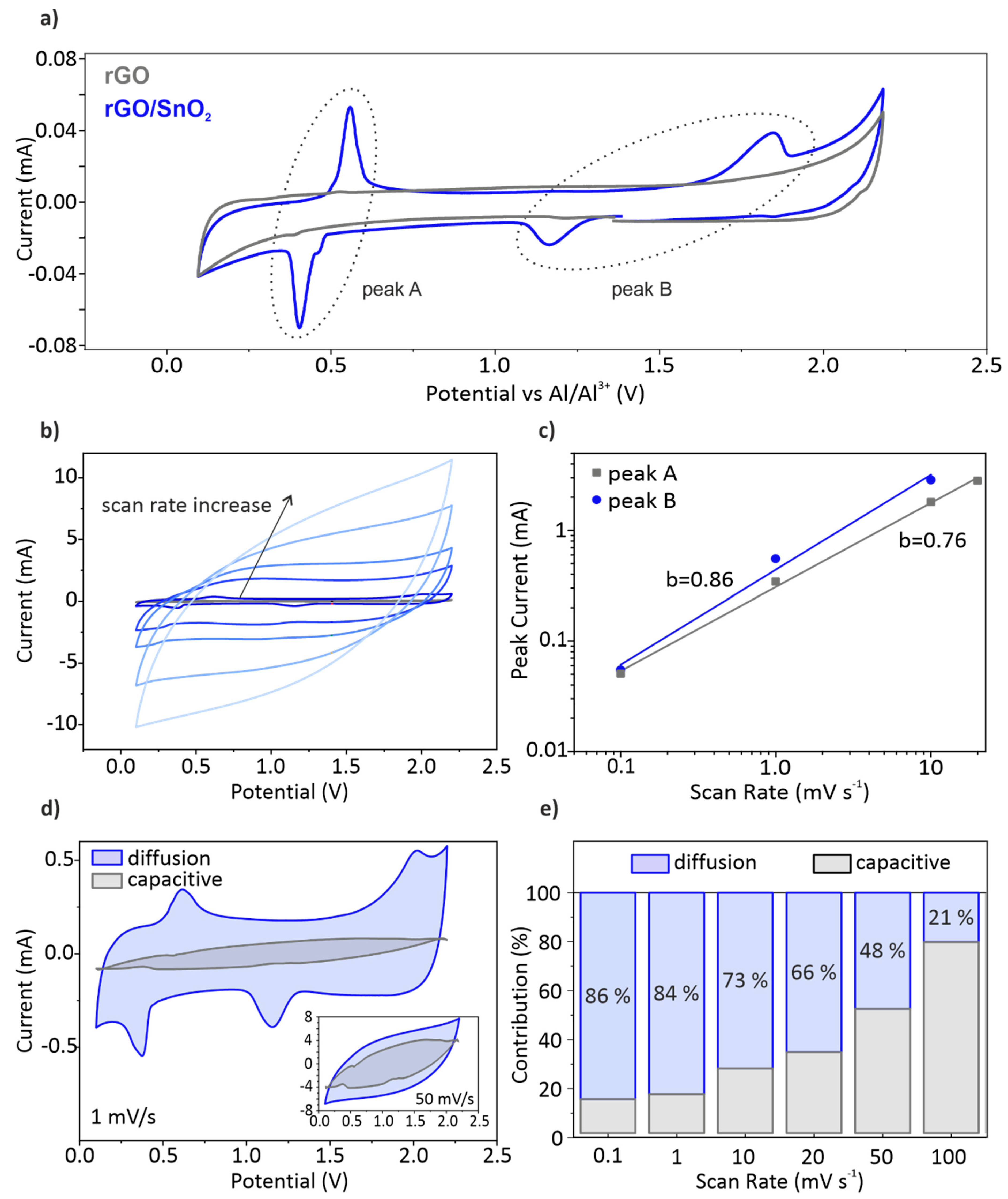
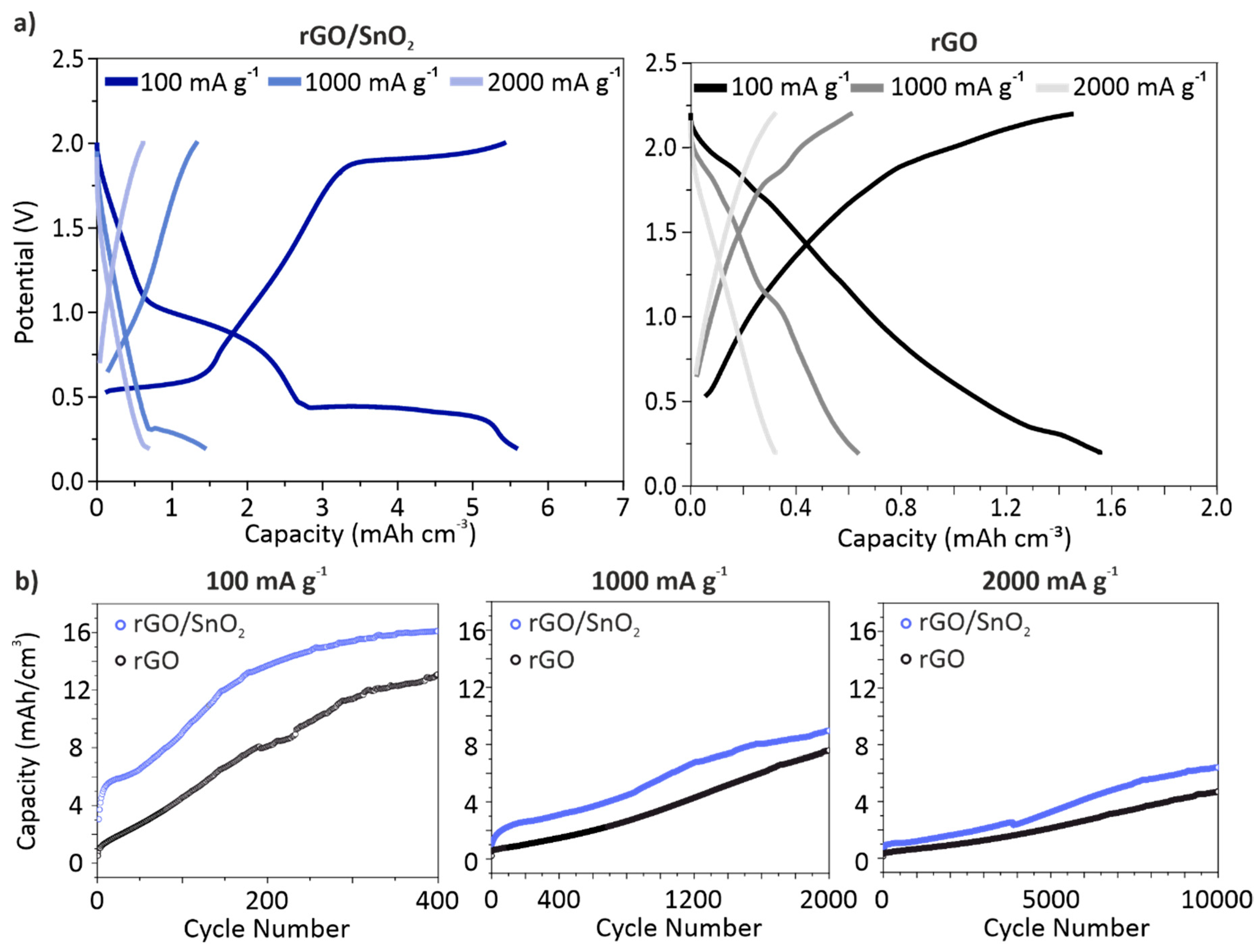
3.3. Electrochemical Performance of the rGO and rGO/SnO2 Aerogels
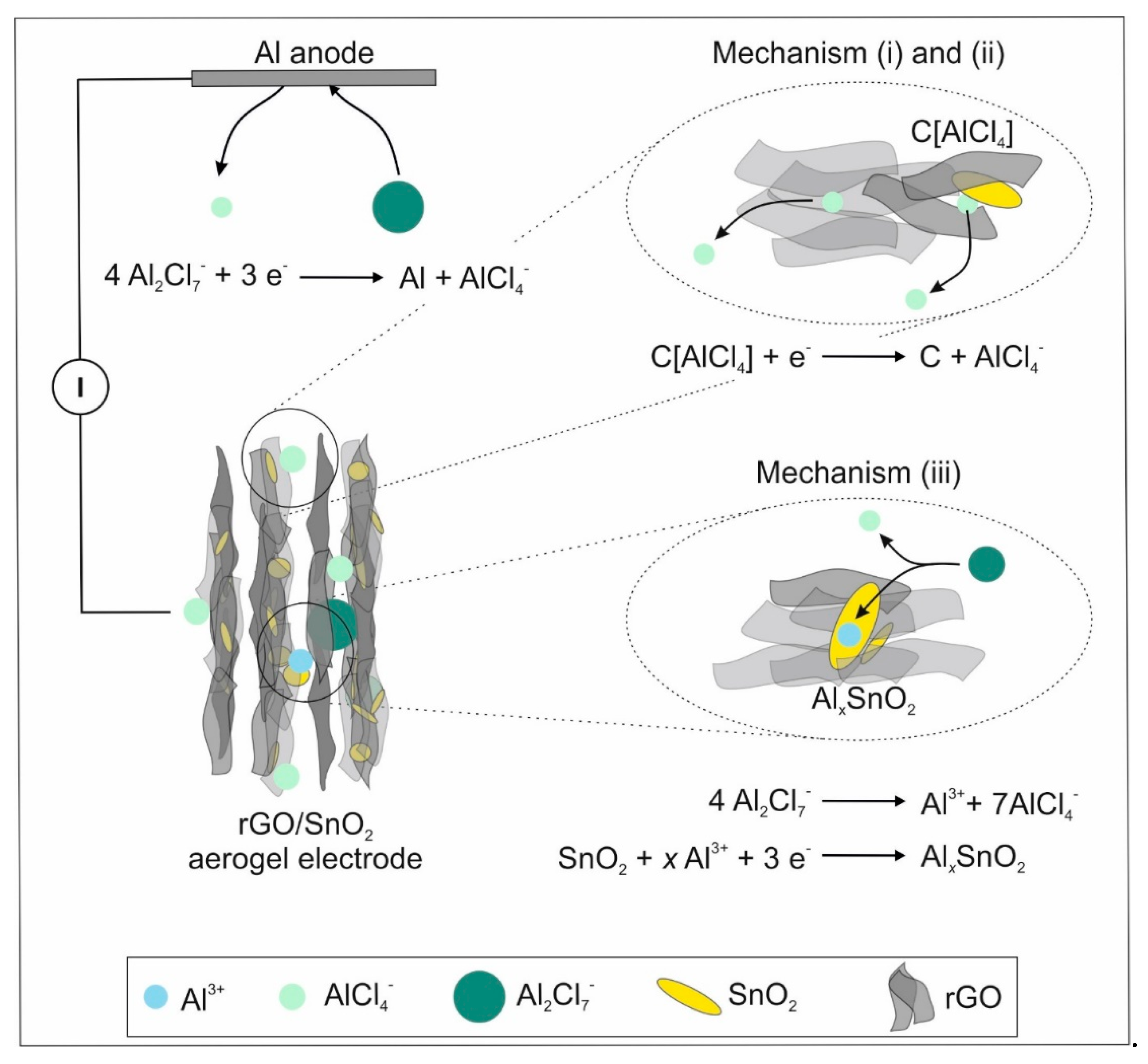
- (i)
- The non-Faradaic contribution is partially correlated with the electrochemical double layer capacitance observed for rGO sheets with crystallite sizes below 10 nm [16]. The chloroaluminate anions are thereby adsorbed at the surface of rGO sheets.
- (ii)
- Peak B is hypothesized to correlate with the intercalation of AlCl4- between SnO2 and rGO. The SnO2 nanoparticles embedded in the rGO aerogel, provide sufficient space for intercalation, as they increase the cumulative pore volume fraction. When considering graphitic materials, intercalation involving AlCl4- results in a characteristic peak around 2 V [13,57]. Additionally, the SnO2 nanocrystals distort the graphitic structure, which could facilitate the intercalation and thus lowers the voltage. This mechanism contributes further to the pseudocapacitive behavior of the hybrid aerogel electrode.
- (iii)
- The Faradaic contribution entails the de-/intercalation of Al3+ into SnO2 (peak A), which was similarly observed for SnO2/C cathodes [28].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Zhang, Q.; Li, L.; Niu, Z.; Chen, J. Design Strategies toward Enhancing the Performance of Organic Electrode Materials in Metal-Ion Batteries. Chemistry 2018, 4, 2786. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Shen, F.; Bommier, C.; Zhu, H.; Ji, X.; Hu, L.N. Na-Ion Battery Anodes: Materials and Electrochemistry. Acc. Chem. Res. 2016, 49, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Valma, C.; Buchholz, D.; Passerini, S. Non-aqueous potassium-ion batteries: A review. Curr. Opin. Electrochem. 2018, 9, 41–48. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Ji, X.; Li, Y.; Shao, Y.; Chen, Z.; Zhong, C.; Amine, K. Design strategies for nonaqueous multivalent-ion and monovalent-ion battery anodes. Nat. Rev. Mater. 2020, 5, 276–294. [Google Scholar] [CrossRef]
- Cui, L.; Zhou, L.; Kang, Y.-M.; An, Q. Recent Advances in the Rational Design and Synthesis of Two-Dimensional Materials for Multivalent Ion Batteries. ChemSusChem 2020, 13, 1071–1092. [Google Scholar] [CrossRef]
- Das, S.K.; Mahapatra, S.; Lahan, H. Aluminium-ion batteries: Developments and challenges. J. Mater. Chem. A 2017, 5, 6347. [Google Scholar] [CrossRef]
- Das, S.K. Graphene: A Cathode Material of Choice for Aluminum-Ion Batteries. Angew. Chem. Int. Ed. 2018, 57, 16606. [Google Scholar] [CrossRef]
- Elia, G.A.; Marquardt, K.; Hoeppner, K.; Fantini, S.; Lin, R.; Knipping, E.; Peters, W.; Drillet, J.-F.; Passerini, S.; Hahn, R. An Overview and Future Perspectives of Aluminum Batteries. Adv. Mater. 2016, 28, 7564. [Google Scholar] [CrossRef]
- Leisegang, T.; Meutzner, F.; Zschornak, M.; Münchgesang, W.; Schmid, R.; Nestler, T.; Eremin, R.A.; Kabanov, A.A.; Blatov, V.A.; Meyer, D.C. The Aluminum-Ion Battery: A Sustainable and Seminal Concept? Front. Chem. 2019, 7, 268. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Li, J.; Sun, Z.; He, K.; Cheng, H.-M.; Li, F. Die wiederaufladbare Aluminiumbatterie: Möglichkeiten und Herausforderungen. Angew. Chem. 2019, 131, 12104. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiao, S.; Tu, J.; Song, W.-L.; Xiao, X.; Li, S.; Wang, M.; Lei, H.; Tian, D.; Chen, H.; et al. Rechargeable ultrahigh-capacity tellurium–aluminum batteries. Energy Env. Sci. 2019, 12, 1918. [Google Scholar] [CrossRef]
- Lin, M.-C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.-Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.-J.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, N.; Huang, L.; Zhang, T.; Fang, S.; Chang, H.; Li, N.; Oh, J.; Lee, J.A.; Kozlov, M.; et al. Three-dimensionally bonded spongy graphene material with super compressive elasticity and near-zero Poisson’s ratio. Nat. Commun. 2015, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Guo, F.; Liu, Y.; Huang, T.; Zheng, B.; Ananth, N.; Xu, Z.; Gao, W.; Gao, C. A Defect-Free Principle for Advanced Graphene Cathode of Aluminum-Ion Battery. Adv. Mater. 2017, 29, 1605958. [Google Scholar] [CrossRef] [PubMed]
- Smajic, J.; Alazmi, A.; Batra, N.; Palanisamy, T.; Anjum, D.H.; Costa, P.M.F.J. Electrochemical Energy Storage: Mesoporous Reduced Graphene Oxide as a High Capacity Cathode for Aluminum Batteries. Small 2018, 14, 1870251. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Gong, M.; Lin, M.-C.; Yuan, C.; Angell, M.; Huang, L.; Wang, D.-Y.; Zhang, X.; Yang, J.; Hwang, B.-J.; et al. 3D Graphitic Foams Derived from Chloroaluminate Anion Intercalation for Ultrafast Aluminum-Ion Battery. Adv. Mater. 2016, 28, 9218. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Lee, T.H.; Cha, J.H.; Jang, H.W.; Shokouhimehr, M.; Choi, J.-W. S@ GO as a High-Performance Cathode Material for Rechargeable Aluminum-Ion Batteries. Electron. Mater. Lett. 2019, 15, 720. [Google Scholar] [CrossRef]
- Gao, T.; Li, X.; Wang, X.; Hu, J.; Han, F.; Fan, X.; Suo, L.; Pearse, A.J.; Lee, S.B.; Rubloff, G.W.; et al. A Rechargeable Al/S Battery with an Ionic-Liquid Electrolyte. Angew. Chem. Int. Ed. 2016, 55, 9898. [Google Scholar] [CrossRef]
- Wang, S.; Jiao, S.; Wang, J.; Chen, H.-S.; Tian, D.; Lei, H.; Fang, D.-N. High-Performance Aluminum-Ion Battery with CuS@C Microsphere Composite Cathode. ACS Nano 2017, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Tu, J.; Zhang, G.; Li, S.; Tian, D.; Jiao, S. Flower-like Vanadium Suflide/Reduced Graphene Oxide Composite: An Energy Storage Material for Aluminum-Ion Batteries. ChemSusChem 2018, 11, 709. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Luo, B.; Ye, D.; Zhu, X.; Lyu, M.; Wang, L. An Innovative Freeze-Dried Reduced Graphene Oxide Supported SnS2 Cathode Active Material for Aluminum-Ion Batteries. Adv. Mater. 2017, 29, 1606132. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, Z.; Tu, J.; Wang, J.; Tian, D.; Liu, Y.; Jiao, S. A Novel Aluminum-Ion Battery: Al/AlCl3-[EMIm]Cl/Ni3S2@Graphene. Adv. Energy Mater. 2016, 6, 1600137. [Google Scholar] [CrossRef]
- Cohn, G.; Ma, L.; Archer, L.A. A novel non-aqueous aluminum sulfur battery. J. Power Sources 2015, 283, 416. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Wang, S.; Li, S.; Jiao, S.J. Porous CuO microsphere architectures as high-performance cathode materials for aluminum-ion batteries. Mater. Chem. A 2018, 6, 3084. [Google Scholar] [CrossRef]
- Wang, H.; Bai, Y.; Chen, S.; Luo, X.; Wu, C.; Wu, F.; Lu, J.; Amine, K. Binder-Free V2O5 Cathode for Greener Rechargeable Aluminum Battery. ACS Appl. Mater. Interfaces 2015, 7, 80. [Google Scholar] [CrossRef]
- Lu, H.; Wan, Y.; Wang, T.; Jin, R.; Ding, P.; Wang, R.; Wang, Y.; Teng, C.; Li, L.; Wang, X.; et al. A high performance SnO2/C nanocomposite cathode for aluminum-ion batteries. J. Mater. Chem. A 2019, 7, 7213. [Google Scholar] [CrossRef]
- Yamazaki, T.; Mizutani, U.; Iwama, Y. Electrical Properties of SnO2 Polycrystalline Thin Films and Single Crystals Exposed to O2- and H2-Gases. Jpn. J. Appl. Phys. 1983, 22, 454. [Google Scholar] [CrossRef]
- Fonstad, C.G.; Rediker, R.H. Electrical Properties of High-Quality Stannic Oxide Crystals. J. Appl. Phys. 1971, 42. [Google Scholar] [CrossRef]
- Jahnke, T.; Knöller, A.; Kilper, S.; Rothenstein, D.; Widenmeyer, M.; Burghard, Z.; Bill, J. Coalescence in Hybrid Materials: The Key to High-Capacity Electrodes. ACS Appl. Energy Mater. 2018, 1, 7085–7092. [Google Scholar] [CrossRef]
- Kwon, O.H.; Oh, J.H.; Gu, B.; Jo, M.S.; Oh, S.H.; Kang, Y.C.; Kim, J.-K.; Jeong, S.M.; Cho, J.S. Lithium Polymer Batteries: Porous SnO2/C Nanofiber Anodes and LiFePO4/C Nanofiber Cathodes with a Wrinkle Structure for Stretchable Lithium Polymer Batteries with High Electrochemical Performance. Adv. Sci. 2020, 7, 2070093. [Google Scholar] [CrossRef]
- Cho, J.S.; Kang, Y.C. Nanofibers Comprising Yolk-Shell Sn@void@SnO/SnO₂ and Hollow SnO/SnO₂ and SnO₂ Nanospheres via the Kirkendall Diffusion Effect and Their Electrochemical Properties. Small 2015, 11, 4673–4681. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Wang, C.; Du, G.; Ståhl, K.; Huang, H.; Zhong, Y.; Jiang, J.Z. Ultrathin SnO2 Nanosheets: Oriented Attachment Mechanism, Nonstoichiometric Defects, and Enhanced Lithium-Ion Battery Performances. J. Phys. Chem. C 2012, 116, 4000. [Google Scholar] [CrossRef] [Green Version]
- Deville, S.; Saiz, E.; Nalla, R.K.; Tomsia, A.P. Freezing as a Path to Build Complex Composites. Science 2006, 311, 515–518. [Google Scholar] [CrossRef] [Green Version]
- Deville, S. Freezing Colloids: Observations, Principles, Control, and Use: Applications in Materials Science, Life Science, Earth Science, Food Science, and Engineering (Engineering Materials and Processes); Deville, S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 351–438. [Google Scholar]
- Ni, N.; Barg, S.; Garcia-Tunon, E.; Perez, F.M.; Miranda, M.; Lu, C.; Mattevi, C.; Saiz, E. Mesoscale assembly of chemically modified graphene into complex cellular networks. Sci. Rep. 2015, 5, 4328. [Google Scholar]
- Zhu, C.; Han, T.Y.-J.; Duoss, E.B.; Golobic, A.M.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A. Highly compressible 3D periodic graphene aerogel microlattices. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nieto, A.; Boesl, B.; Agarwal, A. Multi-scale intrinsic deformation mechanisms of 3D graphene foam. Carbon 2015, 85, 299. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.M.; Chhowalla, M.; Cho, K.; Chabal, Y.J. The Role of Oxygen during Thermal Reduction of Graphene Oxide Studied by Infrared Absorption Spectroscopy. Phys. Chem. C 2011, 115, 19761. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, K.; Zhu, S.; Luo, W.; Wang, Y.; Li, Y.; Hitz, E.; Yao, Y.; Dai, J.; Wan, J.; et al. Reduced Graphene Oxide Films with Ultrahigh Conductivity as Li-Ion Battery Current Collectors. Nano Lett. 2016, 16, 3616. [Google Scholar] [CrossRef] [PubMed]
- Acik, M.; Chabal, Y.J. A Review on Reducing Graphene Oxide for Band Gap Engineering. JMSR 2012, 2, 101. [Google Scholar] [CrossRef]
- Wiberg, N.; Holleman, A.F.; Wiberg, E.; Fischer, G. Lehrbuch der Anorganischen Chemie; 102., Stark Umgearb. u. Verb.; De Gruyter: Berlin, Germany; New York, NY, USA, 2007. [Google Scholar]
- Zhou, X.; Wan, L.-W.; Guo, Y.-G. Binding SnO2 Nanocrystals in Nitrogen-Doped Graphene Sheets as Anode Materials for Lithium-Ion Batteries—Zhou. Adv. Mater. 2013, 25, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Noerochim, L.; Wang, J.-Z.; Chou, S.-L.; Wexler, D.; Liu, H.-K. Free-standing single-walled carbon nanotube/SnO2 anode paper for flexible lithium-ion batteries. Carbon 2012, 50, 1289. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.M.; Lim, Y.-G.; Kim, J.-G.; Heo, Y.-U.; Lim, J.H.; Yamauchi, Y.; Park, M.-S.; Kim, Y.-J.; Dou, S.X.; Kim, J.H. A case study on fibrous porous SnO2 anode for robust, high-capacity lithium-ion batteries. Nano Energy 2014, 10, 53. [Google Scholar] [CrossRef]
- Schneider, P. Adsorption isotherms of microporous-mesoporous solids revisited. Appl. Catal. A Gen. 1995, 129, 157. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wang, J.; Xing, C.; Ge, J.; Fan, L.; Liu, Z.; Lu, X.; Wu, M.; Yu, X.; et al. Low-temperature synthesis of edge-rich graphene paper for high-performance aluminum batteries. Energy Storage Mater. 2018, 15, 361. [Google Scholar] [CrossRef]
- Knöller, A.; Kilper, S.; Diem, A.M.; Widenmeyer, M.; Runčevski, T.; Dinnebier, R.E.; Bill, J.; Burghard, Z. Ultrahigh Damping Capacities in Lightweight Structural Materials. Nano Lett. 2018, 18, 2519. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.F.; Medalist, R.F.M. The mechanical properties of cellular solids. MTA 1983, 14, 1755. [Google Scholar] [CrossRef]
- Qiu, L.; Huang, B.; He, Z.; Wang, Y.; Tian, Z.; Liu, J.Z.; Wang, K.; Song, J.; Gengenbach, T.R.; Li, D. Extremely Low Density and Super-Compressible Graphene Cellular Materials. Adv. Mater. 2017, 29, 1701553. [Google Scholar] [CrossRef] [Green Version]
- Jiao, H.; Wang, J.; Tu, J.; Lei, H.; Jiao, S. Aluminum-Ion Asymmetric Supercapacitor Incorporating Carbon Nanotubes and an Ionic Liquid Electrolyte: Al/AlCl3-[EMIm]Cl/CNTs. Energy Technol. 2016, 4, 1112. [Google Scholar] [CrossRef]
- Raju, V.; Rains, J.; Gates, C.; Luo, W.; Wang, X.; Stickle, W.F.; Stucky, G.D.; Ji, X. Superior Cathode of Sodium-Ion Batteries: Orthorhombic V2O5 Nanoparticles Generated in Nanoporous Carbon by Ambient Hydrolysis Deposition. Nano Lett. 2014, 14, 4119. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wen, Y.; Hu, X.; Ji, X.; Yan, M.; Mai, L.; Hu, P.; Shan, B.; Huang, Y. Na+ intercalation pseudocapacitance in graphene-coupled titanium oxide enabling ultra-fast sodium storage and long-term cycling. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Niu, B.; Li, J.; Kang, F. A Novel Graphite–Graphite Dual Ion Battery Using an AlCl3–[EMIm]Cl Liquid Electrolyte. Small 2018, 14, 1800745. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, M.; Chen, W.; Liu, Y.; Zhang, L.; Dongfang, N.; Ruan, Y.; Zhang, J.; Wang, P.; Dong, L.; et al. Sandwich Vertical-Channeled Thick Electrodes with High Rate and Cycle Performance. Adv. Funct. Mater. 2019, 29, 1809196. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahnke, T.; Raafat, L.; Hotz, D.; Knöller, A.; Diem, A.M.; Bill, J.; Burghard, Z. Highly Porous Free-Standing rGO/SnO2 Pseudocapacitive Cathodes for High-Rate and Long-Cycling Al-Ion Batteries. Nanomaterials 2020, 10, 2024. https://doi.org/10.3390/nano10102024
Jahnke T, Raafat L, Hotz D, Knöller A, Diem AM, Bill J, Burghard Z. Highly Porous Free-Standing rGO/SnO2 Pseudocapacitive Cathodes for High-Rate and Long-Cycling Al-Ion Batteries. Nanomaterials. 2020; 10(10):2024. https://doi.org/10.3390/nano10102024
Chicago/Turabian StyleJahnke, Timotheus, Leila Raafat, Daniel Hotz, Andrea Knöller, Achim Max Diem, Joachim Bill, and Zaklina Burghard. 2020. "Highly Porous Free-Standing rGO/SnO2 Pseudocapacitive Cathodes for High-Rate and Long-Cycling Al-Ion Batteries" Nanomaterials 10, no. 10: 2024. https://doi.org/10.3390/nano10102024
APA StyleJahnke, T., Raafat, L., Hotz, D., Knöller, A., Diem, A. M., Bill, J., & Burghard, Z. (2020). Highly Porous Free-Standing rGO/SnO2 Pseudocapacitive Cathodes for High-Rate and Long-Cycling Al-Ion Batteries. Nanomaterials, 10(10), 2024. https://doi.org/10.3390/nano10102024




