Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications
Abstract
1. Introduction
2. Nanobiomaterials
2.1. Organic Nanobiomaterials
2.1.1. Lipids
2.1.2. Liposomes
2.1.3. Dendrimers
2.1.4. Polymeric Nanomaterials
Chitosan
Collagen
Gelatin
Poly (Lactic-co-glycolic) acid (PLGA)
2.1.5. Carbon Nanostructures
2.1.6. Summary and Statistical Analysis of the Survey on Organic Nanobiomaterials
2.2. Inorganic Nanobiomaterials
2.2.1. Nano Silica
2.2.2. Nano Bioglass
2.2.3. Nano Hydroxyapatite
2.2.4. Silver Nanoparticles
2.2.5. Gold Nanoparticles
2.2.6. Titanium Dioxide
2.2.7. Zirconia
2.2.8. Alumina
2.2.9. Copper
2.2.10. Magnetic Nanoparticles
2.2.11. Summary and Statistical Analysis of the Survey on Inorganic Nanobiomaterials
3. Applications of Nanobiomaterials
3.1. Bone Regeneration
3.2. Drug Delivery
3.3. Gene Delivery
3.4. Anti-Infective Nanobiomaterials
3.5. Nanobiomaterials for Coating
3.6. Nanostructured Scaffolds
3.7. Bone Cancer Therapy
4. Counter-Indications
5. Conclusions
Funding
Conflicts of Interest
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.-S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Parratt, K.; Yao, N. Nanostructured biomaterials and their applications. Nanomaterials 2013, 3, 242–271. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Lijuan, Z.; Webster, T.J. Nanobiomaterials: State of the art and future trends. Adv. Eng. Mater. 2011, 13, B197–B217. [Google Scholar]
- Ramos, A.P.; Cruz, M.A.E.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Capek, I.B.T.-S. Nanocomposite Structures and Dispersions; Elsevier: Amsterdam, The Netherlands, 2006; Chapter 1; Volume 23, pp. 1–69. [Google Scholar]
- Florencio-Silva, R.; Rodrigues, G.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S.; Cells, B. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- McMahon, R.; Wang, L.; Skoracki, R.; Mathur, A. Development of nanomaterials for bone repair and regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Kalenderer, Ö.; Turgut, A. Bone. In Musculoskeletal Research and Basic Science; Korkusuz, F., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Chapter 18; pp. 303–321. [Google Scholar]
- Farbod, K.; Nejadnik, M.R.; Jansen, J.A.; Leeuwenburgh, S.C.G. Interactions between inorganic and organic phases in bone tissue as a source of inspiration for design of novel nanocomposites. Tissue Eng. Part B Rev. 2013, 20, 173–188. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Walmsley, G.G.; Mcardle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomedicine 2015, 11, 1253–1263. [Google Scholar] [CrossRef]
- Mohamed, A.M. An overview of bone cells and their regulating factors of differentiation. Malays. J. Med. Sci. 2008, 15, 4–12. [Google Scholar] [PubMed]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Brouki Milan, P.; Kim, H.-W.; Baino, F. Bone tissue engineering using human cells: A Comprehensive review on recent trends, current prospects, and recommendations. Appl. Sci. 2019, 9, 174. [Google Scholar] [CrossRef]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef]
- Glimcher, M. Bone: Nature of the calcium phosphate crystals and cellular, structural, and physical chemical mechanisms in their formation. Rev. Miner. Geochem. 2006, 64, 223–282. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Liu, S.; Gong, W.; Dong, Y.; Hu, Q.; Chen, X.; Gao, X. The effect of submicron bioactive glass particles on in vitro osteogenesis. RSC Adv. 2015, 5, 38830–38836. [Google Scholar] [CrossRef]
- Cheng, C.W.; Solorio, L.D.; Alsberg, E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol. Adv. 2014, 32, 462–484. [Google Scholar] [CrossRef]
- Nie, X.; Wang, D.-A. Decellularized orthopaedic tissue-engineered grafts: Biomaterial scaffolds synthesised by therapeutic cells. Biomater. Sci. 2018, 6, 2798–2811. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.; Cho, S.W.; Kim, J.; Kim, J.-S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014, 24, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, H.; Tian, Y.; Xie, Y.; Li, A.; Ji, L.; Niu, Z.; Wu, D.; Qiu, D. Bioactive nanoparticle—Gelatin composite scaff old with mechanical performance comparable to cancellous bones. ACS Appl. Mater. Interfaces 2014, 6, 13061–13068. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Ajita, J.; Selvamurugan, N. Metallic nanomaterials for bone tissue engineering. J. Biomed. Nanotechnol. 2015, 11, 1675–1700. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vega, A.I.; Gómez-Quintero, T.; Nuñez-Anita, R.E.; Acosta-Torres, L.S.; Castaño, V. Polymeric and ceramic nanoparticles in biomedical applications. J. Nanotechnol. 2012, 2012, 936041. [Google Scholar] [CrossRef]
- Kim, N.J.; Lee, S.J.; Atala, A. Biomedical nanomaterials in tissue engineering. In Woodhead Publishing Series in Biomaterials; Gaharwar, A.K., Sant, S., Hancock, M.J., Hacking, S.A.B.T.-N., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 1e–25e. [Google Scholar]
- Gajanan, K.; Tijare, S.N. Applications of nanomaterials. Mater. Today Proc. 2018, 5, 1093–1096. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y. Bottom-up and top-down approaches to the synthesis of monodispersed spherical colloids of low melting-point metals. Nano Lett. 2004, 4, 2047–2050. [Google Scholar] [CrossRef]
- Saiz, E.; Zimmermann, E.A.; Lee, J.S.; Wegst, U.G.K.; Tomsia, A.P. Perspectives on the role of nanotechnology in bone tissue engineering. Dent. Mater. 2013, 29, 103–115. [Google Scholar] [CrossRef]
- Gardin, C.; Ferroni, L.; Favero, L.; Stellini, E.; Stomaci, D.; Sivolella, S.; Bressan, E.; Zavan, B. Nanostructured biomaterials for tissue engineered bone tissue reconstruction. Int. J. Mol. Sci. 2012, 13, 737–757. [Google Scholar]
- España-Sánchez, B.L.; Cruz-Soto, M.E.; Elizalde-Pena, E.A.; Sabasflores-Benitez, S.; Roca-Aranda, A.; Esquivel-Escalante, K.; Luna-Barcenas, G. Trends in Tissue Regeneration: Bio-nanomaterials. In Tissue Rigeneration; El-Sayed Kaoud, H.A., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Liang, L.; Fan, X.; Yu, Z.; Hotchkiss, A.T.; Wilk, B.J.; Eliaz, I. The role of modified citrus pectin as an effective chelator of lead in children hospitalized with toxic lead levels. Altern. Ther. Health Med. 2008, 14, 34–38. [Google Scholar]
- Zheng, K.; Balasubramanian, P.; Paterson, T.E.; Stein, R.; MacNeil, S.; Fiorilli, S.; Vitale-Brovarone, C.; Shepherd, J.; Boccaccini, A.R. Ag modified mesoporous bioactive glass nanoparticles for enhanced antibacterial activity in 3D infected skin model. Mater. Sci. Eng. C 2019, 103, 109764. [Google Scholar] [CrossRef]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- John, Ł. Selected developments and medical applications of organic–inorganic hybrid biomaterials based on functionalized spherosilicates. Mater. Sci. Eng. C 2018, 88, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. Biomaterials: A review of organic and inorganic biomaterials for neural interfaces. Adv. Mater. 2014, 26, 1793. [Google Scholar] [CrossRef]
- Virlan, M.J.R.; Miricescu, D.; Radulescu, R.; Sabliov, C.M.; Totan, A.; Calenic, B.; Greabu, M. Organic nanomaterials and their applications in the treatment of oral diseases. Molecules 2016, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Chellan, P.; Sadler, P.J. The elements of life and medicines. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140182. [Google Scholar] [CrossRef] [PubMed]
- Kiaie, N.; Aavani, F.; Razavi, M. 2—particles/fibers/bulk. In Development and Evaluation; Razavi, M., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 7–25. [Google Scholar]
- Wang, G.; Mostafa, N.Z.; Incani, V.; Kucharski, C.; Uludaĝ, H. Bisphosphonate-decorated lipid nanoparticles designed as drug carriers for bone diseases. J. Biomed. Mater. Res. Part A 2012, 100, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Guo, B.; Wu, H.; Tang, T.; Zhang, B.T.; Zheng, L.; He, Y.; Yang, Z.; Pan, X.; Chow, H.; et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat. Med. 2012, 18, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.; Dong, Y.; Jin, Y.; Hu, X.; Cai, K.; Ma, J.; Wu, G. Bi-directionally selective bone targeting delivery for anabolic and antiresorptive drugs: A novel combined therapy for osteoporosis? Med. Hypotheses 2014, 83, 694–696. [Google Scholar] [CrossRef]
- Hirsjärvi, S.; Sancey, L.; Dufort, S.; Belloche, C.; Vanpouille-Box, C.; Garcion, E.; Coll, J.-L.; Hindré, F.; Benoît, J.-P. Effect of particle size on the biodistribution of lipid nanocapsules: Comparison between nuclear and fluorescence imaging and counting. Int. J. Pharm. 2013, 453, 594–600. [Google Scholar] [CrossRef]
- Nahar, M.; Dutta, T.; Murugesan, S.; Asthana, A.; Mishra, D.; Rajkumar, V.; Tare, M.; Saraf, S.; Jain, N.K. Functional polymeric nanoparticles: An efficient and promising tool for active delivery of bioactives. Crit. Rev. Ther. Drug Carr. Syst. 2006, 23, 259–318. [Google Scholar] [CrossRef] [PubMed]
- An, S.Y.; Bui, M.-P.N.; Nam, Y.J.; Han, K.N.; Li, C.A.; Choo, J.; Lee, E.K.; Katoh, S.; Kumada, Y.; Seong, G.H. Preparation of monodisperse and size-controlled poly(ethylene glycol) hydrogel nanoparticles using liposome templates. J. Colloid Interface Sci. 2009, 331, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Majoros, I.; Misra, A.C.; Koch, A.E.; Campbell, P.; Marotte, H.; Bergin, I.L.; Cao, Z.; Goonewardena, S.; Morry, J.; et al. Folate receptor-targeted dendrimer-methotrexate conjugate for inflammatory arthritis. J. Biomed. Nanotechnol. 2014, 11, 1370–1384. [Google Scholar] [CrossRef]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef]
- De la Riva, B.; Sánchez, E.; Hernández, A.; Reyes, R.; Tamimi, F.; López-Cabarcos, E.; Delgado, A.; vora, C. Local controlled release of VEGF and PDGF from a combined brushite-chitosan system enhances bone regeneration. J. Control. Release 2010, 143, 45–52. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef]
- Vedakumari, W.S.; Prabu, P.; Sastry, T.P. Chitosan-fibrin nanocomposites as drug delivering and wound healing materials. J. Biomed. Nanotechnol. 2015, 11, 657–667. [Google Scholar] [CrossRef]
- Shim, K.; Won, H.S.; Sang, Y.L.; Sang, H.L.; Seong, J.H.; Myung, C.L.; Lee, S.J. Chitosan nano-/microfibrous double-layered membrane with rolled-up three-dimensional structures for chondrocyte cultivation. J. Biomed. Mater. Res. Part A 2009, 90, 595–602. [Google Scholar] [CrossRef]
- Poth, N.; Seiffart, V.; Gross, G.; Menzel, H.; Dempwolf, W. Biodegradable chitosan nanoparticle coatings on titanium for the delivery of BMP-2. Biomolecules 2015, 5, 3–19. [Google Scholar] [CrossRef]
- Nagarajan, U.; Kawakami, K.; Zhang, S.; Chandrasekaran, B. Fabrication of solid collagen nanoparticles using electrospray deposition. Chem. Pharm. Bull. 2014, 62, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, Y.; Tian, X.; Cui, G.; Zhao, Y.; Yang, Q.; Yu, S.; Xing, G.; Zhang, B. Segmental bone regeneration using an rhBMP-2-loaded gelatin/nanohydroxyapatite/fibrin scaffold in a rabbit model. Biomaterials 2009, 30, 6276–6285. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Sanati, M.H.; Hajizadeh, S.; Babaei, Z. Gelatin nanoparticle fabrication and optimization of the particle size. Phys. Status Solidi 2008, 205, 2898–2902. [Google Scholar] [CrossRef]
- Miller, D.C.; Thapa, A.; Haberstroh, K.M.; Webster, T.J. Endothelial and vascular smooth muscle cell function on poly(lactic-co-glycolic acid) with nano-structured surface features. Biomaterials 2004, 25, 53–61. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Pattison, M.A.; Wurster, S.; Webster, T.J.; Haberstroh, K.M. Three-dimensional, nano-structured PLGA scaffolds for bladder tissue replacement applications. Biomaterials 2005, 26, 2491–2500. [Google Scholar] [CrossRef]
- Soundrapandian, C.; Mahato, A.; Kundu, B.; Datta, S.; Sa, B.; Basu, D. Development and effect of different bioactive silicate glass scaffolds: In vitro evaluation for use as a bone drug delivery system. J. Mech. Behav. Biomed. Mater. 2014, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baldrighi, M.; Trusel, M.; Tonini, R.; Giordani, S. Carbon Nanomaterials Interfacing with Neurons: An In vivo Perspective. Front. Neurosci. 2016, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Gorain, B.; Choudhury, H.; Pandey, M.; Kesharwani, P.; Abeer, M.M.; Tekade, R.K.; Hussain, Z. Carbon nanotube scaffolds as emerging nanoplatform for myocardial tissue regeneration: A review of recent developments and therapeutic implications. Biomed. Pharmacother. 2018, 104, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z. An overview of carbon nanotubes and graphene for biosensing applications. Nanomicro Lett. 2017, 9, 25. [Google Scholar] [CrossRef]
- Guo, Q.; Shen, X.; Li, Y.; Xu, S. Carbon nanotubes-based drug delivery to cancer and brain. Curr. Med. Sci. 2017, 37, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, S.; Rhodes, C.T. Preparation and characterization of liposomes as therapeutic delivery systems: A review. Pharm. Acta Helv. 1995, 70, 95–111. [Google Scholar] [CrossRef]
- Subramaniam, S.; Fahy, E.; Gupta, S.; Sud, M.; Byrnes, R.W.; Cotter, D.; Dinasarapu, A.R.; Maurya, M.R. Bioinformatics and systems biology of the lipidome. Chem. Rev. 2011, 111, 6452–6490. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Carbone, C.; Leonardi, A.; Cupri, S.; Puglisi, G.; Pignatello, R. Pharmaceutical and biomedical applications of lipid-based nanocarriers. Pharm. Pat. Anal. 2014, 3, 199–215. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Rai, R.; Alwani, S.; Badea, I. Polymeric nanoparticles in gene therapy: New avenues of design and optimization for delivery applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Dolatabadi, J.E.N.; Omidi, Y. Solid lipid-based nanocarriers as efficient targeted drug and gene delivery systems. TrAC Trends Anal. Chem. 2016, 77, 100–108. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Mouzouvi, C.R.A.; Umerska, A.; Bigot, A.K.; Saulnier, P. Surface active properties of lipid nanocapsules. PLoS ONE 2017, 12, e0179211. [Google Scholar] [CrossRef] [PubMed]
- Irby, D.; Du, C.; Li, F. Lipid—Drug conjugate for enhancing drug delivery. Mol. Pharm. 2017, 14, 1325–1338. [Google Scholar] [CrossRef]
- Talegaonkar, S.; Bhattacharyya, A. Potential of lipid nanoparticles (SLNs and NLCs) in enhancing oral bioavailability of drugs with poor intestinal permeability. AAPS PharmSciTech 2019, 20, 121. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238-IN27. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Fenske, D.B.; Chonn, A.; Cullis, P.R. Liposomal nanomedicines: An emerging field. Toxicol. Pathol. 2008, 36, 21–29. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- La, W.-G.; Jin, M.; Park, S.; Yoon, H.-H.; Jeong, G.-J.; Bhang, S.; Park, H.; Char, K.; Kim, B.-S. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 2014, 9 (Suppl. 1), 107–116. [Google Scholar]
- Du, G.-Y.; He, S.-W.; Sun, C.-X.; Mi, L.-D. Bone morphogenic Protein-2 (rhBMP2)-loaded silk fibroin scaffolds to enhance the osteoinductivity in bone tissue engineering. Nanoscale Res. Lett. 2017, 12, 573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, M.; Inafuku, K.; Miyagi, T.; Oku, H.; Wada, K.; Imura, T.; Kitamoto, D. Efficient preparation of liposomes encapsulating food materials using lecithins by a mechanochemical method. J. Oleo Sci. 2007, 56, 35–42. [Google Scholar] [CrossRef]
- Zhu, C.T.; Xu, Y.Q.; Shi, J.; Li, J.; Ding, J. Liposome combined porous β-TCP scaffold: Preparation, characterization, and anti-biofilm activity. Drug Deliv. 2010, 17, 391–398. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Y.; Pan, J.; Wang, H.; Li, H.; Xu, X.; Fu, X.; Shi, R.; Luo, Z.; Li, Y.; et al. A hybrid 3D-printed aspirin-laden liposome composite scaffold for bone tissue engineering. J. Mater. Chem. B 2019, 7, 619–629. [Google Scholar] [CrossRef]
- Sou, K.; Inenaga, S.; Takeoka, S.; Tsuchida, E. Loading of curcumin into macrophages using lipid-based nanoparticles. Int. J. Pharm. 2008, 352, 287–293. [Google Scholar] [CrossRef]
- Mayer, L.D.; Bally, M.B.; Cullis, P.R. Uptake of adriamycin into large unilamellar vesicles in response to a pH gradient. Biochim. Biophys. Acta Biomembr. 1986, 857, 123–126. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J.; Bally, M.B.; Madden, T.D.; Mayer, L.D.; Fenske, D.B. Influence of pH gradients on the transbilayer transport of drugs, lipids, peptides and metal ions into large unilamellar vesicles. Biochim. Biophys. Acta Rev. Biomembr. 1997, 1331, 187–211. [Google Scholar] [CrossRef]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef]
- Kulkarni, M.; Greiser, U.; O’Brien, T.; Pandit, A. Liposomal gene delivery mediated by tissue-engineered scaffolds. Trends Biotechnol. 2010, 28, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Majoral, J.-P.; Caminade, A.-M. Dendrimers containing heteroatoms (Si, P, B, Ge, or Bi). Chem. Rev. 1999, 99, 845–880. [Google Scholar] [CrossRef] [PubMed]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About dendrimers: Structure, physical properties, and applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Grinstaff, M. Applications of dendrimers in tissue engineering. Curr. Top. Med. Chem. 2008, 8, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Ravizzini, G.; Choyke, P.L.; Kobayashi, H. Dendrimers in medical nanotechnology. IEEE Eng. Med. Biol. Mag. 2009, 28, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tee, J.K.T.; Chiu, G.N.C. Dendrimers in oral drug delivery application: Current explorations, toxicity issues and strategies for improvement. Curr. Pharm. Des. 2015, 21, 2629–2642. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, J.C.; Deneke, C.; Lanzoni, E.M.; Costa, C.A.; Bae, Y.; Scott, J.L.; Sharma, R.I. Modulating cell response on cellulose surfaces; tunable attachment and scaffold mechanics. Cellulose 2018, 25, 925–940. [Google Scholar] [CrossRef]
- Zhou, L.; Shan, Y.; Hu, H.; Yu, B.Y.; Cong, H. Synthesis and biomedical applications of dendrimers. Curr. Org. Chem. 2018, 22, 600–612. [Google Scholar] [CrossRef]
- Opina, A.C.; Wong, K.J.; Griffiths, G.L.; Turkbey, B.I.; Bernardo, M.; Nakajima, T.; Kobayashi, H.; Choyke, P.L.; Vasalatiy, O. Preparation and long-term biodistribution studies of a PAMAM dendrimer G5–Gd-BnDOTA conjugate for lymphatic imaging. Nanomedicine 2014, 10, 1423–1437. [Google Scholar] [CrossRef]
- Shadrack, D.M.; Swai, H.S.; Munissi, J.J.E.; Mubofu, E.B.; Nyandoro, S.S. Polyamidoamine dendrimers for enhanced solubility of small molecules and other desirable properties for site specific delivery: Insights from experimental and computational studies. Molecules 2018, 23, 1419. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Pooja, D.; Sistla, R.; Kulhari, H. Chapter 7—Dendrimer-drug conjugates: Synthesis strategies, stability and application in anticancer drug delivery. In Design of Nanostructures for Theranostics Applications; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 277–303. [Google Scholar]
- Yiyun, C.; Na, M.; Tongwen, X.; Rongqiang, F.; Xueyuan, W.; Xiaomin, W.; Longping, W. Transdermal delivery of nonsteroidal anti-inflammatory drugs mediated by polyamidoamine (PAMAM) dendrimers. J. Pharm. Sci. 2007, 96, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.-L.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Dendrimer/DNA complexes encapsulated functional biodegradable polymer for substrate-mediated gene delivery. J. Gene Med. 2008, 10, 1334–1342. [Google Scholar] [CrossRef]
- Grinstaff, M.W. Dendritic macromers for hydrogel formation: Tailored materials for ophthalmic, orthopedic, and biotech applications. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 383–400. [Google Scholar] [CrossRef]
- Grinstaff, M. Biodendrimers: New polymeric biomaterials for tissues engineering. Chem. A Eur. J. 2002, 8, 2838–2846. [Google Scholar] [CrossRef]
- Boduch-Lee, K.A.; Chapman, T.; Petricca, S.E.; Marra, K.G.; Kumta, P. Design and synthesis of hydroxyapatite composites containing an mPEG−Dendritic Poly(l-lysine) star polycaprolactone. Macromolecules 2004, 37, 8959–8966. [Google Scholar] [CrossRef]
- Rajzer, I. Fabrication of bioactive polycaprolactone/hydroxyapatite scaffolds with final bilayer nano-/micro-fibrous structures for tissue engineering application. J. Mater. Sci. 2014, 49, 5799–5807. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Sousa, R.A.; Kotobuki, N.; Tadokoro, M.; Hirose, M.; Mano, J.F.; Reis, R.L.; Ohgushi, H. The osteogenic differentiation of rat bone marrow stromal cells cultured with dexamethasone-loaded carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles. Biomaterials 2009, 30, 804–813. [Google Scholar] [CrossRef]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of nanoparticles in tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K. PEGylation: An approach for drug delivery. A review. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 403–447. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Santo, V.E.; Ratanavaraporn, J.; Sato, K.; Gomes, M.E.; Mano, J.F.; Reis, R.L.; Tabata, Y. Cell engineering by the internalization of bioinstructive micelles for enhanced bone regeneration. Nanomedicine 2015, 10, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef]
- Fàbregas, A.; Miñarro, M.; García-Montoya, E.; Pérez-Lozano, P.; Carrillo, C.; Sarrate, R.; Sánchez, N.; Ticó, J.R.; Suñé-Negre, J.M. Impact of physical parameters on particle size and reaction yield when using the ionic gelation method to obtain cationic polymeric chitosan-tripolyphosphate nanoparticles. Int. J. Pharm. 2013, 446, 199–204. [Google Scholar] [CrossRef]
- Vaculikova, E.; Grunwaldova, V.; Kral, V.; Dohnal, J.; Jampilek, J. Preparation of candesartan and atorvastatin nanoparticles by solvent evaporation. Molecules 2012, 17, 13221–13234. [Google Scholar] [CrossRef]
- Chung, J.W.; Lee, K.; Neikirk, C.; Nelson, C.M.; Priestley, R.D. Photoresponsive coumarin-stabilized polymeric nanoparticles as a detectable drug carrier. Small 2012, 8, 1693–1700. [Google Scholar] [CrossRef]
- Langer, K.; Anhorn, M.G.; Steinhauser, I.; Dreis, S.; Celebi, D.; Schrickel, N.; Faust, S.; Vogel, V. Human serum albumin (HSA) nanoparticles: Reproducibility of preparation process and kinetics of enzymatic degradation. Int. J. Pharm. 2008, 347, 109–117. [Google Scholar] [CrossRef]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of nanoparticle-based carriers for targeted drug delivery. J. Nanomater. 2016, 2016, 1087250. [Google Scholar] [CrossRef] [PubMed]
- Nagavarma, B.V.N.; Yadav, H.K.S.; Ayaz, A.; Vasudha, L.S.; Shivakumar, H.G. Different techniques for preparation of polymeric nanoparticles—A review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation techniques for food bioactive components: A review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Bennet, D.; Kim, S. Polymer nanoparticles for smart drug delivery. In Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; IntechOpen: Rijeka, Croatia, 2014. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Shoichet, M.S. Polymer scaffolds for biomaterials applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- Priya James, H.; John, R.; Alex, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef]
- Kashirina, A.; Yao, Y.; Liu, Y.; Leng, J. Biopolymers as bone substitutes: A review. Biomater. Sci. 2019, 7, 3961–3983. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; He, C.; Tian, H.; Ding, J.; Hsiao, B.S.; Chu, B.; Chen, X. Polymeric nanostructured materials for biomedical applications. Prog. Polym. Sci. 2016, 60, 86–128. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Sharma, K.; Mujawar, M.A.; Kaushik, A. State-of-art functional biomaterials for tissue engineering. Front. Mater. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Tezcaner, A.; Baran, E.; Keskin, D. Nanoparticles based on plasma proteins for drug delivery applications. Curr. Pharm. Des. 2016, 22, 3445–3454. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Comparative study of chitosan and chitosan–gelatin scaffold for tissue engineering. Int. Nano Lett. 2017, 7, 285–290. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Al-Qadi, S.; Grenha, A.; Carrión-Recio, D.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: In vivo evaluation of insulin-loaded formulations. J. Control. Release 2012, 157, 383–390. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Kim, S.K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Oliveira, J.L.; Fraceto, L.F. Poly(ethylene glycol) and cyclodextrin-grafted chitosan: From methodologies to preparation and potential biotechnological applications. Front. Chem. 2017, 5, 93. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar]
- Levengood, S.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Eap, S.; Keller, L.; Schiavi, J.; Huck, O.; Jacomine, L.; Fioretti, F.; Gauthier, C.; Sebastian, V.; Schwinté, P.; Benkirane-Jessel, N. A living thick nanofibrous implant bifunctionalized with active growth factor and stem cells for bone regeneration. Int. J. Nanomed. 2015, 10, 1061–1075. [Google Scholar]
- Shrestha, A.; Kishen, A. The effect of tissue inhibitors on the antibacterial activity of chitosan nanoparticles and photodynamic therapy. J. Endod. 2012, 38, 1275–1278. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Synthesis and characterization of nHA-PEG and nBG-PEG scaffolds for hard tissue engineering applications. Ceram. Int. 2019, 45, 8370–8379. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Umar, A.; Sindhu, A.; Mohammed, H.; Al-Hadeethi, Y.; Guo, Z. Fabrication and in-vitro biocompatibility of freeze-dried CTS-nHA and CTS-nBG scaffolds for bone regeneration applications. Int. J. Biol. Macromol. 2020, 149, 1–10. [Google Scholar] [CrossRef]
- Bhowmick, A.; Banerjee, S.L.; Pramanik, N.; Jana, P.; Mitra, T.; Gnanamani, A.; Das, M.; Kundu, P.P. Organically modified clay supported chitosan/hydroxyapatite-zinc oxide nanocomposites with enhanced mechanical and biological properties for the application in bone tissue engineering. Int. J. Biol. Macromol. 2018, 106, 11–19. [Google Scholar] [CrossRef]
- Yilgor, P.; Tuzlakoglu, K.; Reis, R.L.; Hasirci, N.; Hasirci, V. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials 2009, 30, 3551–3559. [Google Scholar] [CrossRef]
- Mili, B.; Das, K.; Kumar, A.; Saxena, A.C.; Singh, P.; Ghosh, S.; Bag, S. Preparation of NGF encapsulated chitosan nanoparticles and its evaluation on neuronal differentiation potentiality of canine mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2017, 29, 4. [Google Scholar] [CrossRef]
- Kumar, P. Nano-TiO(2) doped chitosan scaffold for the bone tissue engineering applications. Int. J. Biomater. 2018, 2018, 6576157. [Google Scholar] [CrossRef]
- Koosha, M.; Solouk, A.; Ghalei, S.; Sadeghi, D.; Bagheri, S.; Mirzadeh, H. Electrospun chitosan/gum tragacanth/polyvinyl alcohol hybrid nanofibrous scaffold for tissue engineering applications. Bioinspired Biomim. Nanobiomater. 2019, 9, 1–8. [Google Scholar]
- Zugravu, M.; Smith, R.; Reves, B.; Jennings, J.; Cooper, J.; Haggard, W.; Bumgardner, J. Physical properties and in vitro evaluation of collagen-chitosan-calcium phosphate microparticle-based scaffolds for bone tissue regeneration. J. Biomater. Appl. 2012, 28, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.; Czernuszka, J. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cell Mater. 2006, 11, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Krafts, K.P. Tissue repair: The hidden drama. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-S.; Ok, Y.-J.; Hwang, S.-Y.; Kwak, J.-Y.; Yoon, S. Marine collagen as a promising biomaterial for biomedical applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef]
- Khan, R.; Khan, M.H. Use of collagen as a biomaterial: An update. J. Indian Soc. Periodontol. 2013, 17, 539–542. [Google Scholar] [CrossRef]
- D’Mello, S.; Atluri, K.; Geary, S.M.; Hong, L.; Elangovan, S.; Salem, A.K. Bone regeneration using gene-activated matrices. AAPS J. 2017, 19, 43–53. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Schaller, B.; Saulacic, N.; Pippenger, B.E.; Zhang, Y.; Miron, R.J. Absorbable collagen sponges loaded with recombinant bone morphogenetic protein 9 induces greater osteoblast differentiation when compared to bone morphogenetic protein 2. Clin. Exp. Dent. Res. 2017, 3, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Tagawa, T. Ultrastructural study of direct bone formation induced by BMPs-collagen complex implanted into an ectopic site. Oral Dis. 2000, 6, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Baumann, M.J.; McCabe, L.R. Osteoblasts respond to hydroxyapatite surfaces with immediate changes in gene expression. J. Biomed. Mater. Res. Part A 2004, 71, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.; Kuo, S.-M.; Kong, A.M.; Morrison, W.A.; Dusting, G.J.; Mitchell, G.M.; Lim, S.Y.; Liu, G.-S. Three dimensional collagen scaffold promotes intrinsic vascularisation for tissue engineering applications. PLoS ONE 2016, 11, e0149799. [Google Scholar] [CrossRef]
- Vázquez, J.J.; Martín-Martínez, E.S. Collagen and elastin scaffold by electrospinning for skin tissue engineering applications. J. Mater. Res. 2019, 34, 2819–2827. [Google Scholar] [CrossRef]
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-based bioinks for hard tissue engineering applications: A comprehensive review. J. Mater. Sci. Mater. Med. 2019, 30, 32. [Google Scholar] [CrossRef]
- Hoque, M.E.; Hutmacher, D.W.; Feng, W.; Li, S.; Huang, M.-H.; Vert, M.; Wong, Y.S. Fabrication using a rapid prototyping system and in vitro characterization of PEG-PCL-PLA scaffolds for tissue engineering. J. Biomater. Sci. Polym. Ed. 2005, 16, 1595–1610. [Google Scholar] [CrossRef]
- Ba Linh, N.T.; Lee, K.H.; Lee, B.T. Functional nanofiber mat of polyvinyl alcohol/gelatin containing nanoparticles of biphasic calcium phosphate for bone regeneration in rat calvaria defects. J. Biomed. Mater. Res. Part A 2013, 101, 2412–2423. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef]
- Won, Y.-W.; Yoon, S.-M.; Sonn, C.H.; Lee, K.-M.; Kim, Y.-H. Nano self-assembly of recombinant human gelatin conjugated with α-tocopheryl succinate for hsp90 inhibitor, 17-AAG, delivery. ACS Nano 2011, 5, 3839–3848. [Google Scholar] [CrossRef] [PubMed]
- Olsen, D.; Yang, C.; Bodo, M.; Chang, R.; Leigh, S.; Baez, J.; Carmichael, D.; Perälä, M.; Hämäläinen, E.-R.; Jarvinen, M.; et al. Recombinant collagen and gelatin for drug delivery. Adv. Drug Deliv. Rev. 2003, 55, 1547–1567. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Nitta, S.K.; Numata, K. Biopolymer-Based nanoparticles for drug/gene delivery and tissue engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Engbers, G.H.M.; Krijgsveld, J.; Zaat, S.A.J.; Dankert, J.; Feijen, J. Cross-linking and characterisation of gelatin matrices for biomedical applications. J. Biomater. Sci. Polym. Ed. 2000, 11, 225–243. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Wang, H.; Boerman, O.; Sariibrahimoglu, K.; Li, Y.; Jansen, J.; Leeuwenburgh, S. Comparison of micro- vs. nanostructured colloidal gelatin gels for sustained delivery of osteogenic proteins: Bone morphogenetic protein-2 and alkaline phosphatase. Biomaterials 2012, 33, 8695–8703. [Google Scholar] [CrossRef]
- Perez, R.; del Valle, S.; Altankov, G.; Ginebra, M.-P. Porous hydroxyapatite and gelatin/hydroxyapatite microspheres obtained by calcium phosphate cement emulsion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 156–166. [Google Scholar] [CrossRef]
- Gil, E.S.; Frankowski, D.J.; Spontak, R.J.; Hudson, S.M. Swelling behavior and morphological evolution of mixed gelatin/silk fibroin hydrogels. Biomacromolecules 2005, 6, 3079–3087. [Google Scholar] [CrossRef]
- Ulrich, D.; Edwards, S.; Su, K.; Tan, K.; White, J.; Ramshaw, J.; Lo, C.; Rosamilia, A.; Werkmeister, J.; Gargett, C. Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair. Tissue Eng. Part A 2013, 20, 785–798. [Google Scholar] [CrossRef]
- Han, J.; Lazarovici, P.; Pomerantz, C.; Chen, X.; Wei, Y.; Lelkes, P.I. Co-Electrospun blends of PLGA, gelatin, and elastin as potential nonthrombogenic scaffolds for vascular tissue engineering. Biomacromolecules 2011, 12, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Deiwick, A.; van Vlierberghe, S.; Dubruel, P.; Möller, L.; Dräger, G.; Chichkov, B. Laser fabrication of three-dimensional CAD scaffolds from photosensitive gelatin for applications in tissue engineering. Biomacromolecules 2011, 12, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Tielens, S.; Declercq, H.; Gorski, T.; Lippens, E.; Schacht, E.; Cornelissen, M. Gelatin-based microcarriers as embryonic stem cell delivery system in bone tissue engineering: An in-vitro study. Biomacromolecules 2007, 8, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.K.; Goranov, V.; Dash, M.; Russo, A.; Shelyakova, T.; Graziosi, P.; Lungaro, L.; Riminucci, A.; Uhlarz, M.; Bañobre-López, M.; et al. Multilayered magnetic gelatin membrane scaffolds. ACS Appl. Mater. Interfaces 2015, 7, 23098–23109. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Meretoja, V.V.; Tirri, T.; Malin, M.; Seppälä, J.V.; Närhi, T.O. Ectopic bone formation in and soft-tissue response to P(CL/DLLA)/bioactive glass composite scaffolds. Clin. Oral Implants Res. 2014, 25, 159–164. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.-T.; Liu, Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, S.; Zhu, Y.; Huang, Y.; Zhu, M.; Tao, C.; Zhang, C. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014, 10, 2269–2281. [Google Scholar] [CrossRef]
- Padmanabhan, J.; Kyriakides, T.R. Nanomaterials, inflammation, and tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 355–370. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Harris, L.D.; Kim, B.; Mooney, D.J. Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res. 1998, 42, 396–402. [Google Scholar] [CrossRef]
- Mikos, A.G.; Thorsen, A.J.; Czerwonka, L.A.; Bao, Y.; Langer, R.; Winslow, D.N.; Vacanti, J.P. Preparation and characterization of poly(l-lactic acid) foams. Polymer 1994, 35, 1068–1077. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, P.X. Poly (A-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering.I. Preparation and morphology. J. Biomed. Mater. Res. 1999, 44, 446–455. [Google Scholar] [CrossRef]
- Perkins, B.L.; Naderi, N. Carbon nanostructures in bone tissue engineering. Open Orthop. J. 2016, 10, 877–899. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Avouris, P. Introduction to carbon materials research. Carbon Nanotub. 2001, 9, 1–9. [Google Scholar]
- Han, Z.J.; Rider, A.E.; Ishaq, M.; Kumar, S.; Kondyurin, A.; Bilek, M.M.M.; Levchenko, I.; Ostrikov, K. Carbon nanostructures for hard tissue engineering. RSC Adv. 2013, 3, 11058–11072. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef]
- Geetha Bai, R.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar] [CrossRef]
- Kim, H.; Macosko, C.W. Processing-property relationships of polycarbonate/graphene composites. Polymer 2009, 50, 3797–3809. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.B.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Bon, S.B.; Valentini, L.; Verdejo, R.; Fierro, J.L.G.; Peponi, L.; Lopez-Manchado, M.A.; Kenny, J.M. Plasma fluorination of chemically derived graphene sheets and subsequent modification with butylamine. Chem. Mater. 2009, 21, 3433–3438. [Google Scholar] [CrossRef]
- Valentini, L.; Armentano, I.; Biagiotti, J.; Frulloni, E.; Kenny, J.M.; Santucci, S. Frequency dependent electrical transport between conjugated polymer and single-walled carbon nanotubes. Diam. Relat. Mater. 2003, 12, 1601–1609. [Google Scholar] [CrossRef]
- MacDonald, R.A.; Voge, C.M.; Kariolis, M.; Stegemann, J.P. Carbon nanotubes increase the electrical conductivity of fibroblast-seeded collagen hydrogels. Acta Biomater. 2008, 4, 1583–1592. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stabil. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Gigli, M.; Luzi, F.; Trotta, R.; Bicchi, I.; Soccio, M.; Lotti, N.; Munari, A.; Martino, S.; et al. Effect of SWCNT introduction in random copolymers on material properties and fibroblast long term culture stability. Polym. Degrad. Stab. 2016, 132, 220–230. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Mihajlovic, M.; Dankers, P.Y.W.; Masereeuw, R.; Sijbesma, R.P. Carbon nanotube reinforced supramolecular hydrogels for bioapplications. Macromol. Biosci. 2019, 19, 1800173. [Google Scholar] [CrossRef]
- Wu, K.; Tao, J.; Qi, L.; Chen, S.; Wan, W. Intracellular microtubules as nano-scaffolding template self-assembles with conductive carbon nanotubes for biomedical device. Mater. Sci. Eng. C 2020, 113, 11971. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Ju, H.-Z.; Liu, S.-F.; Lee, T.-C.; Shih, Y.-W.; Chuang, L.-Y.; Guh, J.-Y.; Yang, Y.-Y.; Liao, T.-N.; Hung, T.-J.; et al. BMP-2 suppresses renal interstitial fibrosis by regulating epithelial–mesenchymal transition. J. Cell. Biochem. 2011, 112, 2558–2565. [Google Scholar] [CrossRef]
- Minati, L.; Antonini, V.; Dalla Serra, M.; Speranza, G. Multifunctional branched gold–carbon nanotube hybrid for cell imaging and drug delivery. Langmuir 2012, 28, 15900–15906. [Google Scholar] [CrossRef]
- Srikanth, M.; Asmatulu, R.; Cluff, K.; Yao, L. Material characterization and bioanalysis of hybrid scaffolds of carbon nanomaterial and polymer nanofibers. ACS Omega 2019, 4, 5044–5051. [Google Scholar] [CrossRef]
- Singh, R.K.; Patel, K.D.; Kim, J.-J.; Kim, T.-H.; Kim, J.-H.; Shin, U.S.; Lee, E.-J.; Knowles, J.C.; Kim, H.-W. Multifunctional hybrid nanocarrier: Magnetic CNTs ensheathed with mesoporous silica for drug delivery and imaging system. ACS Appl. Mater. Interfaces 2014, 6, 2201–2208. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, J.; Dorn, H.C.; Geohegan, D.; Zhang, C. Assembly of single-walled carbon nanohorn supported liposome particles. Bioconjug. Chem. 2011, 22, 1012–1016. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, C.; Thouas, G.A. Progress and challenges in biomaterials used for bone tissue engineering: Bioactive glasses and elastomeric composites. Prog. Biomater. 2012, 1, 2. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control: An Agency of the European Union. Available online: http://ecdc.europa.eu (accessed on 1 September 2020).
- Wu, X.; Wu, M.; Zhao, J.X. Recent development of silica nanoparticles as delivery vectors for cancer imaging and therapy. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 297–312. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Zhang, J.; Linden, M.; Sahlgren, C. Mesoporous silica nanoparticles in tissue engineering—A perspective. Nanomedicine 2016, 11, 391–402. [Google Scholar] [CrossRef]
- Bitar, A.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Silica-based nanoparticles for biomedical applications. Drug Discov. Today 2012, 17, 1147–1154. [Google Scholar] [CrossRef]
- Türk, M.; Tamer, U.; Alver, E.; Çiftçi, H.; Metin, A.U.; Karahan, S. Fabrication and characterization of gold-nanoparticles/chitosan film: A scaffold for L929-fibroblasts. Artif. Cells Nanomed. Biotechnol. 2013, 41, 395–401. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Zuber, A.; Purdey, M.; Schartner, E.; Forbes, C.; van der Hoek, B.; Giles, D.; Abell, A.; Monro, T.; Ebendorff-Heidepriem, H. Detection of gold nanoparticles with different sizes using absorption and fluorescence based method. Sensors Actuators B Chem. 2016, 227, 117–127. [Google Scholar] [CrossRef]
- Ito, A.; Kamihira, M. Tissue Engineering Using Magnetite Nanoparticles, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 104. [Google Scholar]
- Xiong, F.; Wang, H.; Feng, Y.; Li, Y.; Hua, X.; Pang, X.; Zhang, S.; Song, L.; Zhang, Y.; Gu, N. Cardioprotective activity of iron oxide nanoparticles. Sci. Rep. 2015, 5, 8579. [Google Scholar] [CrossRef]
- Madhumathi, K.; Sampath Kumar, T.S. Regenerative potential and anti-bacterial activity of tetracycline loaded apatitic nanocarriers for the treatment of periodontitis. Biomed. Mater. 2014, 9, 35002. [Google Scholar] [CrossRef]
- Choi, B.; Cui, Z.-K.; Kim, S.; Fan, J.; Wu, B.M.; Lee, M. Glutamine-chitosan modified calcium phosphate nanoparticles for efficient siRNA delivery and osteogenic differentiation. J. Mater. Chem. B 2015, 3, 6448–6455. [Google Scholar] [CrossRef]
- Ding, T.; Luo, Z.J.; Zheng, Y.; Hu, X.Y.; Ye, Z.X. Rapid repair and regeneration of damaged rabbit sciatic nerves by tissue-engineered scaffold made from nano-silver and collagen type I. Injury 2010, 41, 522–527. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Saravanan, S.; Nethala, S.; Pattnaik, S.; Tripathi, A.; Moorthi, A.; Selvamurugan, N. Preparation, characterization and antimicrobial activity of a bio-composite scaffold containing chitosan/nano-hydroxyapatite/nano-silver for bone tissue engineering. Int. J. Biol. Macromol. 2011, 49, 188–193. [Google Scholar] [CrossRef]
- Hirota, M.; Hayakawa, T.; Yoshinari, M.; Ametani, A.; Shima, T.; Monden, Y.; Ozawa, T.; Sato, M.; Koyama, C.; Tamai, N.; et al. Hydroxyapatite coating for titanium fibre mesh scaffold enhances osteoblast activity and bone tissue formation. Int. J. Oral Maxillofac. Surg. 2012, 41, 1304–1309. [Google Scholar] [CrossRef]
- Holtorf, H.L.; Jansen, J.A.; Mikos, A.G. Ectopic bone formation in rat marrow stromal cell/titanium fiber mesh scaffold constructs: Effect of initial cell phenotype. Biomaterials 2005, 26, 6208–6216. [Google Scholar] [CrossRef]
- Fan, X.; Lin, L.; Messersmith, P.B. Surface-initiated polymerization from TiO2 nanoparticle surfaces through a biomimetic initiator: A new route toward polymer–matrix nanocomposites. Compos. Sci. Technol. 2006, 66, 1198–1204. [Google Scholar] [CrossRef]
- Kevin, B.; Chang, Y.; Webster, T.J. Increased chondrocyte adhesion on nanotubular anodized titanium. J. Biomed. Mater. Res. Part A 2008, 88, 561–568. [Google Scholar]
- Liao, D.L.; Liao, B.Q. Shape, size and photocatalytic activity control of TiO2 nanoparticles with surfactants. J. Photochem. Photobiol. A Chem. 2007, 187, 363–369. [Google Scholar] [CrossRef]
- Rakhmatia, Y.D.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Koyano, K. Fibroblast attachment onto novel titanium mesh membranes for guided bone regeneration. Odontology 2015, 103, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Lee, J.; Sieweke, J.H.; Rodriguez, N.A.; Schüpbach, P.; Lindström, H.; Susin, C.; Wikesjö, U.M.E. Evaluation of nano-technology-modified zirconia oral implants: A study in rabbits. J. Clin. Periodontol. 2009, 36, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Opalinska, A.; Malka, I.; Dzwolak, W.; Chudoba, T.; Presz, A.; Lojkowski, W. Size-dependent density of zirconia nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Tadd, E.H.; Zubris, M.; Tannenbaum, R. Size-controlled synthesis of alumina nanoparticles from aluminum alkoxides. Mater. Res. Bull. 2005, 40, 1506–1512. [Google Scholar] [CrossRef]
- Ravindran Girija, A.; Balasubramanian, S. Theragnostic potentials of core/shell mesoporous silica nanostructures. Nanotheranostics 2019, 3, 1–40. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous silica nanoparticle nanocarriers: Biofunctionality and biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, Y.; Wang, X.; Lin, T. Layer-by-layer assembly of silica nanoparticles on 3D fibrous scaffolds: Enhancement of osteoblast cell adhesion, proliferation, and differentiation. J. Biomed. Mater. Res. Part A 2014, 102, 3803–3812. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Menon, D.; Koyakutty, M.; Mohan, C.C.; Nair, S.V.; Nair, M.B. Bioinspired composite matrix containing hydroxyapatite–silica core–shell nanorods for bone tissue engineering. ACS Appl. Mater. Interfaces 2017, 9, 26707–26718. [Google Scholar]
- Ambekar, R.S.; Kandasubramanian, B. Progress in the advancement of porous biopolymer scaffold: Tissue engineering application. Ind. Eng. Chem. Res. 2019, 58, 6163–6194. [Google Scholar] [CrossRef]
- Zhou, P.; Cheng, X.; Xia, Y.; Wang, P.; Zou, K.; Xu, S.; Du, J. Organic/inorganic composite membranes based on poly(l-lactic-co-glycolic acid) and mesoporous silica for effective bone tissue engineering. ACS Appl. Mater. Interfaces 2014, 6, 20895–20903. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Correia, A.; Yang, Y.; Zheng, K.; Liu, D.; Schubert, D.W.; Boccaccini, A.R.; Santos, H.A.; Roether, J.A. Electrospun polyhydroxybutyrate/poly(ε-Caprolactone)/sol–gel-derived silica hybrid scaffolds with drug releasing function for bone tissue engineering applications. ACS Appl. Mater. Interfaces 2018, 10, 14540–14548. [Google Scholar] [CrossRef]
- Cha, C.; Oh, J.; Kim, K.; Qiu, Y.; Joh, M.; Shin, S.R.; Wang, X.; Camci-Unal, G.; Wan, K.; Liao, R.; et al. Microfluidics-assisted fabrication of gelatin-silica core–shell microgels for injectable tissue constructs. Biomacromolecules 2014, 15, 283–290. [Google Scholar] [CrossRef]
- Qiu, K.; Chen, B.; Nie, W.; Zhou, X.; Feng, W.; Wang, W.; Chen, L.; Mo, X.; Wei, Y.; He, C. Electrophoretic deposition of dexamethasone-loaded mesoporous silica nanoparticles onto poly(l-Lactic Acid)/Poly(ε-Caprolactone) composite scaffold for bone tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 4137–4148. [Google Scholar] [CrossRef]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef]
- Boccaccini, A.; Maquet, V. Bioresorbable and bioactive polymer/bioglass composites with tailored pore structure for tissue engineering applications. Compos. Sci. Technol. 2003, 63, 2417–2429. [Google Scholar] [CrossRef]
- Yu, H.; Peng, J.; Xu, Y.; Chang, J.; Li, H. Bioglass activated skin tissue engineering constructs for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive glasses: From parent 45s5 composition to scaffold-assisted tissue-healing therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Felfel, R.M.; Abou Neel, E.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive calcium phosphate-based glasses and ceramics and their biomedical applications: A review. J. Tissue Eng. 2017, 8, 2041731417719170. [Google Scholar] [CrossRef] [PubMed]
- González, P.; Serra, J.; Liste, S.; Chiussi, S.; León, B.; Pérez-Amor, M. Raman spectroscopic study of bioactive silica based glasses. J. Non. Cryst. Solids 2003, 320, 92–99. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A.; Kumar, V. Synthesis and characterization of nano bioglass for the application of bone tissue engineering. J. Nanosci. Technol. 2018, 4, 471–474. [Google Scholar] [CrossRef]
- De Oliveira, A.A.R.; de Souza, D.A.; Dias, L.L.S.; de Carvalho, S.M.; Mansur, H.S.; de Magalhães Pereira, M. Synthesis, characterization and cytocompatibility of spherical bioactive glass nanoparticles for potential hard tissue engineering applications. Biomed. Mater. 2013, 8, 025011. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Boccaccini, A. Poly(D,L-lactic acid) coated 45S5 Bioglass-based scaffolds: Processing and characterization. J. Biomed. Mater. Res. Part A 2006, 77, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Ravarian, R.; Zhong, X.; Barbeck, M.; Ghanaati, S.; Kirkpatrick, C.J.; Murphy, C.M.; Schindeler, A.; Chrzanowski, W.; Dehghani, F. Nanoscale chemical interaction enhances the physical properties of bioglass composites. ACS Nano 2013, 7, 8469–8483. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Desai, M.S.; Jin, H.-E.; Lee, J.H.; Chang, J.; Lee, S.-W. Self-healing elastin–bioglass hydrogels. Biomacromolecules 2016, 17, 2619–2625. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Ghezzi, C.E.; Barralet, J.E.; Boccaccini, A.R.; Nazhat, S.N. Three-dimensional mineralization of dense nanofibrillar collagen−bioglass hybrid scaffolds. Biomacromolecules 2010, 11, 1470–1479. [Google Scholar] [CrossRef]
- Misra, S.K.; Nazhat, S.N.; Valappil, S.P.; Moshrefi-Torbati, M.; Wood, R.J.K.; Roy, I.; Boccaccini, A.R. fabrication and characterization of biodegradable poly(3-hydroxybutyrate) composite containing bioglass. Biomacromolecules 2007, 8, 2112–2119. [Google Scholar] [CrossRef]
- Fernandes, J.S.; Martins, M.; Neves, N.M.; Fernandes, M.H.V.; Reis, R.L.; Pires, R.A. Intrinsic antibacterial borosilicate glasses for bone tissue engineering applications. ACS Biomater. Sci. Eng. 2016, 2, 1143–1150. [Google Scholar] [CrossRef]
- Xu, Y.; Luong, D.; Walker, J.M.; Dean, D.; Becker, M.L. Modification of poly(propylene fumarate)–bioglass composites with peptide conjugates to enhance bioactivity. Biomacromolecules 2017, 18, 3168–3177. [Google Scholar] [CrossRef]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Leventouri, T.; Antonakos, A.; Kyriacou, A.; Venturelli, R.; Liarokapis, E.; Perdikatsis, V. Crystal structure studies of human dental apatite as a function of age. Int. J. Biomater. 2009, 2009, 698547. [Google Scholar] [CrossRef]
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable polymer scaffolds for tissue engineering. Nat. Biotechnol. 1994, 12, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Isikli, C.; Hasirci, V.; Hasirci, N. Development of porous chitosan–gelatin/hydroxyapatite composite scaffolds for hard tissue-engineering applications. J. Tissue Eng. Regen. Med. 2011, 6, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Driessen, A.A.; Degroot, K.; Vandenhooff, A. Biodegradation behavior of various calcium-phosphate materials in bone tissue. J. Biomed. Mater. Res. 1983, 17, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Guzmán Vázquez, C.; Piña Barba, C.; Munguía, N. Stoichiometric hydroxyapatite obtained by precipitation and sol gel processes. Rev. Mex. Fis. 2005, 51, 284–293. [Google Scholar]
- Li, J.; Lu, X.L.; Zheng, Y.F. Effect of surface modified hydroxyapatite on the tensile property improvement of HA/PLA composite. Appl. Surf. Sci. 2008, 255, 494–497. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008, 43, 3028–3057. [Google Scholar] [CrossRef]
- Narasaraju, T.S.B.; Phebe, D.E. Some physico-chemical aspects of hydroxylapatite. J. Mater. Sci. 1996, 31, 1–21. [Google Scholar] [CrossRef]
- Fathi, M.H.; Hanifi, A.; Mortazavi, V. Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopowder. J. Mater. Process. Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Sopyan, I.; Singh, R.; Hamdi, M. Synthesis of nano sized hydroxyapatite powder using sol-gel technique and its conversion to dense and porous bodies. Indian J. Chem. Sect. A 2008, 47, 1626–1631. [Google Scholar]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–-Past, present, and future in bone regeneration. Bone Tissue Regen. Insights 2016, 7, S36138. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Song, J.; Ji, P.; Zhang, X.; Li, X.; Xu, X.; Wang, M.; Zhang, S.; Deng, Y.; Deng, F.; et al. Polydopamine-templated hydroxyapatite reinforced polycaprolactone composite nanofibers with enhanced cytocompatibility and osteogenesis for bone tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 3499–3515. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, Y.; Xiong, C.; Su, S.; Ding, H. Preparation and properties of bamboo fiber/nano-hydroxyapatite/poly(lactic-co-glycolic) composite scaffold for bone tissue engineering. ACS Appl. Mater. Interfaces 2017, 9, 4890–4897. [Google Scholar] [CrossRef] [PubMed]
- Salarian, M.; Xu, W.Z.; Wang, Z.; Sham, T.-K.; Charpentier, P.A. Hydroxyapatite–TiO2-based nanocomposites synthesized in supercritical co2 for bone tissue engineering: Physical and mechanical properties. ACS Appl. Mater. Interfaces 2014, 6, 16918–16931. [Google Scholar] [CrossRef]
- Liang, X.; Duan, P.; Gao, J.; Guo, R.; Qu, Z.; Li, X.; He, Y.; Yao, H.; Ding, J. Bilayered PLGA/PLGA-HAp composite scaffold for osteochondral tissue engineering and tissue regeneration. ACS Biomater. Sci. Eng. 2018, 4, 3506–3521. [Google Scholar] [CrossRef]
- Fu, S.; Wang, X.; Guo, G.; Shi, S.; Liang, H.; Luo, F.; Wei, Y.; Qian, Z. Preparation and characterization of nano-hydroxyapatite/poly(ε-caprolactone)−poly(ethylene glycol)−poly(ε-caprolactone) composite fibers for tissue engineering. J. Phys. Chem. C 2010, 114, 18372–18378. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Sun, S.; Li, X.; Zhou, Y.; Hou, Z.; Lin, J. Multifunctional Hydroxyapatite/Na(Y/Gd)F4:Yb3+,Er3+ composite fibers for drug delivery and dual modal imaging. Langmuir 2014, 30, 1176–1182. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Yin, M.; Yang, X.; Yuan, Q.; Ren, J.; Qu, X. Aptamer-capped multifunctional mesoporous strontium hydroxyapatite nanovehicle for cancer-cell-responsive drug delivery and imaging. Biomacromolecules 2012, 13, 4257–4263. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Wu, C.; Lee, C.; Chen, J.; Kuo, S.; Fan, S.; Cheng, C.; Chang, F.; Ko, F. Microwave-assisted electroless deposition of silver nanoparticles onto multiwalled carbon nanotubes. Int. J. Electrochem. Sci. 2012, 7, 4133–4142. [Google Scholar]
- Ciobanu, C.S.; Iconaru, S.L.; Coustumer, P.L.; Constantin, L.V.; Predoi, D. Antibacterial activity of silver-doped hydroxyapatite nanoparticles against gram-positive and gram-negative bacteria. Nanoscale Res. Lett. 2012, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, O.V.; Gorbenko, O.Y.; Markelova, M.N.; Kaul, A.R.; Atsarkin, V.A.; Demidov, V.V.; Soto, C.; Roy, E.J.; Odintsov, B.M. Ag-doped manganite nanoparticles: New materials for temperature-controlled medical hyperthermia. J. Biomed. Mater. Res. A 2009, 91, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C 2018, 91, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surfaces B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Stetciura, I.Y.; Markin, A.V.; Ponomarev, A.N.; Yakimansky, A.V.; Demina, T.S.; Grandfils, C.; Volodkin, D.V.; Gorin, D.A. New surface-enhanced raman scattering platforms: Composite calcium carbonate microspheres coated with astralen and silver nanoparticles. Langmuir 2013, 29, 4140–4147. [Google Scholar] [CrossRef]
- Zan, X.; Kozlov, M.; McCarthy, T.J.; Su, Z. Covalently attached, silver-doped Poly(vinyl alcohol) hydrogel films on Poly(l-lactic acid). Biomacromolecules 2010, 11, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- García-Astrain, C.; Chen, C.; Burón, M.; Palomares, T.; Eceiza, A.; Fruk, L.; Corcuera, M.Á.; Gabilondo, N. Biocompatible hydrogel nanocomposite with covalently embedded silver nanoparticles. Biomacromolecules 2015, 16, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhang, W.; Meng, F.; Guo, J.; Wang, D.; Qian, S.; Jiang, X.; Liu, X.; Chu, P.K. Osteogenesis Catalyzed by Titanium-Supported Silver Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 5149–5157. [Google Scholar] [CrossRef] [PubMed]
- Patrascu, J.M.; Nedelcu, I.A.; Sonmez, M.; Ficai, D.; Ficai, A.; Vasile, B.S.; Ungureanu, C.; Albu, M.G.; Andor, B.; Andronescu, E.; et al. Composite scaffolds based on silver nanoparticles for biomedical applications. J. Nanomater. 2015, 2015, 587989. [Google Scholar] [CrossRef]
- Huang, X.; Qian, W.; El-Sayed, I.H.; El-Sayed, M.A. The potential use of the enhanced nonlinear properties of gold nanospheres in photothermal cancer therapy. Lasers Surg. Med. 2007, 39, 747–753. [Google Scholar] [CrossRef]
- Vieira, S.; Vial, S.; Maia, F.R.; Carvalho, M.R.; Reis, R.L.; Granja, P.L.; Oliveira, J.M. Gellan gum-coated gold nanorods: An intracellular nanosystem for bone tissue engineering. RSC Adv. 2015, 5, 77996–78005. [Google Scholar] [CrossRef]
- Yuan, H.; Khoury, C.G.; Wilson, C.M.; Grant, G.A.; Bennett, A.J.; Vo-Dinh, T. In vivo particle tracking and photothermal ablation using plasmon-resonant gold nanostars. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1355–1363. [Google Scholar] [CrossRef]
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. Nanomaterials for energy conversion and storage. Chem. Soc. Rev. 2013, 42, 3127–3171. [Google Scholar] [CrossRef]
- Li, H.; Pan, S.; Xia, P.; Chang, Y.; Fu, C.; Kong, W.; Yu, Z.; Wang, K.; Yang, X.; Qi, Z. Advances in the applications of gold nanoparticles in bone tissue engineering. J. Biol. Eng. 2020, 14, 14. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef]
- Morita, M.; Tachikawa, T.; Seino, S.; Tanaka, K.; Majima, T. Controlled synthesis of gold nanoparticles on fluorescent nanodiamond via electron-beam-induced reduction method for dual-modal optical and electron bioimaging. ACS Appl. Nano Mater. 2018, 1, 355–363. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Wang, Y.; Wang, L.; Wang, Y.; Cai, X.; Zhang, C.; Wang, L.V.; Xia, Y. Labeling human mesenchymal stem cells with gold nanocages for in vitro and in vivo tracking by two-photon microscopy and photoacoustic microscopy. Theranostics 2013, 3, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.; Bogatyrev, V.; Dykman, L.; Khlebtsov, B.; Staroverov, S.; Shirokov, A.; Matora, L.; Khanadeev, V.; Pylaev, T.; Tsyganova, N.; et al. Analytical and theranostic applications of gold Na-noparticles and multifunctional nanocomposites. Theranostics 2013, 3, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.-M.; Wang, Y.-C.; Wang, F.; Du, J.; Mao, C.; Sun, C.-Y.; Tang, R.; Liu, Y.; Zhu, J.; Zhu, Y.-H.; et al. Cancer stem cell therapy using doxorubicin conjugated to gold nanoparticles via hydrazone bonds. Biomaterials 2013, 35, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lei, B.; Gao, C.; Yan, J.; Ma, P.X. Optimizing surface-engineered ultra-small gold nanoparticles for highly efficient miRNA delivery to enhance osteogenic differentiation of bone mesenchimal stromal cells. Nano Res. 2017, 10, 49–63. [Google Scholar] [CrossRef]
- Vial, S.; Reis, R.L.; Oliveira, J.M. Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 92–112. [Google Scholar] [CrossRef]
- Khorasani, A.; Goldberg, M.; Doeven, E.; Littlefair, G. Titanium in biomedical applications—Properties and fabrication: A Review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Hong, Y.C.; Kim, J.H.; Bang, C.U.; Uhm, H.S. Gas-phase synthesis of nitrogen-doped TiO2 nanorods by microwave plasma torch at atmospheric pressure. Phys. Plasmas 2005, 12, 114501. [Google Scholar] [CrossRef]
- Dar, M.I.; Chandiran, A.K.; Gratzel, M.; Nazeeruddin, M.K.; Shivashankar, S.A. Controlled synthesis of TiO2 nanospheres using a microwave assisted approach for their application in dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 1662–1667. [Google Scholar] [CrossRef]
- Jeon, S.; Braun, P.V. Hydrothermal synthesis of er-doped luminescent TiO2 nanoparticles. Chem. Mater. 2003, 15, 1256–1263. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Natarajan, M.; Santhanam, A.; Asokan, V.; Velauthapillai, D. Size controlled synthesis of TiO2 nanoparticles by modified solvothermal method towards effective photocatalitic and photovoltaic applications. Mater. Res. 2018, 97, 351–360. [Google Scholar]
- Loryuenyong, V.; Angamnuaysiri, K.; Sukcharoenpong, J.; Suwannasri, A. Sol–gel derived mesoporous titania nanoparticles: Effects of calcination temperature and alcoholic solvent on the photocatalytic behavior. Ceram. Int. 2012, 38, 2233–2237. [Google Scholar] [CrossRef]
- Maheswari, D.; Venkatachalam, P. Enhanced efficiency and improved photocatalytic activity of 1:1 composite mixture of TiO2 nanoparticles and nanotubes in dye-sensitized solar cell. Bull. Mater. Sci. 2014, 37, 1489–1496. [Google Scholar] [CrossRef]
- Cai, Q.; Paulose, M.; Varghese, O.K.; Grimes, C.A. The effect of electrolyte composition on the fabrication of self-organized titanium oxide nanotube arrays by anodic oxidation. J. Mater. Res. 2005, 20, 230–236. [Google Scholar] [CrossRef]
- Tang, Y.; Tao, J.; Zhang, Y.; Wu, T.; Tao, H.; Bao, Z. Preparation and characterization of TiO2 nanotube arrays via anodization of titanium films deposited on FTO conducting glass at room temperature. Acta Physico Chimica Sin. 2008, 24, 2191–2197. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.; Yue, P.; Lu, M.; Greenfield, P. Synthesis of anatase TiO2 supported on porous solids by chemical vapor deposition. Catal. Today 2001, 68, 173–182. [Google Scholar] [CrossRef]
- Lee, H.; Song, M.; Jurng, J.; Park, Y.-K. The synthesis and coating process of TiO2 nanoparticles using CVD process. Powder Technol. 2011, 214, 64–68. [Google Scholar] [CrossRef]
- Arami, H.; Mazloumi, M.; Khalifehzadeh, R.; Sadrnezhaad, S.K. Sonochemical preparation of TiO2 nanoparticles. Mater. Lett. 2007, 61, 4559–4561. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Chen, Z.; Li, Y.; Yu, Z.; Liu, Q.; Li, J.; Feng, C.; Zhang, D. Sonochemical synthesis of TiO2 nanoparticles on graphene for use as photocatalyst. Ultrason. Sonochem. 2011, 18, 1082–1090. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Atarod, M.; Jaleh, B.; Gandomirouzbahani, M. In situ green synthesis of Ag nanoparticles on graphene oxide/TiO2 nanocomposite and their catalytic activity for the reduction of 4-nitrophenol, Congo red and methylene blue. Ceram. Int. 2016, 42, 8587–8596. [Google Scholar] [CrossRef]
- Sivaranjani, V.; Philominathan, P. Synthesize of Titanium dioxide nanoparticles using Moringa oleifera leaves and evaluation of wound healing activity. Wound Med. 2016, 12, 1–5. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Paunesku, T.; Rajh, T.; Wiederrecht, G.; Maser, J.; Vogt, S.; Stojićević, N.; Protić, M.; Lai, B.; Oryhon, J.; Thurnauer, M.; et al. Biology of TiO2–oligonucleotide nanocomposites. Nat. Mater. 2003, 2, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Paunesku, T.; Vogt, S.; Lai, B.; Maser, J.; Stojićević, N.; Thurn, K.T.; Osipo, C.; Liu, H.; Legnini, D.; Wang, Z.; et al. Intracellular distribution of TiO2-DNA oligonucleotide nanoconjugates directed to nucleolus and mitochondria indicates sequence specificity. Nano Lett. 2007, 7, 596–601. [Google Scholar] [CrossRef]
- Cesmeli, S.; Biray Avci, C. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J. Drug Target. 2019, 27, 762–766. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Losic, D.; Aw, M.; Santos, A.; Gulati, K.; Bariana, M. Titania nanotube arrays for local drug delivery: Recent advances and perspectives. Expert Opin. Drug Deliv. 2014, 12, 103–127. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.-Y.; Li, H.-Q.; Chen, Z.; Zhao, A.Z.-J.; Wang, Y.; Zhang, K.-Q.; Sun, H.-T.; Al-Deyab, S.S.; Lai, Y.-K. TiO2 nanotube platforms for smart drug delivery: A review. Int. J. Nanomed. 2016, 11, 4819–4834. [Google Scholar]
- Wu, S.; Weng, Z.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Functionalized TiO2 based nanomaterials for biomedical applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- Tiainen, H.; Monjo, M.; Knychala, J.; Nilsen, O.; Lyngstadaas, S.P.; Ellingsen, J.E.; Haugen, H.J. The effect of fluoride surface modification of ceramic TiO2 on the surface properties and biological response of osteoblastic cells in vitro. Biomed. Mater. 2011, 6, 45006. [Google Scholar] [CrossRef] [PubMed]
- Tiainen, H.; Lyngstadaas, S.P.; Ellingsen, J.E.; Haugen, H.J. Ultra-porous titanium oxide scaffold with high compressive strength. J. Mater. Sci. Mater. Med. 2010, 21, 2783–2792. [Google Scholar] [CrossRef]
- Tabriz, K.R.; Katbab, A.A. Preparation of modified-TiO2/PLA nanocomposite films: Micromorphology, photo-degradability and antibacterial studies. AIP Conf. Proc. 2017, 1914, 70009. [Google Scholar]
- Wu, X.; Liu, X.; Wei, J.; Ma, J.; Deng, F.; Wei, S. Nano-TiO2/PEEK bioactive composite as a bone substitute material: In vitro and in vivo studies. Int. J. Nanomed. 2012, 7, 1215–1225. [Google Scholar]
- Eslami, H.; Azimi Lisar, H.; Jafarzadeh Kashi, T.S.; Tahriri, M.; Ansari, M.; Rafiei, T.; Bastami, F.; Shahin-Shamsabadi, A.; Mashhadi Abbas, F.; Tayebi, L. Poly(lactic-co-glycolic acid)(PLGA)/TiO2 nanotube bioactive composite as a novel scaffold for bone tissue engineering: In vitro and in vivo studies. Biologicals 2018, 53, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Paušová, Š.; Krýsa, J.; Jirkovský, J.; Prevot, V.; Mailhot, G. Preparation of TiO2-SiO2 composite photocatalysts for environmental applications. J. Chem. Technol. Biotechnol. 2014, 89, 1129–1135. [Google Scholar] [CrossRef]
- Liu, S.; Tao, W.; Li, J.; Yang, Z.; Liu, F. Study on the formation process of Al2O3–TiO2 composite powders. Powder Technol. 2005, 155, 187–192. [Google Scholar] [CrossRef]
- Omid-Bakhtiari, M.; Nasr-Esfahani, M.; Nourmohamadi, A. TiO2-Bioactive glass nanostructure composite films produced by a sol-gel method: In vitro behavior and UV-enhanced bioactivity. J. Mater. Eng. Perform. 2014, 23, 285–293. [Google Scholar] [CrossRef]
- Oktar, F.N. Hydroxyapatite–TiO2 composites. Mater. Lett. 2006, 60, 2207–2210. [Google Scholar] [CrossRef]
- Khalid, N.R.; Bilal Tahir, M.; Majid, A.; Ahmed, E.; Ahmad, M.; Khalid, S.; Ahmed, W. TiO2-graphene-based composites: Synthesis, characterization, and application in photocatalysis of organic pollutants. In Micro and Nanomanufacturing Volume II; Jackson, M.J., Ahmed, W., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 95–122. [Google Scholar]
- Roguska, A.; Pisarek, M.; Andrzejczuk, M.; Dolata, M.; Lewandowska, M.; Janik-Czachor, M. Characterization of a calcium phosphate–TiO2 nanotube composite layer for biomedical applications. Mater. Sci. Eng. C 2011, 31, 906–914. [Google Scholar] [CrossRef]
- Rehman, F.U.; Zhao, C.; Jiang, H.; Wang, X. Biomedical applications of nano-titania in theranostics and photodynamic therapy. Biomater. Sci. 2016, 4, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, X.; Zhao, J.; Tang, G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J. Appl. Toxicol. 2009, 29, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Chennell, P.; Feschet-Chassot, E.; Devers, T.; Awitor, K.O.; Descamps, S.; Sautou, V. In vitro evaluation of TiO2 nanotubes as cefuroxime carriers on orthopaedic implants for the prevention of periprosthetic joint infections. Int. J. Pharm. 2013, 455, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Bioceramics for hard tissue engineering applications: A review. Int. J. Appl. Eng. Res. 2018, 13, 2744–2752. [Google Scholar]
- Hentrich, R.L.; Graves, G.A.; Stein, H.G.; Bajpai, P.K. An evaluation of inert and resorbale ceramics for future clinical orthopedic applications. J. Biomed. Mater. Res. 1971, 5, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Christel, P.; Meunier, A.; Heller, M.; Torre, J.P.; Peille, C.N. Mechanical properties and short-term in vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J. Biomed. Mater. Res. 1989, 23, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.A.F.; Kesarwani, P.; Reddy, K.M.; Kalmodia, S.; Basu, B.; Balani, K. Functionally graded hydroxyapatite-alumina-zirconia biocomposite: Synergy of toughness and biocompatibility. Mater. Sci. Eng. C 2012, 32, 1164–1173. [Google Scholar] [CrossRef]
- Cales, B.; Stefani, Y.; Lilley, E. Long-term in vivo and in vivo aging of a zirconia ceramic used in orthopaedy. J. Biomed. Mater. Res. 1994, 28, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Yoon, T.-R. Recent updates for biomaterials used in total hip arthroplasty. Biomater. Res. 2018, 22, 1–12. [Google Scholar] [CrossRef]
- Vagkopoulou, T.; Koutayas, S.O.; Koidis, P.; Strub, J.R. Zirconia in dentistry: Part 1. Discovering the nature of an upcoming bioceramic. Eur. J. Esthet. Dent. 2009, 4, 130–151. [Google Scholar]
- Tosiriwatanapong, T.; Singhatanadgit, W. Zirconia-based biomaterials for hard tissue reconstruction. Bone Tissue Regen. Insights 2018, 9. [Google Scholar] [CrossRef]
- Aboushelib, M.N.; Shawky, R. Osteogenesis ability of CAD/CAM porous zirconia scaffolds enriched with nano-hydroxyapatite particles. Int. J. Implant Dent. 2017, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Mushahary, D.; Sravanthi, R.; Li, Y.; Kumar, M.J.; Harishankar, N.; Hodgson, P.D.; Wen, C.; Pande, G. Zirconium, calcium, and strontium contents in magnesium based biodegradable alloys modulate the efficiency of implant-induced osseointegration. Int. J. Nanomed. 2013, 8, 2887–2902. [Google Scholar]
- Ramaswamy, Y.; Wu, C.; Van Hummel, A.; Combes, V.; Grau, G.; Zreiqat, H. The responses of osteoblasts, osteoclasts and endothelial cells to zirconium modified calcium-silicate-based ceramic. Biomaterials 2008, 29, 4392–4402. [Google Scholar] [CrossRef]
- Kim, H.-W.; Kim, H.-E.; Knowles, J.C. Hard-tissue-engineered zirconia porous scaffolds with hydroxyapatite sol–gel and slurry coatings. J. Biomed. Mater. Res. Part B. 2004, 70, 270–277. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L. Zirconia as a biomaterial. Compr. Biomater. 2011, 1, 95–108. [Google Scholar]
- Alizadeh, A.; Moztarzadeh, F.; Ostad, S.N.; Azami, M.; Geramizadeh, B.; Hatam, G.; Bizari, D.; Tavangar, S.M.; Vasei, M.; Ai, J. Synthesis of calcium phosphate-zirconia scaffold and human endometrial adult stem cells for bone tissue engineering. Artif. Cells Nanomed. Biotechnol. 2016, 44, 66–73. [Google Scholar] [CrossRef]
- Jayakumar, R.; Ramachandran, R.; Sudheesh Kumar, P.T.; Divyarani, V.V.; Srinivasan, S.; Chennazhi, K.P.; Tamura, H.; Nair, S.V. Fabrication of chitin–chitosan/nano ZrO2 composite scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2011, 49, 274–280. [Google Scholar] [CrossRef]
- Teimouri, A.; Ebrahimi, R.; Emadi, R.; Beni, B.H.; Chermahini, A.N. Nano-composite of silk fibroin–chitosan/Nano ZrO2 for tissue engineering applications: Fabrication and morphology. Int. J. Biol. Macromol. 2015, 76, 292–302. [Google Scholar] [CrossRef]
- Bermúdez-Reyes, B.; del Refugio Lara-Banda, M.; Reyes-Zarate, E.; Rojas-Martínez, A.; Camacho, A.; Moncada-Saucedo, N.; Pérez-Silos, V.; García-Ruiz, A.; Guzmán-López, A.; Peña-Martínez, V.; et al. Effect on growth and osteoblast mineralization of hydroxyapatite-zirconia (HA-ZrO2) obtained by a new low temperature system. Biomed. Mater. 2018, 13, 035001. [Google Scholar] [CrossRef]
- Sa, M.-W.; Nguyen, B.-N.B.; Moriarty, R.A.; Kamalitdinov, T.; Fisher, J.P.; Kim, J.Y. Fabrication and evaluation of 3D printed BCP scaffolds reinforced with ZrO2 for bone tissue applications. Biotechnol. Bioeng. 2018, 115, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, A.; Pramanik, N.; Mitra, T.; Gnanamani, A.; Das, M.; Kundu, P.P. Mechanical and biological investigations of chitosan–polyvinyl alcohol based ZrO2 doped porous hybrid composites for bone tissue engineering applications. New J. Chem. 2017, 41, 7524–7530. [Google Scholar] [CrossRef]
- Wang, X.; Chen, D.; Cao, L.; Li, Y.; Boyd, B.J.; Caruso, R.A. Mesoporous titanium zirconium oxide nanospheres with potential for drug delivery applications. ACS Appl. Mater. Interfaces 2013, 5, 10926–10932. [Google Scholar] [CrossRef] [PubMed]
- Abánades Lázaro, I.; Haddad, S.; Rodrigo-Muñoz, J.M.; Marshall, R.J.; Sastre, B.; del Pozo, V.; Fairen-Jimenez, D.; Forgan, R.S. Surface-functionalization of Zr-Fumarate MOF for selective cytotoxicity and immune system compatibility in nanoscale drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 31146–31157. [Google Scholar] [CrossRef]
- Milak, P.; Minatto, F.; De Noni, A., Jr.; Montedo, O. Wear performance of alumina-based ceramics—A review of the influence of microstructure on erosive wear. Cerâmica 2015, 61, 88–103. [Google Scholar] [CrossRef]
- Gaber, A.A.A.-A.; Ibrahim, D.M.; Abd-AImohsen, F.F.; El-Zanati, M.M. Synthesis of alumina, titania, and alumina-titania hydrophobic membranes via sol–gel polymeric route. J. Anal. Sci. Technol. 2013, 4, 18. [Google Scholar] [CrossRef]
- Elsberg, L.; Moore, M. Total hip replacement: Metal-on-metal systems. In Clinical Performance of Skeletal Prostheses; Hench, L.L., Wilson, J., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 57–70. [Google Scholar]
- Al-Sanabani, F.A.; Madfa, A.A.; Al-Qudaimi, N.H. Alumina ceramic for dental applications: A review article. Am. J. Mater. Res. 2014, 1, 26–34. [Google Scholar]
- Lee, K.-L.; Hsu, H.-Y.; You, M.-L.; Chang, C.-C.; Pan, M.-Y.; Shi, X.; Ueno, K.; Misawa, H.; Wei, P.-K. Highly sensitive aluminum-based biosensors using tailorable fano resonances in capped nanostructures. Sci. Rep. 2017, 7, 44104. [Google Scholar] [CrossRef]
- Bartzsch, H.; Glöß, D.; Böcher, B.; Frach, P.; Goedicke, K. Properties of SiO2 and Al2O3 films for electrical insulation applications deposited by reactive pulse magnetron sputtering. Surf. Coat. Technol. 2003, 174, 774–778. [Google Scholar] [CrossRef]
- Elisabet, X.-P.; Josep, F.-B.; Josep, P.; Marsal, F.L. Mesoporous alumina as a biomaterial for biomedical applications. Open Mater. Sci. 2015, 2, 13. [Google Scholar]
- Mohanty, M. Medical Applications of alumina ceramics. Trans. Indian Ceram. Soc. 1995, 54, 200–204. [Google Scholar] [CrossRef]
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Piconi, C.; Condo, S.G.; Kosmac, T. Chapter 11—Alumina- and zirconia-based ceramics for load-bearing applications. In Advanced Ceramics for Dentistry; Shen, J.Z., Kosmač, T., Eds.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 219–253. [Google Scholar]
- Kandpal, B.C.; kumar, J.; Singh, H. Fabrication and characterisation of Al2O3/aluminium alloy 6061 composites fabricated by stir casting. Mater. Today Proc. 2017, 4, 2783–2792. [Google Scholar] [CrossRef]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, F.; Shafiee Afarani, M.; Mohebbi-Kalhori, D.; Shayesteh, M. Fabrication of alumina porous scaffolds with aligned oriented pores for bone tissue engineering applications. Appl. Phys. A 2016, 122, 390. [Google Scholar] [CrossRef]
- Levin, I.; Brandon, D. Metastable alumina polymorphs: Crystal structures and transition sequences. J. Am. Ceram. Soc. 1998, 81, 1995–2012. [Google Scholar] [CrossRef]
- Rahmati, M.; Mozafari, M. Biocompatibility of alumina-based biomaterials–A review. J. Cell. Physiol. 2019, 234, 3321–3335. [Google Scholar] [CrossRef]
- Ferraz, N.; Hong, J.; Santin, M.; Ott, M. Nanoporosity of alumina surfaces induces different patterns of activation in adhering monocytes/macrophages. Int. J. Biomater. 2010, 2010, 402715. [Google Scholar] [CrossRef]
- Gregorová, E.; Pabst, W.; Živcová, Z.; Sedlářová, I.; Holíková, S. Porous alumina ceramics prepared with wheat flour. J. Eur. Ceram. Soc. 2010, 30, 2871–2880. [Google Scholar] [CrossRef]
- Bartonickova, E.; Vojtisek, J.; Tkacz, J.; Pořízka, J.; Masilko, J.; Moncekova, M.; Parizek, L. Porous HA/Alumina composites intended for bone-tissue engineering. Mater. Technol. 2017, 51, 631–636. [Google Scholar] [CrossRef]
- Leary Swan, E.E.; Popat, K.C.; Desai, T.A. Peptide-immobilized nanoporous alumina membranes for enhanced osteoblast adhesion. Biomaterials 2005, 26, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Yang, R.; Ding, J.; Yuan, N.; Zhu, Y. Fabrication of porous anodic alumina with ultrasmall nanopores. Nanoscale Res. Lett. 2010, 5, 1257. [Google Scholar] [CrossRef] [PubMed]
- Zaraska, L.; Jaskula, M.; Sulka, G. Porous anodic alumina layers with modulated pore diameters formed by sequential anodizing in different electrolytes. Mater. Lett. 2016, 171, 315–318. [Google Scholar] [CrossRef]
- Porta-I-Batalla, M.; Xifré-Pérez, E.; Eckstein, C.; Ferré-Borrull, J.; Marsal, L.F. 3D Nanoporous Anodic Alumina Structures for Sustained Drug Release. Nanomaterials 2017, 7, 227. [Google Scholar] [CrossRef]
- Vishal, P. Design and fabrication of ordered mesoporous alumina scaffold for drug delivery of poorly water soluble drug. Austin Ther. 2015, 2, 1–5. [Google Scholar]
- Kang, H.J.; Park, S.J.; Yoo, J.B.; Kim, D.J. Controlled drug release using nanoporous anodic aluminum oxide. Solid State Phenom. 2007, 121, 709–712. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Kumar Panda, S.; Dhupal, D.; Kumar Nanda, B. Experimental study on Ca3(PO4)2-Al2O3 bio-ceramic composite using DPSS laser. Mater. Today Proc. 2018, 5, 24133–24140. [Google Scholar] [CrossRef]
- Toloue, E.B.; Karbasi, S.; Salehi, H.; Rafienia, M. Evaluation of mechanical properties and cell viability of poly (3-Hydroxybutyrate)-Chitosan/Al2O3 nanocomposite scaffold for cartilage tissue engineering. J. Med. Signals Sens. 2019, 9, 111–116. [Google Scholar]
- Venkatesh, N.; Hanumantharaju, H.G.; Aravind, J. A Study of bio-active coating of Al2O3, egg and sea shell powder on Ss316l and Ti-6Al-4V. Mater. Today Proc. 2018, 5, 22687–22693. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Wang, Y.; Liu, Q.; Yang, J.; Zhang, L.; He, F. Fabrication of Al2O3 by anodic oxidation and hydrothermal synthesis of strong-bonding hydroxyapatite coatings on its surface. Appl. Surf. Sci. 2019, 470, 959–969. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Ríos, S.; González, M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell. Biochem. 2002, 85, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Hu, G. Copper stimulates proliferation of human endothelial cells under culture. J. Cell. Biochem. 1998, 69, 326–335. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, P.G.; Traversa, E. Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Gallo, J.; Holinka, M.; Moucha, C.S. Antibacterial surface treatment for orthopaedic implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef]
- Chaudhri, M.A.; Kemmler, W.; Harsch, I.; Watling, R.J. Plasma copper and bone mineral density in osteopenia: An indicator of bone mineral density in osteopenic females. Biol. Trace Elem. Res. 2009, 129, 94–98. [Google Scholar] [CrossRef]
- Das, A.; Sudhahar, V.; Chen, G.-F.; Kim, H.W.; Youn, S.-W.; Finney, L.; Vogt, S.; Yang, J.; Kweon, J.; Surenkhuu, B.; et al. Endothelial antioxidant-1: A Key mediator of copper-dependent wound healing in vivo. Sci. Rep. 2016, 6, 33783. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Tripathi, A.; Saravanan, S.; Pattnaik, S.; Moorthi, A.; Partridge, N.C.; Selvamurugan, N. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/nano-copper–zinc for bone tissue engineering. Int. J. Biol. Macromol. 2012, 50, 294–299. [Google Scholar] [CrossRef]
- Azeena, S.; Subhapradha, N.; Selvamurugan, N.; Narayan, S.; Srinivasan, N.; Murugesan, R.; Chung, T.W.; Moorthi, A. Antibacterial activity of agricultural waste derived wollastonite doped with copper for bone tissue engineering. Mater. Sci. Eng. C 2017, 71, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.J.; Ryan, A.J.; González-Vázquez, A.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.J.; O’Brien, F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Seyedalipour, B.; Shafieyan, S.; Kheime, A.; Mohammadi, P.; Aghdami, N. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem. Biophys. Res. Commun. 2019, 517, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Michels, H.; Moran, W.; Michel, J. Antimicrobial properties of copper alloy surfaces, with a focus on hospital-acquired infections. Int. J. Met. 2008, 2, 47–56. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Sakharkar, M.K.; Nayak, A.; Kishore, U.; Khan, A. Nanoparticles for biomedical applications: An overview. In Nanobiomaterials: Nanostructured Materials for Biomedical Applications, 1st ed.; Narayan, R.B.T.-N., Ed.; Woodhead Publishing: Cambridge, UK, 2017; Chapter 14; pp. 357–384. [Google Scholar]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef]
- Sensenig, R.; Sapir, Y.; MacDonald, C.; Cohen, S.; Polyak, B. Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine 2012, 7, 1425–1442. [Google Scholar] [CrossRef]
- Khalil, M.I. Co-precipitation in aqueous solution synthesis of magnetite nanoparticles using iron(III) salts as precursors. Arab. J. Chem. 2015, 8, 279–284. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials: 1st Nano update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef]
- Daou, T.J.; Pourroy, G.; Bégin-Colin, S.; Grenèche, J.M.; Ulhaq-Bouillet, C.; Legaré, P.; Bernhardt, P.; Leuvrey, C.; Rogez, G. Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem. Mater. 2006, 18, 4399–4404. [Google Scholar] [CrossRef]
- Takai, Z.; Mustafa, M.; Asman, S.; Sekak, K. Preparation and characterization of magnetite (Fe3O4) nanoparticles by sol-gel method. Int. J. Nanoelectron. Mater. 2019, 12, 37–46. [Google Scholar]
- Hachani, R.; Lowdell, M.; Birchall, M.; Hervault, A.; Mertz, D.; Begin-Colin, S.; Thanh, N.T.K. Polyol synthesis, functionalisation, and biocompatibility studies of superparamagnetic iron oxide nanoparticles as potential MRI contrast agents. Nanoscale 2016, 8, 3278–3287. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Muhammed, M.; Zagorodni, A. Novel flow injection synthesis of iron oxide nanoparticles with narrow size distribution. Chem. Eng. Sci. 2006, 61, 4625–4633. [Google Scholar] [CrossRef]
- Hassanjani-Roshan, A.; Vaezi, M.R.; Shokuhfar, A.; Rajabali, Z. Synthesis of iron oxide nanoparticles via sonochemical method and their characterization. Particuology 2011, 9, 95–99. [Google Scholar] [CrossRef]
- Aivazoglou, E.; Metaxa, E.; Hristoforou, E. Microwave-assisted synthesis of iron oxide nanoparticles in biocompatible organic environment. AIP Adv. 2017, 8, 48201. [Google Scholar] [CrossRef]
- Starowicz, M.; Starowicz, P.; Zukrowski, J.; Przewoźnik, J.; Lemański, A.; Kapusta, C. Electrochemical synthesis of magnetic iron oxide nanoparticles with controlled size. J. Nanopart. Res. 2011, 13, 7167–7176. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Huan, W.; Liang, X.; Liu, X.; Yang, Y. A study on synthesis and properties of Fe3O4 nanoparticles by solvothermal method. Glas. Phys. Chem. 2010, 36, 325–331. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Cao, L.; Fu, L.; Li, X.; Wang, Y.; Yu, G. A Magnetism-assisted chemical vapor deposition method to produce branched or iron-encapsulated carbon nanotubes. J. Am. Chem. Soc. 2007, 129, 7364–7368. [Google Scholar] [CrossRef]
- Majidi, S.; Sehrig, F.Z.; Farkhani, S.M.; Goloujeh, M.S.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef]
- Karade, V.C.; Waifalkar, P.P.; Dongle, T.D.; Sahoo, S.C.; Kollu, P.; Patil, P.S.; Patil, P.B. Greener synthesis of magnetite nanoparticles using green tea extract and their magnetic properties. Mater. Res. Express 2017, 4, 96102. [Google Scholar] [CrossRef]
- Kayal, S.; Bandyopadhyay, D.; Mandal, T.; Ramanujan, R. The flow of magnetic nanoparticles in magnetic drug targeting. RSC Adv. 2011, 1, 238–246. [Google Scholar] [CrossRef]
- Safarik, I.; Safarikova, M. Magnetic techniques for the isolation and purification of proteins and peptides. Biomagn. Res. Technol. 2004, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- David, R.; Groebner, M.; Franz, W. Magnetic cell sorting purification of differentiated embryonic stem cells stably expressing truncated human CD4 as surface marker. Stem Cells 2005, 23, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Bajwa, U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef]
- Pan, Y.; Du, X.; Zhao, F.; Xu, B. Magnetic nanoparticles for the manipulation of proteins and cells. Chem. Soc. Rev. 2012, 41, 2912–2942. [Google Scholar] [CrossRef]
- Baroli, B.; Ennas, M.G.; Loffredo, F.; Isola, M.; Pinna, R.; López-Quintela, M.A. Penetration of Metallic Nanoparticles in Human Full-Thickness Skin. J. Investig. Dermatol. 2007, 127, 1701–1712. [Google Scholar] [CrossRef]
- Li, Y.; Ye, D.; Li, M.; Ma, M.; Gu, N. Adaptive materials based on iron oxide nanoparticles for bone regeneration. ChemPhysChem 2018, 19, 1965–1979. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.-Z.; Long, T.; Yu, X.-B.; Tang, T.; Dai, K.-R.; Tian, B.; Guo, Y.-P.; Zhu, Z.-A. Mesoporous bioactive glass as a drug delivery system: Fabrication, bactericidal properties and biocompatibility. J. Mater. Sci. Mater. Med. 2013, 24, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-J.; Wang, Y.-Y.; Chen, T.; Wei, Y.-T.; Chu, L.-F.; Guo, Y.-P. Hollow carbonated hydroxyapatite microspheres with mesoporous structure: Hydrothermal fabrication and drug delivery property. Mater. Sci. Eng. C 2013, 33, 3166–3172. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.E.; Kamei, G.; Harada, Y.; Shimizu, R.; Kamei, N.; Adachi, N.; Misk, N.A.; Ochi, M. Cell magnetic targeting system for repair of severe chronic osteochondral defect in a rabbit model. Cell Transplant. 2016, 25, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, L.M.; Zimmerman, J.B.; Plata, D.L.; Hutchison, J.E.; Anastas, P.T. Designing nanomaterials to maximize performance and minimize undesirable implications guided by the Principles of Green Chemistry. Chem. Soc. Rev. 2015, 44, 5758–5777. [Google Scholar] [CrossRef]
- Uskoković, V. Entering the era of nanoscience: Time to be so small. J. Biomed. Nanotechnol. 2013, 9, 1441–1470. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.-S. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef]
- Singh, S.K.; Kulkarni, P.P.; Dash, D. Biomedical applications of nanomaterials: An overview. Bionanotechnology 2013, 1–32. [Google Scholar] [CrossRef]
- Das, S.; Mitra, S.; Khurana, S.M.P.; Debnath, N. Nanomaterials for biomedical applications. Front. Life Sci. 2013, 7, 90–98. [Google Scholar] [CrossRef]
- Ng, C.-T.; Baeg, G.-H.; Yu, L.E.; Bay, C.-N.O. Biomedical applications of nanomaterials as therapeutics. Curr. Med. Chem. 2018, 25, 1409–1419. [Google Scholar] [CrossRef]
- Gentile, A.; Ruffino, F.; Grimaldi, G.M. Complex-morphology metal-based nanostructures: Fabrication, characterization, and applications. Nanomaterials 2016, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Biener, J.; Wittstock, A.; Baumann, T.F.; Weissmüller, J.; Bäumer, M.; Hamza, A.V. Surface chemistry in nanoscale materials. Materials 2009, 2, 2404–2428. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Singh, A.; Elisseeff, J. Biomaterials for stem cell differentiation. J. Mater. Chem. 2010, 20, 8832–8847. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, X.; Zhang, R.; Feng, Q. In vitro uptake of hydroxyapatite nanoparticles and their effect on osteogenic differentiation of human mesenchymal stem cells. Stem Cells Int. 2018, 2018, 2036176. [Google Scholar] [CrossRef]
- Lavenus, S.; Trichet, V.; Le Chevalier, S.; Hoornaert, A.; Louarn, G.; Layrolle, P. Cell differentiation and osseointegration influenced by nanoscale anodized titanium surfaces. Nanomedicine 2012, 7, 967–980. [Google Scholar] [CrossRef]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surface and materials for dental implants: Surface coating, pattering and functionalization for improved performance. Biomater. Sci. 2018, 6, 1312–1338. [Google Scholar] [CrossRef]
- Bertazzo, S.; Zambuzzi, W.F.; Da Silva, H.A.; Ferreira, C.V.; Bertran, C.A. Bioactivation of alumina by surface modification: A possibility for improving the applicability of alumina in bone and oral repair. Clin. Oral Implants Res. 2009, 20, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Profeta, A.C.; Huppa, C. Bioactive-glass in oral and maxillofacial surgery. Craniomaxillofac. Trauma Reconstr. 2016, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Crovace, M.C.; Souza, M.T.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D. Biosilicate®—A multipurpose, highly bioactive glass-ceramic. In vitro, in vivo and clinical trials. J. Non. Cryst. Solids 2016, 432, 90–110. [Google Scholar] [CrossRef]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and promising applications of strontium (Sr)-containing bioactive glasses in bone tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Vitale-Brovarone, C. Three-dimensional glass-derived scaffolds for bone tissue engineering: Current trends and forecasts for the future. J. Biomed. Mater. Res. Part A 2011, 97, 514–535. [Google Scholar] [CrossRef]
- Tautzenberger, A.; Kovtun, A.; Ignatius, A. Nanoparticles and their potential for application in bone. Int. J. Nanomed. 2012, 7, 4545–4557. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Ibuprofen-loaded CTS/nHA/nBG Scaffolds for the applications of hard tissue engineering. Iran. Biomed. J. 2019, 23, 190–199. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorganic Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size 1. Cancer Res. 2000, 60, 4440–4445. [Google Scholar]
- Trewyn, B.G.; Slowing, I.I.; Chen, H.; Lin, V.S. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singla, R.; Guliani, A.; Yadav, S.K. Nanoencapsulation for drug delivery. EXCLI J. 2014, 13, 265–286. [Google Scholar] [PubMed]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.K.; Vermerris, W. Recent advances in nanomaterials for gene delivery—A review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S. Endgame: Glybera finally recommended for approval as the first gene therapy drug in the European Union. Mol. Ther. 2012, 20, 1831–1832. [Google Scholar] [CrossRef]
- Loh, X.J.; Lee, T.-C.; Dou, Q.; Deen, G.R. Utilising inorganic nanocarriers for gene delivery. Biomater. Sci. 2016, 4, 70–86. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, L. Lipid nanoparticles for gene delivery. Adv. Genet. 2014, 88, 13–36. [Google Scholar]
- Martin, B.; Sainlos, M.; Aissaoui, A.; Oudrhiri, N.; Hauchecorne, M.; Vigneron, J.-P.; Lehn, J.-M.; Lehn, P. The design of cationic lipids for gene delivery. Curr. Pharm. Des. 2005, 11, 375–394. [Google Scholar] [CrossRef]
- Nordling-David, M.M.; Golomb, G. Gene delivery by liposomes. Isr. J. Chem. 2013, 53, 737–747. [Google Scholar] [CrossRef]
- Ropert, C. Liposomes as a gene delivery system. Braz. J. Med. Biol. Res. 1999, 32, 163–169. [Google Scholar] [CrossRef]
- Zylberberg, C.; Gaskill, K.; Pasley, S.; Matosevic, S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 2017, 24, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Santander-Ortega, M.J.; Lozano, M.V.; Uchegbu, I.F.; Schätzlein, A.G. 6—Dendrimers for gene therapy. In Polymers and Nanomaterials for Gene Therapy; Narain, R., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 113–146. [Google Scholar]
- Eliyahu, H.; Barenholz, Y.; Domb, A.J. Polymers for DNA delivery. Molecules 2005, 10, 34–64. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.R.R.; Rekha, C.P. Polymers for gene delivery: Current status and future perspectives. Recent Patents DNA Gene Seq. 2012, 6, 98–107. [Google Scholar]
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Imani, R.; Mohabatpour, F.; Mostafavi, F. Graphene-based nano-carrier modifications for gene delivery applications. Carbon N. Y. 2018, 140, 569–591. [Google Scholar] [CrossRef]
- Dolatabadi, J.E.N.; Omid, Y.O.; Losic, D. Carbon nanotubes as an advanced drug and gene delivery nanosystem. Curr. Nanosci. 2011, 7, 297–314. [Google Scholar] [CrossRef]
- Ramos-Perez, V.; Cifuentes, A.; Coronas, N.; de Pablo, A.; Borrós, S. Modification of carbon nanotubes for gene delivery vectors. In Nanomaterial Interfaces in Biology: Methods and Protocols; Bergese, P., Hamad-Schifferli, K., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 261–268. [Google Scholar]
- Keasberry, N.A.; Yapp, C.W.; Idris, A. Mesoporous silica nanoparticles as a carrier platform for intracellular delivery of nucleic acids. Biochemistry 2017, 82, 655–662. [Google Scholar] [CrossRef]
- Mendes, R.; Fernandes, A.R.; Baptista, P.V. Gold nanoparticle approach to the selective delivery of gene silencing in cancer—The case for combined delivery? Genes 2017, 8, 94. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef]
- Majidi, S.; Zeinali Sehrig, F.; Samiei, M.; Milani, M.; Abbasi, E.; Dadashzadeh, K.; Akbarzadeh, A. Magnetic nanoparticles: Applications in gene delivery and gene therapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1186–1193. [Google Scholar] [CrossRef]
- McBain, S.C.; Yiu, H.H.P.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar]
- Giacca, M.; Zacchigna, S. Virus-mediated gene delivery for human gene therapy. J. Control. Release 2012, 161, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Domvri, K.; Zarogoulidis, P.; Porpodis, K.; Koffa, M.; Lambropoulou, M.; Kakolyris, S.; Minadakis, G.; Zarogoulidis, K.; Chatzaki, E. Gene therapy in liver diseases: State-of-the-art and future perspectives. Curr. Gene Ther. 2012, 12, 463–483. [Google Scholar] [CrossRef] [PubMed]
- Nienhuis, A.W.; Nathwani, A.C.; Davidoff, A.M. Gene therapy for hemophilia. Mol. Ther. 2017, 25, 1163–1167. [Google Scholar] [CrossRef]
- Luo, J.; Sun, M.; Kang, Q.; Peng, Y.; Jiang, W.; Luu, H.; Luo, Q.; Park, J.; Li, Y.; Haydon, R. Gene therapy for bone regeneration. Curr. Gene Ther. 2005, 5, 167–179. [Google Scholar] [CrossRef]
- Pensak, M.J.; Lieberman, J.R. Gene therapy for bone regeneration. Curr. Pharm. Des. 2013, 19, 3466–3473. [Google Scholar] [CrossRef]
- Shapiro, G.; Lieber, R.; Gazit, D.; Pelled, G. Recent advances and future of gene therapy for bone regeneration. Curr. Osteoporos. Rep. 2018, 16, 504–511. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Navya, P.N.; Daima, H.K. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016, 3, 1. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, N. Chapter 13—Nanoparticles in gene therapy: Principles, prospects, and challenges. In Nanoparticles in Translational Science and Medicine; Villaverde, A.B.T.-P., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 104, pp. 509–562. [Google Scholar]
- Islam, M.; Park, T.-E.; Singh, B.; Maharjan, S.; Firdous, J.; Kang, S.-K.; Yun, C.-H.; Choi, Y.; Cho, C. Major degradable polycations as carriers for DNA and siRNA. J. Control. Release 2014, 193, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Weber, T.J.; Hu, D.; Lin, C.-T.; Li, J.; Lin, Y. In Situ Live Cell Sensing of multiple nucleotides exploiting DNA/RNA aptamers and graphene oxide nanosheets. Anal. Chem. 2013, 85, 6775–6782. [Google Scholar] [CrossRef] [PubMed]
- Keles, E.; Song, Y.; Du, D.; Dong, W.-J.; Lin, Y. Recent progress in nanomaterials for gene delivery applications. Biomater. Sci. 2016, 4, 1291–1309. [Google Scholar] [CrossRef] [PubMed]
- Radu, D.R.; Lai, C.-Y.; Jeftinija, K.; Rowe, E.W.; Jeftinija, S.; Lin, V.S.-Y. A Polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J. Am. Chem. Soc. 2004, 126, 13216–13217. [Google Scholar] [CrossRef] [PubMed]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed]
- Kukowska-Latallo, J.F.; Bielinska, A.U.; Johnson, J.; Spindle, R.; Tomalia, D.A.; Baker, J.R. Efficient transfer of genetic material into mammalian cells using starburst polyamidoamine dendrimers. Proc. Natl. Acad. Sci. USA 1996, 93, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef]
- Buhmann, M.T.; Stiefel, P.; Maniura-Weber, K.; Ren, Q. In vitro biofilm models for device-related infections. Trends Biotechnol. 2016, 34, 945–948. [Google Scholar] [CrossRef]
- Lin, G.-L.; McGinley, J.P.; Drysdale, S.B.; Pollard, A.J. Epidemiology and immune pathogenesis of viral sepsis. Front. Immunol. 2018, 9, 2147. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.C.B.B.; Correia, M.T.S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.-T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Ng, V. Risk of Disease Transmission With Bone Allograft. Orthopedics 2012, 35, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Gopalu, K.; Rangaraj, S.; Venkatachalam, R.; Kannan, N. Influence of ZrO2, SiO2, Al2O3 and TiO2 nanoparticles on maize seed germination under different growth conditions. IET Nanobiotechnol. 2016, 10, 171–177. [Google Scholar]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Mozumder, M.S.; Zhu, J.; Perinpanayagam, H. Titania-polymeric powder coatings with nano-topography support enhanced human mesenchymal cell responses. J. Biomed. Mater. Res. Part A 2012, 100, 2695–2709. [Google Scholar] [CrossRef]
- Trentler, T.J.; Denler, T.E.; Bertone, J.F.; Agrawal, A.; Colvin, V.L. Synthesis of TiO2 nanocrystals by nonhydrolytic solution-based reactions. J. Am. Chem. Soc. 1999, 121, 1613–1614. [Google Scholar] [CrossRef]
- Hussian, H.A.R.A.; Hassan, M.A.M.; Agool, I.R. Synthesis of titanium dioxide (TiO2) nanofiber and nanotube using different chemical method. Optik 2016, 127, 2996–2999. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Ashkarran, A.A.; Fakhari, M.; Hamidinezhad, H.; Haddadi, H.; Nourani, M.R. TiO2 nanoparticles immobilized on carbon nanotubes for enhanced visible-light photo-induced activity. J. Mater. Res. Technol. 2015, 4, 126–132. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Müller, L.; Kunze, J.; Müller, F.; Greil, P.; Virtanen, S.; Schmuki, P. Hydroxyapatite growth on anodic TiO2 nanotubes. J. Biomed. Mater. Res. Part A 2006, 77, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Inzunza, D.; Covarrubias, C.; Von Marttens, A.; Leighton, Y.; Carvajal, J.C.; Valenzuela, F.; Diaz-Dosque, M.; Méndez, N.; Martínez, C.; Pino, A.M.; et al. Synthesis of nanostructured porous silica coatings on titanium and their cell adhesive and osteogenic differentiation properties. J. Biomed. Mater. Res. Part A 2013, 102, 37–48. [Google Scholar] [CrossRef]
- Assad, M.; Chernyshov, A.; Leroux, M.; Rivard, C. A new porous titanium-nickel alloy: Part 1. Cytotoxicity and genotoxicity evaluation. Biomed. Mater. Eng. 2002, 12, 225–237. [Google Scholar]
- Gu, Y.W.; Khor, K.; Pan, D.; Cheang, P. Activity of plasma sprayed yttria stabilized zirconia reinforced hydroxyapatite/Ti–6Al–4V composite coatings in simulated body fluid. Biomaterials 2004, 25, 3177–3185. [Google Scholar] [CrossRef]
- Maschhoff, P.M.; Geilich, B.M.; Webster, T.J. Greater fibroblast proliferation on an ultrasonicated ZnO/PVC nanocomposite material. Int. J. Nanomed. 2014, 9, 257–263. [Google Scholar]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of carbon nanotubes in bone tissue regeneration and engineering: Superiority, concerns, current advancements, and prospects. Nanomaterials 2019, 9, 1501. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Du, P.; Van, S.Y.; Suhaeri, M.; Hwang, M.P.; Lee, K.; Kwideok, P. Fibronectin-tethered graphene oxide as an artificial matrix for osteogenesis. Biomed. Mater. 2014, 9, 65003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lu, X.; Zanden, Z.; Liu, J. The promising application of graphene oxide as coating materials in orthopedic implants: Preparation, characterization and cell behavior. Biomed. Mater. 2015, 10, 15019. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the antimicrobial properties of carbon nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Nishida, E.; Miyaji, H.; Takita, H.; Kanayama, I.; Tsuji, M.; Akasaka, T.; Sugaya, T.; Sakagami, R.; Kawanami, M. Graphene oxide coating facilitates the bioactivity of scaffold material for tissue engineering. Jpn. J. Appl. Phys. 2014, 53, 06JD04. [Google Scholar] [CrossRef]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-based nanomaterials for tissue engineering in the dental field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Vera-Sánchez, M.; Aznar-Cervantes, S.; Jover, E.; García-Bernal, D.; Oñate-Sánchez, R.; Hernández-Romero, D.; Moraleda, J.M.; Collado-González, M.; Rodríguez-Lozano, F.J.; Cenis, J. Silk-fibroin and graphene oxide composites promote human periodontal ligament stem cell spontaneous differentiation into osteo/cementoblast-like cells. Stem Cells Dev. 2016, 25, 1742–1754. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]
- Farack, J.; Wolf-Brandstetter, C.; Glorius, S.; Nies, B.; Standke, G.; Quadbeck, P.; Worch, H.; Scharnweber, D. The effect of perfusion culture on proliferation and differentiation of human mesenchymal stem cells on biocorrodible bone replacement material. Mater. Sci. Eng. B 2011, 176, 1767–1772. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, T.; Kong, J.-L. In situ monitoring of intracellular controlled drug release from mesoporous silica nanoparticles coated with pH-responsive charge-reversal polymer. ACS Appl. Mater. Interfaces 2014, 6, 17446–17453. [Google Scholar] [CrossRef]
- Kim, S.-S.; Ahn, K.-M.; Park, M.S.; Lee, J.-H.; Choi, C.Y.; Kim, B.-S. A poly(lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. J. Biomed. Mater. Res. Part A 2007, 80, 206–215. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, S.R.; Yoon, I.-S. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Philp, D.; Hoffman, M.P. Role of the extracellular matrix in morphogenesis. Curr. Opin. Biotechnol. 2003, 14, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for tissue engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Webster, T.J.; Sato, M.; Webster, T.J. Nanobiotechnology: Implications for the future of nanotechnology in orthopedic applications Nanobiotechnology: Implications for the future of nanotechnology in orthopedic applications. Expert Rev. Med. Devices 2004, 1, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Demais, V.; Audrain, C.; Mabilleau, G.; Chappard, D.; Baslé, M.F. Diversity of bone matrix adhesion proteins modulates osteoblast attachment and organization of actin cytoskeleton. Morphologie 2014, 98, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Webster, T.J. Increased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticles. Acta Biomater. 2011, 7, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Ma, P.X. Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B Biointerfaces 2004, 39, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Tuzlakoglu, K.; Bolgen, N.; Salgado, A.J.; Gomes, M.E.; Piskin, E.; Reis, R.L. Nano- and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. J. Mater. Sci. Mater. Med. 2005, 16, 1099–1104. [Google Scholar] [CrossRef]
- Shin, M.; Yoshimoto, H.; Vacanti, J.P. In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng. 2004, 10, 33–41. [Google Scholar] [CrossRef]
- Li, W.J.; Tuli, R.; Huang, X.; Laquerriere, P.; Tuan, R.S. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials 2005, 26, 5158–5166. [Google Scholar] [CrossRef]
- Li, W.J.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 2005, 26, 599–609. [Google Scholar] [CrossRef]
- Woo, K.M.; Chen, V.J.; Ma, P.X. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J. Biomed. Mater. Res. 2003, 67, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.M.; Jun, J.H.; Chen, V.J.; Seo, J.; Baek, J.H.; Ryoo, H.M.; Kim, G.S.; Somerman, M.J.; Ma, P.X. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials 2007, 28, 335–343. [Google Scholar] [CrossRef]
- Bhattarai, N.; Edmondson, D.; Veiseh, O.; Matsen, F.A.; Zhang, M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials 2005, 26, 6176–6184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Xu, C.; Li, Y.; Gao, J.; Wang, W.; Liu, Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater. Chem. 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.; Ruoff, R. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef]
- Kim, S.-S.; Sun Park, M.; Gwak, S.-J.; Choi, C.; Kim, B.-S. Accelerated bonelike apatite growth on porous polymer/ceramic composite scaffolds in vitro. Tissue Eng. 2006, 12, 2997–3006. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Wei, B.; Yao, Q.; Guo, Y.; Mao, F.; Liu, S.; Xu, Y.; Wang, L. Three-dimensional polycaprolactone–hydroxyapatite scaffolds combined with bone marrow cells for cartilage tissue engineering. J. Biomater. Appl. 2015, 30, 160–170. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Cui, F.-Z.; Feng, Q.; Zhu, X.D.; Groot, K. Tissue response to nano-hydroxyapatite/collagen composite implants in marrow cavity. J. Biomed. Mat. Res. 1998, 42, 540–548. [Google Scholar] [CrossRef]
- Li, Z.; Yubao, L.; Aiping, Y.; Xuelin, P.; Xuejiang, W.; Xiang, Z. Preparation and in vitro investigation of chitosan/nano-hydroxyapatite composite used as bone substitute materials. J. Mater. Sci. Mater. Med. 2005, 16, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef]
- Srinivasan, M.; Rajabi, M.A.; Mousa, S. Chapter 3—Nanobiomaterials in cancer therapy. In Nanobiomaterials in Cancer Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 57–89. [Google Scholar]
- Hernandez, R.K.; Wade, S.W.; Reich, A.; Pirolli, M.; Liede, A.; Lyman, G.H. Incidence of bone metastases in patients with solid tumors: Analysis of oncology electronic medical records in the United States. BMC Cancer 2018, 18, 44. [Google Scholar] [CrossRef]
- Adjei, I.M.; Temples, M.N.; Brown, S.B.; Sharma, B. Targeted nanomedicine to treat bone metastasis. Pharmaceutics 2018, 10, 205. [Google Scholar] [CrossRef]
- Serafini, A.N. Therapy of metastatic bone pain. J. Nucl. Med. 2001, 42, 895–906. [Google Scholar]
- Auffinger, B.; Morshed, R.; Tobias, A.; Cheng, Y.; Ahmed, A.U.; Lesniak, M.S. Drug-loaded nanoparticle systems and adult stem cells: A potential marriage for the treatment of malignant glioma? Oncotarget 2013, 4, 378–396. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
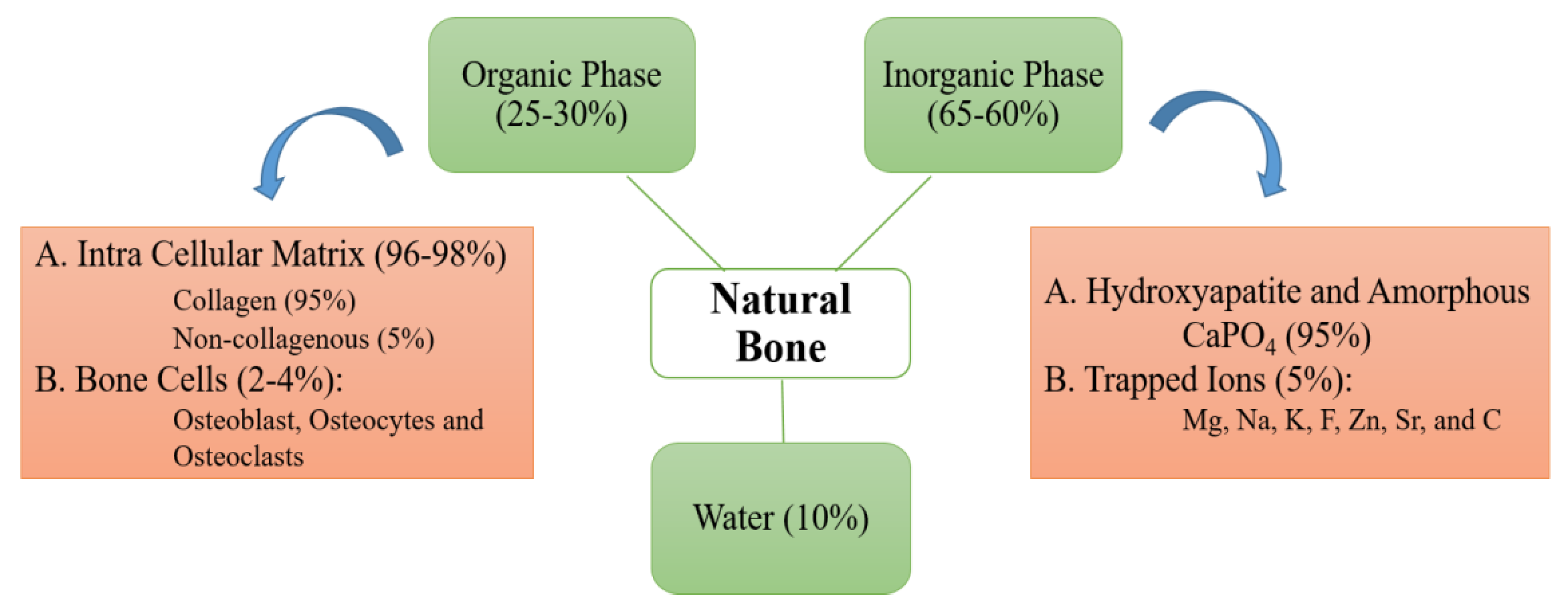
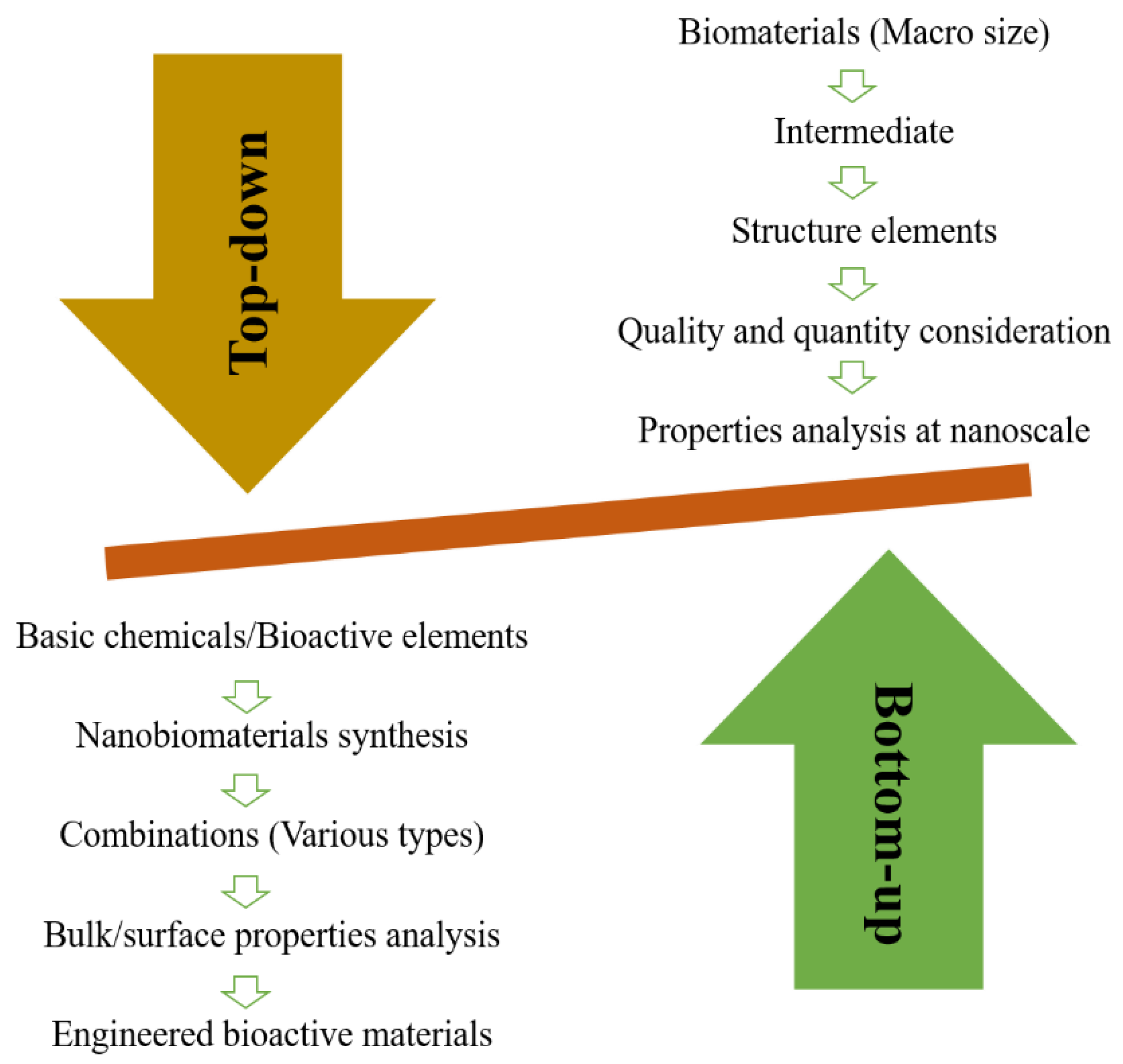
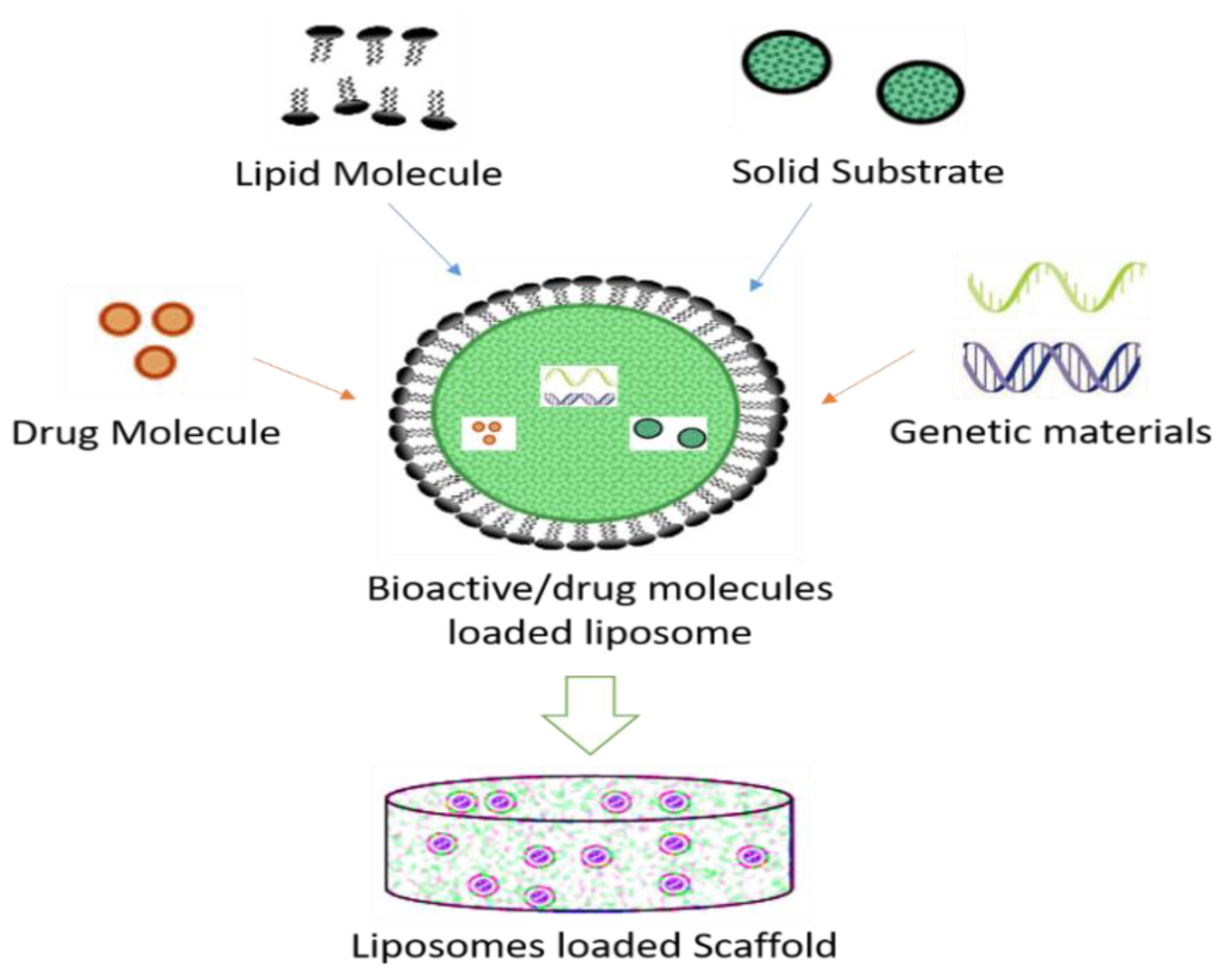
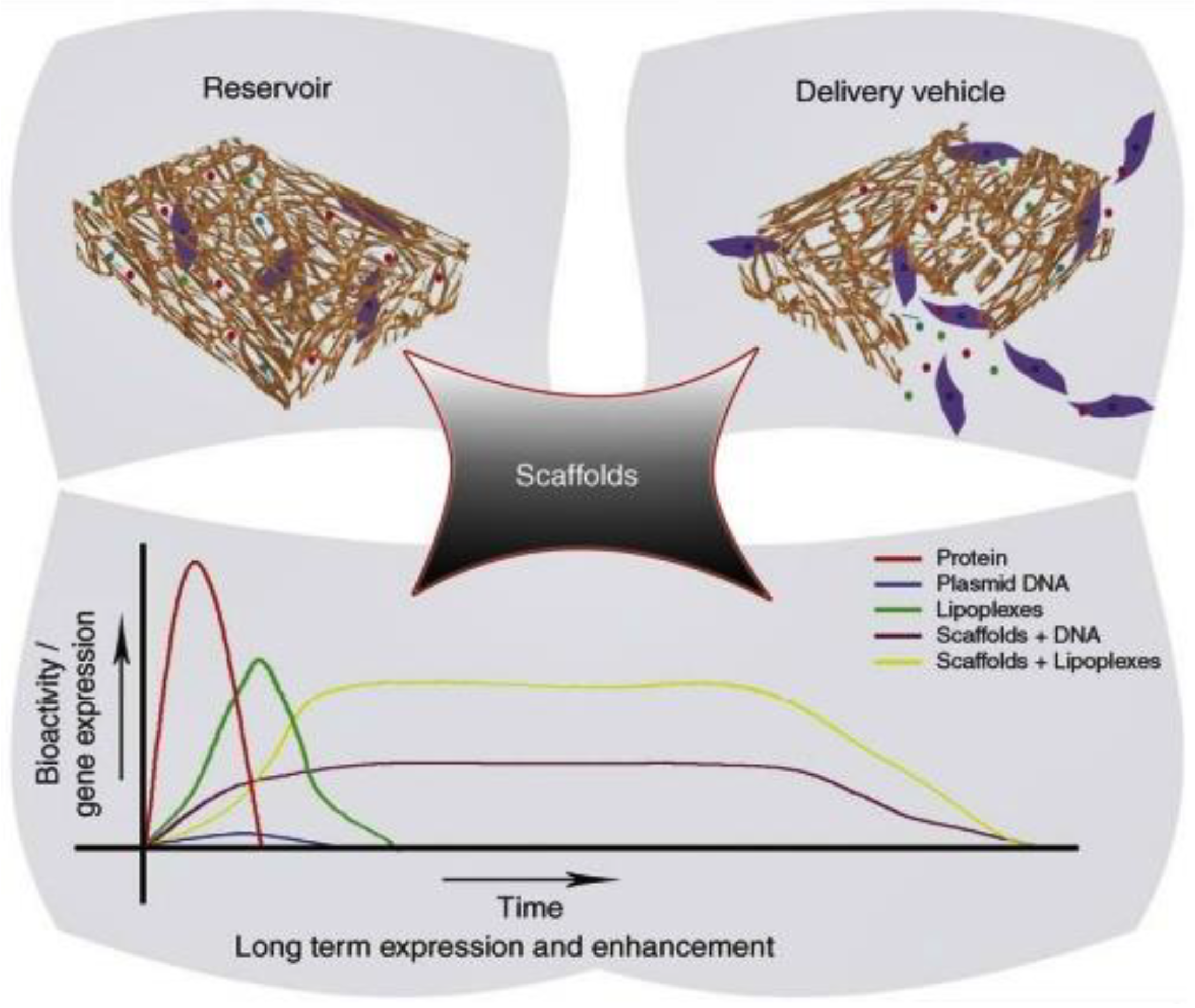

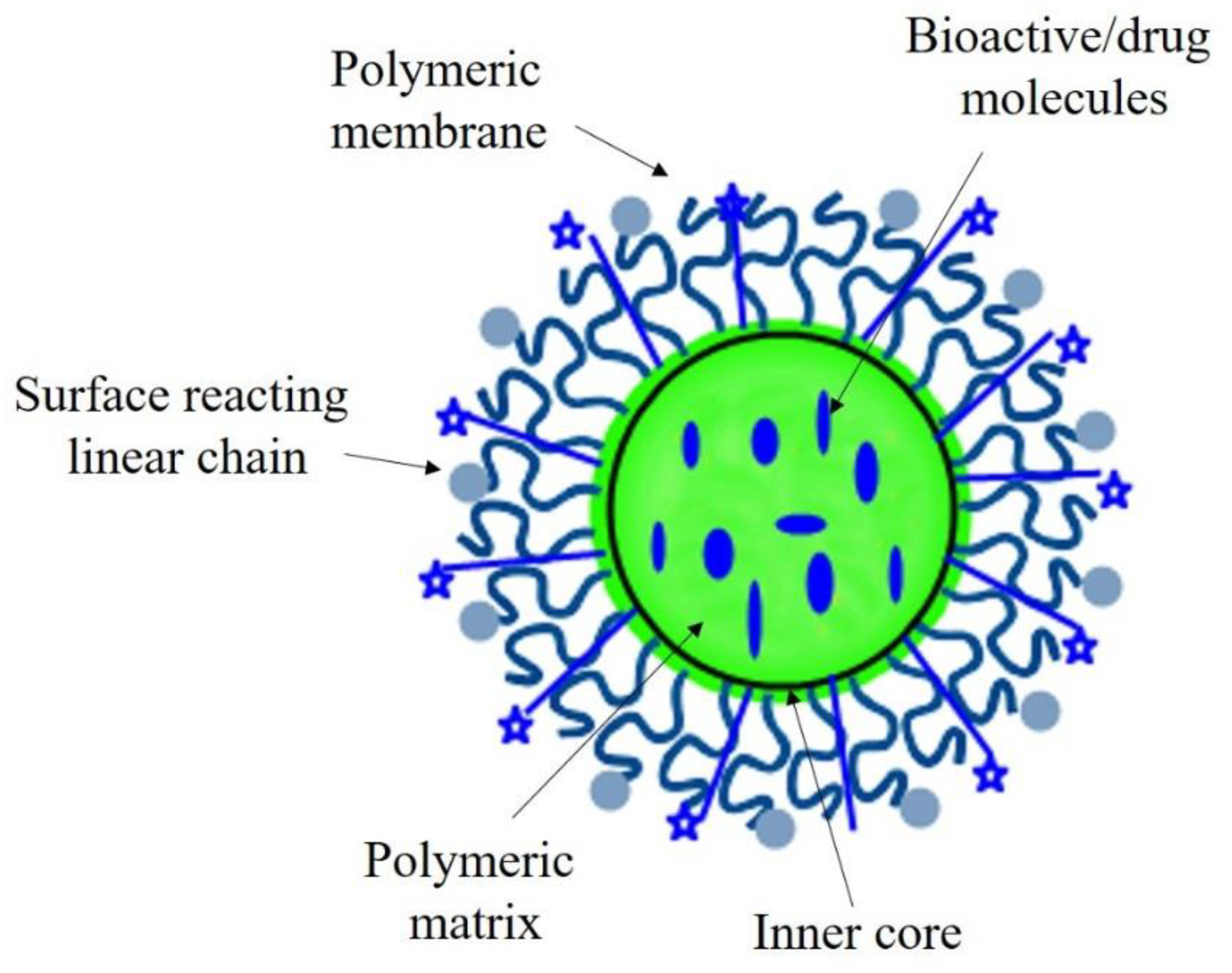
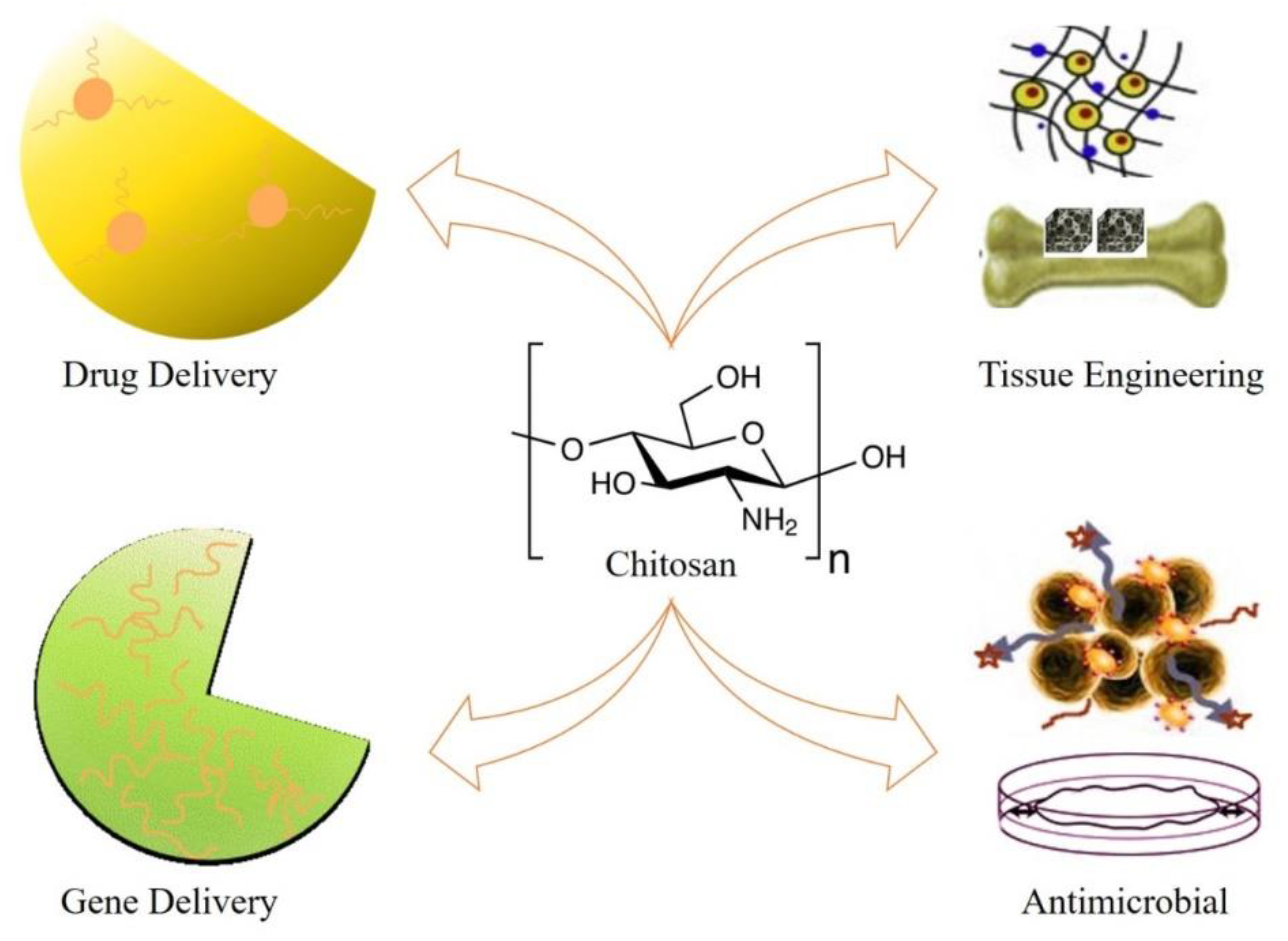
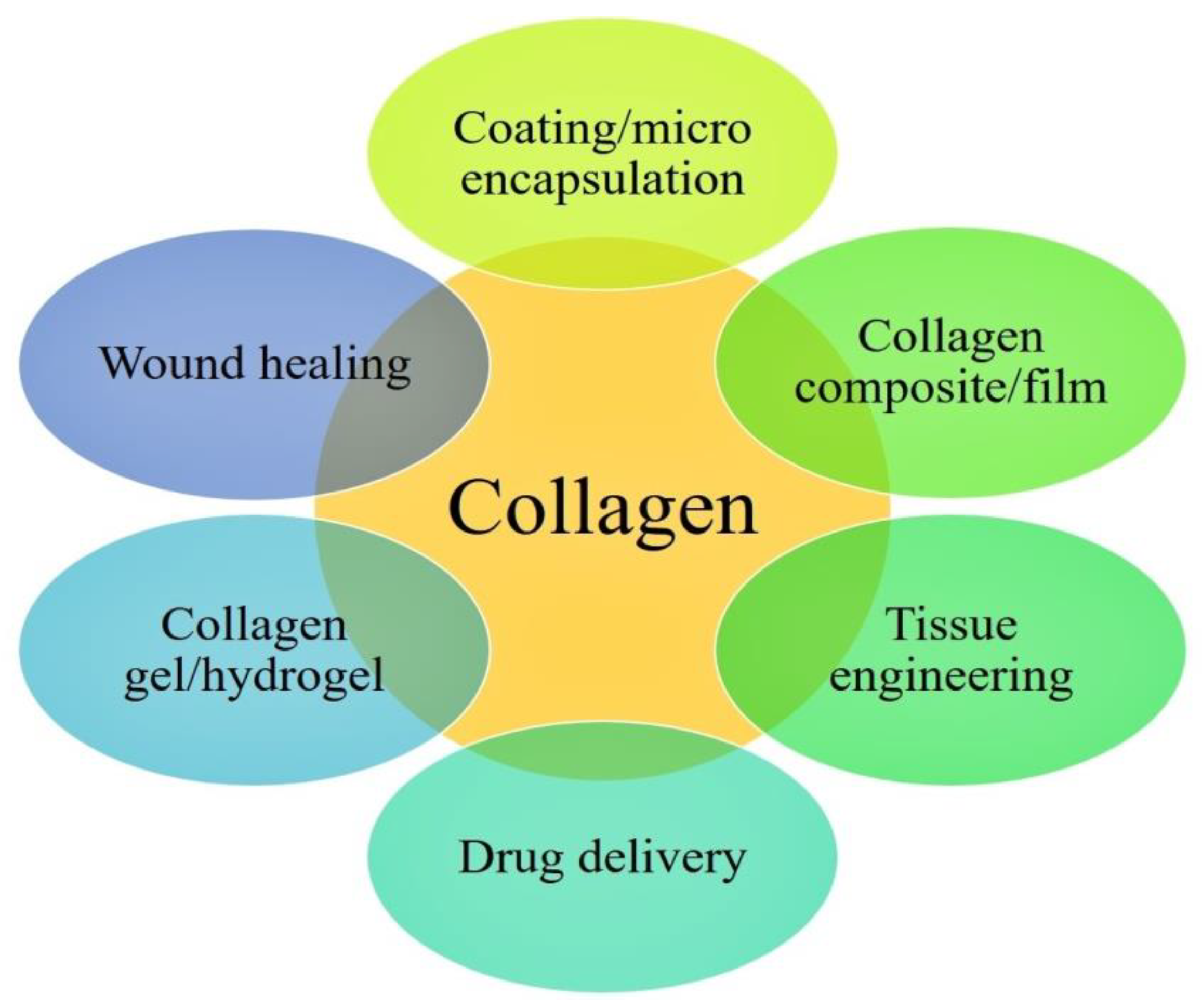
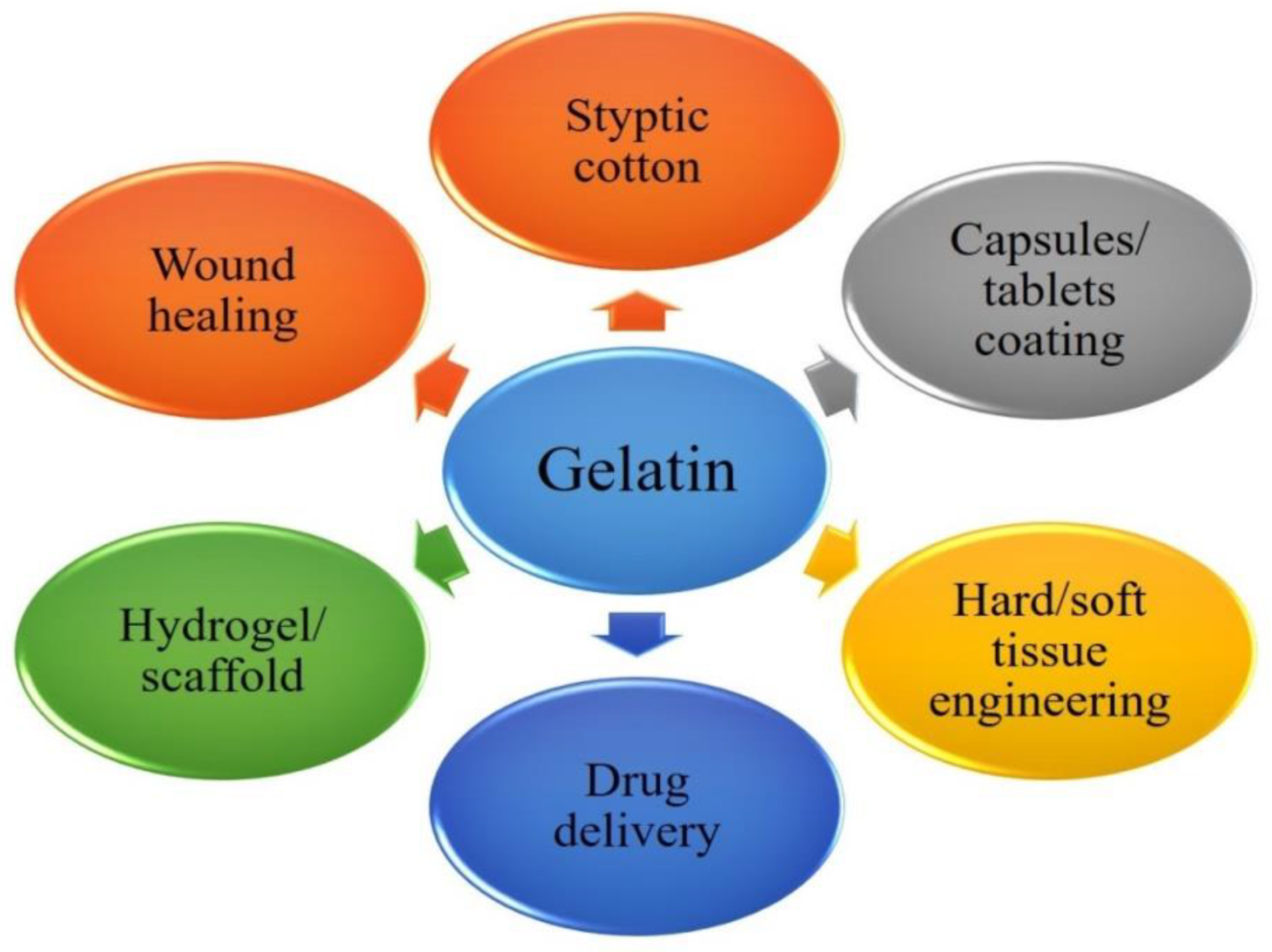
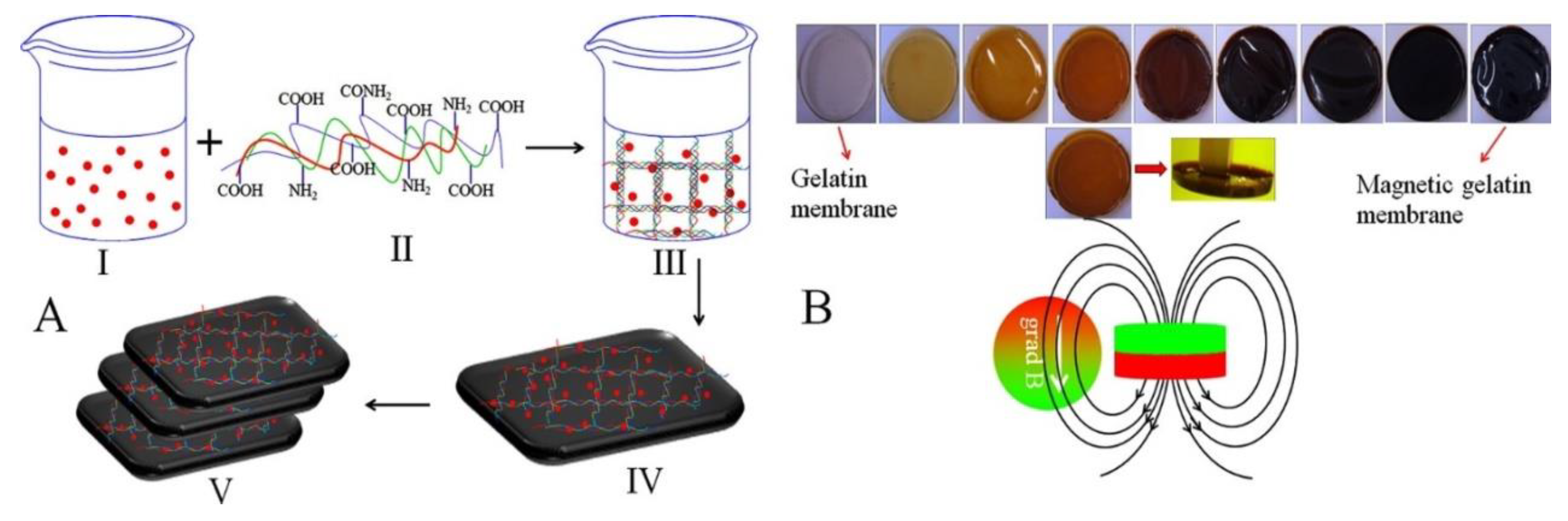
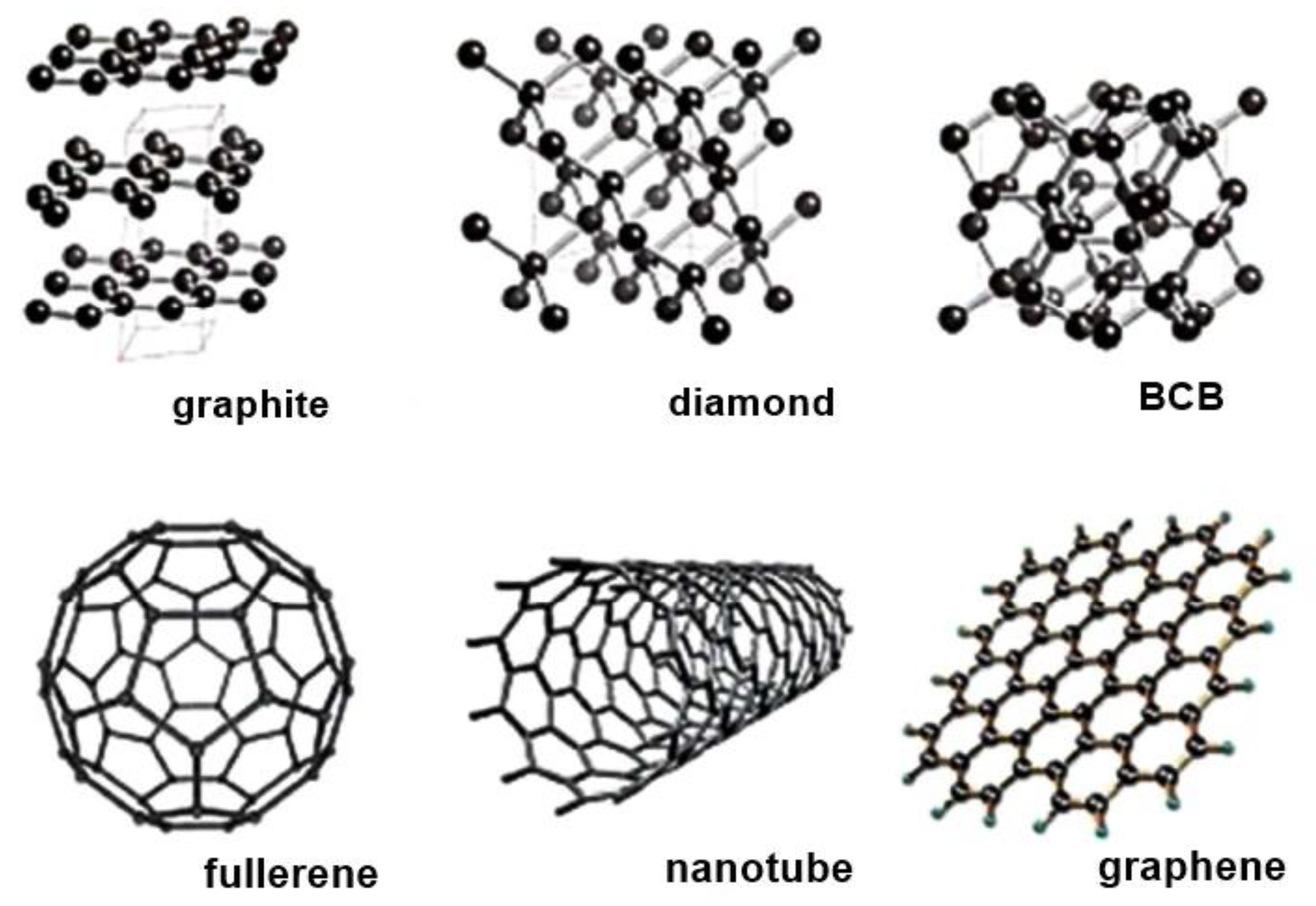
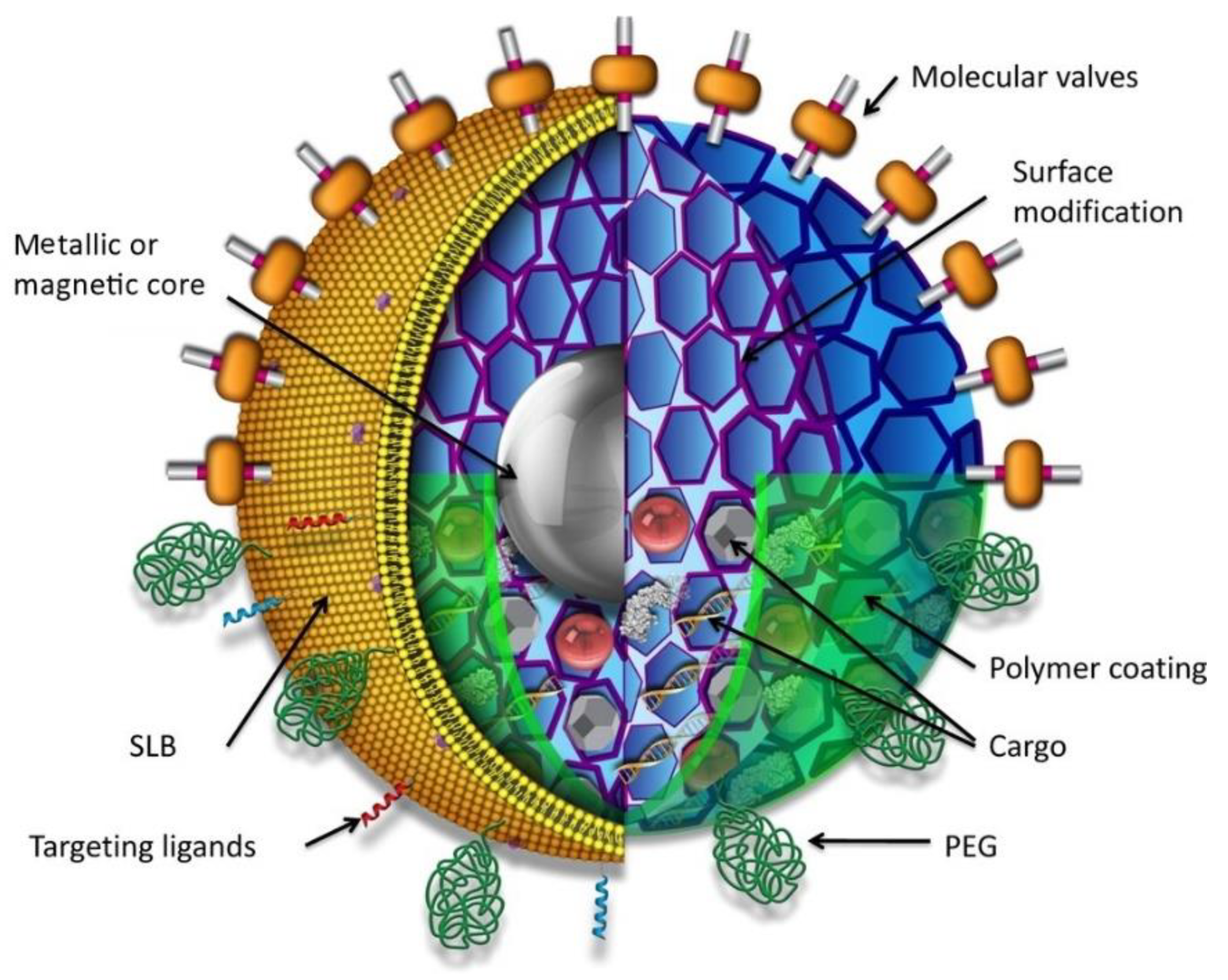
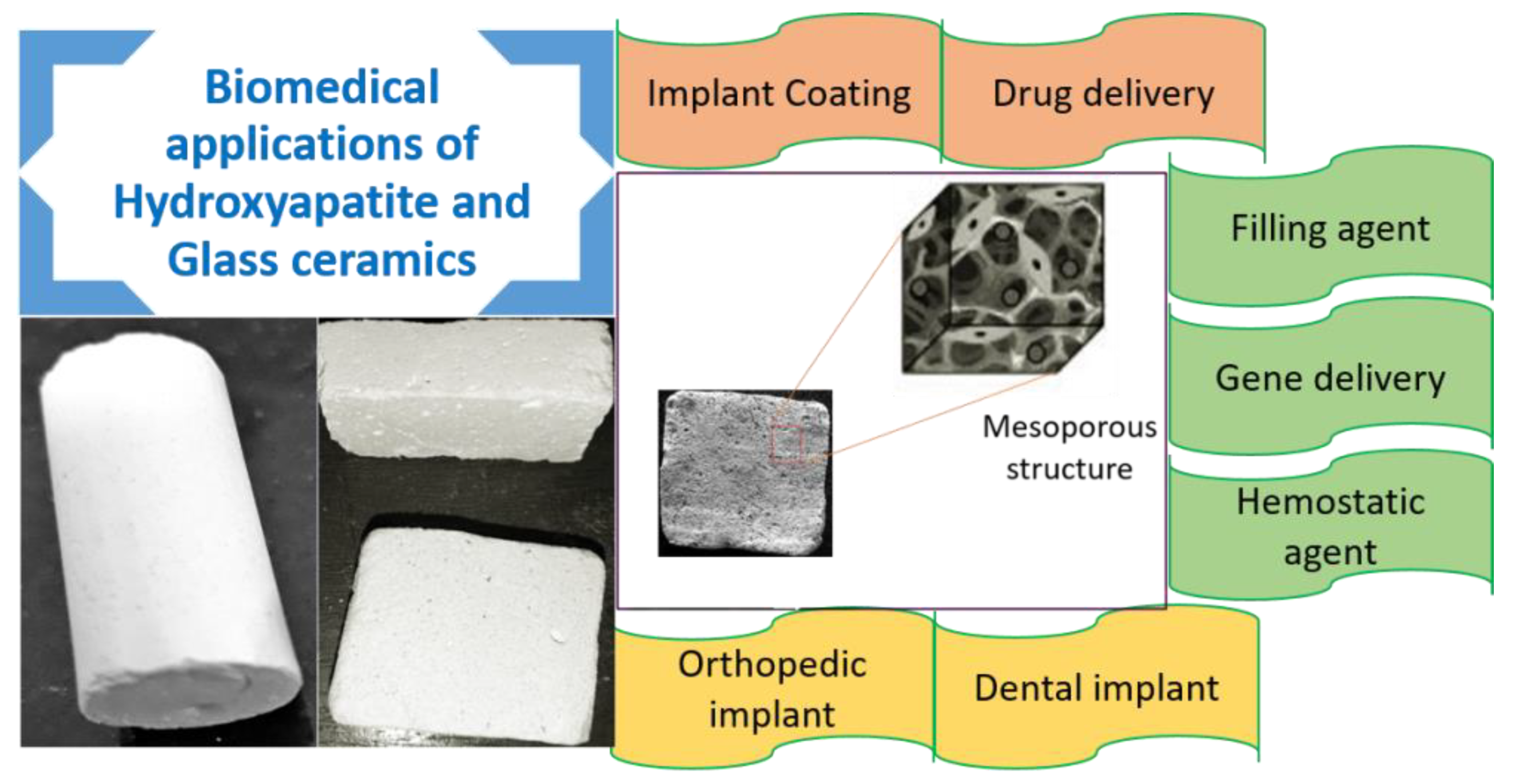
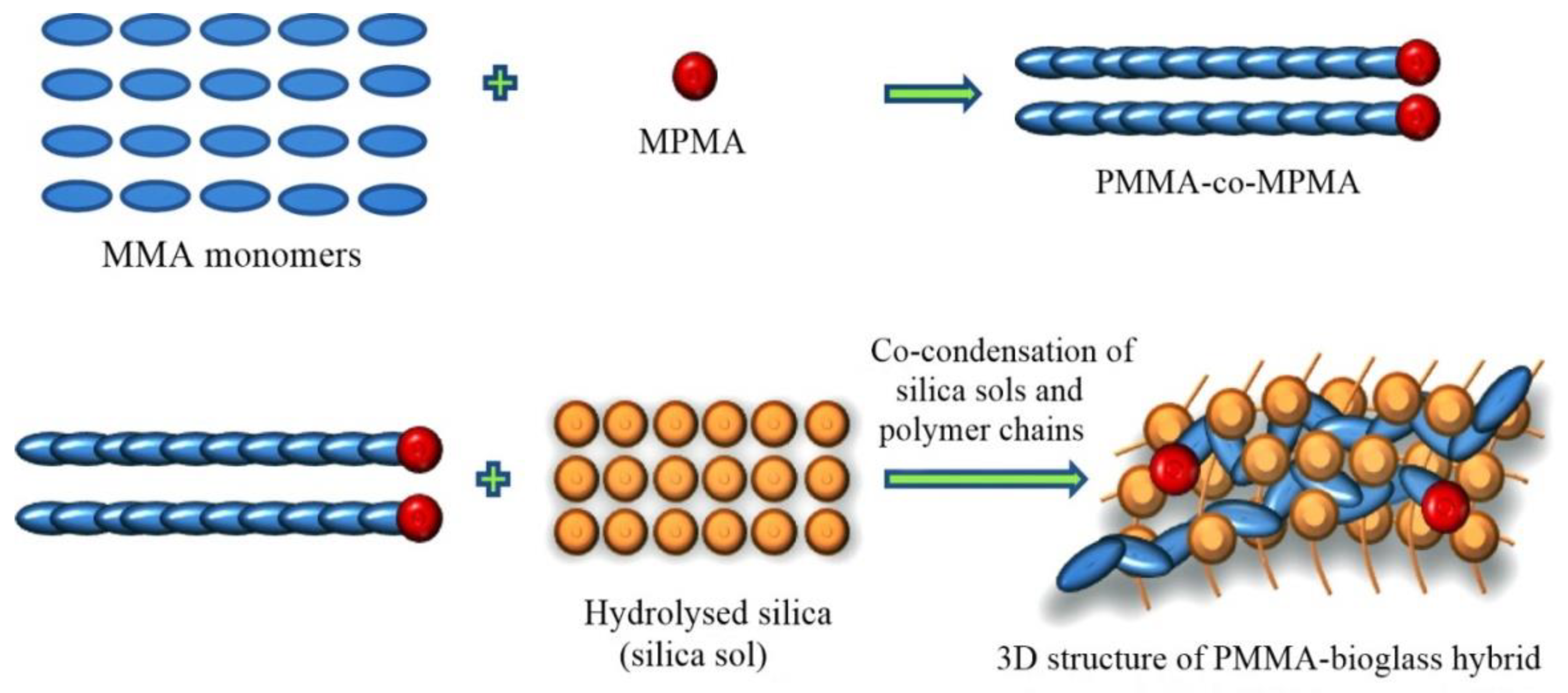

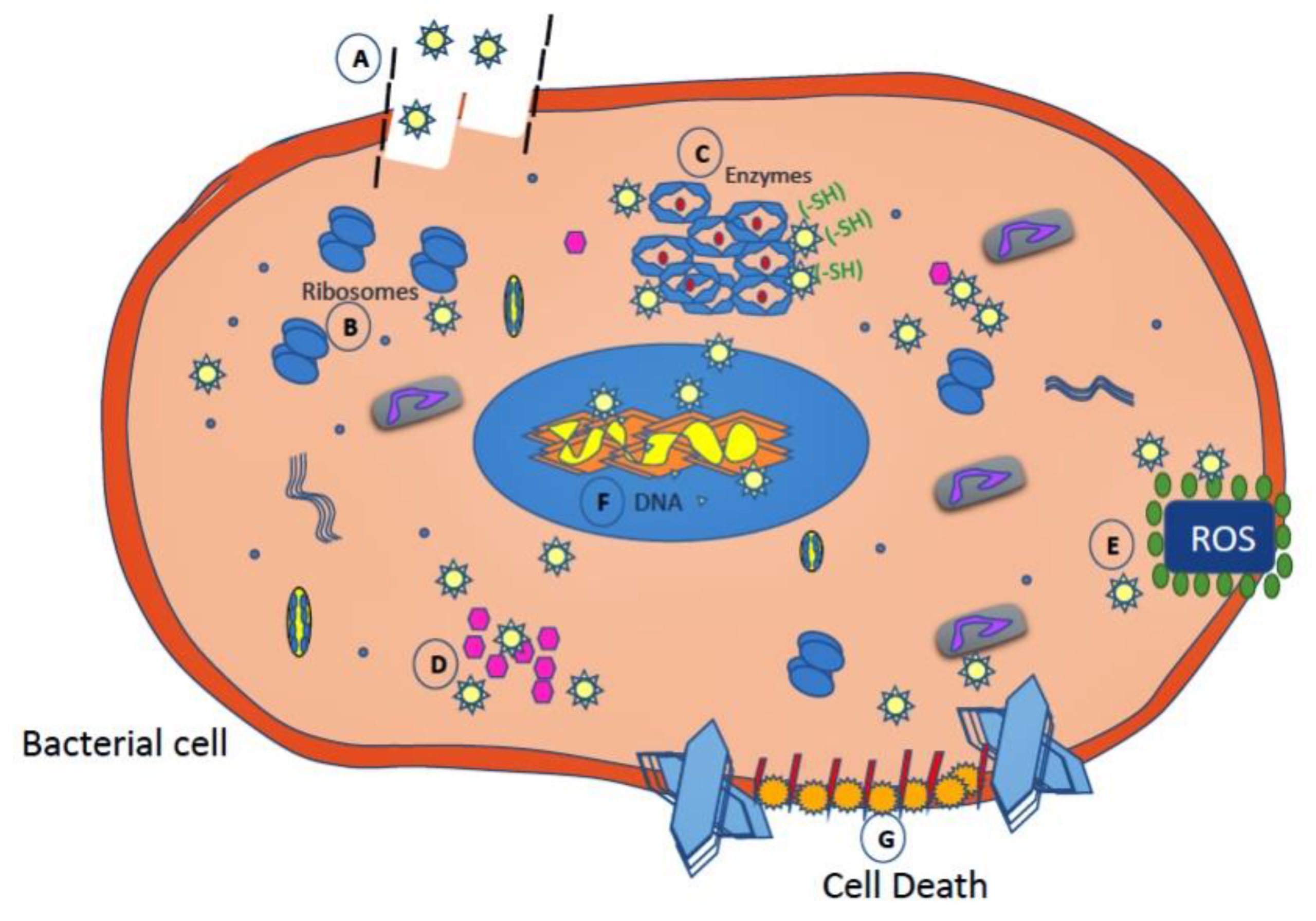
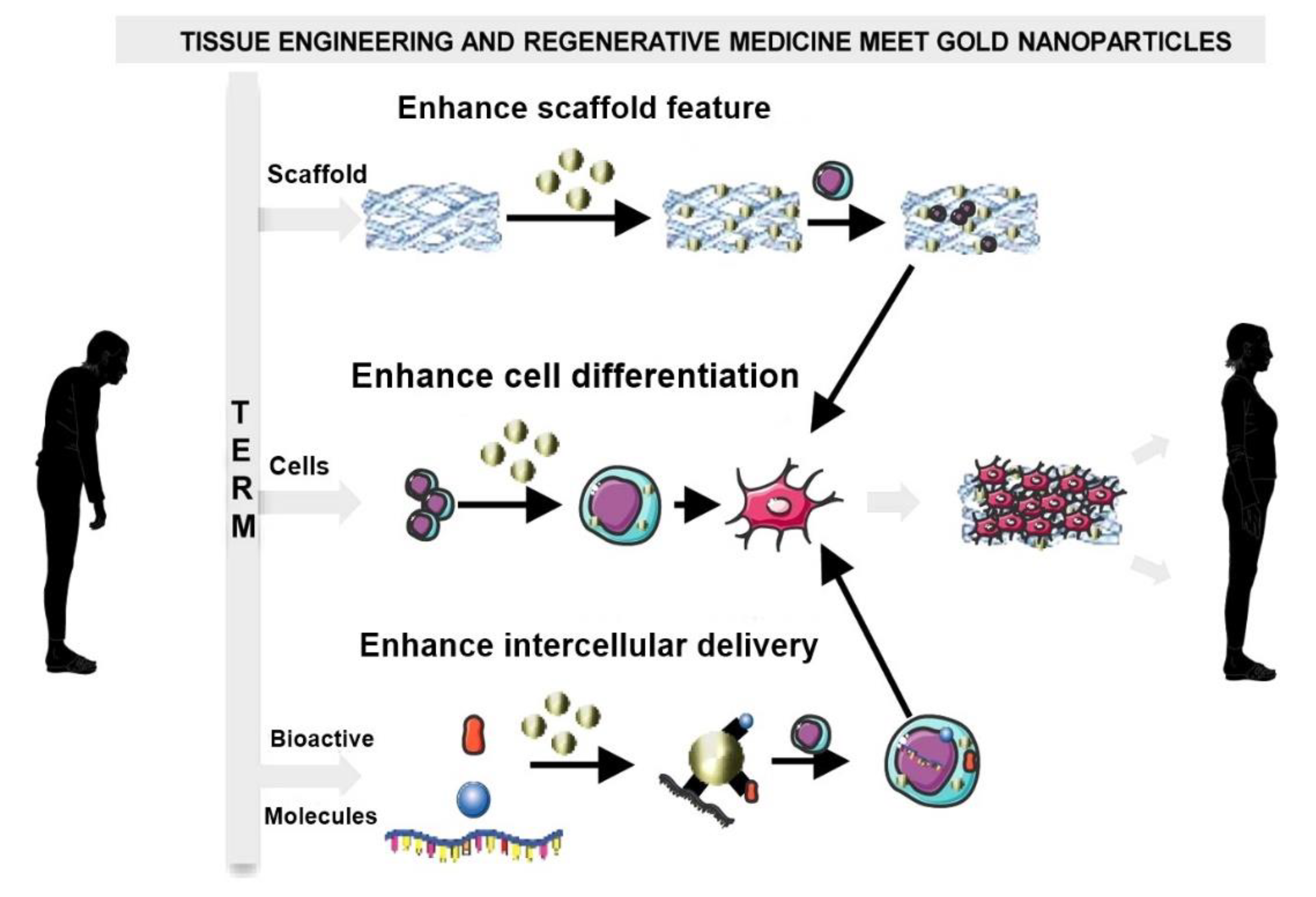
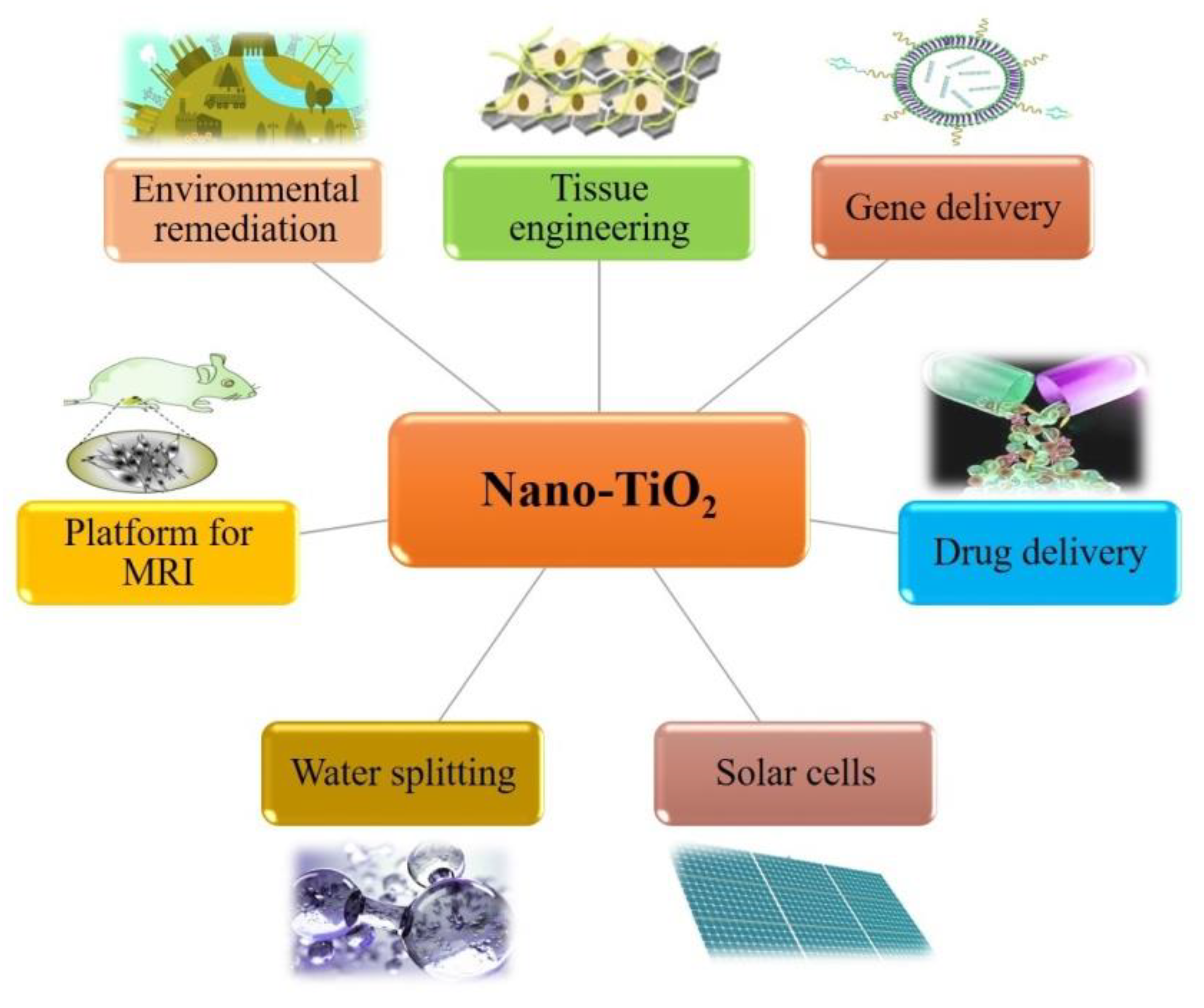
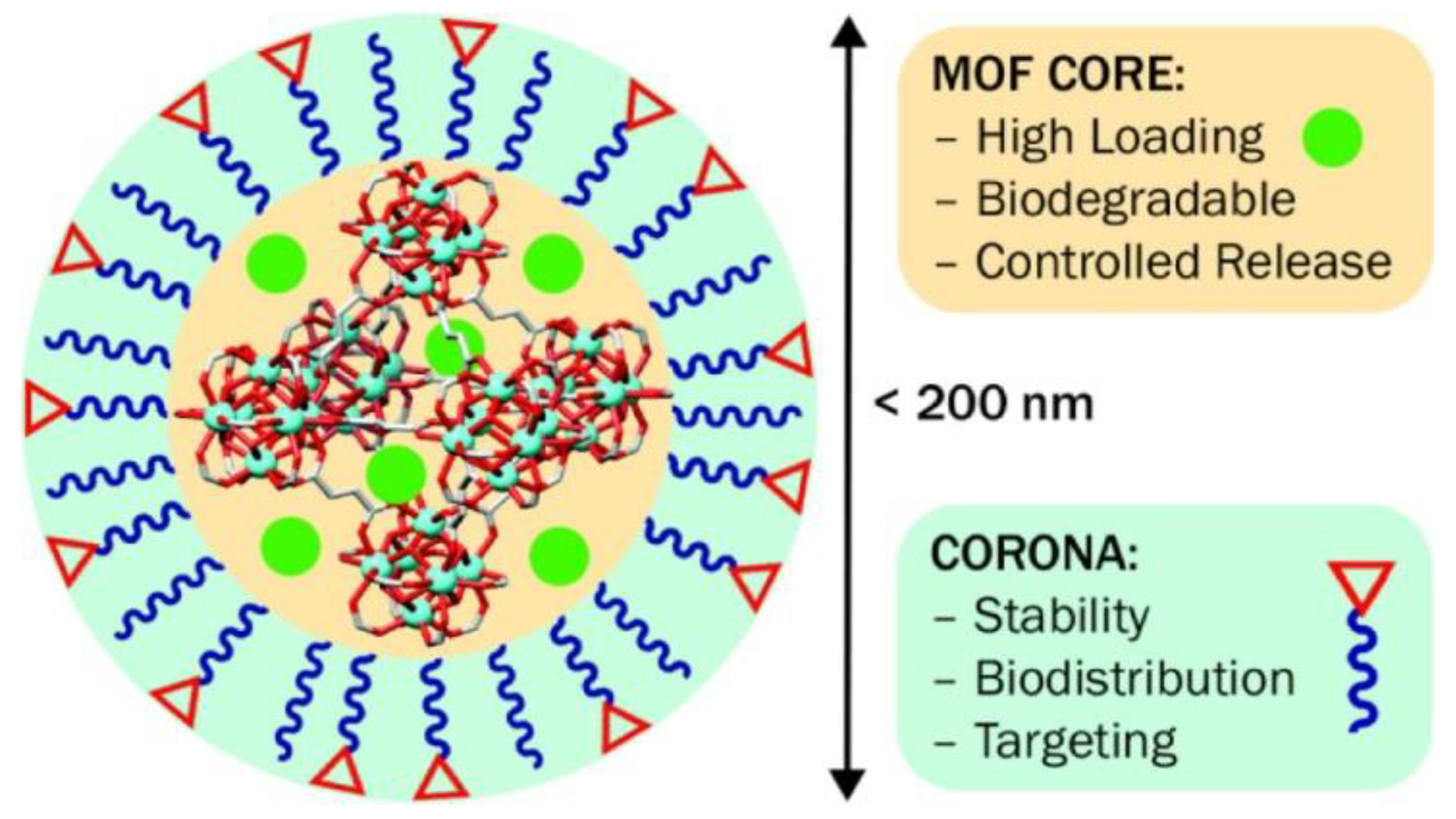
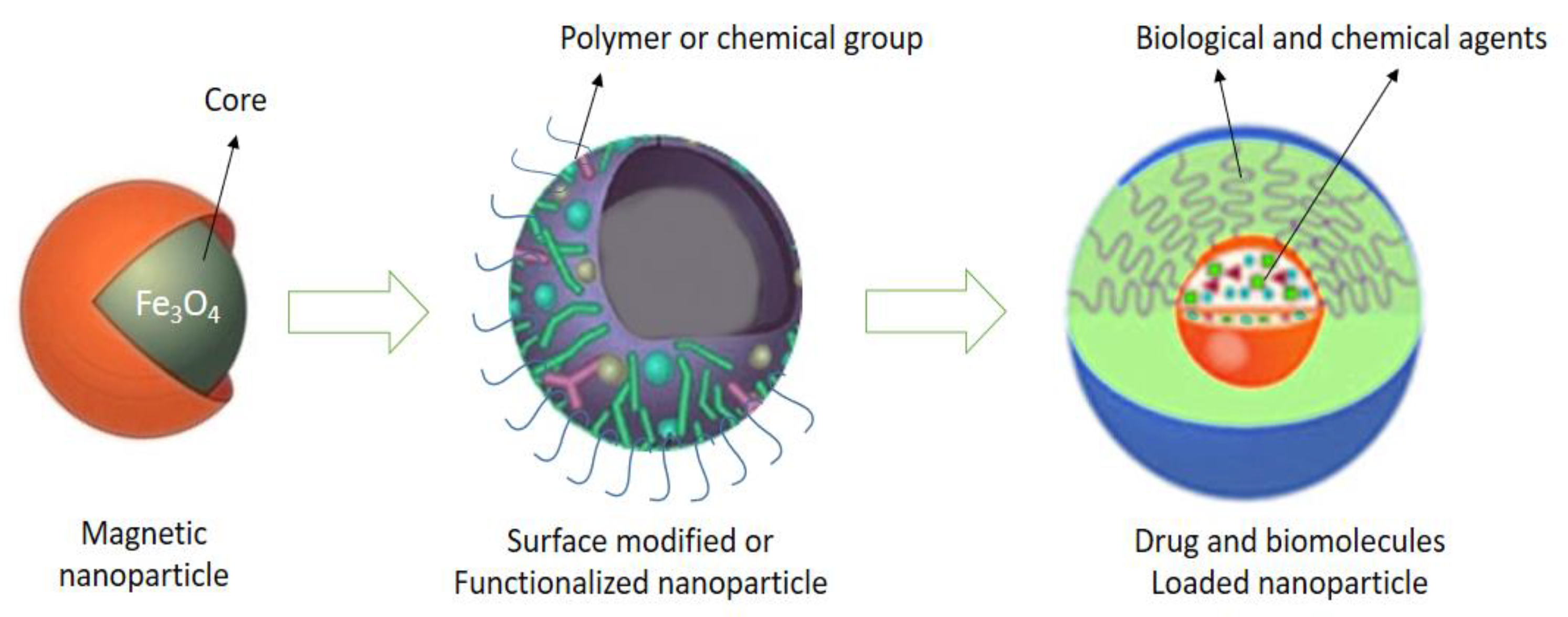
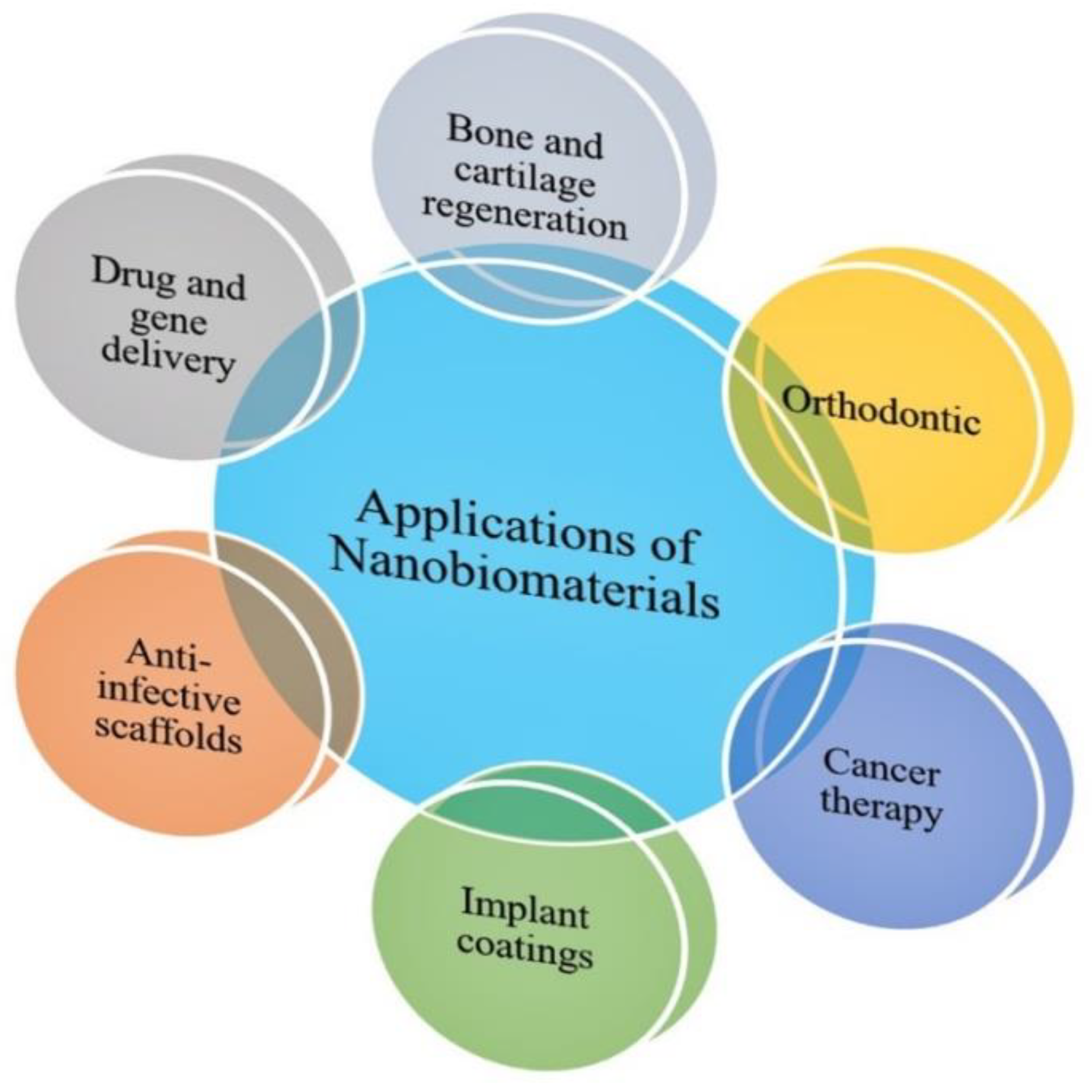
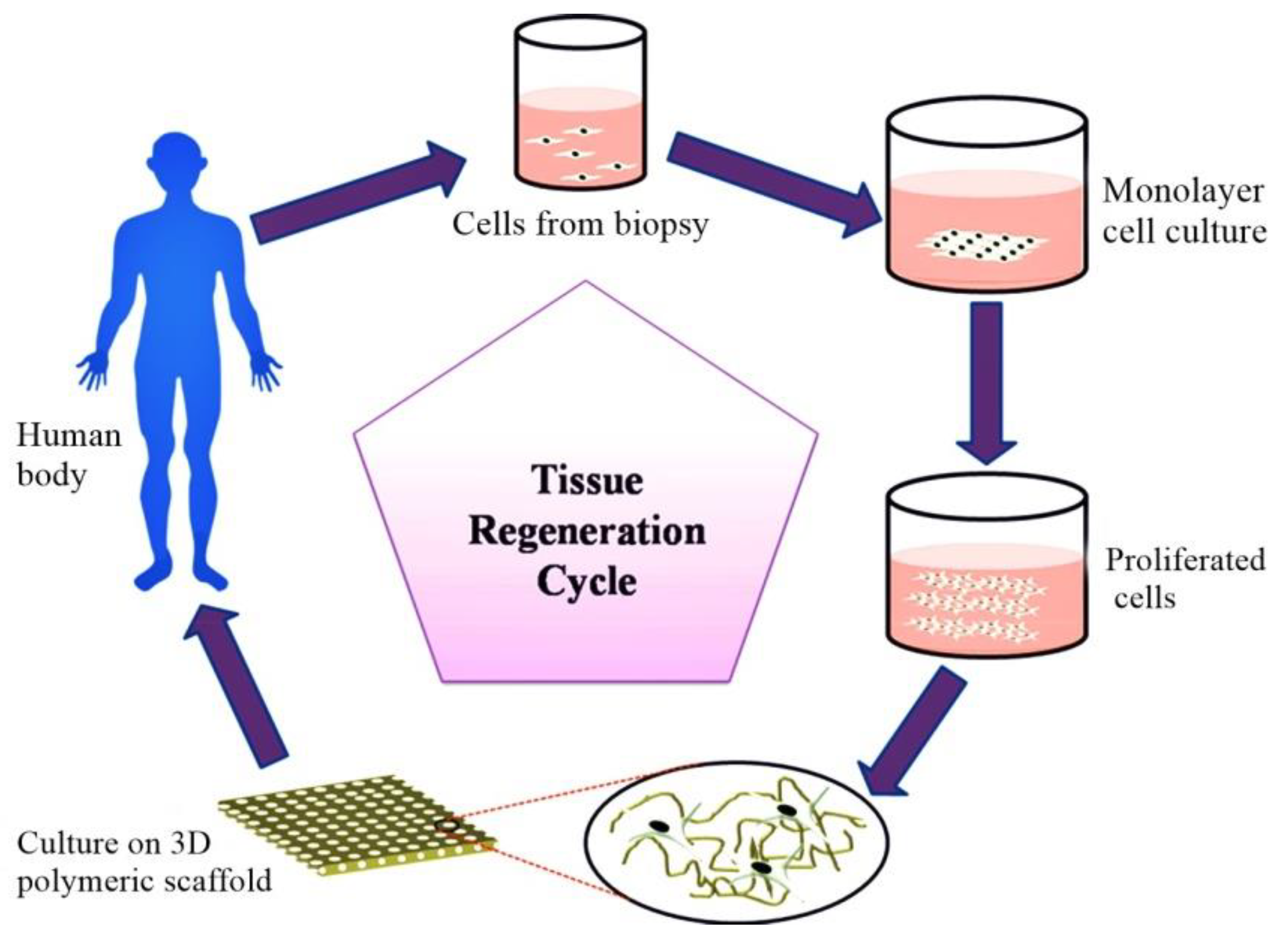
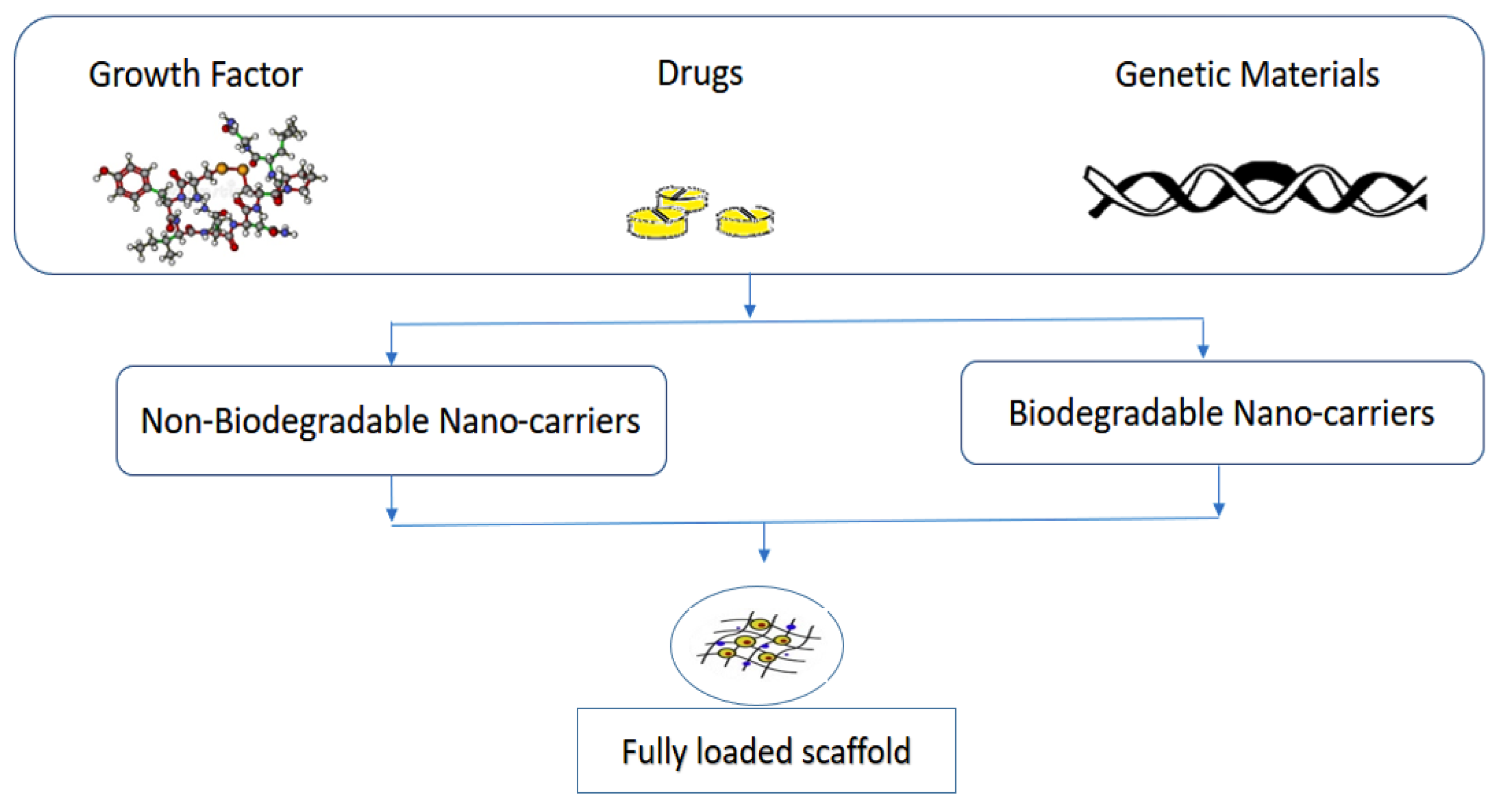
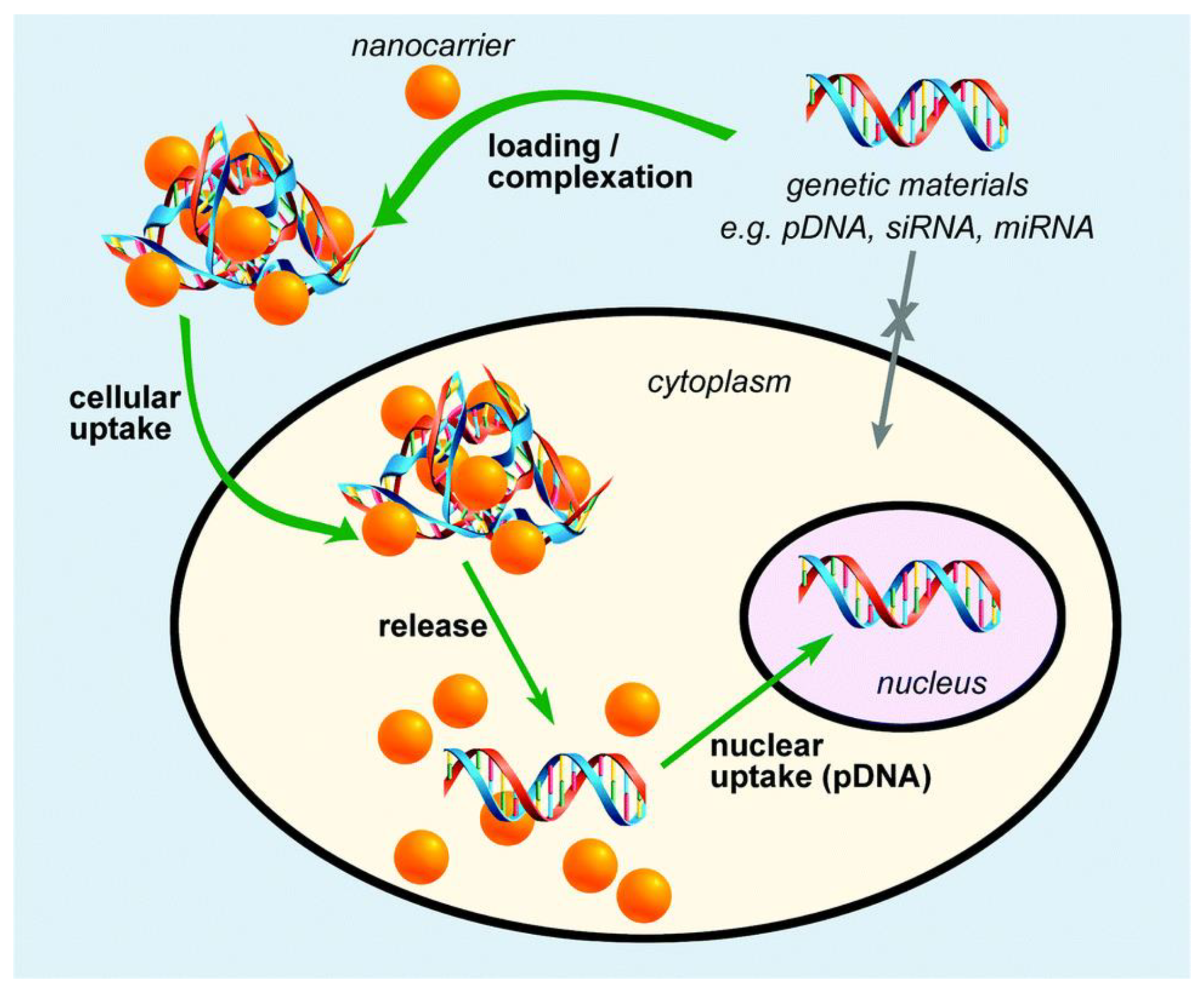

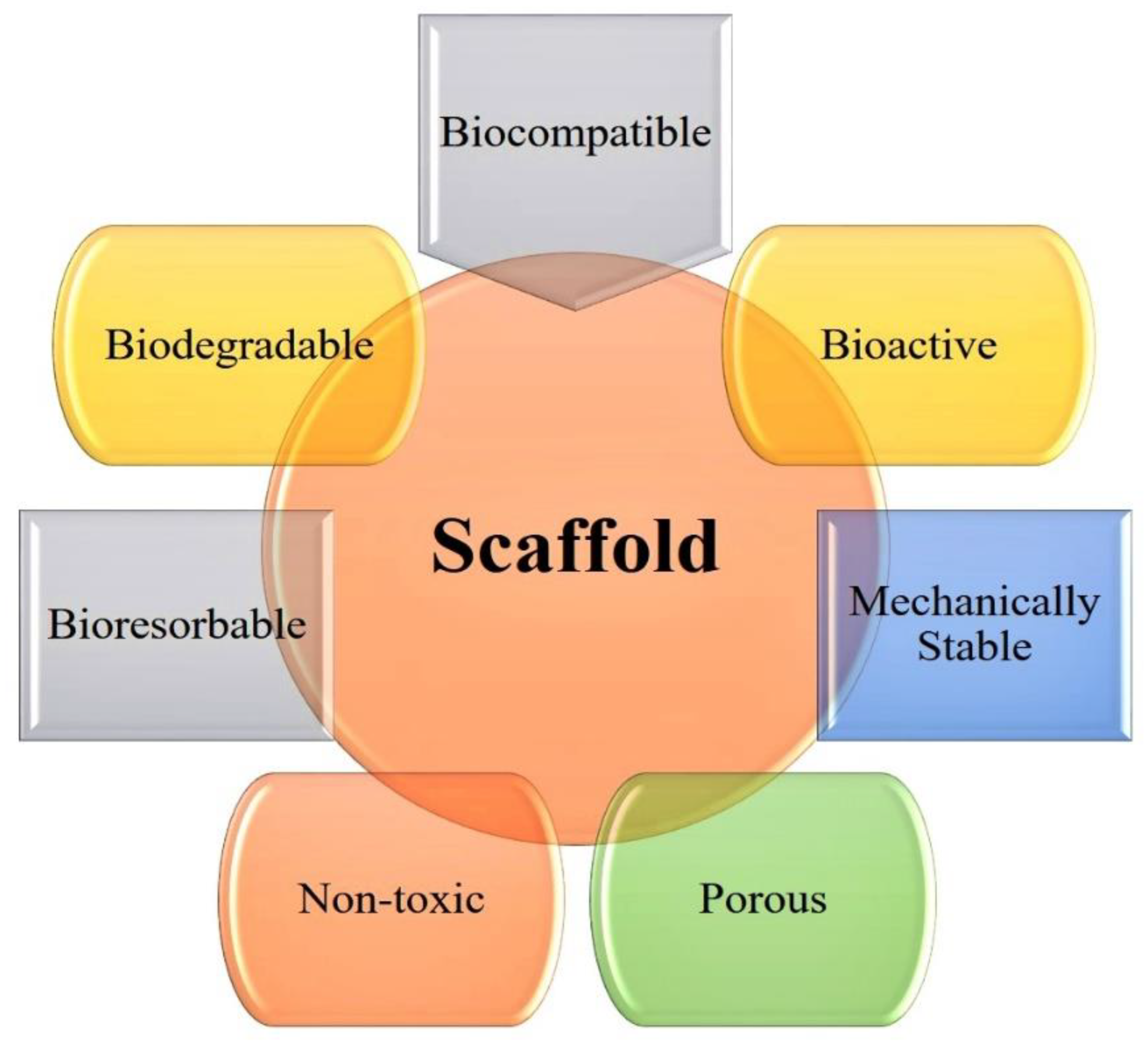
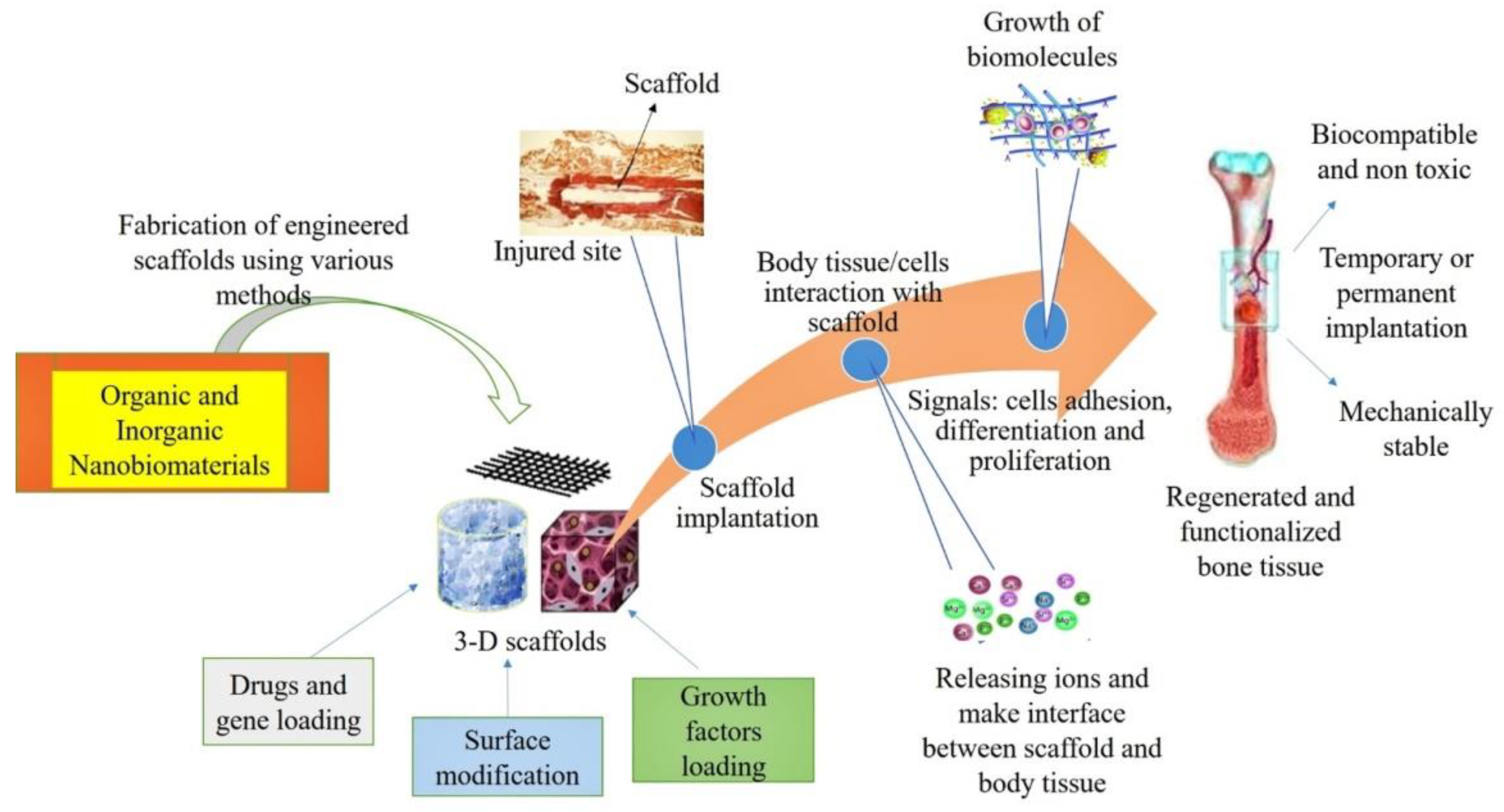
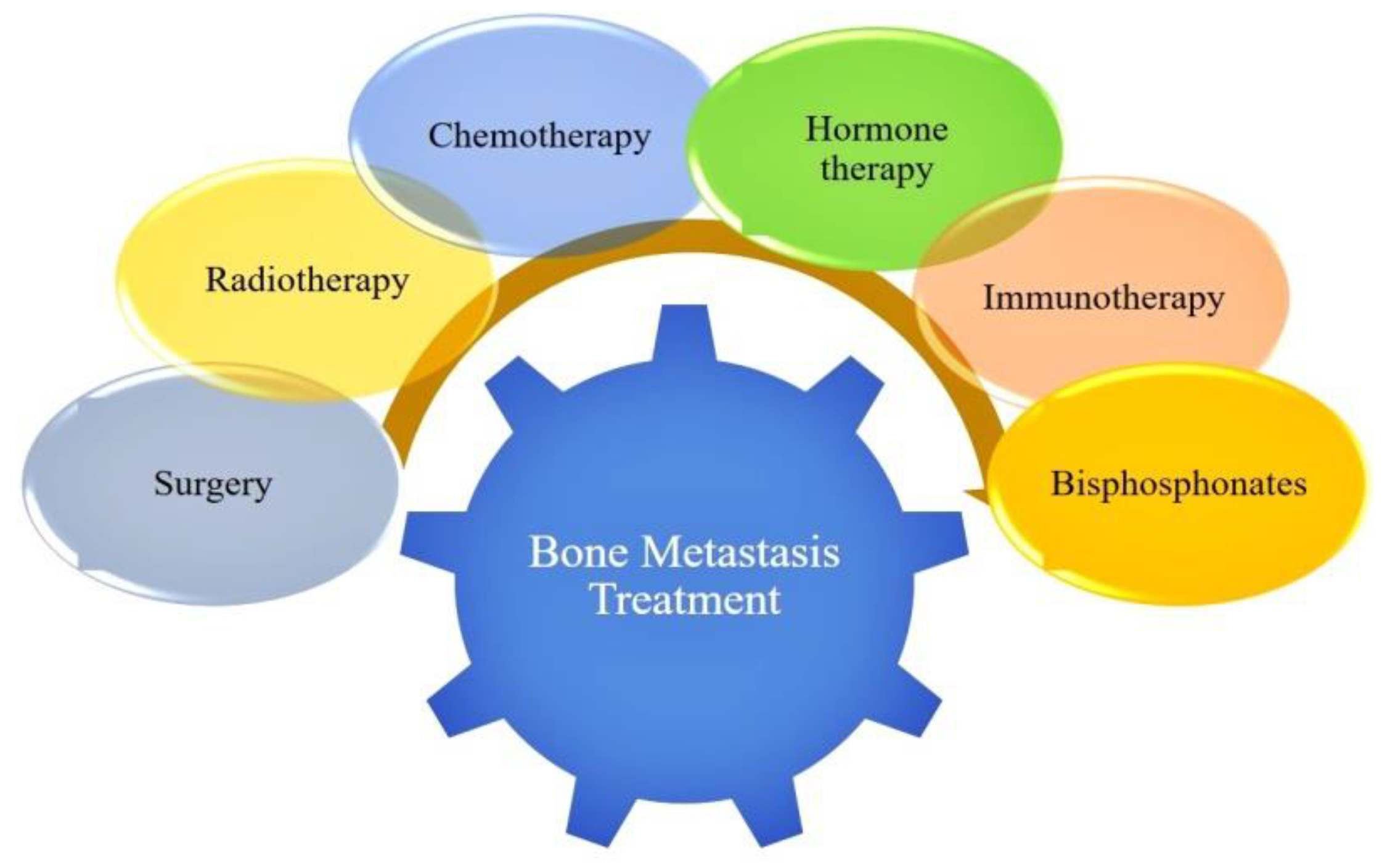
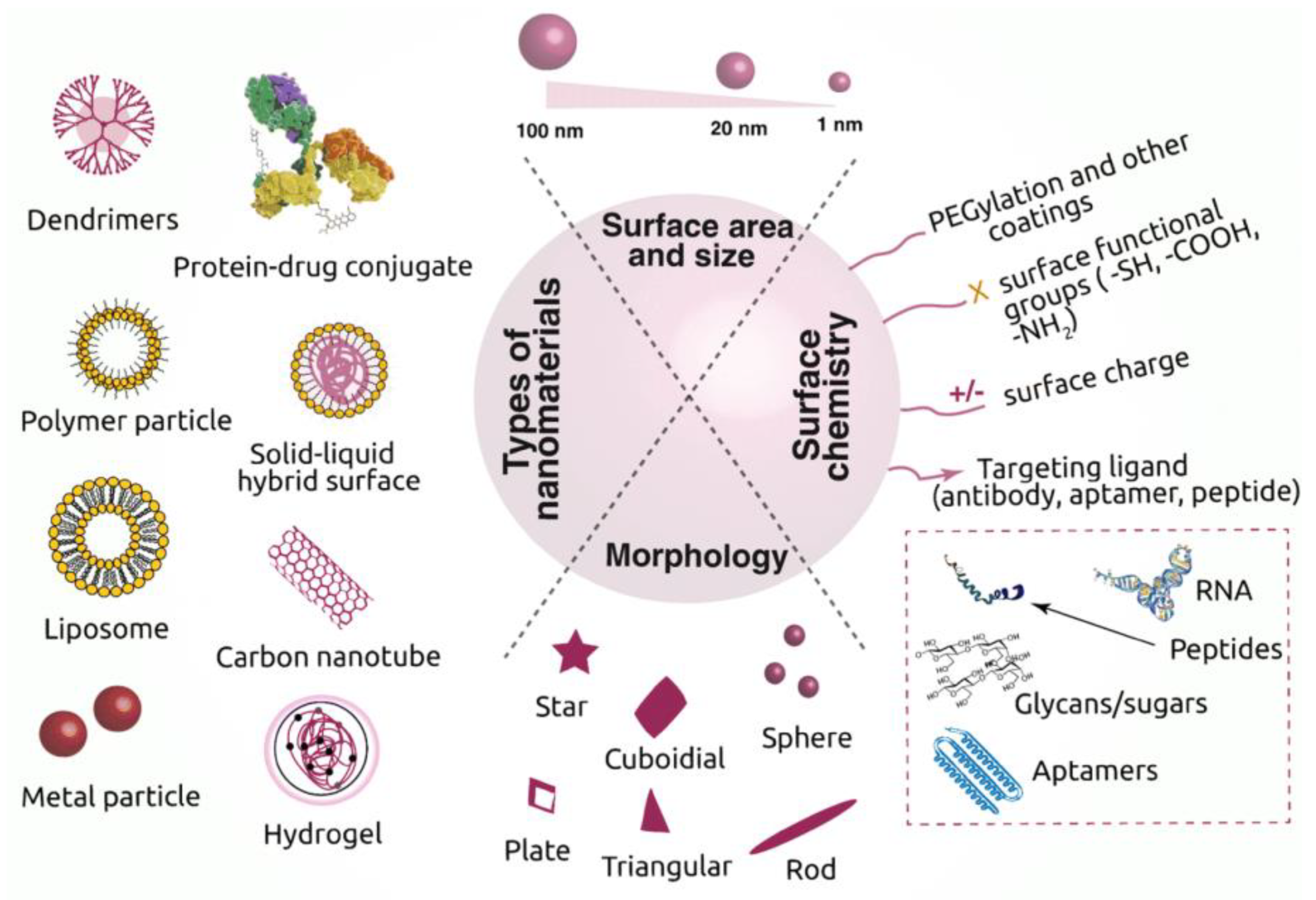
| Types of Nanomaterials | Size (nm) | Applications | References |
|---|---|---|---|
| Lipid | <100 | Nanocarriers for anticancer drug doxorubicin Osteoblastic bone formation Osteoporosis treatment | [42,43,44,45] |
| Liposome | >25 | High encapsulation of hydrophilic drug (drug delivery) Growth factor delivery Therapeutic gene delivery Used as a template | [46,47,48] |
| Dendrimers | <10 | Multidrug delivery system | [46,49,50] |
| Chitosan | 20–200 | Nano/microparticles or fiber-based scaffolds Drug delivery Support chondrocyte adhesion Implant coating | [51,52,53,54,55] |
| Collagen | – | Drug Delivery Scaffolds | [56] |
| Gelatin | <200 | Bone scaffold systems formation Drug-loaded gelatin nanoparticles (DGNPs) Promote cell growth | [24,57,58] |
| Poly(lactic-co-glycolic acid) PLGA | 100–250 | Drug delivery Scaffold system Nanostructured Film Enhanced cell attachment and growth | [59,60,61,62] |
| Carbon Nanotubes | 20–100 | Drug delivery Biosensing Mechanically improved scaffold fabrication Enhanced rat brain neuron response | [63,64,65,66] |
| Types of Nanomaterials | Size (nm) | Applications | References |
|---|---|---|---|
| Nano Silica | 10–100 | Composite-based scaffold Bio-imaging Drug delivery Enhanced osteogenic differentiation | [225,226,227] |
| Gold nanostructured materials | 5–50 | Bioinorganic hybrid nanostructures Thin film scaffold Bio-imaging | [228,229,230] |
| Magnetic nanomaterials and nanoparticles | 10 | Drug and gene delivery Improved cell adhesion Cell tracking | [21,231,232] |
| Bioactive Glasses | 20–500 | Improved scaffolds performance Drug and gene delivery | [233,234] |
| Silver nanoparticles | 1–100 | Tissue repair and regeneration Antibacterial action | [235,236,237] |
| Nanostructured Titanium | <300 | Nano tubular anodized titanium Improved mechanical properties Enhanced chondrocyte adhesion Support osteoblast adhesion and proliferation Orthopedic coating | [238,239,240,241,242,243] |
| Hydroxyapatite | 20–80 ~200–500 | Enhanced osteoblast functioning Increase bone apatite formation | [244,245] |
| Zirconia nanoparticles | <100 | Enhanced osteointegration Antibacterial implants formation | [246,247] |
| Alumina nanoparticles | <80 | Enhanced bone cells adhesion and proliferation Calcium phase deposition | [245,248] |
| Copper nanoparticles | <100 | Antimicrobial implant fabrication | [25] |
| Types | Ca | P | Ca/P | Total Inorganic (%) | Total Organic (%) | Water (%) |
|---|---|---|---|---|---|---|
| HA | 39.6 | 18.5 | 1.67 | 100 | - | - |
| Dentine | 35.1 | 16.9 | 1.61 | 70 | 20 | 10 |
| Bone | 34.8 | 15.2 | 1.71 | 65 | 25 | 10 |
| Enamel | 36.5 | 17.1 | 1.63 | 97 | 1.5 | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.; Saini, M.; Dehiya, B.S.; Sindhu, A.; Kumar, V.; Kumar, R.; Lamberti, L.; Pruncu, C.I.; Thakur, R. Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications. Nanomaterials 2020, 10, 2019. https://doi.org/10.3390/nano10102019
Kumar P, Saini M, Dehiya BS, Sindhu A, Kumar V, Kumar R, Lamberti L, Pruncu CI, Thakur R. Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications. Nanomaterials. 2020; 10(10):2019. https://doi.org/10.3390/nano10102019
Chicago/Turabian StyleKumar, Pawan, Meenu Saini, Brijnandan S. Dehiya, Anil Sindhu, Vinod Kumar, Ravinder Kumar, Luciano Lamberti, Catalin I. Pruncu, and Rajesh Thakur. 2020. "Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications" Nanomaterials 10, no. 10: 2019. https://doi.org/10.3390/nano10102019
APA StyleKumar, P., Saini, M., Dehiya, B. S., Sindhu, A., Kumar, V., Kumar, R., Lamberti, L., Pruncu, C. I., & Thakur, R. (2020). Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications. Nanomaterials, 10(10), 2019. https://doi.org/10.3390/nano10102019








