Effects of Humic Acids on the Ecotoxicity of Fe3O4 Nanoparticles and Fe-Ions: Impact of Oxidation and Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Modification of Fe3O4 Nanoparticles
2.1.1. Synthesis of Fe3O4 and Fe3O4/HA Nanoparticles
2.1.2. Simulation of Oxidation of Fe3O4/HA NPs in Mild and Harsh Conditions
2.1.3. Preparation of Complexes of HA with Fe2+ and/or Fe3+
2.2. Characterisation of the Microstructure of Magnetic NPs (MNPs)
2.3. Characterisation of the Magnetic Properties of Magnetite NPs (MNPs)
2.4. Analysis of the Surface Charge and Hydrodynamic Diameter of Magnetic NPs (MNPs)
2.5. Ecotoxicity Testing of Magnetic NPs (MNPs) and Fe-Ions
2.5.1. Paramecium caudatum Acute Toxicity Test
2.5.2. Sinapis alba L. Acute Toxicity Test
2.6. Statistical Analysis
3. Results and Discussion
3.1. Microstructure of Magnetite Nanoparticles (MNPs)
3.2. Evaluation of the Magnetic Properties of the Studied MNPs
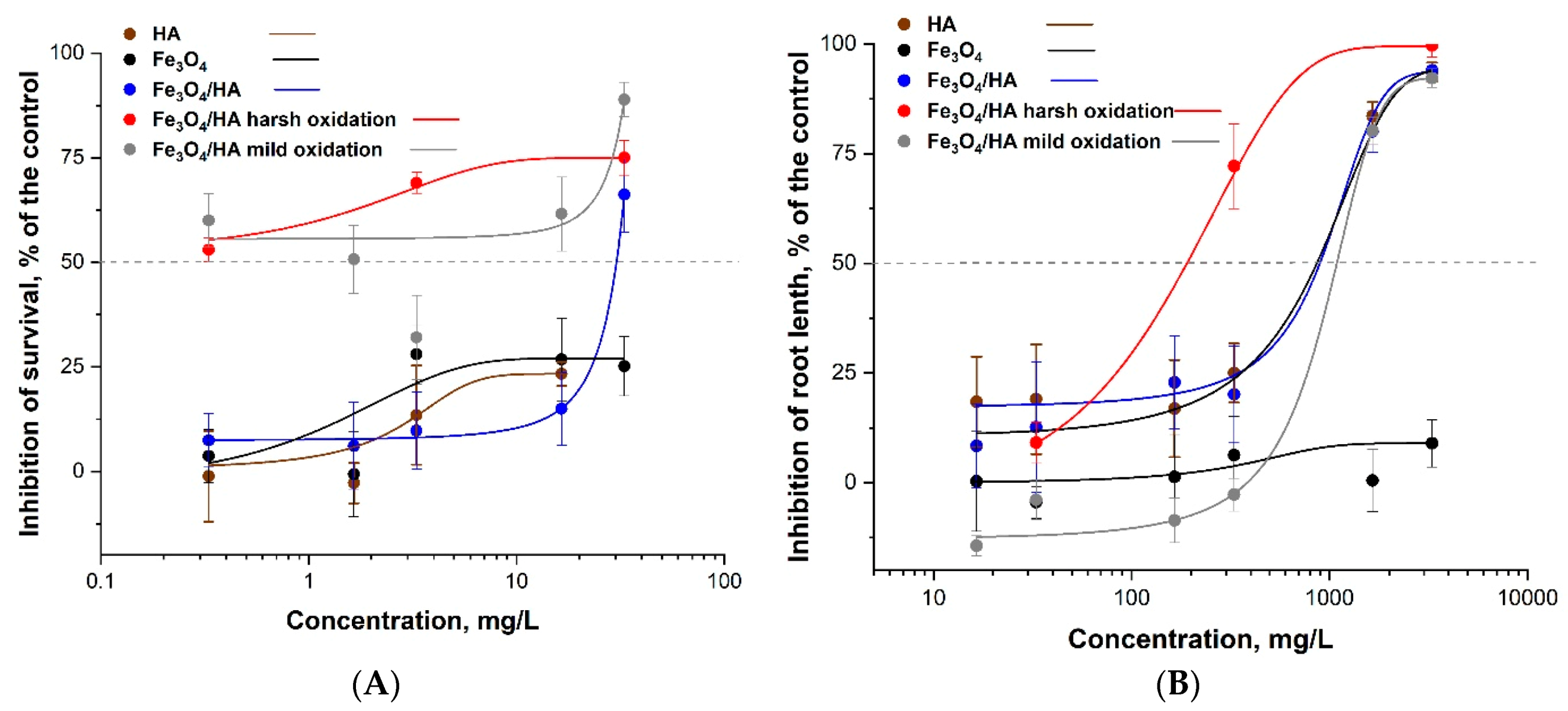
3.3. The Ecotoxicity of Bare Fe3O4 NPs (MNPs) and Humic Acids-Modified MNPs Before and After Oxidation in Mild and Harsh Conditions
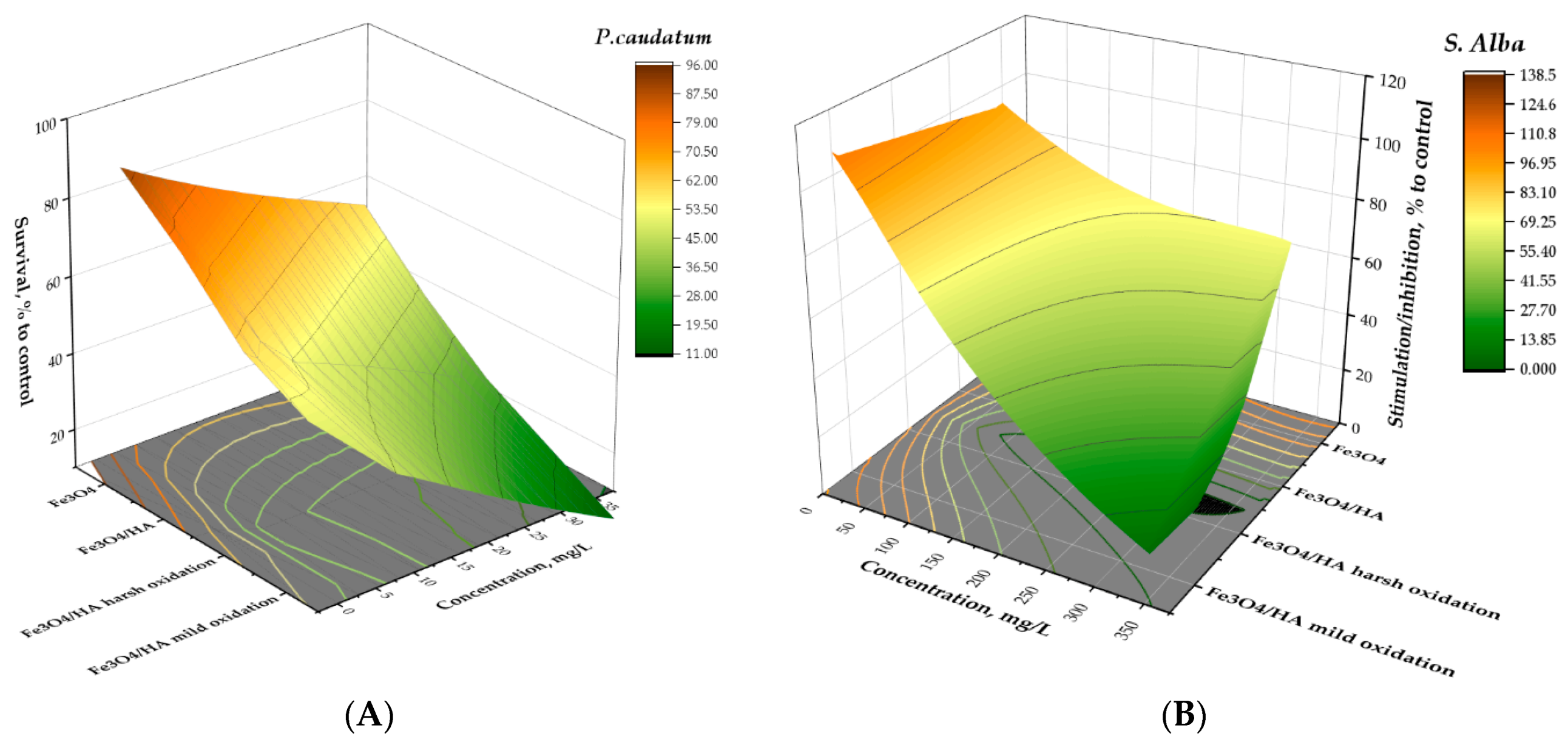
3.4. Structure–Bioactivity Relationship for MNPs
3.5. The Ecotoxicity of Fe-Ions before and after Addition of Humic Acids
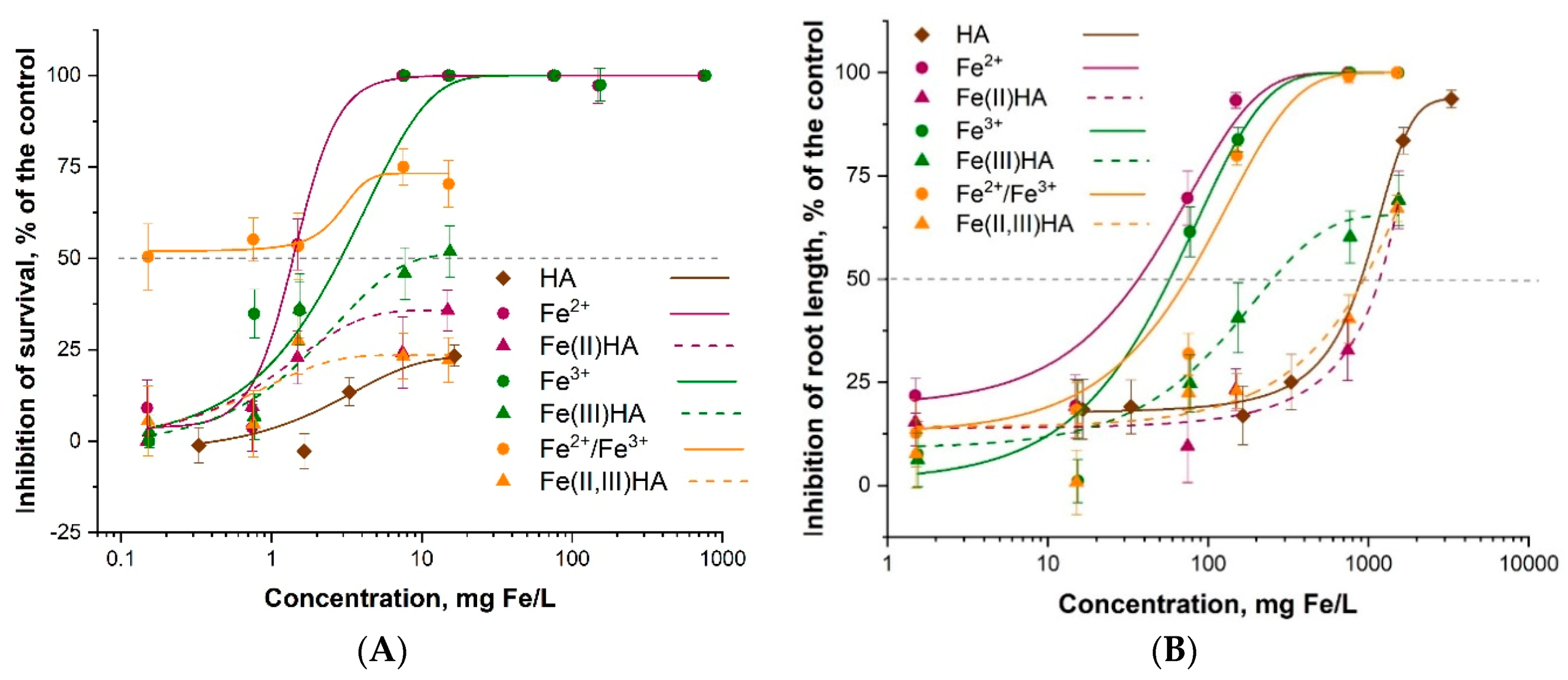
3.5.1. Ecotoxicity of Fe2+/Fe3+
3.5.2. Effects of Humic Acids on the Ecotoxicity of Fe2+ and Fe3+
3.6. Acute Toxicity of Fe2+/Fe3+ with and without Addition of Humic Acids: Effect of Aging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HA | Humic acids |
| HS | Humic substances |
| NPs | Nanoparticles |
| MNPs | Magnetite nanoparticles |
| SPIONs | Superparamagnet iron oxide nanoparticles |
| XRD | X-ray diffraction analysis |
| DOM | Dissolved organic matter |
| nZVI | Nano zerovalent iron |
| NOM | Natural organic matter |
| DLS | Dynamic light scattering |
References
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drenkova-Tuhtan, A.; Mandel, K.; Paulus, A.; Meyer, C.; Hutter, F.; Gellermann, C.; Sextl, G.; Franzreb, M.; Steinmetz, H. Phosphate recovery from wastewater using engineered superparamagnetic particles modified with layered double hydroxide ion exchangers. Water Res. 2013, 47, 5670–5677. [Google Scholar] [CrossRef] [PubMed]
- Bhateria, R.; Singh, R. A review on nanotechnological application of magnetic iron oxides for heavy metal removal. J. Water. Process Eng. 2009, 31, 100845. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wie, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total. Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.; Shipley, H.J.; Kan, A.; Tomson, M.; et al. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef]
- Lefevre, E.; Bossa, N.; Wiesner, M.R.; Gunsch, C.K. A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): Behavior, transport and impacts on microbial communities. Sci. Total Environ. 2016, 565, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem. Rev. 2012, 112, 2323–2338. [Google Scholar] [CrossRef] [Green Version]
- Selmani, A.; Ulm, L.; Kasemets, K.; Kurvet, I.; Erceg, I.; Barbir, R.; Pem, B.; Santini, P.; Delač Marion, I.; Vinković, T.; et al. Stability and toxicity of differently coated selenium nanoparticles under model environmental exposure settings. Chemosphere 2020, 250, 126265. [Google Scholar] [CrossRef]
- Lei, C.; Zhang, L.; Yang, K.; Zhu, L.; Lin, D. Toxicity of iron-based nanoparticles to green algae: Effects of particle size, crystal phase, oxidation state and environmental aging. Environ. Pollut. 2016, 218, 505–512. [Google Scholar] [CrossRef]
- Maity, D.; Agrawal, D.C. Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J. Magn. Magn. Mater. 2007, 308, 46–55. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P.; Wagner, F.E.; Berensmeier, S. Oxidation of magnetite nanoparticles: Impact on surface and crystal properties. CrystEngComm 2016, 19, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Sun, Y.; Tsang, D.C.W.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2018, 232, 10–30. [Google Scholar] [CrossRef]
- Garner, K.L.; Keller, A.A. Emerging patterns for engineered nanomaterials in the environment: A review of fate and toxicity studies. J. Nanopart. Res. 2014, 16, 2503. [Google Scholar] [CrossRef]
- Tiller, C.L.; O’Melia, C.R. Natural organic matter and colloidal stability: Models and measurements. Colloids Surf. A Physicochem. Eng. Asp. 1993, 73, 89–102. [Google Scholar] [CrossRef]
- Slowey, A.J. Rate of formation and dissolution of mercury sulfide nanoparticles: The dual role of natural organic matter. Geochim. Cosmochim. Acta. 2010, 74, 4693–4708. [Google Scholar] [CrossRef]
- Borch, T.; Kretzschmar, R.; Skappler, A.; Van Cappellen, P.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef]
- Philippe, A.; Schaumann, G.E. Interactions of dissolved organic matter with nat-ural and engineered inorganic colloids: A review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The role of variable surface charge and surface complexation in the adsorption of humic acid on magnetite. Colloids Surf. A 2003, 230, 99–109. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Dzhardimalieva, G.I.; Irzhak, V.I.; Bratskaya, S.Y.; Mayorov, V.Y.; Privar, Y.O.; Kasymova, E.D.; Kulyabko, L.S.; Zhorobekova, S.; Kydralieva, K.A. Stabilization of magnetite nanoparticles in humic acids medium and study of their sorption properties. Colloid J. 2020, 82, 1–7. [Google Scholar] [CrossRef]
- Khundzhua, D.A.; Yuzhakov, V.I.; Korvatovskiy, B.N.; Paschenko, V.Z.; Kulyabko, L.S.; Kydralieva, K.A.; Patsaeva, S.V. Spectroscopic manifestation of interaction of humic acids with trivalent iron ions in aqueous solutions. Mosc. Univ. Phys. Bull. 2018, 73, 632–637. [Google Scholar] [CrossRef]
- Yurishcheva, A.A.; Dzhardimalieva, G.I.; Kasymova, E.J.; Pomogailo, S.I.; Kydralieva, K.; Li, S.P. The structure of nanocomposites based on magnetite and humic acids produced by chemical coprecipitation and mechanochemical synthesis. Nanomechanics Sci. Technol. Int. J. 2014, 5, 323–326. [Google Scholar] [CrossRef]
- Sundman, A.; Byrne, J.M.; Bauer, I.; Menguy, N.; Kappler, A. Interactions between magnetite and humic substances: Redox reactions and dissolution processes. Geochem. Trans. 2017, 18, 1–12. [Google Scholar] [CrossRef]
- Velimirovic, M.; Auffan, M.; Carniato, L.; Micić Batka, V.; Schmid, D.; Wagner, S.; Borschneck, D.; Proux, O.; Von der Kammer, F.; Hofmann, T. Effect of field site hydrogeochemical conditions on the corrosion of milled zerovalent iron particles and their dechlorination efficiency. Sci. Total Environ. 2018, 618, 1619–1627. [Google Scholar] [CrossRef]
- Bogart, L.K.; Blanco-Andujar, C.; Pankhurst, Q.A. Environmental oxidative aging of iron oxide nanoparticles. Appl. Phys. Lett. 2018, 113, 133701. [Google Scholar] [CrossRef] [Green Version]
- Kahru, A.; Dubourguier, H.C. From ecotoxicology to nanoecotoxicology. Toxicology 2010, 269, 105–119. [Google Scholar] [CrossRef]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
- Aruoja, V.; Dubourguier, H.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total. Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notter, D.A.; Mitrano, D.M.; Nowack, B. Are nanosized or dissolved metals more toxic in the environment? Environ. Toxicol. Chem. 2014, 33, 2733–2739. [Google Scholar] [CrossRef] [PubMed]
- Juganson, K.; Ivask, A.; Mortimer, M.; Kahru, A. NanoE-Tox: New and in-depth database concerning ecotoxicity of nanomaterials. Beilstein J. Nanotechnol. 2015, 6, 1788–1804. [Google Scholar] [CrossRef] [PubMed]
- Walker, H.W.; Bob, M.M. Stability of particle flocs upon addition of natural organic matter under quiescent conditions. Water Res. 2001, 35, 875–882. [Google Scholar] [CrossRef]
- Pomogailo, A.D.; Kydralieva, K.A.; Zaripova, A.A.; Muratov, V.S.; Dzhardimalieva, G.I.; Pomogailo, S.I.; Golubeva, N.D.; Jorobekova, S.J. Magnetoactive humic-based nanocomposites. Macromol. Symp. 2011, 304, 18–23. [Google Scholar] [CrossRef]
- Garcia-Mina, J.M.; Sanchez-Diaz, M.; Iniguez, J. The ability of several iron (II)—Humic complexes to provide available iron to plants under adverse soil conditions. In Iron Nutrition in Soil and Plant; Abadia, J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 235–239. [Google Scholar]
- Gorski, C.A.; Scherer, M.M. Determination of nanoparticulate magnetite stoichiometry by Mossbauer spectroscopy, acid dissolution, and powder X-ray diffraction: A critical review. Am. Mineral. 2010, 95, 1017–1026. [Google Scholar] [CrossRef]
- Rakhleeva, A.A.; Terekhova, V.A. Method for Assessment of Toxicity of Wastes, Soil, Sewage Sludge, Surface and Ground Water Using Biotesting Techniques with Paramecium caudatum Ehrenberg (FR. 1.39.2006.02506); Moscow University: Moscow, Russia, 2006. [Google Scholar]
- ISO 18763:2016. Soil Quality—Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Nikolaeva, O.V.; Terekhova, V.A. Improvement of laboratory phytotest for the ecological evaluation of soils. Euras. Soil Sci. 2017, 50, 1105–1114. [Google Scholar] [CrossRef]
- Frison, R.; Cernuto, G.; Cervellino, A.; Zaharko, O.; Colonna, G.M.; Guagliardi, A.; Masciocchi, N. Magnetite-maghemite nanoparticles in the 5–15 nm range: Correlating the core-shell composition and the surface structure to the magnetic properties. A total scattering study. Chem. Mater. 2013, 25, 4820–4827. [Google Scholar] [CrossRef]
- Pankratov, D.A.; Anuchina, M.M.; Spiridonov, F.M.; Krivtsov, G.G. Fe3–δO4. Nanoparticles Synthesized in the Presence of Natural Polyelectrolytes. Crystallogr Rep. 2020, 65, 393–397. [Google Scholar] [CrossRef]
- Kim, D.K.; Zhang, Y.; Voit, W.; Rao, K.V.; Muhammed, M. Synthesis and Characterization of Surfactant Coated Superparamagnetic Monodispersed Iron Oxide Nanoparticles. J. Magn. Magn. Mater. 2001, 225, 30–36. [Google Scholar] [CrossRef]
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macêdo, M.A.; Nakamura, M.; Toma, H.E. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Tombácz, E.; Illés, E.; Majzik, A.; Hajdú, A.; Rideg, N.; Szekeres, M. Ageing in the inorganic nanoworld: Example of magnetite nanoparticles in aqueous medium. Croat. Chem. Acta. 2007, 80, 503–515. [Google Scholar]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [Green Version]
- Roth, H.C.; Schwaminger, S.P.; Schindler, M.; Wagner, F.E.; Berensmeier, S. Influencing factors in the CO-precipitation process of superparamagnetic iron oxide nanoparticles: A model based study. J. Magn. Magn. Mater. 2015, 377, 81–89. [Google Scholar] [CrossRef]
- Romano, F.L.; Ambrosano, G.M.B.; Magnani, M.B.B.; Nouer, D.F. Analysis of the coefficient of variation in shear and tensile bond strength tests. J. Appl. Oral. Sci. 2005, 13, 243–246. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.M.; Toumi, M.; Mhiri, T.; Begin-Colin, S. Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J. Phys. Chem. 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- Ko, K.; Kong, I.C. Toxic effects of nanoparticles on bioluminescence activity, seed germination, and gene mutation. Appl. Microbiol. Biotechnol. 2014, 98, 3295–3303. [Google Scholar] [CrossRef]
- Blinova, I.; Kanarbik, L.; Irha, N.; Kahru, A. Ecotoxicity of nanosized magnetite to crustacean Daphnia magna and duckweed Lemna minor. Hydrobiologia 2017, 798, 141–149. [Google Scholar] [CrossRef]
- Aruoja, V.; Pokhrel, S.; Sihtmae, M.; Mortimer, M.; Mädler, L.; Kahru, A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ. Sci. Nano. 2015, 2, 630–644. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances: A novel understanding of humus chemistry and implications in soil science. Adv. Agron. 2002, 75, 57–134. [Google Scholar] [CrossRef]
- Kallianou, C.S.; Yassoglou, N.J. Bonding and oxidation state of iron in humic complexes extracted from some Greek soils. Geoderma 1985, 35, 209–221. [Google Scholar] [CrossRef]
- Fujii, M.; Imaoka, A.; Yoshimura, C.; Waite, T.D. Effects of molecular composition of natural organic matter on ferric iron complexation at circumneutral pH. Environ. Sci Technol. 2014, 48, 4414–4424. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Kurvet, I.; Juganson, K.; Vija, H.; Sihtmäe, M.; Blinova, I.; Syvertsen-Wiig, G.; Kahru, A. Toxicity of Nine (Doped) Rare Earth Metal Oxides and Respective Individual Metals to Aquatic Microorganisms Vibrio fischeri and Tetrahymena thermophila. Materials 2017, 10, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, E.E. Soil and Applied Iron. Univ. Wisconsin Ext. RP08-2004 (I09/92). Available online: http://www.soils.wisc.edu/extension/pubs/A3554.pdf (accessed on 20 July 2020).
- Briat, J.F. Iron from soil to plant products. Bull. Acad. Natl. Med. 2005, 189, 1609–1619. [Google Scholar] [PubMed]
- Schnitzer, M.; Skinner, S. Organo-metallic interactions in soils: 5. stability constants of Cu++-, Fe++-, and Zn++-fulvic acid complexes. Soil Sci. 1966, 102, 361–365. [Google Scholar] [CrossRef]
- Van Dijk, H. Cation binding humic acids. Geoderma 1971, 5, 53–67. [Google Scholar] [CrossRef]
- Catrouillet, C.; Davranche, M.; Dia, A.; Bouhnik-Le Coz, M.; Marsac, R.; Pourret, G.; Gruau, G. Geochemical modeling of Fe(II) binding to humic and fulvic acids. Chem. Geol. 2014, 372, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, N.; Furubayashi, K.; Fukushima, M.; Yamamoto, M.; Komai, T.; Ootsuka, K.; Kawabe, Y. Evaluation of the iron(II)-binding abilities of humic acids by complexometric titration using colorimetry with ortho-phenanthroline. Humic Subst. Res. 2011, 8, 1–6. [Google Scholar]
- Garcia-Mina, J.; Antolín, M.; Sanchez-Diaz, M. Metal-humic complexes and plant micronutrient uptake: A study based on different plant species cultivated in diverse soil types. Plant. Soil. 2004, 258, 57–68. [Google Scholar] [CrossRef]
- Milne, C.J.; Kinniburgh, D.G.; van Riemsdijk, W.H.; Tipping, E. Generic NICA-Donnan model parameters for metal-ion binding by humic substances. Environ. Sci. Technol. 2003, 37, 958–971. [Google Scholar] [CrossRef] [PubMed]
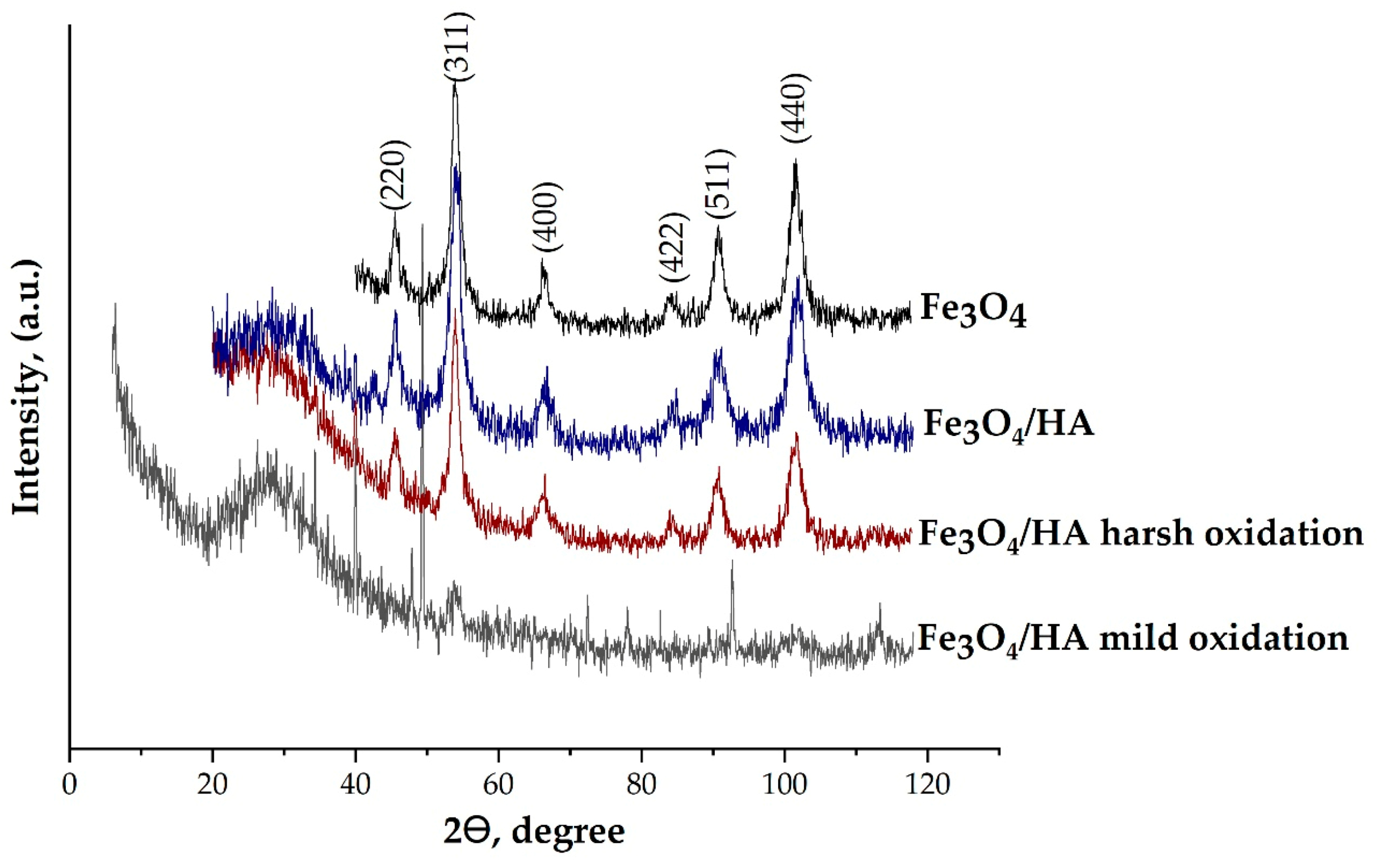
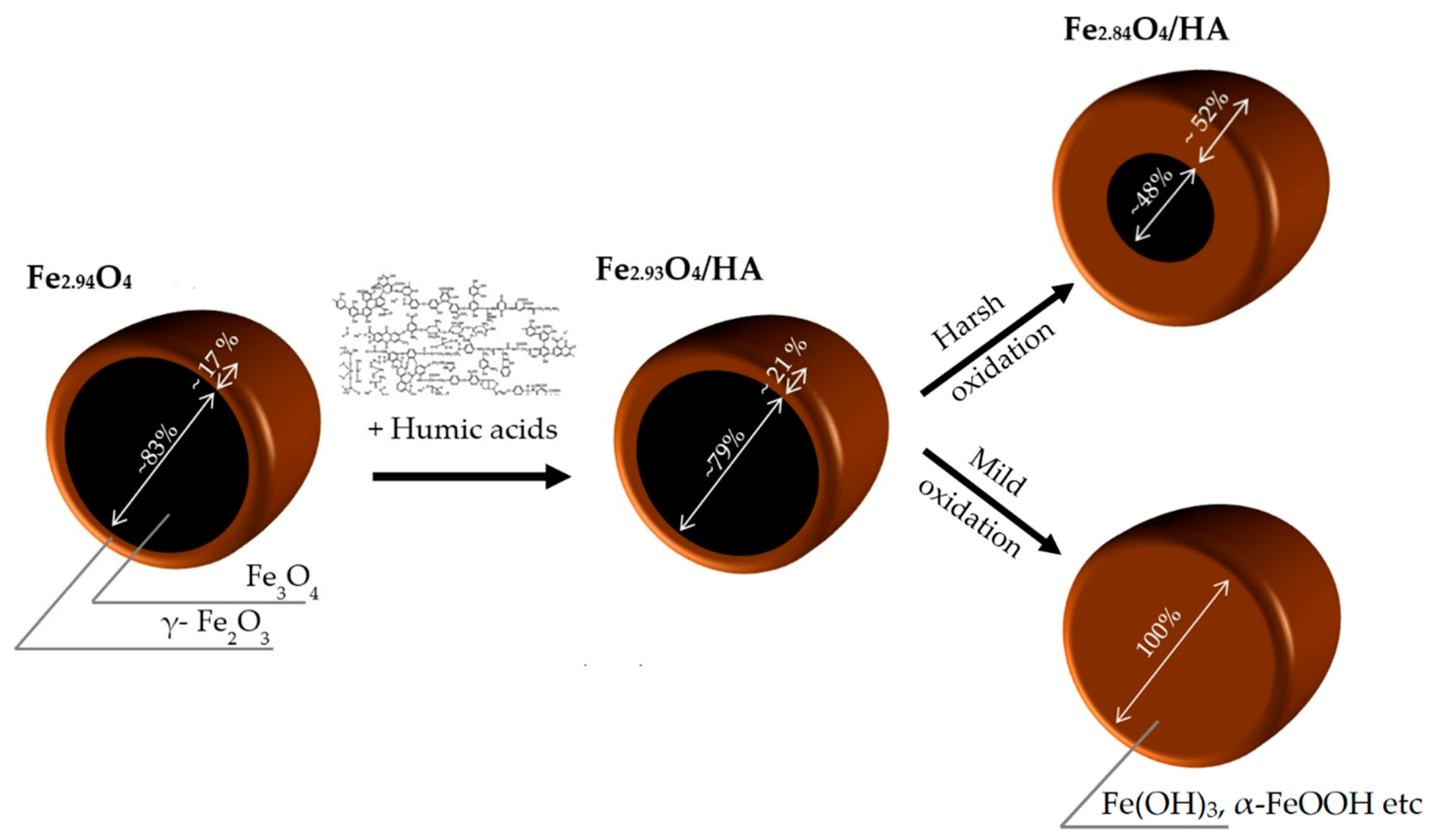

| Sample | Fe3O4 | Fe3O4/HA | Fe3O4/HA Harsh Oxidation | Fe3O4/HA Mild Oxidation | ||||
|---|---|---|---|---|---|---|---|---|
| hkl | 2θ, ° | d, Å | 2θ, ° | d, Å | 2θ, ° | d, Å | 2θ, ° | d, Å |
| 220 | 45.60 | 2.971 | 45.67 | 2.966 | 45.62 | 2.954861 | 49.33 | 2.74502038 |
| 311 | 53.98 | 2.535 | 54.01 | 2.535 | 53.92 | 2.526709 | 53.92 | 2.52670925 |
| 400 | 66.28 | 2.094 | 66.36 | 2.094 | 66.39 | 2.09233 | - | - |
| 422 | 84.25 | 1.714 | 84.25 | 1.714 | 84.38 | 1.705695 | 82.6 | 1.73564713 |
| 511 | 90.77 | 1.610 | 90.71 | 1.612 | 90.62 | 1.61133 | 92.69 | 1.58329633 |
| 440 | 101.52 | 1.476 | 101.4 | 1.476 | 101.68 | 1.477368 | 101.3 | 1.48137719 |
| a, A | 8.383(2) | 8.382(6) | 8.365(5) | 8.250(8) | ||||
| x | 0.387(7) | 0.382(1) | 0.232(7) | - | ||||
| δ | 0.059(4) | 0.062(7) | 0.154(2) | - | ||||
| Fe3-δO4 | Fe2.94O4 | Fe2.93O4 | Fe2.84O4 | - | ||||
| % Fe3O4 | ~82.7 | ~79.2 | ~48.3 | 0 | ||||
| D, nm | 6.9 ± 2.4 | 10.3 ± 1.3 | 7.8 ± 1.9 | 11.03 ± 5.1 | ||||
| CV, % | 34 | 12.6 | 24.6 | 50.2 | ||||
| Sample | Saturation Magnetization Ms, emu/g | Remanent Magnetization Mr, emu/g | Coercive Force Hc, Oe |
|---|---|---|---|
| Fe3O4 (bare) | 68.2 | 6.88 | 74.1 |
| Fe3O4/HA | 30.9 | 6.40 | 160 |
| Fe3O4/HAox (harsh oxidation) | 15.7 | 4.11 | 159 |
| Sample | %Fe3O4 | ξ, mV | Hydrodynamic Diameter, nm | 24 h EC50 for P. caudatum, mg/L | 96 h EC50 for S. alba, mg/L |
|---|---|---|---|---|---|
| Fe3O4 | 83 | −25.5 ± 6.03 | 400.8 | >33 ** | >3300 |
| HA | - | - | - | >33 ** | 900.65 ± 106.2 |
| Fe3O4/HA | 79 | −38.8 ± 7.1 | 153.2 | 32 ± 3.1 | 902.71 ± 211.5 |
| Fe3O4/HA harsh oxidation | 48 | −16.19 ± 2.1 | 886.7 | 0.33 ± 0.01 | 330 ± 10.1 |
| Fe3O4/HA mild oxidation | 0 * | −49.63 ± 2.2 | 586.1 | 1.40 ± 0.5 | 951.6 ± 80.2 |
| Sample | EC50 ± SD (mg/L) |
|---|---|
| Toxicity to ciliates P. caudatum (24 h EC50) | |
| Fe2+ a | 1.15 ± 1.4 |
| Fe3+ a | 3.27 ± 0.8 |
| Fe2+/Fe3+ c | 0.48 |
| Fe3O4 | >33 |
| HA | >33 |
| Fe(II)HA | >33 |
| Fe(III)HA | 24.6 ± 9.3 |
| Fe(II,III)HA b,c | >15.1 |
| Fe3O4/HA b | 32 ± 3.1 |
| Toxicity to plants S. alba (96 h EC50) | |
| Fe2+ | 35.92 ± 18.6 |
| Fe3+ | 58.71 ± 21.2 |
| Fe2+/Fe3+ | 75.21 ± 44.4 |
| Fe3O4 c | >3300 |
| HA | 900.65 ± 106.2 |
| Fe(II)HA | 1402.3 ± 135.1 |
| Fe(III)HA | 256.18 ± 50.4 |
| Fe(II,III)HA | 910.43 ± 270.1 |
| Fe3O4/HA | 902.71 ± 211.5 |
| Sample | EC50 ± SD (mg Fe/L) |
|---|---|
| Toxicity to ciliates P. caudatum (24 h EC50) | |
| HA * | >33 |
| HA * (90 days) | 1.93 ± 0.55 |
| Fe2+/Fe3+ | 0.48 ± 0.02 |
| Fe2+/Fe3+ (90 days) | 0.67 ± 0.02 |
| Fe(II,III)HA | >15.1 |
| Fe(II,III)HA (90 days) | 1.4 ± 0.4 |
| Toxicity to plants S. alba (96 h EC50) | |
| HA * | 900.65 ± 106.2 |
| HA * (90 days) | 880.1 ± 123.1 |
| Fe2+/Fe3+ | 75.21 ± 44.4 |
| Fe2+/Fe3+ (90 days) | 231.41 ± 97.46 |
| Fe(II,III)HA | 910.43 ± 270.1 |
| Fe(II,III)HA (90 days) | 915.23 ± 145.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondarenko, L.; Kahru, A.; Terekhova, V.; Dzhardimalieva, G.; Uchanov, P.; Kydralieva, K. Effects of Humic Acids on the Ecotoxicity of Fe3O4 Nanoparticles and Fe-Ions: Impact of Oxidation and Aging. Nanomaterials 2020, 10, 2011. https://doi.org/10.3390/nano10102011
Bondarenko L, Kahru A, Terekhova V, Dzhardimalieva G, Uchanov P, Kydralieva K. Effects of Humic Acids on the Ecotoxicity of Fe3O4 Nanoparticles and Fe-Ions: Impact of Oxidation and Aging. Nanomaterials. 2020; 10(10):2011. https://doi.org/10.3390/nano10102011
Chicago/Turabian StyleBondarenko, Lyubov, Anne Kahru, Vera Terekhova, Gulzhian Dzhardimalieva, Pavel Uchanov, and Kamila Kydralieva. 2020. "Effects of Humic Acids on the Ecotoxicity of Fe3O4 Nanoparticles and Fe-Ions: Impact of Oxidation and Aging" Nanomaterials 10, no. 10: 2011. https://doi.org/10.3390/nano10102011
APA StyleBondarenko, L., Kahru, A., Terekhova, V., Dzhardimalieva, G., Uchanov, P., & Kydralieva, K. (2020). Effects of Humic Acids on the Ecotoxicity of Fe3O4 Nanoparticles and Fe-Ions: Impact of Oxidation and Aging. Nanomaterials, 10(10), 2011. https://doi.org/10.3390/nano10102011







