Electrochemical and Structural Property of TiSiNb TFSOC on Affordable Interconnects in Proton Exchange Membrane Fuel Cell Applications
Abstract
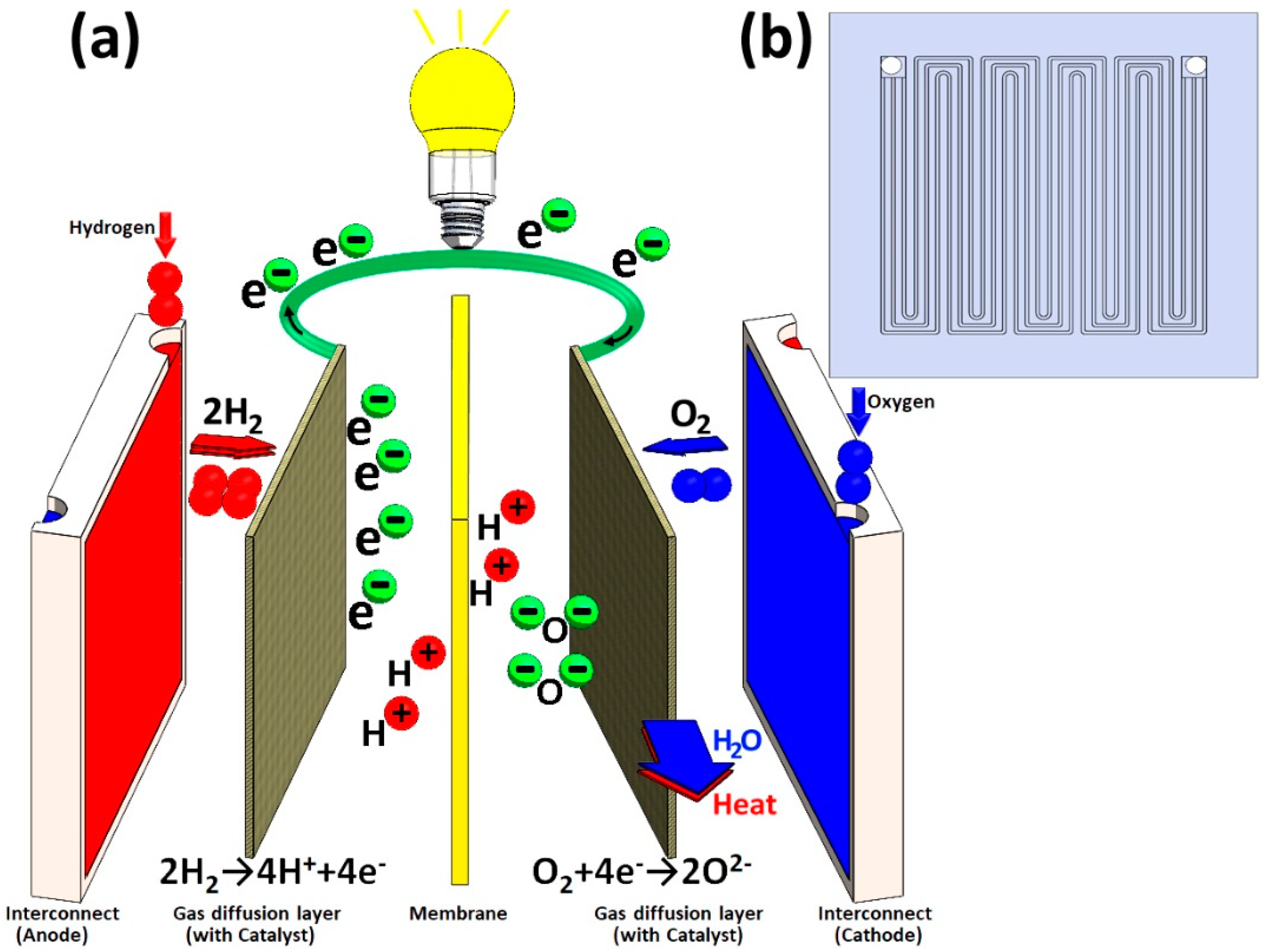
1. Introduction
2. Experimental Procedure
2.1. Materials and Substrate Preparation
2.2. Preparation of TiSiNb Sol
2.3. Preparation of TFSOC on the Substrate
2.4. Characterization of TFSOC
3. Results and Discussions
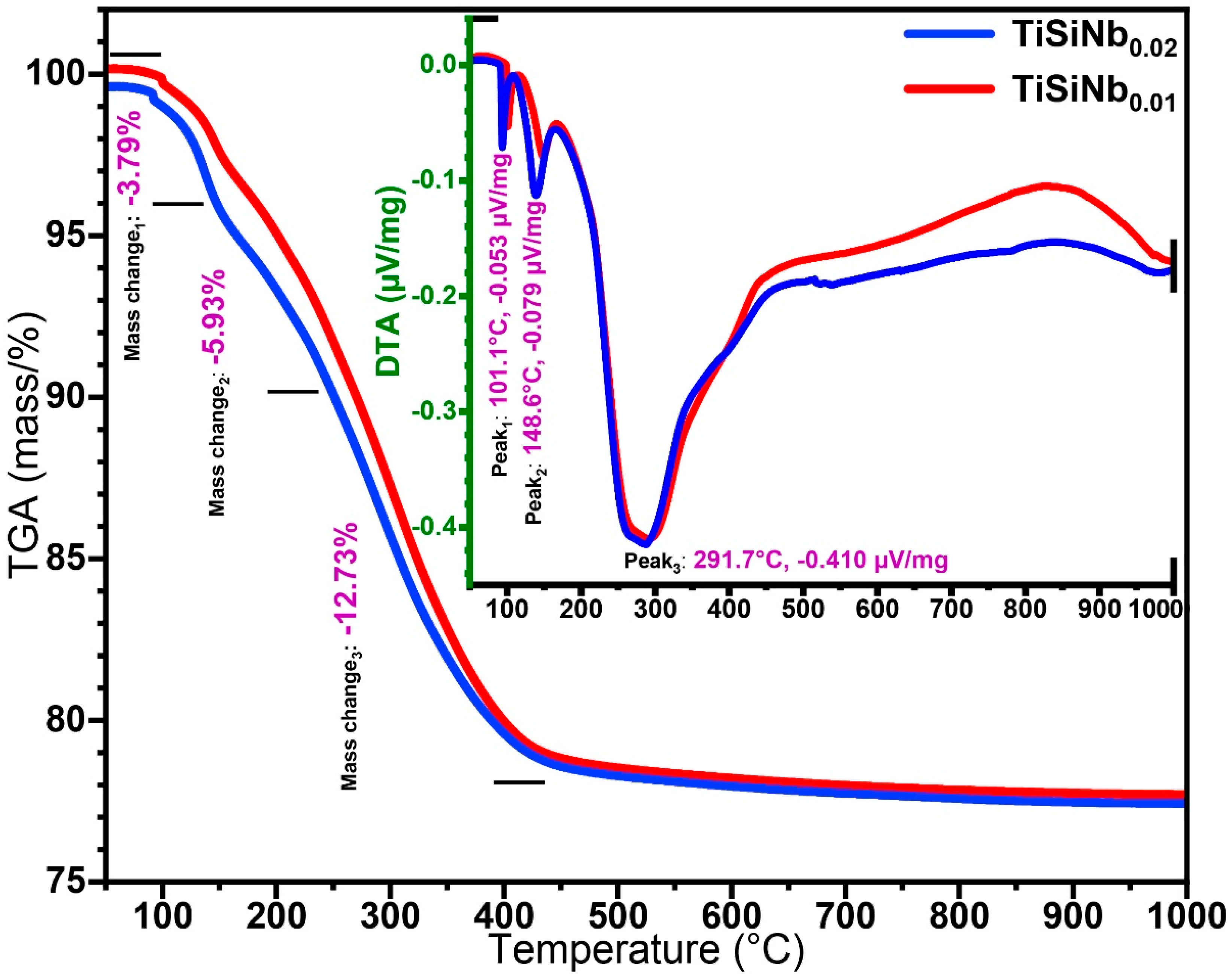
3.1. Thermal Gravimetric and Differential Thermal Analysis
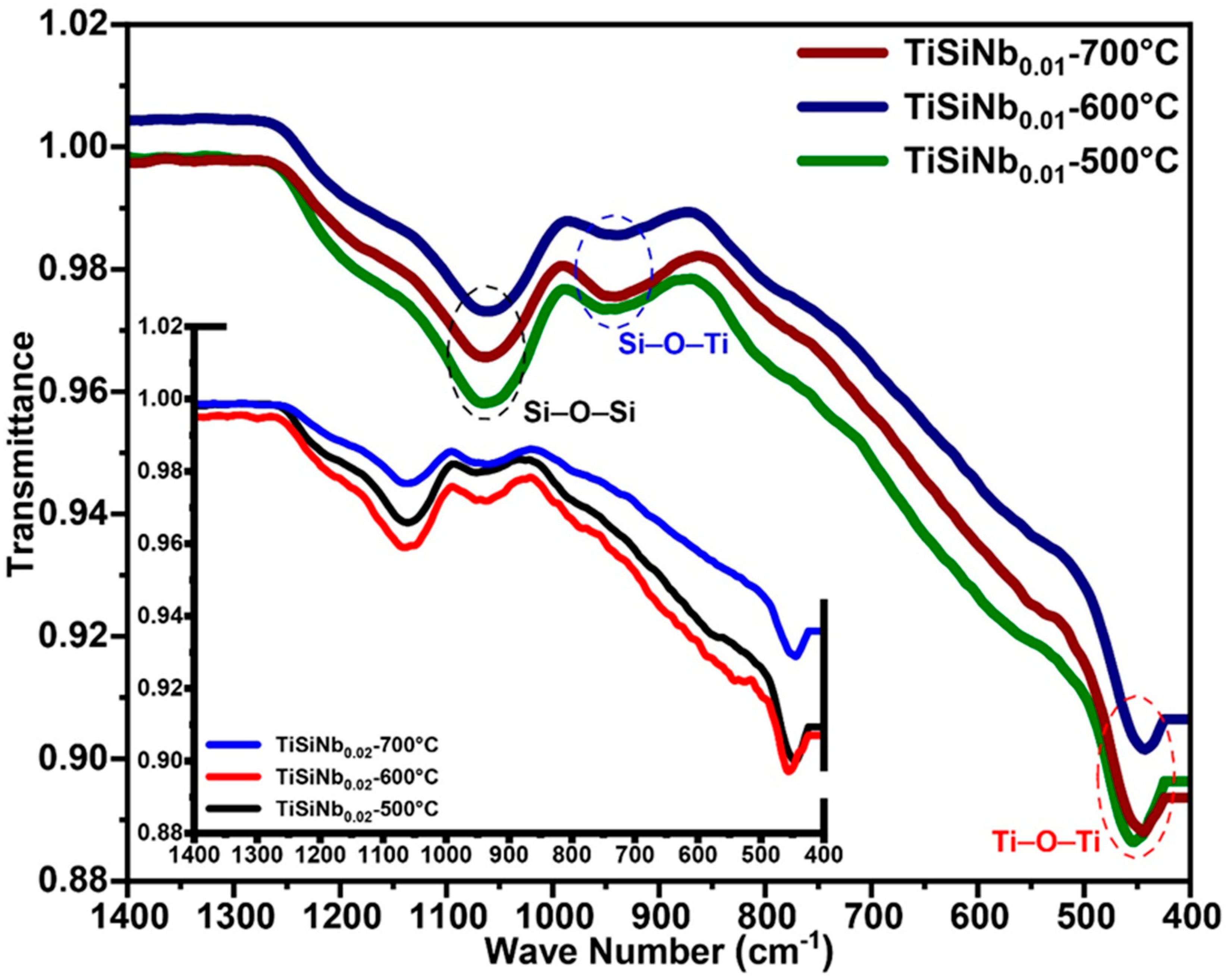
3.2. FT-IR Spectra of TiSiNb TFSOC
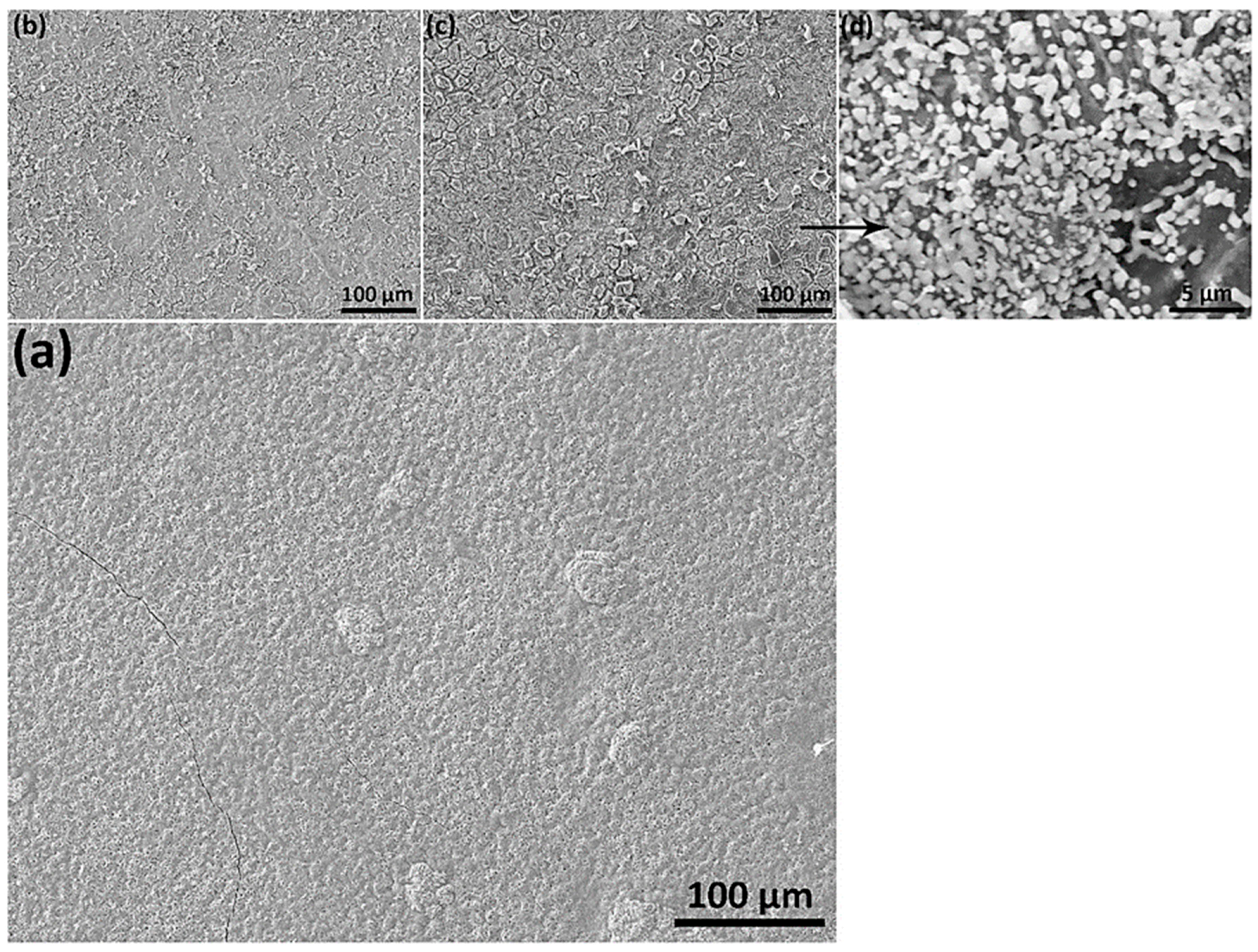
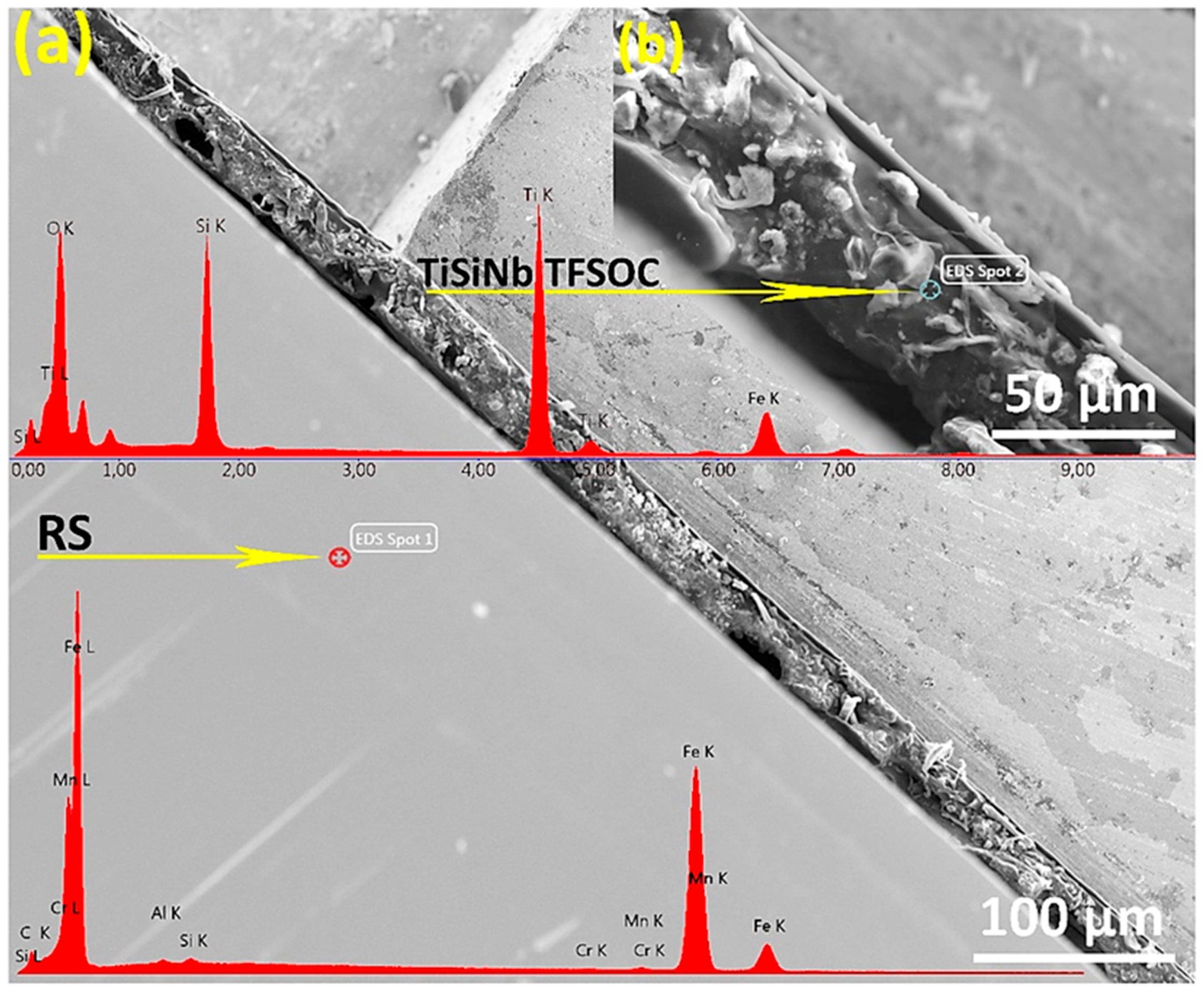
3.3. SEM-EDS Analysis
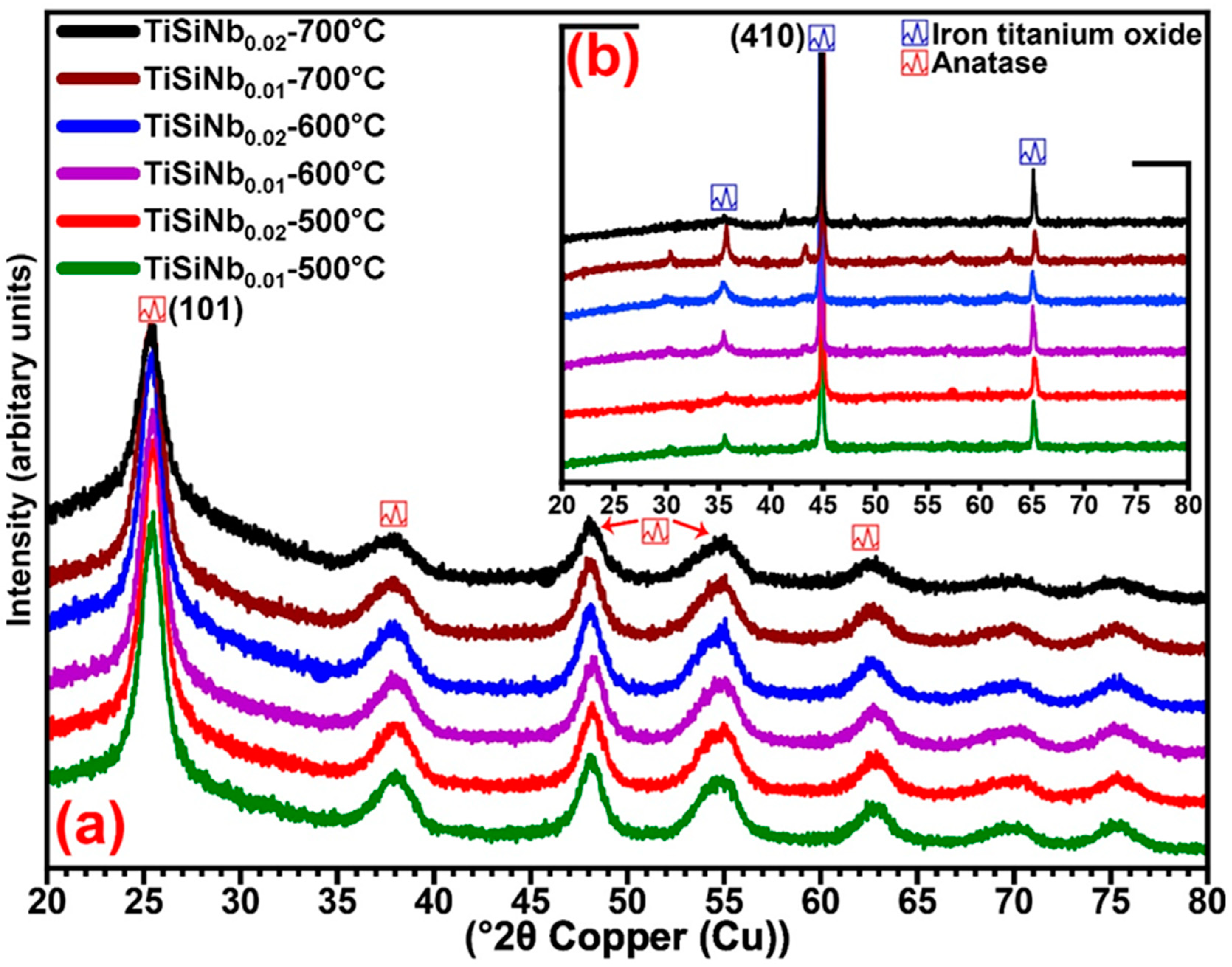
3.4. X-ray Diffraction Analyses
3.5. Studies on Corrosion
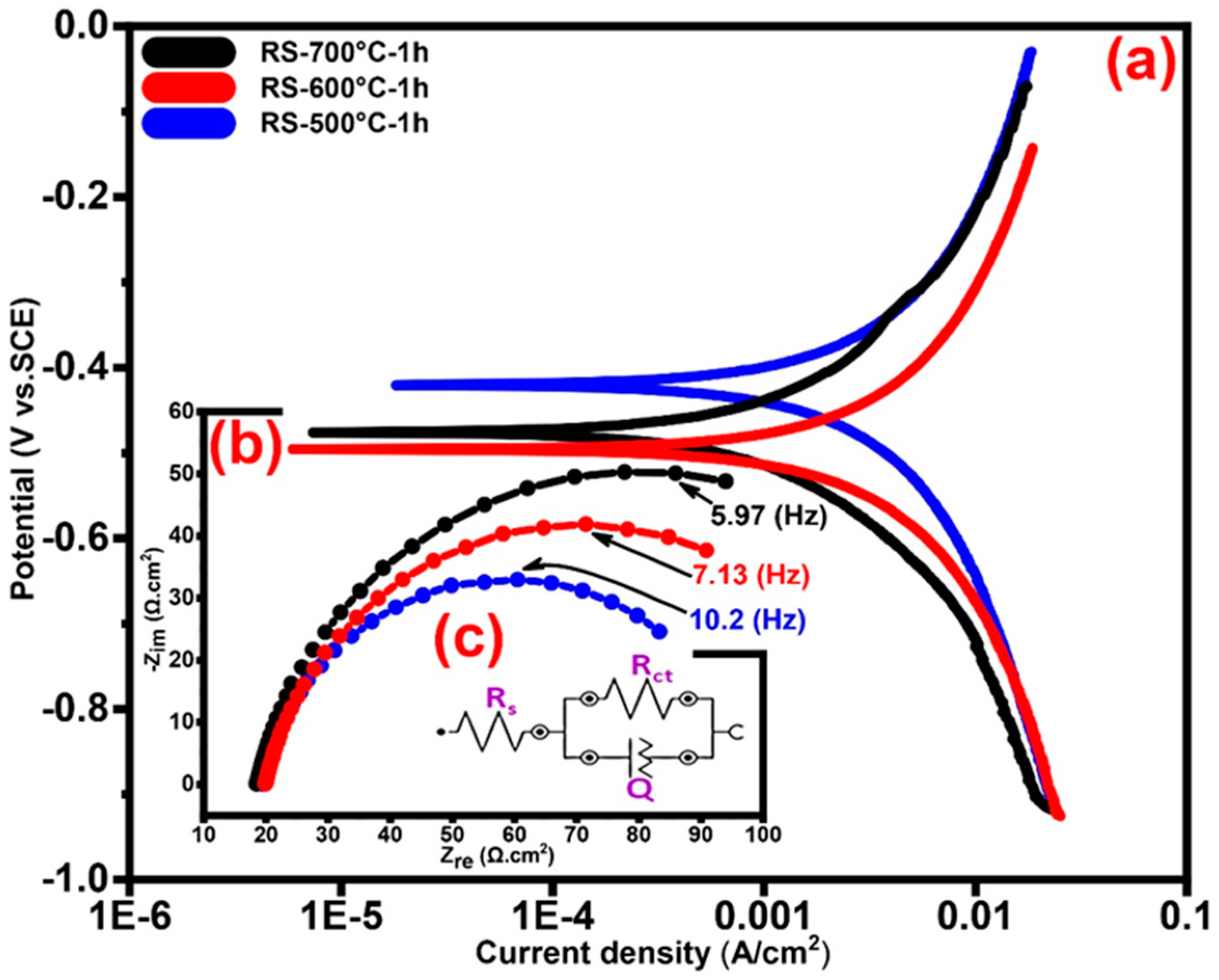
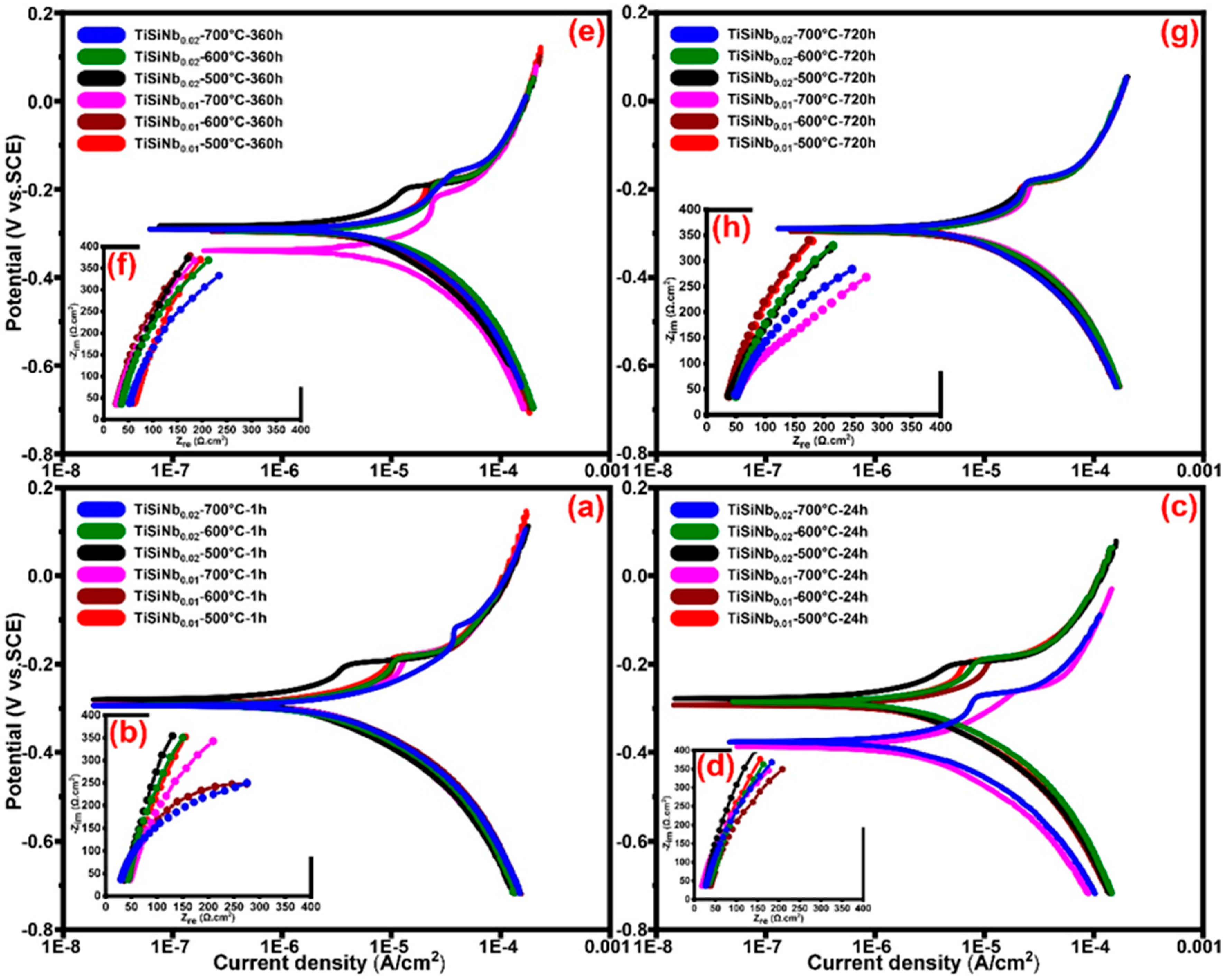
3.5.1. Tafel Polarization Technique and Electrochemical Behavior
3.5.2. Electrochemical Impedance Spectroscopy (EIS)
3.6. Computation and Evaluation of Conductivity and Activation Energy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rivarolo, M.; Rattazzi, D.; Lamberti, T.; Magistri, L. Clean energy production by PEM fuel cells on tourist ships: A time-dependent analysis. Int. J. Hydrog. Energy 2020, 45, 25747–25757. [Google Scholar] [CrossRef]
- Alves-Lima, D.F.; Letizia, R.; Degl’Innocenti, R.; Dawson, R.; Lin, H. Quantitative video-rate hydration imaging of Nafion proton exchange membranes with terahertz radiation. J. Power Sources 2020, 450, 227665. [Google Scholar] [CrossRef]
- Hu, M.; Triulzi, G.; Sharifzadeh, M. Technological change in fuel cell technologies. In Design and Operation of Solid Oxide Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–41. [Google Scholar] [CrossRef]
- Park, H.; Kim, D.-K.; Kim, H.; Oh, S.; Jung, W.S.; Kim, S.-K. Binder-coated electrodeposited PtNiCu catalysts for the oxygen reduction reaction in high-temperature polymer electrolyte membrane fuel cells. Appl. Surf. Sci. 2020, 510, 145444. [Google Scholar] [CrossRef]
- Jin, J.; He, Z.; Zhao, X. Formation of a protective TiN layer by liquid phase plasma electrolytic nitridation on Ti–6Al–4V bipolar plates for PEMFC. Int. J. Hydrog. Energy 2020, 45, 12489–12500. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, P.; Han, Y.; Wang, H.; Wang, X.; Yu, Y.; Sun, J. Investigation on electrochemical behavior and surface conductivity of titanium carbide modified Ti bipolar plate of PEMFC. Int. J. Hydrog. Energy 2020, 45, 10050–10058. [Google Scholar] [CrossRef]
- Deyab, M.A.; Mele, G. Stainless steel bipolar plate coated with polyaniline/Zn-Porphyrin composites coatings for proton exchange membrane fuel cell. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Simaafrookhteh, S.; Khorshidian, M.; Momenifar, M. Fabrication of multi-filler thermoset-based composite bipolar plates for PEMFCs applications: Molding defects and properties characterizations. Int. J. Hydrog. Energy 2020, 45, 14119–14132. [Google Scholar] [CrossRef]
- Kargar-Pishbijari, H.; Hosseinipour, S.J.; Aval, H.J. A novel method for manufacturing microchannels of metallic bipolar plate fuel cell by the hot metal gas forming process. J. Manuf. Process 2020, 55, 268–275. [Google Scholar] [CrossRef]
- Bouziane, K.; Lachat, R.; Zamel, N.; Meyer, Y.; Candusso, D. Impact of cyclic mechanical compression on the electrical contact resistance between the gas diffusion layer and the bipolar plate of a polymer electrolyte membrane fuel cell. Renew. Energy 2020, 153, 349–361. [Google Scholar] [CrossRef]
- Haye, E.; Deschamps, F.; Caldarella, G.; Piedboeuf, M.-L.; Lafort, A.; Cornil, H.; Colomer, J.-F.; Pireaux, J.-J.; Job, N. Formable chromium nitride coatings for proton exchange membrane fuel cell stainless steel bipolar plates. Int. J. Hydrog. Energy 2020, 45, 15358–15365. [Google Scholar] [CrossRef]
- Pilinski, N.; Käding, C.; Dushina, A.; Hickmann, T.; Dyck, A.; Wagner, P. Investigation of Corrosion Methods for Bipolar Plates for High Temperature Polymer Electrolyte Membrane Fuel Cell Application. Energies 2020, 13, 235. [Google Scholar] [CrossRef]
- Alo, O.A.; Otunniyi, I.O.; Pienaar, H.C.; Sadiku, E.R. Electrical and mechanical properties of polypropylene/epoxy blend-graphite/carbon black composite for proton exchange membrane fuel cell bipolar plate. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.-Y.; Han, M.; Yong, R.-Y.; Sun, D.; Chen, J. Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Li, X.; Zhou, P.; Ogle, K.; Proch, S.; Paliwal, M.; Jansson, A.; Westlinder, J. Transient stainless-steel dissolution and its consequences on ex-situ bipolar plate testing procedures. Int. J. Hydrog. Energy 2020, 45, 984–995. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, G.; Yang, W.; Xu, B.; Chen, Y.; Yin, X.; Liu, Y. Superior conducting polypyrrole anti-corrosion coating containing functionalized carbon powders for 304 stainless steel bipolar plates in proton exchange membrane fuel cells. Chem. Eng. J. 2020, 124675. [Google Scholar] [CrossRef]
- Leng, Y.; Ming, P.; Yang, D.; Zhang, C. Stainless steel bipolar plates for proton exchange membrane fuel cells: Materials, flow channel design and forming processes. J. Power Sources 2020, 451, 227783. [Google Scholar] [CrossRef]
- Li, T.; Zhang, P.C.; Liu, K.; Xu, S.; Han, Y.T.; Shi, J.F.; Sun, J.C. Performance of Tantalum Modified 316L Stainless Steel Bipolar Plate for Proton Exchange Membrane Fuel Cell. Fuel Cells 2019, 19, 724–730. [Google Scholar] [CrossRef]
- Kumar, N.; Shaik, G.P.; Pandurangan, S.; Khalkho, B.; Neelakantan, L.; Chetty, R. Corrosion characteristics and fuel cell performance of a cost-effective high Mn–Low Ni austenitic stainless steel as an alternative to SS 316L bipolar plate. Int. J. Energy Res. 2020. [Google Scholar] [CrossRef]
- Chen, G.; Tao, T.; Gao, P.-P.; Xie, Z.-Y.; Wu, X.-B. Corrosion Protection of Amorphous Carbon Coating for the Bipolar Plates of PEMFCs. Surf. Rev. Lett. 2019, 26, 1950059. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; Khatib, F.N.; Leslie, T.; El-Hassan, Z.; Olabi, A.G. Technical evaluation of proton exchange membrane (PEM) fuel cell performance—A review of the effects of bipolar plates coating. Renew. Sustain. Energy Rev. 2019, 113, 109286. [Google Scholar] [CrossRef]
- Jadi, S.B.; El Jaouhari, A.; Aouzal, Z.; El Guerraf, A.; Bouabdallaoui, M.; Wang, R.; Bazzaoui, M. Electropolymerization and corrosion resistance of polypyrrole on nickel bipolar plate for PEM fuel cell application. Mater. Today Proc. 2020, 22, 52–56. [Google Scholar] [CrossRef]
- Hickmann, T.; Zielinski, O. Bipolar Plates: Different Materials and Processing Methods for Their Usage in Fuel Cells. E3S Web Conf. 2020, 160, 1002. [Google Scholar] [CrossRef]
- Liu, S.; Pan, T.J.; Wang, R.F.; Yue, Y.; Shen, J. Anti-corrosion and conductivity of the electrodeposited graphene/polypyrrole composite coating for metallic bipolar plates. Prog. Org. Coat. 2019, 136, 105237. [Google Scholar] [CrossRef]
- Jiang, L.; Syed, J.A.; Lu, H.; Meng, X. In-situ electrodeposition of conductive polypyrrole-graphene oxide composite coating for corrosion protection of 304SS bipolar plates. J Alloys Compd. 2019, 770, 35–47. [Google Scholar] [CrossRef]
- Peng, S.; Xu, J.; Li, Z.; Jiang, S.; Munroe, P.; Xie, Z.-H.; Lu, H. A reactive-sputter-deposited TiSiN nanocomposite coating for the protection of metallic bipolar plates in proton exchange membrane fuel cells. Ceram Int. 2020, 46, 2743–2757. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Raeissi, K.; Karimzadeh, F.; Golozar, M.A. A study on corrosion behavior of graphene oxide coating produced on stainless steel by electrophoretic deposition. Surf. Coat. Technol. 2019, 372, 327–342. [Google Scholar] [CrossRef]
- Lu, J.L.; Abbas, N.; Tang, J.; Hu, R.; Zhu, G.M. Characterization of Ti3SiC2-coating on stainless steel bipolar plates in simulated proton exchange membrane fuel cell environments. Electrochem. Commun. 2019, 105, 106490. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Hadidi, M.R.; Salehzadeh, D.; Nemati, A. Hydrophobic octadecylamine-functionalized graphene/TiO2 hybrid coating for corrosion protection of copper bipolar plates in simulated proton exchange membrane fuel cell environment. Int. J. Hydrog. Energy 2020, 45, 15380–15389. [Google Scholar] [CrossRef]
- Hua, Q.; Zeng, Y.; He, Z.; Xu, Q.; Min, Y. Microstructure, synergistic mechanism and corrosion behavior of tin oxide conversion film modified by chitosan on aluminum alloy surface. Colloid Interface Sci. Commun. 2020, 36, 100262. [Google Scholar] [CrossRef]
- Manso, A.P.; Marzo, F.F.; Garicano, X.; Alegre, C.; Lozano, A.; Barreras, F. Corrosion behavior of tantalum coatings on AISI 316L stainless steel substrate for bipolar plates of PEM fuel cells. Int. J. Hydrog. Energy 2020, 45, 20679–20691. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Lu, Z.; Wang, P.; Ji, X.; Li, W. Preparation and performance of electrically conductive Nb-doped TiO2/polyaniline bilayer coating for 316L stainless steel bipolar plates of proton-exchange membrane fuel cells. RSC Adv. 2018, 8, 19426–19431. [Google Scholar] [CrossRef]
- Yang, L.; Qin, Z.L.; Pan, H.T.; Yun, H.; Min, Y.L.; Xu, Q.J. Corrosion protection of 304 stainless steel bipolar plates of PEMFC by coating SnO2 film. Int. J. Electrochem. Sci. 2017, 12, 10946–10957. [Google Scholar] [CrossRef]
- Park, J.H.; Jeon, B.J.; Lee, J.K. Electrochemical characteristics of fluorine-doped tin oxide film coated on stainless steel bipolar plates. Surf. Coat. Technol. 2015, 277, 1–6. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, D.; Hu, M.; Hu, Q. Cr2O3/C composite coatings on stainless steel 304 as bipolar plate for proton exchange membrane fuel cell. Energy 2014, 76, 816–821. [Google Scholar] [CrossRef]
- Mohammadi, N.; Yari, M.; Allahkaram, S.R. Characterization of PbO2 coating electrodeposited onto stainless steel 316L substrate for using as PEMFC’s bipolar plates. Surf. Coat. Technol. 2013, 236, 341–346. [Google Scholar] [CrossRef]
- Kamnerdkhag, P.; Free, M.L.; Shah, A.A.; Rodchanarowan, A. The effects of duty cycles on pulsed current electrodeposition of ZnNiAl2O3 composite on steel substrate: Microstructures, hardness and corrosion resistance. Int. J. Hydrog. Energy 2017, 42, 20783–20790. [Google Scholar] [CrossRef]
- Khosravi, S.; Veerapandiyan, V.K.; Vallant, R.; Reichmann, K. Effect of processing conditions on the structural properties and corrosion behavior of TiO2–SiO2 multilayer coatings derived via the sol–gel method. Ceram Int. 2020, 46, 17741–17751. [Google Scholar] [CrossRef]
- Mumjitha, M.; Raj, V. Fabrication of TiO2–SiO2 bioceramic coatings on Ti alloy and its synergetic effect on biocompatibility and corrosion resistance. J. Mech. Behav. Biomed. Mater. 2015, 46, 205–221. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wang, P.; Chen, S.; Lu, Z.; Li, W. Electropolymerization and corrosion protection performance of the Nb:TiO2 nanofibers/polyaniline composite coating. J. Taiwan Inst. Chem. Eng. 2019, 103, 190–198. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Lu, Z.; Wang, L.; Li, W. Preparation and performances of electrically conductive Nb-doped TiO2 coatings for 316 stainless steel bipolar plates of proton-exchange membrane fuel cells. Corros Sci. 2018, 142, 249–257. [Google Scholar] [CrossRef]
- Figueira, R.B.; Sousa, R.; Silva, C.J.R. Multifunctional and smart organic–inorganic hybrid sol–gel coatings for corrosion protection applications. Adv. Smart Coatings Thin Film. Futur. Ind. Biomed. Eng. Appl. 2020, 57–97. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Nagode, A.; Jerina, K.; Jerman, I.; Vella, D.; Bizjak, M.; Kosec, B.; Karpe, B.; Zorc, B. The effect of sol–gel boehmite coatings on the corrosion and decarburization of C45 steel. J. sol–gel Sci. Technol. 2018, 86, 568–579. [Google Scholar] [CrossRef]
- Bai, C.-Y.; Wen, T.-M.; Hou, K.-H.; Ger, M.-D. The bipolar plate of AISI 1045 steel with chromized coatings prepared by low-temperature pack cementation for proton exchange membrane fuel cell. J. Power Sources 2010, 195, 779–786. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Cheng, S.-C.; Liang, L.-X.; Zhang, J.-C.; Li, S.-Q.; Wang, Z.-L.; Zeng, R.-C. In vitro corrosion resistance of layer-by-layer assembled polyacrylic acid multilayers induced Ca–P coating on magnesium alloy AZ31. Bioact. Mater. 2020, 5, 153–163. [Google Scholar] [CrossRef]
- Martin, U.; Ress, J.; Bosch, J.; Bastidas, D.M. Stress corrosion cracking mechanism of AISI 316LN stainless steel rebars in chloride contaminated concrete pore solution using the slow strain rate technique. Electrochim. Acta 2020, 335, 135565. [Google Scholar] [CrossRef]
- Abbas, N.; Shao, G.N.; Imran, S.M.; Haider, M.S.; Kim, H.T. Inexpensive synthesis of a high-performance Fe3O4-SiO2-TiO2 photocatalyst: Magnetic recovery and reuse. Front. Chem. Sci. Eng. 2016, 10, 405–416. [Google Scholar] [CrossRef]
- Biglu, Y.F.G.; Taheri-Nassaj, E. Investigation of phase separation of nano-crystalline anatase from TiO2-SiO2 thin film. Ceram Int. 2013, 39, 2511–2518. [Google Scholar] [CrossRef]
- Dihingia, P.J.; Rai, S. Synthesis of TiO2 nanoparticles and spectroscopic upconversion luminescence of Nd3+-doped TiO2–SiO2 composite glass. J. Lumin. 2012, 132, 1243–1251. [Google Scholar] [CrossRef]
- Kim, Y.N.; Shao, G.N.; Jeon, S.J.; Imran, S.M.; Sarawade, P.B.; Kim, H.T. Sol–gel synthesis of sodium silicate and titanium oxychloride based TiO2–SiO2 aerogels and their photocatalytic property under UV irradiation. Chem. Eng. J. 2013, 231, 502–511. [Google Scholar] [CrossRef]
- Zita, J.; Maixner, J.; Krýsa, J. Multilayer TiO2/SiO2 thin sol–gel films: Effect of calcination temperature and Na+ diffusion. J. Photochem. Photobiol. A Chem. 2010, 216, 194–200. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, Y.; Yang, S. Fabrication, characterization, and photocatalytic performance of TiO2 hybridized with SiO2. Russ. J. Appl. Chem. 2016, 89, 2050–2060. [Google Scholar] [CrossRef]
- Yan, S.-R.; Gholami, T.; Amiri, O.; Salavati-Niasari, M.; Seifi, S.; Amiri, M. Effect of adding TiO2, SiO2 and graphene on of electrochemical hydrogen storage performance and coulombic efficiency of CoAl2O4 spinel. J. Alloys Compd. 2020, 828, 154353. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2–SiO2–PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Q. TiO2/SiO2 membrane materials via a sol–gel process: Preparation and characterization calcined under N2 atmosphere. Ferroelectrics 2019, 547, 10–20. [Google Scholar] [CrossRef]
- Koli, V.B.; Mavengere, S.; Kim, J.-S. An efficient one-pot N doped TiO2-SiO2 synthesis and its application for photocatalytic concrete. Appl. Surf. Sci. 2019, 491, 60–66. [Google Scholar] [CrossRef]
- Kirtay, S. Characterization of SiO2-TiO2 Hybrid Corrosion Protective Coatings on Mild Steel. J. Mater. Eng. Perform. 2014, 23, 4309–43015. [Google Scholar] [CrossRef]
- Krzak-Roś, J.; Filipiak, J.; Pezowicz, C.; Baszczuk, A.; Miller, M.; Kowalski, M. The effect of substrate roughness on the surface structure of TiO2, SiO2, and doped thin films prepared by the sol–gel method. Acta Bioeng. Biomech. 2009, 11, 21–29. [Google Scholar]
- Halin, D.S.C.; Abdullah, M.; Mahmed, N.; Malek, S.N.A.A.; Vizureanu, P.; Azhari, A.W. Synthesis and Characterization of TiO2/SiO2 Thin Film via sol–gel Method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 12002. [Google Scholar] [CrossRef]
- Riazian, M.; Montazeri, N.; Biazar, E. Nano structural properties of TiO2-SiO2. Orient. J. Chem. 2011, 27, 903. [Google Scholar]
- Wiranwetchayan, O.; Promnopat, S.; Thongtem, T.; Chaipanich, A.; Thongtem, S. Effect of polymeric precursors on the properties of TiO2 films prepared by sol–gel method. Mater. Chem. Phys. 2020, 240, 122219. [Google Scholar] [CrossRef]
- Gulyaev, A.; Kondrutieva, O.; Koldaev, V.; Koltsov, V. Building a Three-dimensional Purbe Diagram. In Proceedings of the 2020 IEEE Conference of Russian Young Researchers in Electrical and Electronic Engineering (EIConRus), Moscow, Russia, 27–30 January 2020; pp. 1960–1963. [Google Scholar]
- Wongpanya, P.; Saramas, Y.; Chumkratoke, C.; Wannakomol, A. Erosion–corrosion behaviors of 1045 and J55 steels in crude oil. J. Pet. Sci. Eng. 2020, 189, 106965. [Google Scholar] [CrossRef]
- Ziadi, I.; Alves, M.M.; Taryba, M.; El-Bassi, L.; Hassairi, H.; Bousselmi, L.; Akrout, K. Microbiologically influenced corrosion mechanism of 304L stainless steel in treated urban wastewater and protective effect of silane-TiO2 coating. Bioelectrochemistry 2020, 132, 107413. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dong, L.; Yang, Y.; Wu, Z.; Zhu, H.; Dong, Y. Corrosion Behavior of AISI 1045 Carbon Steel in Metalworking Fluids Containing Pseudomonas xiamenensis. Int. J. Electrochem. Sci. 2020, 15, 470–483. [Google Scholar] [CrossRef]
- Aversa, A.; Saboori, A.; Librera, E.; de Chirico, M.; Biamino, S.; Lombardi, M.; Fino, P. The Role of Directed Energy Deposition Atmosphere Mode on the Microstructure and Mechanical Properties of 316L Samples. Addit. Manuf. 2020, 34, 101274. [Google Scholar] [CrossRef]
- Boes, J.; Röttger, A.; Becker, L.; Theisen, W. Processing of gas-nitrided AISI 316L steel powder by laser powder bed fusion–Microstructure and properties. Addit. Manuf. 2019, 30, 100836. [Google Scholar] [CrossRef]
- Gu, W.; Baker, D.R.; Liu, Y.; Gasteiger, H.A. Proton exchange membrane fuel cell (PEMFC) down-the-channel performance model. Handb. Fuel Cells 2010. [Google Scholar] [CrossRef]
- Ghorbani, M.M.; Taherian, R.; Bozorg, M. Investigation on physical and electrochemical properties of TiN-coated Monel alloy used for bipolar plates of proton exchange membrane fuel cell. Mater. Chem. Phys. 2019, 238, 121916. [Google Scholar] [CrossRef]
- He, R.Y.; Jiang, J.; Wang, R.F.; Yue, Y.; Chen, Y.; Pan, T.J. Anti-corrosion and conductivity of titanium diboride coating on metallic bipolar plates. Corros. Sci. 2020, 170, 108646. [Google Scholar] [CrossRef]
- Mani, S.P.; Rajendran, N. Corrosion and interfacial contact resistance behavior of electrochemically nitrided 316L SS bipolar plates for proton exchange membrane fuel cells. Energy 2017, 133, 1050–1062. [Google Scholar] [CrossRef]
- Yang, S.; Brant, A.T.; Giles, N.C.; Halliburton, L.E. Intrinsic small polarons in rutile TiO2. Phys. Rev. B 2013, 87, 125201. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Reduced and n-type doped TiO2: Nature of Ti3+ species. J. Phys. Chem. C 2009, 113, 20543–20552. [Google Scholar] [CrossRef]


| Elements | Mn | C | Si | Cr | Ni | S | P | Fe |
|---|---|---|---|---|---|---|---|---|
| wt.% | 0.65 | 0.45 | 0.27 | 0.25 | 0.25 | 0.02 | 0.04 | balance |
| Annealing Temperature | Annealing Atmosphere | RS | Nb0.01 | Nb0.02 |
|---|---|---|---|---|
| 700 °C | Nitrogen | RS-700 °C | TiSiNb0.01-700 °C | TiSiNb0.02-700 °C |
| 600 °C | RS-600 °C | TiSiNb0.01-600 °C | TiSiNb0.02-600 °C | |
| 500 °C | RS-500 °C | TiSiNb0.01-500 °C | TiSiNb0.02-500 °C |
| Sample | Immersion Time | Parameters | ||||
|---|---|---|---|---|---|---|
| Ecorr (V) | βa (V/dec) | βc (V/dec) | Icorr (A/cm2) | Rp (Ω cm2) | ||
| TiSiNb0.02-700 °C | 720 h | −0.29.4 | 2.8 × 10−2 | 3.2 × 10−2 | 6.9 × 10−7 | 9262 |
| TiSiNb0.02-600 °C | −0.29.5 | 3.3 × 10−2 | 3.3 × 10−2 | 7.1 × 10−7 | 9681 | |
| TiSiNb0.02-500 °C | −0.28.9 | 3.8 × 10−2 | 4.1 × 10−2 | 6.5 × 10−7 | 13,285 | |
| TiSiNb0.02-700 °C | 360 h | −0.29.6 | 2.9 × 10−2 | 3.1 × 10−2 | 7.2 × 10−7 | 9038 |
| TiSiNb0.02-600 °C | −0.29.6 | 4.1 × 10−2 | 4.2 × 10−2 | 9.5 × 10−7 | 9581 | |
| TiSiNb0.02-500 °C | −0.28.4 | 2.7 × 10−2 | 3.1 × 10−2 | 2.8 × 10−7 | 21,050 | |
| TiSiNb0.02-700 °C | 24 h | −0.29.2 | 2.9 × 10−2 | 3.3 × 10−2 | 7.1 × 10−7 | 9345 |
| TiSiNb0.02-600 °C | −0.28.7 | 3.9 × 10−2 | 4.1 × 10−2 | 7.1 × 10−7 | 12,423 | |
| TiSiNb0.02-500 °C | −0.27.9 | 3.6 × 10−2 | 4.2 × 10−2 | 3.7 × 10−7 | 23,152 | |
| TiSiNb0.02-700 °C | 1 h | −0.29.5 | 3.1 × 10−2 | 3.9 × 10−2 | 7.1 × 10−7 | 10,523 |
| TiSiNb0.02-600 °C | −0.29.4 | 3.6 × 10−2 | 4.1 × 10−2 | 6.1 × 10−7 | 13,588 | |
| TiSiNb0.02-500 °C | −0.28.1 | 2.9 × 10−2 | 3.5 × 10−2 | 2.6 × 10−7 | 26,533 | |
| RS-700 °C | −0.47.5 | 4.9 × 10−2 | 5.9 × 10−2 | 5.8 × 10−6 | 2030 | |
| RS-600 °C | −0.47.9 | 5.9 × 10−2 | 6.5 × 10−2 | 1.6 × 10−5 | 854 | |
| RS-500 °C | −0.48.4 | 5.6 × 10−2 | 3.7 × 10−2 | 3.1 × 10−5 | 326 | |
| Sample Name | RS-500 °C | TiSiNb0.02-700 °C | |||
|---|---|---|---|---|---|
| Immersion Time | 1 h | 1 h | 24 h | 360 h | 720 h |
| Rs (Ω cm2) | 19.5 | 16.4 | 18.1 | 18.3 | 18.9 |
| Y0 (Ω−1 cm2 sn) | 2.9 × 10−4 | 2.4 × 10−3 | 6.9 × 10−3 | 6.5 × 10−4 | 1.5 × 10−3 |
| n | 0.9 | 0.6 | 0.6 | 0.8 | 0.7 |
| Rct (Ω cm2) | 179.1 | 3.3 × 103 | 2.8 × 103 | 2.5 × 103 | 2.7 × 103 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khosravi H., S.; Vallant, R.; Ladenstein, L.; Reichmann, K. Electrochemical and Structural Property of TiSiNb TFSOC on Affordable Interconnects in Proton Exchange Membrane Fuel Cell Applications. Nanomaterials 2020, 10, 2010. https://doi.org/10.3390/nano10102010
Khosravi H. S, Vallant R, Ladenstein L, Reichmann K. Electrochemical and Structural Property of TiSiNb TFSOC on Affordable Interconnects in Proton Exchange Membrane Fuel Cell Applications. Nanomaterials. 2020; 10(10):2010. https://doi.org/10.3390/nano10102010
Chicago/Turabian StyleKhosravi H., Saman, Rudolf Vallant, Lukas Ladenstein, and Klaus Reichmann. 2020. "Electrochemical and Structural Property of TiSiNb TFSOC on Affordable Interconnects in Proton Exchange Membrane Fuel Cell Applications" Nanomaterials 10, no. 10: 2010. https://doi.org/10.3390/nano10102010
APA StyleKhosravi H., S., Vallant, R., Ladenstein, L., & Reichmann, K. (2020). Electrochemical and Structural Property of TiSiNb TFSOC on Affordable Interconnects in Proton Exchange Membrane Fuel Cell Applications. Nanomaterials, 10(10), 2010. https://doi.org/10.3390/nano10102010





