Silver Nanoparticles Agglomerate Intracellularly Depending on the Stabilizing Agent: Implications for Nanomedicine Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Silver Nanoparticles
2.2. Incubation Scenarios
2.3. Dynamic Light Scattering
2.4. Transmission Electron Microscopy
2.5. Hepatocyte Cell Culture
2.6. Fluorescence Imaging
2.7. Hyperspectral Imaging
2.8. Inductively Coupled Plasma-Mass Spectrometry
2.9. Ultraviolet-Visible Absorption Spectroscopy
2.10. Statistical Analyses
3. Results
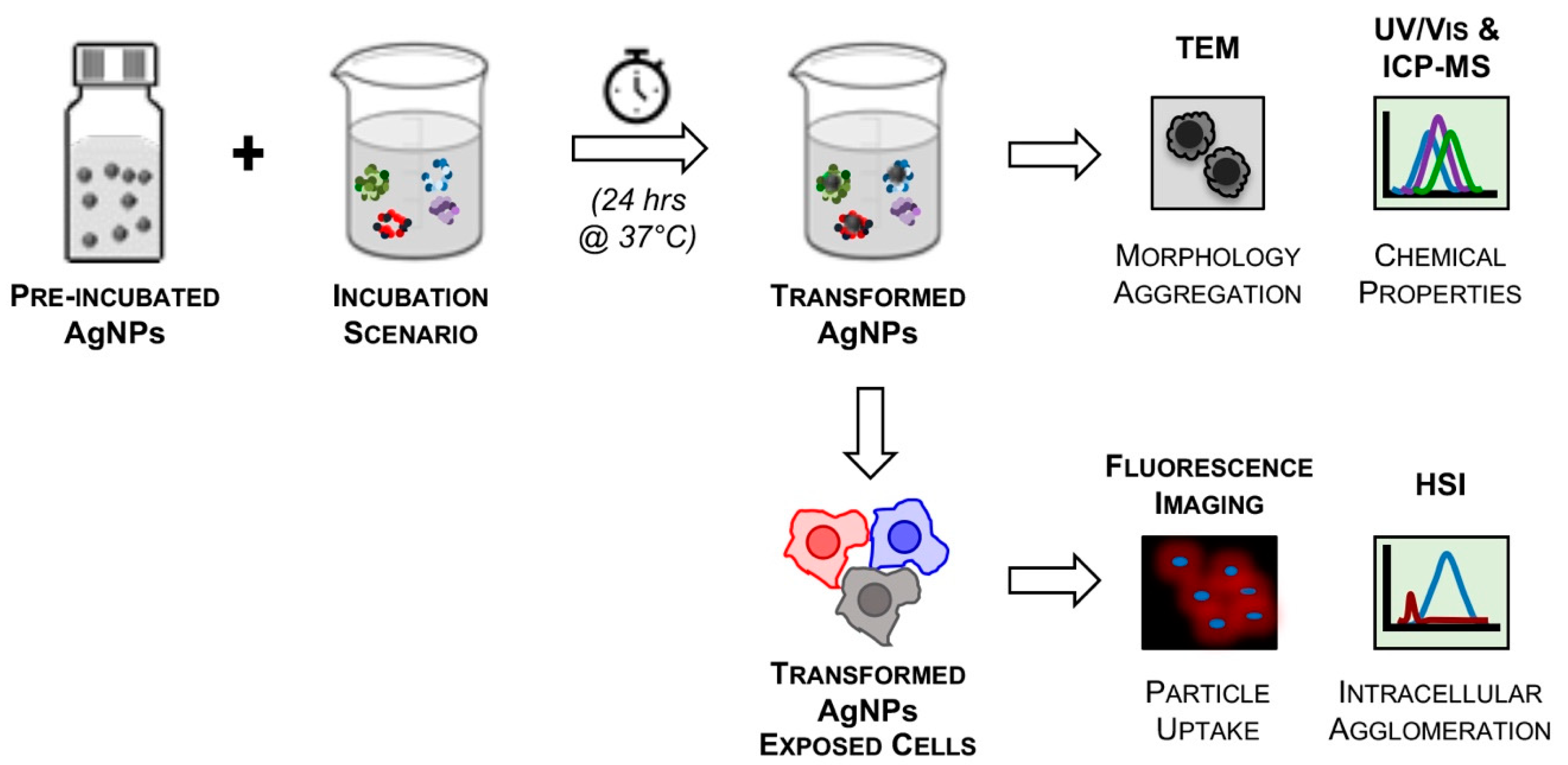
3.1. Experimental Design
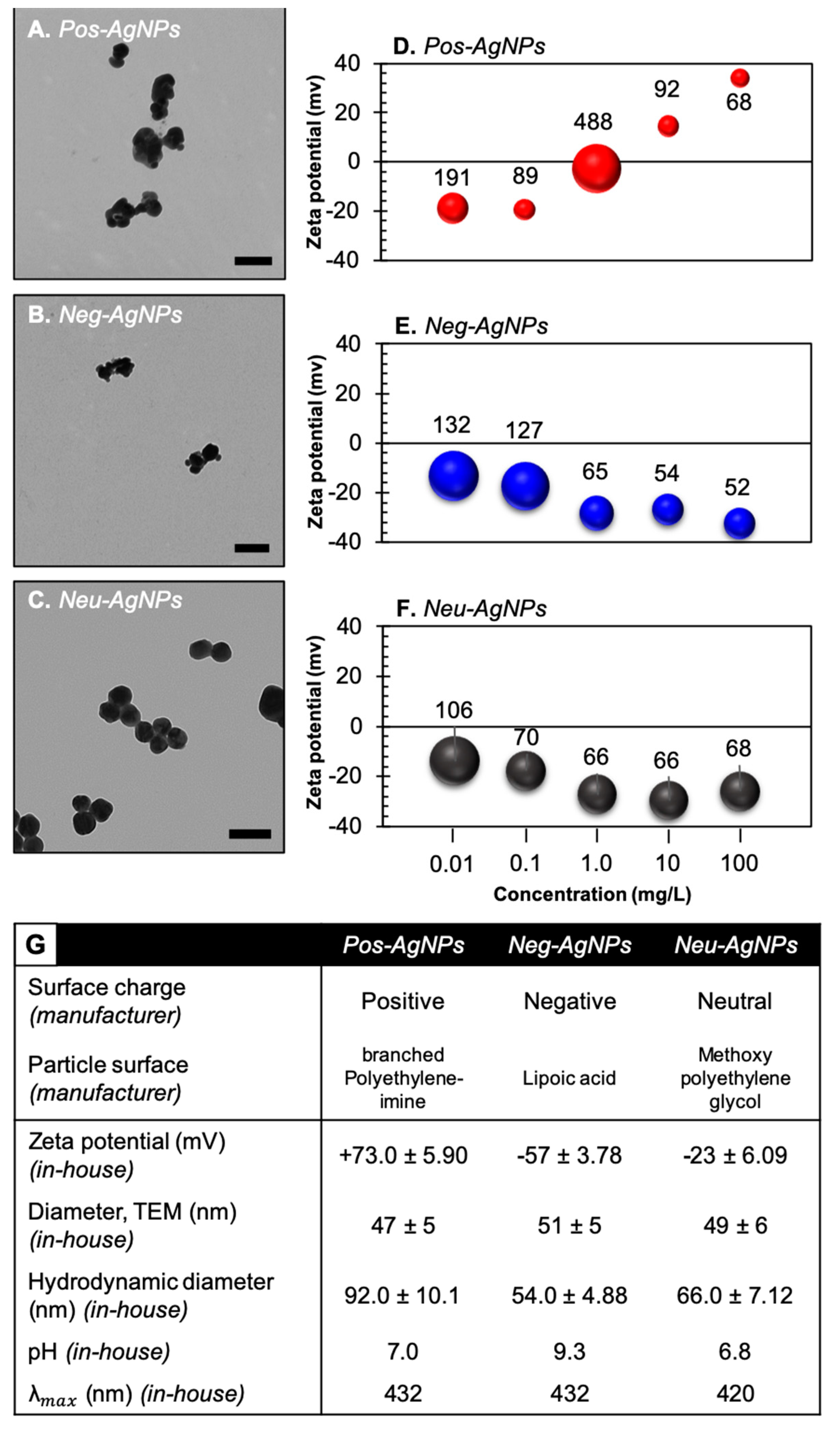
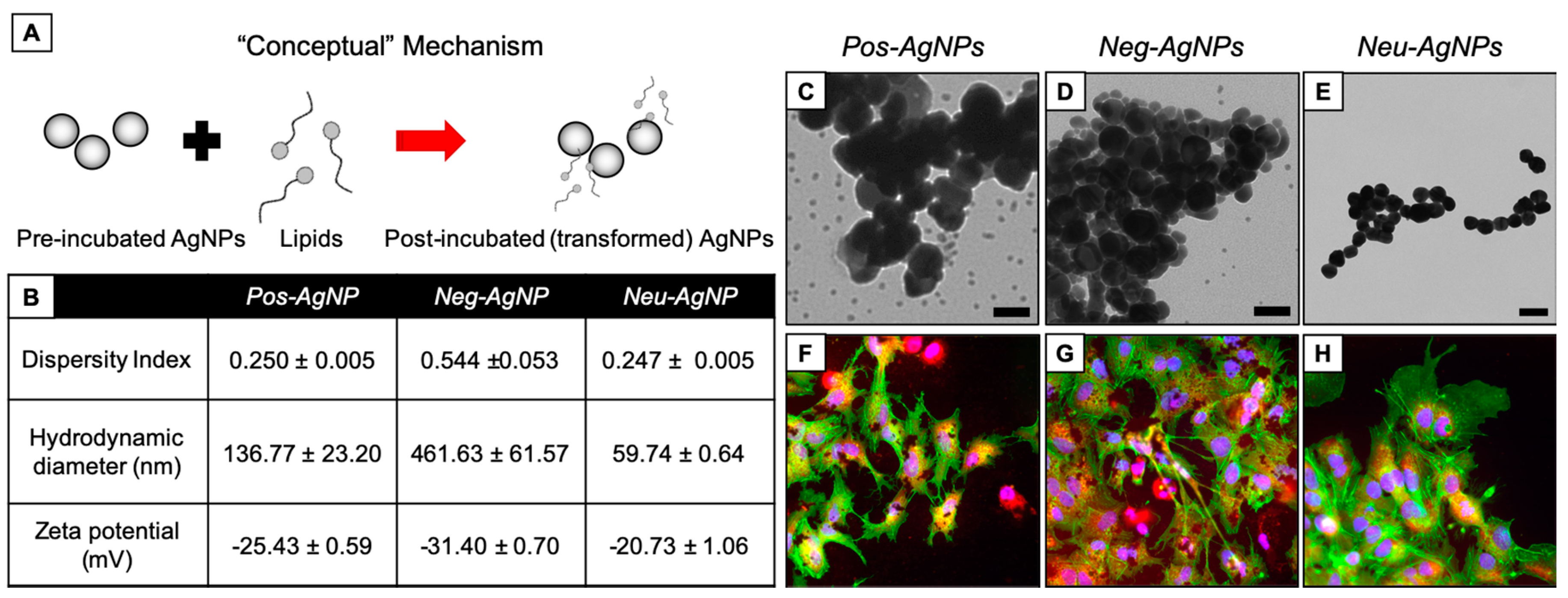
3.2. Engineered Nanoparticles for Nano-Enabled Drug Products
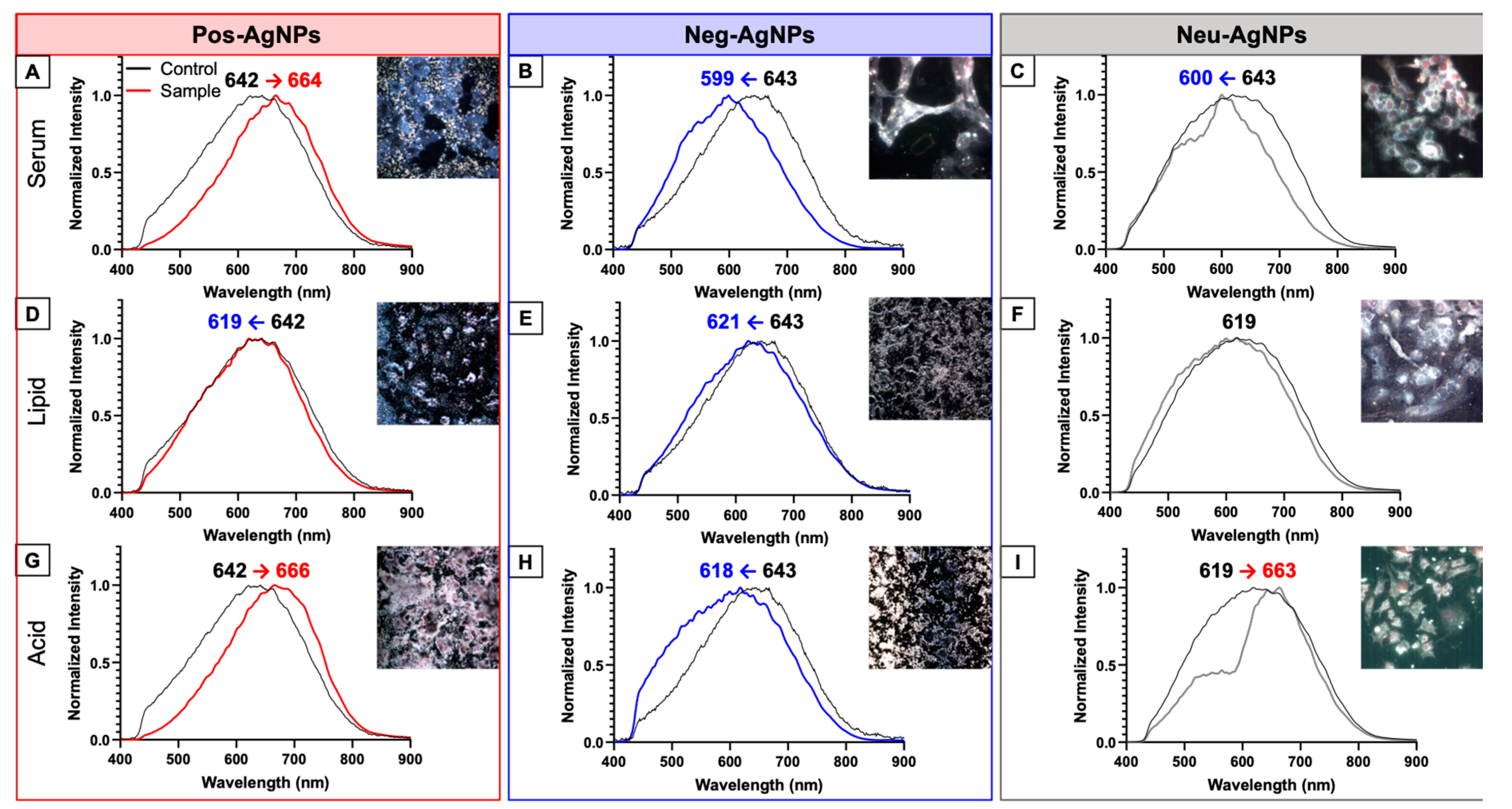
3.3. Hyperspectral Imaging for Intracellular Agglomeration Analysis
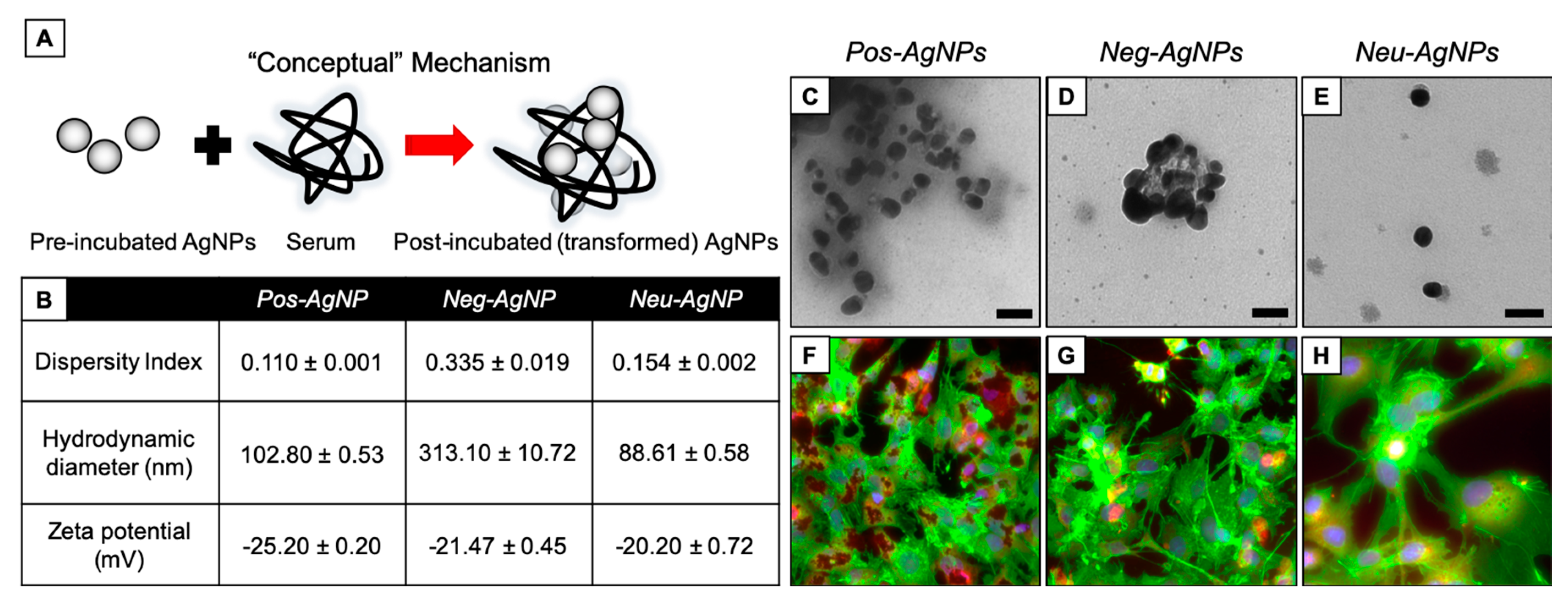
3.4. Biotransformed Silver Nanoparticles Used in Drug Products
3.5. Negatively-Charged Silver Nanoparticles Influence Nanoparticle Biotransformation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medici, S.; Peana, M.; Nurchi, V.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef] [PubMed]
- Yih, T.C.; Al-Fandi, M. Engineered nanoparticles as precise drug delivery systems. J. Cell. Biochem. 2006, 97, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.; Shrestha, N.; Granja, P.L.; Hirvonen, J.; Santos, H.A.; Sarmento, B. Safety and toxicity concerns of orally delivered nanoparticles as drug carriers. Expert Opin. Drug Metab. Toxicol. 2014, 11, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul. Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N.-J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sayes, C.M.; Guo, B.; Pillai, S.; Parsons, J.G.; Wang, C.; Yan, B.; Ma, X. Interactions between silver nanoparticles and other metal nanoparticles under environmentally relevant conditions: A review. Sci. Total Environ. 2019, 653, 1042–1051. [Google Scholar] [CrossRef]
- Mulenos, M.R.; Liu, J.; Lujan, H.; Guo, B.; Lichtfouse, E.; Sharma, V.K.; Sayes, C.M. Copper, silver, and titania nanoparticles do not release ions under anoxic conditions and release only minute ion levels under oxic conditions in water: Evidence for the low toxicity of nanoparticles. Environ. Chem. Lett. 2020, 18, 1319–1328. [Google Scholar] [CrossRef]
- Vrček, I.V.; Žuntar, I.; Petlevski, R.; Pavičić, I.; Sikirić, M.D.; Ćurlin, M.; Goessler, W. Comparison of in vitro toxicity of silver ions and silver nanoparticles on human hepatoma cells. Environ. Toxicol. 2016, 31, 679–692. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Münchow, M.; Pirev, E.; Heβner, F.; Bozkurt, A.; Uciechowski, P.; Pallua, N.; Kröncke, K.-D.; Suschek, C.V. Silver ions induce oxidative stress and intracellular zinc release in human skin fibroblasts. Free. Radic. Biol. Med. 2009, 47, 1570–1577. [Google Scholar] [CrossRef]
- Trickler, W.J.; Lantz, S.M.; Murdock, R.C.; Schrand, A.M.; Robinson, B.; Newport, G.D.; Schlager, J.J.; Oldenburg, S.J.; Paule, M.G.; Slikker, W.; et al. Silver Nanoparticle Induced Blood-Brain Barrier Inflammation and Increased Permeability in Primary Rat Brain Microvessel Endothelial Cells. Toxicol. Sci. 2010, 118, 160–170. [Google Scholar] [CrossRef]
- Duvall, M.N. FDA Regulation of Nanotechnology; Beveridge & Diamond, PG: Washington, DC, USA, 2012. [Google Scholar]
- Zheng, N.; Sun, D.D.; Zou, P.; Jiang, W. Scientific and Regulatory Considerations for Generic Complex Drug Products Containing Nanomaterials. AAPS J. 2017, 19, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Braydich-Stolle, L.K.; Breitner, E.K.; Comfort, K.K.; Schlager, J.J.; Hussain, S.M. Dynamic Characteristics of Silver Nanoparticles in Physiological Fluids: Toxicological Implications. Langmuir 2014, 30, 15309–15316. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; DeLoid, G.; Pyrgiotakis, G.; Demokritou, P. Interactions of engineered nanomaterials in physiological media and implications for in vitro dosimetry. Nanotoxicology 2012, 7, 417–431. [Google Scholar] [CrossRef] [PubMed]
- De Vlieger, J.S.B.; Crommelin, D.J.A.; Tyner, K.; Drummond, D.C.; Jiang, W.; McNeil, S.E.; Neervannan, S.; Crist, R.M.; Shah, V.P. Report of the AAPS Guidance Forum on the FDA Draft Guidance for Industry: “Drug Products, Including Biological Products, that Contain Nanomaterials”. AAPS J. 2019, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Morrison, H.G.; Tao, W.; Trieu, W.; Walker, S.D.; Cui, S.; Huggins, S.; Nagapudi, K. Correlation of Drug Substance Particle Size Distribution with Other Bulk Properties to Predict Critical Quality Attributes. Org. Process. Res. Dev. 2014, 19, 1076–1081. [Google Scholar] [CrossRef]
- Dawidczyk, C.M.; Kim, C.; Park, J.H.; Russell, L.M.; Lee, K.; Pomper, M.G.; Searson, P.C. State-of-the-art in design rules for drug delivery platforms: Lessons learned from FDA-approved nanomedicines. J. Control. Release 2014, 187, 133–144. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Lujan, H.; Griffin, W.C.; Taube, J.H.; Sayes, C.M. Synthesis and characterization of nanometer-sized liposomes for encapsulation and microRNA transfer to breast cancer cells. Int. J. Nanomed. 2019, 14, 5159–5173. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of Environmental Conditions (pH, Ionic Strength, and Electrolyte Type) on the Surface Charge and Aggregation of Silver Nanoparticles Suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Pokhrel, L.R.; Dubey, B.K.; Tolaymat, T.M.; Maier, K.J.; Liu, X. Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: Comparison between general linear model-predicted and observed toxicity. Sci. Total. Environ. 2014, 468, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Saibene, M.; Moschini, E.; Bonfanti, P.; Collini, M.; Kasemets, K.; Mantecca, P. Teratogenic hazard of BPEI-coated silver nanoparticles to Xenopus laevis. Nanotoxicology 2017, 11, 405–418. [Google Scholar] [CrossRef]
- Gitipour, A.; Thiel, S.W.; Scheckel, K.G.; Tolaymat, T. Anaerobic toxicity of cationic silver nanoparticles. Sci. Total. Environ. 2016, 557, 363–368. [Google Scholar] [CrossRef]
- Niska, K.; Knap, N.; Kędzia, A.; Jaskiewicz, M.; Kamysz, W.; Inkielewicz-Stepniak, I. Capping Agent-Dependent Toxicity and Antimicrobial Activity of Silver Nanoparticles: An In Vitro Study. Concerns about Potential Application in Dental Practice. Int. J. Med. Sci. 2016, 13, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Chandran, P.; Riviere, J.E.; Monteiro-Riviere, N.A. Surface chemistry of gold nanoparticles determines the biocorona composition impacting cellular uptake, toxicity and gene expression profiles in human endothelial cells. Nanotoxicology 2017, 11, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, L.M.; Boyd, B.; Karellas, P.; Krippner, G.Y.; Lessene, R.; Kelly, B.; Porter, C.J. The Impact of Molecular Weight and PEG Chain Length on the Systemic Pharmacokinetics of PEGylated Poly l-Lysine Dendrimers. Mol. Pharm. 2008, 5, 449–463. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Giudice, C.L.; Herda, L.M.; Polo, E.; Dawson, K. In situ characterization of nanoparticle biomolecular interactions in complex biological media by flow cytometry. Nat. Commun. 2016, 7, 13475. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Liu, F.D.; Kane, A.B.; Hurt, R.H. Chemical Transformations of Nanosilver in Biological Environments. ACS Nano 2012, 6, 9887–9899. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, Y.; Dana, S.F.; Li, B.; Lee, C.-Y.; Kang, L. Novel engineered systems for oral, mucosal and transdermal drug delivery. J. Drug Target. 2013, 21, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [PubMed]
- Corbo, C.; Molinaro, R.; Parodi, A.; Furman, N.E.T.; Salvatore, F.; Tasciotti, E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, H.S.; Palanikumar, L.; Go, E.M.; Jana, B.; Park, S.A.; Kim, H.Y.; Kim, K.; Seo, J.K.; Kwak, S.K.; et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat. Commun. 2018, 9, 4548. [Google Scholar] [CrossRef]
- Maiolo, D.; Del Pino, P.; Metrangolo, P.; Parak, W.J.; Bombelli, F.B. Nanomedicine delivery: Does protein corona route to the target or off road? Nanomedicine 2015, 10, 3231–3247. [Google Scholar] [CrossRef]
- Elder, A.; Vidyasagar, S.; De Louise, L.A. Physicochemical factors that affect metal and metal oxide nanoparticle passage across epithelial barriers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 434–450. [Google Scholar] [CrossRef]
- Riediker, M.; Zink, D.; Kreyling, W.; Oberdörster, G.; Elder, A.; Graham, U.; Lynch, I.; Duschl, A.; Ichihara, G.; Ichihara, S.; et al. Particle toxicology and health-where are we? Part. Fibre Toxicol. 2019, 16, 19. [Google Scholar] [CrossRef]
- Kang, S.; Char, K.; Kang, Y.S. Novel Application of Partially Positively Charged Silver Nanoparticles for Facilitated Transport in Olefin/Paraffin Separation Membranes. Chem. Mater. 2008, 20, 1308–1311. [Google Scholar] [CrossRef]
- Kang, S.-W.; Choi, J.; Park, J.H.; Mun, S.H.; Kim, J.H.; Cho, J.; Char, K.; Kang, Y.S. Nanocomposite membranes containing positively polarized gold nanoparticles for facilitated olefin transport. J. Membr. Sci. 2008, 321, 90–93. [Google Scholar] [CrossRef]
- Sabella, S.; Carney, R.P.; Brunetti, V.; Malvindi, M.A.; Al-Juffali, N.; Vecchio, G.; Janes, S.M.; Bakr, O.M.; Cingolani, R.; Stellacci, F.; et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale 2014, 6, 7052–7061. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Wang, Y.; Kapronezai, K.; Lorente, L.R.; Zhang, R.; Pyrgiotakis, G.; Konduru, N.V.; Ericsson, M.; White, J.C.; De La Torre-Roche, R.; et al. An integrated methodology for assessing the impact of food matrix and gastrointestinal effects on the biokinetics and cellular toxicity of ingested engineered nanomaterials. Part. Fibre Toxicol. 2017, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Gil-Sánchez, I.; Tamargo, A.; Miralles, B.; Crespo, J.; Bartolomé, B.; Moreno-Arribas, M.V. Gastrointestinal digestion of food-use silver nanoparticles in the dynamic SIMulator of the GastroIntestinal tract (simgi®). Impact on human gut microbiota. Food Chem. Toxicol. 2019, 132, 110657. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.H.; Axson, J.L.; Bergin, I.L.; Ault, A.P. Nanoparticle Digestion Simulator Reveals pH-dependent Aggregation in the Gastrointestinal Tract. Anal. Chem. 2020, 92, 12257–12264. [Google Scholar] [CrossRef] [PubMed]
- Duman, O.; Tunç, S. Electrokinetic and rheological properties of Na-bentonite in some electrolyte solutions. Microporous Mesoporous Mater. 2009, 117, 331–338. [Google Scholar] [CrossRef]
- Michen, B.; Geers, C.; Vanhecke, D.; Endes, C.; Rothen-Rutishauser, B.; Balog, S.; Petri-Fink, A. Avoiding drying-artifacts in transmission electron microscopy: Characterizing the size and colloidal state of nanoparticles. Sci. Rep. 2015, 5, 9793. [Google Scholar] [CrossRef]
- Dieckmann, Y.; CoÖlfen, H.; Hofmann, H.; Petri-Fink, A. Particle Size Distribution Measurements of Manganese-Doped ZnS Nanoparticles. Anal. Chem. 2009, 81, 3889–3895. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar]
- Adabi, M.; Naghibzadeh, M.; Adabi, M.; Zarrinfard, M.A.; Esnaashari, S.S.; Seifalian, A.M.; Faridi-Majidi, R.; Aiyelabegan, H.T.; Ghanbari, H. Biocompatibility and nanostructured materials: Applications in nanomedicine. Artif. Cells Nanomed. Biotechnol. 2016, 45, 833–842. [Google Scholar] [CrossRef]
- Mortimer, M.; Gogos, A.; Bartolomé, N.; Kahru, A.; Bucheli, T.D.; Slaveykova, V.I. Potential of Hyperspectral Imaging Microscopy for Semi-quantitative Analysis of Nanoparticle Uptake by Protozoa. Environ. Sci. Technol. 2014, 48, 8760–8767. [Google Scholar] [CrossRef]
- Zucker, R.M.; Ortenzio, J.; Degn, L.L.; Lerner, J.M.; Boyes, W.K. Biophysical comparison of four silver nanoparticles coatings using microscopy, hyperspectral imaging and flow cytometry. PLoS ONE 2019, 14, e0219078. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of Nanomaterials in the Environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef] [PubMed]
- Hirn, S.; Semmler-Behnke, M.; Schleh, C.; Wenk, A.; Lipka, J.; Schäffler, M.; Takenaka, S.; Möller, W.; Schmid, G.; Simon, U.; et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm. 2011, 77, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Surface-Coating-Dependent Dissolution, Aggregation, and Reactive Oxygen Species (ROS) Generation of Silver Nanoparticles under Different Irradiation Conditions. Environ. Sci. Technol. 2013, 47, 10293–10301. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulenos, M.R.; Lujan, H.; Pitts, L.R.; Sayes, C.M. Silver Nanoparticles Agglomerate Intracellularly Depending on the Stabilizing Agent: Implications for Nanomedicine Efficacy. Nanomaterials 2020, 10, 1953. https://doi.org/10.3390/nano10101953
Mulenos MR, Lujan H, Pitts LR, Sayes CM. Silver Nanoparticles Agglomerate Intracellularly Depending on the Stabilizing Agent: Implications for Nanomedicine Efficacy. Nanomaterials. 2020; 10(10):1953. https://doi.org/10.3390/nano10101953
Chicago/Turabian StyleMulenos, Marina R., Henry Lujan, Lauren R. Pitts, and Christie M. Sayes. 2020. "Silver Nanoparticles Agglomerate Intracellularly Depending on the Stabilizing Agent: Implications for Nanomedicine Efficacy" Nanomaterials 10, no. 10: 1953. https://doi.org/10.3390/nano10101953
APA StyleMulenos, M. R., Lujan, H., Pitts, L. R., & Sayes, C. M. (2020). Silver Nanoparticles Agglomerate Intracellularly Depending on the Stabilizing Agent: Implications for Nanomedicine Efficacy. Nanomaterials, 10(10), 1953. https://doi.org/10.3390/nano10101953





