How Reversible Are the Effects of Fumed Silica on Macrophages? A Proteomics-Informed View
Abstract
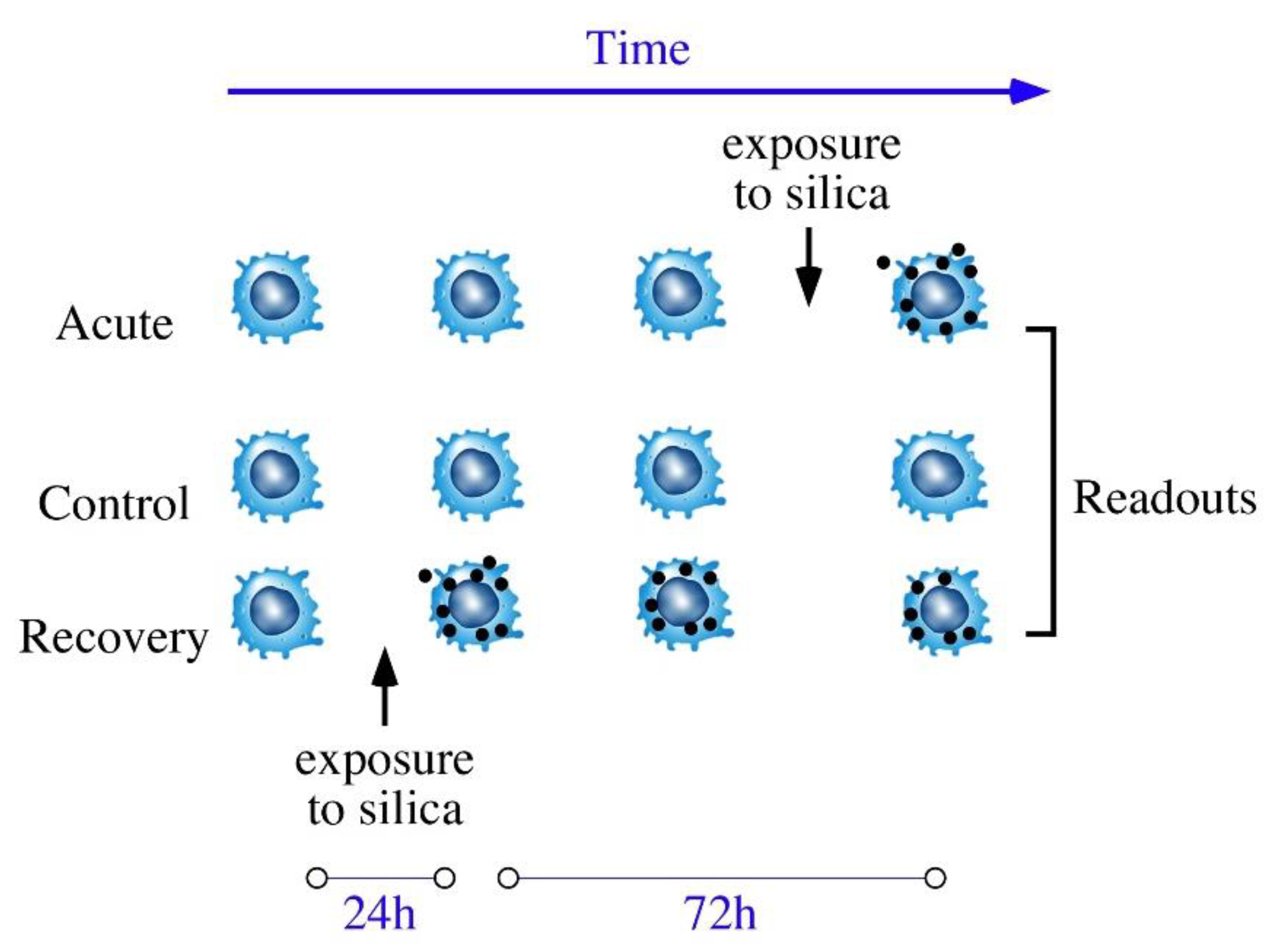
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles
2.2. Cell Culture
2.3. Enzyme Assays
2.4. Phagocytosis and Particle Internalization Assay
2.5. Mitochondrial Transmembrane Potential Measurement
2.6. Lysosomal Function Evaluation
2.7. NO Production and Cytokines Production
2.8. F-Actin Staining
2.9. Proteomics
3. Results
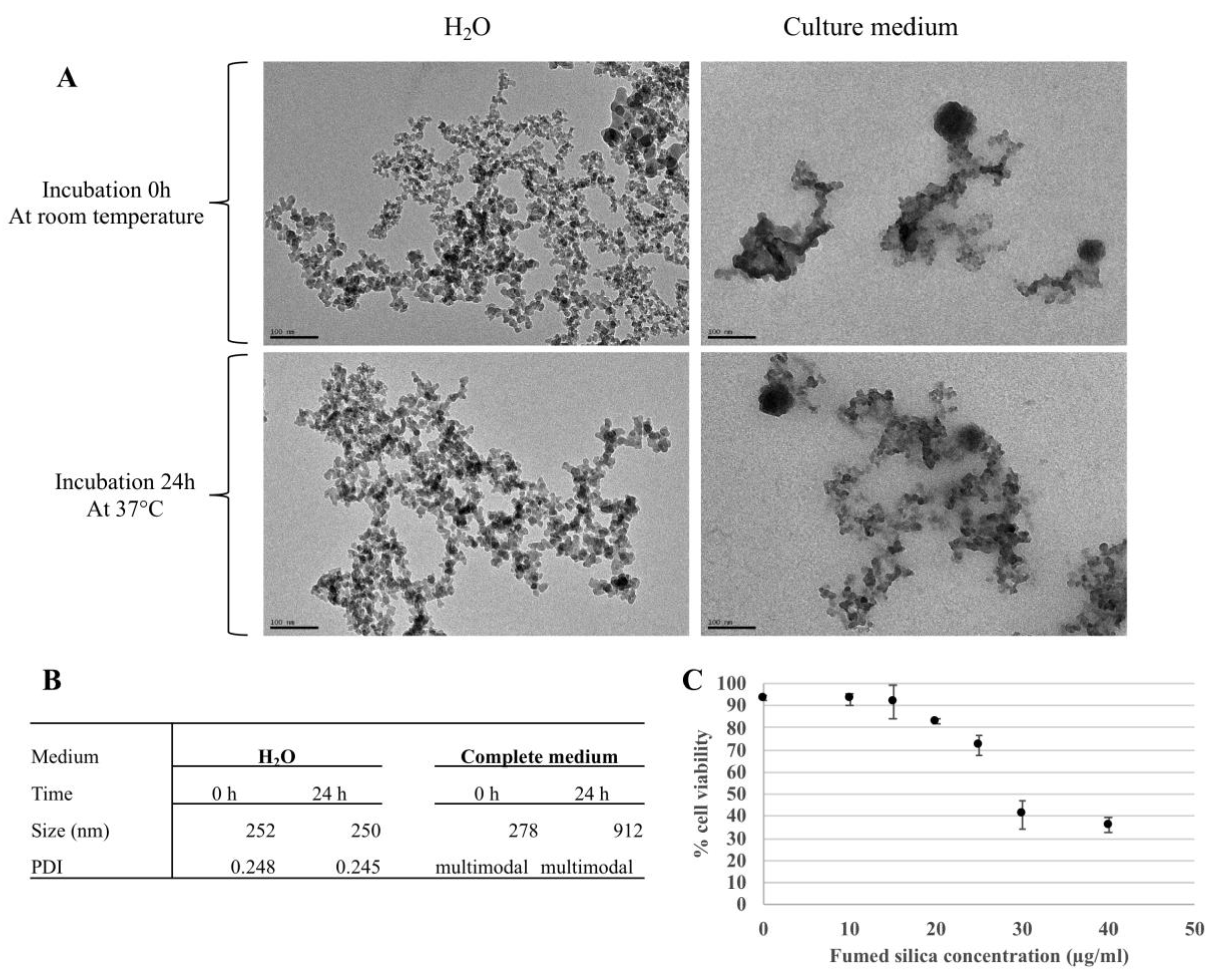
3.1. Nanoparticles Characterization and Determination of the Effective Doses
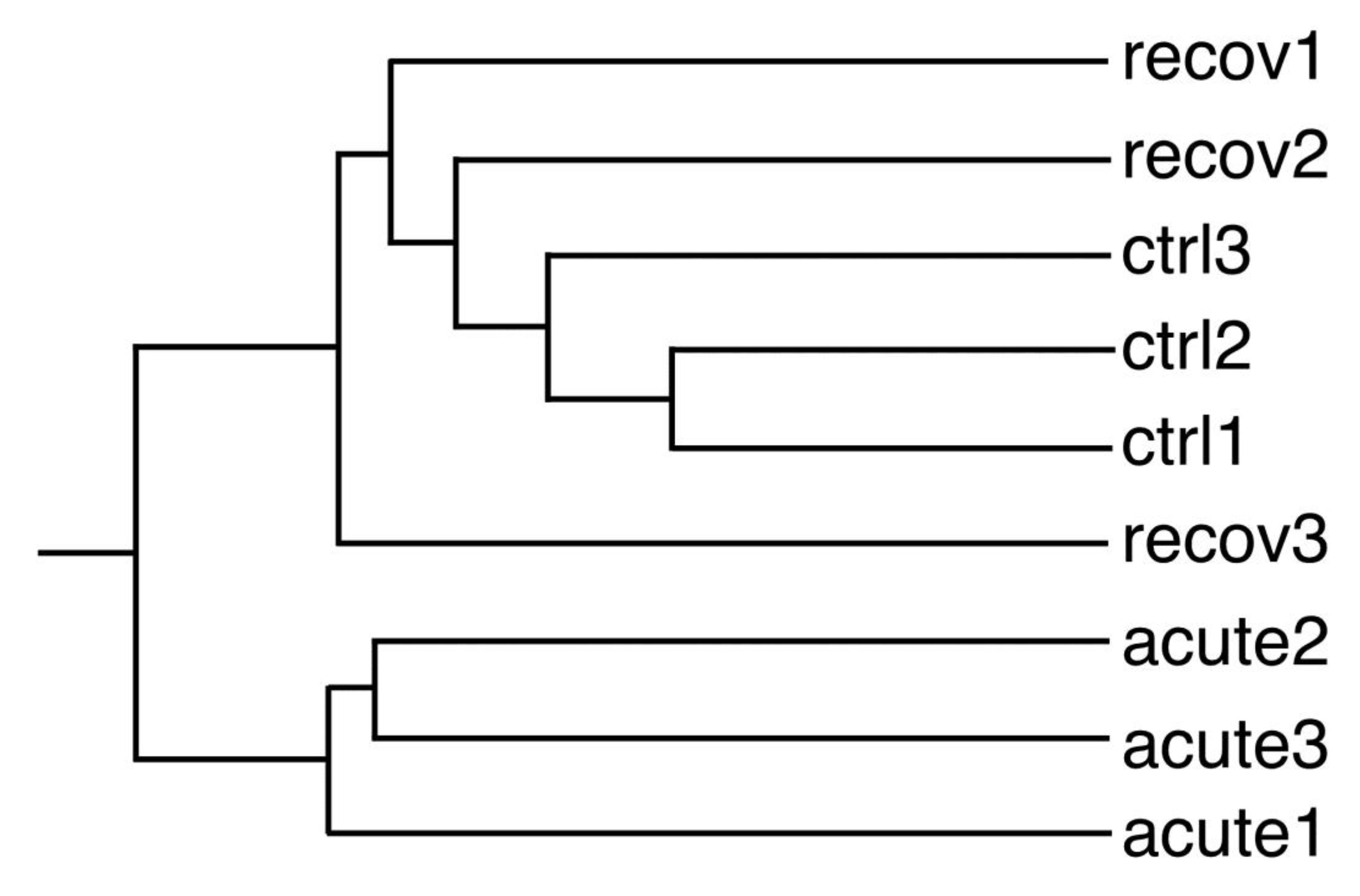
3.2. Proteomic Studies
3.3. Validation Studies
3.3.1. Enzyme Activities
3.3.2. Cytoskeleton and Phagocytosis
3.3.3. Mitochondrial Potential
3.3.4. Lysosomal Integrity
- Control cells: 0.9884 ± 0.0116.
- Cells acutely exposed to 20 µg/mL pyrolytic silica for 24 h and analyzed just after exposure: 0.9888 ± 0.0038.
- Cells acutely exposed to 20 µg/mL pyrolytic silica for 24 h and analyzed after a 72 h recovery period: 1.0001 ± 0.0044.
- These data showed no significant perturbation in the lysosomal integrity under the conditions tested.
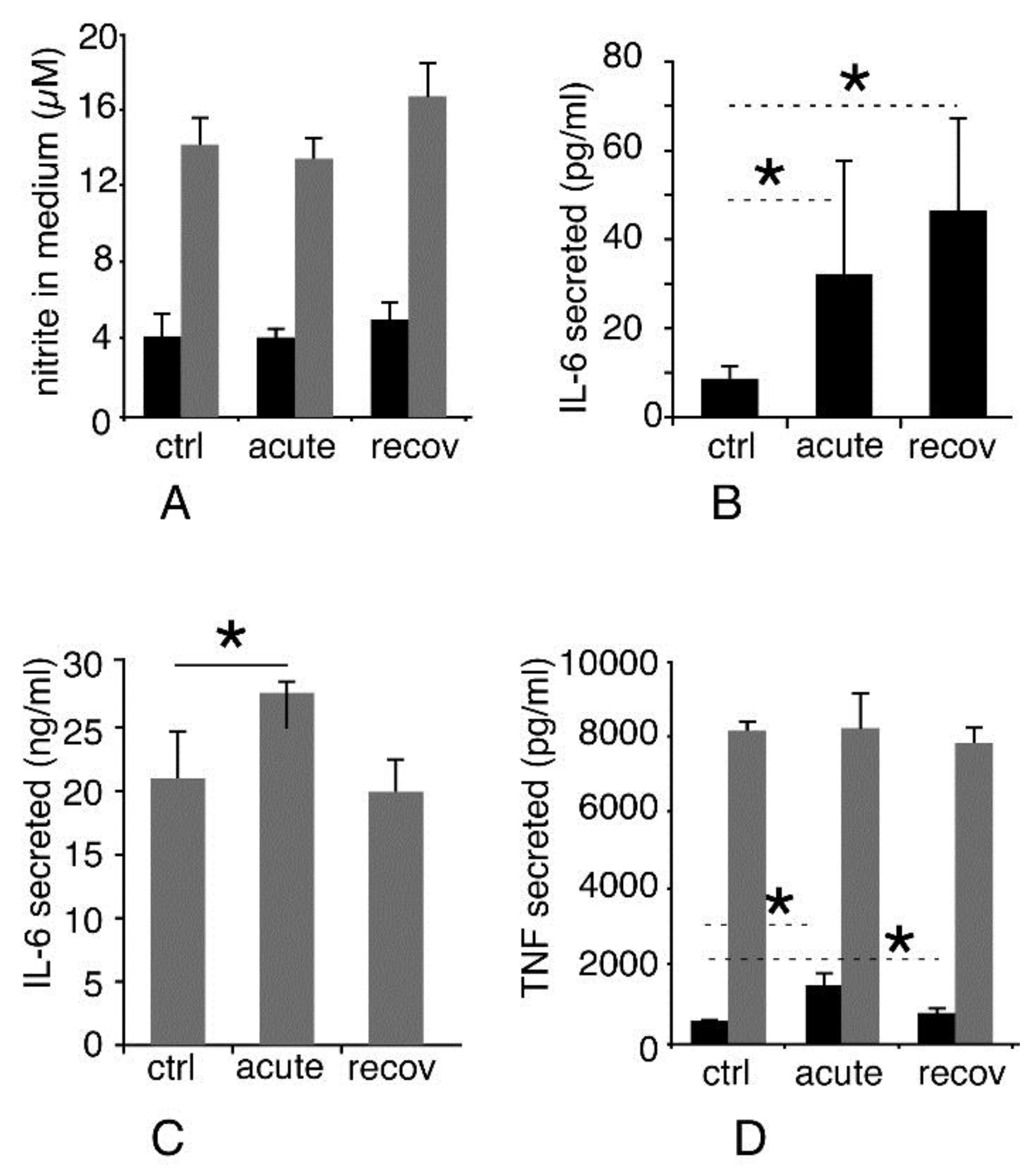
3.3.5. Inflammatory Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamilton, R.F., Jr.; Thakur, S.A.; Holian, A. Silica binding and toxicity in alveolar macrophages. Free. Radic. Biol. Med. 2008, 44, 1246–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayes, C.M.; Reed, K.L.; Warheit, D.B. Assessing toxicity of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol. Sci. 2007, 97, 163–180. [Google Scholar] [CrossRef] [Green Version]
- Arts, J.H.E.; Muijser, H.; Duistermaat, E.; Junker, K.; Kuper, C.F. Five-day inhalation toxicity study of three types of synthetic amorphous silicas in Wistar rats and post-exposure evaluations for up to 3 months. Food Chem. Toxicol. 2007, 45, 1856–1867. [Google Scholar] [CrossRef]
- Turci, F.; Pavan, C.; Leinardi, R.; Tomatis, M.; Pastero, L.; Garry, D.; Anguissola, S.; Lison, D.; Fubini, B. Revisiting the paradigm of silica pathogenicity with synthetic quartz crystals: The role of crystallinity and surface disorder. Part. Fibre Toxicol. 2016, 13, 32. [Google Scholar] [CrossRef] [Green Version]
- Ghiazza, M.; Polimeni, M.; Fenoglio, I.; Gazzano, E.; Ghigo, D.; Fubini, B. Does vitreous silica contradict the toxicity of the crystalline silica paradigm? Chem. Res. Toxicol. 2010, 23, 620–629. [Google Scholar] [CrossRef]
- Gazzano, E.; Ghiazza, M.; Polimeni, M.; Bolis, V.; Fenoglio, I.; Attanasio, A.; Mazzucco, G.; Fubini, B.; Ghigo, D. Physicochemical determinants in the cellular responses to nanostructured amorphous silicas. Toxicol. Sci. 2012, 128, 158–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.Y.; Dunphy, D.R.; Jiang, X.M.; Meng, H.; Sun, B.B.; Tarn, D.; Xue, M.; Wang, X.; Lin, S.J.; Ji, Z.X.; et al. Processing Pathway Dependence of Amorphous Silica Nanoparticle Toxicity: Colloidal vs Pyrolytic. J. Am. Chem. Soc. 2012, 134, 15790–15804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cristo, L.; Movia, D.; Bianchi, M.G.; Allegri, M.; Mohamed, B.M.; Bell, A.P.; Moore, C.; Pinelli, S.; Rasmussen, K.; Riego-Sintes, J.; et al. Proinflammatory Effects of Pyrogenic and Precipitated Amorphous Silica Nanoparticles in Innate Immunity Cells. Toxicol. Sci. 2016, 150, 40–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodali, V.; Littke, M.H.; Tilton, S.C.; Teeguarden, J.G.; Shi, L.; Frevert, C.W.; Wang, W.; Pounds, J.G.; Thrall, B.D. Dysregulation of Macrophage Activation Profiles by Engineered Nanoparticles. Acs Nano 2013, 7, 6997–7010. [Google Scholar] [CrossRef]
- Okoturo-Evans, O.; Dybowska, A.; Valsami-Jones, E.; Cupitt, J.; Gierula, M.; Boobis, A.R.; Edwards, R.J. Elucidation of Toxicity Pathways in Lung Epithelial Cells Induced by Silicon Dioxide Nanoparticles. PLoS ONE 2013, 8, e72363. [Google Scholar] [CrossRef]
- Dalzon, B.; Aude-Garcia, C.; Collin-Faure, V.; Diemer, H.; Beal, D.; Dussert, F.; Fenel, D.; Schoehn, G.; Cianferani, S.; Carriere, M.; et al. Differential proteomics highlights macrophage-specific responses to amorphous silica nanoparticles. Nanoscale 2017, 9, 9641–9658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toybou, D.; Celle, C.; Aude-Garcia, C.; Rabilloud, T.; Simonato, J.-P. A toxicology-informed, safer by design approach for the fabrication of transparent electrodes based on silver nanowires. Environ. Sci. Nano 2019, 6, 684–694. [Google Scholar] [CrossRef]
- Silva, R.M.; Xu, J.; Saiki, C.; Anderson, D.S.; Franzi, L.M.; Vulpe, C.D.; Gilbert, B.; Van Winkle, L.S.; Pinkerton, K.E. Short versus long silver nanowires: A comparison of in vivo pulmonary effects post instillation. Part. Fibre Toxicol. 2014, 11, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triboulet, S.; Aude-Garcia, C.; Armand, L.; Collin-Faure, V.; Chevallet, M.; Diemer, H.; Gerdil, A.; Proamer, F.; Strub, J.M.; Habert, A.; et al. Comparative proteomic analysis of the molecular responses of mouse macrophages to titanium dioxide and copper oxide nanoparticles unravels some toxic mechanisms for copper oxide nanoparticles in macrophages. PLoS ONE 2015, 10, e0124496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalzon, B.; Torres, A.; Diemer, H.; Ravanel, S.; Collin-Faure, V.; Pernet-Gallay, K.; Jouneau, P.-H.; Bourguignon, J.; Cianférani, S.; Carrière, M.; et al. How reversible are the effects of silver nanoparticles on macrophages? A proteomic-instructed view. Environ. Sci. Nano 2019, 6, 3133–3157. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.; Dalzon, B.; Collin-Faure, V.; Rabilloud, T. Repeated vs. Acute Exposure of RAW264.7 Mouse Macrophages to Silica Nanoparticles: A Bioaccumulation and Functional Change Study. Nanomater. Basel 2020, 10, 215. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.J.; Trakshel, G.M.; Maines, M.D. Detection of 10 variants of biliverdin reductase in rat liver by two-dimensional gel electrophoresis. J. Biol. Chem. 1989, 264, 7844–7849. [Google Scholar]
- Kerry, J.A.; Rohde, M.; Kwok, F. Brain pyridoxal kinase. Purification and characterization. Eur. J. Biochem. 1986, 158, 581–585. [Google Scholar] [CrossRef]
- Malcovati, M.; Valentini, G.; Wood, W.A. AMP- and fructose 1, 6-bisphosphate-activated pyruvate kinases from Escherichia coli. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1982; Volume 90, pp. 170–179. [Google Scholar]
- Kuntz, G.n.W.K.; Krietsch, W.K.G. Phosphoglycerate kinase from animal tissue. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1982; Volume 90, pp. 103–110. [Google Scholar]
- Thomé, M.P.; Filippi-Chiela, E.C.; Villodre, E.S.; Migliavaca, C.B.; Onzi, G.R.; Felipe, K.B.; Lenz, G. Ratiometric analysis of Acridine Orange staining in the study of acidic organelles and autophagy. J. Cell Sci. 2016, 129, 4622–4632. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Aude-Garcia, C.; Dalzon, B.; Ravanat, J.L.; Collin-Faure, V.; Diemer, H.; Strub, J.M.; Cianferani, S.; Van Dorsselaer, A.; Carriere, M.; Rabilloud, T. A combined proteomic and targeted analysis unravels new toxic mechanisms for zinc oxide nanoparticles in macrophages. J. Proteom. 2016, 134, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Merkwirth, C.; Langer, T. Prohibitin function within mitochondria: Essential roles for cell proliferation and cristae morphogenesis. Mol. Cell Res. 2009, 1793, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rival, T.; Macchi, M.; Arnauné-Pelloquin, L.; Poidevin, M.; Maillet, F.; Richard, F.; Fatmi, A.; Belenguer, P.; Royet, J. Inner-membrane proteins PMI/TMEM11 regulate mitochondrial morphogenesis independently of the DRP1/MFN fission/fusion pathways. EMBO Rep. 2011, 12, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Cour, J.M.; Winding Gojkovic, P.; Ambjorner, S.E.B.; Bagge, J.; Jensen, S.M.; Panina, S.; Berchtold, M.W. ALG-2 participates in recovery of cells after plasma membrane damage by electroporation and digitonin treatment. PLoS ONE 2018, 13, e0204520. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.B.; Wang, X.; Liao, Y.P.; Ji, Z.X.; Chang, C.H.; Pokhrel, S.; Ku, J.; Liu, X.S.; Wang, M.; Dunphy, D.R.; et al. Repetitive Dosing of Fumed Silica Leads to Profibrogenic Effects through Unique Structure-Activity Relationships and Biopersistence in the Lung. ACS Nano 2016, 10, 8054–8066. [Google Scholar] [CrossRef] [Green Version]
- Sadauskas, E.; Danscher, G.; Stoltenberg, M.; Vogel, U.; Larsen, A.; Wallin, H. Protracted elimination of gold nanoparticles from mouse liver. Nanomedicine 2009, 5, 162–169. [Google Scholar] [CrossRef]
- Aude-Garcia, C.; Villiers, F.; Collin-Faure, V.; Pernet-Gallay, K.; Jouneau, P.H.; Sorieul, S.; Mure, G.; Gerdil, A.; Herlin-Boime, N.; Carriere, M.; et al. Different in vitro exposure regimens of murine primary macrophages to silver nanoparticles induce different fates of nanoparticles and different toxicological and functional consequences. Nanotoxicology 2016, 10, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Mowat, A.M.; Scott, C.L.; Bain, C.C. Barrier-tissue macrophages: Functional adaptation to environmental challenges. Nat. Med. 2017, 23, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Dalzon, B.; Aude-Garcia, C.; Diemer, H.; Bons, J.; Marie-Desvergne, C.; Pérard, J.; Dubosson, M.; Collin-Faure, V.; Carapito, C.; Cianférani, S.; et al. The longer the worse: A combined proteomic and targeted study of the long-term versus short-term effects of silver nanoparticles on macrophages. Environ. Sci. Nano 2019, 7, 2032–2046. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Hurst, G.B.; Wang, W.; Foster, C.M.; Nallathamby, P.D.; Retterer, S.T. Dynamic development of the protein corona on silica nanoparticles: Composition and role in toxicity. Nanoscale 2013, 5, 6372–6380. [Google Scholar] [CrossRef] [PubMed]
- Docter, D.; Bantz, C.; Westmeier, D.; Galla, H.J.; Wang, Q.; Kirkpatrick, J.C.; Nielsen, P.; Maskos, M.; Stauber, R.H. The protein corona protects against size- and dose-dependent toxicity of amorphous silica nanoparticles. Beilstein J. Nanotechnol. 2014, 5, 1380–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wattiez, R.; Hermans, C.; Cruyt, C.; Bernard, A.; Falmagne, P. Human bronchoalveolar lavage fluid protein two-dimensional database: Study of interstitial lung diseases. Electrophoresis 2000, 21, 2703–2712. [Google Scholar] [CrossRef]
- Garmany, T.H.; Moxley, M.A.; White, F.V.; Dean, M.; Hull, W.M.; Whitsett, J.A.; Nogee, L.M.; Hamvas, A. Surfactant composition and function in patients with ABCA3 mutations. Pediatr. Res. 2006, 59, 801–805. [Google Scholar] [CrossRef] [Green Version]
- Leroy, B.; Falmagne, P.; Wattiez, R. Sample preparation of bronchoalveolar lavage fluid. Methods Mol. Biol. 2008, 425, 67–75. [Google Scholar]
- Delaval, M.; Boland, S.; Solhonne, B.; Nicola, M.A.; Mornet, S.; Baeza-Squiban, A.; Sallenave, J.M.; Garcia-Verdugo, I. Acute exposure to silica nanoparticles enhances mortality and increases lung permeability in a mouse model of Pseudomonas aeruginosa pneumonia. Part. Fibre Toxicol. 2015, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; He, H.; Tang, Z.; Hattori, T.; Liu, Y.; Young, M.M.; Serfass, J.M.; Chen, L.; Gebru, M.; Chen, C.; et al. An autophagy assay reveals the ESCRT-III component CHMP2A as a regulator of phagophore closure. Nat. Commun. 2018, 9, 2855. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Sowa, M.E.; Chen, J.; Li, X.; Gygi, S.P.; Harper, J.W. An FTS/Hook/p107FHIP Complex Interacts with and Promotes Endosomal Clustering by the Homotypic Vacuolar Protein Sorting Complex. Mol. Biol. Cell 2008, 19, 5059–5071. [Google Scholar] [CrossRef] [Green Version]
- Fuster, J.J.; Gonzàlez, J.M.; Edo, M.D.; Viana, R.; Boya, P.; Cervera, J.; Verges, M.; Rivera, J.; Andrés, V. Tumor suppressor p27Kip1 undergoes endolysosomal degradation through its interaction with sorting nexin 6. FASEB J. 2010, 24, 2998–3009. [Google Scholar] [CrossRef]
- Damen, E.; Krieger, E.; Nielsen, J.E.; Eygensteyn, J.; van Leeuwen, J.E. The human Vps29 retromer component is a metallo-phosphoesterase for a cation-independent mannose 6-phosphate receptor substrate peptide. Biochem. J. 2006, 398, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Sano, H.; Ishino, M.; Kr√§mer, H.; Shimizu, T.; Mitsuzawa, H.; Nishitani, C.; Kuroki, Y. The microtubule-binding protein Hook3 interacts with a cytoplasmic domain of scavenger receptor A. J. Biol. Chem. 2007, 282, 7973–7981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, R.; Hamilton, R.F.; Li, L.; Holian, A. Silica-induced apoptosis mediated via scavenger receptor in human alveolar macrophages. Toxicol. Appl. Pharmacol. 1996, 141, 84–92. [Google Scholar] [CrossRef]
- Orr, G.A.; Chrisler, W.B.; Cassens, K.J.; Tan, R.; Tarasevich, B.J.; Markillie, L.M.; Zangar, R.C.; Thrall, B.D. Cellular recognition and trafficking of amorphous silica nanoparticles by macrophage scavenger receptor A. Nanotoxicology 2011, 5, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Scott, F.L.; Hirst, C.E.; Sun, J.; Bird, C.H.; Bottomley, S.P.; Bird, P.I. The intracellular serpin proteinase inhibitor 6 is expressed in monocytes and granulocytes and is a potent inhibitor of the azurophilic granule protease, cathepsin G. Blood 1999, 93, 2089–2097. [Google Scholar] [CrossRef]
- Thibodeau, M.S.; Giardina, C.; Knecht, D.A.; Helble, J.; Hubbard, A.K. Silica-Induced Apoptosis in Mouse Alveolar Macrophages Is Initiated by Lysosomal Enzyme Activity. Toxicol. Sci. 2004, 80, 34–48. [Google Scholar] [CrossRef]
- Sohaebuddin, S.K.; Tang, L. A Simple Method to Visualize and Assess the Integrity of Lysosomal Membrane in Mammalian Cells Using a Fluorescent Dye. In Cellular and Subcellular Nanotechnology; Humana Press: Totowa, NJ, USA, 2013; Volume 991, pp. 25–31. [Google Scholar] [CrossRef]
- Shen, Y.; Zhong, L.; Johnson, S.; Cao, D. Human aldo-keto reductases 1B1 and 1B10: A comparative study on their enzyme activity toward electrophilic carbonyl compounds. Chem. Biol. Interact. 2011, 191, 192–198. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, T.; Ireland, L.S.; Harrison, D.J.; Hayes, J.D. Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. Biochem. J. 1999, 343 Pt 2, 487–504. [Google Scholar] [CrossRef]
- Kurahashi, T.; Kwon, M.; Homma, T.; Saito, Y.; Lee, J.; Takahashi, M.; Yamada, K.-i.; Miyata, S.; Fujii, J. Reductive detoxification of acrolein as a potential role for aldehyde reductase (AKR1A) in mammals. Biochem. Biophys. Res. Commun. 2014, 452, 136–141. [Google Scholar] [CrossRef]
- Del Corso, A.; Costantino, L.; Rastelli, G.; Buono, F.; Mura, U. Aldose reductase does catalyse the reduction of glyceraldehyde through a stoichiometric oxidation of NADPH. Exp. Eye Res. 2000, 71, 515–521. [Google Scholar] [CrossRef]
- Vander Jagt, D.L.; Hassebrook, R.K.; Hunsaker, L.A.; Brown, W.M.; Royer, R.E. Metabolism of the 2-oxoaldehyde methylglyoxal by aldose reductase and by glyoxalase-I: Roles for glutathione in both enzymes and implications for diabetic complications. Chem.-Biol. Interact. 2001, 130–132, 549–562. [Google Scholar] [CrossRef]

| Code | Name | Accession | Ratio/t-Test Acute/Control | Ratio/t-Test Recov./Control |
|---|---|---|---|---|
| akr1a1 | Alcohol dehydrogenase [NADP(+)] | Q9JII6 | 1.23/0.39 | 0.75/0.07 |
| akr1b1 | Aldose reductase | P45376 | 0.91/0.34 | 0.83/0.04 |
| ap3m1 | AP-3 complex subunit mu-1 | Q9JKC8 | 2.23/0.01 | 0.79/0.64 |
| atpb | ATP synthase subunit beta, mitochondrial | P56480 | 1.55/0.01 | 1.22/0.27 |
| bpnt1 | 3′(2′),5′-bisphosphate nucleotidase 1 | Q9Z0S1 | 2.09/0.02 | 1.28/0.37 |
| bvra ac | Biliverdin reductase A | Q9CY64 | 0.41/0.04 | 1.01/0.98 |
| cap1 | Adenylyl cyclase-associated protein 1 | P40124 | 0.69/0.03 | 1.01/0.96 |
| capg | Macrophage-capping protein | P24452 | 0.82/0.04 | 0.90/0.31 |
| casp3 | Caspase-3 | P70677 | 0.99/0.99 | 0.68/0.04 |
| catS | Cathepsin S | O70370 | 0.71/0.02 | 0.91/0.33 |
| caza2 | F-actin-capping protein subunit alpha-2 | P47754 | 1.31/0.02 | 0.96/0.56 |
| chm2a | Charged multivesicular body protein 2a | Q9DB34 | 0.95/0.40 | 0.65/0.01 |
| cof | Cofilin-1 | P18760 | 0.52/0.06 | 0.95/0.88 |
| dj1 | Protein deglycase DJ-1 | Q99LX0 | 1.65/0.09 | 0.93/0.77 |
| esd | S-formylglutathione hydrolase | Q9R0P3 | 1.26/0.03 | 0.86/0.10 |
| fkbp4 | Peptidyl-prolyl cis-trans isomerase FKBP4 | P30416 | 1.56/0.11 | 0.91/0.72 |
| fpps | Farnesyl pyrophosphate synthase | Q920E5 | 1.40/0.04 | 1.25/0.35 |
| gapdh | Glyceraldehyde-3-phosphate dehydrogenase | P16858 | 0.56/0.01 | 1.03/0.85 |
| gmfb | Glia maturation factor beta | Q9CQI3 | 0.75/0.03 | 1.22/0.29 |
| grp75 | Stress-70 protein, mitochondrial | O35501 | 1.94/0.007 | 1.02/0.89 |
| hook3 | Protein Hook homolog 3 | Q8BUK6 | 1.07/0.65 | 0.45/0.005 |
| kpym | Pyruvate kinase PKM | P52480 | 0.66/0.048 | 0.98/0.88 |
| ldha | L-lactate dehydrogenase A chain | P06151 | 0.60/0.00675 | 0.91/0.64 |
| ndus3 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 3, mitochondria | Q9DCT2 | 1.76/0.01 | 1.06/0.71 |
| nit1 | Nitrilase homolog 1 | Q8VDK1 | 0.53/0.01 | 0.82/0.10 |
| odo2 | Dihydrolipoyllysine-residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex, mitochondrial | Q9D2G2 | 1.33/0.02 | 0.94/0.80 |
| pdcd6 | Programmed cell death protein 6 | P12815 | 1.32/0.04 | 1.06/0.53 |
| pddc1 | Parkinson disease 7 domain-containing protein 1 | Q8BFQ8 | 0.70/0.20 | 0.77/0.01 |
| pgk1 | Phosphoglycerate kinase 1 | P09411 | 0.70/0.03 | 0.95/0.50 |
| phb | Prohibitin | P67778 | 1.27/0.0018 | 0.98/0.78 |
| prx3 | Thioredoxin-dependent peroxide reductase, mitochondrial | P20108 | 1.83/0.02 | 0.82/0.37 |
| psa1 | Proteasome subunit alpha type-1 | Q9R1P4 | 1.35/0.03 | 0.86/0.06 |
| psb10 | Proteasome subunit beta type-10 | O35955 | 1.45/0.03 | 0.64/0.06 |
| psb4 | Proteasome subunit beta type-4 | P99026 | 2.90/0.04 | 1.02/0.93 |
| psmd2 | 26S proteasome non-ATPase regulatory subunit 2 | Q8VDM4 | 1.69/0.04 | 1.05/0.79 |
| psmd14 | 26S proteasome non-ATPase regulatory subunit 14 | O35593 | 1.51/0.05 | 0.91/0.61 |
| snx6 | Sorting nexin-6 | Q6P8 × 1 | 0.97/0.53 | 0.88/0.007 |
| spb6 | Serpin B6 | Q60854 | 0.83/0.11 | 1.23/0.02 |
| spre | Sepiapterin reductase | Q64105 | 0.76/0.03 | 1.21/0.39 |
| tmem11 | Transmembrane protein 11, mitochondrial | Q8BK08 | 1.50/0.02 | 1.21/0.55 |
| twf2 | Twinfilin-2 | Q9Z0P5 | 0.68/0.03 | 0.92/0.65 |
| vata | V-type proton ATPase catalytic subunit A | P50516 | 1.66/0.01 | 1.01/0.95 |
| vps29 | Vacuolar protein sorting-associated protein 29 | Q9QZ88 | 0.99/0.99 | 0.67/0.03 |
| Term | Count 1 | p-Value 2 | FDR in % 3 |
|---|---|---|---|
| GO:0005925~focal adhesion | 7 | 0.0049 | 0.068 |
| GO:0098609~cell–cell adhesion | 5 | 0.0086 | 0.96 |
| GO:0007005~mitochondrion organization | 5 | 3.94 × 10−4 | 0.22 |
| GO:0005739~mitochondrion | 24 | 1.59 × 10−7 | 5.775 × 10−6 |
| Proteasome | 5 | 4.58 × 10−5 | 0.0016 |
| Hydrolase | 18 | 4.97 × 10−5 | 0.0016 |
| Ubl conjugation | 17 | 6.13 × 10−5 | 0.0016 |
| GO:0000502~proteasome complex | 5 | 1.44 × 10−4 | 0.0033 |
| mmu01200: Carbon metabolism | 7 | 1.64 × 10−4 | 0.011 |
| GO:0006006~glucose metabolic process | 4 | 0.0030 | 0.43 |
| GO:0005975~carbohydrate metabolic process | 6 | 0.0018 | 0.39 |
| Glycolysis | 3 | 0.0068 | 0.083 |
| GO:0006096~glycolytic process | 3 | 0.010 | 0.96 |
| NAD | 6 | 4.77 × 10−4 | 0.0089 |
| Oxidoreductase | 9 | 0.0018 | 0.029 |
| IPR002108: Actin-binding, cofilin/tropomyosin type | 3 | 6.57 × 10−4 | 0.082 |
| Actin-binding | 6 | 0.0019 | 0.029 |
| GO:0051016~barbed-end actin filament capping | 3 | 0.0021 | 0.39 |
| GO:0003779~actin binding | 7 | 0.0040 | 0.27 |
| GO:0031982~vesicle | 5 | 0.0043 | 0.065 |
| GO:0005765~lysosomal membrane | 5 | 0.015 | 0.18 |
| Enzyme | Control | Acute | Recov |
|---|---|---|---|
| BVR | 1.86 ± 0.62 | 0.58 ± 0.41 * | 1.08 ± 0.26 |
| GAPDH | 19.83 ± 3.33 | 1.50 ± 1.00 * | 19.50 ± 1.73 |
| LDH | 119.50 ± 16.26 | 23.17 ± 14.74 * | 134.17 ± 10.27 |
| PDXK | 9.33 ± 2.91 | 15.33 ± 3.05 | 16.67 ± 5.29 |
| PGK | 3547 ± 546 | 2242 ± 1059 | 2950 ± 597 |
| PKM | 4219 ± 607 | 983 ± 581 * | 4998 ± 195 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, A.; Dalzon, B.; Collin-Faure, V.; Diemer, H.; Fenel, D.; Schoehn, G.; Cianférani, S.; Carrière, M.; Rabilloud, T. How Reversible Are the Effects of Fumed Silica on Macrophages? A Proteomics-Informed View. Nanomaterials 2020, 10, 1939. https://doi.org/10.3390/nano10101939
Torres A, Dalzon B, Collin-Faure V, Diemer H, Fenel D, Schoehn G, Cianférani S, Carrière M, Rabilloud T. How Reversible Are the Effects of Fumed Silica on Macrophages? A Proteomics-Informed View. Nanomaterials. 2020; 10(10):1939. https://doi.org/10.3390/nano10101939
Chicago/Turabian StyleTorres, Anaelle, Bastien Dalzon, Véronique Collin-Faure, Hélène Diemer, Daphna Fenel, Guy Schoehn, Sarah Cianférani, Marie Carrière, and Thierry Rabilloud. 2020. "How Reversible Are the Effects of Fumed Silica on Macrophages? A Proteomics-Informed View" Nanomaterials 10, no. 10: 1939. https://doi.org/10.3390/nano10101939
APA StyleTorres, A., Dalzon, B., Collin-Faure, V., Diemer, H., Fenel, D., Schoehn, G., Cianférani, S., Carrière, M., & Rabilloud, T. (2020). How Reversible Are the Effects of Fumed Silica on Macrophages? A Proteomics-Informed View. Nanomaterials, 10(10), 1939. https://doi.org/10.3390/nano10101939






