Nanoparticle-Based Chemotherapy Formulations for Head and Neck Cancer: A Systematic Review and Perspectives
Abstract
:1. Introduction
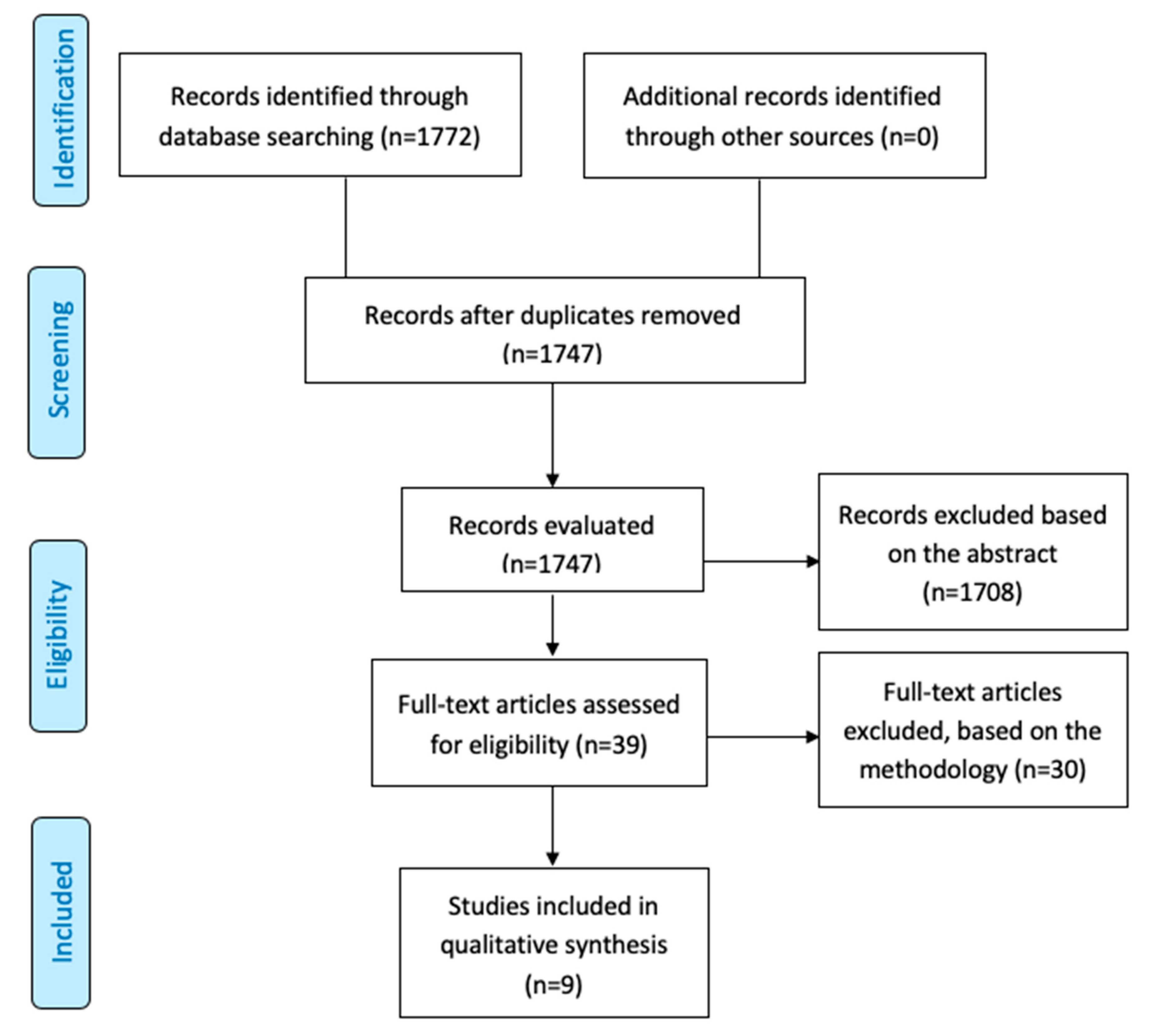
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection: Inclusion and Exclusion Criteria
2.3. Data Extraction and Study Quality Assessment
2.4. Risk of Bias Assessment
2.5. Types of Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Study Overview
3.2. Interventions in HNC Using Chemotherapy Nanoformulations
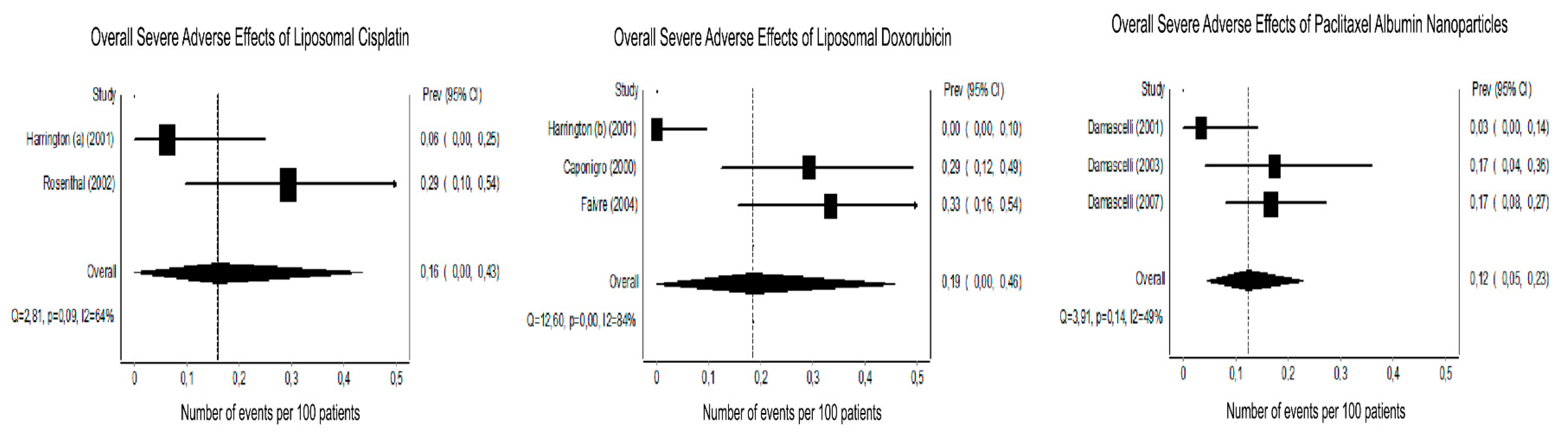
3.3. Tumor Response and Host Toxicity
3.4. Nanoformulation and Chemotherapeutic Agents Used in the Clinical Trials for HNC
3.4.1. Platinum-Based Chemotherapy
3.4.2. Doxorubicin
3.4.3. Paclitaxel
3.5. Ongoing Clinical Trials
3.6. Outcomes
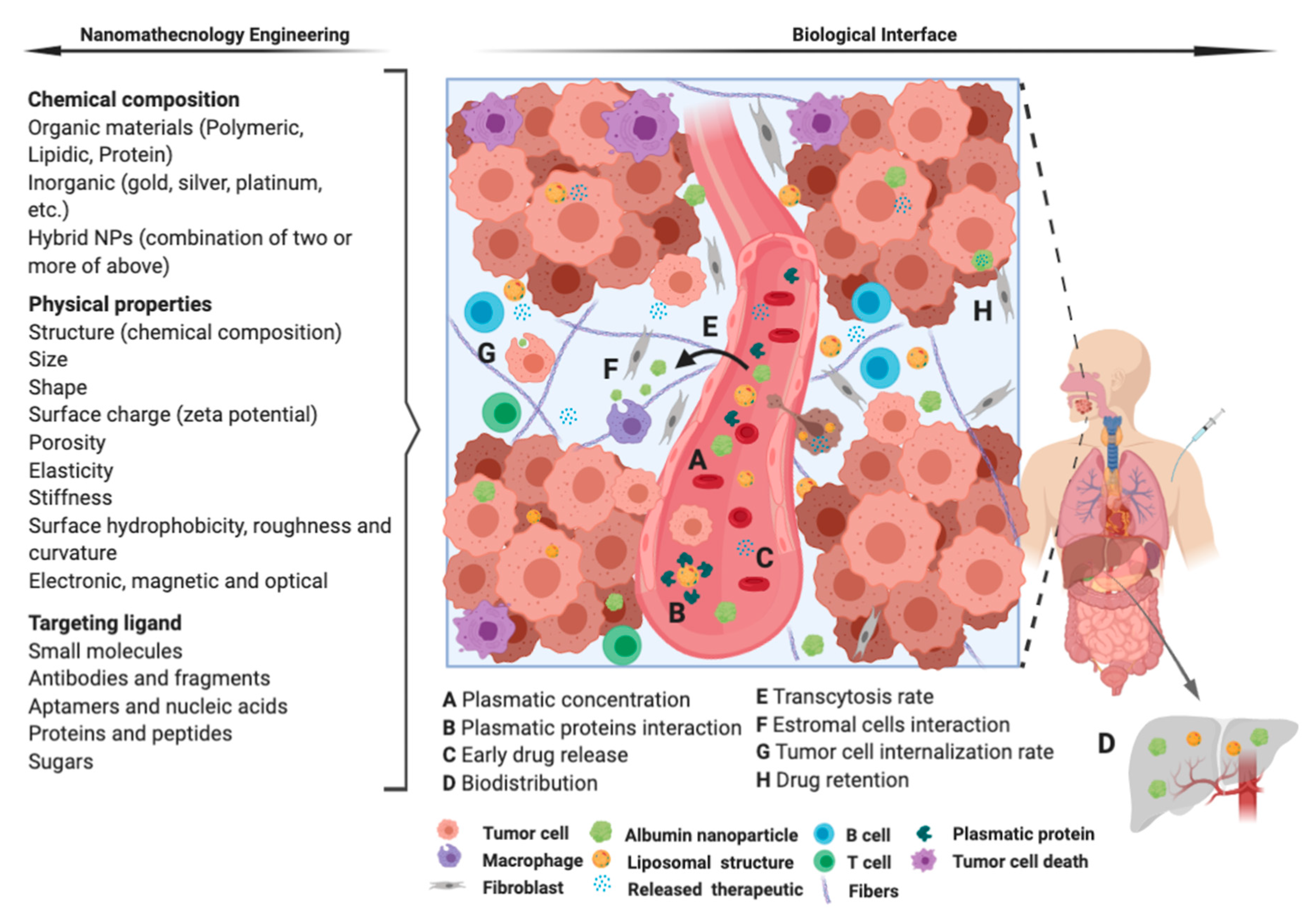
4. Remarks and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and neck cancers—Major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA A Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Groome, P.A.; Schulze, K.M.; Mackillop, W.J.; Grice, B.; Goh, C.; Cummings, B.J.; Hall, S.F.; Liu, F.F.; Payne, D.; Rothwell, D.M. A comparison of published head and neck stage groupings in carcinomas of the tonsillar region. Cancer 2001, 92, 1484–1494. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and neck squamous cell carcinoma: Update on epidemiology, diagnosis, and treatment. Proc. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef]
- Chan, K.K.; Glenny, A.M.; Weldon, J.C.; Furness, S.; Worthington, H.V.; Wakeford, H. Interventions for the treatment of oral and oropharyngeal cancers: Targeted therapy and immunotherapy. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.; Krapcho, M. Previous Version: SEER cancer statistics review, 1975–2010. Natl. Cancer Inst. 2013, 21, 12. [Google Scholar]
- Forastiere, A.; Koch, W.; Trotti, A.; Sidransky, D. Head and neck cancer. N. Engl. J. Med. 2001, 345, 1890–1900. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Nishimoto, I.N.; Califano, J.A.; Kowalski, L.P. Trends in incidence and prognosis for head and neck cancer in the United States: A site-specific analysis of the SEER database. Int. J. Cancer 2005, 114, 806–816. [Google Scholar] [CrossRef]
- Lytton-Jean, A.K.; Kauffman, K.J.; Kaczmarek, J.C.; Langer, R. Cancer nanotherapeutics in clinical trials. Cancer Treat. Res. 2015, 293–322. [Google Scholar]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjugate Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Chan, K.K.W.; Glenny, A.M.; Furness, S.; Worthington, H.V. Interventions for the treatment of oral and oropharyngeal cancers: Targeted therapy and immunotherapy. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Percy, C.; Holten, V.V.; Muir, C.S. International Classification of Diseases for Oncology; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Durieux, N.; Pasleau, F.; Howick, J. OCEBM Levels of Evidence Working Group. Rev. Med. Liege 2013, 68, 644–649. [Google Scholar] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotti, A.; Byhardt, R.; Stetz, J.; Gwede, C.; Corn, B.; Fu, K.; Gunderson, L.; McCormick, B.; Morris, M.; Rich, T. Common toxicity criteria: Version 2.0. An improved reference for grading the acute effects of cancer treatment: Impact on radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 13–47. [Google Scholar] [CrossRef]
- Harrington, K.J.; Lewanski, C.R.; Northcote, A.D.; Whittaker, J.; Wellbank, H.; Vile, R.G.; Peters, A.M.; Stewart, J.S.W. Phase I-II study of pegylated liposomal cisplatin (SPI-077TM) in patients with inoperable head and neck cancer. Ann. Oncol. 2001, 12, 493–496. [Google Scholar] [CrossRef]
- Friedrich, J.O.; Adhikari, N.K.; Beyene, J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res. Methodol. 2007, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenthal, D.I.; Yom, S.S.; Liu, L.; Machtay, M.; Algazy, K.; Weber, R.S.; Weinstein, G.S.; Chalian, A.A.; Mille, L.K.; Rockwell, K., Jr.; et al. A phase I study of SPI-077 (Stealth liposomal cisplatin) concurrent with radiation therapy for locally advanced head and neck cancer. Investig. New Drugs 2002, 20, 343–349. [Google Scholar] [CrossRef]
- Caponigro, F.; Comella, P.; Budillon, A.; Bryce, J.; Avallone, A.; De Rosa, V.; Ionna, F.; Comella, G. Phase I study of Caelyx (doxorubicin HCL, pegylated liposomal) in recurrent or metastatic head and neck cancer. Ann. Oncol. 2000, 11, 339–342. [Google Scholar] [CrossRef]
- Faivre, S.; Alsabe, H.; Djafari, L.; Janot, F.; Julieron, M.; Domenge, C.; Djazouli, K.; Armand, J.P.; Luboinski, B.; Raymond, E. Locoregional effects of pegylated liposomal doxorubicin (Caelyx) in irradiated area: A phase I-II study in patients with recurrent squamous cell carcinoma of the head and neck. Eur. J. Cancer 2004, 40, 1517–1521. [Google Scholar] [CrossRef]
- Damascelli, B.; Patelli, G.; Ticha, V.; Di Tolla, G.; Frigerio, L.F.; Garbagnati, F.; Lanocita, R.; Marchiano, A.; Spreafico, C.; Mattavelli, F.; et al. Feasibility and efficacy of percutaneous transcatheter intraarterial chemotherapy with paclitaxel in albumin nanoparticles for advanced squamous-cell carcinoma of the oral cavity, oropharynx, and hypopharynx. J. Vasc. Interv. Radiol. 2007, 18, 1395–1403. [Google Scholar] [CrossRef]
- Damascelli, B.; Patelli, G.L.; Lanocita, R.; Di Tolla, G.; Frigerio, L.F.; Marchiano, A.; Garbagnati, F.; Spreafico, C.; Ticha, V.; Gladin, C.R.; et al. A novel intraarterial chemotherapy using paclitaxel in albumin nanoparticles to treat advanced squamous cell carcinoma of the tongue: Preliminary findings. Am. J. Roentgenol. 2003, 181, 253–260. [Google Scholar] [CrossRef]
- Harrington, K.J.; Lewanski, C.; Northcote, A.D.; Whittaker, J.; Peters, A.M.; Vile, R.G.; Stewart, J.S. Phase II study of pegylated liposomal doxorubicin (Caelyx) as induction chemotherapy for patients with squamous cell cancer of the head and neck. Eur. J. Cancer 2001, 37, 2015–2022. [Google Scholar] [CrossRef]
- Damascelli, B.; Cantuand, G.; Mattavelli, F.; Tamplenizza, P.; Bidoli, P.; Leo, E.; Dosio, F.; Cerrotta, A.M.; Tolla, G.D.; Frigerio, L.F.; et al. Intraarterial chemotherapy with polyoxyethylated castor oil free paclitaxel, incorporated in albumin nanoparticles (ABI-007): Phase I study of patients with squamous cell carcinoma of the head and neck and anal canal: Preliminary evidence of clinical activity. Cancer 2001, 92, 2592–2602. [Google Scholar] [PubMed] [Green Version]
- Strieth, S.; Dunau, C.; Michaelis, U.; Jager, L.; Gellrich, D.; Wollenberg, B.; Dellian, M. Phase I/II clinical study on safety and antivascular effects of paclitaxel encapsulated in cationic liposomes for targeted therapy in advanced head and neck cancer. Head Neck 2014, 36, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Bjornmalm, M.; Thurecht, K.J.; Michael, M.; Scott, A.M.; Caruso, F. Bridging bio–nano science and cancer nanomedicine. ACS Nano 2017, 11, 9594–9613. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Hassan, S.; Prakash, G.; Ozturk, A.B.; Saghazadeh, S.; Sohail, M.F.; Seo, J.; Dokmeci, M.R.; Zhang, Y.S.; Khademhosseini, A. Evolution and clinical translation of drug delivery nanomaterials. Nano Today 2017, 15, 91–106. [Google Scholar] [CrossRef]
- Park, K. Facing the truth about nanotechnology in drug delivery. ACS Nano 2013, 7, 7442–7447. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, I.H. Nanotechnology in head and neck cancer: The race is on. Curr. Oncol. Rep. 2010, 12, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Stern, S.T.; Hall, J.B.; Lee, L.Y.; Wood, L.J.; Paciotti, G.F.; Tamarkin, L.; Long, S.E.; McNeil, S.E. Translational considerations for cancer nanomedicine. J. Control. Release 2010, 146, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Bogner, J.R.; Kronawitter, U.; Rolinski, B.; Truebenbach, K.; Goebel, F.-D. Liposomal doxorubicin in the treatment of advanced AIDS-related Kaposi-sarcoma. J. Acquir. Immune Defic. Syndr. 1994, 4634–4668. [Google Scholar]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Leserman, L.D.; Barbet, J.; Kourilsky, F.; Weinstein, J.N. Targeting to cells of fluorescent liposomes covalently coupled with monoclonal antibody or protein A. Nature 1980, 288, 602–604. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Smith, A. Big Moment for Nanotech: Oncology Therapeutics Poised for a Leap. OncLive 2013. Available online: https://www.onclive.com/view/big-moment-for-nanotech-oncology-therapeutics-poised-for-a-leap (accessed on 26 September 2020).
- Butts, C.; Socinski, M.A.; Mitchell, P.L.; Thatcher, N.; Havel, L.; Krzakowski, M.; Nawrocki, S.; Ciuleanu, T.-E.; Bosquée, L.; Trigo, J.M. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.; Cortes, J.; Hurvitz, S.A.; Krop, I.E.; Tripathy, D.; Verma, S.; Riahi, K.; Reynolds, J.G.; Wickham, T.J.; Molnar, I. HERMIONE: A randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer 2016, 16, 352. [Google Scholar] [CrossRef] [Green Version]

| Patient Characteristics | n |
|---|---|
| Mean age | 59.23 (range 25–87) years |
| Total patients recruited | 229 (range 7–60) |
| Number of patients analyzed | 216 (94.3%) |
| Gender | |
| Male | 179 (78.17%) |
| Female | 50 (21.83%) |
| Previously treated | |
| Yes | 88 (37.9%) |
| No | 141 (62.1%) |
| Tumor Size * | |
| T1 + T2 | 11 (7.43%) |
| T3 + T4 | 137 (92.57%) |
| Lymph nodes metastasis * | |
| Positive | 108 (72.48%) |
| Negative | 41 (27.52%) |
| Distant metastasis * | |
| Yes | 19 (10.86%) |
| No | 156 (89.14%) |
| Died of disease * | |
| Yes | 61 (44.20%) |
| No | 77 (55.80%) |
| Tumor Location | |
| Oral cavity | 97 (41.99%) |
| Larynx | 43 (18.62%) |
| Oropharynx | 38 (16.45%) |
| Hypopharynx | 28 (12.12%) |
| Maxilary sinus | 9 (3.89%) |
| Others | 16 (6.92%) |
| Study (Year) | Phase | Number of Participants/ Number Analyzed | CT | Vehicle/Carrier | Dose | Previous Treatments | Median Follow-Up (Months) a/ Definitive Treatment | Tumor Response b (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| CR | SD | PD | ||||||||
| Harrington (a) (2001) [15] | I-II | 18/16 | Cisplatin | Pegylated Liposome | 2 cycles of 200 mg/m2 every 3 weeks. The last 8 patients received 260 mg/m2 | No | 17/RT after the second dose | 11.1 | 55.6 | 33.3 |
| Rosenthal (2002) [17] | I | 20/17 | Cisplatin | Pegylated Liposome | Escalated from 20–200 mg/m2 in six dose levels | Yes | 36/Concurrent with RT | 40 | 0 | 60 |
| Harrington (b) (2001) [22] | II | 20/18 | Doxorubicin | Pegylated Liposome | Escalated doses starting at 10 mg/m2 and increasing through 15 mg/m2 to 20 mg/m2. | No | 13/RT began after the last dose | 57 | 31 | 13 |
| Caponigro (2000) [18] | I | 24/24 | Doxorubicin | Pegylated Liposome | Initial dose of 30 mg/m2 and subsequently escalated by 5 mg/m2 | Yes | 1/Not stated | 33.33 | 62.5 | 4.16 |
| Faivre (2004) [19] | I-II | 26/24 | Doxorubicin | Pegylated Liposome | 15 patients received a dose of 35 mg/m2 every 3 weeks. The following 11 patients group was treated at 45 mg/m2. | Yes | 34/Not stated | 17 | 33 | 50d |
| Damascelli (2001) [23] | I | 31/28 | Paclitaxel | Albumin nanoparticle | Starting dose of 120 mg/m2 was increased by 30 mg/m2 during three treatment cycles. | Yes | 3-13/Not stated | 75.85 | 17.24 | 6.88 |
| Damascelli (2003) [21] | I | 23/23 | Paclitaxel | Albumin nanoparticle | Starting dose of 120 mg/m2 was increased by 30 mg/m2 at 3 subsequent levels each 4 weeks. | No | 5-12/Not stated | 78 | 13 | 9 |
| Damascelli (2007) [20] | II | 60/60 | Paclitaxel | Albumin nanoparticle | Starting dose of 230 mg/m2 and subsequently a reduced dose of 150 mg/m2. | No | 0.5/Surgery, CT and/or RT | 75 | 11.67 | 13.33 |
| Strieth (2014) [24] | I-II | 07/05 | Paclitaxel | Liposome | One group received 3 infusions of 0.55 mg/kg and another received 1.1 mg/kg. | Yes | 0.75/Not stated | 0 | 80 | 20 |
| Study | CT and Vehicle | Number of Analyzed Patients | Main Severe Adverse Effects (n) | |||||
|---|---|---|---|---|---|---|---|---|
| Hematological | Neutro-/Leucopenia | Gastrointestinal b | Mucocutaneous | Neurological | Allergy | |||
| Harrington [15] | Cisplatin (Liposome) | 16 | 1 | |||||
| Rosenthal [17] | Cisplatin (Liposome) | 17 | 2 | 1 | 2 | |||
| Total (events per 100 patients) | .. | 33 | 3 (9) | 1 (3) | 2 (6) | 0 | 0 | 0 |
| Harrington [22] | Doxorubicin (Liposome) | 18 | ||||||
| Caponigro [18] | Doxorubicin (Liposome) | 24 | 2 | 5 | ||||
| Faivre [19] | Doxorubicin (Liposome) | 24 | 3 | 2 | 1 | 2 | ||
| Total (events per 100 patients) | .. | 66 | 3 (5) | 4 (6) | 1 (2) | 5 (8) | 0 | 2 (4) |
| Damascelli [23] | Paclitaxel (Albumin nanoparticle) | 29 | 1 | |||||
| Damascelli [21] | Paclitaxel (Albumin nanoparticle) | 23 | 2 | 2 | ||||
| Damascelli [20] | Paclitaxel (Albumin nanoparticle) | 60 | 4 | 6 | ||||
| Strieth [24] | Paclitaxel (Liposome) | 05 | ||||||
| Total (events per 100 patients) | .. | 117 | 2 (2) | 5 (4) | 0 | 0 | 8 (7) | 0 |
| Phase | Year | NCC | Identifier Number |
|---|---|---|---|
| I | 2016 | Cisplatin polymeric micelle | NCT02817113 |
| I | 2013 | Paclitaxel albumin- nanoparticle | NCT01847326 |
| I | 2008 | Paclitaxel albumin- nanoparticle | NCT00736619 |
| II | 2014 | Paclitaxel albumin- nanoparticle | NCT02033538 |
| II | 2009 | Paclitaxel albumin- nanoparticle | NCT00851877 |
| II | 2012 | Paclitaxel albumin- nanoparticle | NCT01566435 |
| na * | 2007 | Paclitaxel albumin- nanoparticle | NCT00499291 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima, J.M.; Bonan, P.R.; da Cruz Perez, D.E.; Hier, M.; Alaoui-Jamali, M.A.; da Silva, S.D. Nanoparticle-Based Chemotherapy Formulations for Head and Neck Cancer: A Systematic Review and Perspectives. Nanomaterials 2020, 10, 1938. https://doi.org/10.3390/nano10101938
de Lima JM, Bonan PR, da Cruz Perez DE, Hier M, Alaoui-Jamali MA, da Silva SD. Nanoparticle-Based Chemotherapy Formulations for Head and Neck Cancer: A Systematic Review and Perspectives. Nanomaterials. 2020; 10(10):1938. https://doi.org/10.3390/nano10101938
Chicago/Turabian Stylede Lima, Jefferson Muniz, Paulo Rogerio Bonan, Danyel Elias da Cruz Perez, Michael Hier, Moulay A. Alaoui-Jamali, and Sabrina Daniela da Silva. 2020. "Nanoparticle-Based Chemotherapy Formulations for Head and Neck Cancer: A Systematic Review and Perspectives" Nanomaterials 10, no. 10: 1938. https://doi.org/10.3390/nano10101938
APA Stylede Lima, J. M., Bonan, P. R., da Cruz Perez, D. E., Hier, M., Alaoui-Jamali, M. A., & da Silva, S. D. (2020). Nanoparticle-Based Chemotherapy Formulations for Head and Neck Cancer: A Systematic Review and Perspectives. Nanomaterials, 10(10), 1938. https://doi.org/10.3390/nano10101938







