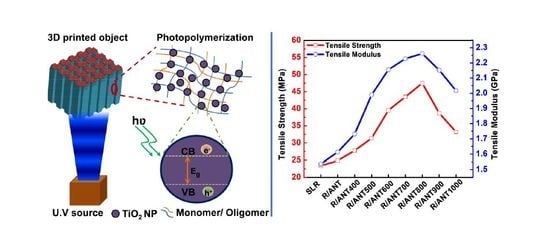
Enhanced Mechanical and Thermal Properties of Stereolithography 3D Printed Structures by the Effects of Incorporated Controllably Annealed Anatase TiO2 Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Anatase TNPs and Calcinations at the Different Temperatures
2.2.2. Preparation of Nanocomposites for SLA 3D Printing.
2.2.3. Fabrication of Three-Dimensional Structures by SLA 3D Printer
2.3. Measurements and Characterization
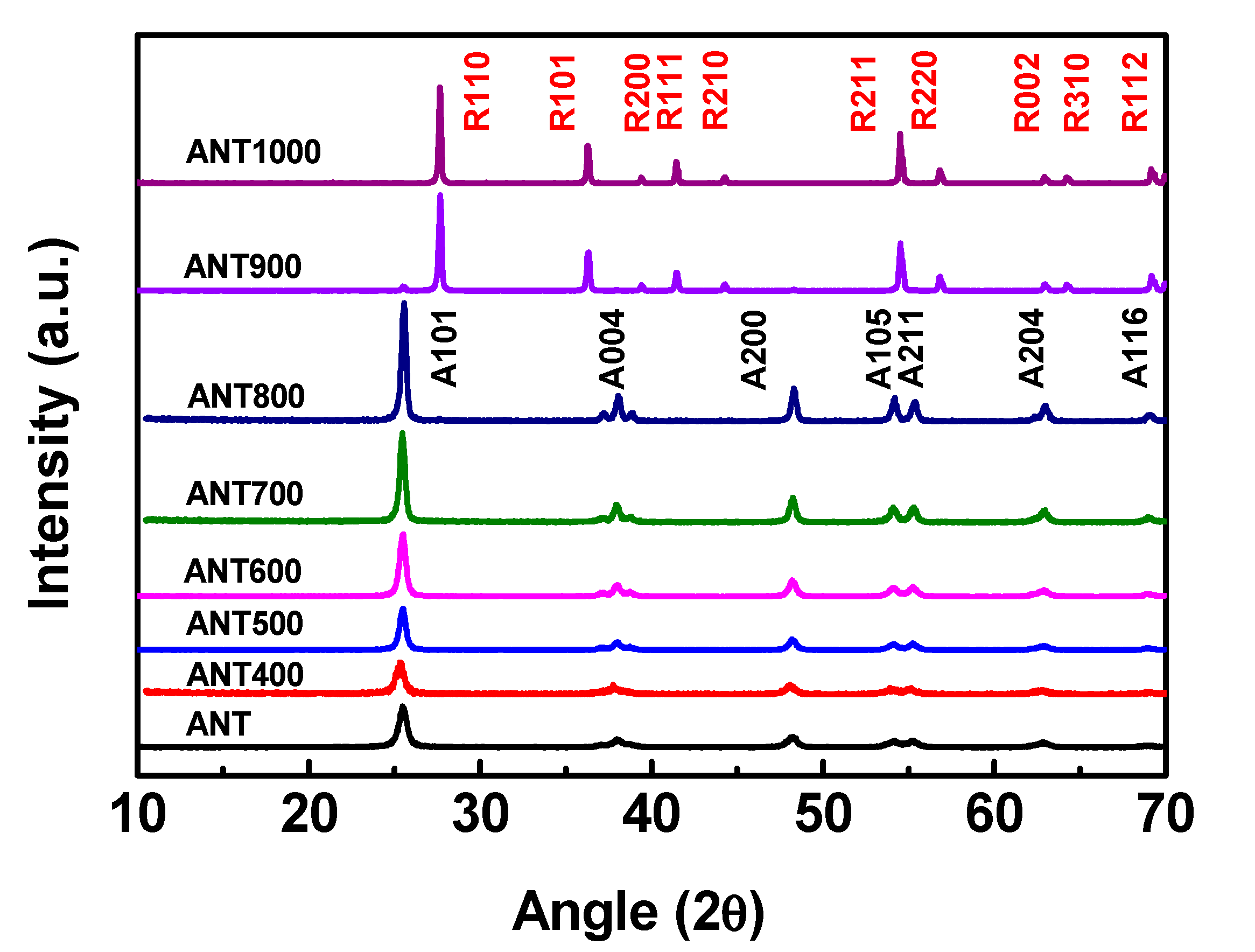
2.3.1. X-ray Diffraction (XRD) Measurements
2.3.2. Raman Spectroscopy Analysis
2.3.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.3.4. FTIR Spectroscopy
2.3.5. Electron Microscope Analysis
2.3.6. Diffusive Reflectance Spectroscopy (DRS)
2.3.7. UV Rheological Analysis
2.3.8. Mechanical Properties
2.3.9. Nanoindentation Test
2.3.10. Dynamic Mechanical Properties
2.3.11. Thermal Gravimetric Analysis (TGA)
2.3.12. Thermal Conductivity Analysis
3. Results and Discussion
3.1. Characterization of As-Prepared Different Temperature-Treated Anatase TNPs
3.2. Characterization of SLA 3D Printed Samples
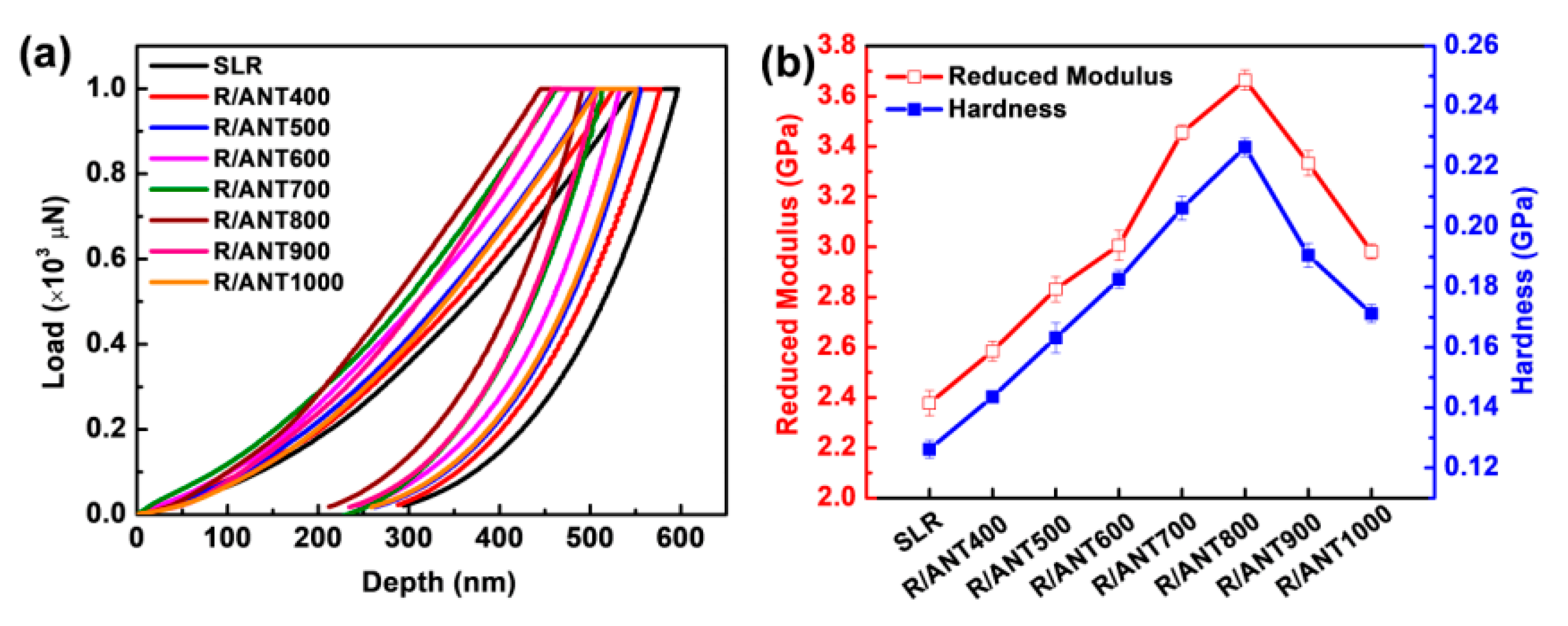
3.2.1. Mechanical Properties
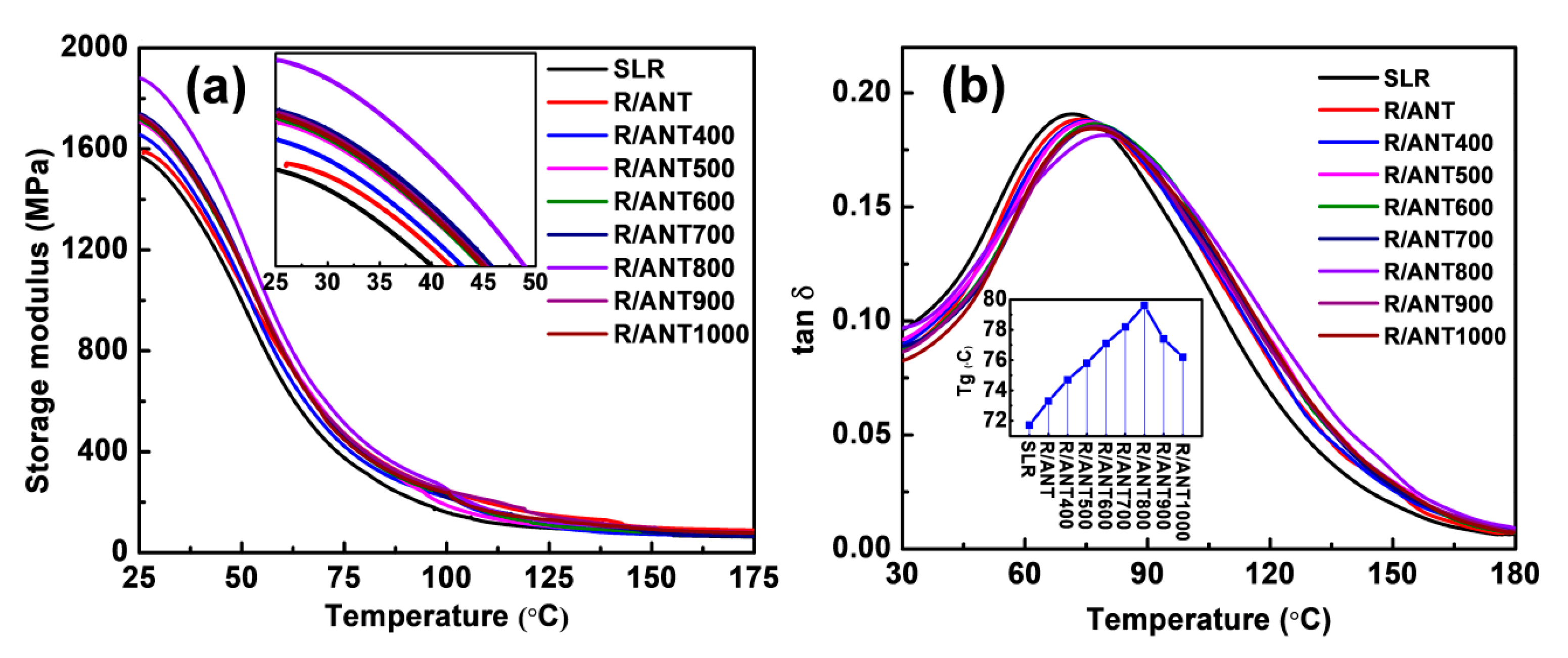
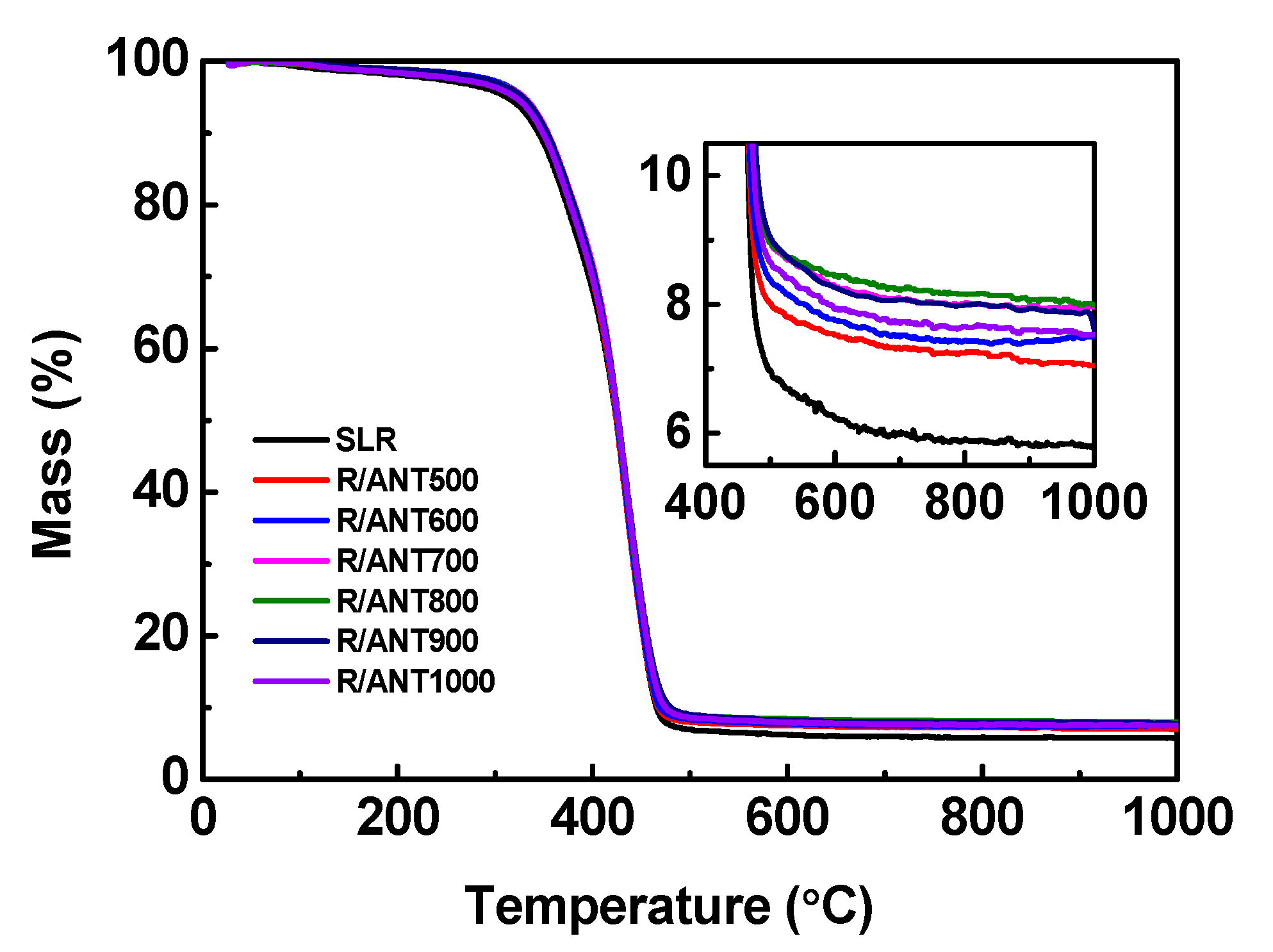
3.2.2. Thermal Properties
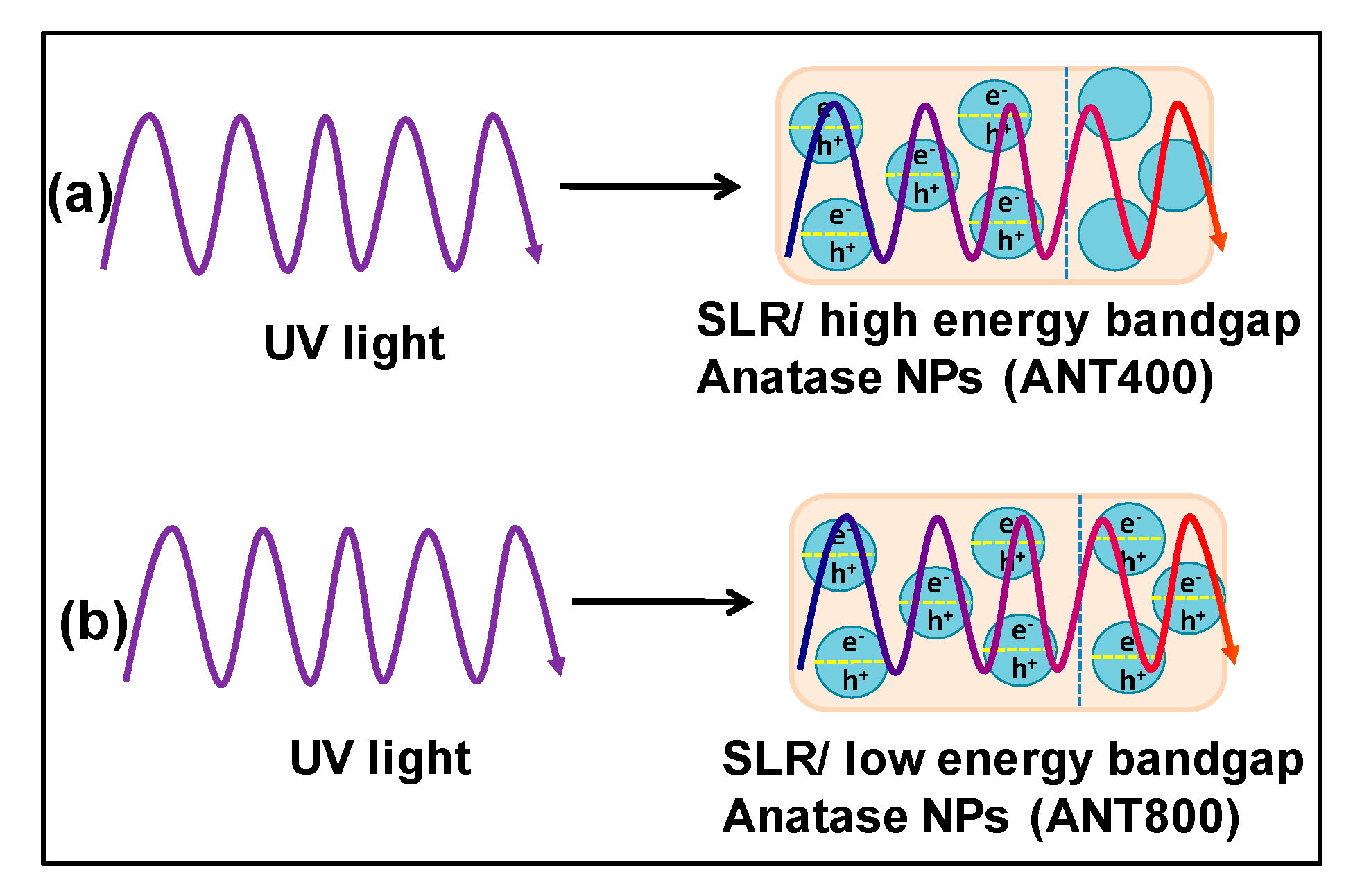
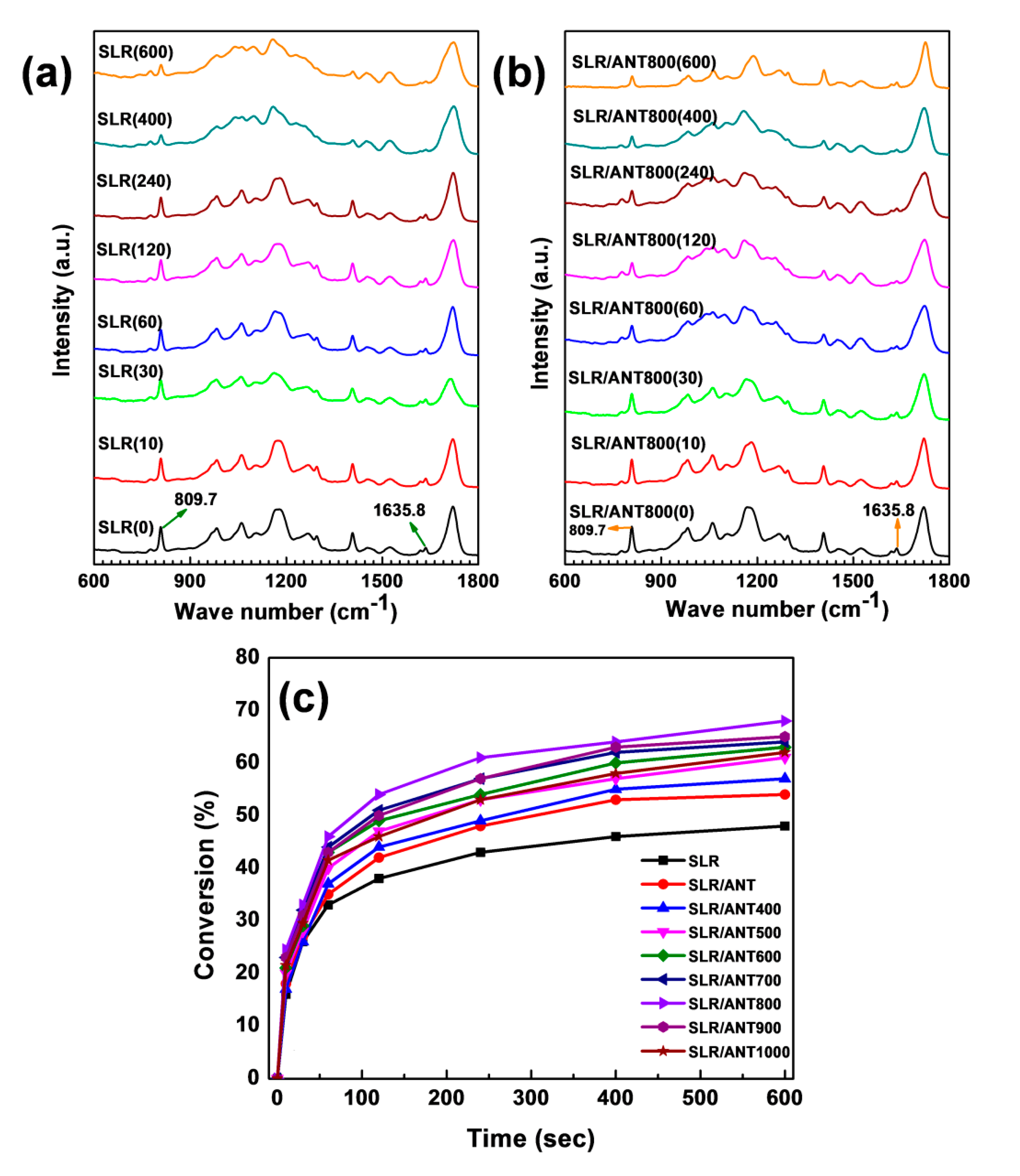
3.3. Mechanical and Thermal Enhancement Mechanism of SLR/Nanofiller 3D Printed Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Derby, B. Printing and Prototyping of Tissues and Scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Kamat, A.M.; Pei, Y.; Kottapalli, A.G.P. Bioinspired Cilia Sensors with Graphene Sensing Elements Fabricated Using 3D Printing and Casting. Nanomaterials 2019, 9, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Valk, D.C.; van der Ven, C.F.T.; Blaser, M.C.; Grolman, J.M.; Wu, P.J.; Fenton, O.S.; Lee, L.H.; Tibbitt, M.W.; Andresen, J.L.; Wen, J.R.; et al. Engineering a 3d-bioprinted model of human heart valve disease using nanoindentation-based biomechanics. Nanomaterials 2018, 8, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chizari, K.; Daoud, M.A.; Ravindran, A.R.; Therriault, D. 3D printing of highly conductive nanocomposites for the functional optimization of liquid sensors. Small 2016, 12, 6076–6082. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Shi, H.; Li, G.; Xue, Q.; Zhao, L.; Wang, F.; Hu, B. 3D-printed concentration-controlled microfluidic chip with diffusion mixing pattern for the synthesis of alginate drug delivery microgels. Nanomaterials 2019, 9, 1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.C.; Sheikh, A.A. 3D printing in aerospace and its long-term sustainability. Virtual Phys. Prototyp. 2015, 10, 175–185. [Google Scholar] [CrossRef]
- Sun, W.; Starly, B.; Nam, J.; Darling, A. Bio-CAD modeling and its applications in computer-aided tissue engineering. Comput.-Aided Des. 2005, 37, 1097–1114. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [Green Version]
- Bártolo, P.J. Stereolithography: Materials, Processes and Applications, 1st ed.; Springer: New York, NY, USA, 2011; pp. 37–52. [Google Scholar]
- Zhang, F.; Li, C.; Wang, Z.; Zhang, J.; Wang, Y. Multimaterial 3D Printing for Arbitrary Distribution with Nanoscale Resolution. Nanomaterials 2019, 9, 1108. [Google Scholar] [CrossRef] [Green Version]
- Vaezi, M.; Seitz, H.; Yang, S.A. A review on 3D micro-additive manufacturing technologies. Int. J. Adv. Manuf. Technol. 2013, 67, 1721–1754. [Google Scholar] [CrossRef]
- Cunico, M.W.M.; Carvalho, J. Development of acrylate-based material using a multivariable approach: Additive manufacturing applications. Rapid Prototyp. J. 2014, 20, 121–132. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Wang, P.H.; Wang, T.L.; Lin, W.C.; Lin, H.Y.; Lee, M.H.; Yang, C.H. Crosslinked polymer ionic liquid/ionic liquid blends prepared by photopolymerization as solid-state electrolytes in supercapacitors. Nanomaterials 2018, 8, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Graziola, F.; Girardi, F.; Bauer, M.; Di Maggio, R.; Rovezzi, M.; Bertagnolli, H.; Sada, C.; Rossetto, G.; Gross, S. UV-photopolymerisation of poly (methyl methacrylate)-based inorganic–organic hybrid coatings and bulk samples reinforced with methacrylate-modified zirconium oxocluster. Polymer 2008, 49, 4332–4343. [Google Scholar] [CrossRef]
- Decker, C. Photo initiated curing of multifunctional monomers. Acta Polym. 1994, 45, 333–347. [Google Scholar] [CrossRef]
- Fantino, E.; Vitale, A.; Quaglio, M.; Cocuzza, M.; Pirri, C.F.; Bongiovanni, R. Blue and UV combined photolithographic polymerization for the patterning of thick structures. Chem. Eng. J. 2015, 267, 65–72. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; AtillaTasdelen, M.; Mohamed Asiri, A.; Bahadar Khan, S.; Yagci, Y. Photo induced atom transfer radical polymerization using semiconductor nanoparticles. Macromol. Rapid Commun. 2014, 35, 454–459. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Allonas, X.; Burget, D. Photopolymerization reactions under visible lights: Principle, mechanisms and examples of applications. Prog. Org. Coat. 2003, 47, 16–36. [Google Scholar] [CrossRef]
- Song, S.Y.; Park, M.S.; Lee, J.W.; Yun, J.S. A study on the rheological and mechanical properties of photo-curable ceramic/polymer composites with different silane coupling agents for SLA 3D printing technology. Nanomaterials 2018, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Leon, A.C.; Chen, Q.; Palaganas, N.B.; Palaganas, J.O.; Manapat, J.Z.; Advincula, R.C. High performance polymer nanocomposites for additive manufacturing applications. React. Funct. Polym. 2016, 103, 141–155. [Google Scholar] [CrossRef]
- Ligon, S.C.; Husar, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to reduce oxygen inhibition in photoinduced polymerization. Chem. Rev. 2013, 114, 557–589. [Google Scholar] [CrossRef] [PubMed]
- Dadashi-Silab, S.; Asiri, A.M.; Khan, S.B.; Alamry, K.A.; Yagci, Y. Semiconductor nanoparticles for photoinitiation of Free radical polymerization in aqueous and organic media. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1500–1507. [Google Scholar] [CrossRef]
- Bartholomew, R.F.; Davidson, R.S. The photosensitised oxidation of amines. Part II. The use of dyes as photosensitisers: Evidence that singlet oxygen is not involved. J. Chem. Soc. C Org. 1971, 2347–2351. [Google Scholar] [CrossRef]
- El-Roz, M.; Lalevée, J.; Allonas, X.; Fouassier, J.P. Mechanistic investigation of the silane, germane, and stannane behavior when incorporated in type I and type II photoinitiators of polymerization in aerated media. Macromolecules 2009, 42, 8725–8732. [Google Scholar] [CrossRef]
- Malas, A.; Isakov, D.; Couling, K.; Gibbons, G.J. Fabrication of High Permittivity Resin Composite for Vat Photopolymerization 3D Printing: Morphology, Thermal, Dynamic Mechanical and Dielectric Properties. Materials 2019, 12, 3818. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Dai, L.; Zhong, L.; Liu, B.; Ren, J.; Xu, Y. Synthesis and characterization of reinforced acrylate photosensitive resin by 2-hydroxyethyl methacrylate-functionalized graphenenanosheets for 3D printing. J. Mater. Sci. 2018, 53, 1874–1886. [Google Scholar] [CrossRef]
- Manapat, J.Z.; Chen, Q.; Ye, P.; Advincula, R.C. 3D printing of polymer nanocomposites via stereolithography. Macromol. Mater. Eng. 2017, 302, 1600553. [Google Scholar] [CrossRef]
- Manapat, J.Z.; Mangadlao, J.D.; Tiu, B.D.; Tritchler, G.C.; Advincula, R.C. High-strength Stereolithographic 3D printed nanocomposites: Graphene oxide meta-stability. ACS Appl. Mater. Interfaces 2017, 9, 10085–10093. [Google Scholar] [CrossRef]
- Lin, D.; Jin, S.; Zhang, F.; Wang, C.; Wang, Y.; Zhou, C.; Cheng, G.J. 3D stereolithography printing of graphene oxide reinforced complex architectures. Nanotechnology 2015, 26, 434003. [Google Scholar] [CrossRef] [Green Version]
- Makvandi, P.; Esposito Corcione, C.; Paladini, F.; Gallo, A.L.; Montagna, F.; Jamaledin, R.; Pollini, M.; Maffezzoli, A. Antimicrobial modified hydroxyapatite composite dental bite by stereolithography. Polym. Adv. Technol. 2018, 29, 364–371. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Striani, R.; Montagna, F.; Cannoletta, D. Organically modified montmorillonite polymer nanocomposites for stereolithography building process. Polym. Adv. Technol. 2015, 26, 92–98. [Google Scholar] [CrossRef]
- Corcione, C.E. Development and characterization of novel photopolymerizable formulations for stereolithography. J. Polym. Eng. 2014, 34, 85–93. [Google Scholar] [CrossRef]
- Corcione, C.E.; Montagna, F.; Greco, A.; Licciulli, A.; Maffezzoli, A. Free form fabrication of silica moulds for aluminium casting by stereolithography. Rapid Prototyp. J. 2006, 12, 184–188. [Google Scholar] [CrossRef]
- Corcione, C.E.; Greco, A.; Maffezzoli, A. Temperature evolution during stereolithography building with a commercial epoxy resin. Polym. Eng. Sci. 2006, 46, 493–502. [Google Scholar] [CrossRef]
- Licciulli, A.; Corcione, C.E.; Greco, A.; Amicarelli, V.; Maffezzoli, A. Laser stereolithography of ZrO2 toughened Al2O3. J. Eur. Ceram. Soc. 2004, 24, 3769–3777. [Google Scholar] [CrossRef]
- Esposito, C.C.; Greco, A.; Maffezzoli, A. Photopolymerization kinetics of an epoxy based resin for stereolithography. J. Therm. Anal. Calorim. 2003, 72, 687–693. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- O’regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737. [Google Scholar] [CrossRef]
- Ortiz, G.F.; Hanzu, I.; Djenizian, T.; Lavela, P.; Tirado, J.L.; Knauth, P. Alternative Li-Ion Battery Electrode Based on Self-Organized Titania Nanotubes. Chem. Mater. 2009, 21, 63–67. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Zhu, W.; Qu, X.; Aaronson, C.; McCall, W.R.; Chen, S.; Sirbuly, D.J. 3D optical printing of piezoelectric nanoparticles-polymer composite materials. ACS Nano 2014, 8, 9799–9806. [Google Scholar] [CrossRef] [PubMed]
- Tien, M.-S.; Lin, L.-Y.; Xiao, B.-C.; Hong, S.-T. Enhancing the Contact Area of Ti Wire as Photoanode Substrate of Flexible Fiber-Type Dye-Sensitized Solar Cells Using the TiO2 Nanotube Growth and Removal Technique. Nanomaterials 2019, 9, 1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.G.; Yang, J.; Jia, Y.-G.; Lu, B.; Ren, L. TiO2 and PEEK Rein forced 3D Printing PMMA Composite Resin for Dental Denture Base Applications. Nanomaterials 2019, 9, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zan, L.; Wang, S.; Fa, W.; Hu, Y.; Tian, L.; Deng, K. Solid-phase photocatalytic degradation of polystyrene with modified nano-TiO2 catalyst. Polymer 2006, 47, 8155–8162. [Google Scholar] [CrossRef]
- Sun, M.; Kong, W.; Zhao, Y.; Liu, X.; Xuan, J.; Liu, Y.; Jia, F.; Yin, G.; Wang, J.; Zhang, J. Improving Photocatalytic Degradation Activity of Organic Pollutant by Sn4+ Doping of Anatase TiO2 Hierarchical Nanospheres with Dominant {001} Facets. Nanomaterials 2019, 9, 1603. [Google Scholar] [CrossRef] [Green Version]
- Morselli, D.; Bondioli, F.; Sangermano, M.; Messori, M. Photo-cured epoxy networks reinforced with TiO2 in-situ generated by means of non-hydrolytic sol–gel process. Polymer 2012, 53, 283–290. [Google Scholar] [CrossRef]
- Maira, A.J.; Yeung, K.L.; Lee, C.Y.; Yue, P.L.; Chan, C.K. Size Effects in Gas-Phase Photo-oxidation of Trichloroethylene Using Nanometer-Sized TiO2 Catalysts. J. Catal. 2002, 192, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Zuñiga-Ibarra, V.A.; Shaji, S.; Krishnan, B.; Johny, J.; Sharma Kanakkillam, S.; Avellaneda, D.A.; Aguilar Martinez, J.A.; Das Roy, T.K.; Ramos-Delgado, N.A. Synthesis and characterization of black TiO2 nanoparticles by pulsed laser irradiation in liquid. Appl. Surf. Sci. 2019, 483, 156–164. [Google Scholar] [CrossRef]
- Yang, M.; Lu, D.; Wang, H.; Wu, P.; Fang, P. A facile one-pot hydrothermal synthesis of two-dimensional TiO2-based nanosheets loaded with surface-enriched NixOy nanoparticles for efficient visible-light-driven photocatalysis. Appl. Surf. Sci. 2019, 467–468, 124–134. [Google Scholar] [CrossRef]
- Giannuzzi, R.; Manca, M.; De Marco, L.; Belviso, M.R.; Cannavale, A.; Sibillano, T.; Giannini, C.; Cozzoli, P.D.; Gigli, G. Ultrathin TiO2 (B) nano-rods with superior lithium-ion storage performance. ACS Appl. Mater. Interfaces 2014, 6, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.T.; Liu, M.; Wang, D.W.; Sun, T.; Guan, D.S.; Li, F.; Zhou, J.G.; Sham, T.; Cheng, H.M. Comparison of the rate capability of nanostructured amorphous and anatase TiO2 for lithium insertion using anodic TiO2 nano-tube arrays. Nanotechnology 2009, 20, 225701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; He, H.Y. Synthesis and photocatalytic property of N-doped TiO2nano-rods and nanotubes with high nitrogen content. Appl. Surf. Sci. 2011, 258, 972–976. [Google Scholar] [CrossRef]
- Hao, B.; Yan, Y.; Wang, X.; Chen, G. Synthesis of anatase TiO2nanosheets with enhanced pseudo capacitive contribution for fast lithium storage. ACS Appl. Mater. Interfaces 2013, 5, 6285–6291. [Google Scholar] [CrossRef]
- Lee, K.H.; Song, S.W. One-Step Hydrothermal Synthesis of Meso-porous Anatase TiO2 Microsphere and Interfacial Control for Enhanced Lithium Storage Performance. ACS Appl. Mater. Interfaces 2011, 3, 3697–3703. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Zhu, H.; Zhang, D.; Chen, J. Elongated TiO2 nanotubes directly grown on graphenenanosheets as an efficient material for supercapacitors and absorbents. Compos. Part A-Appl. Sci. Manuf. 2017, 10, 297–305. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, J.; Wu, P.; Ji, X. Immobilization of TiO2 films on activated carbon fiber and their photocatalytic degradation properties for dye compounds with different molecular size. Catal. Commun. 2008, 9, 1846–1850. [Google Scholar] [CrossRef]
- Khan, M.M.; Lee, J.; Cho, M.H. Au@TiO2nanocomposites for the catalytic degradation of methyl orange and methylene blue: An electron relay effect. J. Ind. Eng. Chem. 2013, 20, 1584–1590. [Google Scholar] [CrossRef]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikandan, G.; Anandi, V. Cu-doped TiO2 nanoparticles for photocatalytic disinfection of bacteria under visible light. J. Colloid Interface Sci. 2010, 352, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Waiskopf, N.; Ben-Shahar, Y.; Galchenko, M.; Carmel, I.; Moshitzky, G.; Soreq, H.; Banin, U. Photocatalytic reactive oxygen species formation by semiconductor–metal hybrid nanoparticles. Toward light-induced modulation of biological processes. Nano Lett. 2016, 16, 4266–4273. [Google Scholar] [CrossRef]
- Amirav, L.; Alivisatos, A.P. Luminescence Studies of Individual Quantum Dot Photocatalysts. J. Am. Chem. Soc. 2013, 135, 13049–13053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawar, A.A.; Halivni, S.; Waiskopf, N.; Ben-Shahar, Y.; Soreni-Harari, M.; Bergbreiter, S.; Banin, U.; Magdassi, S. Rapid three-dimensional printing in water using semiconductor–metal hybrid nanoparticles as photoinitiators. Nano Lett. 2017, 17, 4497–4501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorn, M.; Bae, W.K.; Kwak, J.; Lee, H.; Lee, C.; Zentel, R.; Char, K. Quantum Dot Block Copolymer Hybrids with Improved Properties and Their Application to Quantum Dot Light-Emitting Devices. ACS Nano 2009, 3, 1063–1068. [Google Scholar] [CrossRef]
- Landmann, M.; Rauls, E.W.; Schmidt, W.G. The electronic structure and optical response of rutile, anatase and brookite TiO2. J. Phys. Condens. Matter. 2012, 24, 195503. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.V.; Gayathri, P.K.; Gummaluri, V.S.; Nambissan, P.M.; Vijayan, C. Large bandgap narrowing in rutile TiO2 aimed towards visible light applications and its correlation with vacancy-type defects history and transformation. J. Phys. D Appl. Phys. 2018, 51, 045107. [Google Scholar] [CrossRef]
- Tezuka, Y.; Shin, S.; Ishii, T.; Ejima, T.; Suzuki, S.; Sato, S. Photoemission and bremsstrahlung isochromat spectroscopy studies of TiO2 (rutile) and SrTiO3. J. Phys. Soc. Jpn. 1994, 63, 347–357. [Google Scholar] [CrossRef]
- Rangan, S.; Katalinic, S.; Thorpe, R.; Bartynski, R.A.; Rochford, J.; Galoppini, E. Energy level alignment of a Zinc (II) tetraphenylporphyrin dye adsorbed onto TiO2 (110) and ZnO (1120) surfaces. J. Phys. Chem. C 2009, 114, 1139–1147. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Gao, C.; Cao, L. The synthesis of nano-sized TiO2 powder using a sol-gel method with TiCl4 as a precursor. J. Mater. Sci. 2000, 35, 4049–4054. [Google Scholar] [CrossRef]
- Yugang, D.; Yuan, Z.; Yiping, T.; Dichen, L. Nano-TiO2-modified photosensitive resin for RP. Rapid Prototyp. J. 2011, 17, 247–252. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, J.; Bao, S.; Wu, Q.; Wang, Q. Semiconductor nanoparticles-based hydrogels prepared via self-initiated polymerization under sunlight, even visible light. Sci. Rep. 2013, 3, 1399. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, M. ZnO Nano-particle Induced Photo-Kolbe Reaction, Fragment Stabilization and Effect on Photopolymerization Monitored by Raman-UV-Vis Measurements. Macromol. Chem. Phys. 2012, 23, 1953–1962. [Google Scholar] [CrossRef]
- Ni, X.; Ye, J.; Dong, C. Kinetics studies of methyl methacrylate photopolymerization initiated by titanium dioxide semiconductor nanoparticles. J. Photochem. Photobiol. A Chem. 2006, 181, 19–27. [Google Scholar] [CrossRef]
- Wang, W.K.; Chen, J.J.; Zhang, X.; Huang, Y.X.; Li, W.W.; Yu, H.Q. Self-induced synthesis of phase junction TiO2 with a tailored rutile to anatase ratio below phase transition temperature. Sci. Rep. 2016, 6, 20491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, R.; Gangwar, J.; Srivastava, A.K. Multiphase TiO2 nanostructures: A review of efficient synthesis, growth mechanism, probing capabilities, and applications in bio-safety and health. RSC Adv. 2017, 7, 44199. [Google Scholar] [CrossRef] [Green Version]
- Ren, R.; Wen, Z.; Cui, S.; Hou, Y.; Guo, X.; Chen, J. Controllable Synthesis and Tunable Photocatalytic Properties of Ti3+-doped TiO2. Sci. Rep. 2014, 5, 10714. [Google Scholar] [CrossRef] [Green Version]
- Riaz, N.; Bustam, M.A.; Chong, F.K.; Man, Z.B.; Khan, M.S. Photocatalytic Degradation of DIPA Using Bimetallic Cu-Ni/TiO2Photocatalyst under Visible Light Irradiation. Sci. World J. 2014, 2014, 342020. [Google Scholar] [CrossRef]
- Abboud, M.; Turner, M.; Duguet, E.; Fontanille, M. PMMA-based composite materials with reactive ceramic fillers Part 1. Chemical modification and characterization of ceramic particles. J. Mater. Chem. 1997, 7, 1527–1532. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Radovici, C.; Ghiurea, M.; Paven, H.; Iorga, M.D. Influence of Rutile and Anatase TiO2 Nanoparticles on Polyethylene Properties. Polym.-Plast. Technol. Eng. 2011, 50, 196–202. [Google Scholar] [CrossRef]
- Weng, Z.; Zhou, Y.; Lin, W.; Senthil, T.; Wu, L. Structure-property relationship of nano enhanced stereolithography resin for desktop SLA 3D printer. Compos. Part A-Appl. Sci. Manuf. 2016, 88, 234–242. [Google Scholar] [CrossRef]
- Chiappone, A.; Roppolo, I.; Naretto, E.; Fantino, E.; Calignano, F.; Sangermano, M.; Pirri, F. Study of graphene oxide-based 3D printable composites: Effect of the in situ reduction. Compos. Part B Eng. 2017, 124, 9–15. [Google Scholar] [CrossRef]
- Papastergiou, M.; Kanellou, A.; Chriti, D.; Raptopoulos, G.; Paraskevopoulou, P. Poly (Urethane-Acrylate) Aerogels via Radical Polymerization of Dendritic Urethane-Acrylate Monomers. Materials 2018, 11, 2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, R.; Liang, Y.; Ge, X.; Du, M.; Li, S.; Li, T.; Li, Z. Preparation and photocatalytic degradation activity of TiO2/rGO/polymer composites. Colloid Polym. Sci. 2015, 293, 1151–1157. [Google Scholar] [CrossRef]
- Glass, S.; Trinklein, B.; Abel, B.; Schulze, A. TiO2 as Photosensitizer and Photoinitiator for Synthesis of Photoactive TiO2-PEGDA Hydrogel without Organic Photoinitiator. Front. Chem. 2018, 6, 340. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Nimo, J.; Pastoriza-Gallego, M.J.; Piñeiro, M.M.; Legido, J.L.; Lugo, L. Thermal conductivity of dry anatase and rutile nano-powders and ethylene and propylene glycol-based TiO2nano-fluids. J. Chem. Thermodyn. 2015, 83, 67–76. [Google Scholar] [CrossRef]
- Decker, C.; Moussa, K. Real-time kinetic study of laser-induced polymerization. Macromolecules 1989, 22, 4455–4462. [Google Scholar] [CrossRef]
- Decker, C.; Moussa, K. A new method for monitoring ultra-fast photopolymerizations by real-time infra-red (RTIR) spectroscopy. Die Makromol. Chem. Macromol. Chem. Phys. 1988, 189, 2381–2394. [Google Scholar] [CrossRef]
- Esen, D.S.; Karasu, F.; Arsu, N. The investigation of photoinitiated polymerization of multifunctional acrylates with TX-BT by Photo-DSC and RT-FTIR. Prog. Org. Coat. 2011, 70, 102–107. [Google Scholar] [CrossRef]
- Channei, D.; Inceesungvorn, B.; Wetchakun, N.; Ukritnukun, S.; Nattestad, A.; Chen, J.; Phanichphant, S. Photocatalytic Degradation of Methyl Orange by CeO2 and Fe–doped CeO2 Films under Visible Light Irradiation. Sci. Rep. 2014, 4, 5757. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Jaegermann, W.; Rockstroh, N.; Junge, H.; Toupance, T. Band alignment investigations of hetero-structure NiO/TiO2 nanomaterial’s used as efficient hetero-junction earth-abundant metal oxide photocatalysts for hydrogen production. Phys. Chem. Chem. Phys. 2017, 19, 19279–19288. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?-Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangermano, M.; Marino, F.; Reuel, N.; Strano, M.S. Semiconducting Single-Walled Carbon Nanotubes as Radical Photoinitiators. Macromol. Chem. Phys. 2011, 212, 1469–1473. [Google Scholar] [CrossRef]



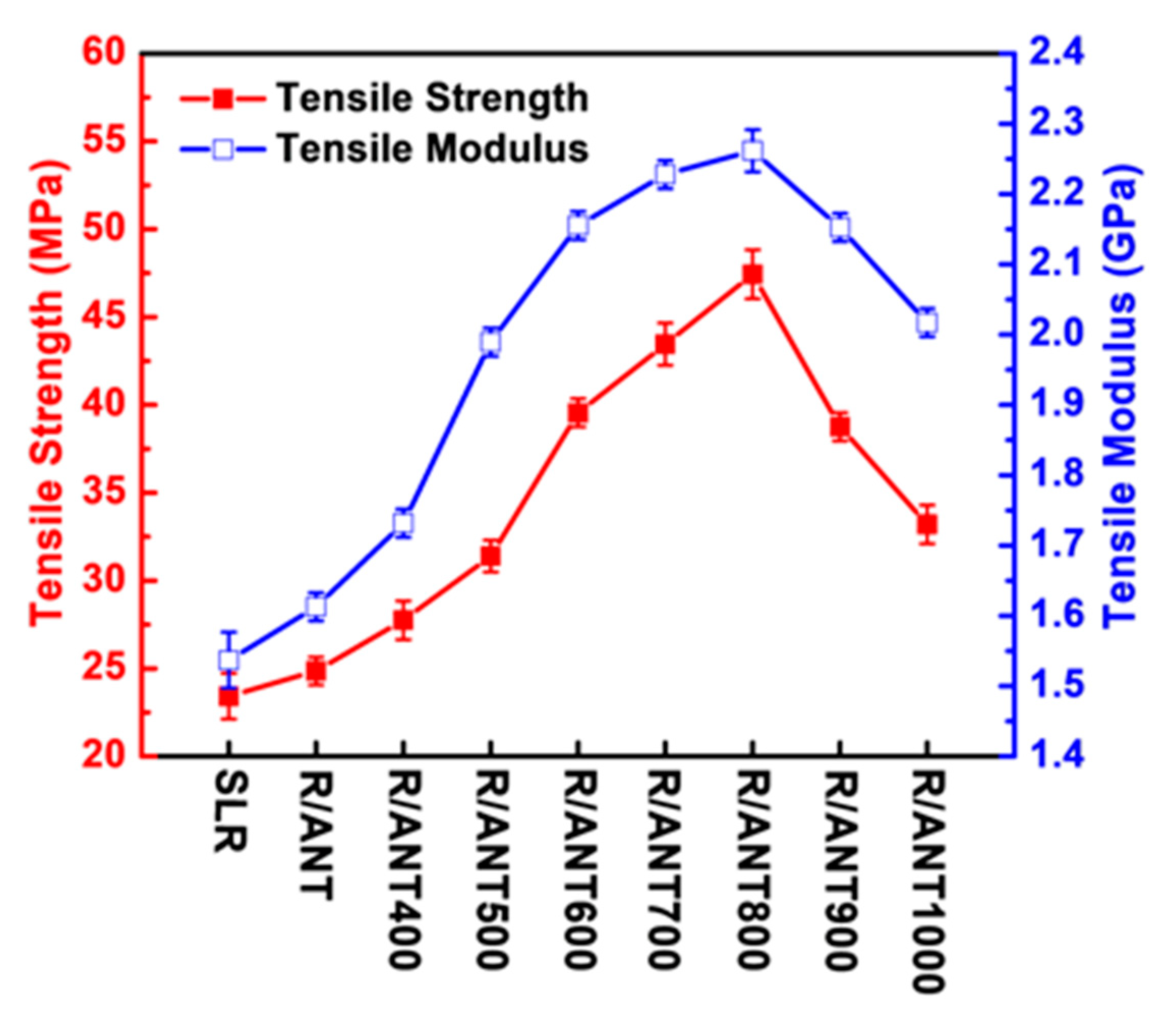



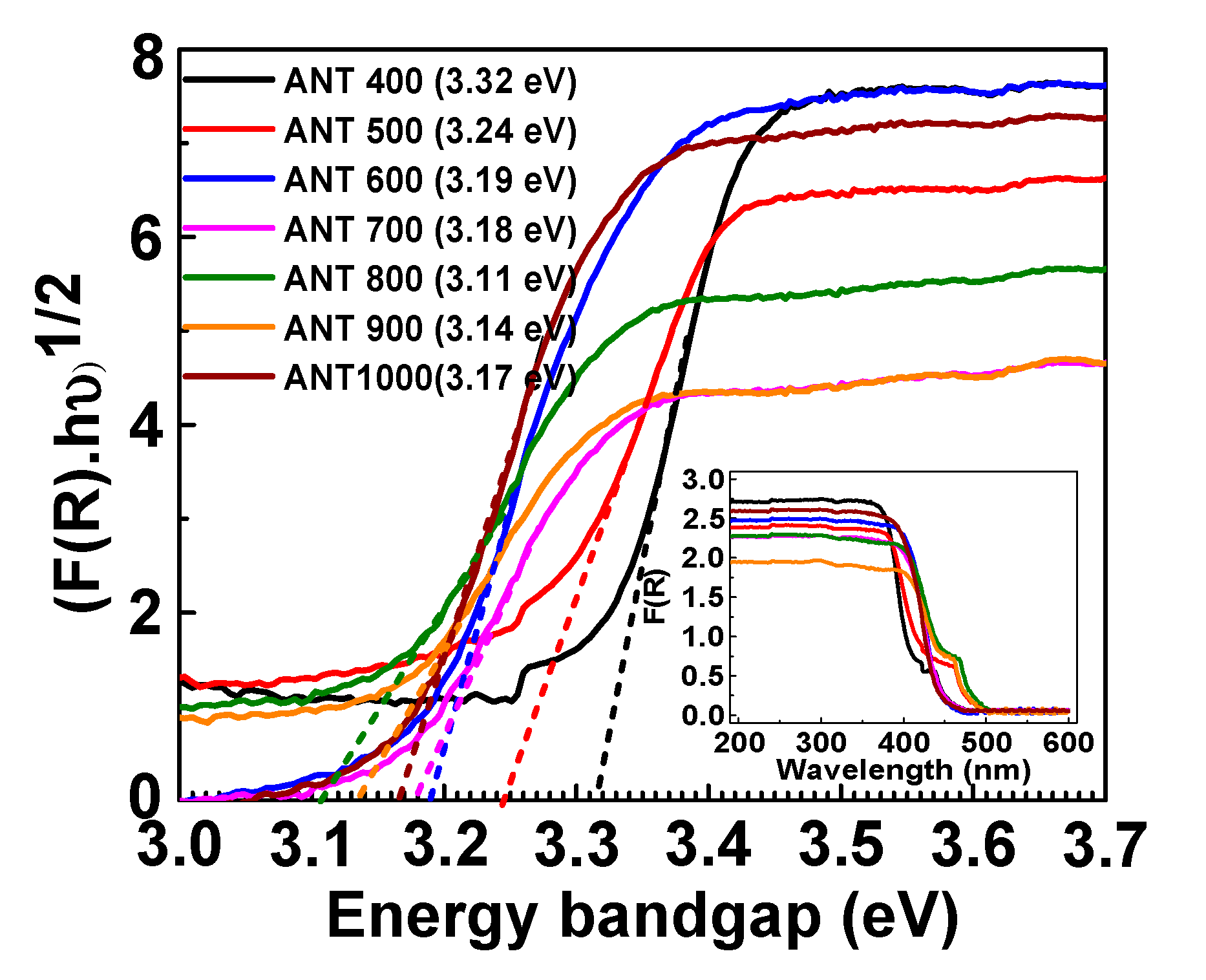
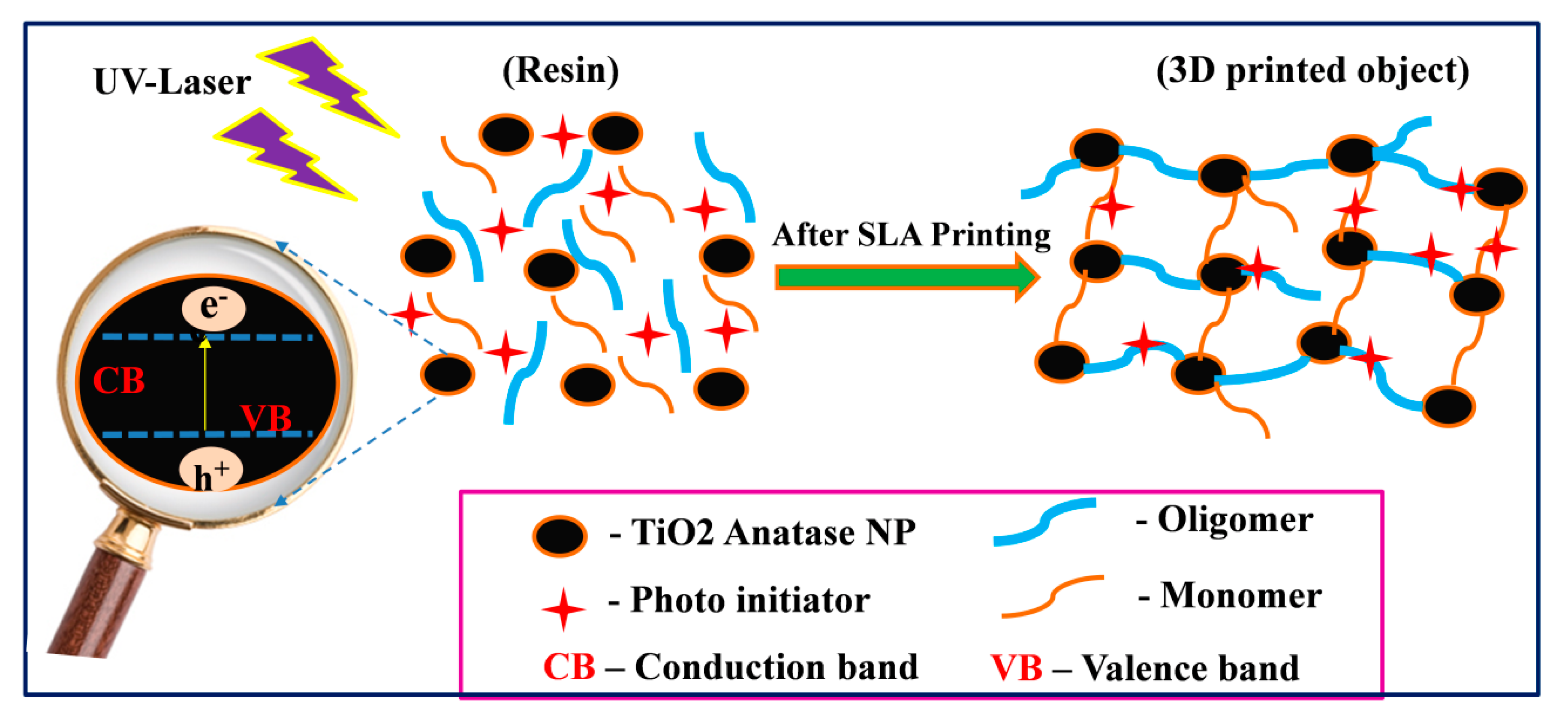
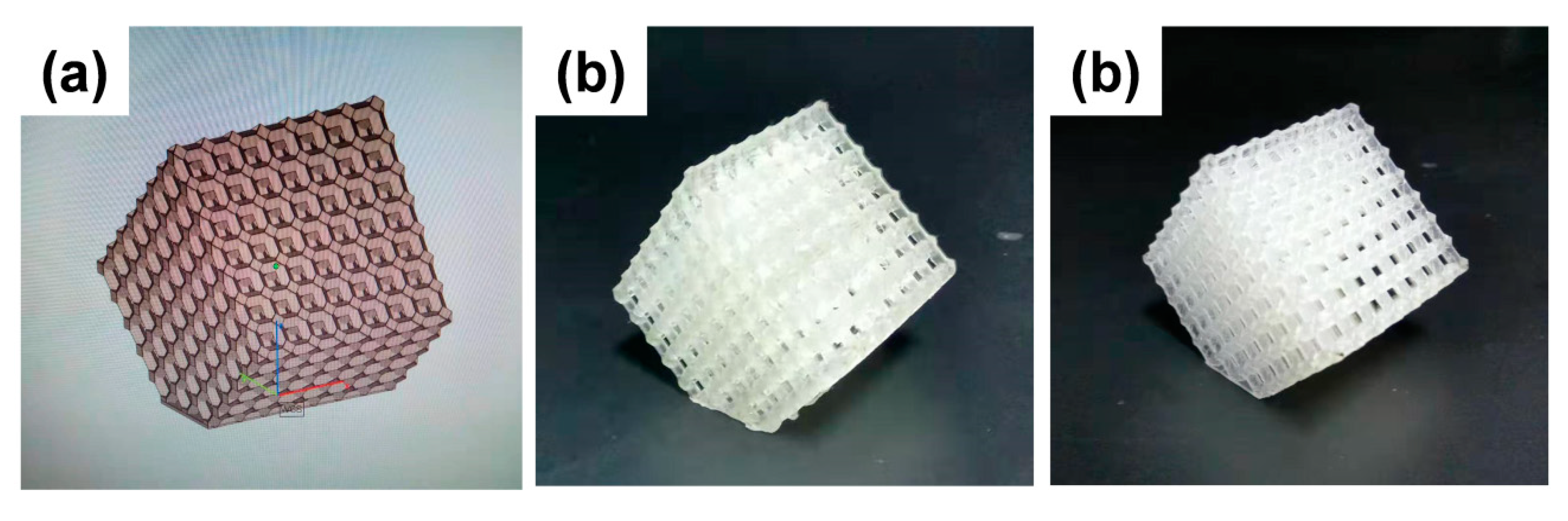
| Sample | Tensile Strength (MPa) | Tensile Modulus (GPa) | Strain (%) |
|---|---|---|---|
| Neat SLR | 23.42 ± 1.3 | 1.53 ± 0.04 | 4.55 ± 0.4 |
| R/ANT | 24.87 ± 0.8 | 1.61 ± 0.02 | 1.79 ± 0.3 |
| R/ANT400 | 27.76 ± 1.1 | 1.73 ± 0.02 | 1.98 ± 0.2 |
| R/ANT500 | 31.40 ± 0.9 | 1.98 ± 0.02 | 2.22 ± 0.4 |
| R/ANT600 | 39.55 ± 0.8 | 2.15 ± 0.02 | 2.42 ± 0.3 |
| R/ANT700 | 43.46 ± 1.2 | 2.22 ± 0.02 | 2.63 ± 0.2 |
| R/ANT800 | 47.43 ± 1.4 | 2.26 ± 0.03 | 3.06 ± 0.4 |
| R/ANT900 | 38.73 ± 0.8 | 2.15 ± 0.02 | 2.59 ± 0.2 |
| R/ANT1000 | 33.20 ± 1.1 | 2.01 ± 0.02 | 2.27 ± 0.2 |
| Sample | Max Indentation Depth (nm) | Reduced Modulus (GPa) | Hardness (GPa) |
|---|---|---|---|
| Neat SLR | 596.60 ± 32.2 | 2.3776 ± 0.01 | 0.1262 ± 0.003 |
| R/ANT400 | 577.35 ± 26.3 | 2.5856 ± 0.02 | 0.1436 ± 0.002 |
| R/ANT500 | 555.55 ± 20.2 | 2.8318 ± 0.02 | 0.1632 ± 0.005 |
| R/ANT600 | 532.97 ± 18.4 | 3.0062 ± 0.02 | 0.1826 ± 0.003 |
| R/ANT700 | 513.56 ± 30.3 | 3.4552 ± 0.01 | 0.2062 ± 0.004 |
| R/ANT800 | 490.87 ± 24.2 | 3.6645 ± 0.02 | 0.2264 ± 0.003 |
| R/ANT900 | 507.74 ± 26.3 | 3.3328 ± 0.03 | 0.1906 ± 0.004 |
| R/ANT1000 | 550.60 ± 31.2 | 2.9814 ± 0.01 | 0.1712 ± 0.003 |
| Sample | Storage 30 °C | Modulus (MPa) 100 °C | Tan δ Peak Height | Tg (°C) |
|---|---|---|---|---|
| Neat SLR | 1515.72 | 160.63 | 0.191 | 71.7 |
| R/ANT | 1555.24 | 188.18 | 0.189 | 73.3 |
| R/ANT400 | 1601.18 | 220.65 | 0.188 | 74.7 |
| R/ANT500 | 1652.33 | 230.02 | 0.187 | 75.8 |
| R/ANT600 | 1659.90 | 238.82 | 0/186 | 77.1 |
| R/ANT700 | 1684.91 | 245.08 | 0.185 | 78.2 |
| R/ANT800 | 1827.31 | 252.08 | 0.181 | 79.6 |
| R/ANT900 | 1675.70 | 247.39 | 0.184 | 77.4 |
| R/ANT1000 | 1665.93 | 236.84 | 0.185 | 76.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubarak, S.; Dhamodharan, D.; Divakaran, N.; Kale, M.B.; Senthil, T.; Wu, L.; Wang, J. Enhanced Mechanical and Thermal Properties of Stereolithography 3D Printed Structures by the Effects of Incorporated Controllably Annealed Anatase TiO2 Nanoparticles. Nanomaterials 2020, 10, 79. https://doi.org/10.3390/nano10010079
Mubarak S, Dhamodharan D, Divakaran N, Kale MB, Senthil T, Wu L, Wang J. Enhanced Mechanical and Thermal Properties of Stereolithography 3D Printed Structures by the Effects of Incorporated Controllably Annealed Anatase TiO2 Nanoparticles. Nanomaterials. 2020; 10(1):79. https://doi.org/10.3390/nano10010079
Chicago/Turabian StyleMubarak, Suhail, Duraisami Dhamodharan, Nidhin Divakaran, Manoj B. Kale, T. Senthil, Lixin Wu, and Jianlei Wang. 2020. "Enhanced Mechanical and Thermal Properties of Stereolithography 3D Printed Structures by the Effects of Incorporated Controllably Annealed Anatase TiO2 Nanoparticles" Nanomaterials 10, no. 1: 79. https://doi.org/10.3390/nano10010079
APA StyleMubarak, S., Dhamodharan, D., Divakaran, N., Kale, M. B., Senthil, T., Wu, L., & Wang, J. (2020). Enhanced Mechanical and Thermal Properties of Stereolithography 3D Printed Structures by the Effects of Incorporated Controllably Annealed Anatase TiO2 Nanoparticles. Nanomaterials, 10(1), 79. https://doi.org/10.3390/nano10010079