Efficient Hydrogenation of Xylose and Hemicellulosic Hydrolysate to Xylitol over Ni-Re Bimetallic Nanoparticle Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. The Preparation and Purification of Hydrolysate
2.4. Catalytic Reaction
3. Results and Discussion
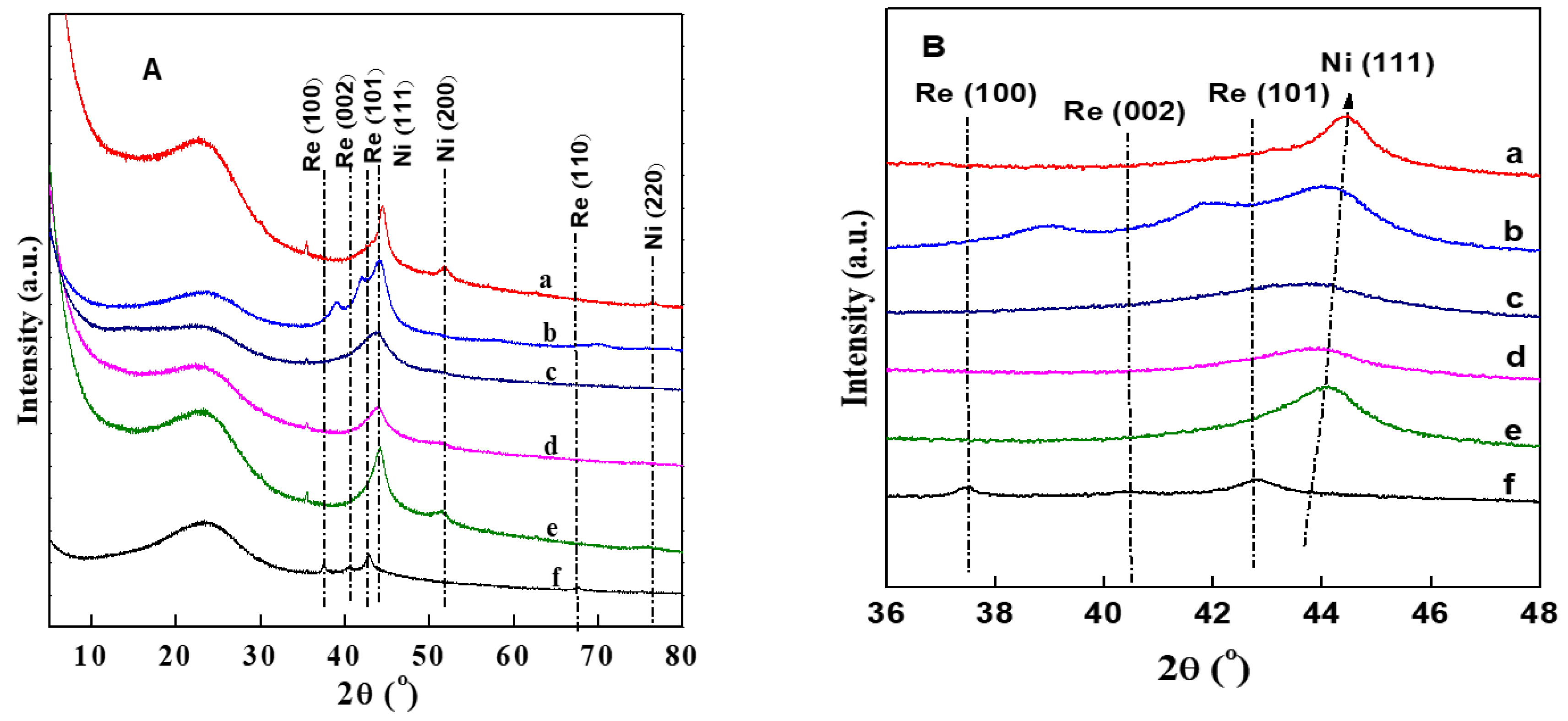
3.1. XRD Results
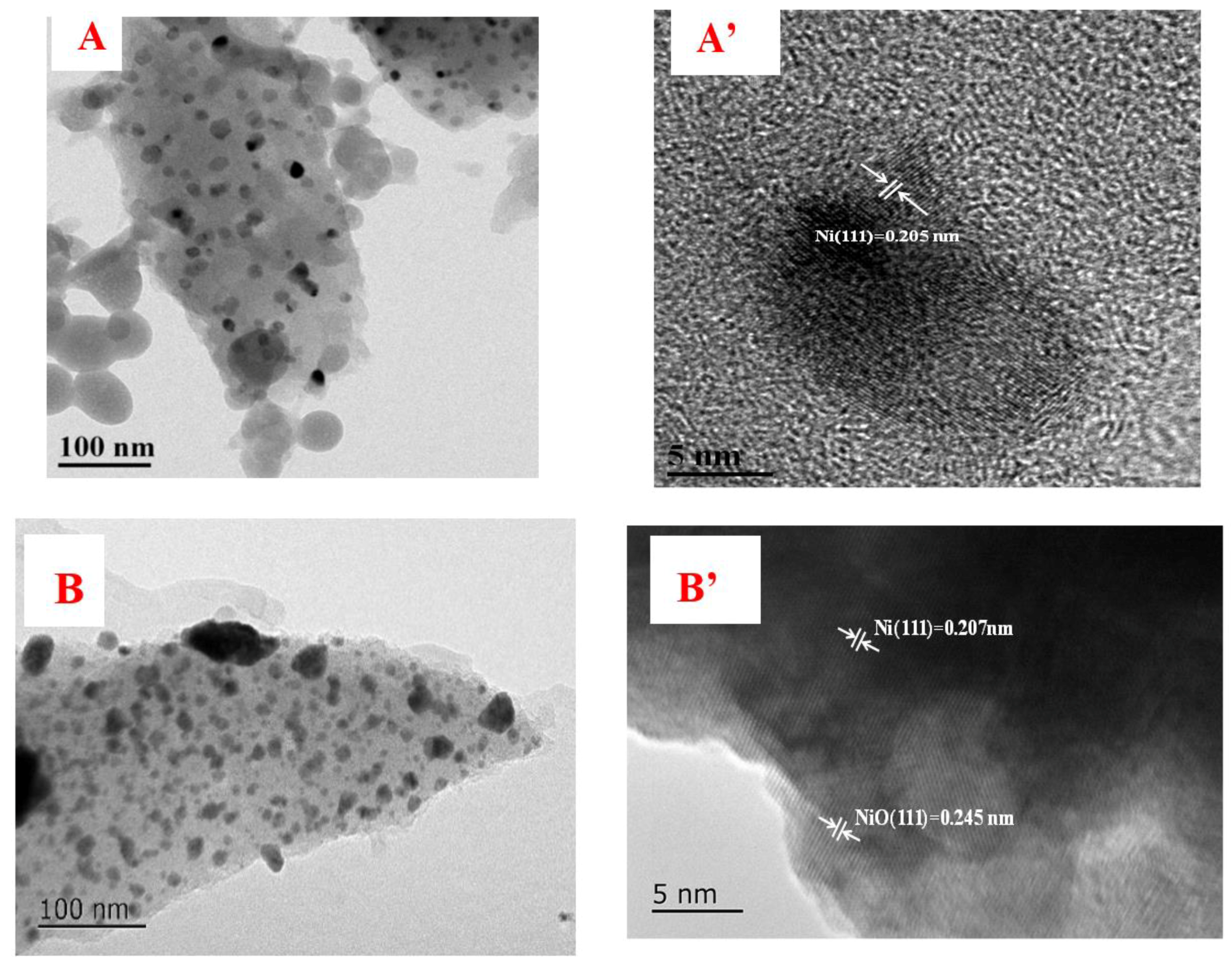
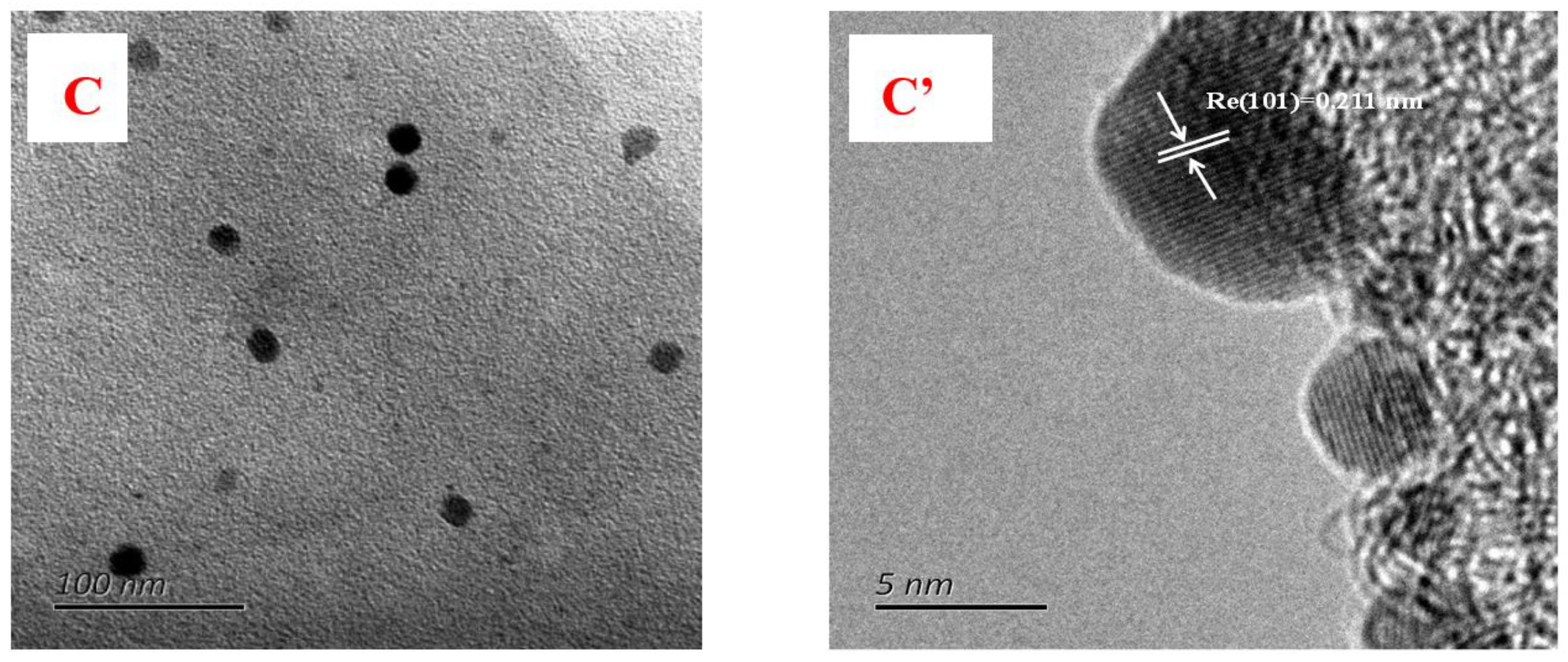
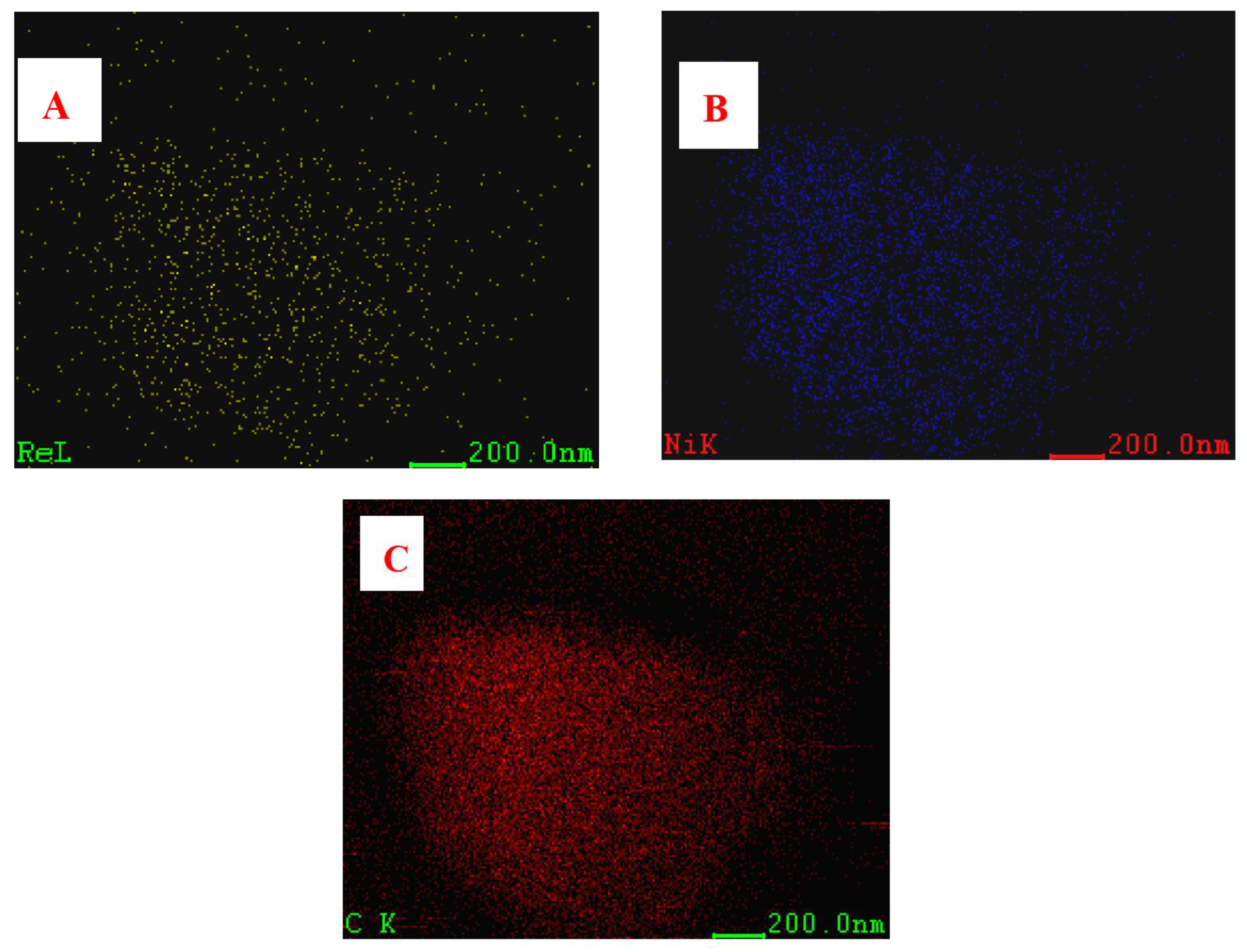
3.2. TEM Results
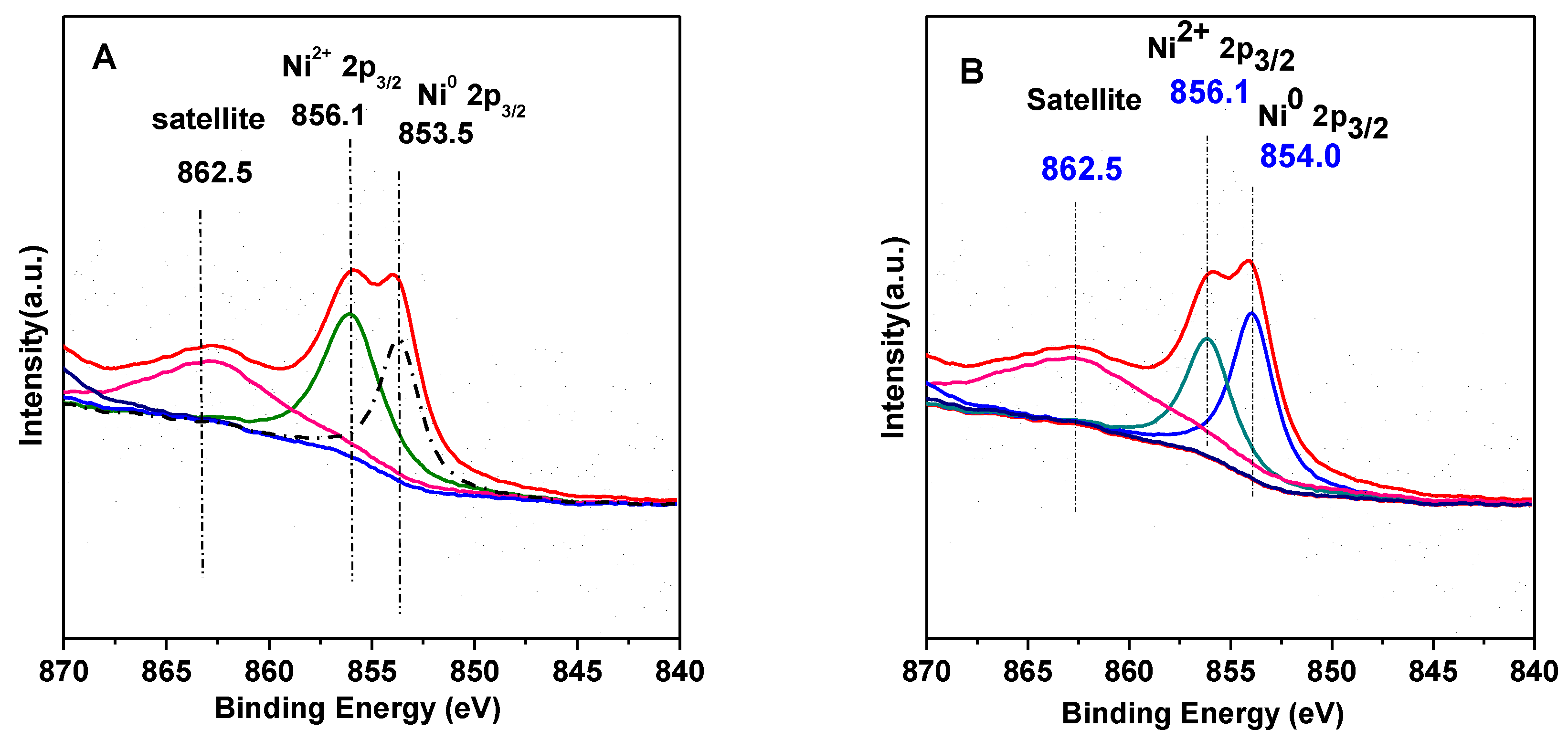
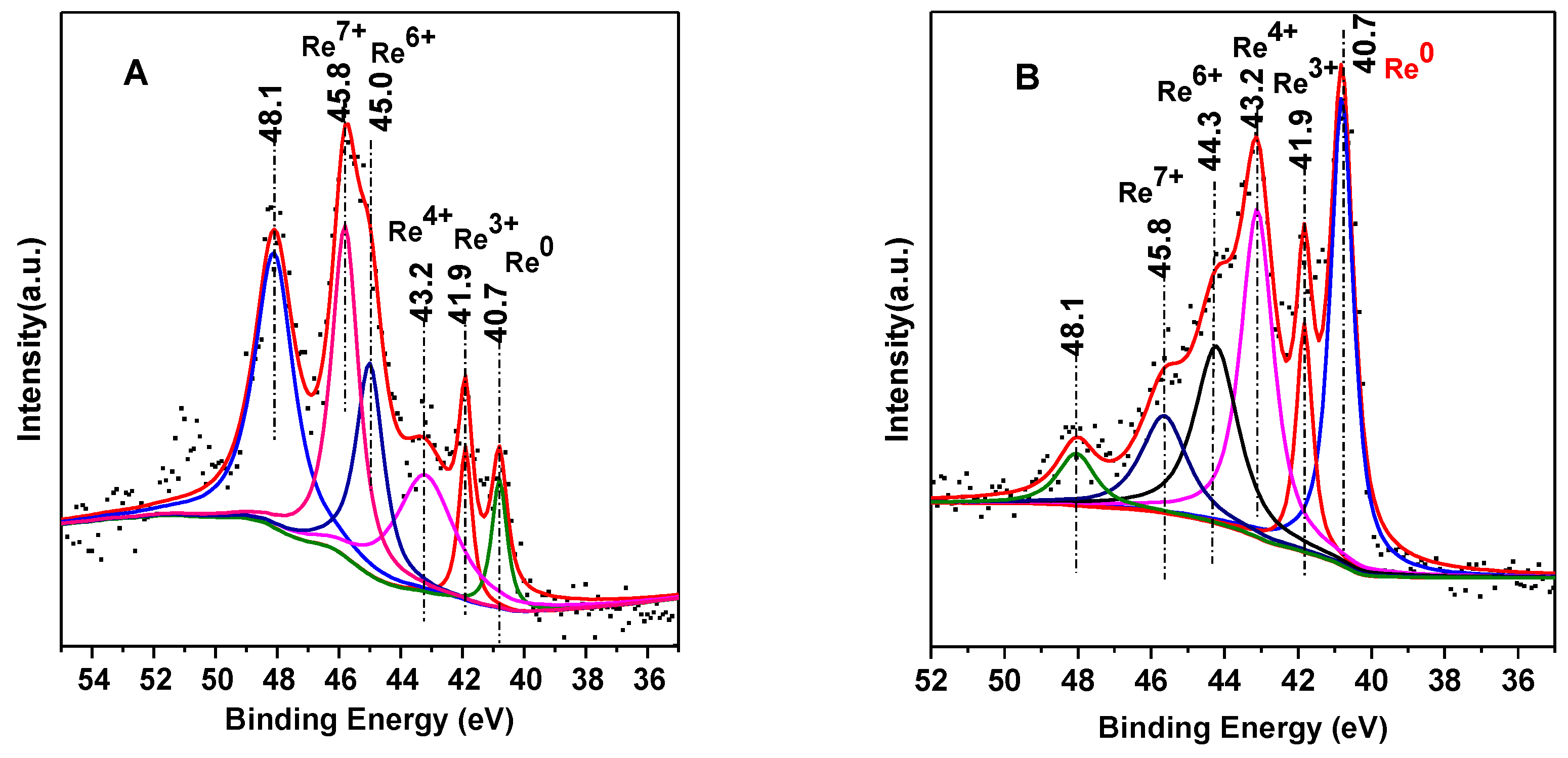
3.3. XPS Results
3.4. CO Chemisorption
3.5. Catalytic Performances
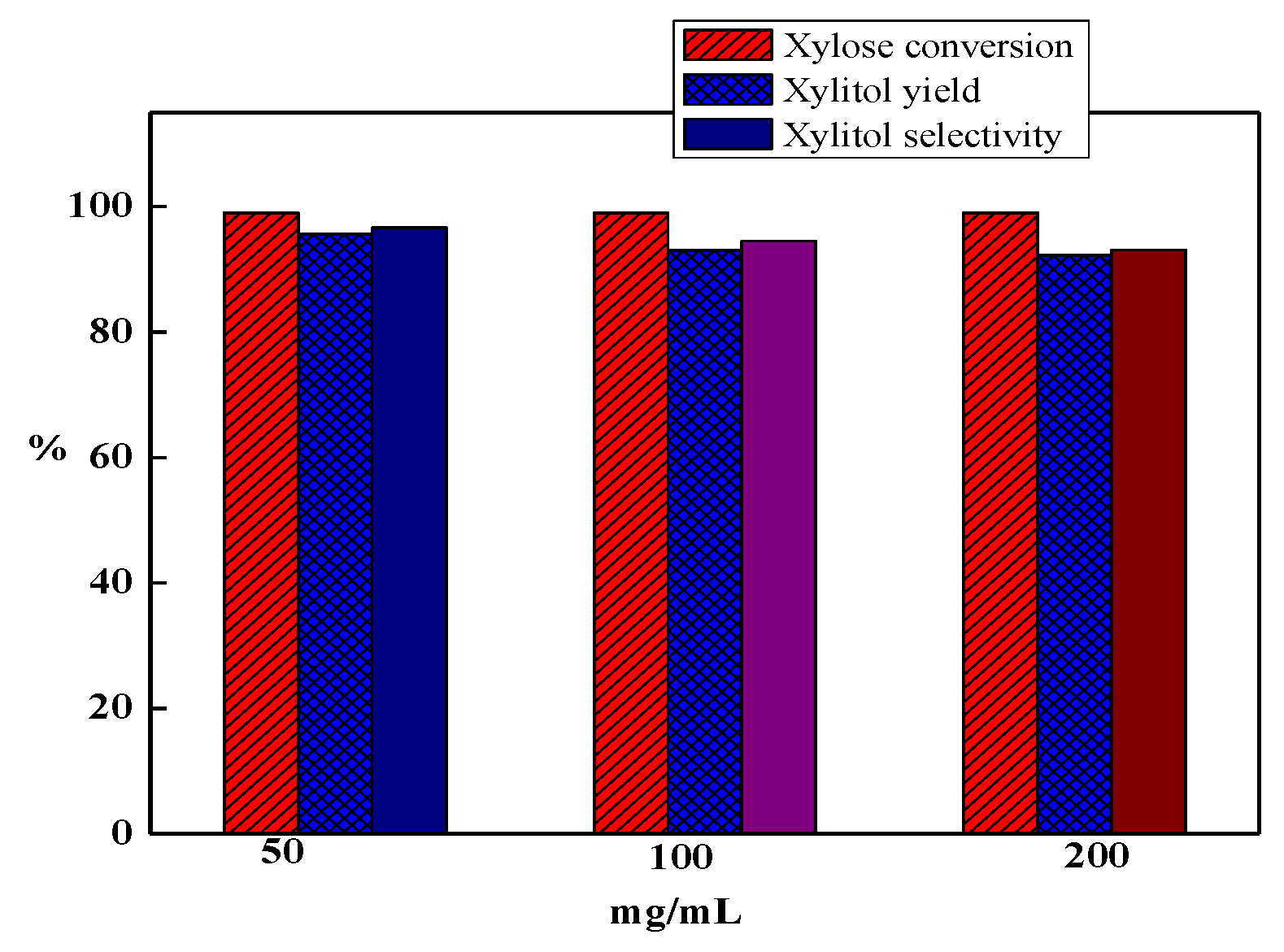
3.6. Hemicellulosic Hydrolysate Hydrogenation Reaction
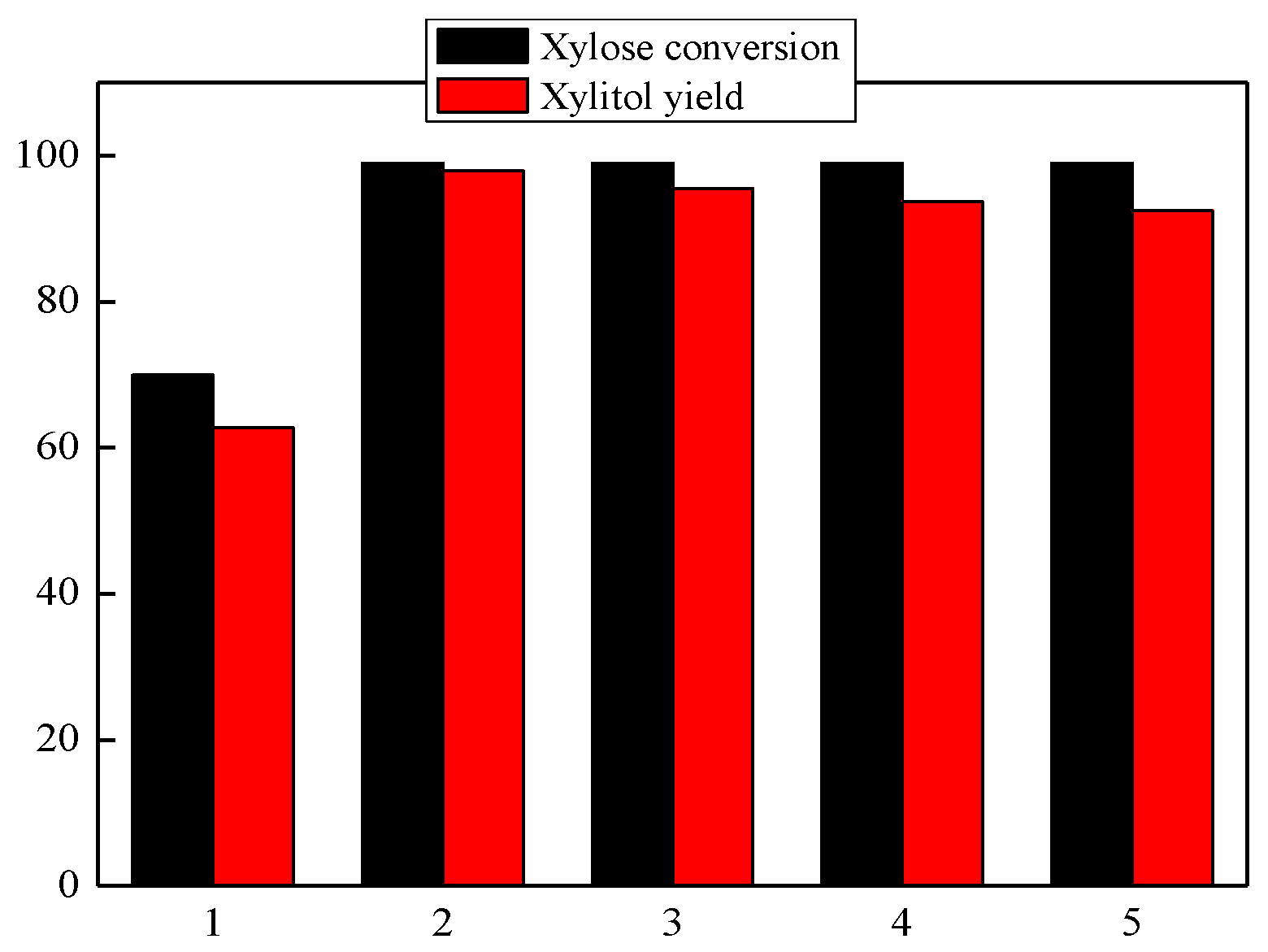
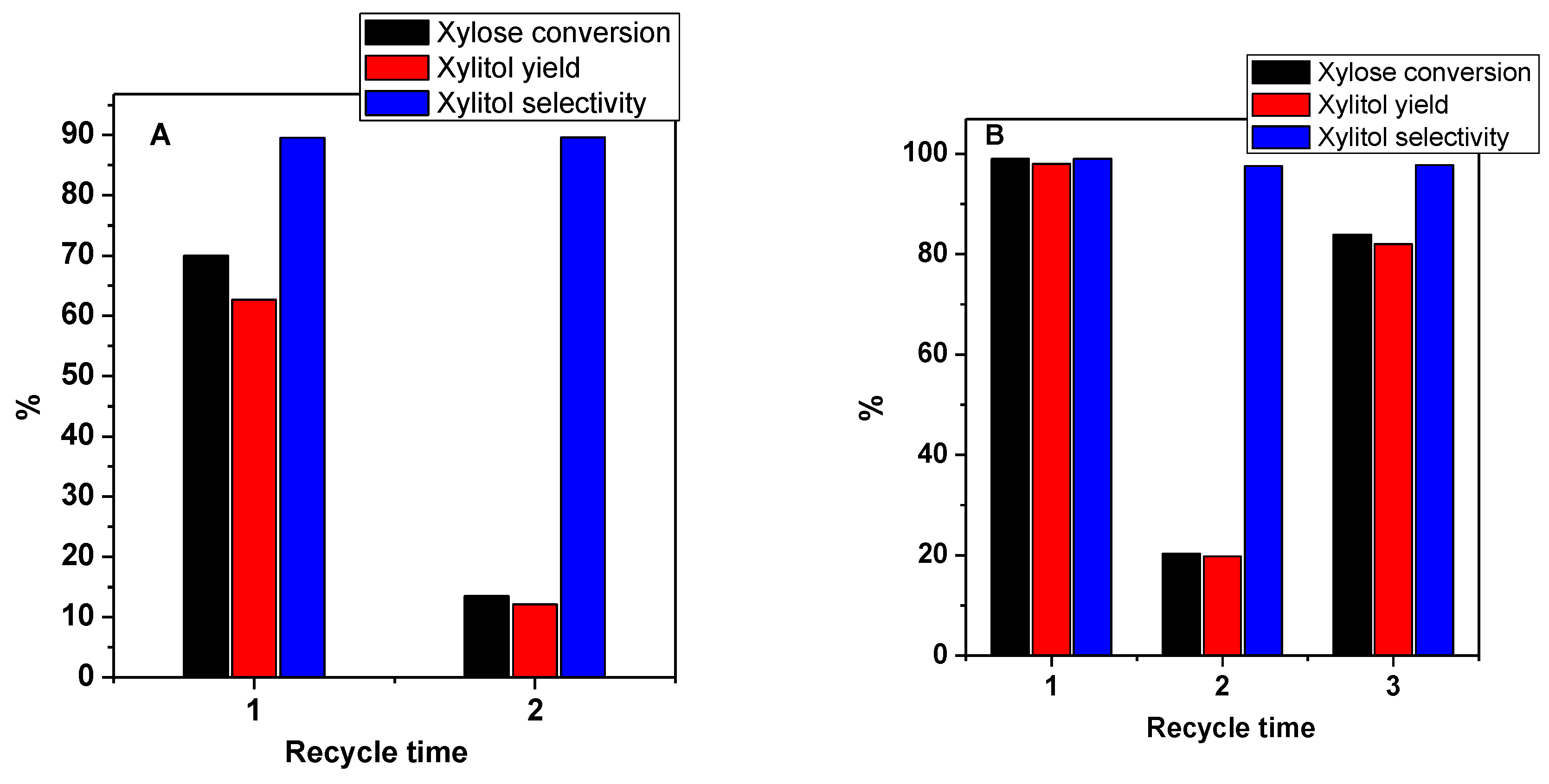
3.7. The Recycling Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.M., Jr.; Bu, Q.; Liang, J.; Liu, Y.; Mao, H.; Shi, A.; Lei, H.; Ruan, R. A review of catalytic microwave pyrolysis of lignocellulosic biomass for value-added fuel and chemicals. Bioresour. Technol. 2017, 230, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, J.; Han, B. Catalytic Transformation of Lignocellulose into Chemicals and Fuel Products in Ionic Liquids. Chem. Rev. 2017, 117, 6834–6880. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef]
- Hong, M.; Min, J.; Wang, S. Metal-Free Epoxidation of Internal and Terminal Alkenes with tert-Butyl Hydroperoxide/Isobutyraldehyde/Oxygen System. Chemistryselect 2018, 3, 4818–4821. [Google Scholar] [CrossRef]
- Mika, L.T.; Csefalvay, E.; Nemeth, A. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Xiang, Z.; Liang, J.; Morgan, H.M., Jr.; Liu, Y.; Mao, H.; Bu, Q. Thermal behavior and kinetic study for co-pyrolysis of lignocellulosic biomass with polyethylene over Cobalt modified ZSM-5 catalyst by thermogravimetric analysis. Bioresour. Technol. 2018, 247, 804–811. [Google Scholar] [CrossRef]
- Xia, H.; Xu, S.; Hu, H.; An, J.; Li, C. Efficient conversion of 5-hydroxymethylfurfural to high-value chemicals by chemo- and bio-catalysis. RSC Adv. 2018, 8, 30875–30886. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, G.W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals. Chem. Soc. Rev. 2018, 47, 1351–1390. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, G.L.; Miao, C.X. Green and renewable bio-diesel produce from oil hydrodeoxygenation: Strategies for catalyst development and mechanism. Renew. Sustain. Energy Rev. 2019, 101, 568–589. [Google Scholar] [CrossRef]
- Sun, G.; An, J.; Hu, H.; Li, C.; Zuo, S.; Xia, H. Green catalytic synthesis of 5-methylfurfural by selective hydrogenolysis of 5-hydroxymethylfurfural over size-controlled Pd nanoparticle catalysts. Catal. Sci. Technol. 2019, 9, 1238–1244. [Google Scholar] [CrossRef]
- Mikkola, J.P.; Salmi, T. Three-phase catalytic hydrogenation of xylose to xylitol prolonging the catalyst activity by means of on-line ultrasonic treatment. Catal. Today 2001, 64, 271–277. [Google Scholar] [CrossRef]
- Mikkola, J.P.; Salmi, T.; Villela, A.; Vainio, H.; Maki-Arvela, P.; Kalantar, A.; Ollonqvist, T.; Vayrynen, J.; Sjoholm, R. Hydrogenation of xylose to xylitol on sponge nickel catalyst—A study of the process and catalyst deactivation kinetics. Braz. J. Chem. Eng. 2003, 20, 263–271. [Google Scholar] [CrossRef]
- Yadav, M.; Mishra, D.K.; Hwang, J.-S. Catalytic hydrogenation of xylose to xylitol using ruthenium catalyst on NiO modified TiO2 support. Appl. Catal. A Gen. 2012, 425, 110–116. [Google Scholar] [CrossRef]
- Hilpmann, G.; Steudler, S.; Ayubi, M.M.; Pospiech, A.; Walther, T.; Bley, T.; Lange, R. Combining Chemical and Biological Catalysis for the Conversion of Hemicelluloses: Hydrolytic Hydrogenation of Xylan to Xylitol. Catal. Lett. 2019, 149, 69–76. [Google Scholar] [CrossRef]
- Tangale, N.P.; Niphadkar, P.S.; Joshi, P.N.; Dhepe, P.L. Hierarchical K/LTL zeolite as solid base for aqueous phase hydrogenation of xylose to xylitol. Micropor. Mesopor. Mater. 2019, 278, 70–80. [Google Scholar] [CrossRef]
- Huang, C.; Chu, Q.; Xie, Y.; Li, X.; Jin, Y.; Min, D.; Yong, Q. Effect of Kraft Pulping Pretreatment on the Chemical Composition, Enzymatic Digestibility, and Sugar Release of Moso Bamboo Residues. Bioresources 2015, 10, 240–255. [Google Scholar] [CrossRef]
- Li, X.; Gu, X.; Lai, C.; Ouyang, J.; Yong, Q. Production of Fumaric Acid by Rhizopus oryzae in Simultaneous Saccharification and Fermentation using Xylo-Oligosaccharides Manufacturing Waste Residue. Bioresources 2016, 11, 8831–8843. [Google Scholar] [CrossRef][Green Version]
- Ren, J.; Liu, L.; Xu, Q.; Li, X.; Yong, Q.; Ouyang, J. Comparative Evaluation of Magnesium Bisulfite Pretreatment under Different pH Values for Enzymatic Hydrolysis of Corn Stover. Bioresources 2016, 11, 7258–7270. [Google Scholar] [CrossRef][Green Version]
- Lai, C.; Li, X.; Zhu, J.; Yu, S.; Yong, Q. Detoxification of Steam-Exploded Corn Stover Prehydrolyzate with Organobentonite Enhances Ethanol Fermentation by Pichia stipites. Bioresources 2016, 11, 1905–1918. [Google Scholar] [CrossRef][Green Version]
- Huang, C.; Huang, C.; Lai, C.; Wu, X.; Huang, Y.; He, J.; Li, X.; Yong, Q. Strategy to Utilize the High Ash Content Biomass Feedstock for Fermentable Sugars. Bioresources 2017, 12, 8306–8319. [Google Scholar]
- Zhang, T.; Kumar, R.; Tsai, Y.-D.; Elander, R.T.; Wyman, C.E. Xylose yields and relationship to combined severity for dilute acid post- hydrolysis of xylooligomers from hydrothermal pretreatment of corn stover. Green Chem. 2015, 17, 394–403. [Google Scholar] [CrossRef]
- Ma, W.; Li, K.; Guo, H.; Yan, L.; Dai, Y.; Luo, X.; Yao, Y. Fabrication of porous carbon microspheres with numerous spherical microstructures directly from waste Camellia oleifera shells and their application in sustained-release of 5-fluorouracil. Micropor. Mesopor. Mater. 2017, 250, 195–202. [Google Scholar] [CrossRef]
- Fan, F.; Zheng, Y.; Huang, Y.; Lu, Y.; Wang, Z.; Chen, B.; Zheng, Z. Preparation and Characterization of Biochars from Waste Camellia oleifera Shells by Different Thermochemical Processes. Energy Fuels 2017, 31, 8146–8151. [Google Scholar] [CrossRef]
- Liang, J.; Qu, T.; Kun, X.; Zhang, Y.; Chen, S.; Cao, Y.-C.; Xie, M.; Guo, X. Microwave assisted synthesis of camellia oleifera shell-derived porous carbon with rich oxygen functionalities and superior supercapacitor performance. Appl. Surf. Sci. 2018, 436, 934–940. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Zhu, Y.; Liu, Y.; Wang, X. Camellia oleifera shell as an alternative feedstock for furfural production using a high surface acidity solid acid catalyst. Bioresour. Technol. 2018, 249, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, J.-P.; Hanna, V.; Tapio, S.; Rainer, S.; Tapio, O.; Juhani, V. Deactivation kinetics of Mo-supported Raney Ni catalyst in the hydrogenation of xylose to xylitol. Appl. Catal. A Gen. 2000, 196, 143–155. [Google Scholar] [CrossRef]
- Feher, A.; Feher, C.; Rozbach, M.; Racz, G.; Fekete, M.; Hegedus, L.; Barta, Z. Treatments of Lignocellulosic Hydrolysates and Continuous-Flow Hydrogenation of Xylose to Xylitol. Chem. Eng. Technol. 2018, 41, 496–503. [Google Scholar] [CrossRef]
- Mishra, D.K.; Dabbawala, A.A.; Hwang, J.-S. Ruthenium nanoparticles supported on zeolite Y as an efficient catalyst for selective hydrogenation of xylose to xylitol. J. Mol. Catal. A Chem. 2013, 376, 63–70. [Google Scholar] [CrossRef]
- Morales, R.; Campos, C.H.; Fierro, J.L.G.; Fraga, M.A.; Pecchi, G. Stable reduced Ni catalysts for xylose hydrogenation in aqueous medium. Catal. Today 2018, 310, 59–67. [Google Scholar] [CrossRef]
- Morales, R.; Campos, C.H.; Fierro, J.L.G.; Fraga, M.A.; Pecchi, G. Perovskite as nickel catalyst precursor - impact on catalyst stability on xylose aqueous-phase hydrogenation. RSC Adv. 2016, 6, 67817–67826. [Google Scholar] [CrossRef]
- Morales, R.; Campos, C.H.; Fierro, J.L.G.; Fraga, M.A.; Pecchi, G. Enhancing xylose aqueous-phase hydrogenation catalytic performance of A-site Ce substituted and B-site Rh doped reduced perovskites. Mol. Catal. 2017, 436, 182–189. [Google Scholar] [CrossRef]
- Scholz, D.; Aellig, C.; Mondelli, C.; Perez-Ramirez, J. Continuous Transfer Hydrogenation of Sugars to Alditols with Bioderived Donors over Cu-Ni-Al Catalysts. ChemCatChem 2015, 7, 1551–1558. [Google Scholar] [CrossRef]
- Mishra, D.K.; Dabbawala, A.A.; Hwang, J.-S. Poly (styrene-co-divinylbenzene) amine functionalized polymer supported ruthenium nanoparticles catalyst active in hydrogenation of xylose. Catal. Commun. 2013, 41, 52–55. [Google Scholar] [CrossRef]
- Tung Ngoc, P.; Samikannu, A.; Rautio, A.-R.; Juhasz, K.L.; Konya, Z.; Warna, J.; Kordas, K.; Mikkola, J.-P. Catalytic Hydrogenation of D-Xylose Over Ru Decorated Carbon Foam Catalyst in a SpinChem (R) Rotating Bed Reactor. Top. Catal. 2016, 59, 1165–1177. [Google Scholar]
- Hernandez-Mejia, C.; Gnanakumar, E.S.; Olivos-Suarez, A.; Gascon, J.; Greer, H.F.; Zhou, W.; Rothenberg, G.; Shiju, N.R. Ru/TiO2-catalysed hydrogenation of xylose: The role of the crystal structure of the support. Catal. Sci. Technol. 2016, 6, 577–582. [Google Scholar] [CrossRef]
- Xu, S.; Yan, X.; Bu, Q.; Xia, H. Catalytic conversion of cellulose into polyols using carbon-nanotube-supported monometallic Pd and bimetallic Pd-Fe catalysts. Cellulose 2017, 24, 2403–2413. [Google Scholar] [CrossRef]
- Yang, F.; Liu, D.; Wang, H.; Liu, X.; Han, J.; Ge, Q.; Zhu, X. Geometric and electronic effects of bimetallic Ni-Re catalysts for selective deoxygenation of m-cresol to toluene. J. Catal. 2017, 349, 84–97. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Kang, H.; Li, X.; Xia, C.; Chen, J.; Liu, H. Efficient bimetallic NiCu-SiO2 catalysts for selective hydrogenolysis of xylitol to ethylene glycol and propylene glycol. Appl. Catal. B Environ. 2018, 220, 251–263. [Google Scholar] [CrossRef]
- Xia, H.; An, J.; Hong, M.; Xu, S.; Zhang, L.; Zuo, S. Aerobic oxidation of 5-hydroxymethylfurfural to 2,5-difurancarboxylic acid over Pd-Au nanoparticles supported on Mg-Al hydrotalcite. Catal. Today 2019, 319, 113–120. [Google Scholar] [CrossRef]
- Miao, M.; Zuo, S.; Zhao, Y.; Wang, Y.; Xia, H.; Tan, C.; Gao, H. Selective oxidation rapidly decomposes biomass-based activated carbons into graphite-like crystallites. Carbon 2018, 140, 504–507. [Google Scholar] [CrossRef]
- Xia, D.; Chen, Y.; Li, C.; Liu, C.; Zhou, G. Carbon dioxide reforming of methane to syngas over ordered mesoporous Ni/KIT-6 catalysts. Int. J. Hydrog. Energy 2018, 43, 20488–20499. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, G.; Xie, H.; Jiao, Z.; Zhang, X. Hydrodeoxygenation of methyl laurate over the sulfur-free Ni/gamma-Al2O3 catalysts. Appl. Catal. A Gen. 2019, 569, 35–44. [Google Scholar] [CrossRef]
- Ma, L.; Sun, K.; Luo, M.; Yan, L.; Jiang, Z.; Lu, A.-H.; Ding, Y. Role of ReOx Species in Ni-Re/Al2O3 Catalyst for Amination of Monoethanolamine. J. Phys. Chem. C 2018, 122, 23011–23025. [Google Scholar] [CrossRef]

| Entry | Catalyst | Ni Particle Size (nm) a | Ni Dispersion (CO/Ni) |
|---|---|---|---|
| 1 | Re/AC | 10.6 ± 5.0 | Nd b |
| 2 | Ni-Re/AC (nNi:nRe = 1:1) | 13.0 ± 5.0 | 0.080 |
| 3 | Ni-Re/AC (nNi:nRe = 2:1) | 15.1 ± 6.1 | 0.027 |
| 4 | Ni-Re/AC (nNi:nRe = 4:1) | 15.8 ± 6.1 | / |
| 5 | Ni-Re/AC (nNi:nRe = 10:1) | 16.0 ± 5.2 | / |
| 6 | Ni/AC | 22.0 ± 6.2 | 0.020 |
| Entry | Temperature (°C) | Time (h) | Xylose Conversion (%) | Xylitol Yield (%) |
|---|---|---|---|---|
| 1 | 120 | 2.0 | 79 | 77.6 |
| 2 | 140 | 0.5 | 59 | 57.2 |
| 3 | 140 | 1.0 | 99 | 98.0 |
| 4 | 140 | 2.0 | 99 | 95.6 |
| 5 | 160 | 2.0 | 99 | 93.0 |
| 6 | 180 | 2.0 | 99 | 85.8 |
| 7 | 200 | 2.0 | 99 | 64.8 |
| Catalyst | Tem. (°C) | Time (h) | H2 pre. (bar) | Conversion (%) | Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|
| Ru/AC | 110 | 1.5 | 50 | 100 | 98.7 | [35] |
| Rh/perovskite | 100 | 2.0 | 25 | 100 | 51.0 | [32] |
| Ru/polymer | 120 | 2.0 | 55 | 99.8 | 94.1 | [34] |
| Ru/TiO2 | 120 | 1.0 | 20 | 100 | 98.0 | [36] |
| Ru/Y | 120 | 1.0 | 55 | 62 | 98.0 | [29] |
| NdCeAl0.162Ni0.838 | 100 | 2.0 | / | 20 | 50.0 | [36] |
| Raney Ni | 120 | 2.0 | 55 | 96.9 | 96.7 | [14] |
| Ni-Re/AC | 140 | 1.0 | 20 | 99 | 98 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, H.; Zhang, L.; Hu, H.; Zuo, S.; Yang, L. Efficient Hydrogenation of Xylose and Hemicellulosic Hydrolysate to Xylitol over Ni-Re Bimetallic Nanoparticle Catalyst. Nanomaterials 2020, 10, 73. https://doi.org/10.3390/nano10010073
Xia H, Zhang L, Hu H, Zuo S, Yang L. Efficient Hydrogenation of Xylose and Hemicellulosic Hydrolysate to Xylitol over Ni-Re Bimetallic Nanoparticle Catalyst. Nanomaterials. 2020; 10(1):73. https://doi.org/10.3390/nano10010073
Chicago/Turabian StyleXia, Haian, Lei Zhang, Hong Hu, Songlin Zuo, and Li Yang. 2020. "Efficient Hydrogenation of Xylose and Hemicellulosic Hydrolysate to Xylitol over Ni-Re Bimetallic Nanoparticle Catalyst" Nanomaterials 10, no. 1: 73. https://doi.org/10.3390/nano10010073
APA StyleXia, H., Zhang, L., Hu, H., Zuo, S., & Yang, L. (2020). Efficient Hydrogenation of Xylose and Hemicellulosic Hydrolysate to Xylitol over Ni-Re Bimetallic Nanoparticle Catalyst. Nanomaterials, 10(1), 73. https://doi.org/10.3390/nano10010073