Peroxide Electrochemical Sensor and Biosensor Based on Nanocomposite of TiO2 Nanoparticle/Multi-Walled Carbon Nanotube Modified Glassy Carbon Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Functionalization of Carbon Nanotubes
2.3. Synthesis of TiO2–CNT Nanostructures
2.4. Material Characterization
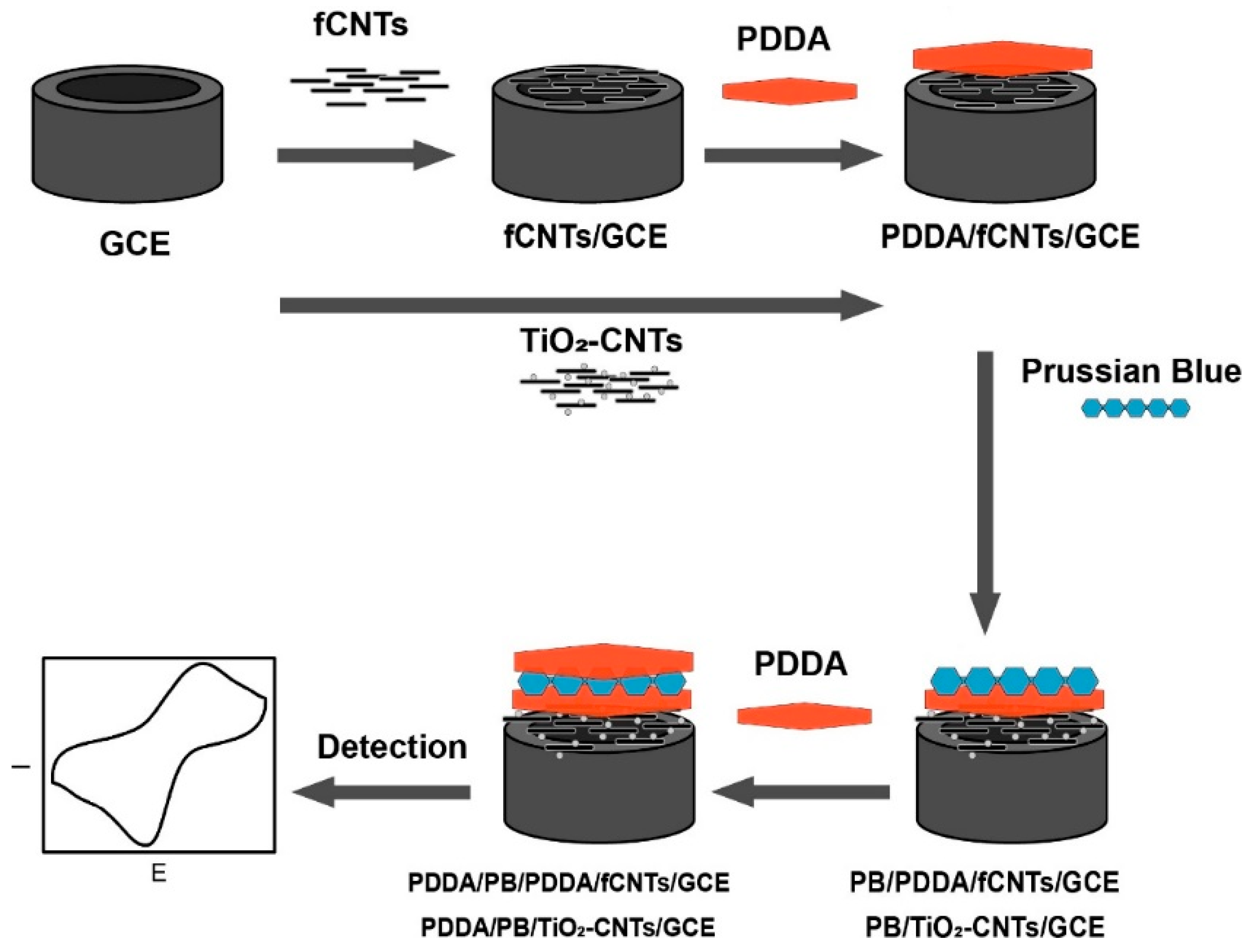
2.5. Electrode Modification
2.6. Electrodeposition and Activation of Prussian Blue at the Modified Electrode and HRP Immobilization at Modified Electrodes
2.7. Electrochemical Characterization
2.8. Peroxide Detection
3. Results and Discussion
3.1. Nanomaterial Characterization
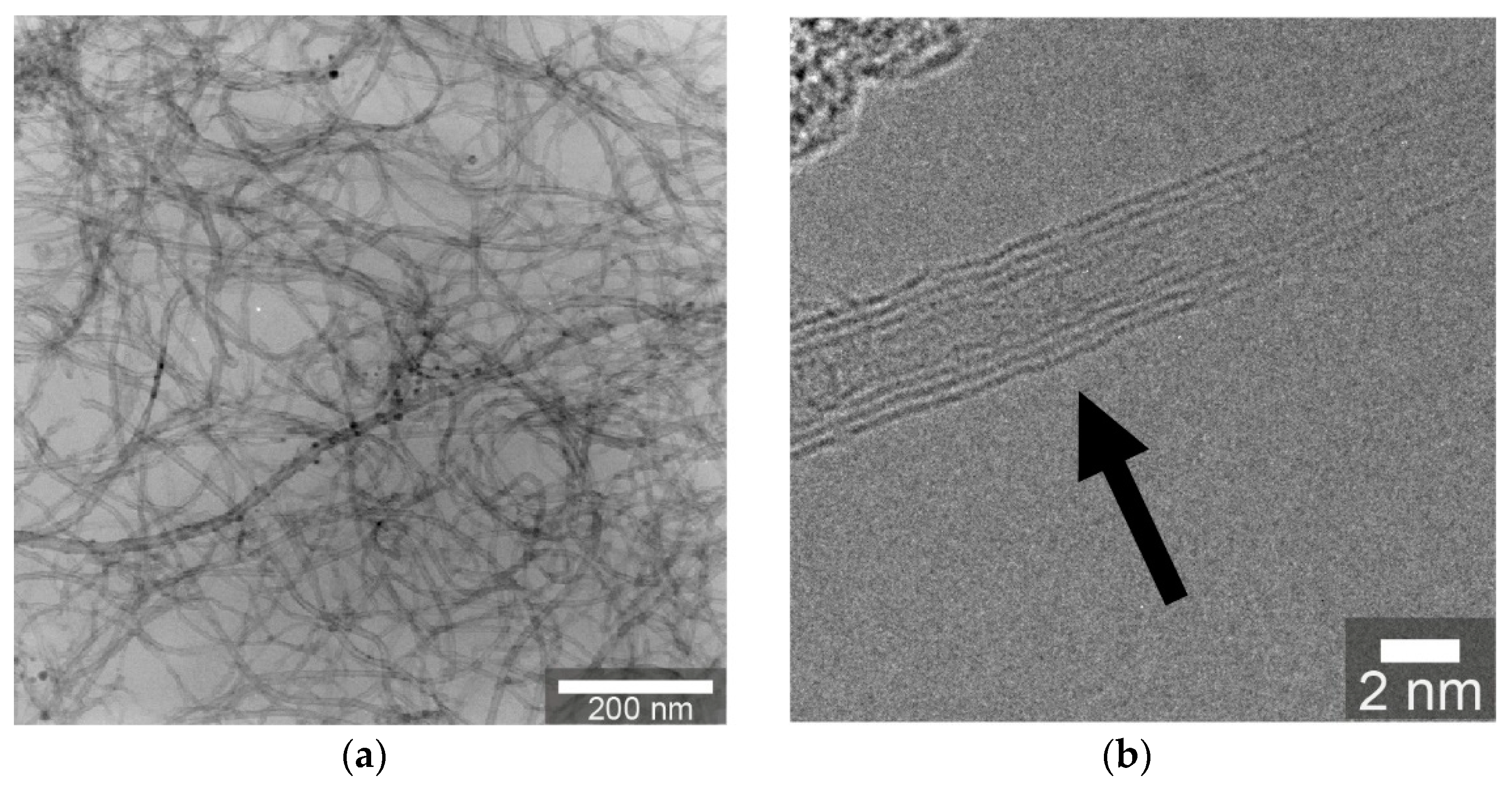
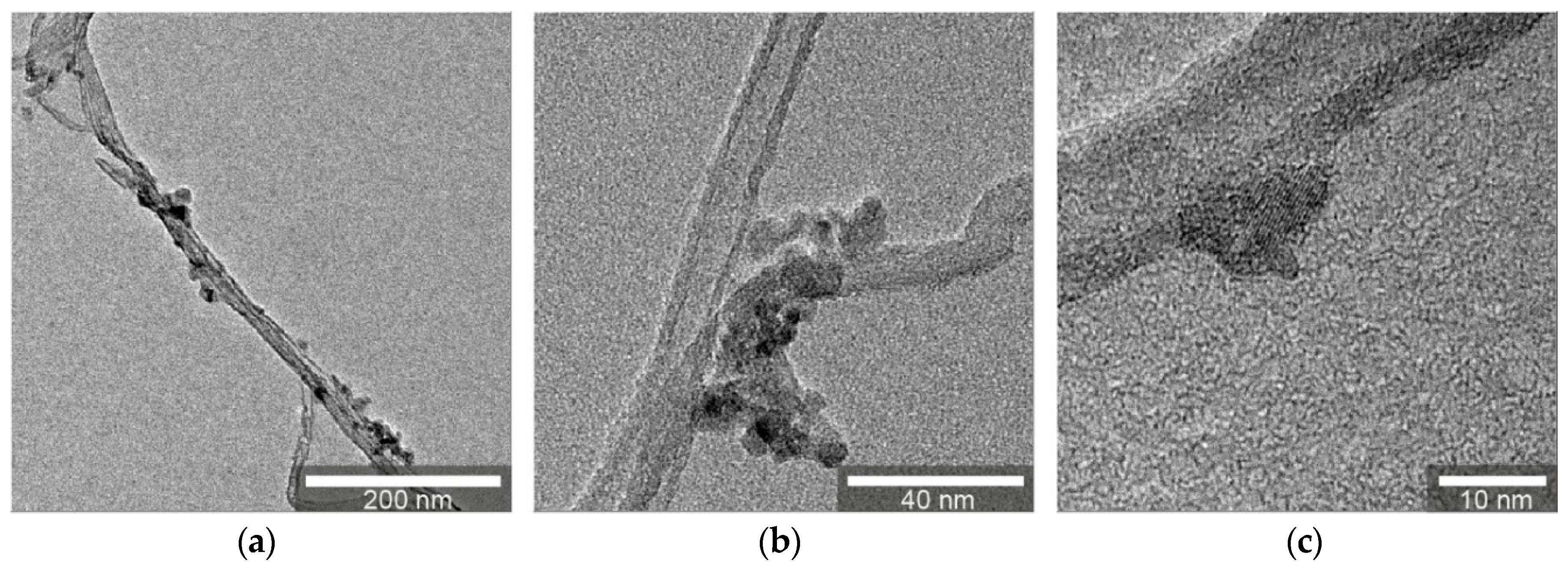
3.1.1. Transmission Electron Microscopy (TEM)
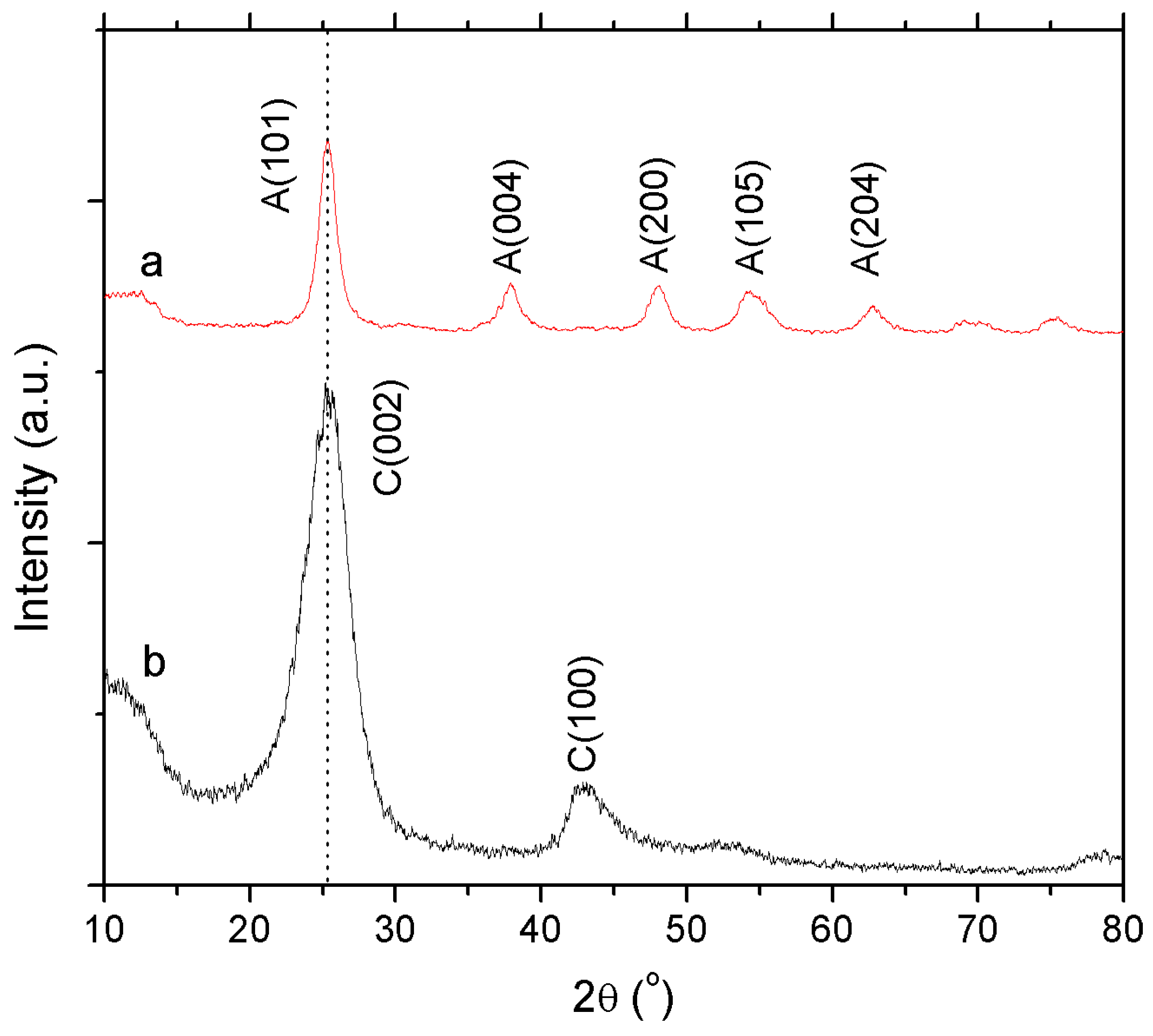
3.1.2. X-ray Diffraction (XRD)
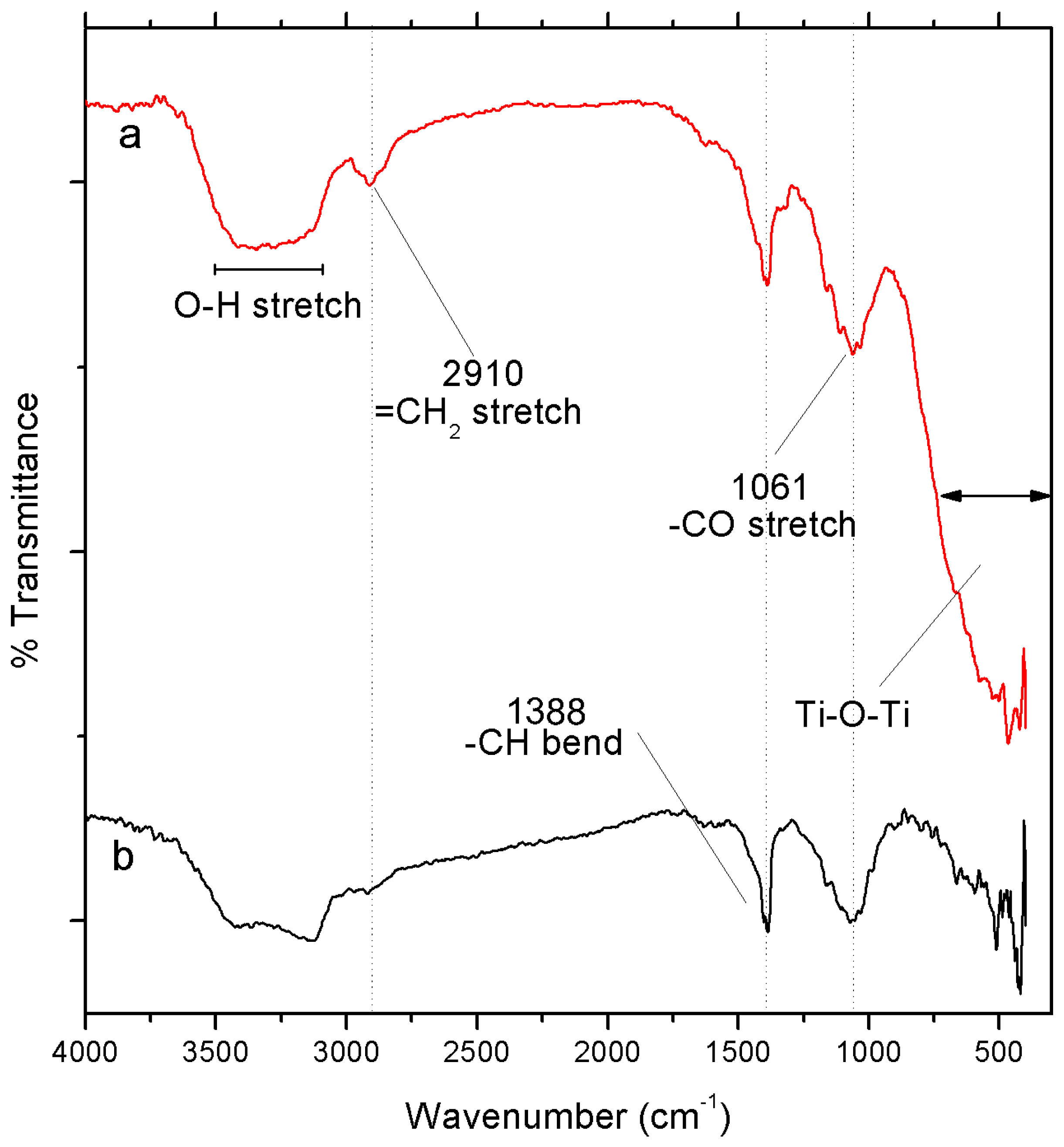
3.1.3. Fourier-Transform Infrared (FTIR) Spectroscopy
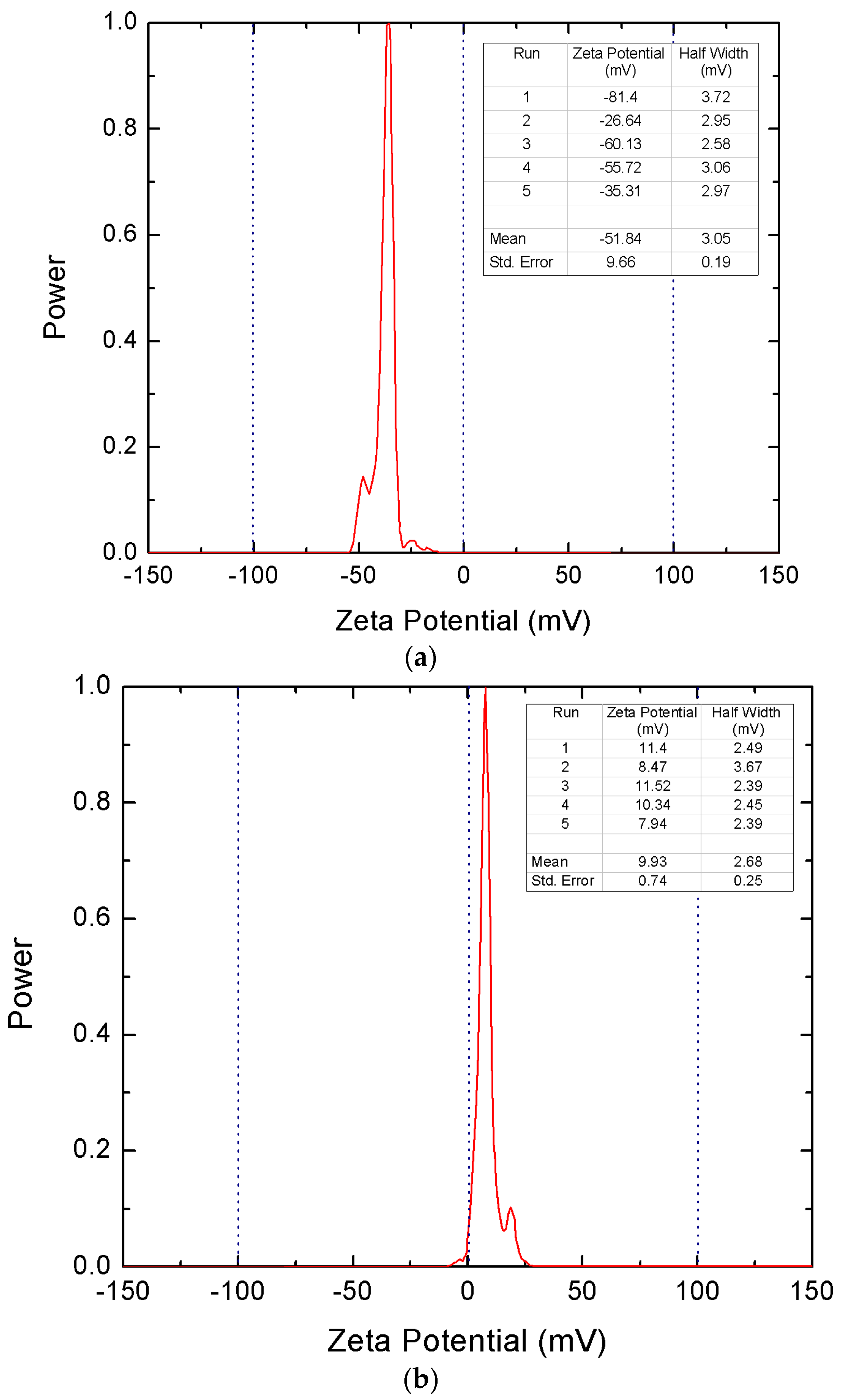
3.1.4. Zeta Potential
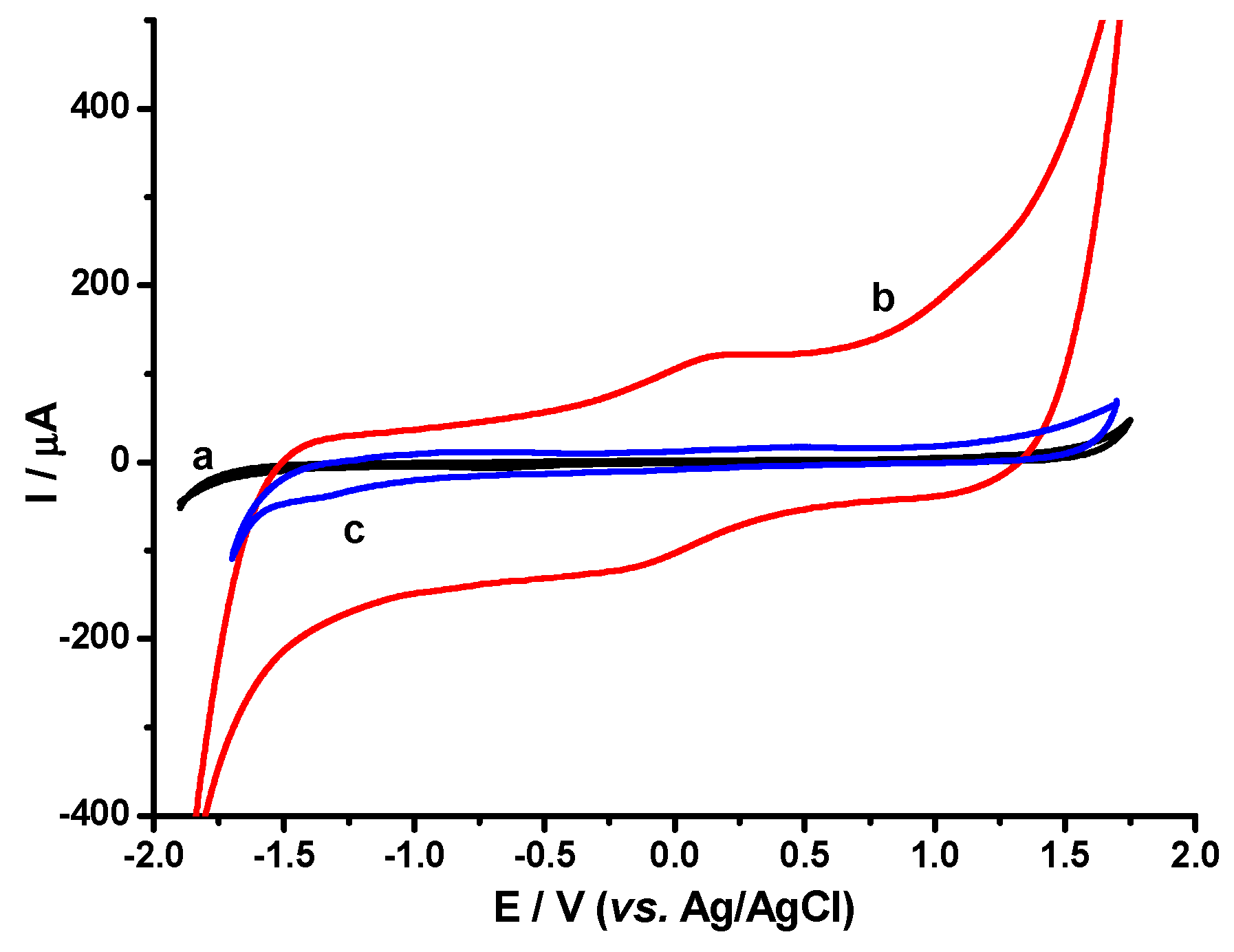
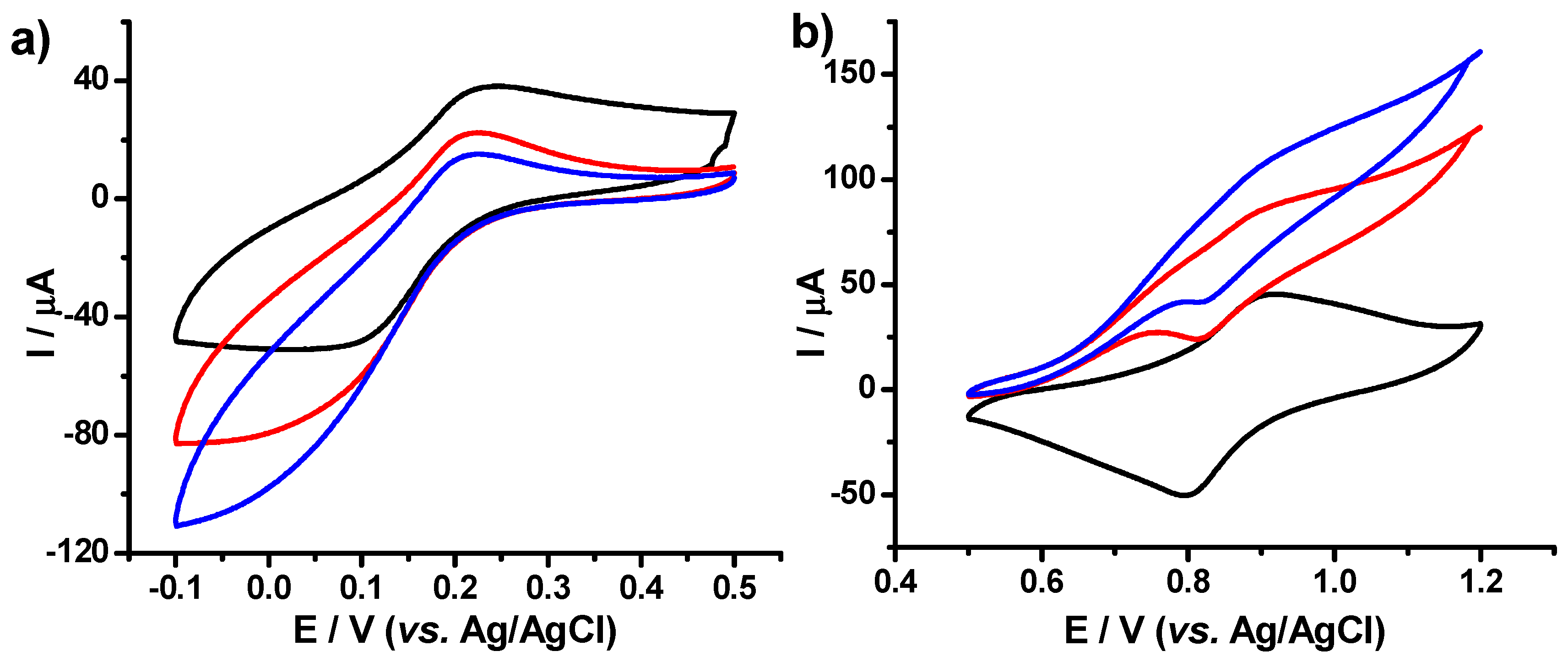
3.1.5. Cyclic Voltammetry (CV)
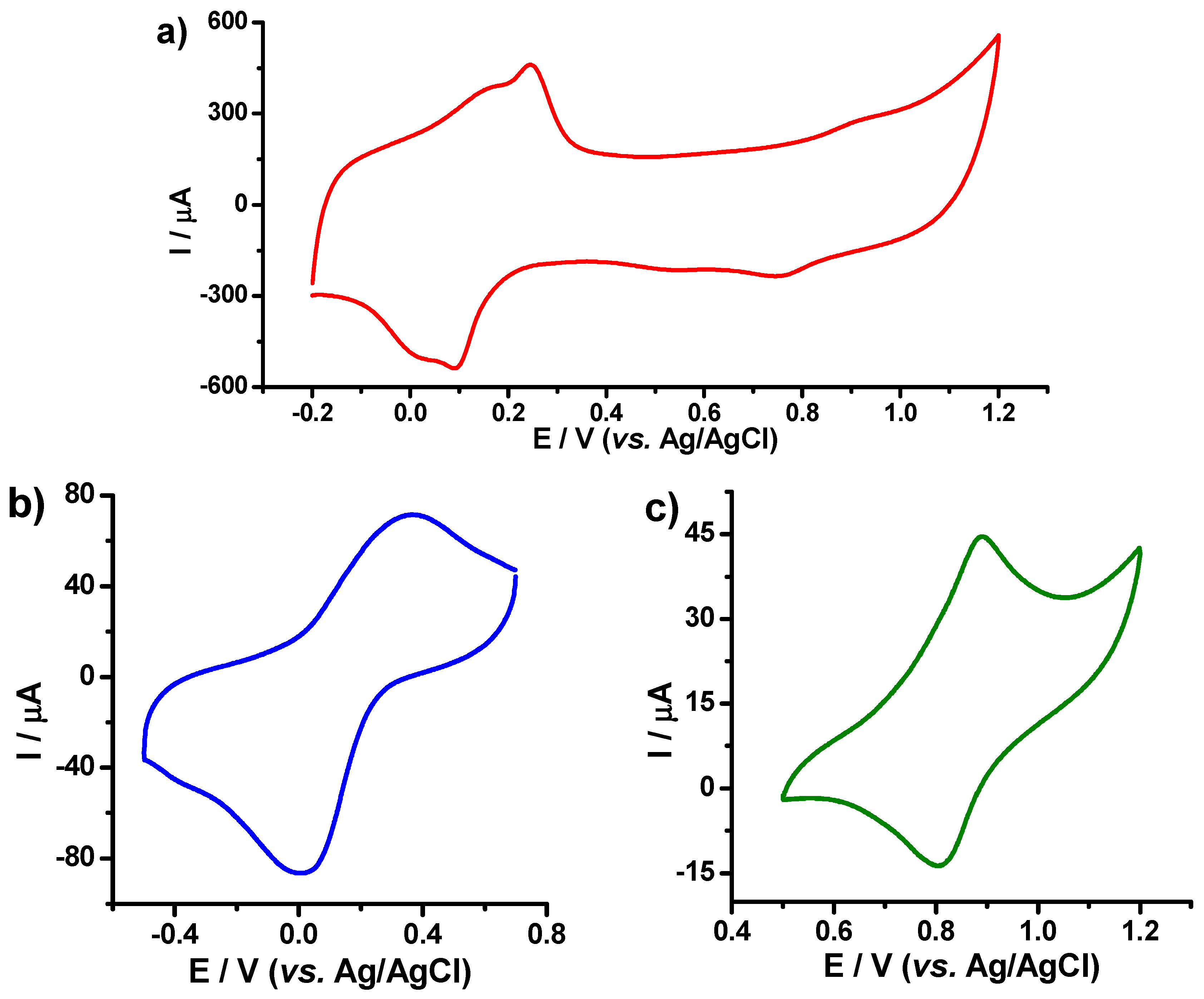
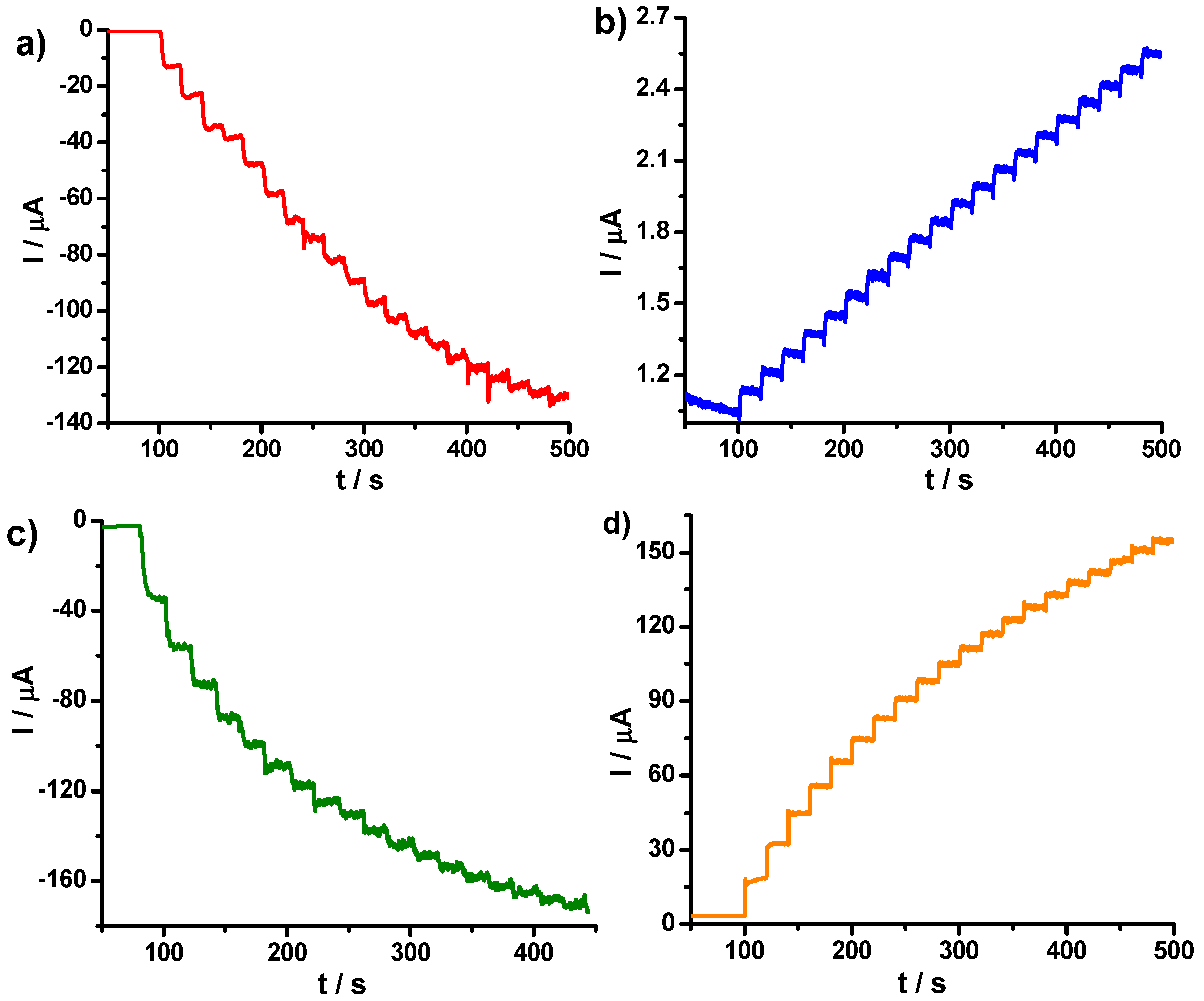
3.2. Electrochemical Performances of the PB–fCNT/GC and PB–TiO2/fCNT/GC Modified Electrodes toward Hydrogen Peroxide
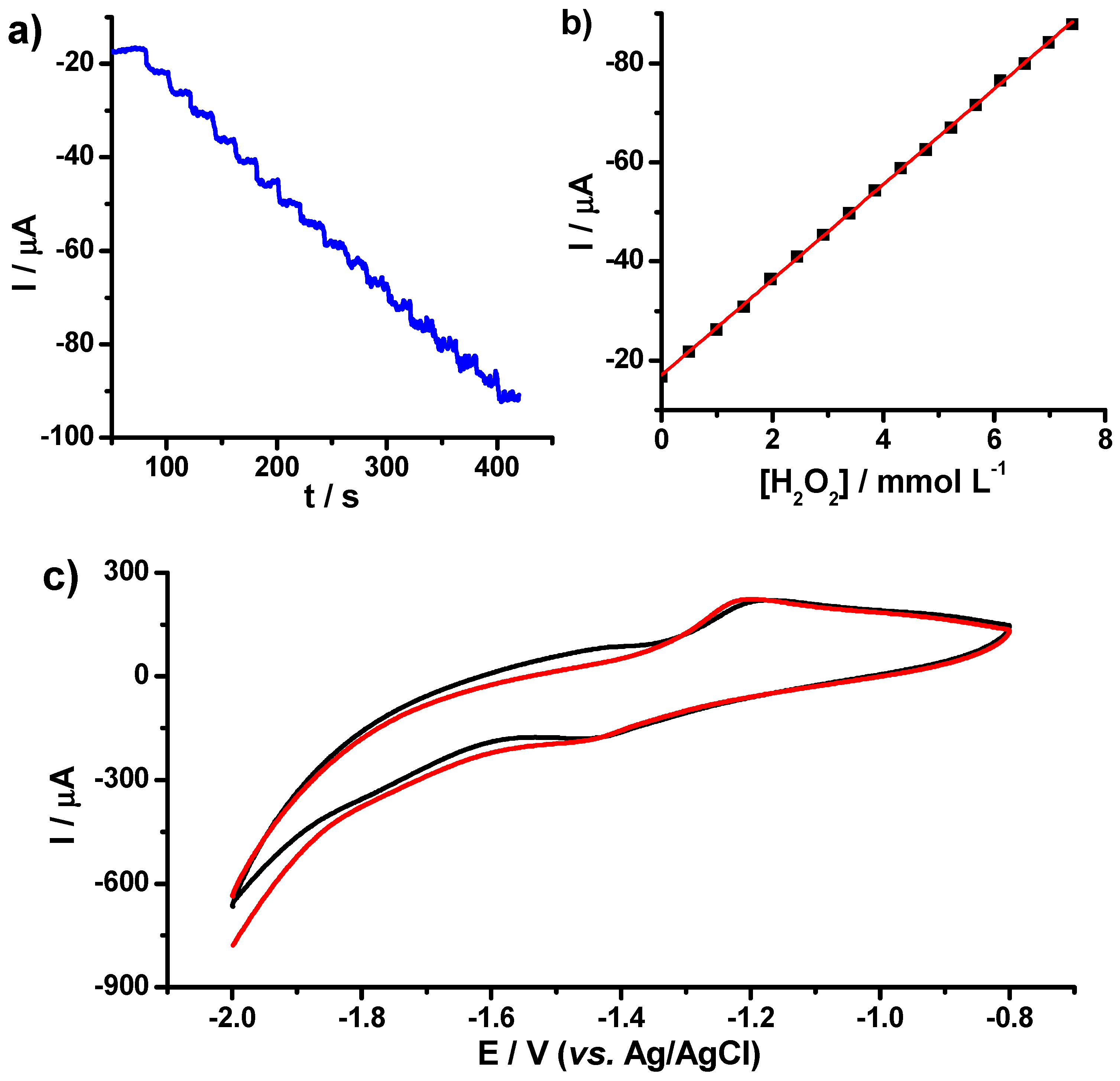
3.3. Electrochemical Performance of TiO2/fCNT/GC Modified Electrode as an Enzyme-Based Electrochemical Biosensor
3.4. Comparison of Results with Previous Sensors Published in the Literature
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhat, S.; Kumar, A. Biomaterials and Bioengineering Tomorrow’s Healthcare. Biomatter 2013, 3, e24717. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Carbon-Nanotube Based Electrochemical Biosensors: A Review. Electroanal. 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon Nanotubes: Properties, Synthesis, Purification, and Medical Applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Stobbe, S.; Lindelof, P.E.; Nygård, J. Integration of Carbon Nanotubes with Semiconductor Technology: Fabrication of Hybrid Devices by III-V Molecular Beam Epitaxy. Semicond. Sci. Technol. 2006, 21, S10–S16. [Google Scholar] [CrossRef][Green Version]
- Maehashi, K.; Katsura, T.; Kerman, K.; Takamura, Y.; Matsumoto, K.; Tamiya, E. Label-Free Protein Biosensor Based on Aptamer-Modified Carbon Nanotube Field-Effect Transistors. Anal. Chem. 2007, 79, 782–787. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Kuzmych, O.; Star, A. Interactions between Single-Walled Carbon Nanotubes and Tetraphenyl Metalloporphyrins: Correlation between Spectroscopic and FET Measurements. J. Phys. Chem. C 2007, 111, 3539–3543. [Google Scholar] [CrossRef]
- Bottini, M.; Cerignoli, F.; Dawson, M.I.; Magrini, A.; Rosato, N.; Mustelin, T. Full-Length Single-Walled Carbon Nanotubes Decorated with Streptavidin-Conjugated Quantum Dots as Multivalent Intracellular Fluorescent Nanoprobes. Biomacromolecules 2006, 7, 2259–2263. [Google Scholar] [CrossRef]
- Du, D.; Huang, X.; Cai, J.; Zhang, A.; Ding, J.; Chen, S. An Amperometric Acetylthiocholine Sensor Based on Immobilization of Acetylcholinesterase on a Multiwall Carbon Nanotube—Cross-Linked Chitosan Composite. Anal. Bioanal. Chem. 2007, 387, 1059–1065. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y. Functionalized Carbon Nanotubes and Nanofibers for Biosensing Applications. Trends Analyt. Chem. 2008, 27, 619–626. [Google Scholar] [CrossRef]
- Montes, R.; Céspedes, F.; Baeza, M. Highly Sensitive Electrochemical Immunosensor for IgG Detection Based on Optimized Rigid Biocomposites. Biosens. Bioelectron. 2016, 78, 505–512. [Google Scholar] [CrossRef]
- Montes, R.; Bartrolí, J.; Baeza, M.; Céspedes, F. Improvement of the Detection Limit for Biosensors: Advances on the Optimization of Biocomposite Composition. Microchem. J. 2015, 119, 66–74. [Google Scholar] [CrossRef]
- Gong, K.; Yan, Y.; Zhang, M.; Su, L.; Xiong, S.; Mao, L. Electrochemistry and Electroanalytical Applications of Carbon Nanotubes: A Review. Anal. Sci. 2005, 21, 1383–1393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Z.; Liu, J.; Liang, Q.; Wang, Y.; Luo, G. Carbon Nanotube-Modified Electrodes for the Simultaneous Determination of Dopamine and Ascorbic Acid. Analyst 2002, 127, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Jiang, H.; Zhai, J.; Liu, B.; Dong, S. A Simple Route to Incorporate Redox Mediator into Carbon nanotubes/Nafion Composite Film and Its Application to Determine NADH at Low Potential. Talanta 2007, 74, 132–139. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.; Chen, D.; Zhang, W.; Li, C.; Fang, B. Fabrication of polythionine/NPAu/MWNTs Modified Electrode for Simultaneous Determination of Adenine and Guanine in DNA. Sensors Actuators B Chem. 2008, 128, 414–421. [Google Scholar] [CrossRef]
- Li, J.; Qiu, J.D.; Xu, J.J.; Chen, H.Y.; Xia, X.H. The Synergistic Effect of Prussian-Blue-Grafted Carbon Nanotube/Poly(4-Vinylpyridine) Composites for Amperometric Sensing. Adv. Funct. Mater. 2007, 17, 1574–1580. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chang, T.S.; Jeong, W.; Kang, D. Methods for Detection and Measurement of Hydrogen Peroxide Inside and Outside of Cells. Mol. Cells 2010, 29, 539–549. [Google Scholar] [CrossRef]
- Grisham, M.B. Methods to Detect Hydrogen Peroxide in Living Cells: Possibilities and Pitfalls. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 165, 429–438. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Gitelmacher, O.V.; Karyakina, E.E. A High-Sensitive Glucose Amperometric Biosensor Based on Prussian Blue Modified Electrodes. Anal. Lett. 1994, 27, 2861–2869. [Google Scholar] [CrossRef]
- Sajjadi, S.; Keihan, A.H.; Norouzi, P.; Habibi, M.M.; Eskandari, K.; Hadizadeh Shirazi, N. Fabrication of an Amperometric Glucose Biosensor Based on a Prussian Blue/Carbon Nanotube/Ionic Liquid Modified Glassy Carbon Electrode. J. Appl. Biotechnol. Rep. 2017, 4, 603–608. [Google Scholar]
- Evans, S.A.G.; Elliott, J.M.; Andrews, L.M.; Bartlett, P.N.; Doyle, P.J.; Denuault, G. Detection of Hydrogen Peroxide at Mesoporous Platinum Microelectrodes. Anal. Chem. 2002, 74, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Fu, K.; Lin, Y.; Huang, W. Functionalized Carbon Nanotubes: Properties and Applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Lee, O.S.; Deria, P.; Kim, S.H.; Park, T.H.; Bonnell, D.A.; Saven, J.G.; Therien, M.J. Helical Wrapping of Single-Walled Carbon Nanotubes by Water Soluble Poly(p-Phenyleneethynylene). Nano Lett. 2009, 9, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Wang, Q.; Qiu, G. Polymer-Encapsulated Carbon Nanotubes Prepared through Ultrasonically Initiated In Situ Emulsion Polymerization. Chem. Mater. 2003, 15, 3879–3886. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured Metal Oxide-Based Biosensors. NPG Asia Mater. 2011, 3, 17. [Google Scholar] [CrossRef]
- Han, F.; Kambala, V.S.R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored Titanium Dioxide Photocatalysts for the Degradation of Organic Dyes in Wastewater Treatment: A Review. Appl. Catal. A Gen. 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- Gonzalez, G.; Albano, C.; Herman, V.; Boyer, I.; Monsalve, A.; Brito, J.A. Nanocomposite Building Blocks of TiO2-MWCNTf and ZrO2-MWCNTf. Mater. Charact. 2012, 64, 96–106. [Google Scholar] [CrossRef]
- Kiema, G.K.; Aktay, M.; McDermott, M.T. Preparation of Reproducible Glassy Carbon Electrodes by Removal of Polishing Impurities. J. Electroanal. Chem. 2003, 540, 7–15. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. On the Mechanism of H2O2 Reduction at Prussian Blue Modified Electrodes. Electrochem. Commun. 1999, 1, 78–82. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, R.; Chai, Y.; Li, W.; Zhong, H.; Wang, C. Glucose Biosensor Based on Titanium Dioxide-Multiwall Carbon Nanotubes-Chitosan Composite and Functionalized Gold Nanoparticles. Bioprocess Biosyst. Eng. 2011, 34, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, J.M.; Patel, N.N.; Kim, D.W.; Rugutt, J.K.; Wanekaya, A.K. Glucose Determination in Beverages Using Carbon Nanotube Modified Biosensor: An Experiment for the Undergraduate Laboratory. J. Chem. Educ. 2013, 90, 1222–1226. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I. Variations of Interlayer Spacing in Carbon Nanotubes. RSC Adv. 2014, 4, 30807–30815. [Google Scholar] [CrossRef]
- Sharon, M.; Modi, F.; Sharon, M. Titania Based Nanocomposites as a Photocatalyst: A Review. Mater. Sci. 2016, 3, 1236–1254. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Atieh, M.A.; Bakather, O.; Al-Tawbini, B.; Bukhari, A.; Abuilaiwi, F.; Fettouhi, M. Effect of Carboxylic Group Functionalized on Carbon Nanotubes Surface on the Removal of Lead From Water. Bioinorg. Chem. Appl. 2010, 2010, 603978. [Google Scholar] [CrossRef]
- do Amaral Montanheiro, T.L.; Cristóvan, F.H.; Machado, J.P.B.; Tada, D.B.; Durán, N.; Lemes, A.P. Effect of MWCNT Functionalization on Thermal and Electrical Properties of PHBV/MWCNT Nanocomposites. J. Mater. Res. 2015, 30, 55–65. [Google Scholar] [CrossRef]
- Albano, C.; Sarmiento, Y.; Gonzalez, G. Synthesis and Characterization of Nanostructures: MWCNTf/TiO2 and MWCNTf/TiO2/HAp. Macromol. Symp. 2012, 321–322, 76–79. [Google Scholar] [CrossRef]
- Kaszuba, M.; Corbett, J.; Watson, F.M.; Jones, A. High-Concentration Zeta Potential Measurements Using Light-Scattering Techniques. Philos. Trans. A. Math. Phys. Eng. Sci. 2010, 368, 4439–4451. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement BT—Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. [Google Scholar] [CrossRef]
- Pramanik, C.; Gissinger, J.R.; Kumar, S.; Heinz, H. Carbon Nanotube Dispersion in Solvents and Polymer Solutions: Mechanisms, Assembly, and Preferences. ACS Nano 2017, 11, 12805–12816. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The Role of Surfactants in Dispersion of Carbon Nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Biesheuvel, P.M.; Dykstra, J.E. The Difference between Faradaic and Nonfaradaic Processes in Electrochemistry. arXiv 2018, arXiv:1809.02930. [Google Scholar]
- Chi, Q.; Dong, S. Amperometric Biosensors Based on the Immobilization of Oxidases in a Prussian Blue Film by Electrochemical Codeposition. Anal. Chim. Acta 1995, 310, 429–436. [Google Scholar] [CrossRef]
- Pan, D.; Chen, J.; Nie, L.; Tao, W.; Yao, S. Amperometric Glucose Biosensor Based on Immobilization of Glucose Oxidase in Electropolymerized O-Aminophenol Film at Prussian Blue-Modified Platinum Electrode. Electrochim. Acta 2004, 49, 795–801. [Google Scholar] [CrossRef]
- Itaya, K.; Ataka, T.; Toshima, S.; Shinohara, T. Electrochemistry of Prussian Blue. An in Situ Moessbauer Effect Measurement. J. Phys. Chem. 1982, 86, 2415–2418. [Google Scholar] [CrossRef]
- Tang, H.; Prasad, K.; Sanjinès, R.; Schmid, P.E.; Lévy, F. Electrical and Optical Properties of TiO2 Anatase Thin Films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Haghighi, B.; Hamidi, H.; Gorton, L. Electrochemical Behavior and Application of Prussian Blue Nanoparticle Modified Graphite Electrode. Sensors Actuators B Chem. 2010, 147, 270–276. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z. Analytical Chemistry; Trans. Tech. Publ.; VCH: New York, NY, USA, 1994. [Google Scholar]
- Centeno, D.A.; Solano, X.H.; Castillo, J.J. A New Peroxidase from Leaves of Guinea Grass (Panicum Maximum): A Potential Biocatalyst to Build Amperometric Biosensors. Bioelectrochemistry 2017, 116, 33–38. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Y.; Yang, X.; Li, C. Photoelectrochemical Detection of H2O2 Based on Flower-Like CuInS2-Graphene Hybrid. Electroanalysis 2014, 26, 573–580. [Google Scholar] [CrossRef]
- Cao, L.; Liu, Y.; Zhang, B.; Lu, L. In Situ Controllable Growth of Prussian Blue Nanocubes on Reduced Graphene Oxide: Facile Synthesis and Their Application as Enhanced Nanoelectrocatalyst for H2O2 Reduction. ACS Appl. Mater. Interfaces 2010, 2, 2339–2346. [Google Scholar] [CrossRef]
- Teepoo, S.; Dawan, P.; Barnthip, N. Electrospun Chitosan-Gelatin Biopolymer Composite Nanofibers for Horseradish Peroxidase Immobilization in a Hydrogen Peroxide Biosensor. Biosensors 2017, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, W.; Yin, G.; Ma, X. Nonenzymatic H2O2 Sensor Based on Pt Nanoflower Electrode. J. Clust. Sci. 2012, 23, 1061–1068. [Google Scholar] [CrossRef]
| Modified Electrode | Sensitivity (μA∙mM−1) | Detection Limit (mM) | Detection Potential (V) | References |
|---|---|---|---|---|
| PB–fCNT/GC | 163.01 | 0.015 | 0.00 | This work |
| HRP–TiO2/fCNT/GC | 963 | 0.81 | −1.50 | This work |
| HRP from leaves of Guinea grass/graphene | 39.93 | 0.15. | −0.65 | [50] |
| CuInS2–graphene/HRP | 11.2 | 0.047 | −0.2 | [51] |
| Prussian blue nanocubes on reduced graphene oxide | Not reported | 0.04 | 0.2 | [52] |
| HRP/chitosan–gelatin composite biopolymer nanofiber/graphite electrode | 44 | 0.05 | −0.30 | [53] |
| Pt/Au | 20 | 0.06 | −0.20 | [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero, L.A.; Fernández, L.; González, G.; Montero-Jiménez, M.; Uribe, R.; Díaz Barrios, A.; Espinoza-Montero, P.J. Peroxide Electrochemical Sensor and Biosensor Based on Nanocomposite of TiO2 Nanoparticle/Multi-Walled Carbon Nanotube Modified Glassy Carbon Electrode. Nanomaterials 2020, 10, 64. https://doi.org/10.3390/nano10010064
Guerrero LA, Fernández L, González G, Montero-Jiménez M, Uribe R, Díaz Barrios A, Espinoza-Montero PJ. Peroxide Electrochemical Sensor and Biosensor Based on Nanocomposite of TiO2 Nanoparticle/Multi-Walled Carbon Nanotube Modified Glassy Carbon Electrode. Nanomaterials. 2020; 10(1):64. https://doi.org/10.3390/nano10010064
Chicago/Turabian StyleGuerrero, L. Andrés, Lenys Fernández, Gema González, Marjorie Montero-Jiménez, Rafael Uribe, Antonio Díaz Barrios, and Patricio J. Espinoza-Montero. 2020. "Peroxide Electrochemical Sensor and Biosensor Based on Nanocomposite of TiO2 Nanoparticle/Multi-Walled Carbon Nanotube Modified Glassy Carbon Electrode" Nanomaterials 10, no. 1: 64. https://doi.org/10.3390/nano10010064
APA StyleGuerrero, L. A., Fernández, L., González, G., Montero-Jiménez, M., Uribe, R., Díaz Barrios, A., & Espinoza-Montero, P. J. (2020). Peroxide Electrochemical Sensor and Biosensor Based on Nanocomposite of TiO2 Nanoparticle/Multi-Walled Carbon Nanotube Modified Glassy Carbon Electrode. Nanomaterials, 10(1), 64. https://doi.org/10.3390/nano10010064







