Luminophore and Magnetic Multicore Nanoassemblies for Dual-Mode MRI and Fluorescence Imaging
Abstract
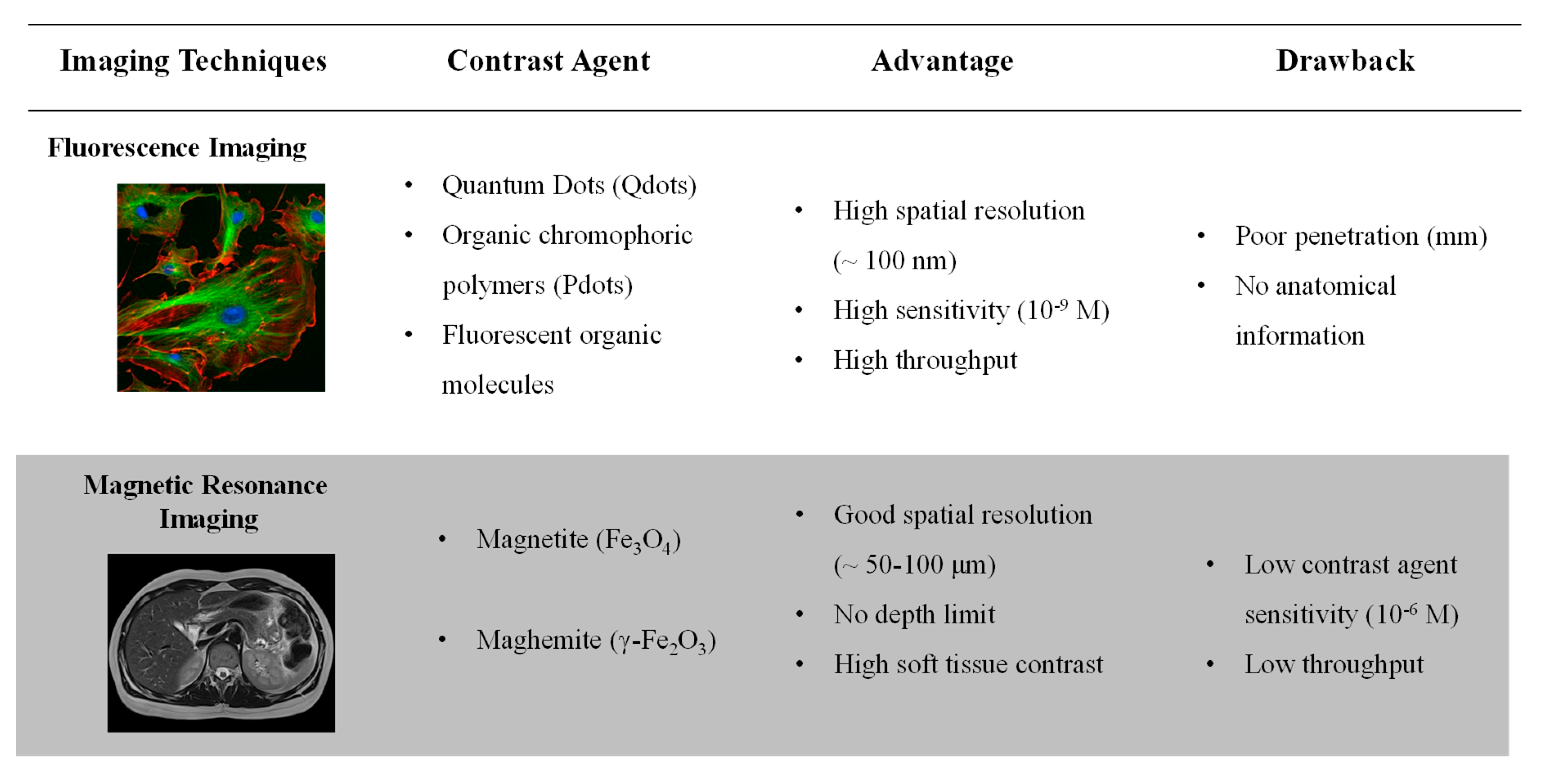
1. Introduction
2. Active Units
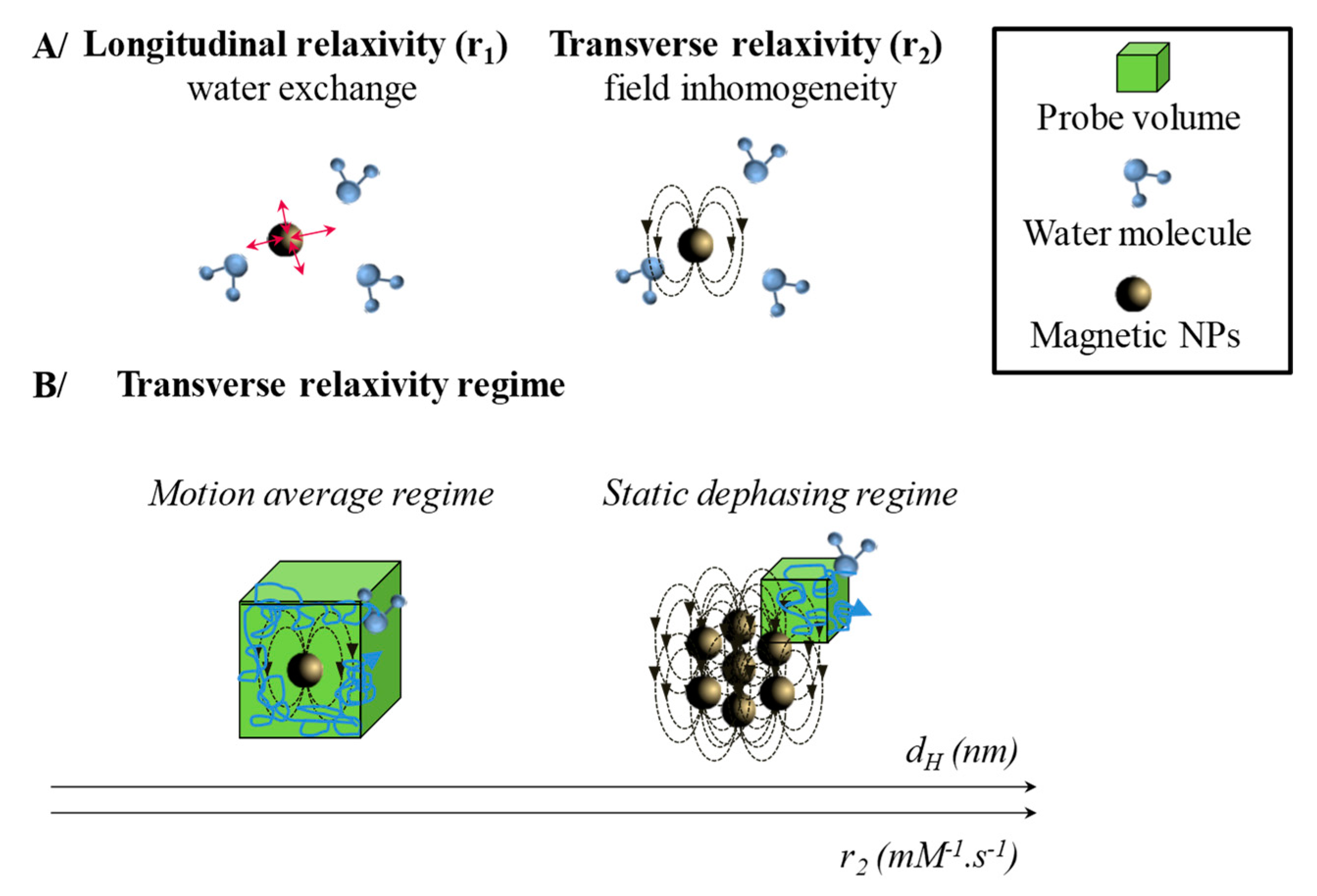
2.1. Magnetic Entities for Magnetic Resonance Imaging (MRI)
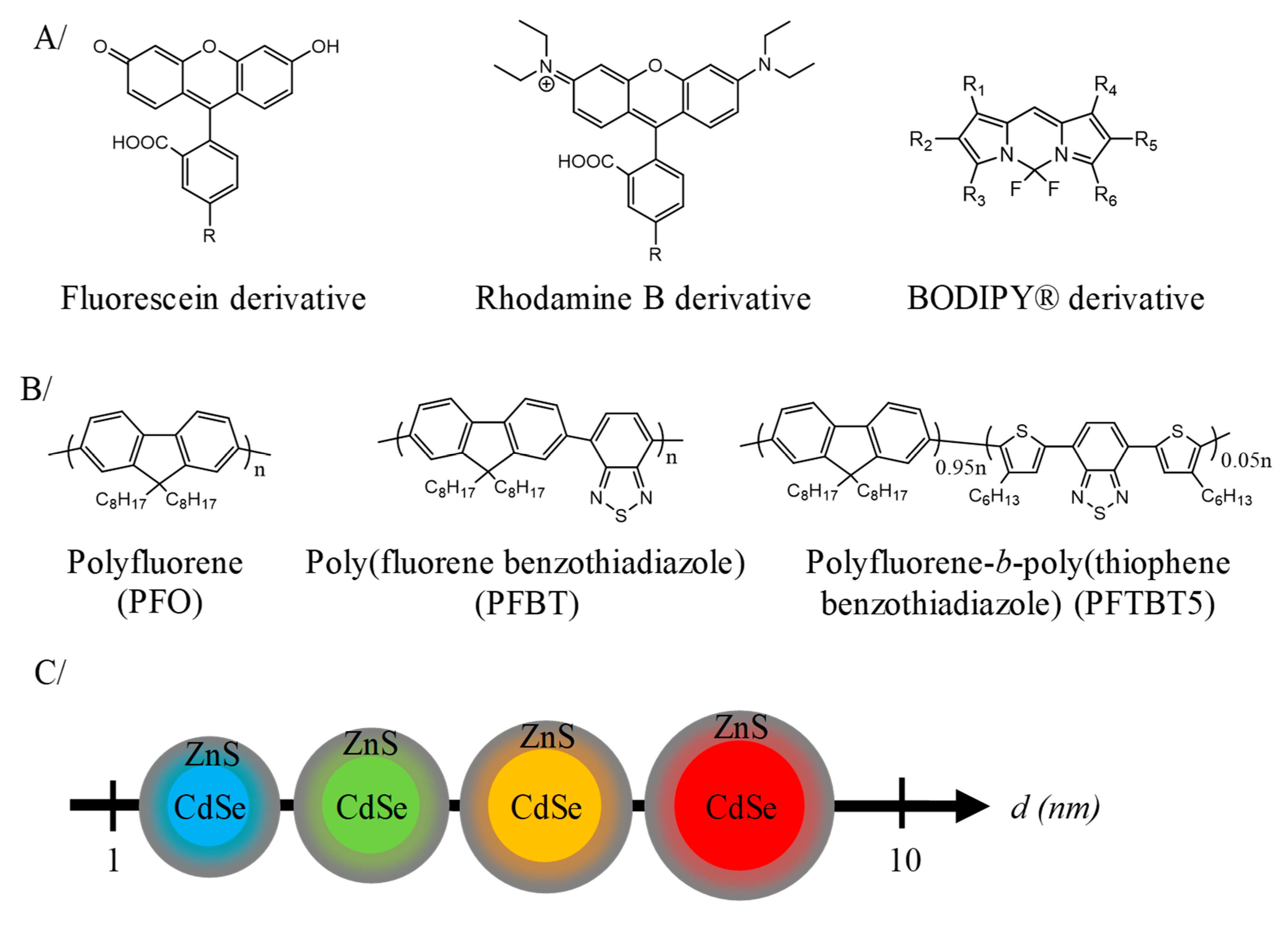
2.2. Nanoparticles Composed of Luminophore for In Vivo Fluorescence Imaging
3. Magneto-Fluorescent Nanosystems
3.1. Association by Covalent Bonding (Nanoparticles)
3.2. Encapsulation in Silica Matrix (Nanostructure)
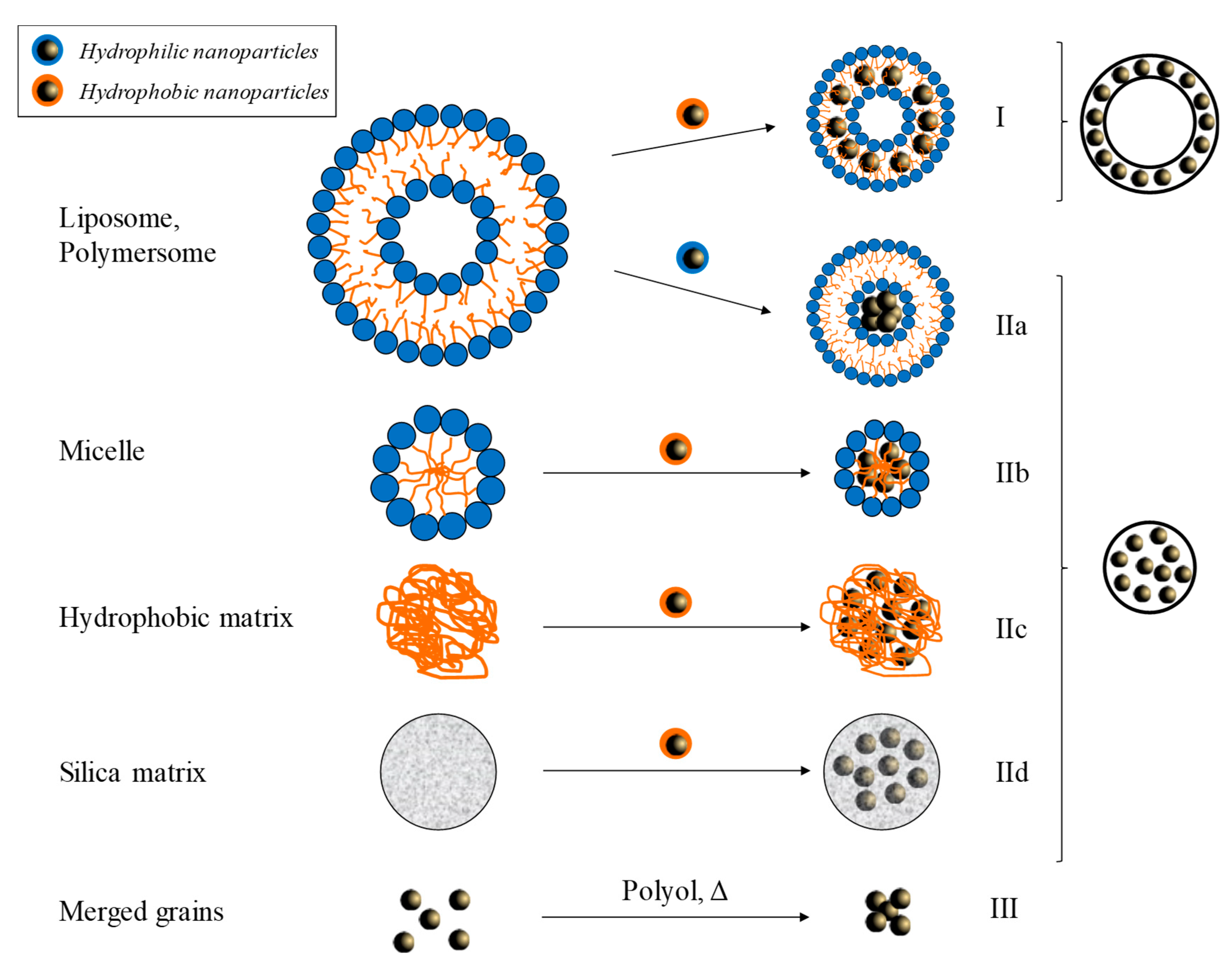
3.3. Dispersion in Nanoassemblies (Supraparticles)
4. Conclusions and Future Outlook
Funding
Conflicts of Interest
References
- Bushberg, J.T.; Seibert, J.A.; Leidholdt, E.M., Jr.; Boone, J.M. The Essential Physics of Medical Imaging, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, UK, 2011. [Google Scholar]
- Pagel, M.D. The Hope and Hype of Multimodality Imaging Contrast Agents. Nanomedicine 2011, 6, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Caschera, L.; Lazzara, A.; Piergallini, L.; Ricci, D.; Tuscano, B.; Vanzulli, A. Contrast Agents in Diagnostic Imaging: Present and Future. Pharm. Res. 2016, 110, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Bigall, N.C.; Parak, W.J.; Dorfs, D. Fluorescent, Magnetic and Plasmonic—Hybrid Multifunctional Colloidal Nano Objects. Nano Today 2012, 7, 282–296. [Google Scholar] [CrossRef]
- Corr, S.A.; Rakovich, Y.P.; Gun’Ko, Y.K. Multifunctional Magnetic-Fluorescent Nanocomposites for Biomedical Applications. Nanoscale Res. Lett. 2008, 3, 87–104. [Google Scholar] [CrossRef]
- Shen, Z.; Wu, A.; Chen, X. Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging. Mol. Pharm. 2017, 14, 1352–1364. [Google Scholar] [CrossRef]
- Amoroso, A.J.; Pope, S.J.A. Using Lanthanide Ions in Molecular Bioimaging. Chem. Soc. Rev. 2015, 44, 4723–4742. [Google Scholar] [CrossRef]
- Ersoy, H.; Rybicki, F.J. Biochemical Safety Profiles of Gadolinium-Based Extracellular Contrast Agents and Nephrogenic Systemic Fibrosis. J. Magn. Reson. Imaging 2007, 26, 1190–1197. [Google Scholar] [CrossRef]
- Perazella, M.A. Current Status of Gadolinium Toxicity in Patients with Kidney Disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 461–469. [Google Scholar] [CrossRef]
- Food and Drug Administration. Available online: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM589442.pdf (accessed on 14 November 2019).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/gadolinium-containing-contrast-agents (accessed on 14 November 2019).
- Wu, L.; Mendoza-Garcia, A.; Li, Q.; Sun, S. Organic Phase Syntheses of Magnetic Nanoparticles and Their Applications. Chem. Rev. 2016, 116, 10473–11512. [Google Scholar] [CrossRef]
- Wang, Y.-X.J. Superparamagnetic Iron Oxide Based MRI Contrast Agents: Current Status of Clinical Application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [CrossRef]
- Gossuin, Y.; Gillis, P.; Hocq, A.; Vuong, Q.L.; Roch, A. Magnetic Resonance Relaxation Properties of Superparamagnetic Particles. WIREs Nanomed. Nanobiotechnol. 2009, 1, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.H.; Choi, Y.; Kim, S.; Cheon, J. Recent Advances in Magnetic Nanoparticle-Based Multi-Modal Imaging. Chem. Soc. Rev. 2015, 44, 4501–4516. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Liu, Q.; Zhang, J.; Chen, J.; Chen, S.; Zhao, Y.; Du, J. Rationally Separating the Corona and Membrane Functions of Polymer Vesicles for Enhanced T2 MRI and Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 14043–14052. [Google Scholar] [CrossRef] [PubMed]
- Sanson, C.; Diou, O.; Thévenot, J.; Ibarboure, E.; Soum, A.; Brûlet, A.; Miraux, S.; Thiaudière, E.; Tan, S.; Brisson, A.; et al. Doxorubicin Loaded Magnetic Polymersomes: Theranostic Nanocarriers for MR Imaging and Magneto-Chemotherapy. ACS Nano 2011, 5, 1122–1140. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.S.; Fortin, J.P.; Ménager, C.; Clément, O.; Barratt, G.; Grabielle-Madelmont, C.; Gazeau, F.; Cabuil, V.; Lesieur, S. Generation of Superparamagnetic Liposomes Revealed as Highly Efficient MRI Contrast Agents for in Vivo Imaging. J. Am. Chem. Soc. 2005, 127, 10676–10685. [Google Scholar] [CrossRef]
- Ren, T.; Liu, Q.; Lu, H.; Liu, H.; Zhang, X.; Du, J. Multifunctional Polymer Vesicles for Ultrasensitive Magnetic Resonance Imaging and Drug Delivery. J. Mater. Chem. 2012, 22, 12329–12338. [Google Scholar] [CrossRef]
- Pothayee, N.; Balasubramaniam, S.; Pothayee, N.; Jain, N.; Hu, N.; Lin, Y.; Davis, R.M.; Sriranganathan, N.; Koretsky, A.P.; Riffle, J.S. Magnetic Nanoclusters with Hydrophilic Spacing for Dual Drug Delivery and Sensitive Magnetic Resonance Imaging. J. Mater. Chem. B 2013, 1, 1142–1149. [Google Scholar] [CrossRef]
- Hickey, R.J.; Koski, J.; Meng, X.; Riggleman, R.A.; Zhang, P.; Park, S.-J.J. Size-Controlled Self-Assembly of Superparamagnetic Polymersomes. ACS Nano 2014, 8, 495–502. [Google Scholar] [CrossRef]
- Mikhaylov, G.; Mikac, U.; Magaeva, A.A.; Itin, V.I.; Naiden, E.P.; Psakhye, I.; Babes, L.; Reinheckel, T.; Peters, C.; Zeiser, R.; et al. Ferri-Liposomes as an MRI-Visible Drug-Delivery System for Targeting Tumours and Their Microenvironment. Nat. Nanotechnol. 2011, 6, 594–602. [Google Scholar] [CrossRef]
- Bleul, R.; Thiermann, R.; Marten, G.U.; House, M.J.; Pierre, T.G.S.; Häfeli, U.O.; Maskos, M. Continuously Manufactured Magnetic Polymersomes-a Versatile Tool (Not Only) for Targeted Cancer Therapy. Nanoscale 2013, 5, 11385–11393. [Google Scholar] [CrossRef] [PubMed]
- Prashant, C.; Dipak, M.; Yang, C.T.; Chuang, K.H.; Jun, D.; Feng, S.S. Superparamagnetic Iron Oxide—Loaded Poly (Lactic Acid)-d-α-Tocopherol Polyethylene Glycol 1000 Succinate Copolymer Nanoparticles as MRI Contrast Agent. Biomaterials 2010, 31, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Flask, C.; Weinberg, B.; Farrell, D.; Pagel, M.D.; Ai, H.; Shuai, X.-T.; Gao, J.; Duerk, J.; Gao, J. Magnetite-Loaded Polymeric Micelles as Ultrasensitive Magnetic-Resonance Probes. Adv. Mater. 2005, 17, 1949–1952. [Google Scholar] [CrossRef]
- Roch, A.; Gossuin, Y.; Muller, R.N.; Gillis, P. Superparamagnetic Colloid Suspensions: Water Magnetic Relaxation and Clustering. J. Magn. Magn. Mater. 2005, 293, 532–539. [Google Scholar] [CrossRef]
- Tanaka, K.; Narita, A.; Kitamura, N.; Uchiyama, W.; Morita, M.; Inubushi, T.; Chujo, Y. Preparation for Highly Sensitive MRI Contrast Agents Using Core/Shell Type Nanoparticles Consisting of Multiple SPIO Cores with Thin Silica Coating. Langmuir 2010, 26, 11759–11762. [Google Scholar] [CrossRef]
- Ebert, S.; Bannwarth, M.B.; Musyanovych, A.; Landfester, K.; Münnemann, K. How Morphology Influences Relaxivity—Comparative Study of Superparamagnetic Iron Oxide-Polymer Hybrid Nanostructures. Contrast Media Mol. Imaging 2015, 10, 456–464. [Google Scholar] [CrossRef]
- Bulte, J.W.M.; De Cuyper, M. Magnetoliposomes as Contrast Agents. Methods Enzymol. 2003, 373, 175–198. [Google Scholar] [CrossRef]
- Pflipsen, C.; Forge, D.; Benali, S.; Gossuin, Y. Improved Stability and Relaxivity of a Commercial Magnetic Ferrofluid. J. Phys. Chem. C 2013, 117, 20919–20926. [Google Scholar] [CrossRef]
- Hobson, N.J.; Weng, X.; Siow, B.; Veiga, C.; Ashford, M. Clustering Superparamagnetic Iron Oxide Nanoparticles Produces Organ-Targeted High-Contrast Magnetic Resonance Images. Nanomedicine 2019, 14, 1135–1152. [Google Scholar] [CrossRef]
- Yang, J.; Lee, C.H.; Ko, H.J.; Suh, J.S.; Yoon, H.G.; Lee, K.; Huh, Y.M.; Haam, S. Multifunctional Magneto-Polymeric Nanohybrids for Targeted Detection and Synergistic Therapeutic Effects on Breast Cancer. Angew. Chem. Int. Ed. 2007, 46, 8836–8839. [Google Scholar] [CrossRef]
- Meledandri, C.J.; Ninjbadgar, T.; Brougham, D.F. Size-Controlled Magnetoliposomes with Tunable Magnetic Resonance Relaxation Enhancements. J. Mater. Chem. 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Taboada, E.; Solanas, R.; Rodríguez, E.; Weissleder, R.; Roig, A. Supercritical-Fluid-Assisted One-Pot Synthesis of Biocompatible Core(γ-Fe2O3)/Shell(SiO2) Nanoparticles as High Relaxivity T2-Contrast Agents for Magnetic Resonance Imaging. Adv. Funct. Mater. 2009, 19, 2319–2324. [Google Scholar] [CrossRef]
- Poselt, E.; Kloust, H.; Tromsdorf, U.; Janschel, M.; Hahn, C.; Masslo, C.; Weller, H. Relaxivity Optimization of a PEGylated Iron-Oxide-Based Negative Magnetic Resonance Contrast Agent for T2-Weighted Spin- Echo Imaging. ACS Nano 2012, 6, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Thévenot, J.; Orlando, T.; Orsini, F.; Corti, M.; Mariani, M.; Bordonali, L.; Innocenti, C.; Sangregorio, C.; Oliveira, H.; et al. Hybrid Iron Oxide-Copolymer Micelles and Vesicles as Contrast Agents for MRI: Impact of the Nanostructure on the Relaxometric Properties. J. Mater. Chem. B 2013, 1, 5317–5328. [Google Scholar] [CrossRef]
- He, J.; Liu, X.; Niu, D.; Chen, J.; Qin, X.; Li, Y. Supramolecular-Based PEGylated Magnetic Hybrid Vesicles with Ultra-High Transverse Relaxivity. Appl. Mater. Today 2018, 11, 238–245. [Google Scholar] [CrossRef]
- Paquet, C.; De Haan, H.W.; Leek, D.M.; Lin, H.Y.; Xiang, B.; Tian, G.; Kell, A.; Simard, B. Clusters of Superparamagnetic Iron Oxide Nanoparticles Encapsulated in a Hydrogel: A Particle Architecture Generating a Synergistic Enhancement of the T2 Relaxation. ACS Nano 2011, 5, 3104–3112. [Google Scholar] [CrossRef]
- Yuan, Y.; He, Y.; Bo, R.; Ma, Z.; Wang, Z.; Dong, L.; Lin, T.Y.; Xue, X.; Li, Y. A Facile Approach to Fabricate Self-Assembled Magnetic Nanotheranostics for Drug Delivery and Imaging. Nanoscale 2018, 10, 21634–21639. [Google Scholar] [CrossRef]
- Berret, J.-F.; Schonbeck, N.; Gazeau, F.; El Kharrat, D.; Sandre, O.; Vacher, A.; Airiau, M. Controlled Clustering of Superparamagnetic Nanoparticles Using Block Copolymers: Design of New Contrast Agents for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2006, 128, 1755–1761. [Google Scholar] [CrossRef]
- Schmidtke, C.; Eggers, R.; Zierold, R.; Feld, A.; Kloust, H.; Wolter, C.; Ostermann, J.; Merkl, J.P.; Schotten, T.; Nielsch, K.; et al. Polymer-Assisted Self-Assembly of Superparamagnetic Iron Oxide Nanoparticles into Well-Defined Clusters: Controlling the Collective Magnetic Properties. Langmuir 2014, 30, 11190–11196. [Google Scholar] [CrossRef]
- Liu, Q.; Song, L.; Chen, S.; Gao, J.; Zhao, P.; Du, J. A Superparamagnetic Polymersome with Extremely High T2 Relaxivity for MRI and Cancer-Targeted Drug Delivery. Biomaterials 2017, 114, 23–33. [Google Scholar] [CrossRef]
- Schaller, V.; Wahnström, G.; Sanz-Velasco, A.; Enoksson, P.; Johansson, C. Monte Carlo Simulation of Magnetic Multi-Core Nanoparticles. J. Magn. Magn. Mater. 2009, 321, 1400–1403. [Google Scholar] [CrossRef]
- Schaller, V.; Wahnström, G.; Sanz-Velasco, A.; Gustafsson, S.; Olsson, E.; Enoksson, P.; Johansson, C. Effective Magnetic Moment of Magnetic Multicore Nanoparticles. Phys. Rev. B 2009, 80, 092406. [Google Scholar] [CrossRef]
- Pedrosa, S.S.; Martins, S.M.S.B.; Souza, R.M.; Dantas, J.T.S.; Souza, C.M.; Rebouças, G.O.G.; de Araújo, J.M.; Dantas, A.L.; Carriço, A.S. Dipolar Effects on the Magnetic Phases of Superparamagnetic Clusters. J. Appl. Phys. 2018, 123, 233902. [Google Scholar] [CrossRef]
- Allia, P.; Tiberto, P.; Coisson, M.; Chiolerio, A.; Celegato, F.; Vinai, F.; Sangermano, M.; Suber, L.; Marchegiani, G. Evidence for Magnetic Interactions among Magnetite Nanoparticles Dispersed in Photoreticulated PEGDA-600 Matrix. J. Nanopart. Res. 2011, 13, 5615–5626. [Google Scholar] [CrossRef]
- Bae, C.J.; Angappane, S.; Park, J.G.; Lee, Y.; Lee, J.; An, K.; Hyeon, T. Experimental Studies of Strong Dipolar Interparticle Interaction in Monodisperse Fe3O4 Nanoparticles. Appl. Phys. Lett. 2007, 91, 102502. [Google Scholar] [CrossRef]
- Cha, J.; Kwon, Y.S.; Yoon, T.J.; Lee, J.K. Relaxivity Control of Magnetic Nanoclusters for Efficient Magnetic Relaxation Switching Assay. Chem. Commun. 2013, 49, 457–459. [Google Scholar] [CrossRef]
- Vuong, Q.L.; Berret, J.-F.; Fresnais, J.; Gossuin, Y.; Sandre, O. A Universal Scaling Law to Predict the Efficiency of Magnetic Nanoparticles as MRI T2-Contrast Agents. Adv. Healthc. Mater. 2012, 1, 502–512. [Google Scholar] [CrossRef]
- Massart, R.; Cabuil, V. Synthèse En Milieu Alcalin de Magnétite Colloïdale: Contrôle Du Rendement et de La Taille Des Particules. J. Chim. Phys. 1987, 84, 967–973. [Google Scholar] [CrossRef]
- Bacri, J.C.; Perzynski, R.; Salin, D.; Cabuil, V.; Massart, R. Magnetic Colloidal Properties of Ionic Ferrofluids. J. Magn. Magn. Mater. 1986, 62, 36–46. [Google Scholar] [CrossRef]
- Gribanov, N.M.; Bibik, E.E.; Buzunov, O.V.; Naumov, V.N. Physico-Chemical Regularities of Obtaining Highly Dispersed Magnetite by the Method of Chemical Condensation. J. Magn. Magn. Mater. 1990, 85, 7–10. [Google Scholar] [CrossRef]
- Lefebure, S.; Dubois, E.; Cabuil, V.; Neveu, S.; Massart, R.; Lefebure, S.; Dubois, E.; Neveu, S. Monodisperse Magnetic Nanoparticles: Preparation and Dispersion in Water and Oils. J. Mater. Res. 1998, 13, 2975–2981. [Google Scholar] [CrossRef]
- Lucas, I.T.; Durand-Vidal, S.; Dubois, E.; Chevalet, J.; Turq, P. Surface Charge Density of Maghemite Nanoparticles: Role of Electrostatics in the Proton Exchange. J. Phys. Chem. C 2007, 111, 18568–18576. [Google Scholar] [CrossRef][Green Version]
- Bacri, J.C.; Perzynski, R.; Salin, D.; Cabuil, V.; Massart, R. Ionic Ferrofluids: A Crossing of Chemistry and Physics. J. Magn. Magn. Mater. 1990, 85, 27–32. [Google Scholar] [CrossRef]
- Bee, A.; Massart, R.; Neveu, S. Synthesis of Very Fine Maghemite Particles. J. Magn. Magn. Mater. 1995, 149, 6–9. [Google Scholar] [CrossRef]
- Chanteau, B.; Fresnais, J.; Berret, J.F. Electrosteric Enhanced Stability of Functional Sub-10 Nm Cerium and Iron Oxide Particles in Cell Culture Medium. Langmuir 2009, 25, 9064–9070. [Google Scholar] [CrossRef] [PubMed]
- Berret, J.F.; Sandre, O.; Mauger, A. Size Distribution of Superparamagnetic Particles Determined by Magnetic Sedimentation. Langmuir 2007, 23, 2993–2999. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakash, G.; Philip, J.; Jayakumar, T.; Raj, B. Effect of Digestion Time and Alkali Addition Rate on Physical Properties of Magnetite Nanoparticles. J. Phys. Chem. B 2007, 111, 7978–7986. [Google Scholar] [CrossRef]
- Santoyo Salazar, J.; Perez, L.; De Abril, O.; Truong Phuoc, L.; Ihiawakrim, D.; Vazquez, M.; Greneche, J.M.; Begin-Colin, S.; Pourroy, G. Magnetic Iron Oxide Nanoparticles in 10–40 Nm Range: Composition in Terms of Magnetite/Maghemite Ratio and Effect on the Magnetic Properties. Chem. Mater. 2011, 23, 1379–1386. [Google Scholar] [CrossRef]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Na, H.B. Synthesis of Highly Crystalline and Monodisperse Maghemite Nanocrystallites without a Size-Selection Process. J. Am. Chem. Soc. 2001, 123, 12798–12801. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Falkner, J.C.; Yavuz, C.T.; Colvin, V.L. Synthesis of Monodisperse Iron Oxide Nanocrystals by Thermal Decomposition of Iron Carboxylate Salts. Chem. Commun. 2004, 20, 2306–2307. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, E.; Hwang, N.-M.; Kang, M.; Kim, S.C.; Hwang, Y.; Hyeon, T. One-Nanometer-Scale Size-Controlled Synthesis of Monodisperse Magnetic Iron Oxide Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 2873–2877. [Google Scholar] [CrossRef] [PubMed]
- Demortière, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Bégin-Colin, S. Size-Dependent Properties of Magnetic Iron Oxide Nanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, L.; Innocenti, C.; Kalaivani, T.; Awwad, A.; Sanchez Duque, M.D.M.; Guari, Y.; Arosio, P. Water-Dispersible Sugar-Coated Iron Oxide Nanoparticles. An Evaluation of Their Relaxometric and Magnetic Hyperthermia Properties. J. Am. Chem. Soc. 2011, 133, 10459–10472. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-R.; Chiang, R.-K.; Wang, J.-S.; Sung, T.-W. Magnetic Properties of Monodisperse Iron Oxide Nanoparticles. J. Appl. Phys. 2006, 99, 08N710. [Google Scholar] [CrossRef]
- Hugounenq, P.; Levy, M.; Alloyeau, D.; Lartigue, L.; Dubois, E.; Cabuil, V.; Ricolleau, C.; Roux, S.; Wilhelm, C.; Gazeau, F.; et al. Iron Oxide Monocrystalline Nanoflowers for Highly Efficient Magnetic Hyperthermia. J. Phys. Chem. C 2012, 116, 15702–15712. [Google Scholar] [CrossRef]
- Cheng, C.; Xu, F.; Gu, H. Facile Synthesis and Morphology Evolution of Magnetic Iron Oxide Nanoparticles in Different Polyol Processes. New J. Chem. 2011, 35, 1072. [Google Scholar] [CrossRef]
- Ge, J.; Hu, Y.; Biasini, M.; Beyermann, W.P.; Yin, Y. Superparamagnetic Magnetite Colloidal Nanocrystal Clusters. Angew. Chem. Int. Ed. 2007, 46, 4342–4345. [Google Scholar] [CrossRef]
- Lartigue, L.; Hugounenq, P.; Alloyeau, D.; Clarke, S.P.; Levy, M.; Bazzi, R.; Brougham, D.F.; Wilhelm, C.; Gazeau, F.; Lévy, M.; et al. Cooperative Organization in Iron Oxide Multi-Core Nanoparticles Potentiates Their Efficiency as Heating Mediators and MRI Contrast Agents. ACS Nano 2012, 6, 10935–10949. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, A.; Velu, S.K.P.; Thangavel, K.; Orsini, F.; Brintakis, K.; Psycharakis, S.; Ranella, A.; Bordonali, L.; Lappas, A.; Lascialfari, A. Colloidal Assemblies of Oriented Maghemite Nanocrystals and Their NMR Relaxometric Properties. Dalt. Trans. 2014, 43, 8395–8404. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.; Wittmann, T. Fluorescence Live Cell Imaging. Methods Cell Biol. 2014, 123, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.J.; Smith, I.; Parker, I.; Bootman, M.D. Fluorescence Microscopy. Cold Spring Harb. Protoc. 2014, 1042–1065. [Google Scholar] [CrossRef]
- Hell, S.W. Toward Fluorescence Nanoscopy. Nat. Biotechnol. 2003, 21, 1347–1355. [Google Scholar] [CrossRef]
- Palmer, A.E.; Tsien, R.Y. Measuring Calcium Signaling Using Genetically Targetable Fluorescent Indicators. Nat. Protoc. 2006, 1, 1057–1065. [Google Scholar] [CrossRef]
- Paige, J.S.; Nguyen-Duc, T.; Song, W.; Jaffrey, S.R. Fluorescence Imaging of Cellular Metabolites with RNA. Science 2012, 335, 1194. [Google Scholar] [CrossRef]
- Weiss, S. Fluorescence Spectroscopy of Single Biomolecules. Science 1999, 283, 1676–1683. [Google Scholar] [CrossRef]
- Anderson, N.G.; Mann, M.; Meng, C.K.; Wong, S.F.; Hillenkamp, F.; Goodlet, D.R.; Mann, R.M.; Glish, G.L.; Mcluckey, S.A.; Kaiser, R.E. The Fluorescent Toolbox for Assessing Protein Location and Function. Science 2006, 312, 217–224. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum Dots versus Organic Dyes as Fluorescent Labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative Fluorescence Quantum Yields Using a Computer-Controlled Luminescence Spectrometer. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
- Wu, C.; Szymanski, C.; Cain, Z.; McNeill, J. Conjugated Polymer Dots for Multiphoton Fluorescence Imaging. J. Am. Chem. Soc. 2007, 129, 12904–12905. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated Quantum Dots for in Vivo Molecular and Cellular Imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Brown, S.; Walter, G.; Santra, S.; Moudgil, B. Nanoparticles for Bioimaging. Adv. Colloid Interface Sci. 2006, 123–126, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Terai, T.; Nagano, T. Small-Molecule Fluorophores and Fluorescent Probes for Bioimaging. Pflugers Arch. Eur. J. Physiol. 2013, 465, 347–359. [Google Scholar] [CrossRef]
- Wang, L.; Frei, M.S.; Salim, A.; Johnsson, K. Small-Molecule Fluorescent Probes for Live-Cell Super-Resolution Microscopy. J. Am. Chem. Soc. 2019, 141, 2770–2781. [Google Scholar] [CrossRef]
- Johnson, I.; Spence, M.T.Z. The Molecular Probe® Handbook: A Guide to Fluorescent Probes and Labeling Technologies, 11th ed.; Life Technologies Corporation: Carlsbad, CA, USA, 2010. [Google Scholar]
- Johnson, L.V.; Walsh, M.L.; Chen, L.B. Localization of Mitochondria in Living Cells with Rhodamine 123. Proc. Natl. Acad. Sci. USA 1980, 77, 990–994. [Google Scholar] [CrossRef]
- Anderson, R.G. A View of Acidic Intracellular Compartments. J. Cell Biol. 2004, 106, 539–543. [Google Scholar] [CrossRef]
- Crissman, H.A.; Hirons, G.T. Staining of DNA in Live and Fixed Cells. Methods Cell Biol. 1994, 41, 195–209. [Google Scholar] [CrossRef]
- Ishow, E.; Brosseau, A.; Clavier, G.; Nakatani, K.; Tauc, P.; Neveu, S.; Sandre, O.; Léaustic, A. Multicolor Emission of Small Molecule-Based Amorphous Thin Films and Nanoparticles with a Single Excitation Wavelength. Chem. Mater. 2008, 20, 6597–6599. [Google Scholar] [CrossRef][Green Version]
- Patra, A.; Chandaluri, C.G.; Radhakrishnan, T.P. Optical Materials Based on Molecular Nanoparticles. Nanoscale 2012, 4, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Pei, J. Solution-Processed Organic Nano- and Micro-Materials: Design Strategy, Growth Mechanism and Applications. J. Mater. Chem. 2012, 22, 785–798. [Google Scholar] [CrossRef]
- Cui, Q.H.; Zhao, Y.S.; Yao, J. Controlled Synthesis of Organic Nanophotonic Materials with Specific Structures and Compositions. Adv. Mater. 2014, 26, 6852–6870. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.S.; Fu, H.; Peng, A.; Ma, Y.; Xiao, D.; Yao, J. Low-Dimensional Nanomaterials Based on Small Organic Molecules: Preparation and Optoelectronic Properties. Adv. Mater. 2008, 20, 2859–2876. [Google Scholar] [CrossRef]
- Fischer, I.; Kaeser, A.; Peters-Gumbs, M.A.M.; Schenning, A.P.H.J. Fluorescent π-Conjugated Polymer Dots versus Self-Assembled Small-Molecule Nanoparticles: What’s the Difference? Chem. A Eur. J. 2013, 19, 10928–10934. [Google Scholar] [CrossRef]
- Gaiduk, A.; Yorulmaz, M.; Ishow, E.; Orrit, M. Absorption, Luminescence, and Sizing of Organic Dye Nanoparticles and of Patterns Formed Upon Dewetting. ChemPhysChem 2012, 13, 946–951. [Google Scholar] [CrossRef]
- Faucon, A.; Benhelli-Mokrani, H.; Córdova, L.A.W.; Brulin, B.; Heymann, D.; Hulin, P.; Nedellec, S.; Ishow, E. Are Fluorescent Organic Nanoparticles Relevant Tools for Tracking Cancer Cells or Macrophages? Adv. Healthc. Mater. 2015, 4, 2727–2734. [Google Scholar] [CrossRef]
- Day, R.N.; Davidson, M.W. The Fluorescent Protein Palette: Tools for Cellular Imaging. Chem. Soc. Rev. 2009, 38, 2887–2921. [Google Scholar] [CrossRef]
- Shimomura, O. The discovery of aequorin and green fluorescent protein. J. Microsc. 2005, 217, 3–15. [Google Scholar] [CrossRef]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward William, W.; Prasher Douglas, C. Green Fluorescent Protein as a Marker for Gene Expression. Science 1994, 263, 802–805. [Google Scholar] [CrossRef]
- Shimomura, O. Structure of the Chromophore of Aequorea Green Fluorescent Protein. FEBS Lett. 1979, 104, 220–222. [Google Scholar] [CrossRef]
- Yang, F.; Moss, L.G.; Phillips, G.N., Jr. The Molecular Structure of Green Fluorescent Protein. Nat. Biotechnol. 1996, 14, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. The Green Fluorescent Protein. Ann. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.C. Use of Fluorescent Probes: Their Effect on Cell Biology and Limitations. Anat. Rec. (Hoboken) 2012, 295, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Wu, C.; Ye, F.; Jin, Y.; Smith, P.B.; Chiu, D.T. Development of Ultrabright Semiconducting Polymer Dots for Ratiometric PH Sensing. Anal. Chem. 2011, 83, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, D.; Demir, H.V. Conjugated Polymer Nanoparticles. Nanoscale 2010, 2, 484–494. [Google Scholar] [CrossRef]
- Burnham, D.R.; Zeigler, M.; Wu, C.; McNeill, J.D.; Yu, J.; Chiu, D.T.; Schneider, T.; Schiro, P.G. Bioconjugation of Ultrabright Semiconducting Polymer Dots for Specific Cellular Targeting. J. Am. Chem. Soc. 2010, 132, 15410–15417. [Google Scholar] [CrossRef]
- Wu, C.; Bull, B.; Szymanski, C.; Christensen, K.; McNeill, J. Multicolor Conjugated Polymer Dots for Biological Fluorescence Imaging. ACS Nano 2008, 2, 2415–2423. [Google Scholar] [CrossRef]
- Wu, C.; Chiu, D.T. Highly Fluorescent Semiconducting Polymer Dots for Biology and Medicine. Angew. Chem. Int. Ed. 2013, 52, 3086–3109. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated Polymer Nanoparticles: Preparation, Properties, Functionalization and Biological Applications. Chem. Soc. Rev. 2013, 42, 6620–6633. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-Soluble Conjugated Polymers for Imaging, Diagnosis, and Therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hansen, S.J.; Hou, Q.; Yu, J.; Zeigler, M.; Jin, Y.; Burnham, D.R.; McNeill, J.D.; Olson, J.M.; Chiu, D.T. Design of Highly Emissive Polymer Dot Bioconjugates for in Vivo Tumor Targeting. Angew. Chem. Int. Ed. 2011, 50, 3430–3434. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Li, R.; Shan, C.; Zeng, Z.; Xue, B.; Yuan, W.; Mo, C.; Xi, P.; Wu, C.; et al. Multicolor Super-Resolution Fluorescence Microscopy with Blue and Carmine Small Photoblinking Polymer Dots. ACS Nano 2017, 11, 8084–8091. [Google Scholar] [CrossRef] [PubMed]
- Dubertret, B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In Vivo Imaging of Quantum Dots Encapsulated in Phospholipid Micelles. Science 2002, 298, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.R.; Zipfel, W.R.; Williams, R.M.; Clark, S.W.; Bruchez, M.P.; Wise, F.W.; Webb, W.W. Water-Soluble Quantum Dots for Multiphoton Fluorescence Imaging in Vivo. Science 2003, 300, 1434–1436. [Google Scholar] [CrossRef]
- Åkerman, M.E.; Chan, W.C.W.; Laakkonen, P.; Bhatia, S.N.; Ruoslahti, E. Nanocrystal Targeting in Vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 12617–12621. [Google Scholar] [CrossRef]
- Bruchez, M., Jr.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science 1998, 281, 2013–2015. [Google Scholar] [CrossRef]
- Christodoulou, S.; Vaccaro, G.; Pinchetti, V.; De Donato, F.; Grim, J.Q.; Casu, A.; Genovese, A.; Vicidomini, G.; Diaspro, A.; Brovelli, S.; et al. Synthesis of Highly Luminescent Wurtzite CdSe/CdS Giant-Shell Nanocrystals Using a Fast Continuous Injection Route. J. Mater. Chem. C 2014, 2, 3439–3447. [Google Scholar] [CrossRef]
- Chen, O.; Zhao, J.; Chauhan, V.P.; Cui, J.; Wong, C.; Harris, D.K.; Wei, H.; Han, H.; Fukumura, D.; Jain, R.K.; et al. Compact High-Quality CdSe–CdS Core–shell Nanocrystals with Narrowemission Linewidths and Suppressed Blinking. Nat. Mater. 2013, 12, 445–451. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Zhang, C.Y. Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application. Chem. Rev. 2015, 115, 11669–11717. [Google Scholar] [CrossRef]
- Hoshino, A.; Fujioka, K.; Oku, T.; Suga, M. Physicochemical Properties and Cellular Toxicity of Nanocrystal Quantum Dots Depend on Their Surface Modification. Nano Lett. 2004, 4, 2163–2169. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 2163–2169. [Google Scholar] [CrossRef]
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the Biological Windows: Current Perspectives on Fluorescent Bioprobes Emitting above 1000 nm. Nanoscale Horiz. 2016, 1, 168–184. [Google Scholar] [CrossRef]
- Xi, L.; Jiang, H. Image-Guided Surgery Using Multimodality Strategy and Molecular Probes. WIREs Nanomed. Nanobiotechnol. 2016, 8, 46–60. [Google Scholar] [CrossRef]
- Senders, J.T.; Muskens, I.S.; Schnoor, R.; Karhade, A.V.; Cote, D.J.; Smith, T.R.; Broekman, M.L.D. Agents for Fluorescence-Guided Glioma Surgery: A Systematic Review of Preclinical and Clinical Results. Acta Neurochir. 2016, 1, 151–167. [Google Scholar] [CrossRef]
- Bertorelle, F.; Wilhelm, C.; Roger, J.; Gazeau, F.; Ménager, C.; Cabuil, V. Fluorescence-Modified Superparamagnetic Nanoparticles: Intracellular Uptake and Use in Cellular Imaging. Langmuir 2006, 22, 5385–5391. [Google Scholar] [CrossRef]
- Alcantara, D.; Guo, Y.; Yuan, H.; Goergen, C.J.; Chen, H.H.; Cho, H.; Sosnovik, D.E.; Josephson, L. Fluorochrome-Functionalized Magnetic Nanoparticles for High-Sensitivity Monitoring of the Polymerase Chain Reaction by Magnetic Resonance. Angew. Chem. Int. Ed. 2012, 51, 6904–6907. [Google Scholar] [CrossRef]
- Yen, S.K.; Jańczewski, D.; Lakshmi, J.L.; Dolmanan, S.B.; Tripathy, S.; Ho, V.H.B.; Vijayaragavan, V.; Hariharan, A.; Padmanabhan, P.; Bhakoo, K.K.; et al. Design and Synthesis of Polymer-Functionalized NIR Fluorescent Dyes-Magnetic Nanoparticles for Bioimaging. ACS Nano 2013, 7, 6796–6805. [Google Scholar] [CrossRef]
- Santra, S.; Kaittanis, C.; Grimm, J.; Perez, J.M. Drug/Dye-Loaded, Multifunctional Iron Oxide Nanoparticles for Combined Targeted Cancer Therapy and Dual Optical/Magnetic Resonance Imaging. Small 2009, 5, 1862–1868. [Google Scholar] [CrossRef]
- Redl, F.X.; Cho, K.S.; Murray, C.B.; O’Brien, S. Three-Dimensional Binary Superlattices of Magnetic Nanocrystals and Semiconductor Quantum Dots. Nature 2003, 423, 968–971. [Google Scholar] [CrossRef]
- Cho, M.; Contreras, E.Q.; Lee, S.S.; Jones, C.J.; Jang, W.; Colvin, V.L. Characterization and Optimization of the Fluorescence of Nanoscale Iron Oxide/Quantum Dot Complexes. J. Phys. Chem. C 2014, 118, 14606–14616. [Google Scholar] [CrossRef]
- Shibu, E.S.; Ono, K.; Sugino, S.; Nishioka, A.; Yasuda, A.; Shigeri, Y.; Wakida, S.I.; Sawada, M.; Biju, V. Photouncaging Nanoparticles for MRI and Fluorescence Imaging in Vitro and in Vivo. ACS Nano 2013, 7, 9851–9859. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, W.; Huang, P.; Zhang, B.; Zhang, X.; Xu, B. Intracellular Spatial Control of Fluorescent Magnetic Nanoparticles. J. Am. Chem. Soc. 2008, 130, 3710–3711. [Google Scholar] [CrossRef]
- Selvan, S.T.; Patra, P.K.; Ang, C.Y.; Ying, J.Y. Synthesis of Silica-Coated Semiconductor and Magnetic Quantum Dots and Their Use in the Imaging of Live Cells. Angew. Chem. Int. Ed. 2007, 46, 2448–2452. [Google Scholar] [CrossRef]
- Pahari, S.K.; Olszakier, S.; Kahn, I.; Amirav, L. Magneto-Fluorescent Yolk-Shell Nanoparticles. Chem. Mater. 2018, 30, 775–780. [Google Scholar] [CrossRef]
- Wang, D.; He, J.; Rosenzweig, N.; Rosenzweig, Z. Superparamagnetic Fe2O3 Beads−CdSe/ZnS Quantum Dots Core−Shell Nanocomposite Particles for Cell Separation. Nano Lett. 2004, 4, 409–413. [Google Scholar] [CrossRef]
- Lee, J.; Lee, N.; Kim, H.; Kim, J. Mesoporous Dye-Doped Silica Nanoparticles Decorated With Multiple Magnetite Nanocrystals for Simultaneous Enhanced Magnetic Resonance Imaging, Fluorescence. J. Am. Chem. Soc. 2010, 132, 552–557. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jun, Y.W.; Yeon, S.-I.; Shin, J.-S.; Cheon, J. Dual-Mode Nanoparticle Probes for High-Performance Magnetic Resonance and Fluorescence Imaging of Neuroblastoma. Angew. Chem. Int. Ed. 2006, 45, 8160–8162. [Google Scholar] [CrossRef]
- Chekina, N.; Horák, D.; Jendelová, P.; Trchová, M.; Bene, M.J.; Hrubý, M.; Herynek, V.; Turnovcová, K.; Syková, E. Fluorescent Magnetic Nanoparticles for Biomedical Applications. J. Mater. Chem. 2011, 21, 7630–7639. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Zhao, Z.; Tang, S.; Huang, X.; Lin, C.; Cai, C.; Zheng, N. Synthesis of Magnetic, Fluorescent and Mesoporous Core-Shell-Structured Nanoparticles for Imaging, Targeting and Photodynamic Therapy. J. Mater. Chem. 2011, 21, 11244–11252. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Rahman, M.T.; Ghosh, S.; Hossain, M.Z.; Shi, J.; Hidajat, K.; Uddin, M.S. β-Cyclodextrin Conjugated Magnetic, Fluorescent Silica Core-Shell Nanoparticles for Biomedical Applications. Carbohydr. Polym. 2013, 95, 449–457. [Google Scholar] [CrossRef]
- Li, L.; Choo, E.S.G.; Liu, Z.; Ding, J.; Xue, J. Double-Layer Silica Core-Shell Nanospheres with Superparamagnetic and Fluorescent Functionalities. Chem. Phys. Lett. 2008, 461, 114–117. [Google Scholar] [CrossRef]
- Insin, N.; Tracy, J.B.J.; Lee, H.; Zimmer, J.P.J.; Westervelt, R.M.; Bawendi, M.G. Incorporation of Iron Oxide Nanoparticles and Quantum Dots into Silica Microspheres. ACS Nano 2008, 2, 197–202. [Google Scholar] [CrossRef]
- Sathe, T.R.; Agrawal, A.; Nie, S. Mesoporous Silica Beads Embedded with Semiconductor Quantum Dots and Iron Oxide Nanocrystals: Dual-Function Microcarriers for Optical Encoding and Magnetic Separation. Anal. Chem. 2006, 78, 5627–5632. [Google Scholar] [CrossRef]
- Yi, D.K.; Selvan, S.T.; Lee, S.S.; Papaefthymiou, G.C.; Kundaliya, D.; Ying, J.Y. Silica-Coated Nanocomposites of Magnetic Nanoparticles and Quantum Dots. J. Am. Chem. Soc. 2005, 127, 4990–4991. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, J.E.; Yu, J.H.; Kim, B.C.; An, K.; Hwang, Y.; Shin, C.H.; Park, J.G. Magnetic Fluorescent Delivery Vehicle Using Uniform Mesoporous Silica Spheres Embedded with Monodisperse Magnetic and Semiconductor Nanocrystals. J. Am. Chem. Soc. 2006, 128, 688–689. [Google Scholar] [CrossRef]
- He, X.; Shen, X.; Li, D.; Liu, Y.; Jia, K.; Liu, X. Dual-Mode Fluorescence and Magnetic Resonance Imaging Nanoprobe Based on Aromatic Amphiphilic Copolymer Encapsulated CdSe@CdS and Fe3O4. ACS Appl. Bio Mater. 2018, 1, 520–528. [Google Scholar] [CrossRef]
- Chen, O.; Riedemann, L.; Etoc, F.; Herrmann, H.; Coppey, M.; Barch, M.; Farrar, C.T.; Zhao, J.; Bruns, O.T.; Wei, H.; et al. Magneto-Fluorescent Core-Shell Supernanoparticles. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Pinkerton, N.M.; Gindy, M.E.; Calero-Ddelc, V.L.; Wolfson, T.; Pagels, R.F.; Adler, D.; Gao, D.; Li, S.; Wang, R.; Zevon, M.; et al. Single-Step Assembly of Multimodal Imaging Nanocarriers: MRI and Long-Wavelength Fluorescence Imaging. Adv. Healthc. Mater. 2015, 4, 1376–1385. [Google Scholar] [CrossRef]
- Das, M.; Solanki, A.; Joshi, A.; Devkar, R.; Seshadri, S.; Thakore, S. Β-Cyclodextrin Based Dual-Responsive Multifunctional Nanotheranostics for Cancer Cell Targeting and Dual Drug Delivery. Carbohydr. Polym. 2019, 206, 694–705. [Google Scholar] [CrossRef]
- Bixner, O.; Gal, N.; Zaba, C.; Scheberl, A.; Reimhult, E. Fluorescent Magnetopolymersomes: A Theranostic Platform to Track Intracellular Delivery. Materials 2017, 10, 1303. [Google Scholar] [CrossRef]
- Di Corato, R.; Bigall, N.C.; Ragusa, A.; Dorfs, D.; Genovese, A.; Marotta, R.; Manna, L.; Pellegrino, T. Multifunctional Nanobeads Based on Quantum Dots and Magnetic Nanoparticles: Synthesis and Cancer Cell Targeting and Sorting. ACS Nano 2011, 5, 1109–1121. [Google Scholar] [CrossRef]
- Ling, D.; Park, W.; Park, S.J.; Lu, Y.; Kim, K.S.; Hackett, M.J.; Kim, B.H.; Yim, H.; Jeon, Y.S.; Na, K.; et al. Multifunctional Tumor PH-Sensitive Self-Assembled Nanoparticles for Bimodal Imaging and Treatment of Resistant Heterogeneous Tumors. J. Am. Chem. Soc. 2014, 136, 5647–5655. [Google Scholar] [CrossRef]
- Demillo, V.G.; Zhu, X. Zwitterionic Amphiphile Coated Magnetofluorescent Nanoparticles - Synthesis, Characterization and Tumor Cell Targeting. J. Mater. Chem. B 2015, 3, 8328–8336. [Google Scholar] [CrossRef]
- Feld, A.; Merkl, J.P.; Kloust, H.; Flessau, S.; Schmidtke, C.; Wolter, C.; Ostermann, J.; Kampferbeck, M.; Eggers, R.; Mews, A.; et al. A Universal Approach to Ultrasmall Magneto-Fluorescent Nanohybrids. Angew. Chem. Int. Ed. 2015, 54, 12468–12471. [Google Scholar] [CrossRef]
- Béalle, G.; Di Corato, R.; Kolosnjaj-Tabi, J.; Dupuis, V.; Clément, O.; Gazeau, F.; Wilhelm, C.; Ménager, C. Ultra Magnetic Liposomes for MR Imaging, Targeting, and Hyperthermia. Langmuir 2012, 28, 11834–11842. [Google Scholar] [CrossRef]
- Beaune, G.; Dubertret, B.; Clément, O.; Vayssettes, C.; Cabuil, V.; Ménager, C. Giant Vesicles Containing Magnetic Nanoparticles and Quantum Dots: Feasibility and Tracking by Fiber Confocal Fluorescence Microscopy. Angew. Chem. Int. Ed. 2007, 46, 5421–5424. [Google Scholar] [CrossRef]
- Scheffold, A.; Miltenyi, S.; Radbruch, A. Magnetofluorescent Liposomes for Increased Sensitivity of Immunofluorescence. Immunotechnology 1995, 1, 127–137. [Google Scholar] [CrossRef]
- Beaune, G.; Ménager, C.; Cabuil, V. Location of Magnetic and Fluorescent Nanoparticles Encapsulated inside Giant Liposomes. J. Phys. Chem. B 2008, 112, 7424–7429. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Liu, Y.; Hu, Z.; Mei, X.; Uvdal, K. Magneto-Fluorescent Nanoparticles with High-Intensity NIR Emission, T1- and T2-Weighted MR for Multimodal Specific Tumor Imaging. J. Mater. Chem. B 2015, 3, 3072–3080. [Google Scholar] [CrossRef]
- Li, K.; Ding, D.; Huo, D.; Pu, K.Y.; Thao, N.N.P.; Hu, Y.; Li, Z.; Liu, B. Conjugated Polymer Based Nanoparticles as Dual-Modal Probes for Targeted in Vivo Fluorescence and Magnetic Resonance Imaging. Adv. Funct. Mater. 2012, 22, 3107–3115. [Google Scholar] [CrossRef]
- Vijayan, V.M.; Ereath Beeran, A.; Shenoy, S.J.; Muthu, J.; Thomas, V. New Magneto-Fluorescent Hybrid Polymer Nanogel for Theranostic Applications. ACS Appl. Bio Mater. 2019, 2, 757–768. [Google Scholar] [CrossRef]
- Howes, P.; Green, M.; Bowers, A.; Parker, D.; Varma, G.; Kallumadil, M.; Hughes, M.; Warley, A.; Brain, A.; Botnar, R. Magnetic Conjugated Polymer Nanoparticles as Bimodal Imaging Agents. J. Am. Chem. Soc. 2010, 132, 9833–9842. [Google Scholar] [CrossRef] [PubMed]
- Faucon, A.; Maldiney, T.; Clément, O.; Hulin, P.; Nedellec, S.; Robard, M.; Gautier, N.; De Meulenaere, E.; Clays, K.; Orlando, T.; et al. Highly Cohesive Dual Nanoassemblies for Complementary Multiscale Bioimaging. J. Mater. Chem. B 2014, 2, 7747–7755. [Google Scholar] [CrossRef]
- Faucon, A.; Fresnais, J.; Brosseau, A.; Hulin, P.; Nedellec, S.; Hémez, J.; Ishow, E. Photoactive Chelating Organic Nanospheres as Central Platforms of Bimodal Hybrid Nanoparticles. J. Mater. Chem. C 2013, 1, 3879–3886. [Google Scholar] [CrossRef]
- Faucon, A.; Benhelli-Mokrani, H.; Fleury, F.; Dubreil, L.; Hulin, P.; Nedellec, S.; Doussineau, T.; Antoine, R.; Orlando, T.; Lascialfari, A.; et al. Tuning the Architectural Integrity of High-Performance Magneto-Fluorescent Core-Shell Nanoassemblies in Cancer Cells. J. Colloid Interface Sci. 2016, 479, 139–149. [Google Scholar] [CrossRef]
- Fresnais, J.; Ishow, E.; Sandre, O.; Berret, J.-F. Electrostatic Co-Assembly of Magnetic Nanoparticles and Fluorescent Nanospheres: A Versatile Approach towards Bimodal Nanorods. Small 2009, 5, 2533–2536. [Google Scholar] [CrossRef]
- Linot, C.; Poly, J.; Boucard, J.; Pouliquen, D.; Nedellec, S.; Hulin, P.; Lecouvey, M.; Marec, N.; Arosio, P.; Lascialfari, A.; et al. PEGylated Anionic Magneto Fl Uorescent Nanoassemblies: Impact of Their Interface Structure on Magnetic Resonance Imaging Contrast and Cellular Uptake. ACS Appl. Mater. Interfaces 2017, 9, 14242–14257. [Google Scholar] [CrossRef]
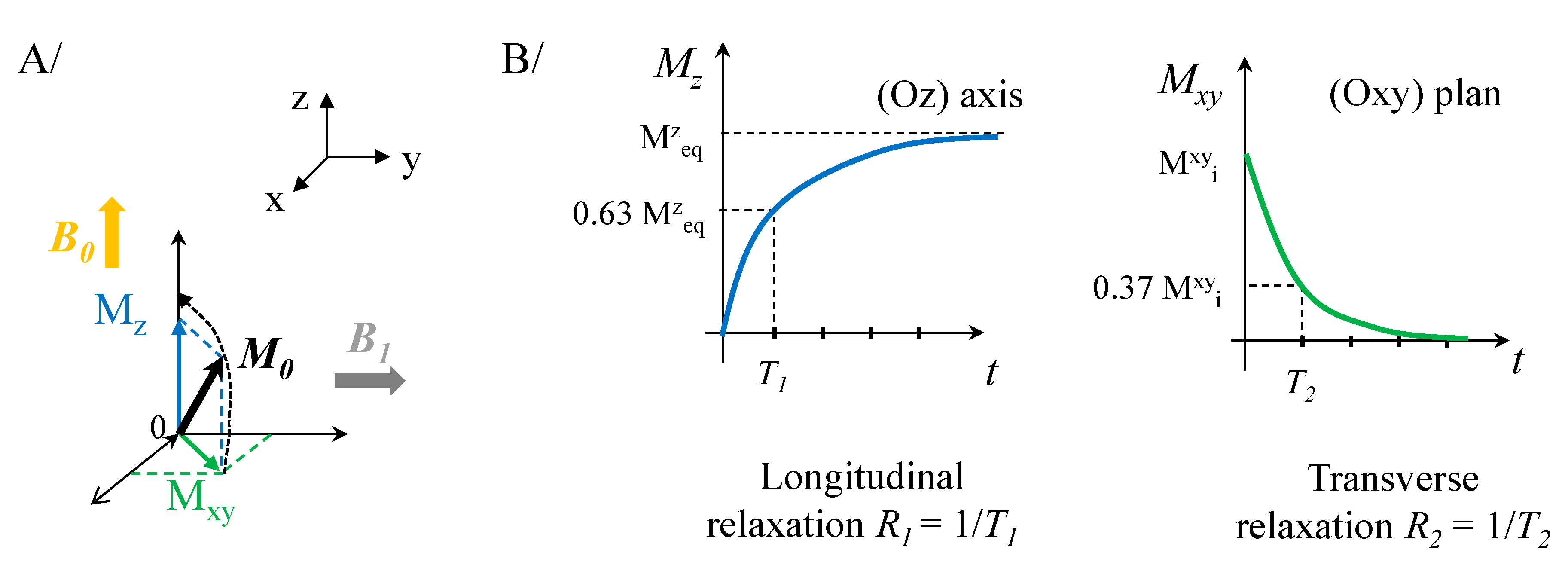

| Name | Classe | dH in nm/Coating | r2 in s−1 mM−1/(r2/r1) | Approval (withdrawn) | Company |
|---|---|---|---|---|---|
| Endorem® or Feridex I.V | ferumoxides | 120–180/dextran 10 kDa | 158 (16) | 1994 (2012) or 1996 (2008) | Guerbet S.A. or Berlex Laboratories |
| Sinerem® or Combidex ® | ferumoxtran-10 | 20–40/dextran 10 kDa | 88 (5) | n.a. (2007) or 2005 (2007) | Guerbet S.A. or AMAG pharmaceuticals, Inc. |
| Resovist® | ferucarbotran | 45–60/carboxydextran 1.8 kDa | 189 (19) | 2001 (2009) | Bayer Healthcare |
| Feraheme® | ferumoxytol | 30/semi-synthetic carbohydrate | 89 (6) | 2009 | AMAG pharmaceuticals, Inc. |
| Lumirem® or GastroMARK® | ferumoxsil | 400/poly [N-(2-aminoethyl)-3-aminopropyl]siloxane | 47 (23) | 1993 (2014) or 1996 (2010) | Guerbet S.A. or AMAG pharmaceuticals, Inc. |
| Type | Dispersant | dcore in nm | Synthesis Route (Provider) | dH in nm | wt% IO | Field/T | r2 in mM−1 s−1 (r2/r1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| I | PAA-b-PS | 5.6 | TD | 513 | 25 | 1.41 | 295(n.a) | [39] |
| 6.4 | 400 | 378 (n.a) | ||||||
| 10.8 | 300 | 561 (n.a) | ||||||
| 15.5 | 241 | 555 (n.a) | ||||||
| I | PTMC-b-PGA | 6.3 | CP | 50 | 20 | 4.7 | 81 (29) | [18] |
| 45 | 35 | 134 (37) | ||||||
| 47 | 50 | 173 (48) | ||||||
| 52 | 70 | 182 (52) | ||||||
| I | PTMC-b-PGA | 6–7 | CP | 125 | 5 | 1.41 | 71 (14) | [30] |
| 6–7 | 109 | 51.6 | 114 (25) | |||||
| 8–10 | 67 | 33.8 | 128 (22) | |||||
| 8–10 | 79 | 50.5 | 167 (25) | |||||
| 10–15 | 87 | 5.1 | 219 (71) | |||||
| 10–15 | 148 | 20 | 280 (103) | |||||
| I | PR-PAA in organosilica matrice | 6 | TD | 76 | 10 | 3 | 642 (n.a) | [31] |
| I | PEG-b-PCL-b-PAA | 1.9 | CP | 140 | 3 | 1.41 | 108 (n.a) | [17] |
| I | Pluronic® L-121 | 10 | n.a. (Webcraft GmbH) | 126 | 7.1 | 1.41 | 682 (68) | [41] |
| I | DOPG or DOPC | 13.8 | CP | 90 | n.a | 1 | 166 (~20) | [26] |
| 110 | 919 (~20) | |||||||
| I | folic acid-PGA-b-PCL | 7 | CP | 174 | n.a | 1.41 | 612 (20) | [36] |
| I | PEG-b-poly(tert-butyl acrylate-stat-PAA | 6 | CP | 175 | 4.8 | 3 | 211 (n.a) | [37] |
| IIa | EPC and DSPE-PEG-methoxy 2000 | 7.7 | CP | 16 | 100 | 0.47 | 108 (3) | [29] |
| 200 | 351 | 116 (6) | ||||||
| 195 | 631 | 130 (17) | ||||||
| IIb | SDS | 9.1 | TD (Ferrotec) | 53 | 75 | 3 | 295 (n.a) | [32] |
| 80 | 350 (n.a) | |||||||
| 99 | 410 (n.a) | |||||||
| IIb | PCL-b-PEG | 4 | TD | 17 | 12.4 | 1.5 | 25 (19) | [43] |
| 4 | 75 | 19.5 | 169 (58) | |||||
| 8 | 97 | 38.1 | 318 (199) | |||||
| 16 | 110 | 54.2 | 471 (236) | |||||
| IIb | PEG-b-PAA | 8.2 | TD | 105 | 34 | 1.41 | 255 (6) | [38] |
| 139 | 444 (6) | |||||||
| 181 | 604 (14) | |||||||
| IIb | PI-b-PEG | 8 | TD | 54 | n.a. | 1.41 | 131 (n.a) | [35] |
| 89 | 250 (n.a) | |||||||
| 96 | 353 (n.a) | |||||||
| 216 | 16 (n.a) | |||||||
| IIb | PEG-b-PBLG | 6–7 | CP | 157 | 5 | 1.41 | 180 (90) | [30] |
| 8–10 | 63 | 20.1 | 95 (20) | |||||
| 8–10 | 73 | 25 | 90 (19) | |||||
| 8–10 | 87 | 29.7 | 105 30) | |||||
| 10–15 | 109 | 20 | 500 (126) | |||||
| IIb | GCPQ | 4.8 | TD | 140 | n.a | 1 | 52 (79) | [24] |
| IIb | PEG-b-PLGA | 7 | TD | 73 | 41 | 1.5 | 333 (n.a) | [25] |
| IIb | PTEA-b-PAM | 6.3 | CP | 11 | 12 | 0.47 | 39 (2) | [34] |
| 70 | 322 | 74 (3) | ||||||
| 170 | 1502 | 162 (9) | ||||||
| IIb | PEI-b-PCL-b-PEG | 4 | TD | 60 | n.a | 1.41 | 20 (n.a) | [28] |
| 4 | 130 | 56 (n.a) | ||||||
| 4 | 170 | 72 (n.a) | ||||||
| 7.5 | 45 | 100 (n.a) | ||||||
| 7.5 | 80 | 200 (n.a) | ||||||
| 7.5 | 130 | 175 (n.a) | ||||||
| 8.7 | 45 | 115 (n.a) | ||||||
| 8.7 | 80 | 235 (n.a) | ||||||
| 8.7 | 180 | 70 (n.a) | ||||||
| 9.8 | 55 | 50 (n.a) | ||||||
| 9.8 | 120 | 375 (n.a) | ||||||
| 9.8 | 190 | 350 (n.a) | ||||||
| 11.8 | 50 | 200 (n.a) | ||||||
| 11.8 | 100 | 420 (n.a) | ||||||
| 11.8 | 220 | 100 (n.a) | ||||||
| IIc | lauric acid-irinotecan prodrug | 20 | TD (Sigma-Aldrich) | 117 | 6 | 7 | 189 (n.a) | [33] |
| IId | silica | 7 | CP | 24 | 25 | 7 | 179 (n.a) | [20] |
| 41 | 27 | 779 (n.a) | ||||||
| 26 | 42 | 1395 (n.a) | ||||||
| IId | silica | 6.1 | TD | 160 | 5 | 0.47 | 148 (510) | [27] |
| 120 | 7.4 | 164 (607) | ||||||
| 313 | 5.9 | 326 (1917) | ||||||
| III | PAA | n.a | Polyol | 79 | 100 | 1.41 | 405 (n.a) | [75] |
| 122 | 508 (n.a) | |||||||
| III | PAA | 7.5 | Polyol | 15 | 100 | 0.47 | 247 (n.a) | [49] |
| 9 | 30 | 340 (n.a) | ||||||
| 11.6 | 50 | 364 (n.a) | ||||||
| 19.7 | 100 | 100 (n.a) | ||||||
| III | PAA | 15.6 | Polyol | 37 | 100 | 0.23 | 361 (3.5) | [74] |
| 12 | 38.5 | 365 (3.4) | ||||||
| 13.5 | 44.3 | 319 (3.1) | ||||||
| 11 | 27 | 289 (3.1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lartigue, L.; Coupeau, M.; Lesault, M. Luminophore and Magnetic Multicore Nanoassemblies for Dual-Mode MRI and Fluorescence Imaging. Nanomaterials 2020, 10, 28. https://doi.org/10.3390/nano10010028
Lartigue L, Coupeau M, Lesault M. Luminophore and Magnetic Multicore Nanoassemblies for Dual-Mode MRI and Fluorescence Imaging. Nanomaterials. 2020; 10(1):28. https://doi.org/10.3390/nano10010028
Chicago/Turabian StyleLartigue, Lénaïc, Marina Coupeau, and Mélanie Lesault. 2020. "Luminophore and Magnetic Multicore Nanoassemblies for Dual-Mode MRI and Fluorescence Imaging" Nanomaterials 10, no. 1: 28. https://doi.org/10.3390/nano10010028
APA StyleLartigue, L., Coupeau, M., & Lesault, M. (2020). Luminophore and Magnetic Multicore Nanoassemblies for Dual-Mode MRI and Fluorescence Imaging. Nanomaterials, 10(1), 28. https://doi.org/10.3390/nano10010028






