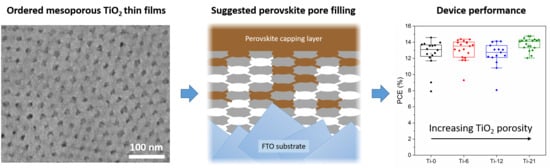
Investigation of Well-Defined Pinholes in TiO2 Electron Selective Layers Used in Planar Heterojunction Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
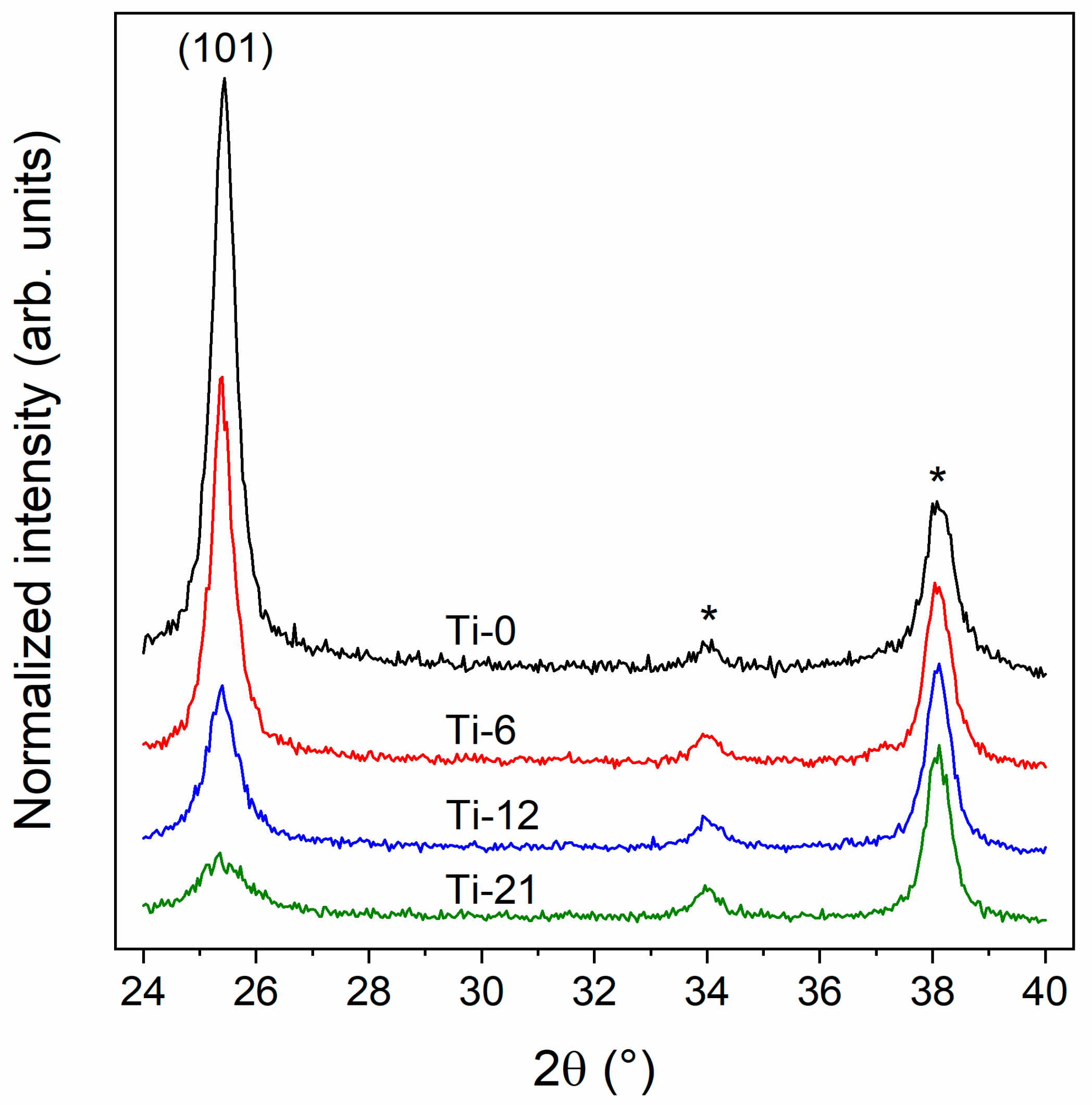
3.1. Structural Properties of the TiO2 Thin Films
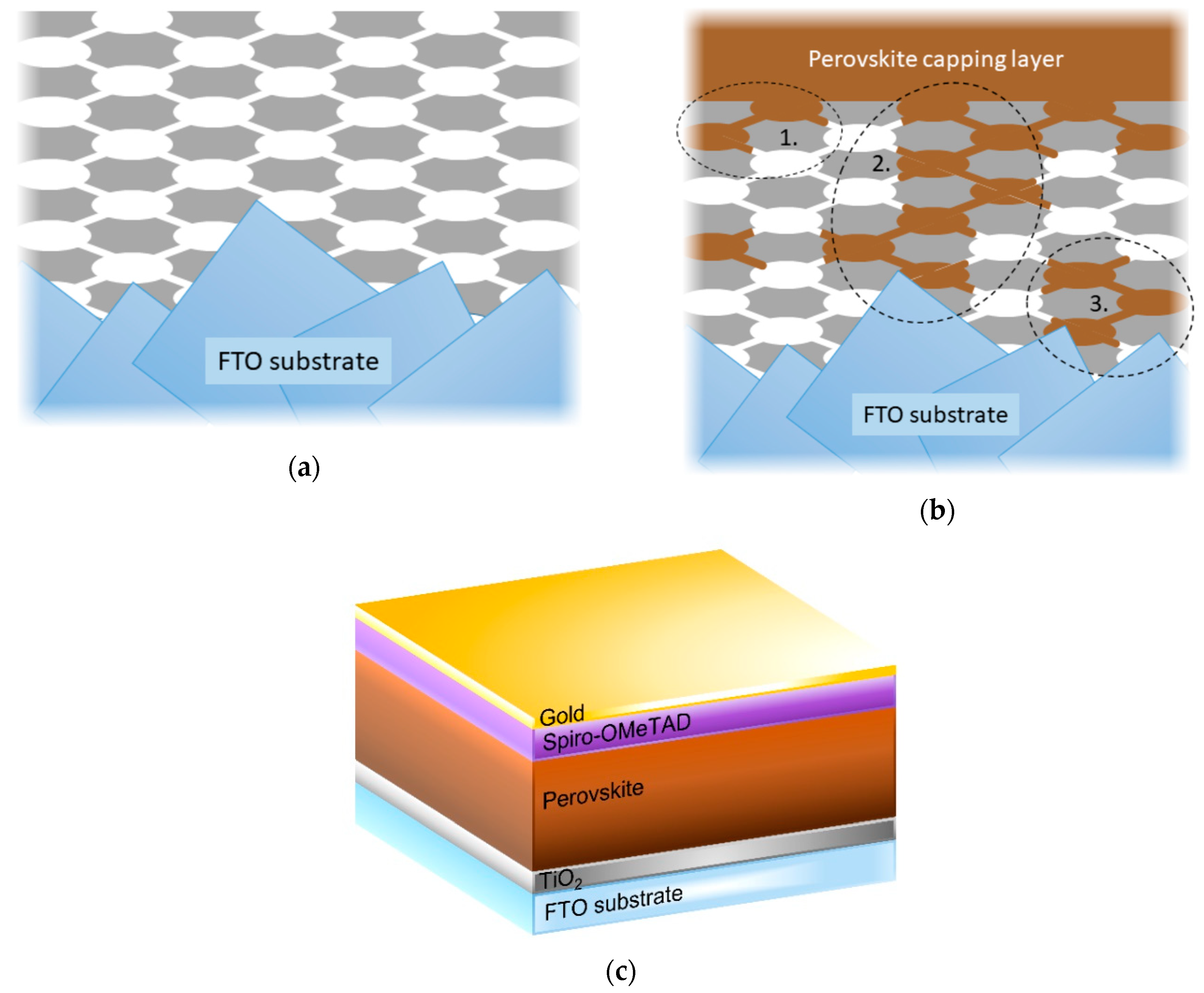
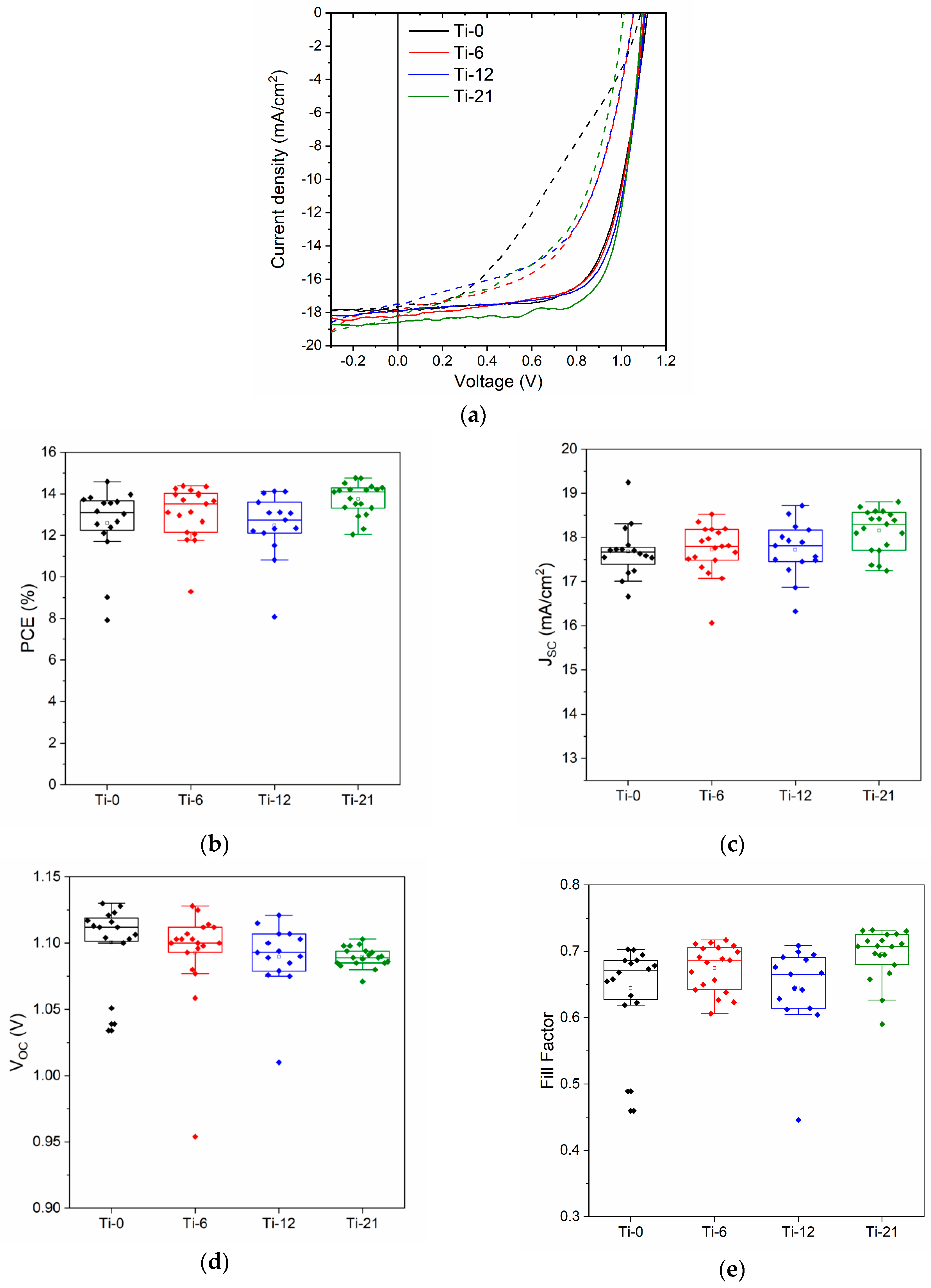
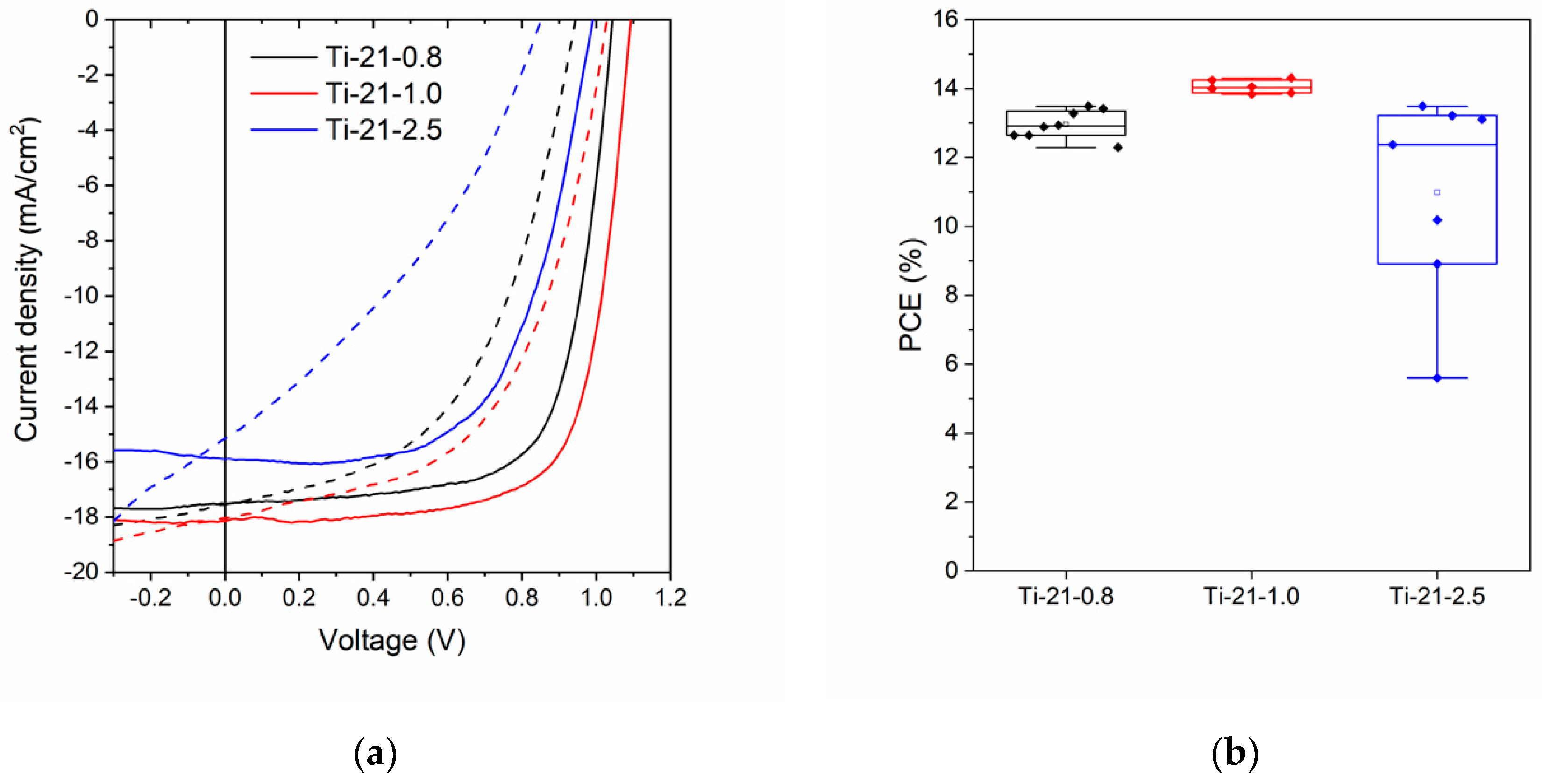
3.2. Device Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mahmood, K.; Sarwar, S.; Mehran, M.T. Current status of electron transport layers in perovskite solar cells: Materials and properties. RSC Adv. 2017, 7, 1744–1762. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Seo, J.; Domanski, K.; Correa-Baena, J.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Best Research Cell-Efficiencies. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20190802.pdf (accessed on 15 December 2019).
- Shen, H.; Wu, Y.; Peng, J.; Duong, T.; Fu, X.; Barugkin, C.; White, T.P.; Weber, K.; Catchpole, K.R. Improved Reproducibility for Perovskite Solar Cells with 1 cm2 Active Area by a Modified Two-Step Process. ACS Appl. Mater. Interfaces 2017, 9, 5974–5981. [Google Scholar] [CrossRef]
- Ramirez, D.; Velilla, E.; Montoya, J.F.; Jaramillo, F. Mitigating scalability issues of perovskite photovoltaic technology through a p-i-n meso-superstructured solar cell architecture. Sol. Energy Mater. Sol. Cells 2019, 195, 191–197. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Yang, Y. Addressing the stability issue of perovskite solar cells for commercial applications. Nat. Commun. 2018, 9, 5265. [Google Scholar] [CrossRef] [PubMed]
- Correa Baena, J.P.; Steier, L.; Tress, W.; Saliba, M.; Neutzner, S.; Matsui, T.; Giordano, F.; Jacobsson, T.J.; Srimath Kandada, A.R.; Zakeeruddin, S.M.; et al. Highly efficient planar perovskite solar cells through band alignment engineering. Energy Environ. Sci. 2015, 8, 2928–2934. [Google Scholar] [CrossRef]
- Mohamad Noh, M.F.; Teh, C.H.; Daik, R.; Lim, E.L.; Yap, C.C.; Ibrahim, M.A.; Ahmad Ludin, N.; bin Mohd Yusoff, A.R.; Jang, J.; Mat Teridi, M.A. The architecture of the electron transport layer for a perovskite solar cell. J. Mater. Chem. C 2018, 6, 682–712. [Google Scholar] [CrossRef]
- Guo, Y.; Sato, W.; Inoue, K.; Zhang, W.; Yu, G.; Nakamura, E. n-Type doping for efficient polymeric electron-transporting layers in perovskite solar cells. J. Mater. Chem. A 2016, 4, 18852–18856. [Google Scholar] [CrossRef]
- Yang, D.; Sano, T.; Yaguchi, Y.; Sun, H.; Sasabe, H.; Kido, J. Achieving 20% Efficiency for Low-Temperature-Processed Inverted Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1807556. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, X.; Wang, K.; Wu, C.; Yang, R.; Hou, Y.; Jiang, Y.; Liu, S.; Priya, S. Stable Efficiency Exceeding 20.6% for Inverted Perovskite Solar Cells through Polymer-Optimized PCBM Electron-Transport Layers. Nano Lett. 2019, 19, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Nelson, C.T.; Scott, M.C.; Minor, A.; Mathews, N.; Wong, L.H. Influence of void-free perovskite capping layer on the charge recombination process in high performance CH3NH3PbI3 perovskite solar cells. Nanoscale 2016, 8, 4181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, E.; Yan, J.; Ai, Y.; Li, N.; Yan, B.; Zeng, Y.; Sheng, J.; Ye, J. Defect engineering of oxygen vacancies in SnOx electron transporting layer for perovskite solar cells. Mater. Today Energy 2019, 12, 389–397. [Google Scholar] [CrossRef]
- Yang, G.; Tao, H.; Qin, P.; Ke, W.; Fang, G. Recent progress in electron selective layers for efficient perovskite solar cells. J. Mater. Chem. A 2016, 4, 3970–3990. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Chen, H.; Zhang, K.; Qin, C.; Liu, J.; Peng, W.; Islam, A.; Bi, E.; Ye, F.; et al. Highly compact TiO2 layer for efficient hole-blocking in perovskite solar cells. Appl. Phys. Express 2014, 7, 052301. [Google Scholar] [CrossRef]
- Jeong, E.; Bae, S.; Park, J.B.; Yu, S.M.; Kim, D.; Lee, H.; Rha, J.; Cho, Y.; Yun, J. Pinhole-free TiO2/Ag(O)/ZnO configuration for flexible perovskite solar cells with ultralow optoelectrical loss. RSC Adv. 2019, 9, 9160–9170. [Google Scholar] [CrossRef]
- Lan, F.; Jiang, M.; Li, G. The characterization of defects states and charge injection barriers in perovskite solar cells. In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology, Pittsburg, PA, USA, 25–28 July 2017; pp. 379–382. [Google Scholar] [CrossRef]
- Ren, H.; Zou, X.; Cheng, J.; Ling, T.; Bai, X.; Chen, D. Facile Solution Spin-Coating SnO2 Thin Film Covering Cracks of TiO2 Hole Blocking Layer for Perovskite Solar Cells. Coatings 2018, 8, 314. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, P.; Ren, G.; Chen, F.; Nan, H.; Liu, R.; Wang, D.; Tan, X.; Liu, X.; Zhang, H.; et al. Low-Temperature Atomic Layer Deposition of Metal Oxide Layers for Perovskite Solar Cells with High Efficiency and Stability under Harsh Environmental Conditions. ACS Appl. Mater. Interfaces 2018, 10, 23928–23937. [Google Scholar] [CrossRef]
- Jiménez, J.M.; Bourret, G.R.; Berger, T.; McKenna, K.P. Modification of Charge Trapping at Particle/Particle Interfaces by Electrochemical Hydrogen Doping of Nanocrystalline TiO2. J. Am. Chem. Soc. 2016, 138, 15956–15964. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, J.; Jiang, C.; Liu, T.; Que, X.; Zhu, R.; Gong, Q. Engineering of Electron-Selective Contact for Perovskite Solar Cells with Efficiency Exceeding 15%. ACS Nano 2014, 8, 10161–10167. [Google Scholar] [CrossRef]
- Masood, M.T.; Weinberger, C.; Sarfraz, J.; Rosqvist, E.; Sandén, S.; Sandberg, O.J.; Vivo, P.; Hashmi, G.; Lund, P.D.; Österbacka, R.; et al. Impact of Film Thickness of Ultrathin Dip-Coated Compact TiO2 Layers on the Performance of Mesoscopic Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 17906–17913. [Google Scholar] [CrossRef] [PubMed]
- Grosso, D.; Cagnol, F.; Soler-Illia, G.; Crepaldi, E.; Amenitsch, H.; Brunet-Bruneau, A.; Bourgeois, A.; Sanchez, C. Fundamentals of Mesostructuring Through Evaporation-Induced Self-Assembly. Adv. Funct. Mater. 2004, 14, 309–322. [Google Scholar] [CrossRef]
- Sarkar, A.; Jeon, N.J.; Noh, J.H.; Seok, S.I. Well-Organized Mesoporous TiO2 Photoelectrodes by Block Copolymer-Induced Sol–Gel Assembly for Inorganic–Organic Hybrid Perovskite Solar Cells. J. Phys. Chem. C 2014, 118, 16688–16693. [Google Scholar] [CrossRef]
- Ortel, E.; Fischer, A.; Chuenchom, L.; Polte, J.; Emmerling, F.; Smarsly, B.; Kraehnert, R. New Triblock Copolymer Templates, PEO-PB-PEO, for the Synthesis of Titania Films with Controlled Mesopore Size, Wall Thickness, and Bimodal Porosity. Small 2012, 8, 298–309. [Google Scholar] [CrossRef]
- Weidenthaler, C. Pitfalls in the characterization of nanoporous and nanosized materials. Nanoscale 2011, 3, 792. [Google Scholar] [CrossRef]
- Saliba, M.; Correa-Baena, J.; Wolff, C.M.; Stolterfoht, M.; Phung, N.; Albrecht, S.; Neher, D.; Abate, A. How to Make over 20% Efficient Perovskite Solar Cells in Regular (n–i–p) and Inverted (p–i–n) Architectures. Chem. Mater. 2018, 30, 4193–4201. [Google Scholar] [CrossRef]
- Manser, J.S.; Kamat, P.V. Band filling with free charge carriers in organometal halide perovskites. Nat. Photonics 2014, 8, 737–743. [Google Scholar] [CrossRef]
- Liu, M.; Endo, M.; Shimazaki, A.; Wakamiya, A.; Tachibana, Y. Identifying an Optimum Perovskite Solar Cell Structure by Kinetic Analysis: Planar, Mesoporous Based, or Extremely Thin Absorber Structure. ACS Appl. Energy Mater. 2018, 1, 3722–3732. [Google Scholar] [CrossRef]
- Coakley, K.M.; Liu, Y.; McGehee, M.D.; Frindell, K.L.; Stucky, G.D. Infiltrating semiconducting polymers into self-assembled mesoporous titania films for photostatic application. Adv. Funct. Mater. 2003, 13, 301–306. [Google Scholar] [CrossRef]
- Abrusci, A.; Ding, I.; Al-Hashimi, M.; Segal-Peretz, T.; McGehee, M.D.; Heeney, M.; Frey, G.L.; Snaith, H.J. Facile infiltration of semiconducting polymer into mesoporous electrodes for hybrid solar cells. Energy Environ. Sci. 2011, 4, 3051. [Google Scholar] [CrossRef]
- Rawolle, M.; Sarkar, K.; Niedermeier, M.A.; Schindler, M.; Lellig, P.; Gutmann, J.S.; Moulin, J.; Haese-Seiller, M.; Wochnik, A.S.; Scheu, C.; et al. Infiltration of Polymer Hole-Conductor into Mesoporous Titania Structures for Solid-State Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, O.J.; Sandén, S.; Sundqvist, A.; Smått, J.H.; Österbacka, R. Determination of Surface Recombination Velocities at Contacts in Organic Semiconductor Devices Using Injected Carrier Reservoirs. Phys. Rev. Lett. 2017, 118, 076601. [Google Scholar] [CrossRef] [PubMed]
- Lantiat, D.; Vivier, V.; Laberty-Robert, C.; Grosso, D.; Sanchez, C. Gold Nanoelectrode Arrays and their Evaluation by Impedance Spectroscopy and Cyclic Voltammetry. ChemPhysChem 2010, 11, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.; Kuemmel, M.; Järn, M.; Linden, M.; Boissière, C.; Nicole, L.; Sanchez, C.; Grosso, D. Surface Nanopatterning by Organic/Inorganic Self-Assembly and Selective Local Functionalization. Small 2006, 2, 587. [Google Scholar] [CrossRef]
- Kaewprajak, A.; Kumnorkaew, P.; Sagawa, T. Improved photovoltaic performance and device stability of planar heterojunction perovskite solar cells using TiO2 and TiO2 mixed with AgInS2 quantum dots as dual electron transport layers. Org. Electron. 2019, 69, 26–33. [Google Scholar] [CrossRef]
- Jeong, I.; Park, Y.H.; Bae, S.; Park, M.; Jeong, H.; Lee, P.; Ko, M.J. Solution-Processed Ultrathin TiO2 Compact Layer Hybridized with Mesoporous TiO2 for High-Performance Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 36865–36874. [Google Scholar] [CrossRef]
- Macdonald, T.J.; Batmunkh, M.; Lin, C.T.; Kim, J.; Tune, D.D.; Ambroz, F.; Li, X.; Xu, S.; Sol, C.; Papakonstantinou, I.; et al. Origin of Performance Enhancement in TiO2-Carbon Nanotube Composite Perovskite Solar Cells. Small Methods 2019, 3, 1900164. [Google Scholar] [CrossRef]
- Du, T.; Kim, J.; Ngiam, J.; Xu, S.; Barnes, P.R.F.; Durrant, J.R.; McLachlan, M.A. Elucidating the Origins of Subgap Tail States and Open-Circuit Voltage in Methylammonium Lead Triiodide Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1801808. [Google Scholar] [CrossRef]
- Kim, J.; Godin, R.; Dimitrov, S.D.; Du, T.; Bryant, D.; McLachlan, M.A.; Durrant, J.R. Excitation Density Dependent Photoluminescence Quenching and Charge Transfer Efficiencies in Hybrid Perovskite/Organic Semiconductor Bilayers. Adv. Energy Mater. 2018, 8, 1802474. [Google Scholar] [CrossRef]
- Makuta, S.; Liu, M.; Endo, M.; Nishimura, H.; Wakamiya, A.; Tachibana, Y. Photo-excitation intensity dependent electron and hole injections from lead iodide perovskite to nanocrystalline TiO2 and spiro-OMeTAD. Chem. Commun. 2016, 52, 673–676. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Yang, W.; Judin, L.; Hukka, T.I.; Priimagi, A.; Deng, Z.; Vivo, P. Thionation Enhances the Performance of Polymeric Dopant-Free Hole-Transporting Materials for Perovskite Solar Cells. Adv. Mater. Interfaces 2019, 6, 1901036. [Google Scholar] [CrossRef]
- Tress, W.; Yavari, M.; Domanski, K.; Yadav, P.; Niesen, B.; Correa Baena, J.P.; Hagfeldt, A.; Graetzel, M. Interpretation and evolution of open-circuit voltage, recombination, ideality factor and subgap defect states during reversible light-soaking and irreversible degradation of perovskite solar cells. Energy Environ. Sci. 2018, 11, 151–165. [Google Scholar] [CrossRef]
- Saliba, M.; Orlandi, S.; Matsui, T.; Aghazada, S.; Cavazzini, M.; Correa-Baena, J.P.; Gao, P.; Scopelliti, R.; Masconi, E.; Dahmen, K.; et al. A molecularly engineered hole-transporting material for efficient perovskite solar cells. Nat. Energy 2016, 1, 15017. [Google Scholar] [CrossRef]
- Unger, E.L.; Hoke, E.T.; Bailie, C.D.; Nguyen, W.H.; Bowring, A.R.; Heumüller, T.; Christoforo, M.G.; McGehee, M.D. Hysteresis and transient behavior in current–voltage measurements of hybrid-perovskite absorber solar cells. Energy Environ. Sci. 2014, 7, 3690. [Google Scholar] [CrossRef]
- Lu, H.; Ma, Y.; Gu, B.; Tian, W.; Li, L. Identifying the optimum thickness of electron transport layers for highly efficient perovskite planar solar cells. J. Mater. Chem. A 2015, 3, 16445–16452. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Xing, Y.; Zhang, Z.; Zhang, X.; Li, Z.; Shi, Y.; Ma, T.; Ma, R.; Wang, K.; et al. Perovskite Solar Cell Using a Two-Dimensional Titania Nanosheet Thin Film as the Compact Layer. ACS Appl. Mater. Interfaces 2015, 7, 15117–15122. [Google Scholar] [CrossRef]

| Sol/Sample 1 | TiCl4 | EtOH | H2O | P2952_BdEO | |
|---|---|---|---|---|---|
| Porosity series | |||||
| Ti-0 | 1 | 32.5 | 8.4 | 0 | |
| Ti-6 | 1 | 36.6 | 9.5 | 5.88 × 10−6 | |
| Ti-12 | 1 | 48.5 | 12.6 | 11.8 × 10−6 | |
| Ti-21 | 1 | 67.9 | 17.6 | 21.2 × 10−6 | |
| Thickness series | |||||
| Ti-21-0.8 | 1 | 56.2 | 14.6 | 21.2 × 10−6 | |
| Ti-21-1 | 1 | 67.9 | 17.6 | 21.2 × 10−6 | |
| Ti-21-2.5 | 1 | 169 | 43.7 | 21.2 × 10−6 |
| Sample | Crystallite Size (nm) | Thickness (nm) | Density (g/cm3) | Porosity (%) 1 |
|---|---|---|---|---|
| Ti-0 | 20 | 75 | 3.21 | 0 |
| Ti-6 | 21 | 71 | 2.76 | 14.0 |
| Ti-12 | 14 | 61 | 2.39 | 25.5 |
| Ti-21 | 7 | 50 | 1.70 | 47.0 |
| Sample | Thickness (nm) | No of Devices | JSC (mA/cm2) | VOC (V) | FF | PCE (%) |
|---|---|---|---|---|---|---|
| Ti-0 | 75 | 13 | 17.6 ± 0.4 | 1.11 ± 0.02 | 0.67 ± 0.03 | 13.1 ± 0.7 |
| Ti-6 | 71 | 18 | 17.8 ± 0.4 | 1.10 ± 0.02 | 0.68 ± 0.03 | 13.3 ± 0.9 |
| Ti-12 | 61 | 13 | 17.8 ± 0.5 | 1.10 ± 0.01 | 0.66 ± 0.03 | 13.0 ± 0.8 |
| Ti-21 | 50 | 19 | 18.1 ± 0.5 | 1.09 ± 0.01 | 0.70 ± 0.03 | 13.8 ± 0.7 |
| Ti-21-0.8 | 75 | 8 | 17.8 ± 0.3 | 1.05 ± 0.01 | 0.69 ± 0.02 | 12.9 ± 0.4 |
| Ti-21-1.0 | 50 | 6 | 18.1 ± 0.1 | 1.08 ± 0.01 | 0.72 ± 0.004 | 14.1 ± 0.2 |
| Ti-21-2.5 | 20 | 7 | 17.2 ± 2.0 | 1.01 ± 0.09 | 0.63 ± 0.11 | 11.0 ± 2.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masood, M.T.; Qudsia, S.; Hadadian, M.; Weinberger, C.; Nyman, M.; Ahläng, C.; Dahlström, S.; Liu, M.; Vivo, P.; Österbacka, R.; et al. Investigation of Well-Defined Pinholes in TiO2 Electron Selective Layers Used in Planar Heterojunction Perovskite Solar Cells. Nanomaterials 2020, 10, 181. https://doi.org/10.3390/nano10010181
Masood MT, Qudsia S, Hadadian M, Weinberger C, Nyman M, Ahläng C, Dahlström S, Liu M, Vivo P, Österbacka R, et al. Investigation of Well-Defined Pinholes in TiO2 Electron Selective Layers Used in Planar Heterojunction Perovskite Solar Cells. Nanomaterials. 2020; 10(1):181. https://doi.org/10.3390/nano10010181
Chicago/Turabian StyleMasood, Muhammad Talha, Syeda Qudsia, Mahboubeh Hadadian, Christian Weinberger, Mathias Nyman, Christian Ahläng, Staffan Dahlström, Maning Liu, Paola Vivo, Ronald Österbacka, and et al. 2020. "Investigation of Well-Defined Pinholes in TiO2 Electron Selective Layers Used in Planar Heterojunction Perovskite Solar Cells" Nanomaterials 10, no. 1: 181. https://doi.org/10.3390/nano10010181
APA StyleMasood, M. T., Qudsia, S., Hadadian, M., Weinberger, C., Nyman, M., Ahläng, C., Dahlström, S., Liu, M., Vivo, P., Österbacka, R., & Smått, J.-H. (2020). Investigation of Well-Defined Pinholes in TiO2 Electron Selective Layers Used in Planar Heterojunction Perovskite Solar Cells. Nanomaterials, 10(1), 181. https://doi.org/10.3390/nano10010181