Structural and Electrical Studies for Birnessite-Type Materials Synthesized by Solid-State Reactions
Abstract
1. Introduction
2. Results and Discussion
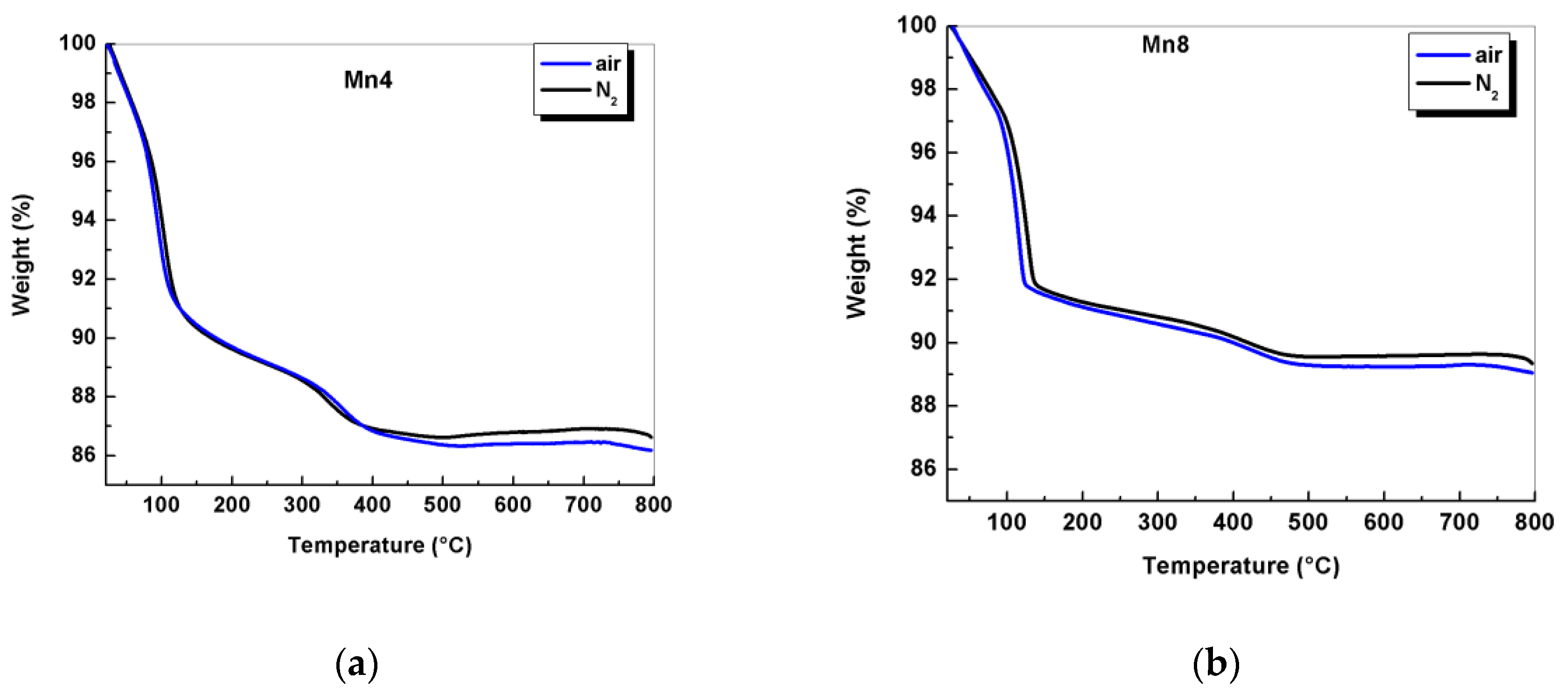
2.1. Chemical Composition, Thermogravimetric Analysis (TGA), and Average Oxidation State (AOS)
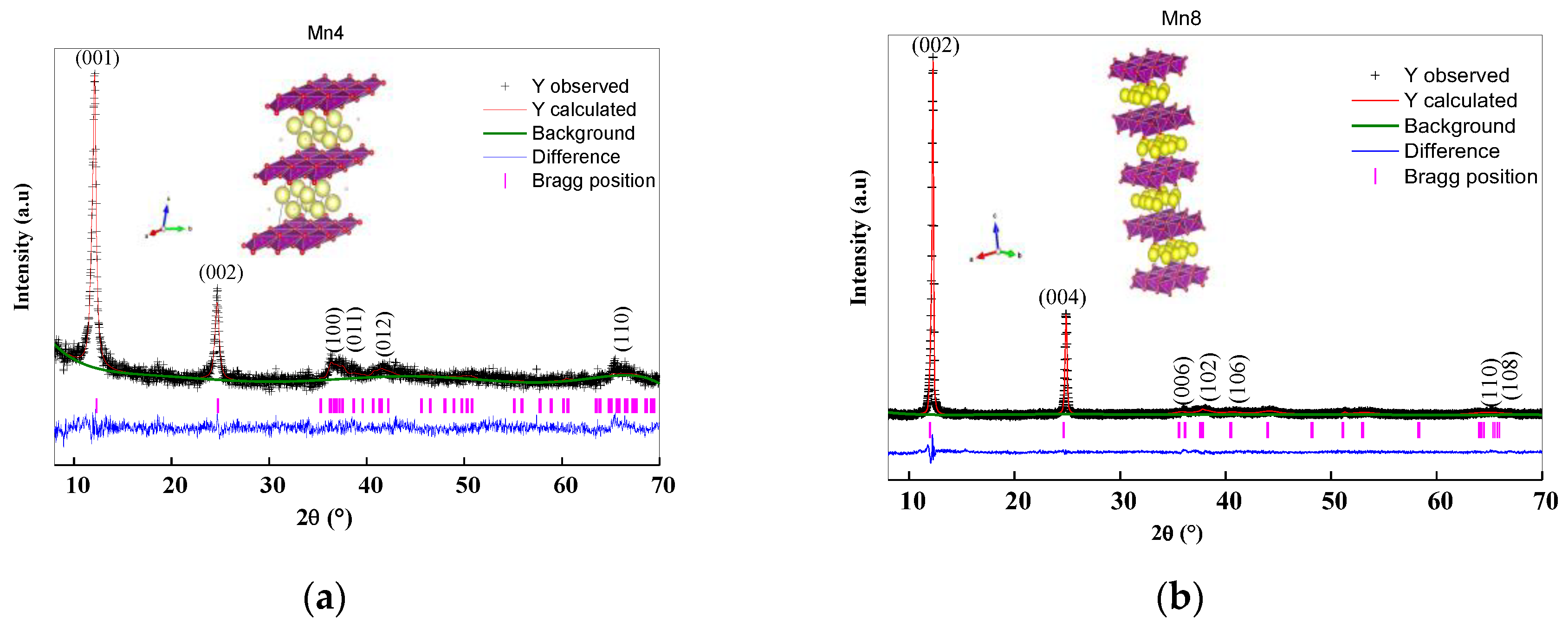
2.2. Structural Analysis and Rietveld Refinement
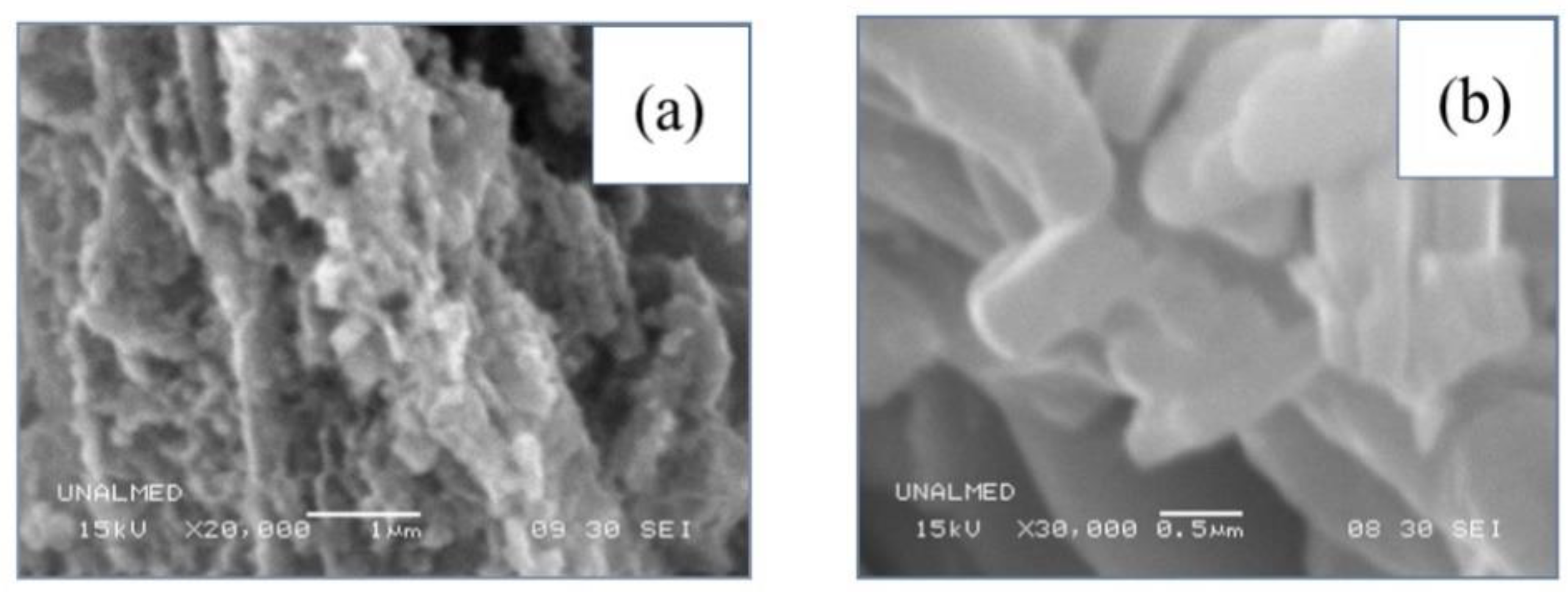
2.3. Morphological Analysis
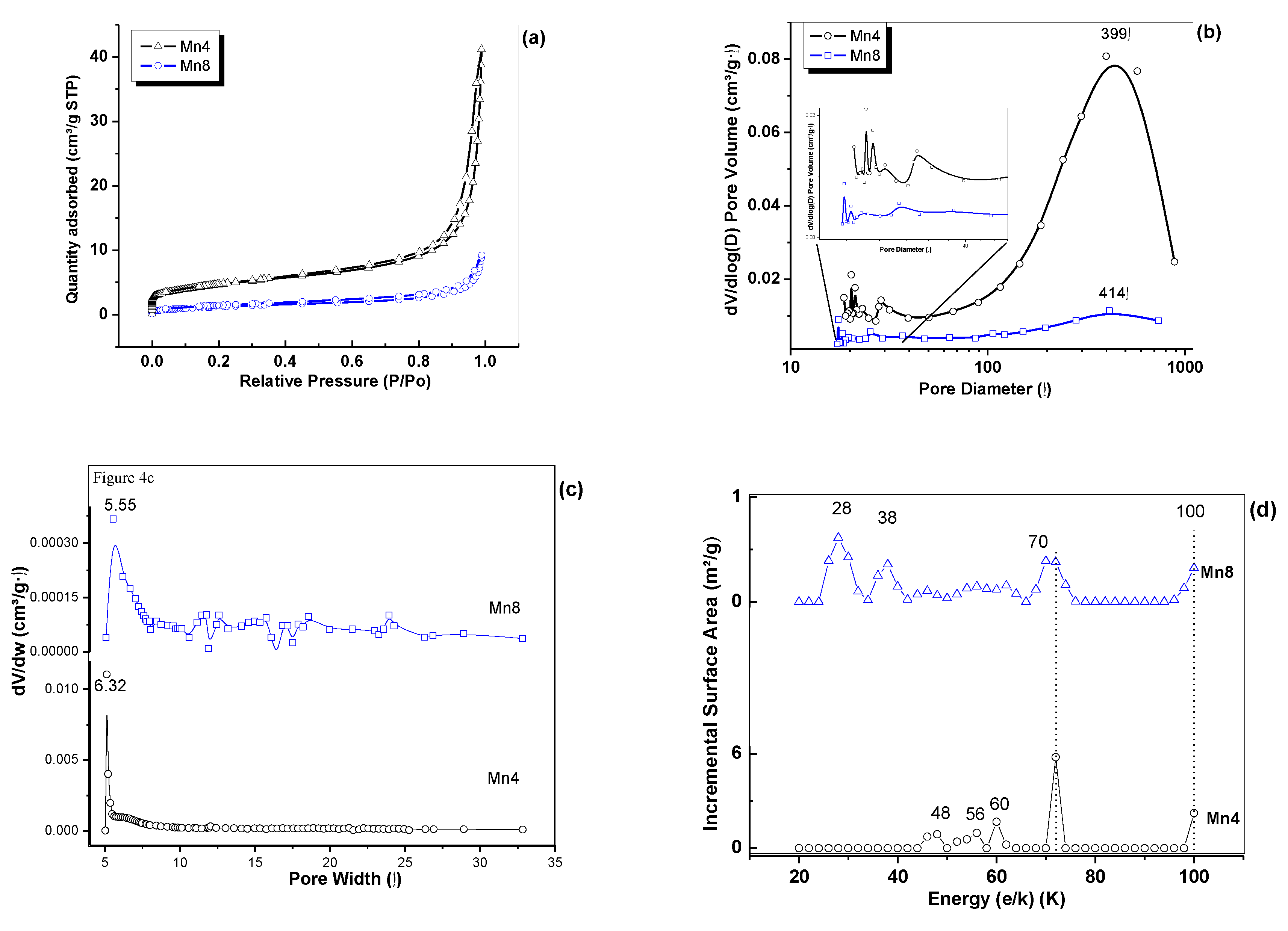
2.4. N2 Adsorption–Desorption Analysis
2.5. Electrical Analysis of Nyquist Plots
2.6. Alternant Current (AC) conductivity
3. Materials and Methods
3.1. Synthesis
3.2. Elemental Analysis and Average Oxidation State of Manganese
3.3. X-ray Diffraction (XRD) Data Collection and Structural Refinement
3.4. Thermal Analysis
3.5. Surface Area and Porosity
3.6. Scanning Electron Microscopy (SEM)
3.7. Impedance Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Mn4 | Mn8 | ||
|---|---|---|---|
| Fractional Coordinates Mn1 | x | 0 | 0 |
| y | 0 | 0 | |
| z | 0 | 0 | |
| Fractional Coordinates K1 | x | 1.625160 | 0.127491 |
| y | 0.835220 | 0.872509 | |
| z | 0.407410 | 0.250000 | |
| Fractional Coordinates O1 | x | 0.223160 | 0.666700 |
| y | 0.821360 | 0.333300 | |
| z | 0.094050 | 0.059257 | |
| Fractional Coordinates O2 | x | 0.032800 | 0.333300 |
| y | −0.059160 | 0.666700 | |
| z | 0.554910 | 0.250000 | |
| T (K) | CPE1-T (F) | CPE1-P | Rg (Ω) | CPE2-T (F) | CPE2-P | Rgb (Ω) | Wo-R | Wo-T | WoP |
|---|---|---|---|---|---|---|---|---|---|
| 298 | 1.40 × 10−11 | 1.04 | 1.67 × 105 | 3.18 × 10−10 | 0.94 | 8.95 × 103 | 3.05 × 104 | 3.45 × 10−1 | 0.22 |
| 323 | 5.54 × 10−11 | 0.95 | 4.85 × 104 | 6.23 × 10−12 | 0.12 | 3.02 × 102 | 3.98 × 103 | 2.86 × 10−2 | 0.22 |
| 348 | 1.58 × 10−9 | 0.76 | 1.30 × 103 | 6.18 × 10−19 | 0.94 | 157 × 102 | 8.63 × 102 | 5.41 × 10−3 | 0.20 |
| 373 | 3.06 × 10−9 | 0.70 | 2.13 × 103 | 2.09 × 10−17 | 2.00 | 1.11 × 101 | 1.22 × 102 | 8.23 × 10−5 | 0.17 |
| 398 | 9.65 × 10−10 | 0.78 | 8.84 × 102 | 1.00 × 10−20 | 2.91 | 1.28 × 102 | 1.04 × 10−1 | 1.49 × 10−12 | 0.17 |
| 423 | 2.08 × 10−9 | 0.77 | 6.74 × 102 | 1.00 × 10−20 | 2.92 | 1.03 × 102 | 8.45 × 10−2 | 5.15 × 10−14 | 0.12 |
| T (K) | CPE1-T (F) | CPE1-P | Rg (Ω) | CPE2-T (F) | CPE2-P | Rgb (Ω) | Wo-R (Ω) | Wo-T (s) | WoP |
|---|---|---|---|---|---|---|---|---|---|
| 298 | 2.51 × 10−11 | 0.98 | 2.08 × 105 | 4.61 × 10−8 | 0.634 | 1.98 × 105 | 1.07 × 106 | 39.38 | 0.34 |
| 323 | 1.67 × 10−11 | 1.00 | 8.87 × 104 | 8.56 × 10−8 | 0.618 | 6.75 × 104 | 6.13 × 105 | 45.61 | 0.32 |
| 348 | 1.37 × 10−11 | 1.02 | 4.34 × 104 | 2.01 × 10−7 | 0.572 | 3.09 × 104 | 2.10 × 105 | 126.6 | 0.26 |
| 373 | 1.13 × 10−11 | 1.04 | 2.68 × 104 | 6.93 × 10−8 | 0.680 | 8.53 × 103 | 4.37 × 104 | 91.41 | 0.19 |
| 398 | 2.10 × 10−11 | 1.00 | 8.29 × 103 | 6.24 × 10−4 | 0.068 | 1.50 × 1017 | N.A | N.A | N.A |
| 423 | 4.36 × 10−11 | 0.97 | 3.69 × 103 | 1.19 × 10−3 | 0.042 | 5.00 × 107 | N.A | N.A | N.A |
References
- Wolkenstein, T. The electron theory of catalysis on semiconductors. In Advances in Catalysis; Eley, D.D., Selwood, P.W., Weisz, P.B., Eds.; Academic Press: Cambridge, MA, USA, 1960; Volume 12, pp. 189–264. [Google Scholar]
- Swaminathan, M. Chapter 10—Semiconductor oxide nanomaterials as catalysts for multiple applications. In Handbook of Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 197–207. [Google Scholar] [CrossRef]
- Venkatanarayanan, A.; Spain, E. 13.03—Review of recent developments in sensing materials. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 47–101. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Paszkiewicz-Gawron, M.; Zaleska-Medynska, A. 3—Metal oxide photocatalysts. In Metal Oxide-Based Photocatalysis; Zaleska-Medynska, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 51–209. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Dutta, B.; Clarke, R.; Raman, S.; Shaffer, T.D.; Achola, L.; Nandi, P.; Suib, S.L. Lithium promoted mesoporous manganese oxide catalyzed oxidation of allyl ethers. Nat. Commun. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Cheng, Y.; Peng, X.; Pan, W. Copper modified manganese oxide with tunnel structure as efficient catalyst for low-temperature catalytic combustion of toluene. Chem. Eng. J. 2019, 369, 758–765. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Feng, N.; Chen, L.; Yu, J.; Meng, J.; Fang, F.; Wang, L.; Wan, H.; Guan, G. Construction of substrate-dependent 3D structured MnO2 catalysts for diesel soot elimination. Appl. Surf. Sci. 2019, 484, 197–208. [Google Scholar] [CrossRef]
- Becerra, M.E.; Arias, N.P.; Giraldo, O.H.; López Suárez, F.E.; Illán Gómez, M.J.; Bueno López, A. Soot combustion manganese catalysts prepared by thermal decomposition of KMnO4. Appl. Catal. B Environ. 2011, 102, 260–266. [Google Scholar] [CrossRef]
- Becerra, M.E.; Arias, N.P.; Giraldo, O.H.; López-Suárez, F.E.; Illán-Gómez, M.J.; Bueno-López, A. Alumina-supported manganese catalysts for soot combustion prepared by thermal decomposition of KMnO4. Catalysts 2012, 2, 352–367. [Google Scholar] [CrossRef]
- Becerra, M.E.; Suarez, A.M.; Arias, N.P.; Giraldo, O. Decomposition of the methylene blue dye using layered manganese oxide materials synthesized by solid state reactions. Int. J. Chem. Eng. 2018, 2018, 11. [Google Scholar] [CrossRef]
- Arias, N.P.; Dávila, M.T.; Giraldo, O. Electrical behavior of an octahedral layered OL-1-type manganese oxide material. Ionics 2013, 19, 201–214. [Google Scholar] [CrossRef]
- Arias, N.P.; Becerra, M.E.; Giraldo, O. Caracterización eléctrica de un oxido de manganeso laminar tipo birnesita. Revista Mexicana de Física 2015, 61, 380–387. [Google Scholar]
- Giraldo, O.; Arias, N.P.; Becerra, M.E. Electrical properties of TiO2-pillared bidimensional manganese oxides. Appl. Clay Sci. 2017, 141, 157–170. [Google Scholar] [CrossRef]
- Suib, S.L. Porous manganese oxide octahedral molecular sieves and octahedral layered materials. Acc. Chem. Res. 2008, 41, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Singh, P.; Carter, M.; Prince, K. The Zn–MnO2 battery: The influence of aqueous LiOH and KOH electrolytes on the intercalation mechanism. Electrochem. Solid State Lett. 2008, 11, A145–A149. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Issa, T.B.; Thurgate, S.; Marco, R.D. Lithium insertion into manganese dioxide electrode in MnO2/Zn aqueous battery: Part II. Comparison of the behavior of EMD and battery grade MnO2 in Zn|MnO2|aqueous LiOH electrolyte. J. Power Sources 2004, 138, 319–322. [Google Scholar] [CrossRef]
- Brock, S.L.; Duan, N.; Tian, Z.R.; Giraldo, O.; Zhou, H.; Suib, S.L. A review of porous manganese oxide materials. Chem. Mater. 1998, 10, 2619–2628. [Google Scholar] [CrossRef]
- Atribak, I.; Bueno-López, A.; García-García, A.; Navarro, P.; Frías, D.; Montes, M. Catalytic activity for soot combustion of birnessite and cryptomelane. Appl. Catal. B Environ. 2010, 93, 267–273. [Google Scholar] [CrossRef]
- Kappenstein, C.; Pirault-Roy, L.; Guérin, M.; Wahdan, T.; Ali, A.A.; Al-Sagheer, F.A.; Zaki, M.I. Monopropellant decomposition catalysts: V. Thermal decomposition and reduction of permanganates as models for the preparation of supported MnOx catalysts. Appl. Catal. A Gen. 2002, 234, 145–153. [Google Scholar] [CrossRef]
- Herbstein, F.H.; Ron, G.; Weissman, A. The thermal decomposition of potassium permanganate and related substances. Part, I. Chemical aspects. J. Chem. Soc. Inorg. Phys. Theor. 1971, 1821–1826. [Google Scholar] [CrossRef]
- Gaillot, A.C.; Flot, D.; Drits, V.A.; Manceau, A.; Burghammer, M.; Lanson, B. Structure of synthetic K-rich birnessite obtained by high-temperature decomposition of KMnO4. I. Two-layer polytype from 800 °C experiment. Chem. Mater. 2003, 15, 4666–4678. [Google Scholar] [CrossRef]
- Cai, J.; Liu, J.; Suib, S.L. Preparative parameters and framework dopant effects in the synthesis of layer-structure birnessite by air oxidation. Chem. Mater. 2002, 14, 2071–2077. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Q.; Huang, A.; Giraldo, O.; Suib, S.L. Double-aging method for preparation of stabilized Na-buserite and transformations to todorokites incorporated with various metals. Inorg. Chem. 1999, 38, 6106–6113. [Google Scholar] [CrossRef]
- Shen, Y.F.; Suib, S.L.; O’Young, C.L. Effects of inorganic cation templates on octahedral molecular sieves of manganese oxide. J. Am. Chem. Soc. 1994, 116, 11020–11029. [Google Scholar] [CrossRef]
- Yang, D.S.; Wang, M.K. Syntheses and Characterization of Well-Crystallized Birnessite. Chem. Mater. 2001, 13, 2589–2594. [Google Scholar] [CrossRef]
- Julien, C.M.M.; Baddour-Hadjean, R.; Frager, S.; Bach, S.; Pereira-Ramos, J.P. Raman spectra of birnessite manganese dioxides. Solid State Ion. 2003, 159, 345–356. [Google Scholar] [CrossRef]
- Drits, V.A.; Tchoubar, C. X-ray Diffraction by Disordered Lamellar Structures: Theory and Applications to Microdivided Silicates and Carbons; Springer Verlag: Berlin/Heidelberg, Germany, 1990; p. 371. [Google Scholar]
- Ching, S.W.E.; Hughes, S.; Bahadoor, A.; Suib, S.L. Nonaqueous Sol-Gel Syntheses of Microporous Manganese Oxides. Chem. Mater. 2002, 14, 1292–1299. [Google Scholar] [CrossRef]
- Victor, A.; Drits, E.S.; Anatoli, I.; Gorshkov, A.; Alain, M. Structure of monoclinic Na-rich birnessite and hexagonal birnessite: I. results from X-ray diffraction and selected-area electron diffraction. Am. Mineral. 1997, 82, 946–961. [Google Scholar]
- Kim, S.H.; Kim, S.J.; Oh, S.M. Preparation of layered MnO2 via thermal decomposition of KMnO4 and its electrochemical characterizations. Chem. Mater. 1999, 11, 557–563. [Google Scholar] [CrossRef]
- Drits Victor, A.; Lanson, B.; Gaillot, A.C. Birnessite polytype systematics and identiÞ cation by powder X-ray diffraction. Am. Mineral. 2007, 92, 771–788. [Google Scholar] [CrossRef]
- Lopano, C.L.; Heaney, P.J.; Post, J.E.; Hanson, J.; Komarneni, S. Time-resolved structural analysis of K-and Ba-exchange reactions with synthetic Na-birnessite using synchrotron X-ray diffraction. Am. Mineral. 2007, 92, 380–387. [Google Scholar] [CrossRef]
- Post, J.E.; Veblen, D.R. Crystal structure determinations of synthetic sodium, magnesium, and potassium birnessite using TEM and the Rietveld method. Am. Mineral. 1990, 75, 477–489. [Google Scholar]
- Nandy, S.; Mallick, S.; Ghosh, P.K.; Das, G.C.; Mukherjee, S.; Mitra, M.K.; Chattopadhyay, K.K. Impedance spectroscopic studies of nickel nanocluster in silica matrix synthesized by sol–gel method. J. Alloys Compd. 2008, 453, 1–6. [Google Scholar] [CrossRef]
- Klobes, P.; Klaus, M.; Munro, G.M. Porosity and Specific Surface Area Mearuments for Solid Materials; NIST, Ed.; NIST: Gaithersburg, MD, USA, 2006.
- Jagiello, J.; Olivier, J.P. A simple two-dimensional NLDFT model of gas adsorption in finite carbon pores. Application to pore structure analysis. J. Phys. Chem. C 2009, 113, 19382–19385. [Google Scholar] [CrossRef]
- Maddox, M.W.; Olivier, J.P.; Gubbins, K.E. Characterization of MCM-41 using molecular simulation: Heterogeneity effects. Langmuir 1997, 13, 1737–1745. [Google Scholar] [CrossRef]
- Minakshi Sundaram, M.; Biswal, A.; Mitchell, D.; Jones, R.; Fernandez, C. Correlation among physical and electrochemical behaviour of nanostructured electrolytic manganese dioxide from leach liquor and synthetic for aqueous asymmetric capacitor. Phys. Chem. Chem. Phys. 2016, 18, 4711–4720. [Google Scholar] [CrossRef] [PubMed]
- Kidner, N.J.; Perry, N.H.; Mason, T.O.; Garboczi, E.J. The brick layer model revisited: Introducing the nano-grain composite model. J. Am. Ceram. Soc. 2008, 91, 1733–1746. [Google Scholar] [CrossRef]
- Swider, K.E.; Merzbacher, C.I.; Hagans, P.L.; Rolison, D.R. Synthesis of ruthenium dioxide-titanium dioxide aerogels: Redistribution of electrical properties on the nanoscale. Chem. Mater. 1997, 9, 1248–1255. [Google Scholar] [CrossRef]
- Korotcenkov, G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R Rep. 2008, 61, 1–39. [Google Scholar] [CrossRef]
- Andrew, K.J. Dielectric relaxation in solids. J. Phys. D Appl. Phys. 1999, 32, R57. [Google Scholar]
- McNealy, B.E.; Hertz, J.L. On the use of the constant phase element to understand variation in grain boundary properties. Solid State Ion. 2014, 256, 52–60. [Google Scholar] [CrossRef]
- Córdoba-Torres, P.; Mesquita, T.J.; Nogueira, R.P. Influence of geometry-induced current and potential distributions on the characterization of constant-phase element behavior. Electrochim. Acta 2013, 87, 676–685. [Google Scholar] [CrossRef]
- Shoar Abouzari, M.R.; Berkemeier, F.; Schmitz, G.; Wilmer, D. On the physical interpretation of constant phase elements. Solid State Ion. 2009, 180, 922–927. [Google Scholar] [CrossRef]
- Jonscher, A.K. Relaxation in low-loss dielectrics. J. Mol. Liq. 2000, 86, 259–268. [Google Scholar] [CrossRef]
- Jonscher, A.K. Dielectric characterisation of semiconductors. Solid State Electron. 1990, 33, 737–742. [Google Scholar] [CrossRef]
- Jonscher, A.K. Frequency-dependence of conductivity in hopping systems. J. Non Cryst. Solids 1972, 8, 293–315. [Google Scholar] [CrossRef]
- Hill, R.M.; Jonscher, A.K. DC and AC conductivity in hopping electronic systems. J. Non Cryst. Solids 1979, 32, 53–69. [Google Scholar] [CrossRef]
- Bucheli, W.; Jiménez, R.; Sanz, J.; Várez, A. The log(σ) vs. log(ω) derivative plot used to analyze the ac conductivity. Application to fast Li+ ion conductors with perovskite structure. Solid State Ion. 2012, 227, 113–118. [Google Scholar] [CrossRef]
- Giraldo, O.; Marquez, M.; Brock, S.L.; Suib, S.L.; Hillhouse, H.; Tsapatsis, M. Spontaneous formation of inorganic helical fibers and rings. J. Am. Chem. Soc. 2000, 122, 12158–12163. [Google Scholar] [CrossRef]
- Henn, F.; Durand, C.; Cerepi, A.; Brosse, E.; Giuntini, J.C. DC conductivity, cationic exchange capacity, and specific surface area related to chemical composition of pore lining chlorites. J. Colloid Interface Sci. 2007, 311, 571–578. [Google Scholar] [CrossRef]
- Le Caër, S.; Lima, M.; Gosset, D.; Simeone, D.; Bergaya, F.; Pommeret, S.; Renault, J.P.; Righini, R. Dynamics of water confined in clay minerals. J. Phys. Chem. C 2012, 116, 12916–12925. [Google Scholar] [CrossRef]
- Madelung, O. Introduction to Solid-State Theory; Springer: Berlin/Heidelberg, Germany, 1978; Volume 2, p. 490. [Google Scholar]
- Díez, A.; Schmidt, R.; Sagua, A.E.; Frechero, M.A.; Matesanz, E.; Leon, C.; Morán, E. Structure and physical properties of nickel manganite NiMn2O4 obtained from nickel permanganate precursor. J. Eur. Ceram. Soc. 2010, 30, 2617–2624. [Google Scholar] [CrossRef]
- Ngala, J.K.; Alia, S.; Dobley, A.; Crisostomo, V.M.B.; Suib, S.L. Characterization and electrocatalytic behavior of layered Li2MnO3 and its acid-treated form. Chem. Mater. 2007, 19, 229–234. [Google Scholar] [CrossRef]
- Ghosh, R.; Shen, X.; Villegas, J.C.; Ding, Y.; Malinger, K.; Suib, S.L. Role of manganese oxide octahedral molecular sieves in styrene epoxidation. J. Phys. Chem. B 2006, 110, 7592–7599. [Google Scholar] [CrossRef]
- Bhide, V.G.; Dani, R.H. Electrical conductivity in oxides of manganese and related compounds. Physica 1961, 27, 821–826. [Google Scholar] [CrossRef]
- De Guzman, R.N.; Awaluddin, A.; Shen, Y.F.; Tian, Z.R.; Suib, S.L.; Ching, S.; O’Young, C.L. Electrical resistivity measurements on manganese oxides with layer and tunnel structures: Birnessites, todorokites, and cryptomelanes. Chem. Mater. 1995, 7, 1286–1292. [Google Scholar] [CrossRef]
- Klose, P.H. Electrical properties of manganese dioxide and manganese sesquioxide. J. Electrochem. Soc. 1970, 117, 854–858. [Google Scholar] [CrossRef]
- Hong, F.; Yue, B.; Hirao, N.; Liu, Z.; Chen, B. Significant improvement in Mn2O3 transition metal oxide electrical conductivity via high pressure. Sci. Rep. 2017, 7, 44078. [Google Scholar] [CrossRef]
- Tan, F.K.; Hassan, J.; Wahab, Z.A.; Azis, R.A.S. Electrical conductivity and dielectric behaviour of manganese and vanadium mixed oxide prepared by conventional solid state method. Eng. Sci. Technol. Int. J. 2016, 19, 2081–2087. [Google Scholar] [CrossRef]
- Hedden, M.; Francis, N.; Haraldsen, J.T.; Ahmed, T.; Constantin, C. Thermoelectric properties of nano-meso-micro β-MnO2 powders as a function of electrical resistance. Nanoscale Res. Lett. 2015, 10, 292. [Google Scholar] [CrossRef]
- Bose, V.C.; Maniammal, K.; Madhu, G.; Veenas, C.L.; Raj, A.S.A.; Biju, V. DC electrical conductivity of nanocrystalline Mn3O4 synthesized through a novel sol-gel route. Iop Conf. Ser. Mater. Sci. Eng. 2015, 73, 012084. [Google Scholar] [CrossRef]
- Byles, B.W.; Palapati, N.K.R.; Subramanian, A.; Pomerantseva, E. The role of electronic and ionic conductivities in the rate performance of tunnel structured manganese oxides in Li-ion batteries. APL Mater. 2016, 4, 046108. [Google Scholar] [CrossRef]
- Gaillot, A.C.; Drits, V.A.; Manceau, A.; Lanson, B. Structure of the synthetic K-rich phyllomanganate birnessite obtained by high-temperature decomposition of KMnO4: Substructures of K-rich birnessite from 1000 °C experiment. Microporous Mesoporous Mater. 2007, 98, 267–282. [Google Scholar] [CrossRef]
- Xia, G.G.; Tong, W.; Tolentino, E.N.; Duan, N.G.; Brock, S.L.; Wang, J.Y.; Suib, S.L.; Ressler, T. Synthesis and characterization of nanofibrous sodium manganese oxide with a 2 × 4 tunnel structure. Chem. Mater. 2001, 13, 1585–1592. [Google Scholar] [CrossRef]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory: Santa Fe, NM, USA, 2004; pp. 86–748.
- Toby, B. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Gražulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Le Bail, A. crystallography open database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quiros, M.; Le Bail, A. Crystallography open database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Mn4 and Mn8 are available from the authors. |
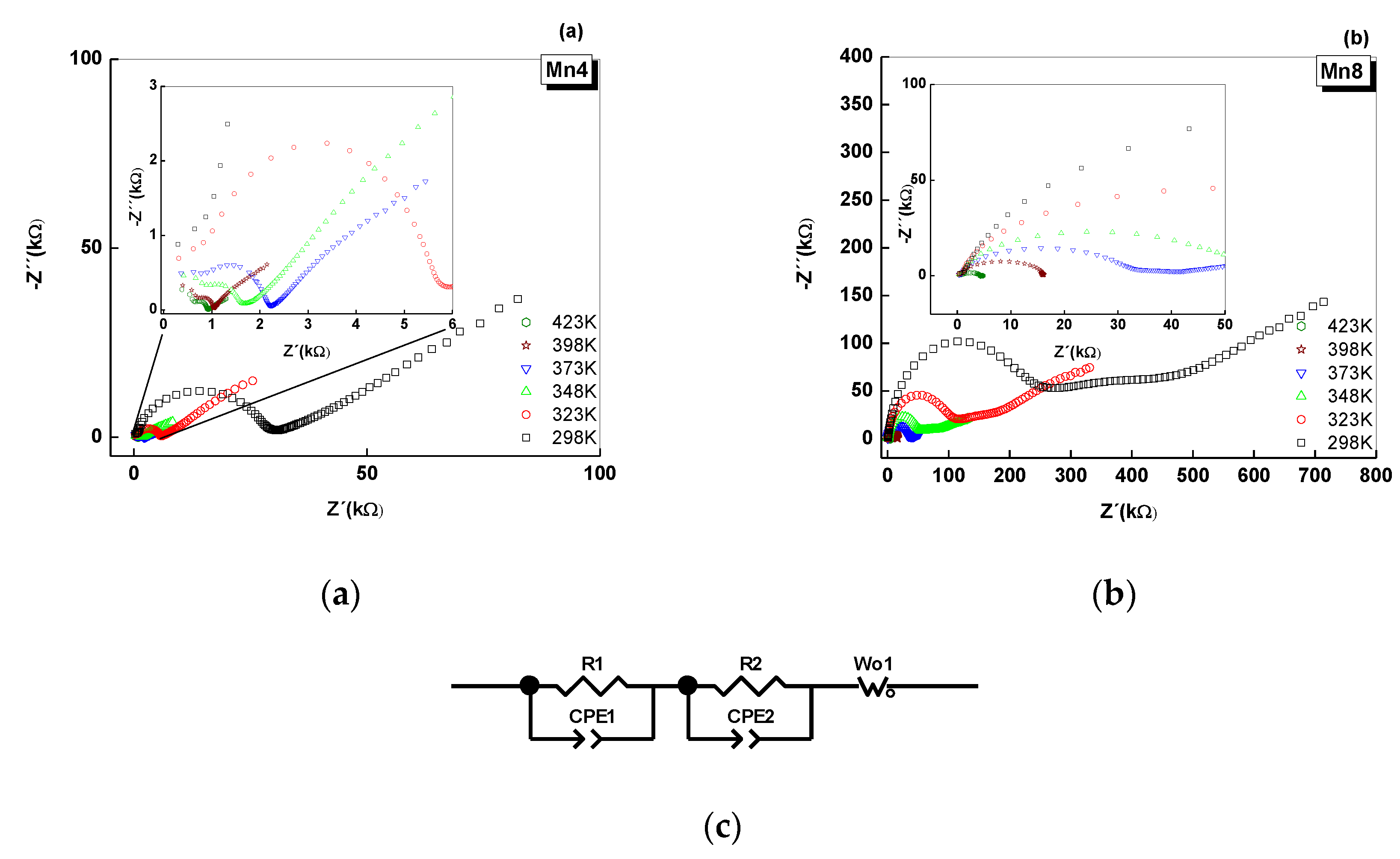
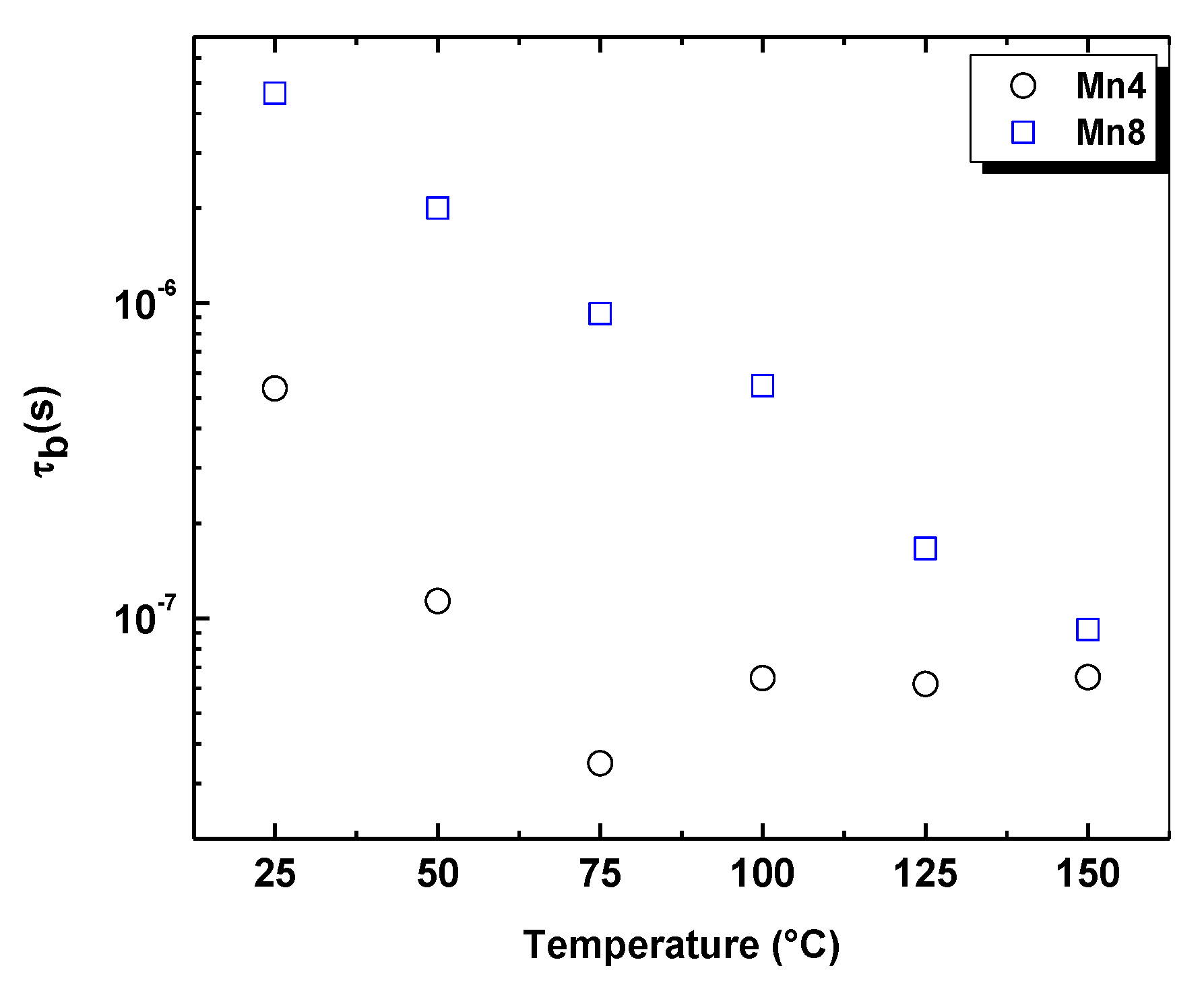
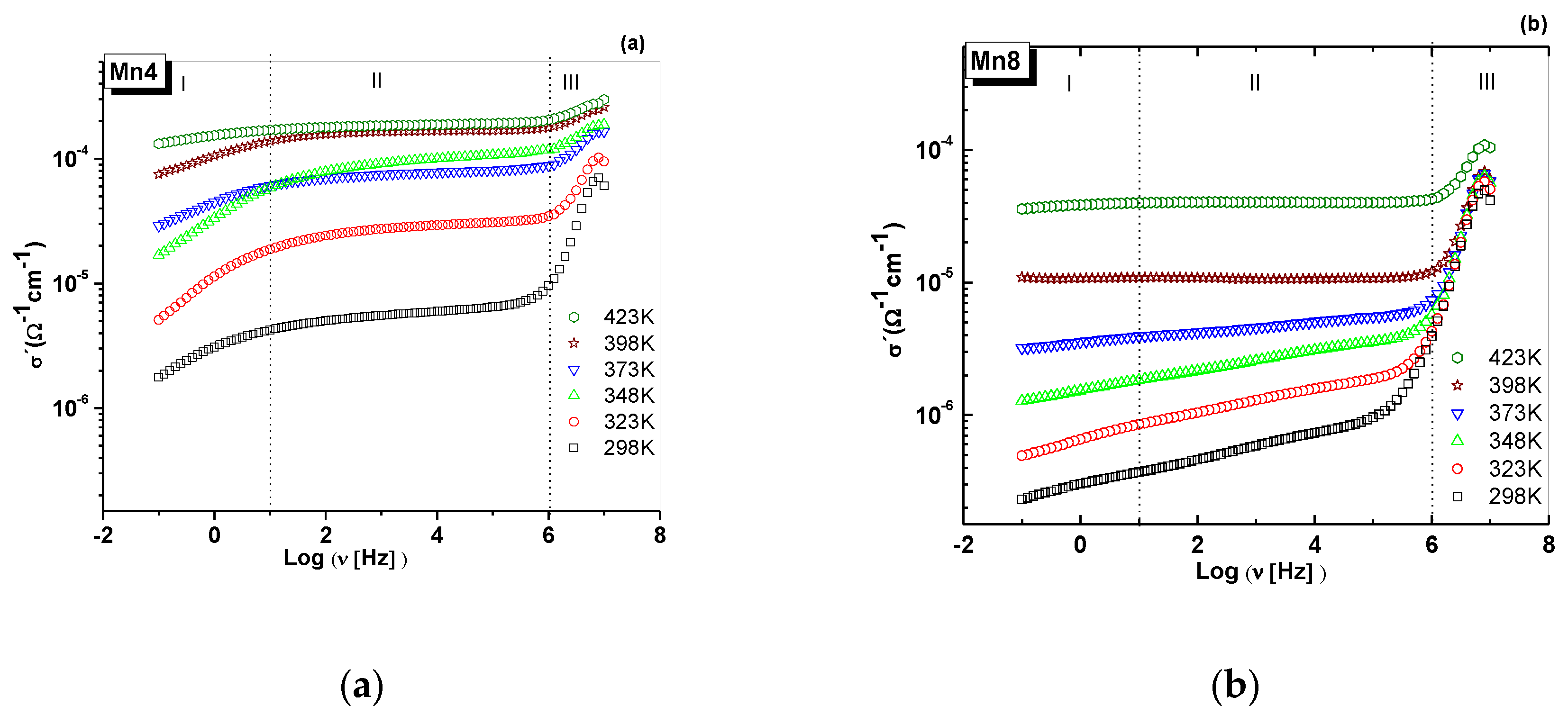
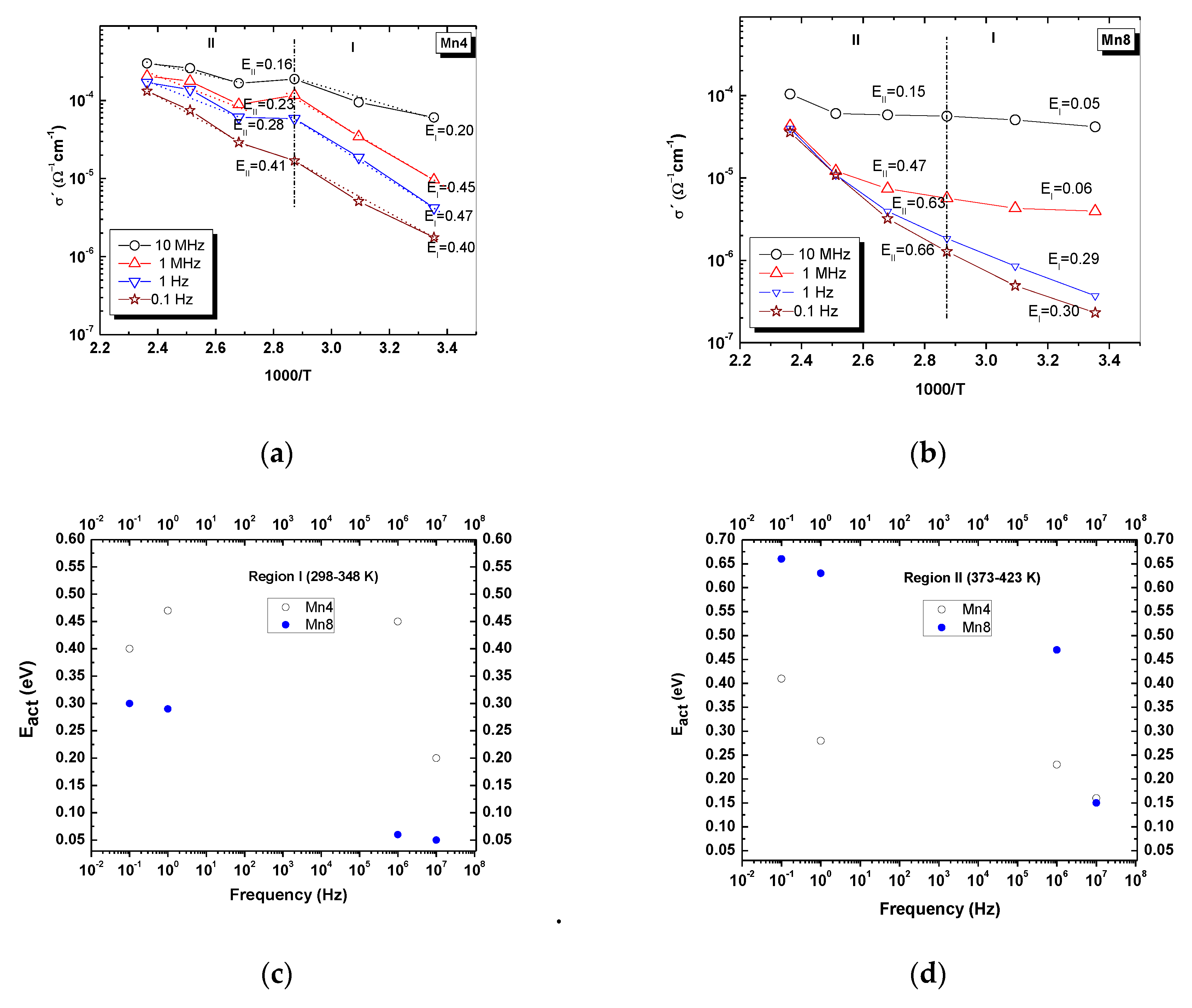
| Material | Structural Formula | K/Mn | Average Oxidation State (AOS) | d001 (Å) | Crystal Size * (nm) | Specific Surface Area (m2/g) | Bulk Conductivity ** (Ω−1cm−1) × 10−5 |
|---|---|---|---|---|---|---|---|
| Mn4 | 0.29 | 3.84 | 7.12 | 25 | 16.26 ± 0.17 | 2.106 | |
| Mn8 | 0.48 | 3.64 | 7.11 | 61 | 4.56 ± 0.04 | 2.135 |
| Sample Parameter | Mn4 | Mn8 | |
|---|---|---|---|
| Space group | P-1 | P63/mmc | |
| Data/Parameters | 3074/26 | 3249/26 | |
| Mn1 (Occ) | 1 | 1 | |
| K1 (Occ) | 0.29 | 0.09 | |
| O1 (Occ) | 1.02 | 0.95 | |
| O2 (Occ) | 0.27 | 0.56 | |
| Mn1(Uiso) | 0.005800 | 0.012370 | |
| K1 (Uiso) | 0.067300 | 0.094000 | |
| O1 (Uiso) | 0.031800 | 0.017300 | |
| O2 (Uiso) | 0.067300 | 0.080000 | |
| Lattice parameters | a (Å) | 2.91846 ± 0.005645 | 2.874708 ± 0.002132 |
| b (Å) | 2.93104 ± 0.005117 | 2.874708 ± 0.002132 | |
| c (Å) | 7.43240 ± 0.006679 | 14.168819 ± 0.000357 | |
| α (°) | 78.2025 ± 0.184 | 90 | |
| β (°) | 103.5718 ± 0.166 | 90 | |
| γ (°) | 121.7815 ± 0.093 | 120 | |
| Volume (Å3) | 52.284 ± 0.140 | 101.403 ± 0.123 | |
| Calculated Unit Cell Molecular Weight | 108.114 | 214.884 | |
| Crystallographic density (g/cm3) | 3.434 | 3.531 | |
| Perpendicular crystal size (nm) | 18 | 53 | |
| Parallel crystal size (nm) | 15 | 198 | |
| Aspect ratio | 0.83 | 3.74 | |
| χ2 | 0.9317 | 1.460 | |
| Rp | 0.0209 | 0.0224 | |
| Rwp | 0.0266 | 0.0293 | |
| Rf | 0.4121 | 0.0315 | |
| Rexp | 0.0276 | 0.0242 | |
| Phase | Conditions | Conductivity | Reference |
|---|---|---|---|
| MnO | Room temperature | 10−9 Ω−1cm−1 | [59] |
| birnessite | 298 K | 10−5to 10−6 Ω−1cm−1 | [60] |
| Todorokite | 298 K | 2.083 × 10−6 Ω−1cm−1 (4.8 × 105 Ωcm) | [60] |
| MnO2 | Room temperature | Resistivity 0.5 ohm-cm (2 Ω−1cm−1) | [61] |
| Mn2O3 | Room temperature | Resistivity 0.0028 ohm-cm | |
| Mn2O3 | Room temperature Under pressure 43 GPa | ~10−3 Ω−1m−1 | [62] |
| MnO2 | Room temperature | Resistivity 6.8 × 10−7 Ωm | [63] |
| B-MnO2 | Room temperature | 1.27 × 10−4 Ω−1cm−1 3.18 × 10−5 Ω−1cm−1 | [64] |
| Mn3O4 | 323 K | (3.68 ± 0.03) × 10−8 Ω−1m−1 | [65] |
| Mn3O4 | 423 K | (2.04 ± 0.001) × 10−5 Ω−1m−1 | [65] |
| MnO2 todorokite | 4.9 × 10−2 Ω−1cm−1 for nanowires | [66] | |
| MnO2 criptomelane | 10−3 to 10−4 Ω−1cm−1 | [15] | |
| MnO2 criptomelane | 10−6 Ω−1cm−1 | [52] | |
| K-birnessite | 10−6 Ω−1cm−1 | ||
| Na Birnessite | 298 K, 1 MHz | 8.39 × 10−6 Ω−1 cm−1 | [12] |
| K-birnessite | 298 K, 10 Hz | Mn4: 4.1250 × 10−6 Ω−1cm−1, Mn8: 3.7074 × 10−7 Ω−1cm−1 | This Work |
| 298 K, 1 MHz | Mn4: 6.555 × 10−5 Ω−1cm−1 Mn8: 3.944 × 10−6 Ω−1cm−1 | This Work | |
| 423 K, 10 Hz | Mn4: 1.6870 × 10−4 Ω−1cm−1 Mn8: 3.85 × 10−5 Ω−1cm−1 | This Work | |
| 423 K, 1 MHz | Mn4: 2.050 × 10−4 Ω−1cm−1 Mn8: 4.270 × 10−5 Ω−1cm−1 | This Work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, N.P.; Becerra, M.E.; Giraldo, O. Structural and Electrical Studies for Birnessite-Type Materials Synthesized by Solid-State Reactions. Nanomaterials 2019, 9, 1156. https://doi.org/10.3390/nano9081156
Arias NP, Becerra ME, Giraldo O. Structural and Electrical Studies for Birnessite-Type Materials Synthesized by Solid-State Reactions. Nanomaterials. 2019; 9(8):1156. https://doi.org/10.3390/nano9081156
Chicago/Turabian StyleArias, Nayda P., María E. Becerra, and Oscar Giraldo. 2019. "Structural and Electrical Studies for Birnessite-Type Materials Synthesized by Solid-State Reactions" Nanomaterials 9, no. 8: 1156. https://doi.org/10.3390/nano9081156
APA StyleArias, N. P., Becerra, M. E., & Giraldo, O. (2019). Structural and Electrical Studies for Birnessite-Type Materials Synthesized by Solid-State Reactions. Nanomaterials, 9(8), 1156. https://doi.org/10.3390/nano9081156





