The Effects of Metal Complexes of Nano-Graphene Oxide to Thermal Decomposition of FOX-7
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Preparation of nGO-Metal Complexes
2.3. Preparation of nGO-Metal-FOX-7 Composites
3. Results and Discussion
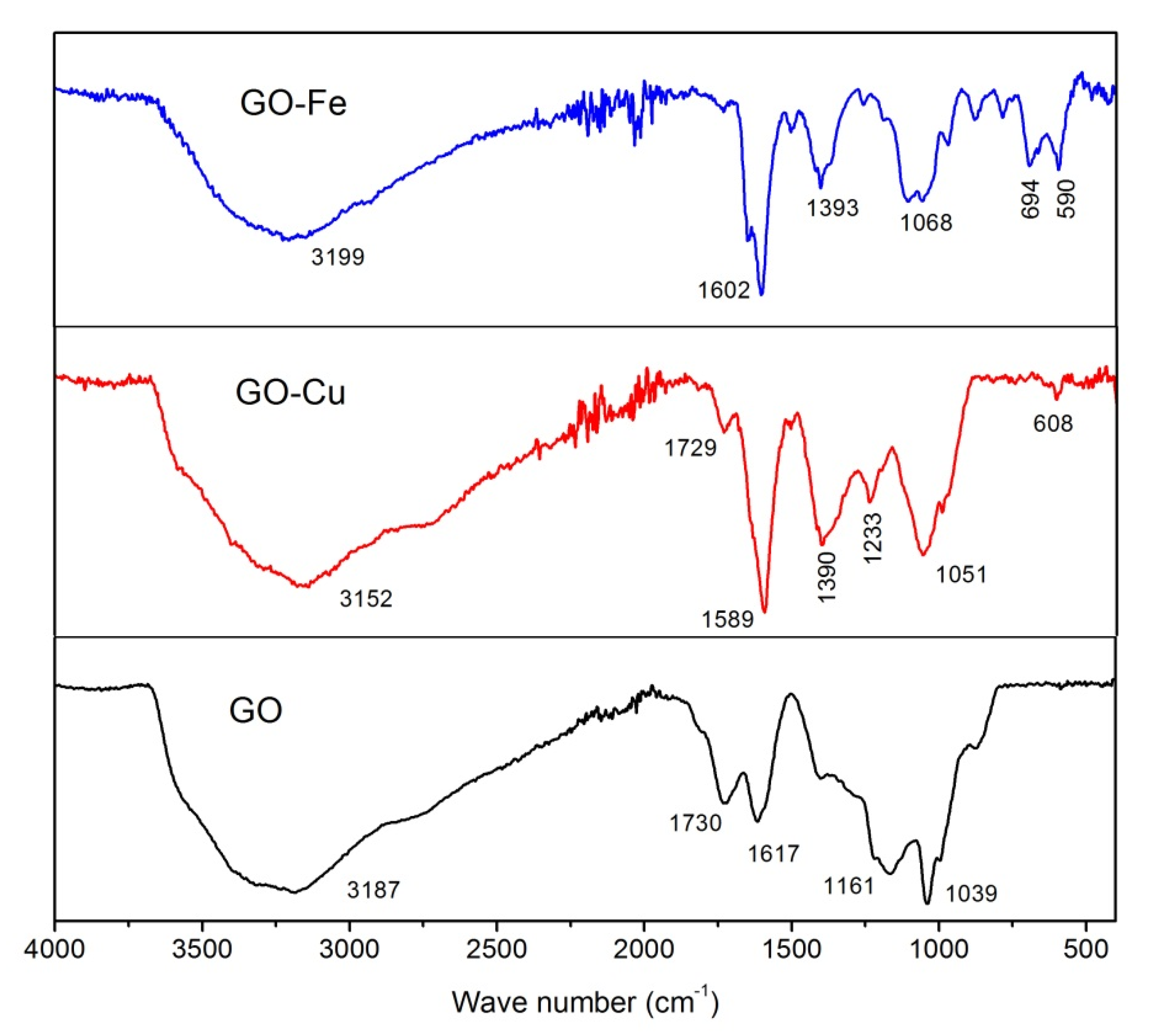
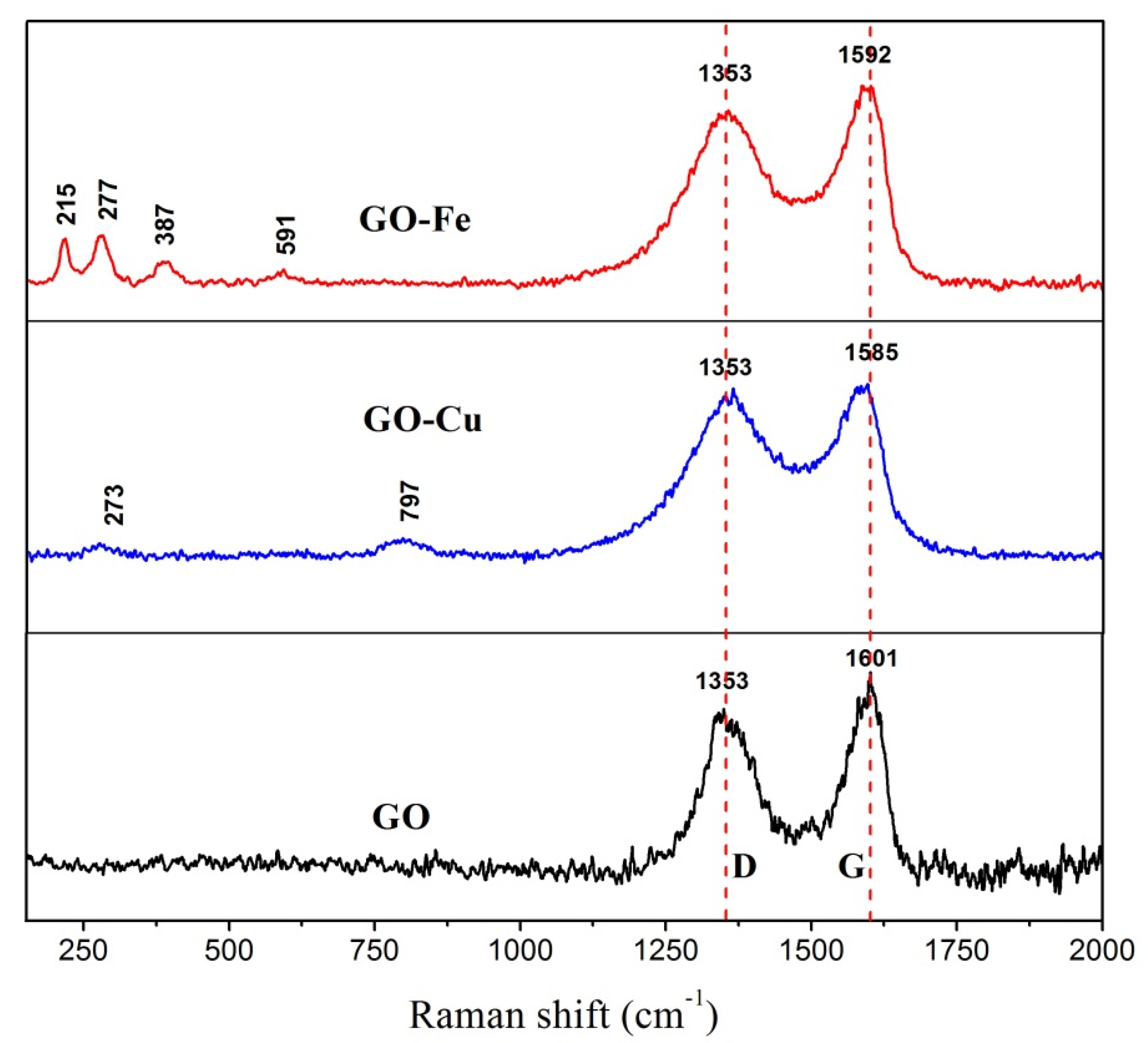
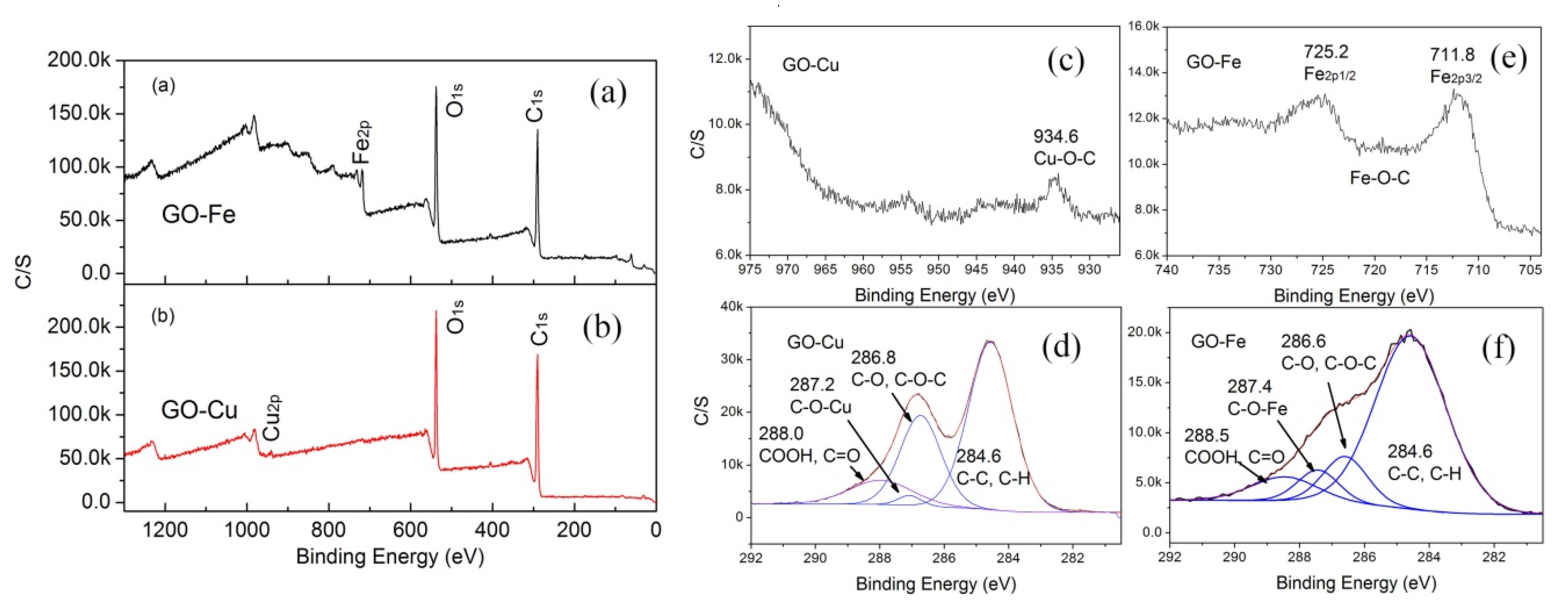
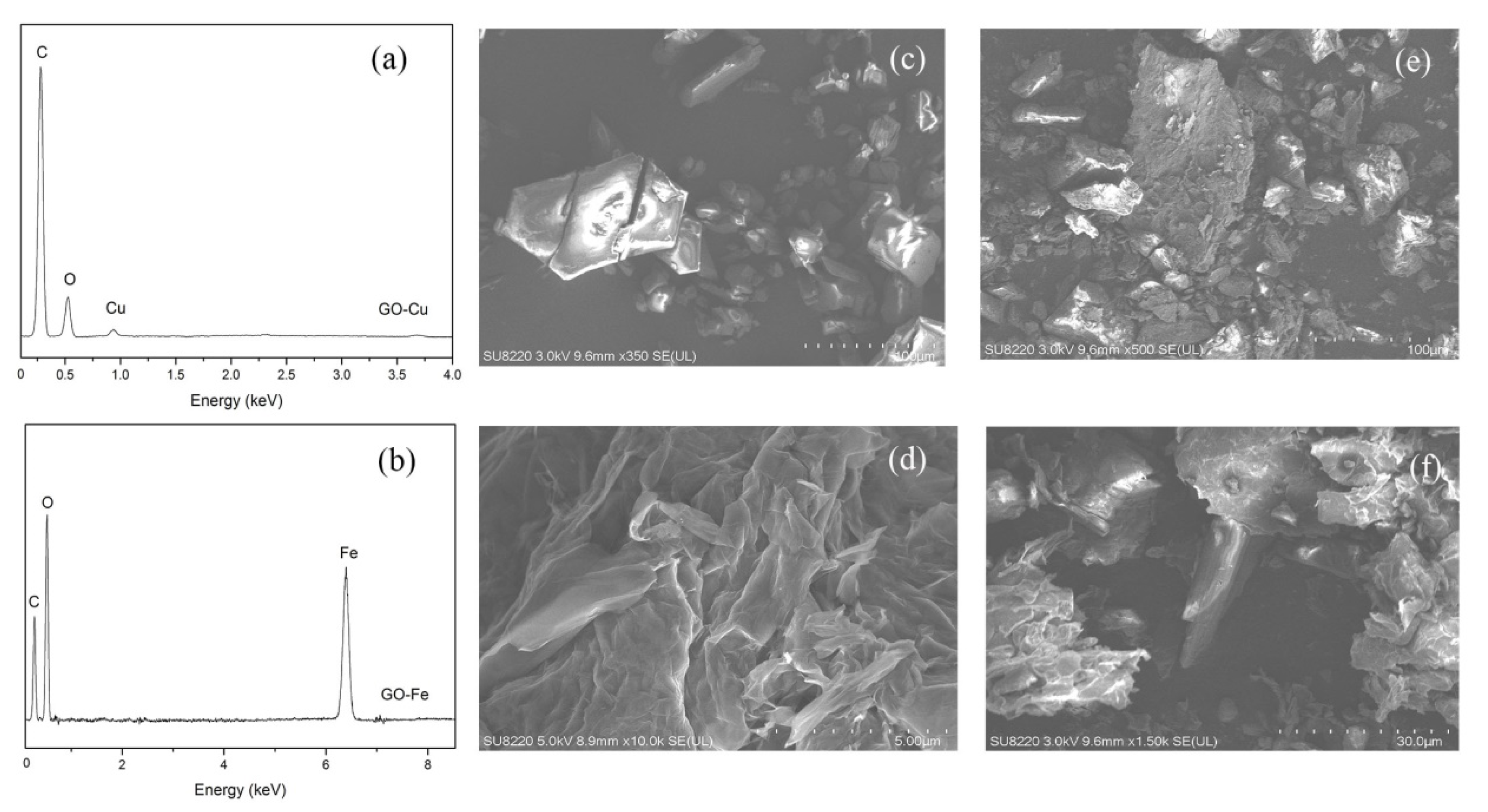
3.1. Chemical Bonding and Morphology
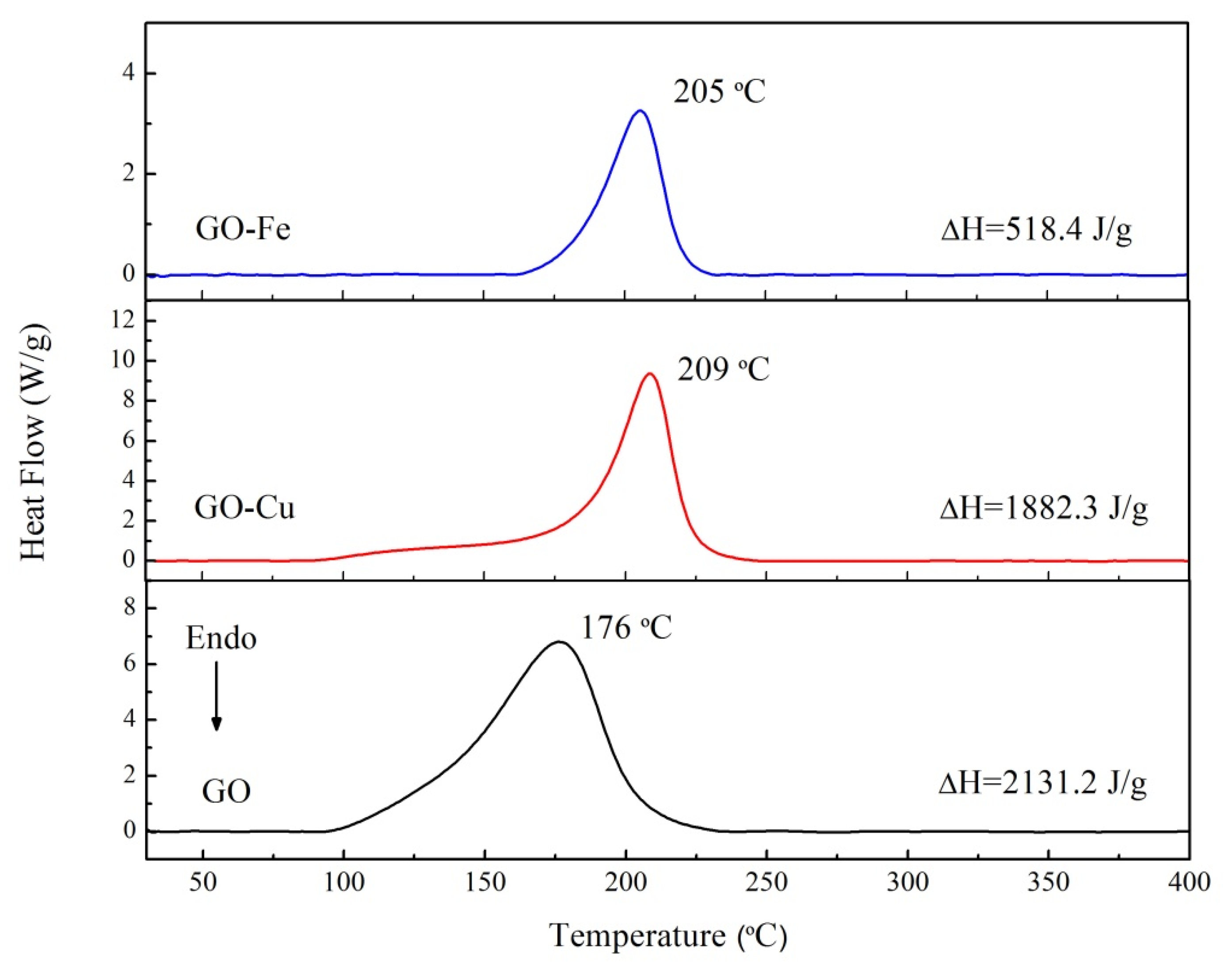
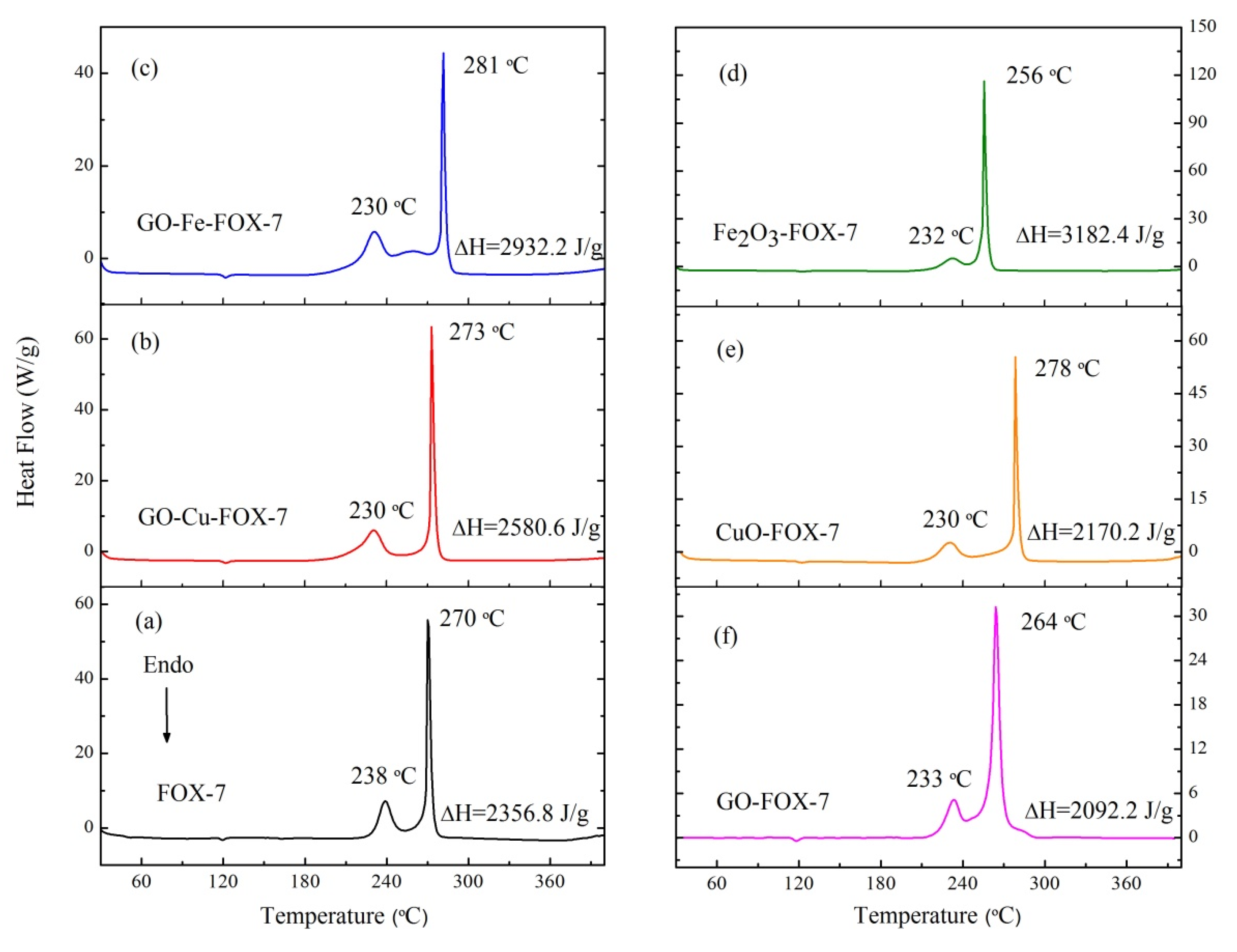
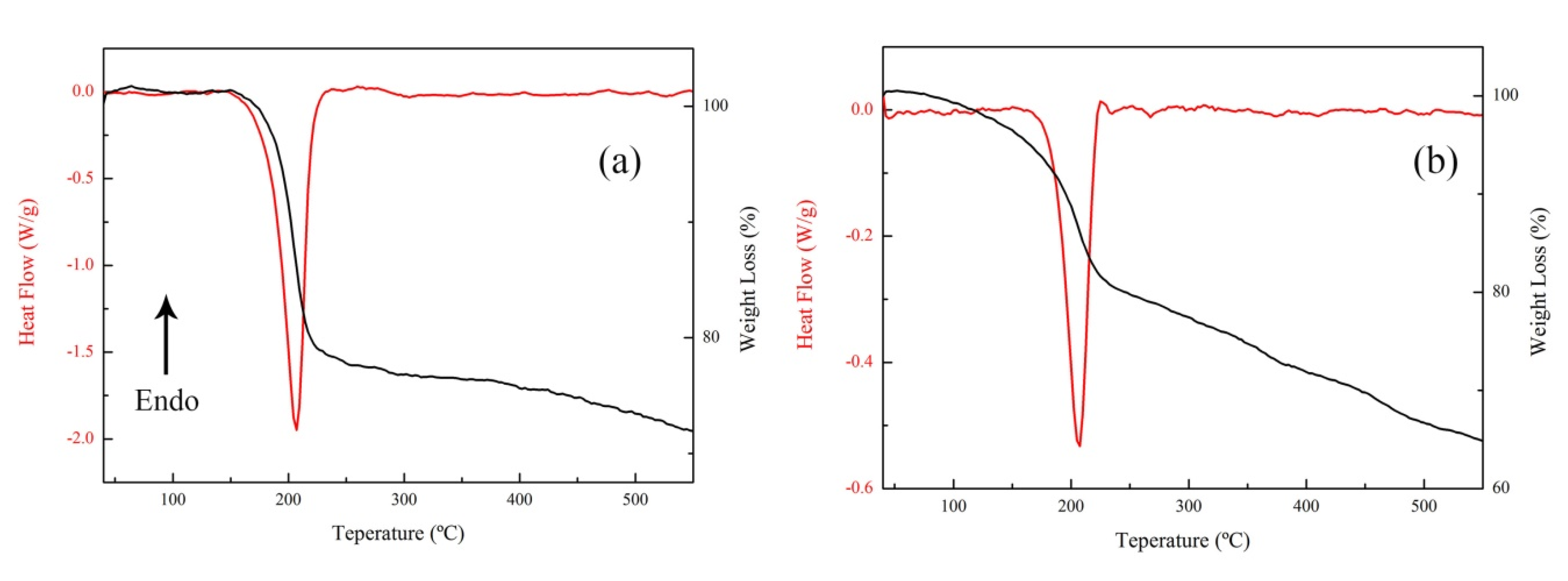
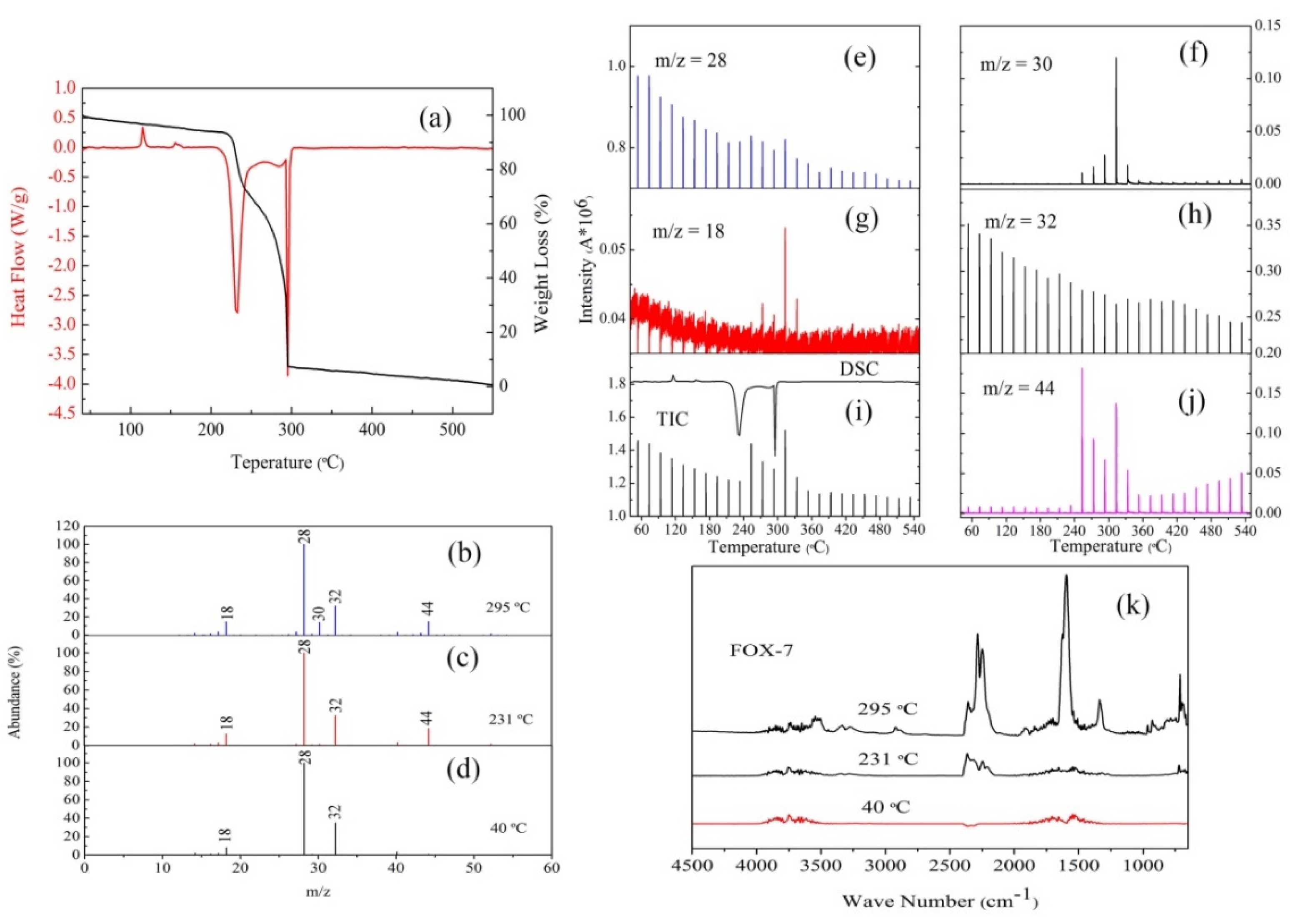
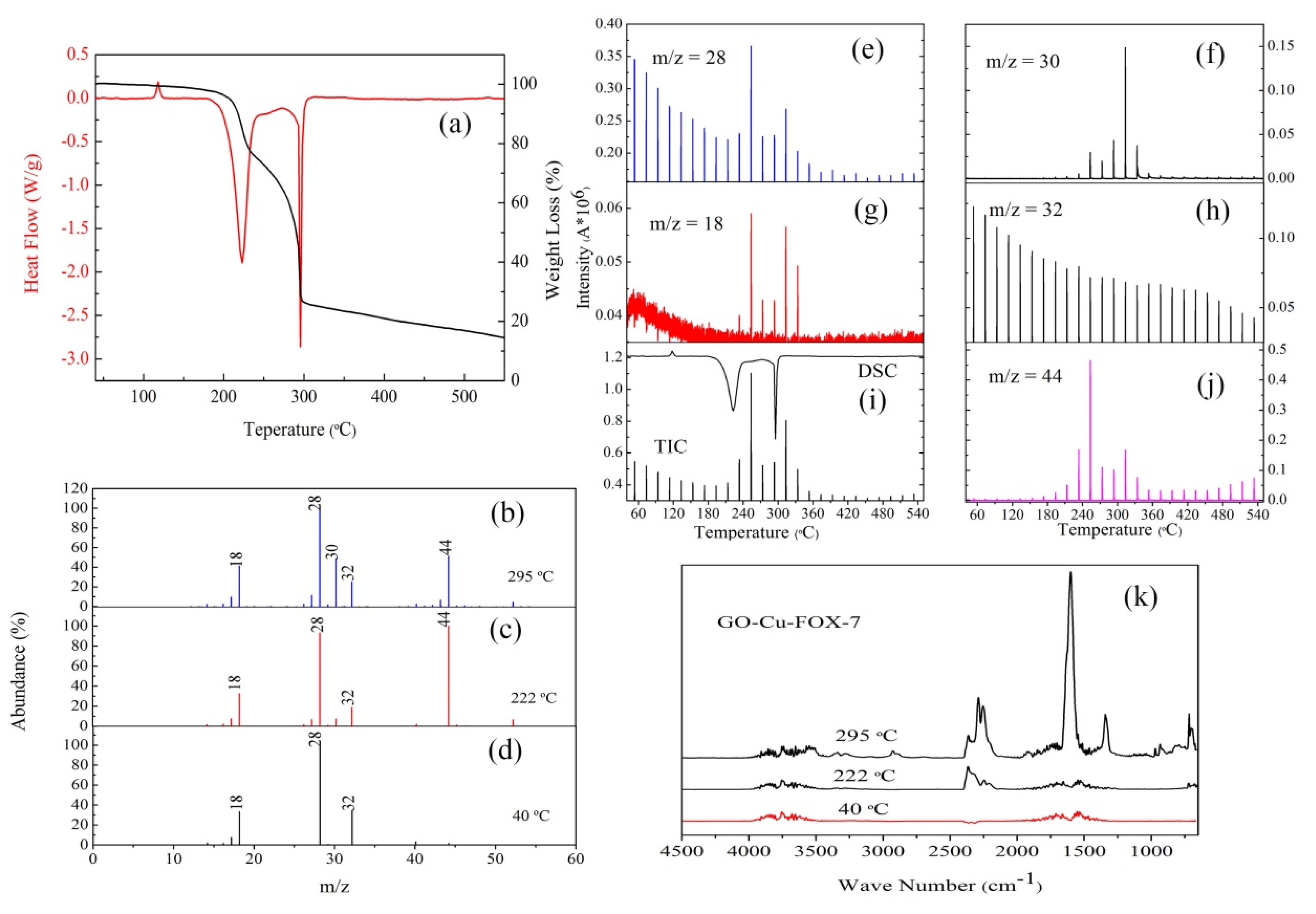
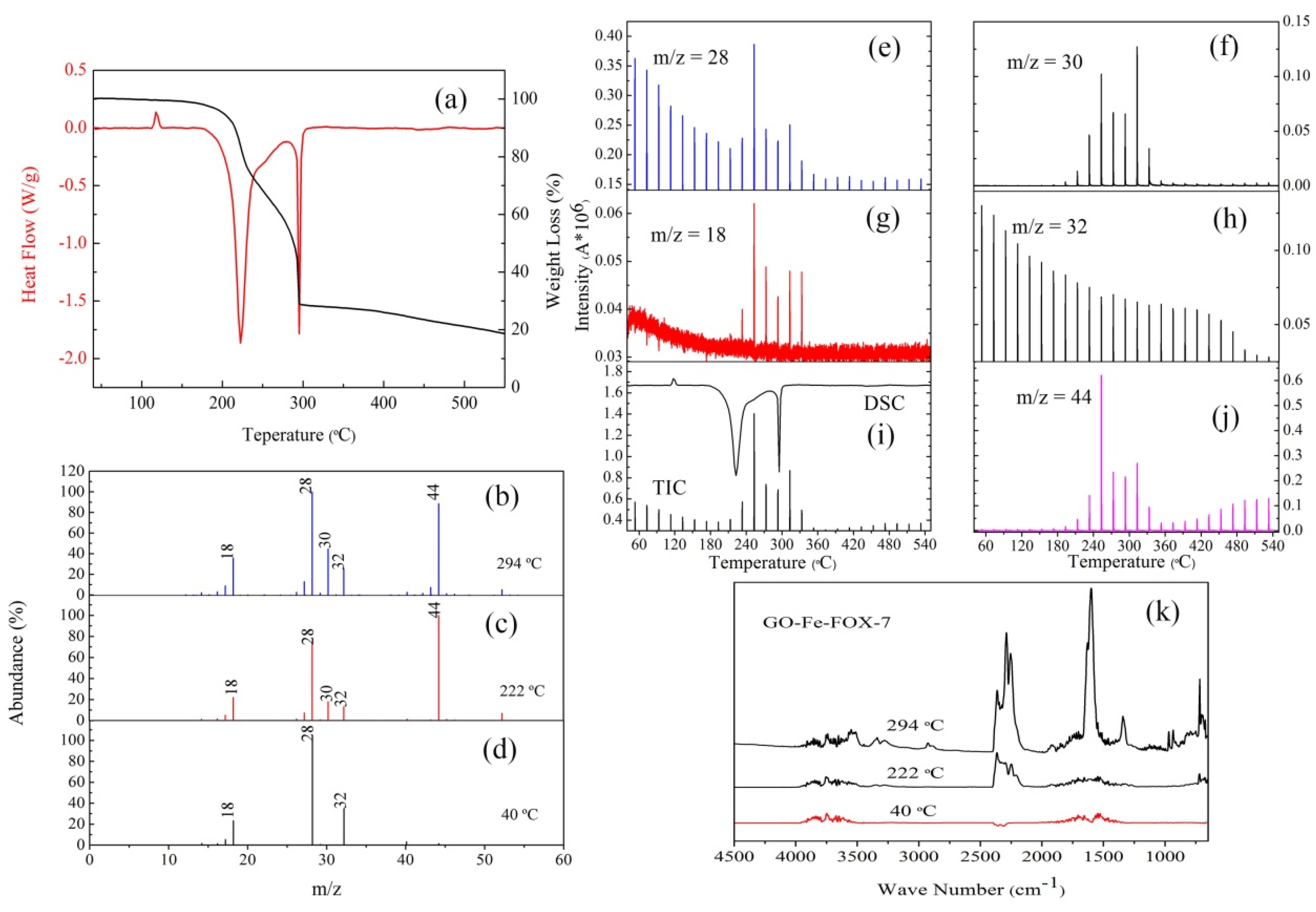
3.2. The Thermal Decomposition Behavior
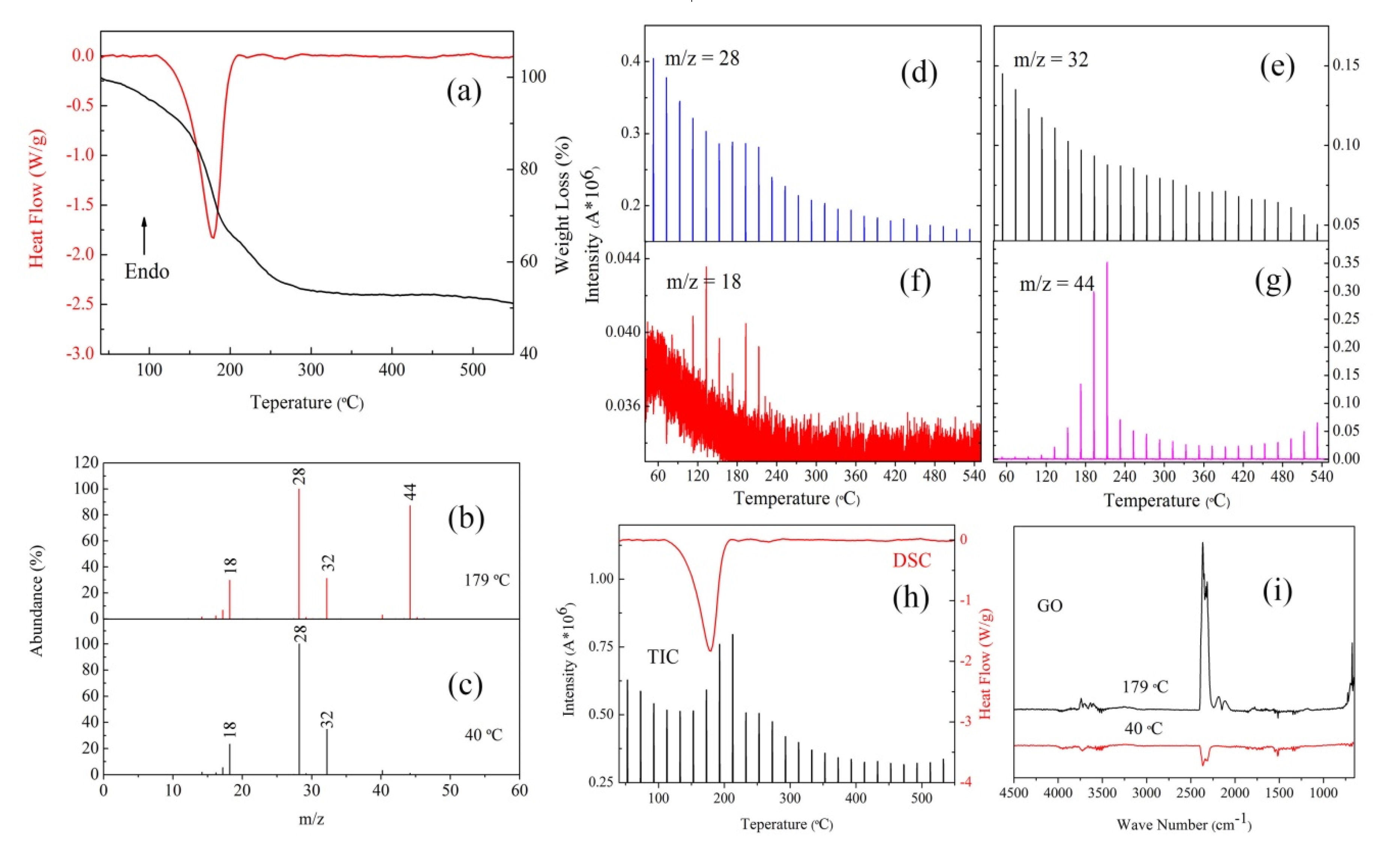
3.3. The Thermal Decomposition Process
4. Conclusions
- (1)
- nGO is used as a ligand to coordinated with Cu2+ and Fe3+. The C-O-Cu (Fe) were confirmed to exist in the metal (Cu2+ and Fe3+) complexes of nGO by IR and Raman spectroscopy and XPS and EDS techniques, indicating nGO successfully coordinated with Cu2+ and Fe3+.
- (2)
- It can be seen from the SEM images that the nGO-metal complexes is uniformly mixed with FOX-7. After mixing with nGO-metal complexes, the thermal decomposition temperature of FOX-7 is decreased, and the heat release is increased, especially after the addition of Fe3+. This indicates that nGO-metal complexes affect obviously on the decomposition process of FOX-7.
- (3)
- After complexing with metal ions, the thermal decomposition temperature of nGO was delayed by 30 °C, but the type and proportion of decomposition products did not change. FOX-7 produces N2O, NO and a small amount of H2O during the decomposition process. After mixing with nGO-metal complexes, the formation of nitrogen during the decomposition of FOX-7 has been increased, indicating the complete decomposition of that.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Davenas, A. Development of modern solid propellants. J. Propuls. Power 2003, 19, 1108–1128. [Google Scholar] [CrossRef]
- Luman, J.; Wehrman, B.; Kuo, K.; Yetter, R.; Masoud, N.; Manning, T.; Harris, L.; Bruck, H. Development and characterization of high performance solid propellants containing nano-sized energetic ingredients. Proc. Combust. Inst. 2007, 31, 2089–2096. [Google Scholar] [CrossRef]
- Ou, Y.; Chen, B.; Dong, S.; Jia, H.; Li, J.; Hong, Y. Development of energetic additives for propellants in China. J. Propuls. Power 2012, 11, 838–847. [Google Scholar] [CrossRef]
- Xin, W. Development and Application of Green Propellants and Explosives and Related Technologies. Chin. J. Explos. Propellants 2006, 5, 67–71. [Google Scholar]
- Wang, Q.-L.; Liu, S.-W.; Tang, H.-M. Study on the Clear-burning Gun Propellant. Chin. J. Explos. Propellants 2003, 26, 5–7. [Google Scholar]
- Xin, W. Current situation of study on insensitive composite explosives in USA. Chin. J. Explos. Propellants 2007, 2, 78–80. [Google Scholar]
- Pagoria, P.F.; Lee, G.S.; Mitchell, A.R.; Schmidt, R.D. A review of energetic materials synthesis. Thermochim. Acta 2002, 384, 187–204. [Google Scholar] [CrossRef]
- Fried, L.E.; Manaa, M.R.; Pagoria, P.F.; Simpson, R.L. Design and synthesis of energetic materials. Annu. Rev. Mater. Res. 2001, 31, 291–321. [Google Scholar] [CrossRef]
- Zhang, G.T.; Zhou, Z.N.; Zhang, T.L.; Yang, L.; Gao, H.X. Advances on energetic catalysts for solid propellant. J. Solid Rocket Technol. 2011, 34, 319–323. [Google Scholar]
- Meda, L.; Marra, G.; Galfetti, L.; Inchingalo, S.; Severini, F.; De Luca, L. Nano-composites for rocket solid propellants. Compos. Sci. Technol. 2005, 65, 769–773. [Google Scholar] [CrossRef]
- Lempert, D.B.; Manelis, G.B.; Nechiporenko, G.N. The ways for development of environmentally safe solid composite propellants. Prog. Propuls. Phys. 2011, 1, 63–80. [Google Scholar]
- Nanda, J.K.; Ramakrishna, P.A. Development of AP/HTPB based Fuel-rich Propellant for Solid Propellant Ramjet. In Proceedings of the AIAA/ASME/SAE/ASEE Joint Propulsion Conference, San Jose, CA, USA, 14–17 July 2013. [Google Scholar]
- Stephens, M.A.; Petersen, E.L.; Carro, R.; Reid, D.L.; Seal, S. Multi-parameter study of nanoscale TiO2 and CeO2 additives in composite AP/HTPB solid propellants. Propellants Explos. Pyrotech. 2010, 35, 143–152. [Google Scholar] [CrossRef]
- Risha, G.; Evans, B.; Boyer, E.; Kuo, K. Metals, energetic additives, and special binders used in solid fuels for hybrid rockets. Prog. Astronaut. Aeronaut. 2007, 218, 413. [Google Scholar]
- Armstrong, R.; Baschung, B.; Booth, D.; Samirant, M. Enhanced propellant combustion with nanoparticles. Nano Lett. 2003, 3, 253–255. [Google Scholar] [CrossRef]
- Pang, W.; Decula, L.T.; Fan, X.; Maggi, F.; Xu, H.; Xie, W.; Shi, X. Effects of Different Nano-Sized Metal Oxide Catalysts on the Properties of Composite Solid Propellants. Combust. Sci. Technol. 2016, 188, 315–328. [Google Scholar] [CrossRef]
- Isert, S.; Groven, L.J.; Lucht, R.P.; Son, S.F. The effect of encapsulated nanosized catalysts on the combustion of composite solid propellants. Combust. Flame 2015, 162, 1821–1828. [Google Scholar] [CrossRef]
- Galfetti, L.; DeLuca, L.; Severini, F.; Colombo, G.; Meda, L.; Marra, G. Pre and post-burning analysis of nano-aluminized solid rocket propellants. Aerosp. Sci. Technol. 2007, 11, 26–32. [Google Scholar] [CrossRef]
- Yetter, R.A.; Risha, G.A.; Son, S.F. Metal particle combustion and nanotechnology. Proc. Combust. Inst. 2009, 32, 1819–1838. [Google Scholar] [CrossRef]
- Lempert, D.B.; Nechiporenko, G.N.; Manelis, G.B. Energetic characteristics of solid composite propellants and ways for energy increasing. Cent. Eur. J. Energ. Mater. 2006, 3, 73–87. [Google Scholar]
- Zhao, N.; He, C.; Liu, J.; Gong, H.; An, T.; Xu, H.; Zhao, F.; Hu, R.; Ma, H.; Zhang, J. Dependence of catalytic properties of Al/Fe2O3 thermites on morphology of Fe2O3 particles in combustion reactions. J. Solid State Chem. 2014, 219, 67–73. [Google Scholar] [CrossRef]
- Alizadeh-Gheshlaghi, E.; Shaabani, B.; Khodayari, A.; Azizian-Kalandaragh, Y.; Rahimi, R. Investigation of the catalytic activity of nano-sized CuO, Co3O4 and CuCo2O4 powders on thermal decomposition of ammonium perchlorate. Powder Technol. 2012, 217, 330–339. [Google Scholar] [CrossRef]
- DeLuca, L.; Galfetti, L.; Colombo, G.; Maggi, F.; Bandera, A.; Babuk, V.; Sinditskii, V. Microstructure effects in aluminized solid rocket propellants. J. Propuls. Power 2010, 26, 724–732. [Google Scholar] [CrossRef]
- DeLuca, L.T.; Marchesi, E.; Spreafico, M.; Reina, A.; Maggi, F.; Rossettini, L.; Bandera, A.; Colombo, L.P.M.; Kosowski, B.M. Aggregation versus agglomeration in metallized solid rocket propellants. Int. J. Energ. Mater. Chem. Propuls. 2010, 9, 91–105. [Google Scholar] [CrossRef]
- Sippel, T.R.; Son, S.F.; Groven, L.J. Aluminum agglomeration reduction in a composite propellant using tailored Al/PTFE particles. Combust. Flame 2014, 161, 311–321. [Google Scholar] [CrossRef]
- Yu, L.; Ren, H.; Guo, X.Y.; Jiang, X.B.; Jiao, Q.J. A novel ε-HNIW-based insensitive high explosive incorporated with reduced graphene oxide. J. Therm. Anal. Calorim. 2014, 117, 1187–1199. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Shen, J.P.; Hua, C.; Yang, G.C. Preparation and Characterization of Insensitive HMX/Graphene Oxide Composites. Propellants Explos. Pyrotech. 2013, 38, 798–804. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, X.; Xiang, B. Sandwich Complex of TATB/Graphene: An Approach to Molecular Monolayers of Explosives. J. Phys. Chem. C 2010, 114, 22684–22687. [Google Scholar] [CrossRef]
- Sabourin, J.L.; Dabbs, D.M.; Yetter, R.A.; Dryer, F.L.; Aksay, I.A. Functionalized graphene sheet colloids for enhanced fuel/propellant combustion. ACS Nano 2009, 3, 3945–3954. [Google Scholar] [CrossRef]
- Memon, N.K.; McBain, A.W.; Son, S.F. Graphene oxide/ammonium perchlorate composite material for use in solid propellants. J. Propuls. Power 2016, 32, 682–686. [Google Scholar] [CrossRef]
- He, W.; Guo, J.-H.; Cao, C.-K.; Liu, X.-K.; Lv, J.-Y.; Chen, S.-W.; Liu, P.-J.; Yan, Q.-L. Catalytic reactivity of graphene oxide stabilized transition metal complexes of triaminoguanidine on thermolysis of RDX. J. Phys. Chem. C 2018, 122, 14714–14724. [Google Scholar] [CrossRef]
- Feng, W.C.; Xu-Ran, X.U.; Yang, Y.; Zheng, W.F.; Lin, X.Y. Research Process on Preparation and Application in Propellant of Graphene. J. Ordnance Equip. Eng. 2016, 37, 89–94. [Google Scholar]
- Lan, Y.; Jin, M.; Luo, Y. Preparation and characterization of graphene aerogel/Fe2O3/ammonium perchlorate nanostructured energetic composite. J. Sol-Gel Sci. Technol. 2015, 74, 161–167. [Google Scholar] [CrossRef]
- Zu, Y.; Zhao, Y.; Xu, K.; Tong, Y.; Zhao, F. Preparation and comparison of catalytic performance for nano MgFe2O4, GO-loaded MgFe2O4 and GO-coated MgFe2O4 nanocomposites. Ceram. Int. 2016, 42, 18844–18850. [Google Scholar] [CrossRef]
- Li, N.; Geng, Z.; Cao, M.; Ren, L.; Zhao, X.; Liu, B.; Tian, Y.; Hu, C. Well-dispersed ultrafine Mn3O4 nanoparticles on graphene as a promising catalyst for the thermal decomposition of ammonium perchlorate. Carbon 2013, 54, 124–132. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, W.; Wang, Y.; Shen, P.; Li, F.; Li, P.; Zhao, F.; Gao, H. Hydrothermal preparation of Fe2O3/graphene nanocomposite and its enhanced catalytic activity on the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2014, 303, 354–359. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, L.; Xu, K.; Song, J.; Zhao, F. Graphene oxide-enveloped Bi2WO6 composites as a highly efficient catalyst for the thermal decomposition of cyclotrimethylenetrinitramine. RSC Adv. 2016, 6, 42428–42434. [Google Scholar] [CrossRef]
- Lin, C.; He, G.; Liu, J.; Pan, L.; Liu, S. Enhanced Non-linear Viscoelastic Properties of Polymer Bonded Explosives Based on Graphene and a Neutral Polymeric Bonding Agent. Cent. Eur. J. Energ. Mater. 2017, 14, 788–805. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef]
- Kholmanov, I.N.; Domingues, S.H.; Chou, H.; Wang, X.; Tan, C.; Kim, J.-Y.; Li, H.; Piner, R.; Zarbin, A.J.; Ruoff, R.S. Reduced graphene oxide/copper nanowire hybrid films as high-performance transparent electrodes. ACS Nano 2013, 7, 1811–1816. [Google Scholar] [CrossRef]
- Na, L.; Cao, M.; Wu, Q.; Hu, C. A facile one-step method to produce Ni/graphene nanocomposites and their application to the thermal decomposition of ammonium perchlorate. Crystengcomm 2011, 14, 428–434. [Google Scholar]
- Anniyappan, M.; Talawar, M.; Gore, G.; Venugopalan, S.; Gandhe, B. Synthesis, characterization and thermolysis of 1,1-diamino-2,2-dinitroethylene (FOX-7) and its salts. J. Hazard. Mater. 2006, 137, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, H. High-pressure behavior of crystalline FOX-7 by density functional theory calculations. Comput. Mater. Sci. 2008, 42, 698–703. [Google Scholar] [CrossRef]
- Florczak, B. Investigation of an aluminized binder/AP composite propellant containing FOX-7. Cent. Eur. J. Energ. Mater. 2008, 5, 65–75. [Google Scholar]
- Vo, T.T.; Zhang, J.; Parrish, D.A.; Twamley, B.; Shreeve, J.N.M. New roles for 1,1-diamino-2,2-dinitroethene (FOX-7): Halogenated FOX-7 and azo-bis (diahaloFOX) as energetic materials and oxidizers. J. Am. Chem. Soc. 2013, 135, 11787–11790. [Google Scholar] [CrossRef]
- Bellamy, A.J. FOX-7 (1,1-Diamino-2,2-dinitroethene). In High Energy Density Material; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–33. [Google Scholar]
- Trzciński, W.A.; Cudziło, S.; Chyłek, Z.; Szymańczyk, L. Detonation Properties and Thermal Behavior of FOX-7-Based Explosives. J. Energ. Mater. 2013, 31, 72–85. [Google Scholar] [CrossRef]
- Haixiang, G.; Shreeve, J.N.M. The Many Faces of FOX-7: A Precursor to High-Performance Energetic Materials. Angew. Chem. 2015, 127, 6433–6436. [Google Scholar]
- Zhang, Y.; Sun, Q.; Xu, K.; Song, J.; Zhao, F. Review on the Reactivity of 1,1-Diamino-2,2-dinitroethylene (FOX-7). Propellants Explos. Pyrotech. 2016, 41, 35–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, T.; Xu, K.; Ren, Z.; Xiao, L.; Song, J.; Zhao, F. Catalytic decomposition action of hollow CuFe2O4 nanospheres on RDX and FOX-7. RSC Adv. 2015, 5, 75630–75635. [Google Scholar] [CrossRef]
- Chaiyakun, S.; Witit-Anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Mohan, R.; Kim, S.-J. Investigation of Raman and photoluminescence studies of reduced graphene oxide sheets. Appl. Phys. A 2012, 106, 501–506. [Google Scholar] [CrossRef]
- Kang, D.; Kwon, J.Y.; Cho, H.; Sim, J.-H.; Hwang, H.S.; Kim, C.S.; Kim, Y.J.; Ruoff, R.S.; Shin, H.S. Oxidation resistance of iron and copper foils coated with reduced graphene oxide multilayers. ACS Nano 2012, 6, 7763–7769. [Google Scholar] [CrossRef] [PubMed]
- Niaura, G. Surface-enhanced Raman spectroscopic observation of two kinds of adsorbed OH-ions at copper electrode. Electrochim. Acta 2000, 45, 3507–3519. [Google Scholar] [CrossRef]
- Chou, M.H.; Liu, S.B.; Huang, C.Y.; Wu, S.Y.; Cheng, C.-L. Confocal Raman spectroscopic mapping studies on a single CuO nanowire. Appl. Surf. Sci. 2008, 254, 7539–7543. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, X.; Li, J.; Fan, X.; Zhang, G. Desensitizing Effect of Graphene Oxide on Thermolysis Mechanisms of 4,4′-Azo-1,2,4-triazole Studied by Reactive Molecular Dynamics Simulations. J. Phys. Chem. A 2019, 123, 1285–1294. [Google Scholar] [CrossRef] [PubMed]

| Sample | Apparent Decomposition Heat/J·g−1 | |
|---|---|---|
| Test Value | Standardized Value | |
| nGO | 2132.2 | 2132.2 |
| nGO-Cu | 1882.3 | 1882.3 |
| nGO-Fe | 518.4 | 518.4 |
| FOX-7 | 2356.8 | 2356.8 |
| nGO-FOX-7 | 2092.2 | 2082.4 |
| nGO-Cu-FOX-7 | 2580.6 | 2755.2 |
| nGO-Fe-FOX-7 | 2932.2 | 3535.6 |
| CuO-FOX-7 | 2170.2 | 2712.7 |
| Fe2O3-FOX-7 | 3182.4 | 3978.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Fu, X.; Zhang, X.; Li, J.; Fan, X.; Zhang, G. The Effects of Metal Complexes of Nano-Graphene Oxide to Thermal Decomposition of FOX-7. Nanomaterials 2020, 10, 144. https://doi.org/10.3390/nano10010144
Zhang C, Fu X, Zhang X, Li J, Fan X, Zhang G. The Effects of Metal Complexes of Nano-Graphene Oxide to Thermal Decomposition of FOX-7. Nanomaterials. 2020; 10(1):144. https://doi.org/10.3390/nano10010144
Chicago/Turabian StyleZhang, Chongmin, Xiaolong Fu, Xuexue Zhang, Jizhen Li, Xuezhong Fan, and Guofang Zhang. 2020. "The Effects of Metal Complexes of Nano-Graphene Oxide to Thermal Decomposition of FOX-7" Nanomaterials 10, no. 1: 144. https://doi.org/10.3390/nano10010144
APA StyleZhang, C., Fu, X., Zhang, X., Li, J., Fan, X., & Zhang, G. (2020). The Effects of Metal Complexes of Nano-Graphene Oxide to Thermal Decomposition of FOX-7. Nanomaterials, 10(1), 144. https://doi.org/10.3390/nano10010144






