Convenient Real-Time Monitoring of the Contamination of Surface Ion Trap
Abstract
1. Introduction
2. Experiment and Simulation of Trap Surface Adatoms
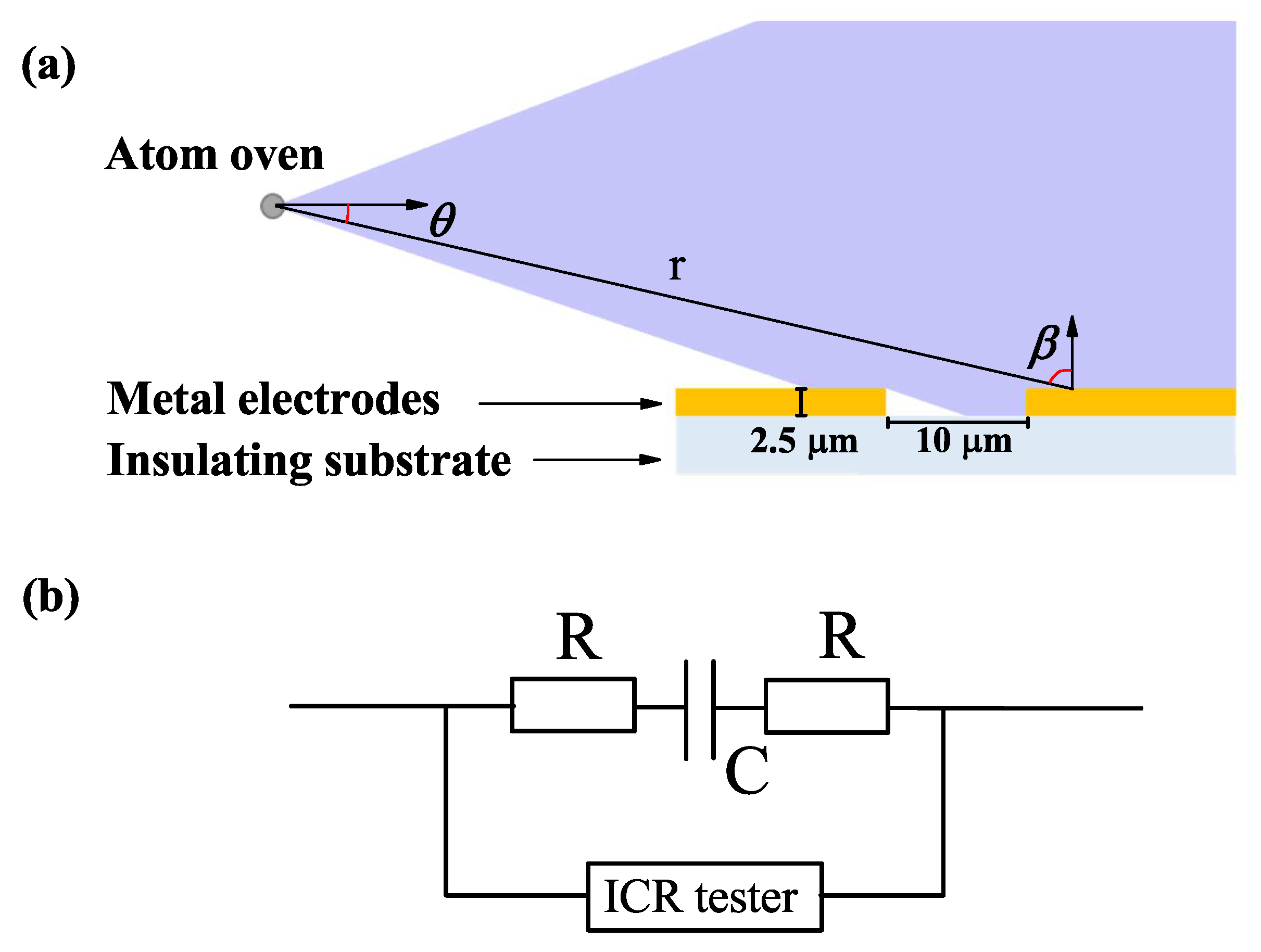
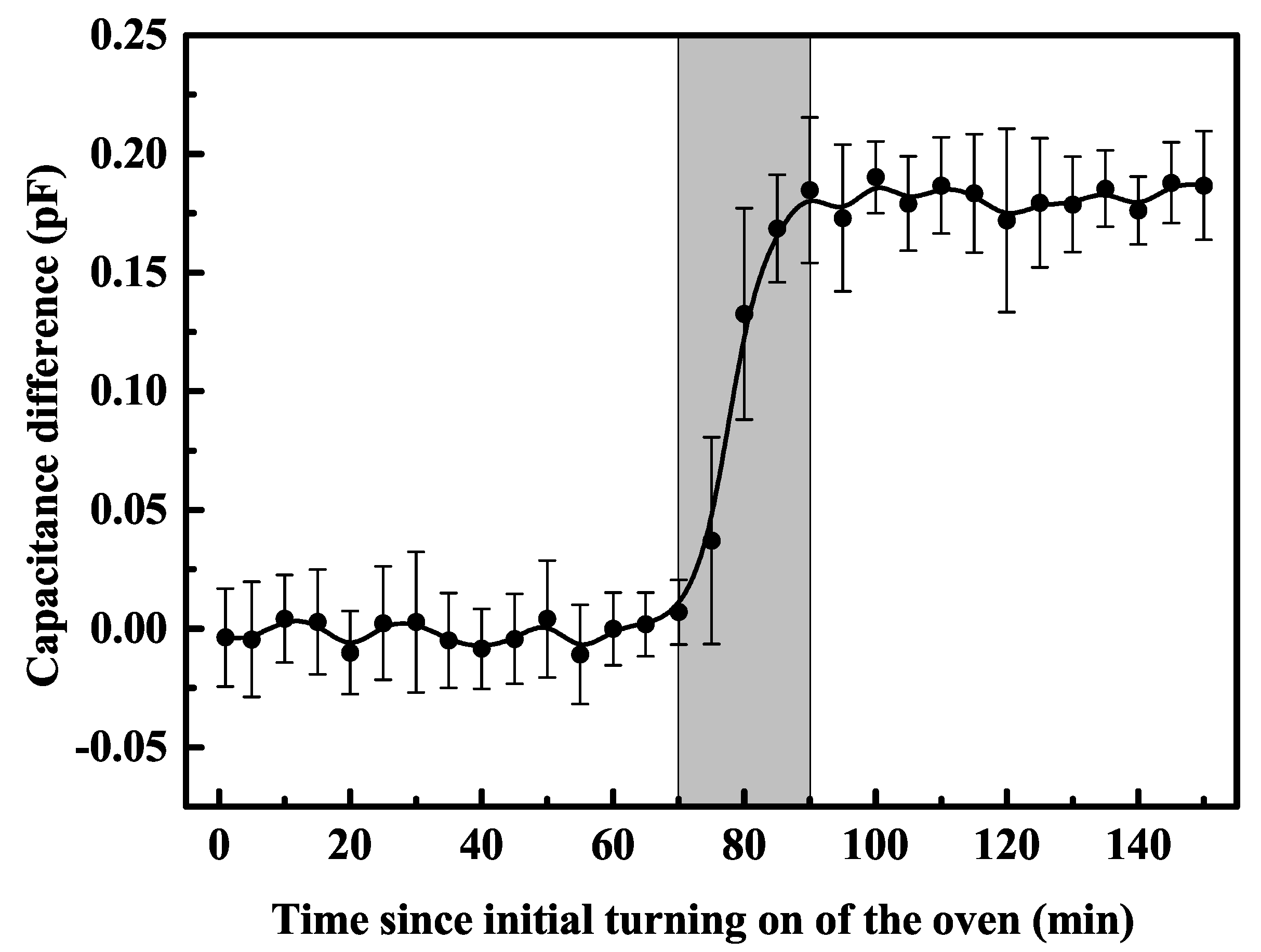
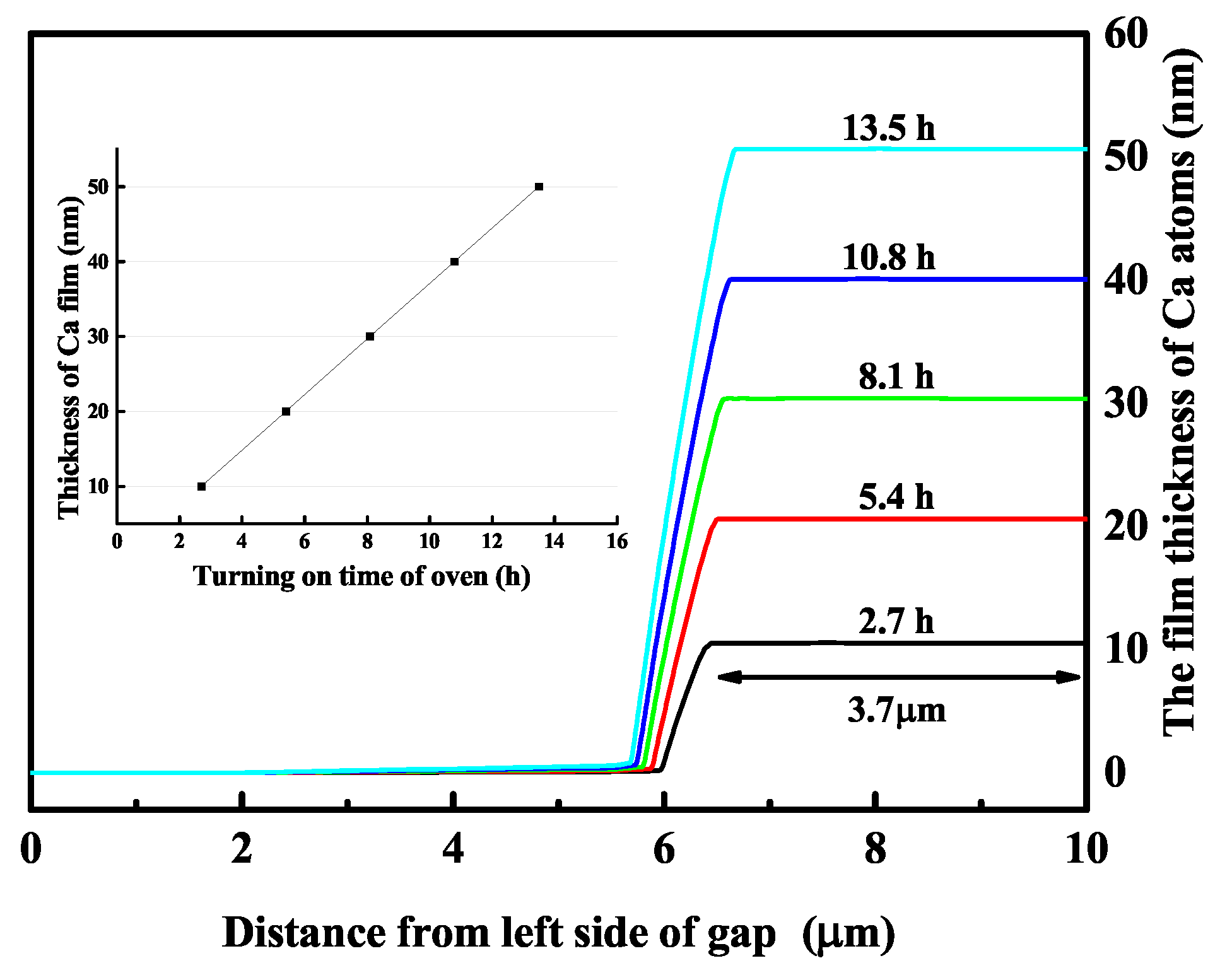
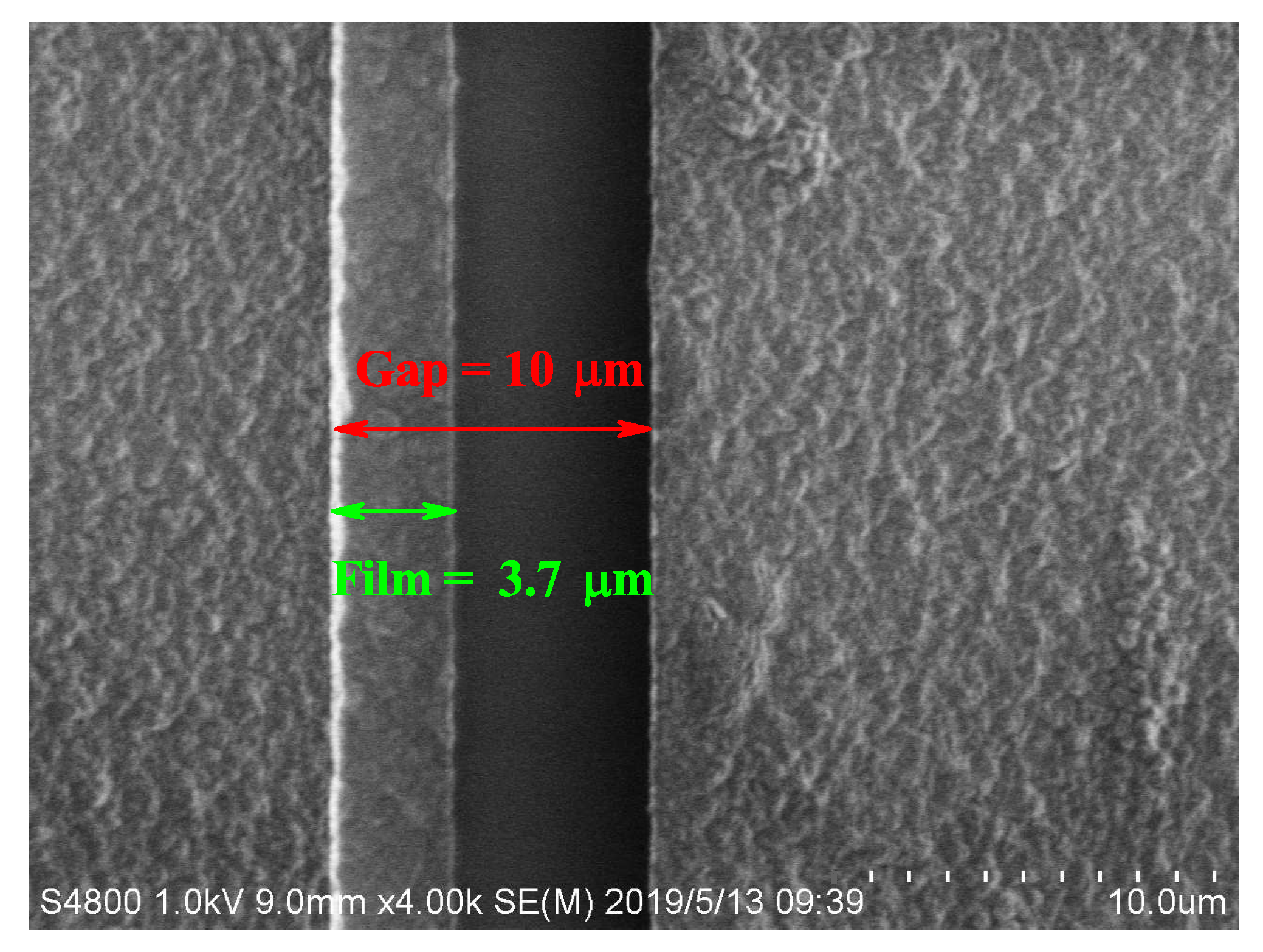
2.1. Experimental Setup and Results
2.2. Theoretical Model and Numerical Simulation
3. Analysis and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Labaziewicz, J.; Ge, Y.; Antohi, P.; Leibrandt, D.; Brown, K.R.; Chuang, I.L. Suppression of heating rates in cryogenic surface-electrode ion traps. Phys. Rev. Lett. 2008, 100, 013001. [Google Scholar] [CrossRef]
- Turchette, Q.; King, B.; Leibfried, D.; Meekhof, D.; Myatt, C.; Rowe, M.; Sackett, C.; Wood, C.; Itano, W.; Monroe, C.; et al. Heating of trapped ions from the quantum ground state. Phys. Rev. A 2000, 61, 063418. [Google Scholar] [CrossRef]
- Deslauriers, L.; Olmschenk, S.; Stick, D.; Hensinger, W.; Sterk, J.; Monroe, C. Scaling and suppression of anomalous heating in ion traps. Phys. Rev. Lett. 2006, 97, 103007. [Google Scholar] [CrossRef]
- Daniilidis, N.; Narayanan, S.; Moller, S.A.; Clark, R.; Lee, T.E.; Leek, P.J.; Wallraff, A.; Schulz, S.; Schmidtkaler, F.; Häffner, H. Fabrication and heating rate study of microscopic surface electrode ion traps. New J. Phys. 2011, 13, 13032. [Google Scholar] [CrossRef]
- Safavi-Naini, A.; Rabl, P.; Weck, P.; Sadeghpour, H. Microscopic model of electric-field-noise heating in ion traps. Phys. Rev. A 2011, 84, 023412. [Google Scholar] [CrossRef]
- Brownnutt, M.; Kumph, M.; Rabl, P.; Blatt, R. Ion-trap measurements of electric-field noise near surfaces. Rev. Mod. Phys. 2015, 87, 1419. [Google Scholar] [CrossRef]
- Allcock, D.; Guidoni, L.; Harty, T.; Ballance, C.; Blain, M.; Steane, A.; Lucas, D. Reduction of heating rate in a microfabricated ion trap by pulsed-laser cleaning. New J. Phys. 2011, 13, 123023. [Google Scholar] [CrossRef]
- DeVoe, R.G.; Kurtsiefer, C. Experimental study of anomalous heating and trap instabilities in a microscopic 137Ba ion trap. Phys. Rev. A 2002, 65, 063407. [Google Scholar] [CrossRef]
- Narayanan, S.; Daniilidis, N.; Möller, S.; Clark, R.; Ziesel, F.; Singer, K.; Schmidt-Kaler, F.; Häffner, H. Electric field compensation and sensing with a single ion in a planar trap. J. App. Phys. 2011, 110, 114909. [Google Scholar] [CrossRef]
- Doret, S.C.; Amini, J.M.; Wright, K.; Volin, C.; Killian, T.; Ozakin, A.; Denison, D.; Hayden, H.; Pai, C.; Slusher, R.E.; et al. Controlling trapping potentials and stray electric fields in a microfabricated ion trap through design and compensation. New J. Phys. 2012, 14, 073012. [Google Scholar] [CrossRef]
- Härter, A.; Krükow, A.; Brunner, A.; Denschlag, J.H. Long-term drifts of stray electric fields in a Paul trap. Appl. Phys. B 2014, 114, 275–281. [Google Scholar] [CrossRef]
- McKay, K.S.; Hite, D.A.; Colombe, Y.; Jördens, R.; Wilson, A.C.; Slichter, D.H.; Allcock, D.T.C.; Leibfried, D.; Wineland, D.J.; Pappas, D.P. Ion-trap electrode preparation with Ne+ bombardment. arXiv 2014, arXiv:1406.1778. [Google Scholar]
- Hite, D.A.; Colombe, Y.; Wilson, A.C.; Brown, K.R.; Warring, U.; Jördens, R.; Jost, J.D.; McKay, K.S.; Pappas, D.P.; Leibfried, D.; et al. 100-fold reduction of electric-field noise in an ion trap cleaned with in situ argon-ion-beam bombardment. Phys. Rev. Lett. 2012, 109, 103001. [Google Scholar] [CrossRef]
- McConnell, R.; Bruzewicz, C.; Chiaverini, J.; Sage, J. Reduction of trapped-ion anomalous heating by in situ surface plasma cleaning. Phys. Rev. A 2015, 92, 020302. [Google Scholar] [CrossRef]
- Bermudez, A.; Xu, X.; Nigmatullin, R.; O’Gorman, J.; Negnevitsky, V.; Schindler, P.; Monz, T.; Poschinger, U.; Hempel, C.; Home, J.; et al. Assessing the progress of trapped-ion processors towards fault-tolerant quantum computation. Phys. Rev. X 2017, 7, 041061. [Google Scholar] [CrossRef]
- An, D.; Matthiesen, C.; Abdelrahman, A.; Berlin-Udi, M.; Gorman, D.; Möller, S.; Urban, E.; Häffner, H. Surface trap with dc-tunable ion-electrode distance. Rev. Sci. Instrum. 2018, 89, 093102. [Google Scholar] [CrossRef]
- Allcock, D.T.C.; Sherman, J.A.; Stacey, D.N.; Burrell, A.H.; Curtis, M.J.; Imreh, G.; Linke, N.M.; Szwer, D.J.; Webster, S.C.; Steane, A.M.; et al. Implementation of a symmetric surface-electrode ion trap with field compensation using a modulated Raman effect. New J. Phys. 2010, 12, 053026. [Google Scholar] [CrossRef]
- Labaziewicz, J.; Ge, Y.; Leibrandt, D.R.; Wang, S.X.; Shewmon, R.; Chuang, I.L. Temperature dependence of electric field noise above gold surfaces. Phys. Rev. Lett. 2008, 101, 180602. [Google Scholar] [CrossRef]
- Bird, G.A.; Brady, J.M. Molecular Gas Dynamics and the Direct Simulation of Gas Flows; Clarendon Press Oxford: Oxford, UK, 1994; Volume 5. [Google Scholar]
- Andersen, H.C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef]
- Hirth, J.P.; Pound, G.M. Coefficients of evaporation and condensation. J. Phys. Chem. 1960, 64, 619–626. [Google Scholar] [CrossRef]
- Leibrandt, D.R.; Clark, R.J.; Labaziewicz, J.; Antohi, P.; Bakr, W.; Brown, K.R.; Chuang, I.L. Laser ablation loading of a surface-electrode ion trap. Phys. Rev. A 2007, 76, 055403. [Google Scholar] [CrossRef]
- Sedlacek, J.A.; Greene, A.; Stuart, J.; McConnell, R.; Bruzewicz, C.D.; Sage, J.; Chiaverini, J. Distance scaling of electric-field noise in a surface-electrode ion trap. Phys. Rev. A 2018, 97, 020302. [Google Scholar] [CrossRef]
- Boldin, I.A.; Kraft, A.; Wunderlich, C. Measuring anomalous heating in a planar ion trap with variable ion-surface separation. Phys. Rev. Lett. 2018, 120, 023201. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hou, Y.; Chen, T.; Wu, W.; Chen, P. Convenient Real-Time Monitoring of the Contamination of Surface Ion Trap. Nanomaterials 2020, 10, 109. https://doi.org/10.3390/nano10010109
Zhang X, Hou Y, Chen T, Wu W, Chen P. Convenient Real-Time Monitoring of the Contamination of Surface Ion Trap. Nanomaterials. 2020; 10(1):109. https://doi.org/10.3390/nano10010109
Chicago/Turabian StyleZhang, Xinfang, Yizhu Hou, Ting Chen, Wei Wu, and Pingxing Chen. 2020. "Convenient Real-Time Monitoring of the Contamination of Surface Ion Trap" Nanomaterials 10, no. 1: 109. https://doi.org/10.3390/nano10010109
APA StyleZhang, X., Hou, Y., Chen, T., Wu, W., & Chen, P. (2020). Convenient Real-Time Monitoring of the Contamination of Surface Ion Trap. Nanomaterials, 10(1), 109. https://doi.org/10.3390/nano10010109




