Covalent Immobilization of β-Glucosidase into Mesoporous Silica Nanoparticles from Anhydrous Acetone Enhances Its Catalytic Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticle Synthesis
2.2. BG Covalent Immobilization Procedure
2.3. BG Physical Immobilization Procedure
2.4. Material Characterization
2.5. Catalytic Assays
2.6. Thermal Stability
2.7. Operational Stability
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koeller, K.M.; Wong, C.H. Enzymes for chemical synthesis. Nature 2001, 409, 232. [Google Scholar] [CrossRef] [PubMed]
- Arsalan, A.; Younus, H. Enzymes and nanoparticles: Modulation of enzymatic activity via nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Bommarius, A.S.; Paye, M.F. Stabilizing biocatalysts. Chem. Soc. Rev. 2013, 42, 6534–6565. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Hartmann, M.; Kostrov, X. Immobilization of enzymes on porous silicas—Benefits and challenges. Chem. Soc. Rev. 2013, 42, 6277–6289. [Google Scholar] [CrossRef]
- Desai, U.R.; Klibanov, A.M. Assessing the structural integrity of a lyophilized protein in organic solvents. J. Am. Chem. Soc. 1995, 117, 3940–3945. [Google Scholar] [CrossRef]
- Fishman, A.; Levy, I.; Cogan, U.; Shoseyov, O. Stabilization of horseradish peroxidase in aqueous-organic media by immobilization onto cellulose using a cellulose-binding-domain. J. Mol. Catal. B Enzym. 2002, 18, 121–131. [Google Scholar] [CrossRef]
- Gupta, M.N. Thermostabilization of proteins. Appl. Biochem. Biotech. 1991, 14, 1–11. [Google Scholar]
- Stark, M.B.; Holmberg, K. Covalent immobilization of lipase in organic solvents. Biotechnol. Bioeng. 1989, 34, 942–950. [Google Scholar] [CrossRef]
- Stolarow, J.; Heinzelmann, M.; Yeremchuk, W.; Syldatk, C.; Hausmann, R. Immobilization of trypsin in organic and aqueous media for enzymatic peptide synthesis and hydrolysis reactions. BMC Biotechnol. 2015, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhou, T.; Wu, X.; Cai, Y.; Yao, D.; Xie, C.; Liu, D. Covalent immobilization of enzymes within micro-aqueous organic media. J. Mol. Catal. B Enzym. 2011, 72, 145–149. [Google Scholar] [CrossRef]
- De Castrom, H.F.; de Oliveira, P.C.; Soares, C.M.; Zanin, G.M. Immobilization of porcine pancreatic lipase on celite for application in the synthesis of butyl butyrate in a nonaqueous system. J. Am. Oil Chem. Soc. 1999, 76, 147–152. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, Y.; Zhou, L.; Gao, J. Immobilization of Candida antarctica lipase B by adsorption in organic medium. New Biotechnol. 2010, 27, 53–58. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Enzyme-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 707–738. [Google Scholar]
- Zhao, J.; Wang, X.; Sun, W.; Mou, Y.; Peng, Y.; Zhou, L. Medium optimization for palmarumycin C13 production in liquid culture of endophytic fungus Berkleasmium sp. Dzf12 using response surface methodology. Electron. J. Biotechnol. 2013, 16, 16. [Google Scholar] [CrossRef]
- González-Pombo, P.; Fariña, L.; Carrau, F.; Batista-Viera, F.; Brena, B.M. A novel extracellular β-glucosidase from Issatchenkia terricola: Isolation, immobilization and application for aroma enhancement of white Muscat wine. Process. Biochem. 2011, 46, 385–389. [Google Scholar] [CrossRef]
- Jung, Y.R.; Shin, H.Y.; Song, Y.S.; Kim, S.B.; Kim, S.W. Enhancement of immobilized enzyme activity by pretreatment of β-glucosidase with cellobiose and glucose. J. Ind. Eng. Chem. 2012, 18, 702–706. [Google Scholar] [CrossRef]
- Singh, R.K.; Zhang, Y.W.; Jeya, M.; Lee, J.K. Covalent immobilization of β-1, 4-glucosidase from Agaricus arvensis onto functionalized silicon oxide nanoparticles. Appl. Microbiol. Biotechnol. 2011, 89, 337–344. [Google Scholar] [CrossRef]
- Sannino, F.; Pansini, M.; Marocco, A.; Bonelli, B.; Garrone, E.; Esposito, S. The role of outer surface/inner bulk Brønsted acidic sites in the adsorption of a large basic molecule (simazine) on HY zeolite. Phys. Chem. Chem. Phys. 2015, 17, 28950–28957. [Google Scholar] [CrossRef] [PubMed]
- De Andrades, D.; Graebin, N.G.; Kadowaki, M.K.; Ayub, M.A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Immobilization and stabilization of different β-glucosidases using the glutaraldehyde chemistry: Optimal protocol depends on the enzyme. Int. J. Biol. Macromol. 2019, 129, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, B.; Pezzella, A.; Luciani, G.; Costantini, A.; Tescione, F.; Branda, F. Heparin conjugated silica nanoparticle synthesis. Mater. Sci. Eng. C 2012, 32, 2037–2041. [Google Scholar] [CrossRef]
- Silvestri, B.; Guarnieri, D.; Luciani, G.; Costantini, A.; Netti, P.A.; Branda, F. Fluorescent (rhodamine), folate decorated and doxorubicin charged, PEGylated nanoparticles synthesis. J. Mater. Sci. Mater. Med. 2012, 23, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Branda, F.; Malucelli, G.; Durante, M.; Piccolo, A.; Mazzei, P.; Costantini, A.; Silvestri, B.; Pennetta, M.; Bifulco, A. Silica treatments: A fire retardant strategy for hemp fabric/epoxy composites. Polymers 2016, 8, 313. [Google Scholar] [CrossRef] [PubMed]
- Branda, F.; Silvestri, B.; Costantini, A.; Luciani, G. Effect of exposure to growth media on size and surface charge of silica based Stöber nanoparticles: A DLS and ζ-potential study. J. Sol Gel Sci. Technol. 2015, 73, 54–61. [Google Scholar] [CrossRef]
- Verma, M.L.; Chaudhary, R.; Tsuzuki, T.; Barrow, C.J.; Puri, M. Immobilization of β-glucosidase on a magnetic nanoparticle improves thermostability: Application in cellobiose hydrolysis. Bioresour. Technol. 2013, 135, 2–6. [Google Scholar] [CrossRef]
- Zheng, P.; Wang, J.; Lu, C.; Xu, Y.; Sun, Z. Immobilized β-glucosidase on magnetic chitosan microspheres for hydrolysis of straw cellulose. Process. Biochem. 2013, 48, 683–687. [Google Scholar] [CrossRef]
- Agrawal, R.; Verma, A.K.; Satlewal, A. Application of nanoparticle-immobilized thermostable β-glucosidase for improving the sugarcane juice properties. Innov. Food Sci. Emerg. Technol. 2016, 33, 472–482. [Google Scholar] [CrossRef]
- Faridi, S.; Bose, H.; Satyanarayana, T. Utility of immobilized recombinant carbonic anhydrase of Bacillus halodurans TSLV1 on the surface of modified iron magnetic nanoparticles in carbon sequestration. Energy Fuels 2017, 31, 3002–3009. [Google Scholar] [CrossRef]
- Magner, E. Immobilisation of enzymes on mesoporous silicate materials. Chem. Soc. Rev. 2013, 42, 6213–6222. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Jung, D. Biocatalysis with enzymes immobilized on mesoporous hosts: The status quo and future trends. J. Mater. Chem. 2010, 20, 844–857. [Google Scholar] [CrossRef]
- Califano, V.; Sannino, F.; Costantini, A.; Avossa, J.; Cimino, S.; Aronne, A. Wrinkled silica nanoparticles: Efficient matrix for β-glucosidase immobilization. J. Phys. Chem. C 2018, 122, 8373–8379. [Google Scholar] [CrossRef]
- Califano, V.; Costantini, A.; Silvestri, B.; Venezia, V.; Cimino, S.; Sannino, F. The effect of pore morphology on the catalytic performance of β-glucosidase immobilized into mesoporous silica. Pure Appl. Chem. 2019, 91, 1583–1592. [Google Scholar] [CrossRef]
- Gomori, G. Preparation of buffers for use in enzyme studies. Methods Enzymol. 1955, 1, 138–146. [Google Scholar]
- Moon, D.S.; Lee, J.K. Formation of wrinkled silica mesostructures based on the phase behavior of pseudoternary systems. Langmuir 2014, 30, 15574–15580. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Bernt, E. UV-assay with pyruvate and NADH. In Methods of Enzymatic Analysis, 2nd ed.; Academic Press: Cambridge, MA, USA, 1974; Volume 2, pp. 574–579. [Google Scholar]
- Illanes, A. Enzyme biocatalysis. In Principles and Applications; Editorial Springer-Verlag New York Inc.: New York, NY, USA, 2008. [Google Scholar]
- Khan, S.; Lindahl, S.; Turner, C.; Karlsson, E.N. Immobilization of thermostable β-glucosidase variants on acrylic supports for biocatalytic processes in hot water. J. Mol. Catal. B Enzym. 2012, 80, 28–38. [Google Scholar] [CrossRef]
- Benessere, V.; Cucciolito, M.E.; Dal Poggetto, G.; Di Serio, M.; Granados, M.L.; Ruffo, F.; Vitagliano, A.; Vitiello, R. Strategies for immobilizing homogeneous zinc catalysts in biodiesel production. Catal. Commun. 2014, 56, 81–85. [Google Scholar] [CrossRef]
- Benessere, V.; Cucciolito, M.E.; Esposito, R.; Lega, M.; Turco, R.; Ruffo, F.; Di Serio, M. A novel and robust homogeneous supported catalyst for biodiesel production. Fuel 2016, 171, 1–4. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Manoel, E.A.; Fernandez-Lafuente, R.; Freire, D.M.G. Support engineering: Relation between development of new supports for immobilization of lipases and their applications. Biotech. Res. Innovation. 2017, 1, 26–34. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.M.; Rokicki, G. Investigations on the synthesis and properties of biodegradable poly (ester-carbonate-urea-urethane) s. Pol. J. Chem. Technol. 2013, 15, 80–88. [Google Scholar] [CrossRef]
- Javni, I.; Song, K.; Lin, J.; Petrovic, Z.S. Structure and properties of flexible polyurethane foams with nano-and micro-fillers. J. Cell. Plast. 2011, 47, 357–372. [Google Scholar] [CrossRef]
- Califano, V.; Bloisi, F.; Perretta, G.; Aronne, A.; Ausanio, G.; Costantini, A.; Vicari, L. Frozen Microemulsions for MAPLE Immobilization of Lipase. Molecules 2017, 22, 2153. [Google Scholar] [CrossRef]
- Serefoglou, E.; Litina, K.; Gournis, D.; Kalogeris, E.; Tzialla, A.A.; Pavlidis, I.V.; Stamatis, H.; Maccallini, E.; Lubomska, M.; Rudolf, P. Smectite clays as solid supports for immobilization of β-glucosidase: Synthesis, characterization, and biochemical properties. Chem. Mater. 2008, 20, 4106–4115. [Google Scholar] [CrossRef]
- Dekker, R.F. Application of a magnetic immobilizedβ-glucosidase in the enzymatic saccharification of steam-exploded lignocellulosic residues. J. Mater. Sci. Mater. Med. 1990, 23, 25–39. [Google Scholar] [CrossRef]
- Figueira, J.A.; Sato, H.H.; Fernandes, P. Establishing the feasibility of using β-glucosidase entrapped in Lentikats and in sol–gel supports for cellobiose hydrolysis. J. Agric. Food Chem. 2013, 61, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Rajkhowa, R.; Wang, X.; Barrow, C.J.; Puri, M. Exploring novel ultrafine Eri silk bioscaffold for enzyme stabilisation in cellobiose hydrolysis. Bioresour. Technol. 2013, 145, 302–306. [Google Scholar] [CrossRef]
- Ahmed, S.A.; El-Shayeb, N.M.A.; Hashem, A.M.; Saleh, S.A.; Abdel-Fattah, A.F. Biochemical studies on immobilized fungal β-glucosidase. Braz. J. Chem. Eng. 2013, 30, 747–758. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, V.; Dubey, A.; Verma, A.K. Kinetic characterization and effect of immobilized thermostable β-glucosidase in alginate gel beads on sugarcane juice. ISRN Biochem. 2014, 2014, 178498. [Google Scholar]
- Iborra, J.L.; Castellar, M.R.; Cánovas, M.; Manjón, A. Picrocrocin hydrolysis by immobilized β-glucosidase. Biotechnol. Lett. 1992, 14, 475–480. [Google Scholar] [CrossRef]
- Fan, G.; Xu, Y.; Zhang, X.; Lei, S.; Yang, S.; Pan, S. Characteristics of immobilised β-glucosidase and its effect on bound volatile compounds in orange juice. Int. J. Food Sci. Technol. 2011, 46, 2312–2320. [Google Scholar] [CrossRef]
- Bloisi, F.; Califano, V.; Perretta, G.; Nasti, L.; Aronne, A.; Di Girolamo, R.; Auriemma, F.; De Rosa, C.; Vicari, L.R.M. Lipase immobilization for catalytic applications obtained using fumed silica deposited with MAPLE technique. Appl. Surf. Sci. 2016, 374, 346–352. [Google Scholar] [CrossRef]
- Sakai, K.; Tamura, M.; Umezawa, S.; Takamatsu, Y.; Torigoe, K.; Yoshimura, T.; Esumi, K.; Sakai, H.; Abe, M. Adsorption characteristics of sugar-based monomeric and gemini surfactants at the silica/aqueous solution interface. Colloids Surf. A 2008, 328, 100–106. [Google Scholar] [CrossRef]
- Chen, B.; Qiu, J.; Mo, H.; Yu, Y.; Ito, K.; Sakai, E.; Feng, H. Synthesis of mesoporous silica with different pore sizes for cellulase immobilization: Pure physical adsorption. New J.Chem. 2017, 41, 9338–9345. [Google Scholar] [CrossRef]
- Mateo, C.; Abian, O.; Fernandez–Lafuente, R.; Guisan, J.M. Increase in conformational stability of enzymes immobilized on epoxy-activated supports by favoring additional multipoint covalent attachment. Enzyme Microb. Technol. 2000, 26, 509–515. [Google Scholar] [CrossRef]
- Wang, P.; Hu, X.; Cook, S.; Hwang, H.M. Influence of silica-derived nano-supporters on cellobiase after immobilization. Appl. Biochem. Biotechnol. 2009, 158, 88–96. [Google Scholar] [CrossRef] [PubMed]
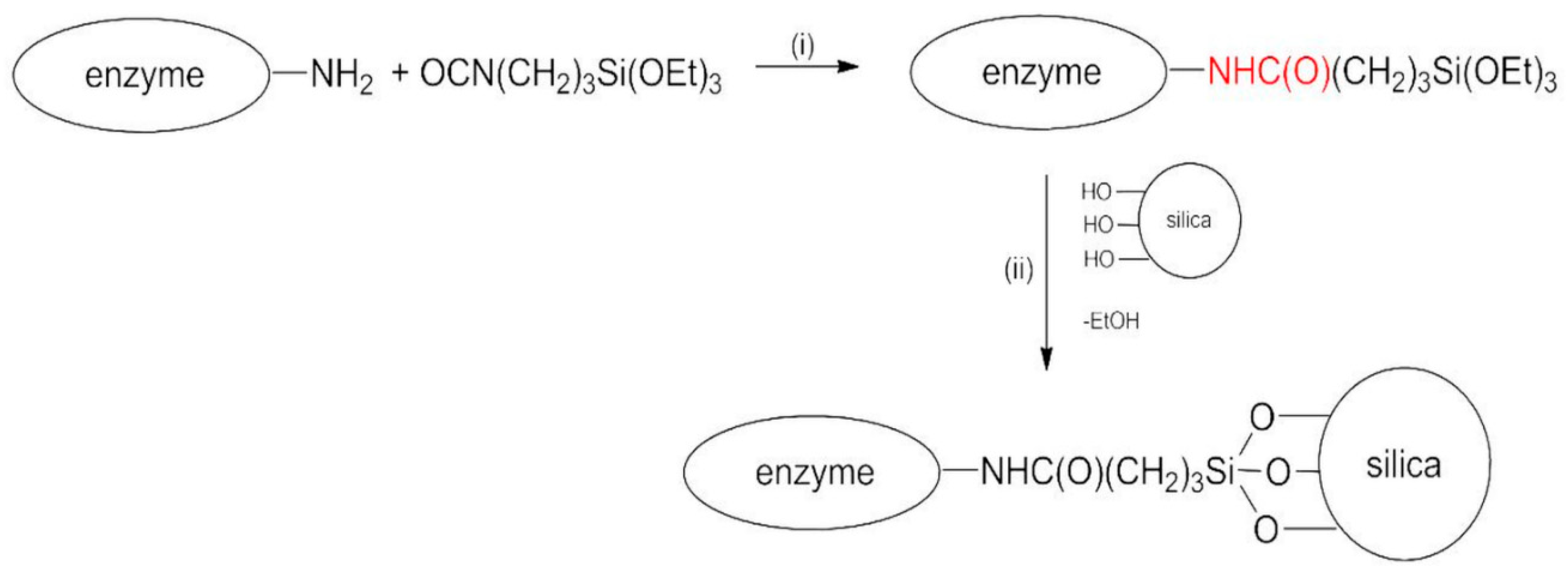
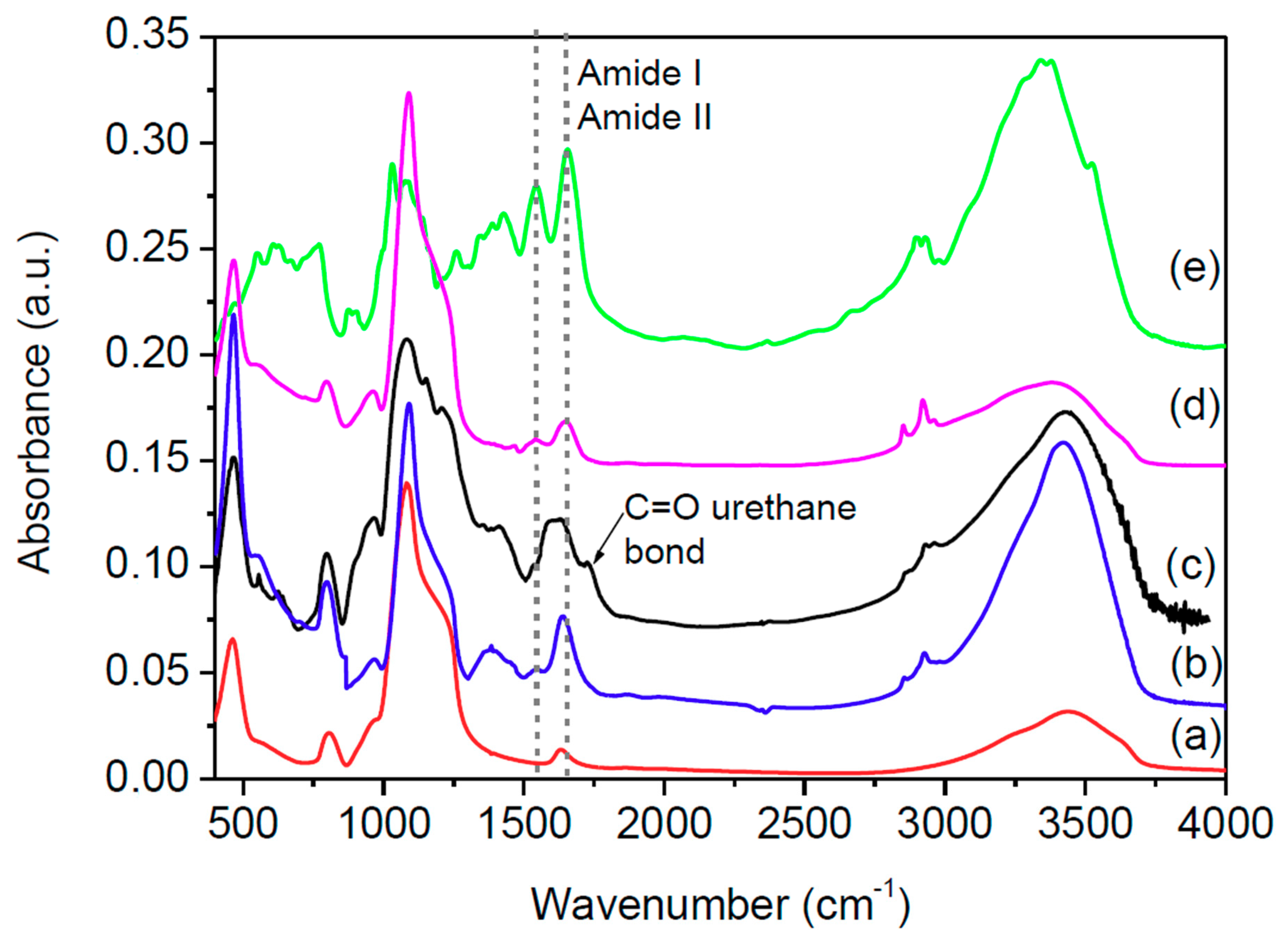
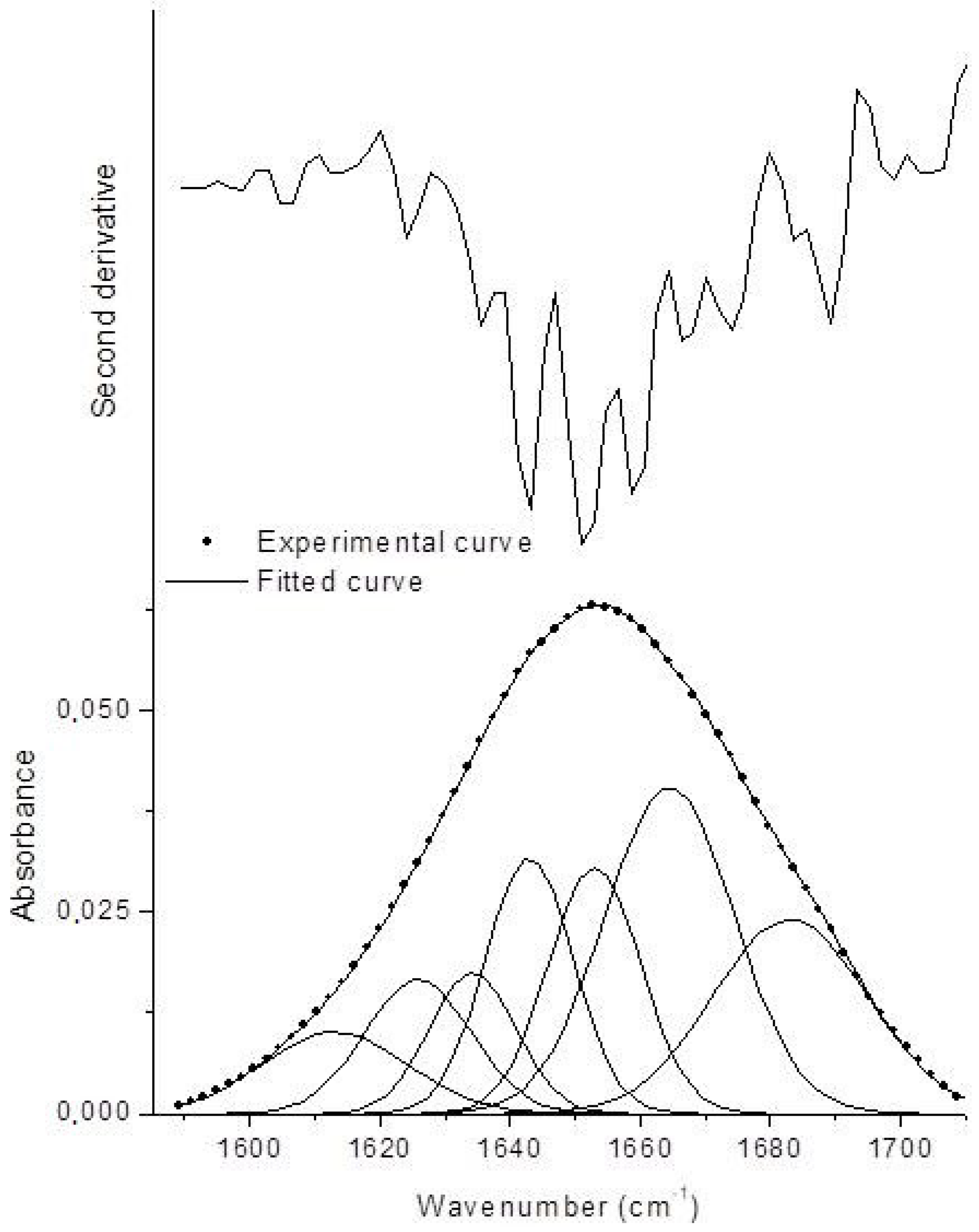
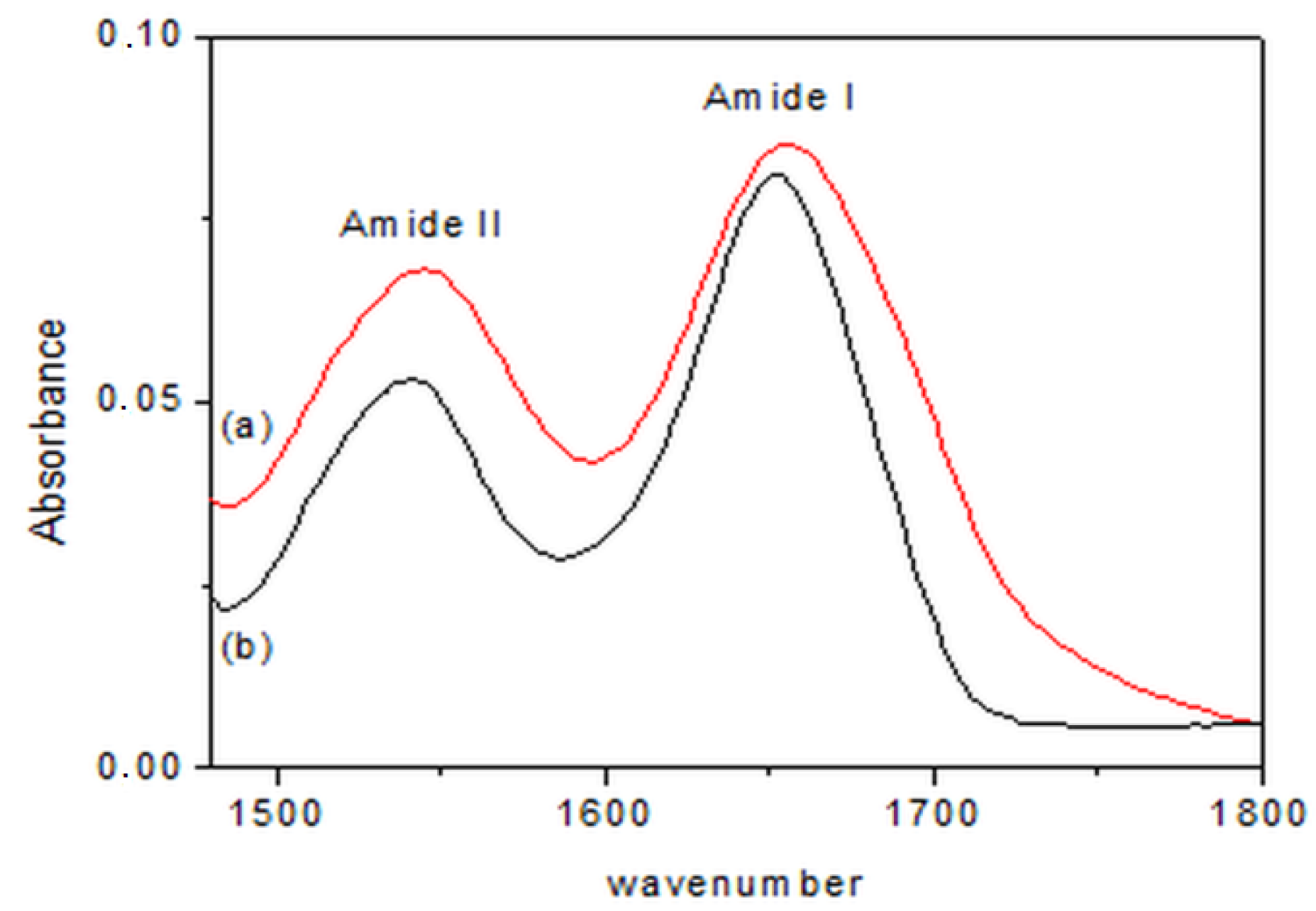
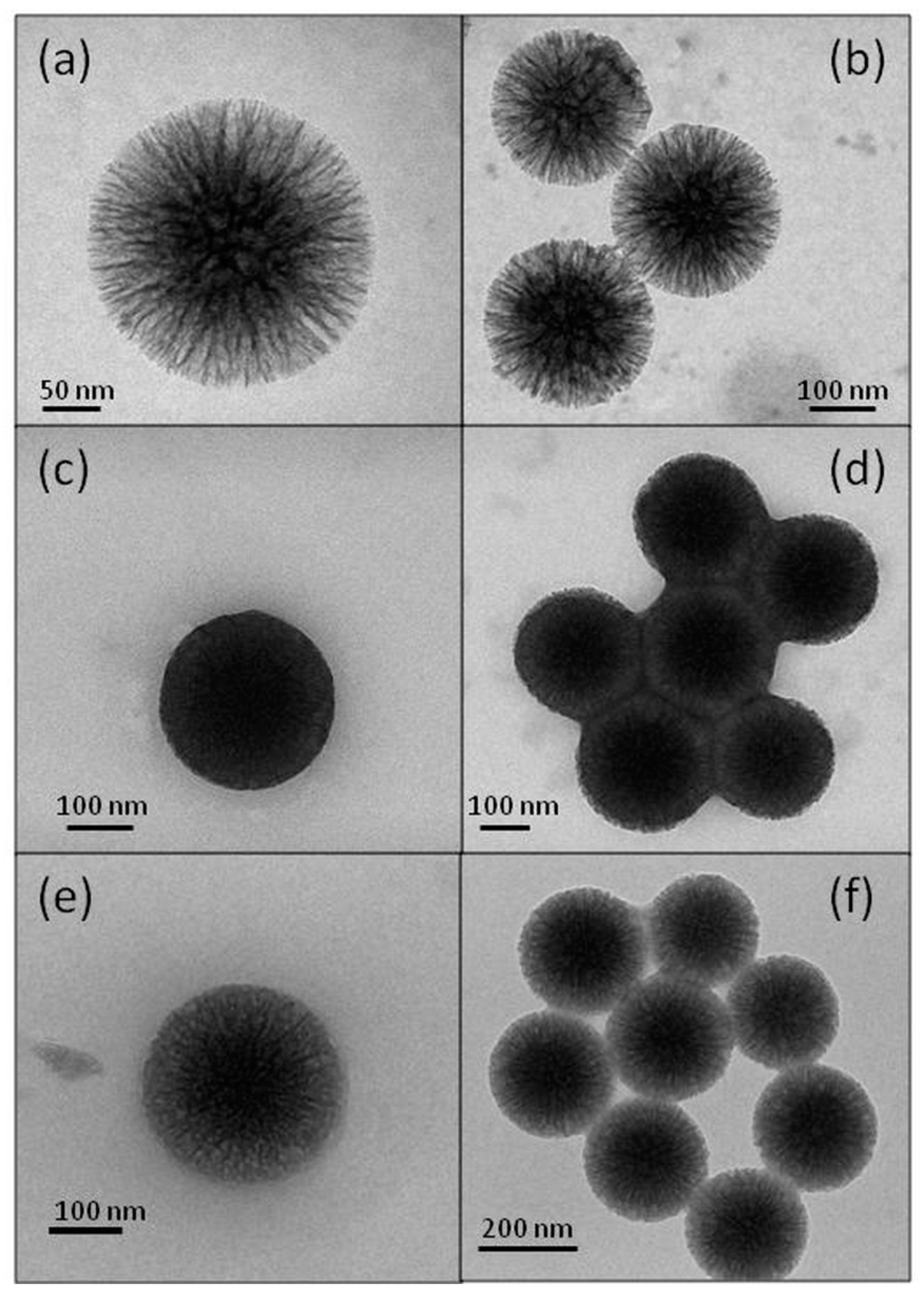
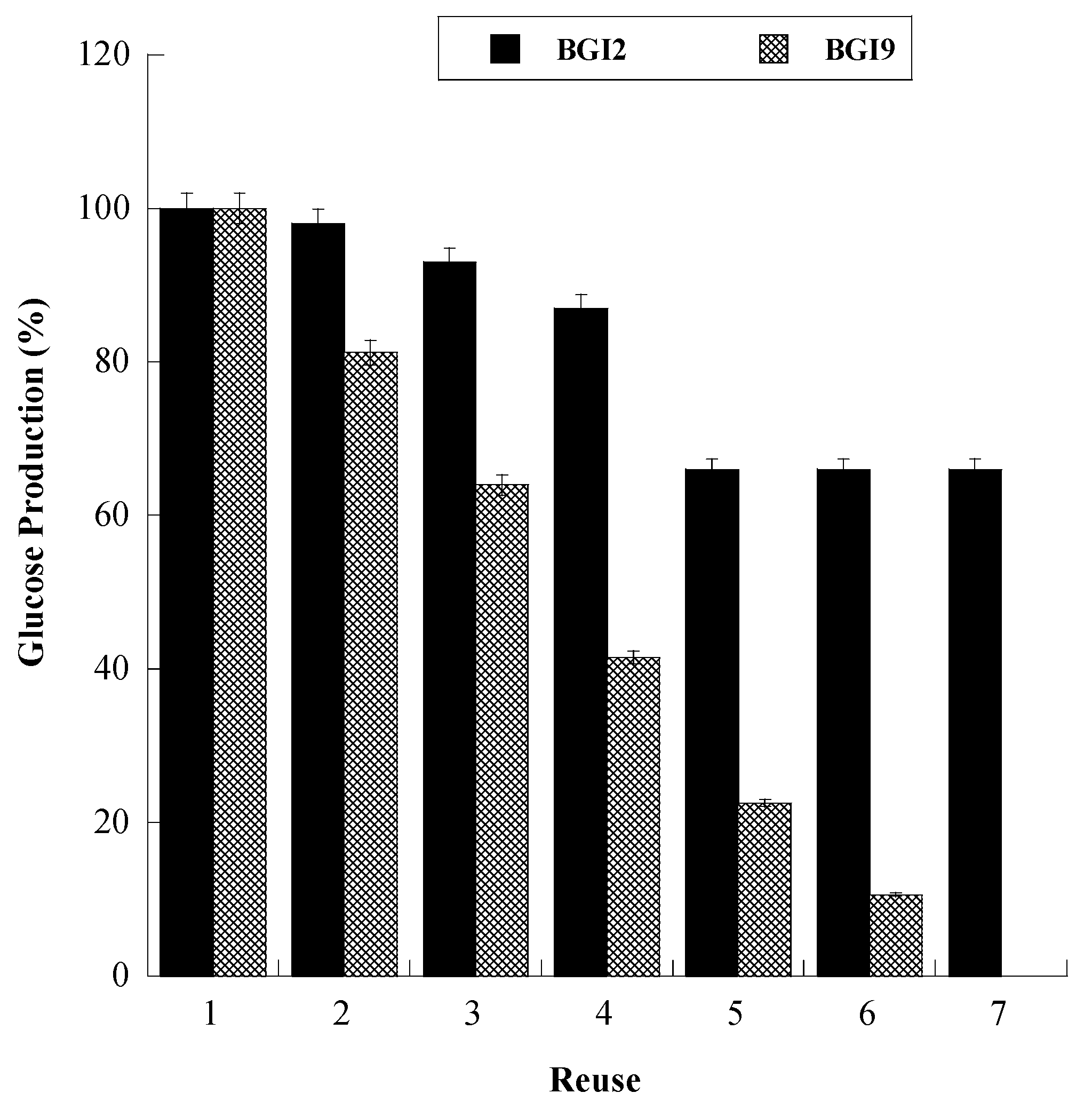
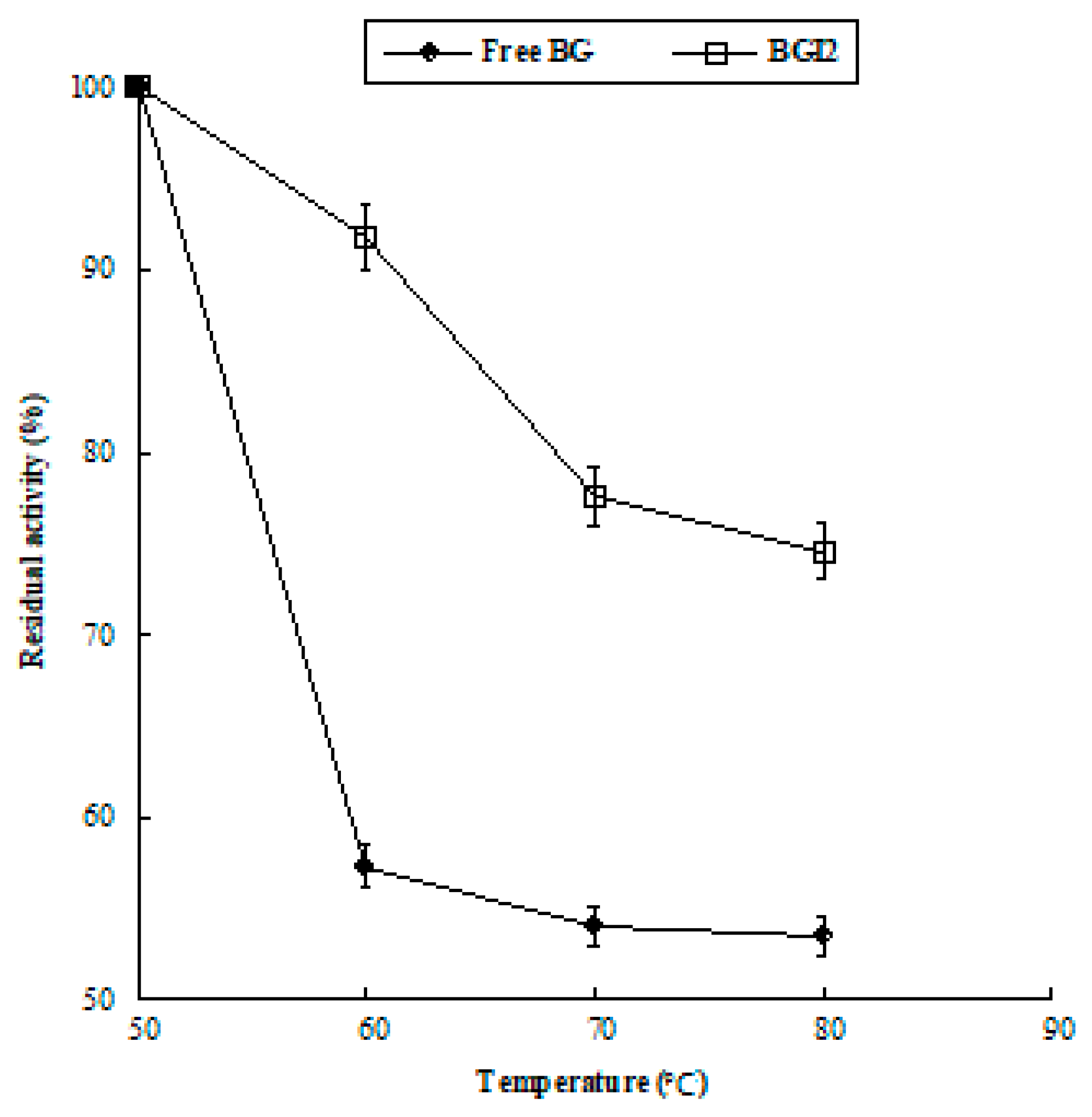
| Structure | Lyophilized BG | BGI2 |
|---|---|---|
| α-Helices | 28.9 | 14.6 |
| β-Sheets | 13.9 | 17 |
| β-Turns | 35.4 | 46.4 |
| Unordered | 16.4 | 14.4 |
| Aggregates | 5.4 | 7.6 |
| Enzyme | KM (mM) | Vmax (μmol/min∙mg) |
|---|---|---|
| Free | 4.74 ± 0.03 | 26.2 ± 0.20 |
| BGI9 | 10.90 ± 0.03 | 13.3 ± 0.01 |
| BGI2 | 2.50 ± 0.01 | 25.0 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sannino, F.; Costantini, A.; Ruffo, F.; Aronne, A.; Venezia, V.; Califano, V. Covalent Immobilization of β-Glucosidase into Mesoporous Silica Nanoparticles from Anhydrous Acetone Enhances Its Catalytic Performance. Nanomaterials 2020, 10, 108. https://doi.org/10.3390/nano10010108
Sannino F, Costantini A, Ruffo F, Aronne A, Venezia V, Califano V. Covalent Immobilization of β-Glucosidase into Mesoporous Silica Nanoparticles from Anhydrous Acetone Enhances Its Catalytic Performance. Nanomaterials. 2020; 10(1):108. https://doi.org/10.3390/nano10010108
Chicago/Turabian StyleSannino, Filomena, Aniello Costantini, Francesco Ruffo, Antonio Aronne, Virginia Venezia, and Valeria Califano. 2020. "Covalent Immobilization of β-Glucosidase into Mesoporous Silica Nanoparticles from Anhydrous Acetone Enhances Its Catalytic Performance" Nanomaterials 10, no. 1: 108. https://doi.org/10.3390/nano10010108
APA StyleSannino, F., Costantini, A., Ruffo, F., Aronne, A., Venezia, V., & Califano, V. (2020). Covalent Immobilization of β-Glucosidase into Mesoporous Silica Nanoparticles from Anhydrous Acetone Enhances Its Catalytic Performance. Nanomaterials, 10(1), 108. https://doi.org/10.3390/nano10010108










