Evaluation of Antibiotic-Releasing Triphasic Bone Void Filler In-Vitro
Abstract
:1. Introduction
2. Results
2.1. Set Time
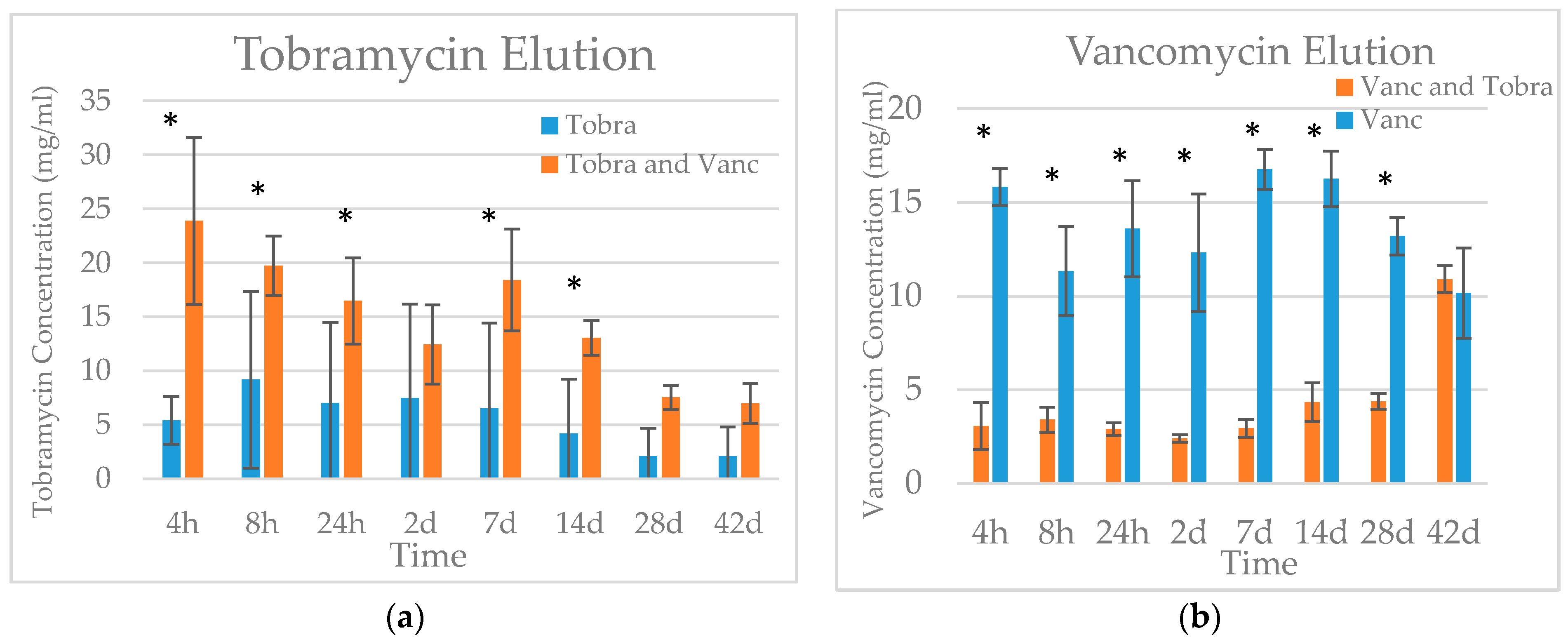
2.2. Elution
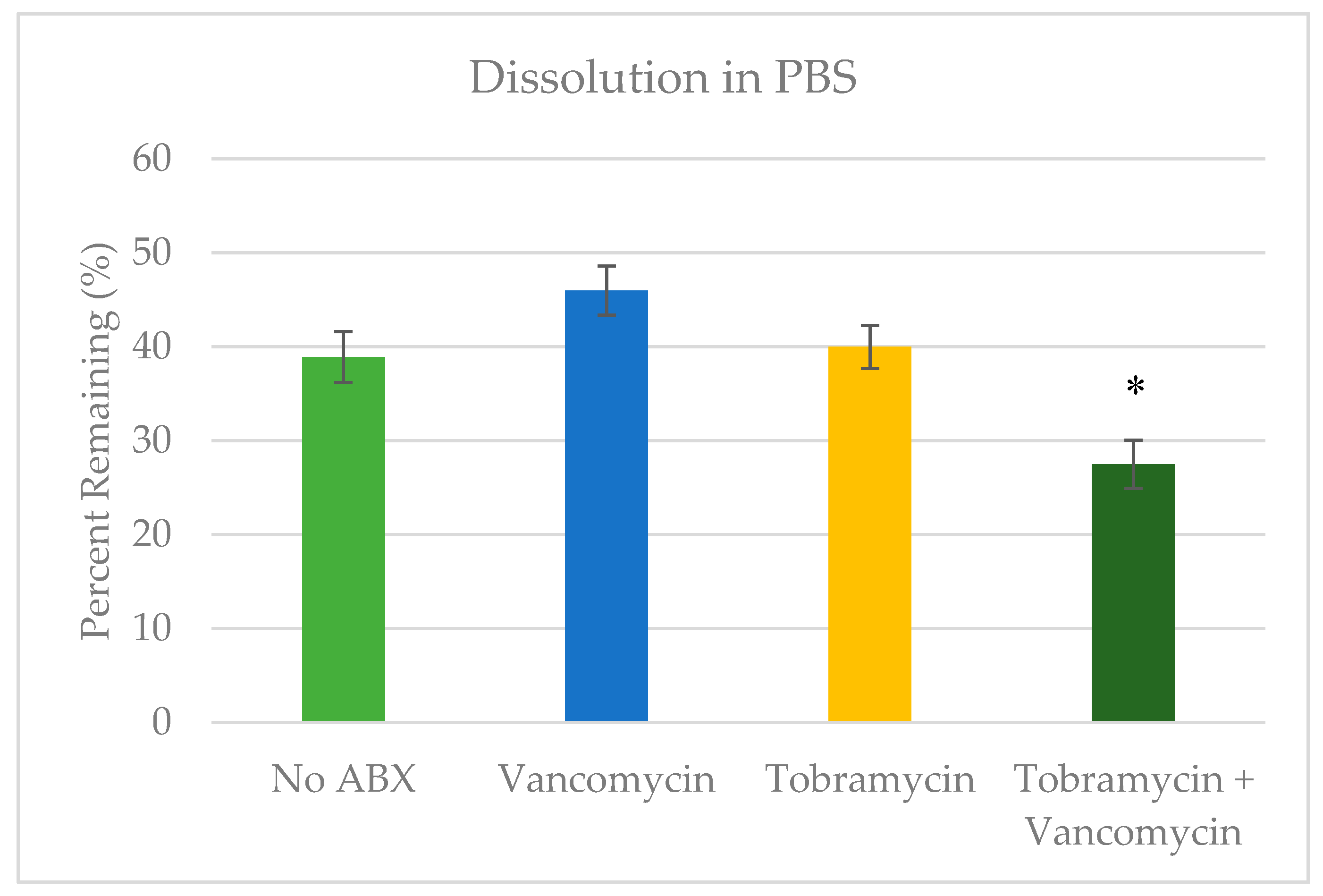
2.3. Dissolution
3. Discussion
4. Materials and Methods
4.1. Bead Preparation
4.2. Elution
4.3. Dissolution
4.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bahn, S.L. Plaster: A bone substitute. Oral Surg. Oral Med. Oral Pathol. 1966, 21, 672–681. [Google Scholar] [CrossRef]
- Behairy, Y.; Jasty, M. Bone grafts and bone substitutes in hip and knee surgery. Orthop. Clin. 1999, 30, 661–671. [Google Scholar] [CrossRef]
- Blitch, E.L.; Ricotta, P.J. Introduction to bone grafting. J. Foot Ankle Surg. 1996, 35, 458–462. [Google Scholar] [CrossRef]
- Ladd, A.L.; Pliam, N.B. Use of bone-graft substitutes in distal radius fractures. J. Am. Acad. Orthop. Surg. 1999, 7, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Tay, B.K.; Patel, V.V.; Bradford, D.S. Calcium sulfate- and calcium phosphate–based bone substitutes: Mimicry of the mineral phase of bone. Orthop. Clin. 1999, 30, 615–623. [Google Scholar] [CrossRef]
- Bajammal, S.S.; Zlowodzki, M.; Lelwica, A.; Tornetta, P., III; Einhorn, T.A.; Buckley, R.; Leighton, R.; Russell, T.A.; Larsson, S.; Bhandari, M. The use of calcium phosphate bone cement in fracture treatment: A meta-analysis of randomized trials. J. Bone Joint Surg. 2008, 90, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Van der Stok, J.; Van Lieshout, E.M.; El-Massoudi, Y.; Van Kralingen, G.H.; Patka, P. Bone substitutes in the netherlands—A systematic literature review. Acta Biomater. 2011, 7, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, W.S.; Ronk, R. Calcium sulfate bone void filler: A review and a look ahead. J. Craniofac. Surg. 2000, 11, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Kurien, T.; Pearson, R.; Scammell, B. Bone graft substitutes currently available in orthopaedic practice: The evidence for their use. Bone Joint J. 2013, 95, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Lerner, T.; Bullmann, V.; Schulte, T.L.; Schneider, M.; Liljenqvist, U. A level-1 pilot study to evaluate of ultraporous β-tricalcium phosphate as a graft extender in the posterior correction of adolescent idiopathic scoliosis. Eur. Spine J. 2009, 18, 170. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008, 43, 3028. [Google Scholar] [CrossRef]
- Larsson, S. Calcium phosphates: What is the evidence? J. Orthop. Trauma 2010, 24, S41–S45. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Zheng, M.H.; Tägil, M. The composite of hydroxyapatite and calcium sulphate: A review of preclinical evaluation and clinical applications. Expert Rev. Med. Devices 2013, 10, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Jiranek, W.A.; Hanssen, A.D.; Greenwald, A.S. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J. Bone Joint Surg. Am. 2006, 88, 2487–2500. [Google Scholar] [CrossRef] [PubMed]
- Hake, M.E.; Young, H.; Hak, D.J.; Stahel, P.F.; Hammerberg, E.M.; Mauffrey, C. Local antibiotic therapy strategies in orthopaedic trauma: Practical tips and tricks and review of the literature. Injury 2015, 46, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.-P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.-P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.V.; Puleo, D.A. Calcium sulfate: Properties and clinical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A.; Wilson, L.F.; Buckland, T. Comparative performance of three ceramic bone graft substitutes. Spine J. 2007, 7, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.; Wilkins, R.M.; Gitelis, S.; Hartjen, C.; Watson, J.T.; Kim, P.T. The use of a surgical grade calcium sulfate as a bone graft substitute: Results of a multicenter trial. Clin. Orthop. Relat. Res. 2001, 382, 42–50. [Google Scholar] [CrossRef]
- Lee, G.H.; Khoury, J.G.; Bell, J.-E.; Buckwalter, J.A. Adverse reactions to osteoset bone graft substitute: The incidence in a consecutive series. Iowa Orthop. J. 2002, 22, 35. [Google Scholar] [PubMed]
- Petruskevicius, J.; Nielsen, S.; Kaalund, S.; Knudsen, P.R.; Overgaard, S. No effect of osteoset®, a bone graft substitute, on bone healing in humans: A prospective randomized double-blind study. Acta Orthop. Scandinav. 2002, 73, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Alk, D.; Sandbank, J.; Farber, R.; Halperin, N. Inflammatory reactions associated with a calcium sulfate bone substitute. Ann. Trans. 1999, 4, 91–97. [Google Scholar]
- Ogose, A.; Hotta, T.; Kawashima, H.; Kondo, N.; Gu, W.; Kamura, T.; Endo, N. Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Walsh, W.R.; Vizesi, F.; Michael, D.; Auld, J.; Langdown, A.; Oliver, R.; Yu, Y.; Irie, H.; Bruce, W. Β-tcp bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials 2008, 29, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Proussaefs, P.; Lozada, J.; Valencia, G.; Rohrer, M.D. Histologic evaluation of a hydroxyapatite onlay bone graft retrieved after 9 years: A clinical report. J. Prosthetic Dent. 2002, 87, 481–484. [Google Scholar] [CrossRef]
- Watanakunakorn, C.; Tisone, J.C. Synergism between vancomycin and gentamicin or tobramycin for methicillin-susceptible and methicillin-resistant staphylococcus aureus strains. Antimicrob. Agents Chemother. 1982, 22, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Penner, M.J.; Masri, B.A.; Duncan, C.P. Elution characteristics of vancomycin and tobramycin combined in acrylic bone—Cement. J. Arthroplast. 1996, 11, 939–944. [Google Scholar] [CrossRef]
- Concia, E.; Prandini, N.; Massari, L.; Ghisellini, F.; Consoli, V.; Menichetti, F.; Lazzeri, E. Osteomyelitis: Clinical update for practical guidelines. Nucl. Med. Commun. 2006, 27, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.K.; Suryanarayanan, R. Solid-state properties of tobramycin. Pharm. Res. 1991, 8, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- McLaren, R.; McLaren, A.; Vernon, B. Generic tobramycin elutes from bone cement faster than proprietary tobramycin. Clin. Orthop. Relat. Res. 2008, 466, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- McLaren, A.C.; McLaren, S.G.; Nelson, C.L.; Wassell, D.L.; Olsen, K.M. The effect of sampling method on the elution of tobramycin from calcium sulfate. Clin. Orthop. Relat. Res. 2002, 403, 54–57. [Google Scholar] [CrossRef]
- Patwardhan, S.; Shyam, A.K.; Mody, R.A.; Sancheti, P.K.; Mehta, R.; Agrawat, H. Reconstruction of bone defects after osteomyelitis with nonvascularized fibular graft: A retrospective study in twenty-six children. J. Bone Joint Surg. 2013, 95, e56. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.; Li-Bland, E.; Wild, L.; Schemitsch, E. A prospective, randomized clinical trial comparing an antibiotic-impregnated bioabsorbable bone substitute with standard antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis and infected nonunion. J. Orthop. Trauma 2010, 24, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Aiken, S.S.; Cooper, J.J.; Florance, H.; Robinson, M.T.; Michell, S. Local release of antibiotics for surgical site infection management using high-purity calcium sulfate: An in vitro elution study. Surg. Infect. 2015, 16, 54–61. [Google Scholar] [CrossRef] [PubMed]
- McConoughey, S.J.; Howlin, R.P.; Wiseman, J.; Stoodley, P.; Calhoun, J.H. Comparing PMMA and calcium sulfate as carriers for the local delivery of antibiotics to infected surgical sites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Sanicola, S.M.; Albert, S.F. The in vitro elution characteristics of vancomycin and tobramycin from calcium sulfate beads. J. Foot Ankle Surg. 2005, 44, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Blom, E.J.; Klein-Nulend, J.; Wolke, J.G.; van Waas, M.A.; Driessens, F.C.; Burger, E.H. Transforming growth factor-beta1 incorporation in a calcium phosphate bone cement: Material properties and release characteristics. J. Biomed. Mater. Res. 2002, 59, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.-P.; Traykova, T.; Planell, J.A. Calcium phosphate cements: Competitive drug carriers for the musculoskeletal system? Biomaterials 2006, 27, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.J.; Florance, H.; McKinnon, J.L.; Laycock, P.A.; Aiken, S.S. Elution profiles of tobramycin and vancomycin from high-purity calcium sulphate beads incubated in a range of simulated body fluids. J. Biomater. Appl. 2016, 31, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Wenisch, S.; Stahl, J.P.; Horas, U.; Heiss, C.; Kilian, O.; Trinkaus, K.; Hild, A.; Schnettler, R. In vivo mechanisms of hydroxyapatite ceramic degradation by osteoclasts: Fine structural microscopy. J. Biomed. Mater. Res. Part A 2003, 67, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Vlad, M.D.; Şindilar, E.; Mariñoso, M.L.; Poeată, I.; Torres, R.; López, J.; Barracó, M.; Fernández, E. Osteogenic biphasic calcium sulphate dihydrate/iron-modified α-tricalcium phosphate bone cement for spinal applications: In vivo study. Acta Biomater. 2010, 6, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Caturla, M.; Cusido, E.; Westerlund, D. High-performance liquid chromatography method for the determination of aminoglycosides based on automated pre-column derivatization with o-phthalaldehyde. J. Chromatogr. A 1992, 593, 69–72. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, M.; Ahmed, H.; Pace, L.; Minter, J.; Neel, M.; Jennings, J. Evaluation of Antibiotic-Releasing Triphasic Bone Void Filler In-Vitro. J. Funct. Biomater. 2018, 9, 55. https://doi.org/10.3390/jfb9040055
Harris M, Ahmed H, Pace L, Minter J, Neel M, Jennings J. Evaluation of Antibiotic-Releasing Triphasic Bone Void Filler In-Vitro. Journal of Functional Biomaterials. 2018; 9(4):55. https://doi.org/10.3390/jfb9040055
Chicago/Turabian StyleHarris, Michael, Hamza Ahmed, Leslie Pace, Jon Minter, Michael Neel, and Jessica Jennings. 2018. "Evaluation of Antibiotic-Releasing Triphasic Bone Void Filler In-Vitro" Journal of Functional Biomaterials 9, no. 4: 55. https://doi.org/10.3390/jfb9040055



