Abstract
Many congenital heart defects and degenerative valve diseases require replacement of heart valves in children and young adults. Transcatheter xenografts degenerate over time. Tissue engineering might help to overcome this limitation by providing valves with ability for self-repair. A transcatheter decellularized tissue-engineered heart valve (dTEHV) was developed using a polyglycolic acid (PGA) scaffold. A first prototype showed progressive regurgitation after 6 months in-vivo due to a suboptimal design and misguided remodeling process. A new geometry was developed accordingly with computational fluid dynamics (CFD) simulations and implemented by adding a polyether-ether-ketone (PEEK) insert to the bioreactor during cultivation. This lead to more belly-shaped leaflets with higher coaptation areas for this second generation dTEHV. Valve functionality assessed via angiography, intracardiac echocardiography, and MRI proved to be much better when compared the first generation dTEHV, with preserved functionality up to 52 weeks after implantation. Macroscopic findings showed no thrombi or signs of acute inflammation. For the second generation dTEHV, belly-shaped leaflets with soft and agile tissue-formation were seen after explantation. No excessive leaflet shortening occurred in the second generation dTEHV. Histological analysis showed complete engraftment of the dTEHV, with endothelialization of the leaflets and the graft wall. Leaflets consisted of collagenous tissue and some elastic fibers. Adaptive leaflet remodeling was visible in all implanted second generation dTEHV, and most importantly no fusion between leaflet and wall was found. Very few remnants of the PGA scaffold were detected even 52 weeks after implantation, with no influence on functionality. By adding a polyether-ether-ketone (PEEK) insert to the bioreactor construct, a new geometry of PGA-scaffold based dTEHV could be implemented. This resulted in very good valve function of the implanted dTEHV over a period of 52 weeks.
1. Introduction
Approximately 9 out of 1000 children are born with a congenital heart defect [1,2]. The Pulmonary valve is most frequently affected by these congenital heart defects, making it the valve that is most often replaced in people born with congenital heart disease [3,4].
Currently, there are two generally different types of valve prostheses being used for heart valve replacement: Mechanical and biological valves. However, these prostheses come with certain limitations. Most common are bleeding complications for mechanical heart valves and degeneration for biological valves, leading to the necessity of new prostheses for pulmonary valve replacement [5,6]. Degeneration of biological valves occurs mainly due to their inability to promote re-endothelialization of autologous cells and tissue remodeling [7,8].
Tissue engineering might help to overcome this limitation by providing valves with the ability for self- repair. The concept of scaffold based tissue-engineering on biodegradable synthetic polymers has become widely accepted for heart valve tissue-engineering [9]. PGA (polyglycolic acid)-based scaffolds have proven themselves as suitable matrices for this purpose [10,11].
However, different scaffold materials can largely influence the successful design of tissue-engineered constructs: PGA-P4HB (poly-4-hydroxybutyrate) scaffolds promote better tissue formation and higher levels of DNA, GAG, and HYP when compared to PGA-PLA (poly-lactic acid) or PGA-PCL (poly-caprolactone) scaffolds, whereas PGA-PCL scaffolds are more robust under biomechanical conditions when compared to PGA-P4HB and PGA-PLA scaffolds [12]. Fibrin-coated bis-urea modified poly-carbonates can be used as scaffolds for in-situ tissue-engineering with promising results [13]. As different types of scaffold promote cell migration into the scaffold fibers and neoformation of tissue differently, the scaffold plays an important role in the guidance of remodeling in tissue-engineered constructs. By inducing an immunoreaction, PGA-based scaffolds might be responsible for early recruitment of blood cells in a tissue-engineered heart valve [14].
In a European consortium, a transcatheter decellularized tissue-engineered heart valve (dTEHV) was developed on a PGA-P4HB-scaffold and implanted transvenously using a self-expanding nitinol-stent. A first generation of these valves showed progressive regurgitation after six months in vivo due to a suboptimal geometrical design. An optimized geometry was then developed using computational fluid dynamics (CFD) simulations, leading to more physiological diastolic load on the leaflets and hence better guidance of the remodeling process of the dTEHV [11,15,16,17]. This novel geometry was implemented by adding a polyether-ether-ketone insert to the bioreactor [18].
Here we present the role of a P4HB-coated PGA-scaffold combined with a polyether-ether-ketone insert for an improved functionality of decellularized tissue-engineered heart valves.
2. Results
After harvesting the dTEHV from the bioreactor, differences in geometry were clearly visible between the first (dTEHV1) and second generation (dTEHV2) of dTE-valves: Leaflets were much more belly-shaped with higher commissures and larger coaptation area in the second generation when compared to the first generation of dTEHV. Tissue formation was thinner and leaflets were subjectively more mobile in the second generation (Figure 1).
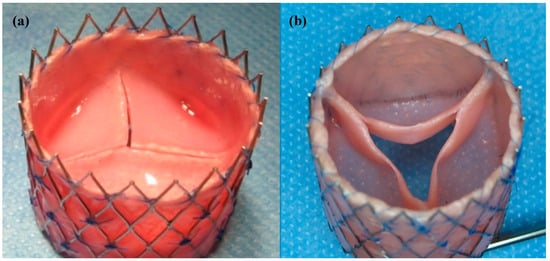
Figure 1.
Decellularized tissue-engineered heart valves after incubation in the bioreactor. (a) First generation transcatheter decellularized tissue-engineered heart valve (dTEHV), grown without the use of a poly-ether-ether-ketone (PEEK) insert; (b) Second generation dTEHV with an improved geometry due to the use of a PEEK insert during cultivation inside the bioreactor leading to more belly-shaped leaflets and larger coaptation areas between the leaflets.
With the transvenous implantation of a first generation of dTE-valves, Schmitt et al. proved the general feasibility of this minimally invasive approach to developing and implanting a tissue-engineered heart valve [15,16]. Their results showed a complete endothelialization of the leaflets and the graft wall 16 weeks after implantation, however remodeling in the implanted valves was somehow misguided. Active leaflet retraction through a-SMA positive cells on the valves surface as well as a fusion between leaflet and the graft wall lead to progressive central regurgitation [16].
Basically it can be said that through the newly developed geometry of the dTEHV, valve functionality over the course of 52 weeks was made possible. A direct comparison between the first and second generation dTEHV can be made, especially concerning results of intracardiac echocardiography (ICE), angiography, and macroscopic, as well as histological findings after explantation.
2.1. ICE
Valve functionality directly after implantation as well as after 24 weeks shows substantial improvement from first to second generation dTEHV. Even after 52 weeks, 6 out of 9 second generation dTEHV still remaining in the experiment showed only mild insufficiency (median insufficiency grade: Severe), whereas for the first generation dTEHV 4 out of 5 valves showed severe regurgitation at 24 weeks already (median insufficiency grade: Severe), mainly due to coaptation deficit. (Figure 2 and Figure 3).
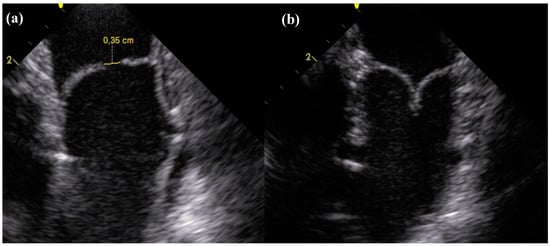
Figure 2.
Longitudinal section of the dTEHV during intracardiac echocardioraphy. (a) Coaptation deficit of 0.35 cm 24 weeks after implantation in a first generation dTEHV; (b) Coaptation 24 weeks after implantation in a second generation dTEHV (Coaptation was 0.5 cm).
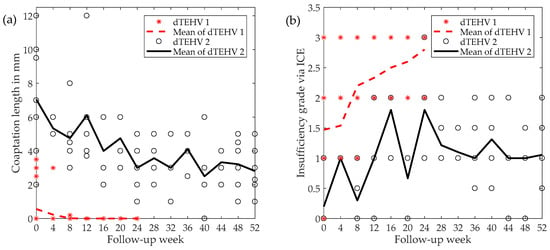
Figure 3.
(a) Development of coaptation length during follow-up for dTEHV 1 and dTEHV 2 assessed via intracardiac echocardiography (ICE); (b) Progression of insufficiency grade via ICE for dTEHV 1 and dTEHV 2. Insufficiencies were graded according to the guidelines by the European Society for Cardiology [19].
Statistical analysis showed significant improvements in pulmonary regurgitation grades and coaptation length directly after implantation, as well as coaptation length 24 weeks after implantation (Table 1). Since in-vivo testing for dTEHV 1 was only conducted over the course of 24 weeks, no comparison between the two generation at 52 weeks after implantation can be made.

Table 1.
Descriptive statistics of the development of insufficiency grade and coaptation length in first and second-generation transcatheter decellularized tissue-engineered heart valve (dTEHV). A Wilcoxon signed-rank test resulted in statistical significant differences (p < 0.05) between the two generations for insufficiency grade and coaptation length directly after implantation (week 0), as well as coaptation length 24 weeks after implantation. Significant results are marked with *.
2.2. Angiography
Directly after implantation, 6 out of 15 (40%) dTEHV 1 showed sufficient valve closure during angiography of the pulmonary artery. Eight out 15 (53.3%) showed slight regurgitation of the applied contrast medium into the RVOT. In one case (7.8%), strong regurgitation with contrast medium staining the whole RV was seen.
All dTEHV 2 showed sufficient closing function in angiography directly after implantation. No contrast medium regurgitated into the RVOT or right ventricle. No paravalvular leakage was recorded either (Figure 4).
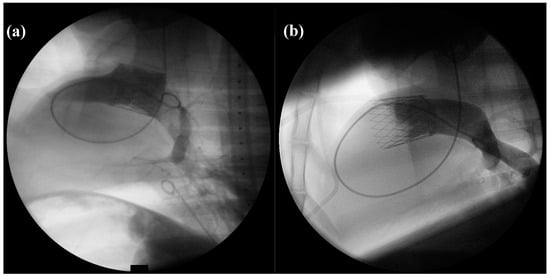
Figure 4.
PA-angiography with the application of contrast medium directly after implantation of the dTEHV (Left: First generation dTEHV; right: Second generation dTEHV). (a) Insufficient closure of the newly implanted dTEHV 1—contrast medium regurgitates back into the RVOT; (b) Sufficient closure of the newly implanted dTEHV 2—no contrast medium regurgitates back into the RVOT or RV. Even the belly-shaped leaflets can be seen in the picture.
2.3. Macroscopic Analysis
After explantation, fusion between the leaflets and the graft wall, as well as leaflet shortening, were macroscopically visible in the first generation of dTEHV. This was more evident after 24 weeks when compared to the explanted specimen after 8 and 16 weeks, leading to the conclusion of a continually progressive process during the recorded follow-up time (Figure 5).
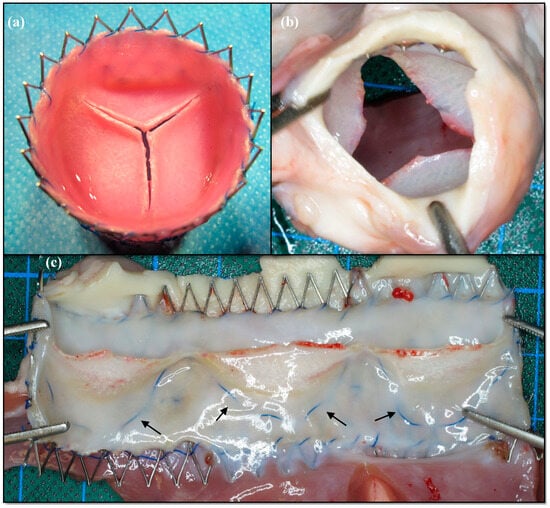
Figure 5.
Macroscopic findings of the first generation dTEHV. (a) Stented dTEHV 1 before implantation; (b) Same dTEHV 1 after explantation. Central coaptation deficit is clearly visible; (c) Same dTEHV1 after explantation cut open longitudinally. Leaflet shortening is present as the blue suture line (black arrows) marked the hinge region before implantation.
Leaflet shortening and fusion between leaflets and graft wall were not present in the second generation dTEHV. Leaflets were still belly-shaped and mobile after explantation. Anatomical features of native pulmonary valves, such as a lunula at the upper rim, as well as a small nodulus in the center of each lunula, were seen. Mean distance from upper rim of the leaflet to the hinge region was 21.9 ± 1.46 mm after explantation. Macroscopically, there were no signs of active endocarditis, calcification, or thrombi visible on the explanted valves. Complete engraftment of the stented valve in the surrounding structures was observed. A thin and shiny layer of endocardium covered the stent struts at the proximal and distal part of the valve. (Figure 6).
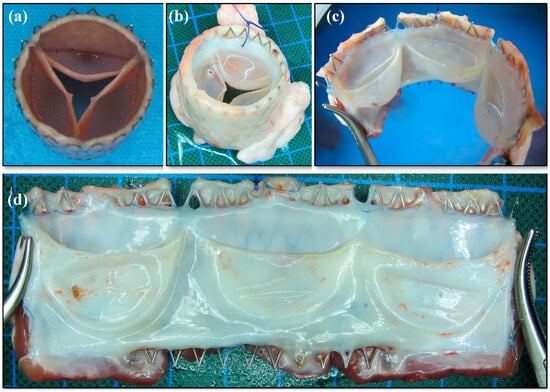
Figure 6.
Macroscopic findings of second generation dTEHV. (a) Stented dTEHV2 before implantation; (b) Same valve after explantation loaded with water inside the leaflets; (c) Same valve after explantation cut open between two leaflets; (d) Same dTEHV after explantation in a lateral view of the opened valve. Soft, mobile and belly-shaped leaflets with high coaptation areas can be seen. Tissue formation seems thin, but strong and covered with a shiny endothelial layer.
2.4. Histology
In the histological analysis, the first generation dTEHV showed partial re-endothelialisation after 8 weeks and complete after 16 weeks. Progressive leaflet shortening, that was visible in the macroscopic analysis, was also confirmed in histology of dTEHV 1 as leaflet length continually decreased from implant to explantation after 8, 16, and 24 weeks (Figure 7). In the lower leaflet area, thrombi and cell conglomerates were found due to insufficient wash-out in this area.
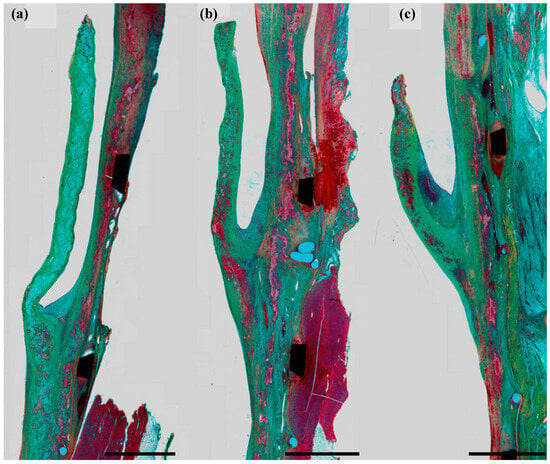
Figure 7.
From left to right: Explanted leaflets of a dTEHV1 at 8 (a), 16 (b), and 24 (c) weeks after implantation. Progressive leaflet shortening is clearly visible. This figure was previously used by Schmitt et al. [16]. The polyglycolic acid (PGA) scaffold (light blue) was continually replaced by collagen ECM (green) and muscular matrix (red). Black scale bar represents 2 mm.
Complete re-endothelialisation was confirmed in dTEHV 2 as all explanted valves presented themselves with an endothelial layer covering leaflets and the graft wall. Leaflets consisted mostly of collagenous fiber with some elastic fibers. Some remnants of the scaffold were seen in the apical portions of the leaflets, however they did not influence functionality. Foreign body reaction against scaffold remnants in the graft wall was present in the form of foreign-body giant-cells.
Chronic foreign body reaction against the stent struts was visible in the form of histiocytic infiltrations surrounding the stent struts. No signs of acute inflammation were found anywhere within the leaflets or graft wall.
In most explanted dTEHV 2 specimens, scaffold remnants were found as shown in Figure 8. These remnants did not have any effect on the functionality of the valves. As scaffold remnants promote rapid cellular infiltration and can induce an immunoreaction [14], these remnants might still have an effect on the remodeling process, even 52 weeks after implantation.
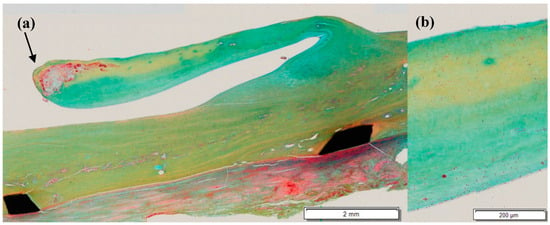
Figure 8.
Movat’s pentachrome stain of an explanted dTEHV2. (a) Overview of the explanted valve. Remntants of the PGA-scaffold were found at the tip of the leaflet; (b) magnified section of the middle part of the leaflet. A multylayered mircostructure is visible.
For second generation dTE-valves, a multi-layered, almost native-like structure of the leaflets was noticeable in histological analyses (Figure 8), implying a very well-directed and physiological remodeling process.
Whereas αSMA-positive cells were found to be mainly responsible for an active leaflet retraction in dTEHV1 [16], different histoligical analyses showed different results for dTEHV 2: Emmert et al. examined the same explanted dTEHV 2 and did not find any αSMA-positive cells on the leaflets surface [20], whereas our histological stains did indeed show some αSMA-positive cells. This may lead to the conclusion of functional irrelevance of the detected αSMA-positive cells.
2.5. MRI
As described, functionality improved substantially from first to second generation dTEHV. As there were no MRI scans performed to assess functionality of the first generation, no accurate comparison can be made concerning MRI-data. However, functionality in MRI proved to be generally good over the course of the set follow-up of 52 weeks. As described above, 9 out of the 10 valves reached the set endpoint of 52 weeks, only one sheep had to be euthanized due to regurgitation exceeding 30% in MRI at 24 weeks according to the study protocol.
Median regurgitation fraction determined by MRI slightly increased from 9% one week after implantation to 14.7% after 52 weeks, however, all remaining valves stayed under the threshold of 30% at the time of explantation, leading to the conclusion of no hemodynamic relevant insufficiencies 52 weeks after implantation (Figure 9).
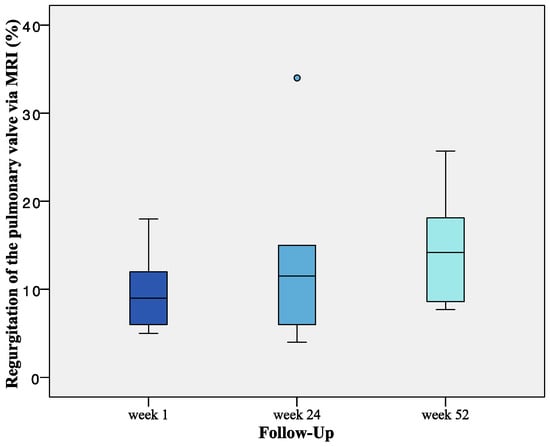
Figure 9.
Progression of Regurgitation fraction assessed via MRI for dTEHV2. Regurgitation increased from a median of 9% (one week after implantation) to 14.7% 52 weeks after implantation (mean regurgitation fraction at 52 weeks was 13.9 ± 5.7%). n = 10 for week 1. n = 6 for week 24. n = 9 for week 52.
3. Discussion
Over the last years, scaffold-based tissue-engineering has proven to provide a suitable alternative to biological heart valve prostheses made from porcine or bovine pericardium in the future. In contrast to these conventional heart valve prostheses, the biological material that is implanted in tissue-engineered heart valves has not been cross-linked with glutaraldehyde and provides an environment for reendothelialization of autologous cells, furthermore inducing a remodeling process. Decellularization plays an important role in the manufacturing process in order to eradicate foreign antibody structures on the grafts surface, as the dTEHV used in our study were grown from homologous and not autologous stem cells. However, using autologous stem cells would not provide a practical alternative to our homologous approach, as infrastructural challenges would increase substantially.
During the in-vivo study of the first generation dTEHV, follow-up of 24 weeks was achieved. However, functionality deteriorated drastically shortly after implantation. Mechanisms for this functional failing of these valves have been identified: Particularly active leaflet shortening conducted through contractile αSMA positive cells and fusion between the leaflets and graft wall due to reduced wash out in the hinge area of the valve were responsible for progressive central regurgitations [16].
In general, it can be said, that by developing a new geometry of these dTEHV, these failing mechanisms could be eradicated in the second generation dTHEV. There were no cell clusters or thrombi found in the hinge areas of the valves. αSMA positive cells were found in our histological analysis. Their functional relevance is at least questionable, though, since Emmert et al. found no αSMA-positive cells on the leaflets surface [20]. However, slight shortening of the leaflets did still occur. Considering that coaptation area slightly decreased between implantation and week 24 of follow-up, but then remained relatively stable, it can be reasoned that after 24 weeks, remodeling inside the valves also remains stable. Hence, leaflet shortening might not become much greater.
Remodeling seems to be much better guided and controlled in the new generation of dTEHV when compared to the results of Schmitt et al. and Driessen-Mol et al. from the first generation dTEHV [16,17]. Computational fluid dynamics simulations predicted a better functional outcome of more belly-shaped dTEHV due to higher radial strain on the leaflets during diastole [16].
As Emmert et al. [20] have pointed out in a recently published paper about the same dTEHV 2, a rather remarkable result is the finding of elastic fibers in most specimen, as there is usually little to no synthesis of elastic fibers in adult tissue-formations of the pulmonary valve after closure of the foramen ovale [21]. Diastolic loading of the leaflets plays a key role for guidance of the remodeling process [22].
Concerning the presence of αSMA-positive cells at the leaflets surface as key contractile agents, dTEHV 2 offered a significant step forward as well. Leaflet shortening in previous studies was mainly conducted by contraction of αSMA-positive cells [16]. Emmert et al. found that αSMA-positive cells were nonexistent in the second generation of dTEHV, similar to native pulmonary valves [20]. Our histological analysis showed some cells that were αSMA-positive, however having no effect on valve functionality.
Hence, the newly developed geometry of dTEHV 2 leading to more physiological hemodynamics surrounding the valve itself, poses a big step towards a more native-like pulmonary valve [20]. The use of an insert during cultivation of these valves functions as a pivot point on the way towards developing a PGA-scaffold based tissue-engineered heart valve with good long-term functionality.
Kluin et al. followed a rather different approach in developing a heart valve capable of remodeling with in-situ tissue-engineering on a thermoplastic elastomer PC-BU scaffold that is implanted and then in-vivo recellularized by autologous cells to form a living heart valve, while the scaffold slowly degrades. Their study also shows promising results with good valve functionality after 12 months. However, a polyether-ether-ketone ring supporting the PC-BU scaffold is vital for mechanical support [13]. A minimally invasive—and therefore less traumatic—implantation has not yet been achieved with in-situ tissue-engineering.
Results of the present study pose the question of finding the optimal time point for adding the polyether-ether-ketone insert to the bioreactor during incubation of a dTEHV 2. Even though functionality improved quite significantly from dTEHV 1 to dTEHV 2, there were still differences in functional performance of the second generation dTE-valves. Since the PEEK-insert was added to the bioreactor at three different time points (Day 0/10/13 of Incubation), tissue-formation was slightly variable between the production batches. Looking at the functional end-points (regurgitaton fraction (RF) via MRI) of each production batch, it is apparent that the time of adding the insert might influence the functional outcome rather substantially. Median RF at 52 weeks was 14.2%. All four dTEHV 2 of the 13 days batch were above the median RF at that point (25.7%; 14.2%; 18.1%; 18.3%), whereas the two valves of the 10 days batch presented a rather good functionality, with both valves at a regurgitation fraction of 8.6%. In the group of the start-batch outcome varied the most, with one animal dropping out due to high regurgitation 24 weeks after implantation and three dTEHV 2 with an RF equally (14.7%) or lower than the median RF (7.7%; 9.5%). In summary, out of nine dTEHV 2 that reached the set follow-up time of 52 weeks, four valves showed RF less than median, one was about median RF, and four valves showed RF higher than the median. The difference of the distance from upper rim of the leaflet to the hinge region before implantation and after explantation provides a suitable macroscopic correlate to the variable functionality. In the group of valves with RF >median, this distance decreased by 3.3 ± 0.94 mm versus 1.1 ± 0.48 mm in the group of valves with RF <median. Due to rather small group size in both cohorts, no statistical significant differences can be identified. However, this result may suggest the importance of finding the right time point of adding the PEEK-insert to the bioreactor. In theory tissue-formation inside the bioreactor is slightly inhibited by the insert, leading to thicker tissue in the leaflets for the 13 days batch when compared to the start-batch. Since radial strain during diastolic loading depends on tissue strength, diastolic pressure, and anisotropy of the tissue fibers [18,22], in-vivo remodeling can be largely influenced by different time points of adding the insert to the bioreactor.
4. Materials and Methods
Valve manufacturing and study protocol of an in-vivo study to test the first generation of decellularized tissue-engineered heart valves have been published before [16]. The following Materials and Methods section will therefore only refer to the manufacturing process and study protocol of the second generation dTEHV.
4.1. Valve Manufacturing
Ten dTEHV with an improved geometry were grown in a bioreactor on a biodegradable polyglygolic acid scaffold. Detailed manufacturing process has been described previously [17]. In short, ovine myofibroblast-derived cells were seeded onto a biodegradable PGA (polyglycolic acid) scaffold coated with P4HB (poly-4-hydroxybutyrate), that was sutured in a self-expanding nitinol stent and placed in a bioreactor equipped with a pulsatile flow. After incubation in a bioreactor for four weeks, the tissue engineered heart valve was harvested and decellularized. A shape-giving insert made from polyether-ether-ketone (PEEK) was added to the bioreactor right from the start of incubation, after 10 or 13 days as described previously [18], leading to different batches of dTE-valves. The inserts were placed on the arterial side of the valve and were equipped with 0.5 mm wide holes in order to ensure circulation of the medium inside the bioreactor (Figure 10).
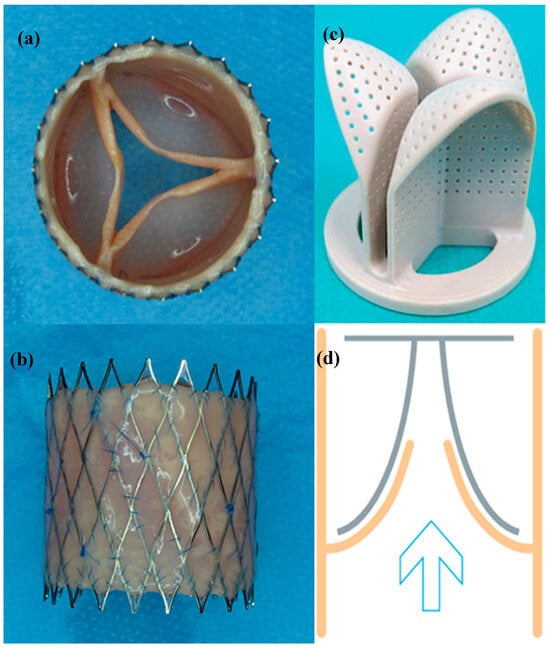
Figure 10.
(a) Top view of a second generation dTEHV after harvesting the valve from the bioreactor; (b) Lateral view of the same valve displayed in (a); (c) Polyether-ether-ketone insert, that was used in the bioreactor to implement the new valve geometry; (d) Schematic sketch of how the PEEK insert was applied to the bioreactor: the tissue-engineered valve is displayed in orange, the insert was added to the arterial side of the leaflets to promote a curved form of the leaflets. The blue indicates the flow direction of the pulsatile flow that was applied inside the bioreactor.
4.2. Delivery System
The dTEHV were implanted minimally invasive into the pulmonary position into sheep, using a custom made delivery system that was introduced through the external jugular vein (Figure 11). This delivery system was developed in order to adequately deploy the stented valve. Specific features have been described in previous publications [23]. In short, the system consisted of an inner sheath made from a steel coil coated with an outer sheath made from a thermoplastic elastomer (Pebax® by Arkema, Colombe, France). At the distal part of these tubes lies the capsule—basically a broadening of the outer sheath—in which the stented valve can be loaded and released. The deployment is performed by pulling back the outer sheath over the inner sheath which functions as a counter bearing for the valve. The process of the deployment can be controlled by the handle which lies on the proximal part of the tube.
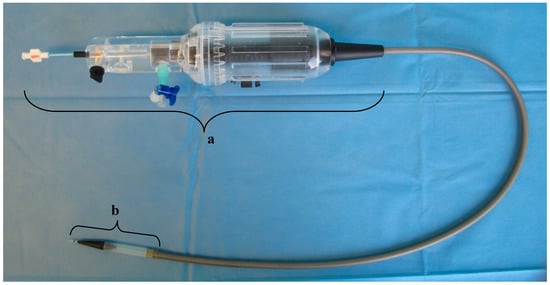
Figure 11.
Delivery system designed for the deployment of the dTEHV. The handle is marked with “a”. At the tip of the delivery system lies the capsule in which the dTEHV can be loaded after crimping (marked with “b”). The middle part consists of a steel coil with an outer sheath made from a thermoplastic elastomer.
4.3. Study Protocoll
CT and MRI scans were performed prior to implantation in order to assess proportions and flow dynamics of the native pulmonary valves. The implantations were framed by intracardiac echocardiography, angiography, as well as pressure measurements of the RA, RV, and PA. After implantation, flow measurements in MRI and intracardiac echocardiography were performed monthly in order to gain detailed data of the valves functionality. After 52 weeks, the valves were explanted and examined macroscopically for thrombi, signs of endocarditis and integrity of the leaflets. Histology was performed subsequently. The study protocol determined that valves exceeding a threshold of 30% regurgitation fraction in MRI (marking the border between moderate and severe regurgitation) should be explanted prematurely.
Valve insufficiency in ICE was graded according to the recommendations for the echocardiographic assessment of native valvular regurgitation by the European Society of Cardiology [19].
The study was approved by the local authorities (Regional Office for Health and Social Affairs Berlin, LAGeSo, Berlin; approval no. G0111/11). Animals were treated in accordance with the guidelines of the European and German Societies of Laboratory Animal Science.
5. Conclusions
A polyether-ether-ketone insert helps improve the geometry of a transcatheter decellularized tissue-engineered pulmonary heart valve, leading to good functionality over the course of 52 weeks. Failing mechanisms of a previously tested, first generation of dTEHV could be eradicated accordingly. Histological findings show remnants of polyglycolic acid scaffold even after 52 weeks, however without any influence on the functionality of the affected valves. Hence, PGA proves to be a suitable scaffold for heart valve tissue-engineering.
Author Contributions
Conceptualization: L.B., B.S., H.S., F.B. Methodology: B.S., H.S., L.B., K.B., M.S. Software: L.B., B.S. Validation: L.B., B.S., H.S. Formal Analysis: L.B. Investigation: L.B., B.S., H.S., M.S. Resources: B.S., F.B. Data Curation: L.B., H.S., V.S., K.B., M.S. Writing-Original Draft Preparation: L.B. Writing-Review & Editing: B.S. Visualization: L.B., B.S. Supervision: B.S., F.B. Project Administration: B.S., F.B. Funding Acquisition: B.S., F.B.
Funding
This research was funded by the European Union’s Seventh Framework Program (FP7/2007-2013) under grant agreement no. 242008.
Acknowledgments
We thank M. Bartosch (Department of Congenital Heart Disease, German Heart Center Berlin, Germany) for developing the minimally invasive delivery system and K. Weber for coordination of animal experiments and man-power. We also gratefully acknowledge the German Heart Foundation (Deutsche Herzstiftung e.V.) for granting the first author their Kaltenbach-scholarship (Kaltenbach-Doktorandenstipendium).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoffman, J.I.E.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Reimer, J.; Syedain, Z.; Haynie, B.; Lahti, M.; Berry, J.; Tranquillo, R. Implantation of a Tissue-Engineered Tubular Heart Valve in Growing Lambs. Ann. Biomed. Eng. 2017, 45, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Henaine, R.; Roubertie, F.; Vergnat, M.; Ninet, J. Valve replacement in children: A challenge for a whole life. Arch. Cardiovasc. Dis. 2012, 105, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Cannegieter, S.C.; Rosendaal, F.R.; Briet, E. Thromboembolic and Bleeding Complications in Patients with Mechanical Heart-Valve Prostheses. Circulation 1994, 89, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Head, S.J.; Celik, M.; Kappetein, A.P. Mechanical versus bioprosthetic aortic valve replacement. Eur. Heart J. 2017, 38, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Rabkin-Aikawa, E.; Mayer, J.E., Jr.; Schoen, F.J. Heart valve regeneration. Adv. Biochem. Eng. Biotechnol. 2005, 94, 141–179. [Google Scholar] [PubMed]
- Schoen, F.J.; Gotlieb, A.I. Heart valve health, disease, replacement, and repair: A 25-year cardiovascular pathology perspective. Cardiovasc. Pathol. 2016, 25, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Fioretta, E.S.; Dijkman, P.E.; Emmert, M.Y.; Hoerstrup, S.P. The future of heart valve replacement: Recent developments and translational challenges for heart valve tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e323–e335. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, A.A.; Mitchell, K.J.; Cesarovic, N.; Schwarzwald, C.C.; Hoerstrup, S.P.; Weber, B. PGA (polyglycolic acid)-P4HB (poly-4-hydroxybutyrate)-Based Bioengineered Valves in the Rat Aortic Circulation. J. Heart Valve Dis. 2016, 25, 380–388. [Google Scholar] [PubMed]
- Dijkman, P.E.; Driessen-Mol, A.; Frese, L.; Hoerstrup, S.P.; Baaijens, F.P.T. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials 2012, 33, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Generali, M.; Kehl, D.; Capulli, A.K.; Parker, K.K.; Hoerstrup, S.P.; Weber, B. Comparative analysis of poly-glycolic acid-based hybrid polymer starter matrices for in vitro tissue engineering. Colloids Surf. B Biointerfaces 2017, 158, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kluin, J.; Talacua, H.; Smits, A.I.; Emmert, M.Y.; Brugmans, M.C.; Fioretta, E.S.; Dijkman, P.E.; Sontjens, S.H.; Duijvelshoff, R.; Dekker, S.; et al. In situ heart valve tissue engineering using a bioresorbable elastomeric implant—From material design to 12 months follow-up in sheep. Biomaterials 2017, 125, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.; Driessen-Mol, A.; Bouten, C.V.C.; Baaijens, F.P.T. The Effects of Scaffold Remnants in Decellularized Tissue-Engineered Cardiovascular Constructs on the Recruitment of Blood Cells. Tissue Eng. Part A 2017, 23, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Spriestersbach, H.; Prudlo, A.; Bartosch, M.; Sanders, B.; Radtke, T.; Baaijens, F.P.T.; Hoerstrup, S.P.; Berger, F.; Schmitt, B. First percutaneous implantation of a completely tissue-engineered self-expanding pulmonary heart valve prosthesis using a newly developed delivery system: A feasibility study in sheep. Cardiovasc. Interv. Ther. 2017, 32, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.; Spriestersbach, H.; Radtke, T.; Bartosch, M.; Peters, H.; Sigler, M.; Frese, L.; Dijkman, P.E.; Baaijens, F.P.; Hoerstrup, S.P.; et al. Percutaneous pulmonary valve replacement using completely tissue-engineered off-the-shelf heart valves: Six-month in vivo functionality and matrix remodelling in sheep. EuroIntervention 2016, 12, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Driessen-Mol, A.; Emmert, M.Y.; Dijkman, P.E.; Frese, L.; Sanders, B.; Weber, B.; Cesarovic, N.; Sidler, M.; Leenders, J.; Jenni, R.; et al. Transcatheter Implantation of Homologous “Off-the-Shelf” Tissue-Engineered Heart Valves With Self-Repair Capacity. J. Am. Coll. Cardiol. 2014, 63, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.; Loerakker, S.; Fioretta, E.S.; Bax, D.J.; Driessen-Mol, A.; Hoerstrup, S.P.; Baaijens, F.P. Improved Geometry of Decellularized Tissue Engineered Heart Valves to Prevent Leaflet Retraction. Ann. Biomed. Eng. 2016, 44, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L.; Scientific Document Committee of the European Association of Cardiovascular. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [PubMed]
- Emmert, M.Y.; Schmitt, B.A.; Loerakker, S.; Sanders, B.; Spriestersbach, H.; Fioretta, E.S.; Bruder, L.; Brakmann, K.; Motta, S.E.; Lintas, V.; et al. Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, E.; Whittaker, P.; Farber, M.; Mendelson, K.; Padera, R.F.; Aikawa, M.; Schoen, F.J. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation 2006, 113, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Loerakker, S.; Argento, G.; Oomens, C.W.; Baaijens, F.P. Effects of valve geometry and tissue anisotropy on the radial stretch and coaptation area of tissue-engineered heart valves. J. Biomech. 2013, 46, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, M.; Peters, H.; Spriestersbach, H.; Darach, O.H.I.; Berger, F.; Schmitt, B. A Universal Delivery System for Percutaneous Heart Valve Implantation. Ann. Biomed. Eng. 2016, 44, 2683–2694. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).