Types of Bone Substitutes and Their Application in Regenerative Medicine: A Systematic Review
Abstract
1. Introduction
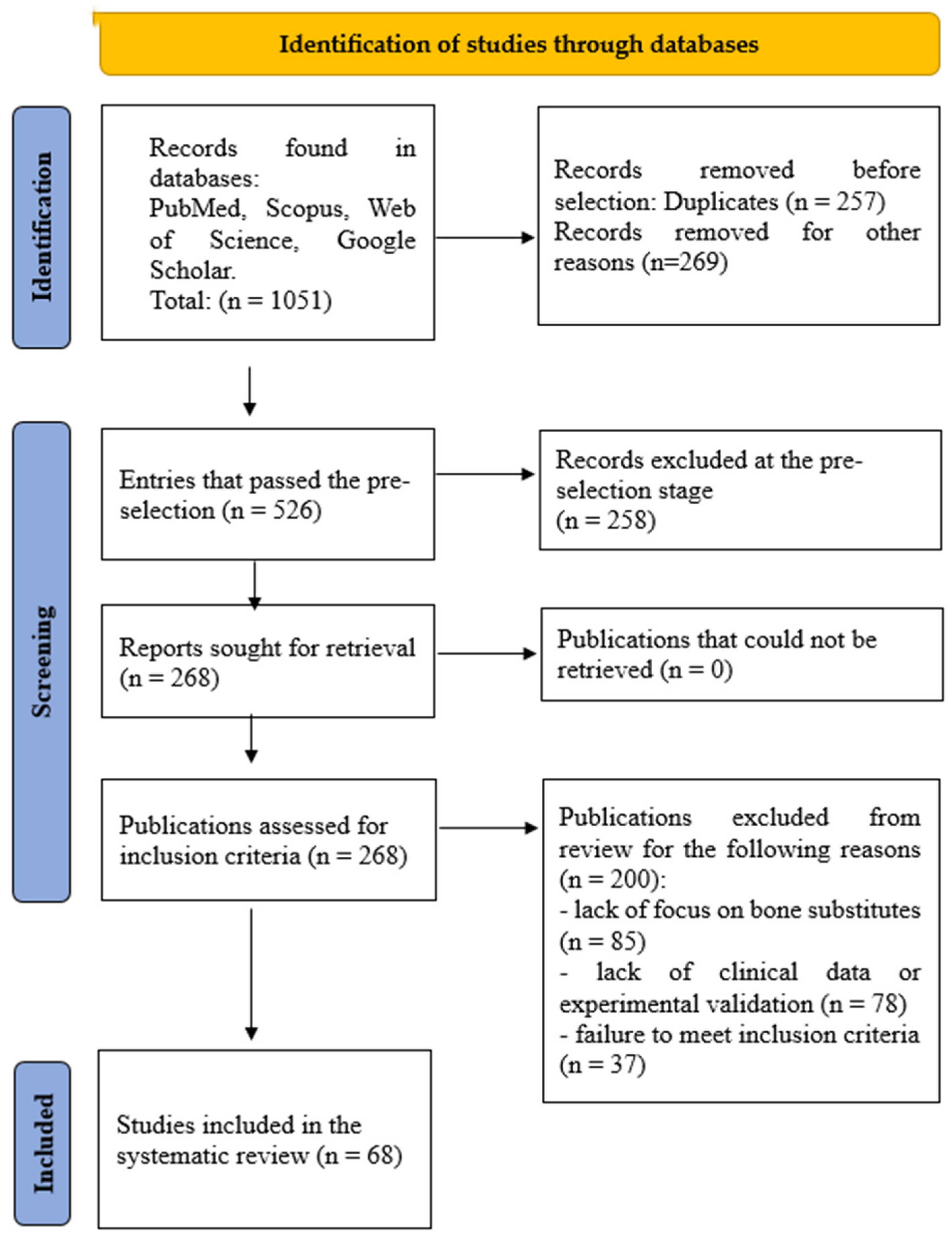
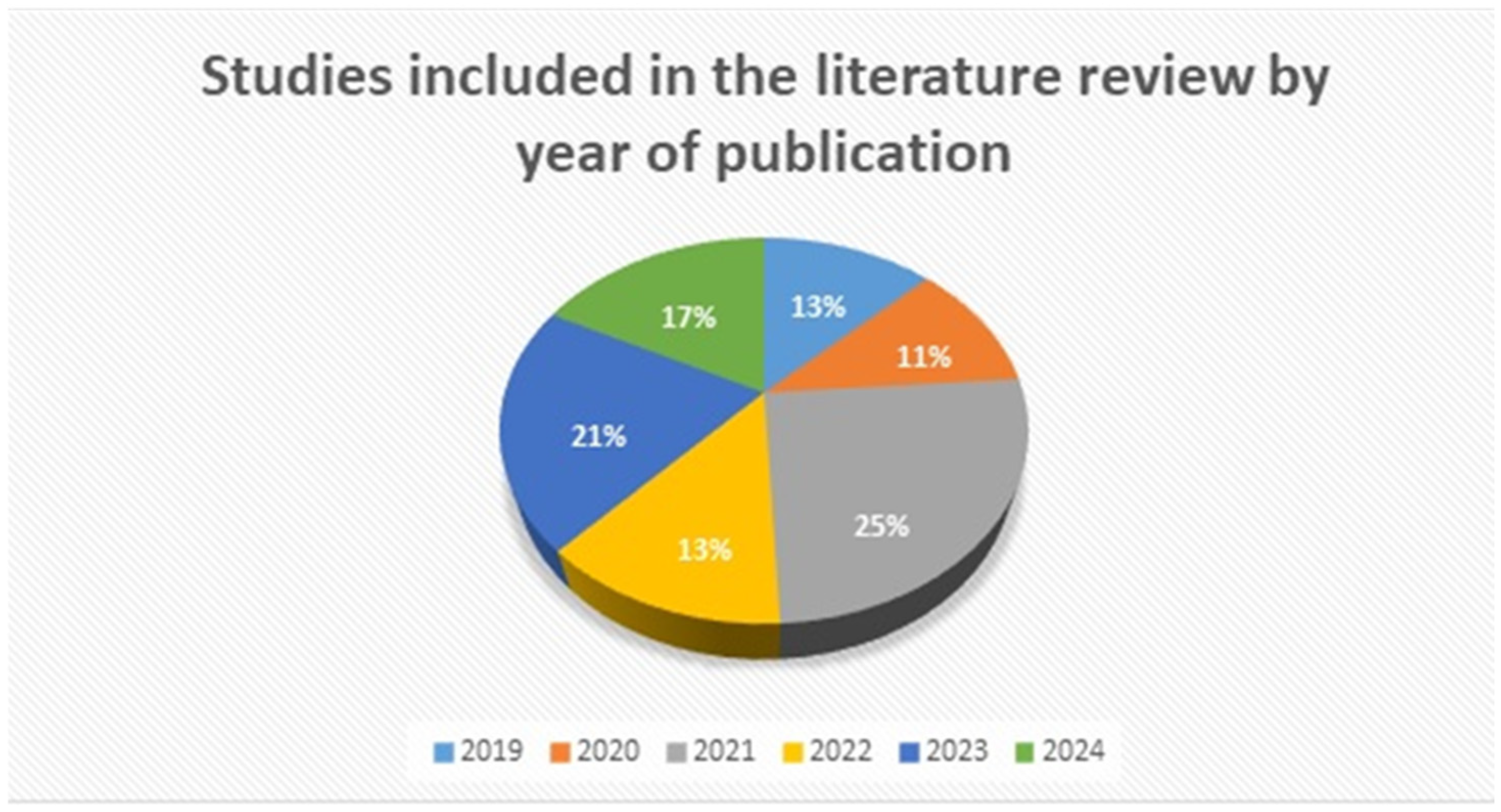
2. Materials and Methods
3. Results and Discussion
3.1. Importance of Bone Substitutes in Regenerative Medicine
3.2. Main Categories and Characteristics of Modern Bone Substitutes
3.2.1. Autologous Bone Grafts
3.2.2. Allogeneic Bone Substitutes
3.2.3. Xenogeneic Bone Substitutes
3.2.4. Synthetic Bone Substitutes
3.3. Biological and Mechanical Properties of Bone Substitutes
3.4. Clinical Applications in Regenerative Medicine
3.5. Three-Dimensional Bioprinting and Future Directions in Bone Substitution
- -
- Extrusion-based bioprinting
- -
- Inkjet-Based Bioprinting
- -
- Pressure-assisted bioprinting
- -
- Laser-assisted bioprinting
3.6. Advanced Bioactive Strategies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| MeSH | Medical Subject Headings |
| BMP | Bone Morphogenetic Proteins |
| CGF | Concentrated Growth Factor |
| BMP-2 | Bone Morphogenetic Protein-2 |
| BMP-7 | Bone Morphogenetic Protein-7 |
| DBM | Demineralised Bone Matrix |
| rhVEGF | Recombinant Human Vascular Endothelial Growth Factor |
| ACL | Anterior Cruciate Ligament |
| GBR | Guided Bone Regeneration |
| PRF | Platelet-Rich Fibrin |
| PRP | Platelet-rich plasma |
| NAAT | Nucleic Acid Amplification Tests |
| AATB | American Association of Tissue Banks |
| Allo-DDM | Allogeneic Demineralised Dentin Matrix |
| HHP | High Hydrostatic Pressure |
| PCMV/PRV | Porcine Cytomegalovirus/Porcine Retrovirus |
| PERVs | Porcine Endogenous Retroviruses |
| β-TCP | β-Tricalcium Phosphate |
| TPMS | Triply Periodic Minimal Surfaces |
| PLA | Polylactic Acid |
| PCL | Polycaprolactone |
| ZrO2 | Zirconium Oxide |
| micro-CT | Micro-Computed Tomography |
| PLGA | Poly(lactic-glycolic acid) |
| BCP | Biphasic Calcium Phosphate |
| DPSCs | Human Dental Pulp Stem Cells |
| EVs | Extracellular Vesicles |
| rhPDGF-BB | Recombinant Human Platelet-Derived Growth Factor-BB |
| CPCs | Calcium phosphate cements |
| PEG | Polyethylene glycol |
| ECM | Extracellular matrix |
| RMAT | Regenerative Medicine Advanced Therapy |
References
- Ferraz, M.P. Bone grafts in dental medicine: An overview of autografts, allografts, and synthetic materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef]
- Heitzer, M.; Modabber, A.; Zhang, X.; Winnand, P.; Zhao, Q.; Bläsius, F.M.; Buhl, E.M.; Wolf, M.; Neuss, S.; Hölzle, F.; et al. In vitro comparison of the osteogenic capability of human pulp stem cells on alloplastic, allogeneic, and xenogeneic bone scaffolds. BMC Oral Health 2023, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Fattahian, H.; Mansouri, K.; Mansouri, N. Biomaterials, substitutes, and tissue engineering in bone repair: Current and future concepts. Comp. Clin. Pathol. 2019, 28, 879–891. [Google Scholar] [CrossRef]
- Dahiya, U.; Mishra, S.; Bano, S. Application of bone substitutes and its future prospective in regenerative medicine. In Biomaterial-Supported Tissue Reconstruction or Regeneration; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Manzini, B.; Machado, L.; Noritomi, P.; Da Silva, J. Advances in bone tissue engineering: A fundamental review. J. Biosci. 2021, 46, 17. [Google Scholar] [CrossRef]
- Miletić, M.; Puač, N.; Škoro, N.; Brković, B.; Andrić, M.; Prokić, B.B.; Danilović, V.; Milutinović-Smiljanić, S.; Mitrović-Ajtić, O.; Mojsilović, S. Bone regeneration potential of periodontal ligament stem cells in combination with cold atmospheric plasma-pretreated beta-tricalcium phosphate: An in vivo assessment. Appl. Sci. 2023, 14, 16. [Google Scholar] [CrossRef]
- Mukhlis, S.; Issabekova, A.; Kudaibergen, G.; Mukhambetova, A.; Nurkina, A.; Altayeva, N.; Ashikbayeva, M.; Temirzhan, A.; Baidarbekov, M.; Ogay, V. The role of pericytes in bone tissue regeneration and therapeutic applications. Eurasian J. Appl. Biotechnol. 2024, 50, 236. [Google Scholar] [CrossRef]
- Dőri, F.; Sculean, A.; Takács, D.; Suba, Z. Histological examination of retrieved ePTFE membranes following regenerative surgery of intrabony defects treated with platelet-rich plasma and bone substitutes. Oral Health Prev. Dent. 2022, 20, 143–148. [Google Scholar] [CrossRef]
- Zhang, Q.; Nettleship, I.; Schmelzer, E.; Gerlach, J.; Zhang, X.; Wang, J.; Liu, C. Tissue engineering and regenerative medicine therapies for cell senescence in bone and cartilage. Tissue Eng. Part B Rev. 2019, 25, 500–526. [Google Scholar] [CrossRef]
- Rupp, M.; Klute, L.; Baertl, S.; Walter, N.; Mannala, G.K.; Frank, L.; Pfeifer, C.; Alt, V.; Kerschbaum, M. The clinical use of bone graft substitutes in orthopedic surgery in Germany—A 10-years survey from 2008 to 2018 of 1,090,167 surgical interventions. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 350–357. [Google Scholar] [CrossRef]
- Laubach, M.; Hildebrand, F.; Suresh, S.; Wagels, M.; Kobbe, P.; Gilbert, F.; Kneser, U.; Holzapfel, B.M.; Hutmacher, D.W. The concept of scaffold-guided bone regeneration for the treatment of long bone defects: Current clinical application and future perspective. J. Funct. Biomater. 2023, 14, 341. [Google Scholar] [CrossRef]
- Santoro, A.; Voto, A.; Fortino, L.; Guida, R.; Laudisio, C.; Cillo, M.; D’Ursi, A.M. Bone defect treatment in regenerative medicine: Exploring natural and synthetic bone substitutes. Int. J. Mol. Sci. 2025, 26, 3085. [Google Scholar] [CrossRef]
- Tournier, P.; Guicheux, J.; Paré, A.; Maltezeanu, A.; Blondy, T.; Veziers, J.; Vignes, C.; André, M.; Lesoeur, J.; Barbeito, A.; et al. A partially demineralized allogeneic bone graft: In vitro osteogenic potential and preclinical evaluation in two different intramembranous bone healing models. Sci. Rep. 2021, 11, 4907. [Google Scholar] [CrossRef]
- Parikh, S.; Pentz, R.; Haight, A.; Adeli, M.; Martin, P.; Driscoll, T.; Page, K.; Kurtzberg, J.; Prasad, V.K.; Barfield, R.C.; et al. Ethical considerations of using a single minor donor for three bone marrow harvests for three HLA-matched siblings with primary immunodeficiency. Pediatr. Blood Cancer 2019, 66, e27602. [Google Scholar] [CrossRef]
- Sharifi, M.; Kheradmandi, R.; Salehi, M.; Alizadeh, M.; Hagen, T.; Falahati, M. Criteria, challenges, and opportunities for acellularized allogeneic/xenogeneic bone grafts in bone repairing. ACS Biomater. Sci. Eng. 2022, 8, 3199–3219. [Google Scholar] [CrossRef] [PubMed]
- Ana, I. Bone substituting materials in dental implantology. In Bone Management in Dental Implantology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 101–119. [Google Scholar] [CrossRef]
- Janjua, O.S.; Qureshi, S.M.; Shaikh, M.S.; Alnazzawi, A.; Rodriguez-Lozano, F.J.; Pecci-Lloret, M.P.; Zafar, M.S. Autogenous tooth bone grafts for repair and regeneration of maxillofacial defects: A narrative review. Int. J. Environ. Res. Public Health 2022, 19, 3690. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Shaikh, A.; Ayub, T.; Rehman, A.; Khan, H.; Haque, A. Efficacy of concentrated growth factor with autograft and xenograft in mandible fractures: A randomized clinical trial. J. Univ. Coll. Med. Dent. 2024, 4, e3519. [Google Scholar] [CrossRef]
- Black, C.; Gibbs, D.; McEwan, J.; Kanczler, J.; Fernández, M.; Tozzi, G.; Dawson, J.; Oreffo, R. Comparison of bone formation mediated by bone morphogenetic protein delivered by nanoclay gels with clinical techniques (autograft and InductOs®) in an ovine bone model. J. Tissue Eng. 2022, 13, 20417314221113746. [Google Scholar] [CrossRef]
- Jin, Y.; Zheng, G.; Lee, J.; Han, S. Comparison of demineralized bone matrix and hydroxyapatite as carriers of Escherichia coli recombinant human BMP-2. Biomater. Res. 2021, 25, 20. [Google Scholar] [CrossRef]
- Tasdemir, U.; Iyilikçi, B.; Aktürk, M.C.; Ozmen, O.; Kizildağ, A.; Elmali, Z. The effect of autogenous bone graft mixed with recombinant human vascular endothelial growth factor on bone regeneration. J. Craniofac. Surg. 2021, 32, 2233–2237. [Google Scholar] [CrossRef]
- Taşdemir, U.; Kirtay, M.; Keleş, A.; Çil, N.; Abban, G.; Dodurga, Y. Autogenous tooth bone graft and simvastatin combination effect on bone healing. J. Craniofac. Surg. 2020, 31, 2350–2354. [Google Scholar] [CrossRef]
- Kunze, K.; Moran, J.; Polce, E.; Pareek, A.; Strickland, S.; Williams, R. Lower donor site morbidity with hamstring and quadriceps tendon autograft compared with bone-patellar tendon-bone autograft after anterior cruciate ligament reconstruction: A systematic review and network meta-analysis of randomized controlled trials. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 3339–3352. [Google Scholar] [CrossRef]
- Attia, A.; Mahmoud, K.; ElSweify, K.; Bariteau, J.; Labib, S. Donor site morbidity of calcaneal, distal tibial, and proximal tibial cancellous bone autografts in foot and ankle surgery. A systematic review and meta-analysis of 2296 bone grafts. Foot Ankle Surg. 2021, 27, 729–735. [Google Scholar] [CrossRef]
- Khalid, M.; Janjua, S.; Sheraz, M.; Kanwal, S.; Ghouri, Q.; Shaheen, U. Quantifying donor site morbidity in anterior cruciate ligament reconstruction using peroneus longus tendon autograft. J. Musculoskelet. Surg. Res. 2024, 8, 165. [Google Scholar] [CrossRef]
- Sun, H.; Yin, X.; Yang, C.; Kuang, H.; Luo, W. Advances in autogenous dentin matrix graft as a promising biomaterial for guided bone regeneration in the maxillofacial region: A review. Medicine 2024, 103, e39422. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Zhang, H.; Zhang, J.Y.; Hu, Y.C. Effects of allogeneic bone substitute configurations on the cell adhesion process in vitro. Orthop. Surg. 2023, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council. Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Off. J. Eur. Union 2004, L102, 48–58. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32004L0023 (accessed on 7 April 2004).
- European Parliament and Council. Commission Directive 2006/86/EC laying down technical requirements for the coding, processing, preservation, storage and distribution of human tissues and cells. Off. J. Eur. Union 2006, L294, 32–50. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006L0086 (accessed on 25 October 2006).
- Ilyas, I.; Al-Rabiah, A.; Alhussainan, T.; Alrumaih, H.; Fallatah, A.; Alsakran, S.; Al-Mohrej, O.A. Principles of bone and tissue banking in Saudi Arabia: 10-year experience report. Cell Tissue Bank 2021, 22, 93–101. [Google Scholar] [CrossRef]
- Regmi, A.; Niraula, B.; Maheshwari, V.; Nongdamba, H.; Karn, R.; Bondarde, P.; Anand, U.; Dhingra, M.; Kandwal, P. Establishing a bone bank within a hospital setting in India: Early insights from a tertiary care center in Northern India—A review article. Cell Tissue Bank 2024, 25, 873–882. [Google Scholar] [CrossRef]
- Asamoto, T.; Osawa, Y.; Takegami, Y.; Takemoto, G.; Komatsu, D.; Seki, T.; Imagama, S. The survey of bone allograft transplantation in a Japanese regional bone bank. J. Orthop. Sci. 2024, 29, 804–809. [Google Scholar] [CrossRef]
- American Association of Tissue Banks. AATB Standards Rebuild Project: Draft 15th Edition Standards For Tissue Banking. 2023. Available online: https://www.aatb.org/news/aatb-standards-rebuild-project-draft-15th-edition-standards-tissue-banking (accessed on 18 December 2023).
- Um, I.; Lee, J.; Kim, J.; Kim, Y.; Bakhshalian, N.; Jeong, Y.; Ku, J.K. Allogeneic dentin graft: A review on its osteoinductivity and antigenicity. Materials 2021, 14, 1713. [Google Scholar] [CrossRef]
- Waletzko-Hellwig, J.; Saemann, M.; Schulze, M.; Frerich, B.; Bader, R.; Dau, M. Mechanical characterization of human trabecular and formed granulate bone cylinders processed by high hydrostatic pressure. Materials 2021, 14, 1069. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Ling, Y.; Chen, L.; Wei, L.; Fan, C.; Lei, D.; Xu, L.; Wang, C. GGTA1/iGb3S double knockout mice: Immunological properties and immunogenicity response to xenogeneic bone matrix. Biomed. Res. Int. 2020, 2020, 9680474. [Google Scholar] [CrossRef] [PubMed]
- Amid, R.; Kheiri, A.; Kheiri, L.; Kadkhodazadeh, M.; Ekhlasmandkermani, M. Structural and chemical features of xenograft bone substitutes: A systematic review of in vitro studies. Biotechnol. Appl. Biochem. 2021, 68, 1432–1452. [Google Scholar] [CrossRef] [PubMed]
- Jerbić Radetić, A.T.; Zoričić Cvek, S.; Tomas, M.; Erjavec, I.; Oguić, M.; Perić Kačarević, Ž.; Cvijanović Peloza, O. CSBD healing in rats after application of bovine xenogeneic biomaterial enriched with magnesium alloy. Int. J. Mol. Sci. 2021, 22, 9089. [Google Scholar] [CrossRef]
- Pröhl, A.; Batinic, M.; Alkildani, S.; Hahn, M.; Radenkovic, M.; Najman, S.; Jung, O.; Barbeck, M. In vivo analysis of the biocompatibility and bone healing capacity of a novel bone grafting material combined with hyaluronic acid. Int. J. Mol. Sci. 2021, 22, 4818. [Google Scholar] [CrossRef]
- Denner, J. The porcine cytomegalovirus (PCMV) will not stop xenotransplantation. Xenotransplantation 2022, 29, e12763. [Google Scholar] [CrossRef]
- Groenendaal, H.; Costard, S.; Ballard, R.; Bienhoff, S.; Challen, D.; Dominguez, B.; Kern, D.R.; Miller, D.; Noordergraaf, J.; Rudenko, L.; et al. Expert opinion on the identification, risk assessment, and mitigation of microorganisms and parasites relevant to xenotransplantation products from pigs. Xenotransplantation 2023, 30, e12815. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Source Animal, Product, Preclinical, And Clinical Issues Concerning the Use of Xenotransplantation Products in Humans: Guidance for Industry. 2016. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/source-animal-product-preclinical-and-clinical-issues-concerning-use-xenotransplantation-products (accessed on 22 April 2024).
- European Medicines Agency. Guideline on Xenogeneic Cell-Based Medicinal Products (EMEA/CHMP/CPWP/83508/2009). 2009. Available online: https://www.ema.europa.eu/en/xenogeneic-cell-based-medicinal-products-scientific-guideline (accessed on 1 December 2019).
- Hawthorne, W.J.; Cowan, P.J.; Ayares, D.; Bührer-Sékula, S.; Buhler, L.H.; Bottino, R.; Pierson, R.N., 3rd; Ahn, C.; Azimzadeh, A.; Cozzi, E.; et al. Third WHO global consultation on regulatory requirements for xenotransplantation clinical trials. Xenotransplantation 2019, 26, e12513. [Google Scholar] [CrossRef]
- Sánchez-Labrador, L.; Molinero-Mourelle, P.; Pérez-González, F.; Saez-Alcaide, L.M.; Brinkmann, J.C.; Martínez, J.L.; Martínez-González, J.M. Clinical performance of alveolar ridge augmentation with xenogeneic bone block grafts versus autogenous bone block grafts: A systematic review. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 293–302. [Google Scholar] [CrossRef]
- Maevskaia, E.; Khera, N.; Ghayor, C.; Bhattacharya, I.; Guerrero, J.; Nicholls, F.; Waldvogel, C.; Bärtschi, R.; Fritschi, L.; Salamon, D.; et al. Three-dimensional printed hydroxyapatite bone substitutes designed by a novel periodic minimal surface algorithm are highly osteoconductive. 3D Print. Addit. Manuf. 2023, 10, 905–916. [Google Scholar] [CrossRef]
- Ding, Y.; Pan, H.; Wang, Z.; Xiao, B. Progress and prospects of 3D printing human skeletal scaffold. Highlights Sci. Eng. Technol. 2024, 70, 123–130. [Google Scholar] [CrossRef]
- Li, X.; Lu, W.; Xu, X.; Wang, Y.; Chen, S. Advanced optical methods and materials for fabricating 3D tissue scaffolds. Light Adv. Manuf. 2022, 3, 26. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Tavtorkin, A.; Komarov, P.; Kretov, E.; Korchagina, S.; Chinova, M.; Gavrilov, D.; Ivchenko, P. Dispersant and protective roles of amphiphilic poly(ethylene phosphate) block copolymers in polyester/bone mineral composites. Int. J. Mol. Sci. 2023, 24, 11175. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.Y.; Seo, J.Y.; Ryu, J.H.; Kim, K.M.; Kwon, J.S. Improvement of the mechanical and biological properties of bioactive glasses by the addition of zirconium oxide (ZrO2) as a synthetic bone graft substitute. J. Biomed. Mater. Res. A 2021, 109, 1196–1208. [Google Scholar] [CrossRef]
- Ferraz, M.P.; Monteiro, F.J.; Manuel, C.M. Hydroxyapatite nanoparticles: A review of preparation methodologies. J. Appl. Biomater. Biomech. 2020, 2, 74–80. [Google Scholar]
- Cheng, L.; Suresh, K.S.; He, H.; Rajput, R.S.; Feng, Q.; Ramesh, S.; Wang, Y.; Krishnan, S.; Ostrovidov, S.; Camci-Unal, G.; et al. 3D printing of micro- and nanoscale bone substitutes: A review on technical and translational perspectives. Int. J. Nanomed. 2021, 16, 4289–4319. [Google Scholar] [CrossRef]
- Liang, D.; Zhong, C.; Jiang, F.; Liao, J.; Ye, H.; Ren, F. Fabrication of porous tantalum with low elastic modulus and tunable pore size for bone repair. ACS Biomater. Sci. Eng. 2023, 9, 2192–2205. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, Y.; Terracciano, A.; Zhao, X.; Su, T.; Kalyon, D.; Katebifar, S.; Kumbar, S.G.; Yu, X. Load-bearing biodegradable polycaprolactone-poly (lactic-co-glycolic acid)- beta tri-calcium phosphate scaffolds for bone tissue regeneration. Polym. Adv. Technol. 2019, 30, 1189–1197. [Google Scholar] [CrossRef]
- Celik, D.; Ustundag, C. Fabrication of biomimetic scaffold through hybrid forming technique. Int. J. Ceram. Eng. Sci. 2024, 6, e10210. [Google Scholar] [CrossRef]
- Marongiu, G.; Verona, M.; Cardoni, G.; Capone, A. Synthetic bone substitutes and mechanical devices for the augmentation of osteoporotic proximal humeral fractures: A systematic review of clinical studies. J. Funct. Biomater. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Makkar, P.; Padalhin, A.; Lee, G.; Im, S.; Lee, B. Comparative study on biodegradation and biocompatibility of multichannel calcium phosphate based bone substitutes. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110694. [Google Scholar] [CrossRef] [PubMed]
- Kowalewicz, K.; Vorndran, E.; Feichtner, F.; Waselau, A.; Brueckner, M.; Meyer-Lindenberg, A. In-vivo degradation behavior and osseointegration of 3D powder-printed calcium magnesium phosphate cement scaffolds. Materials 2021, 14, 946. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, M.; Zhao, Q.; Greven, J.; Winnand, P.; Zhang, X.; Bläsius, F.M.; Buhl, E.M.; Wolf, M.; Neuss, S.; Hildebrand, F.; et al. Evaluation of in vitro biocompatibility of human pulp stem cells with allogeneic, alloplastic, and xenogeneic grafts under the influence of extracellular vesicles. Sci. Rep. 2023, 13, 12475. [Google Scholar] [CrossRef]
- Sallent, I.; Capella-Monsonís, H.; Procter, P.; Bozo, I.Y.; Deev, R.V.; Zubov, D.; Vasyliev, R.; Perale, G.; Pertici, G.; Baker, J.; et al. The few who made it: Commercially and clinically successful innovative bone grafts. Front. Bioeng. Biotechnol. 2020, 8, 952. [Google Scholar] [CrossRef]
- Massari, L.; Saracco, A.; Marchesini, S.; Gambuti, E.; Delorenzi, A.; Caruso, G. Safety of a porous hydroxyapatite bone substitute in orthopedics and traumatology: A multi-centric clinical study. J. Funct. Morphol. Kinesiol. 2024, 9, 71. [Google Scholar] [CrossRef]
- Mahardawi, B.; Jiaranuchart, S.; Dhanesuan, K.; Arunjaroensuk, S.; Mattheos, N.; Pimkhaokham, A. The clinical efficacy of the allogenic demineralized dentin matrix graft for implant placement: A systematic review. Oral Maxillofac. Surg. 2024, 28, 585–593. [Google Scholar] [CrossRef]
- Batra, C.; Goel, A.; Daneshparvar, N.; Hamada, Y. Clinical evaluation of the combination of rhPDGF-BB and xenogeneic bone substites for the treatment of severe periodontal intrabony defects: A case series. Int. J. Periodontics Restor. Dent. 2023, 43, 192–200. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Qi, Y.; Ma, X.; Qiao, S.; Cai, H.; Zhao, B.C.; Jiang, H.B.; Lee, E.S. Comparison of autogenous tooth materials and other bone grafts. Tissue Eng. Regen. Med. 2021, 18, 327–341. [Google Scholar] [CrossRef]
- Jung, K.J.; Sarkar, S.K.; Kim, W.J.; Kim, B.R.; Park, J.S.; Lee, B.T. Bone regeneration by multichannel cylindrical granular bone substitutes for regeneration of bone in cases of tumor, fracture, and arthroplasty. Int. J. Environ. Res. Public Health 2022, 19, 8228. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.; Auston, D.; Mir, H.; Yoon, R.; Koval, K. Autograft, allograft, and bone graft substitutes: Clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Meesuk, L.; Suwanprateeb, J.; Thammarakcharoen, F.; Tantrawatpan, C.; Kheolamai, P.; Palang, I.; Tantikanlayaporn, D.; Manochantr, S. Osteogenic differentiation and proliferation potentials of human bone marrow and umbilical cord-derived mesenchymal stem cells on the 3D-printed hydroxyapatite scaffolds. Sci. Rep. 2022, 14, 19509. [Google Scholar] [CrossRef] [PubMed]
- Adamska, P.; Pylińska-Dąbrowska, D.; Stasiak, M.; Sobczak-Zagalska, H.; Jusyk, A.; Zedler, A.; Studniarek, M. Tooth autotransplantation, autogenous dentin graft, and growth factor application: A method for preserving the alveolar ridge in cases of severe infra occlusion—A case report and literature review. J. Clin. Med. 2024, 13, 3902. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Printing and Prototyping of Tissues and Scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent Advances in Bioprinting Techniques: Approaches, Applications and Future Prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef]
- Rasheed, A.; Azizi, L.; Turkki, P.; Janka, M.; Hytönen, V.P.; Tuukkanen, S. Extrusion-Based Bioprinting of Multilayered Nanocellulose Constructs for Cell Cultivation Using In Situ Freezing and Preprint CaCl2 Cross-Linking. ACS Omega 2021, 6, 569–578. [Google Scholar] [CrossRef]
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Hao, Y.; Cao, B.; Deng, L.; Li, J.; Ran, Z.; Wu, J.; Pang, B.; Tan, J.; Luo, D.; Wu, W. The first 3D-bioprinted personalized active bone to repair bone defects: A case report. Int. J. Bioprint 2022, 9, 654. [Google Scholar] [CrossRef]
- Zan, J.; Qian, G.; Deng, F.; Zhang, J.; Zeng, Z.; Peng, S.; Shuai, C.J. Dilemma and Breakthrough of Biodegradable Poly-L-Lactic Acid in Bone Tissue Repair. J. Mater. Res. Technol. 2022, 17, 2369–2387. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Zheng, Y.; Chen, X.-H.; Yang, J.-A.; Zhu, D.; Ruan, L.; Takashima, K. Challenges in the Use of Zinc and Its Alloys as Biodegradable Metals: Perspective from Biomechanical Compatibility. Acta Biomater. 2019, 97, 23–45. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, B.; He, R.; Huang, P. Advancements of 3D Bioprinting in Regenerative Medicine: Exploring Cell Sources for Organ Fabrication. Heliyon 2024, 10, e24593. [Google Scholar] [CrossRef] [PubMed]
- Mladenovska, T.; Choong, P.F.; Wallace, G.G.; O’Connell, C.D. The Regulatory Challenge of 3D Bioprinting. Regen. Med. 2023, 18, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, Y.; St-Pierre, J.-P.; Bergholt, M.S.; Autefage, H.; Wang, J.; Cai, M.; Yang, G.; Stevens, M.M.; Zhang, S. Bioenergetic-Active Materials Enhance Tissue Regeneration by Modulating Cellular Metabolic State. Sci. Adv. 2020, 6, eaay7608. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Kuzma, M.L.; Bai, X.; Yang, J. Biomaterial-Based Metabolic Regulation in Regenerative Engineering. Adv. Sci. 2019, 6, 1900819. [Google Scholar] [CrossRef]
- Nallusamy, J.; Das, R.K. Hydrogels and Their Role in Bone Tissue Engineering: An Overview. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. S2), S908–S912. [Google Scholar] [CrossRef]
- Utech, S.; Boccaccini, A.R. A Review of Hydrogel-Based Composites for Biomedical Applications: Enhancement of Hydrogel Properties by Addition of Rigid Inorganic Fillers. J. Mater. Sci. 2016, 51, 271–310. [Google Scholar] [CrossRef]
- Shen, C.; Han, Y.; Xiong, H.; Wang, Y.; Tan, Z.; Wei, H.; Ding, Q.; Ma, L.; Ding, C.; Zhao, T. Multifunctional Hydrogel Scaffolds Based on Polysaccharides and Polymer Matrices Promote Bone Repair: A Review. Int. J. Biol. Macromol. 2025, 294, 139418. [Google Scholar] [CrossRef]
- Sun, W.; Ye, B.; Chen, S.; Zeng, L.; Lu, H.; Wan, Y.; Gao, Q.; Chen, K.; Qu, Y.; Wu, B. Neuro–Bone Tissue Engineering: Emerging Mechanisms, Potential Strategies, and Current Challenges. Bone Res. 2023, 11, 65. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, S.; Xu, M.; Hao, L.; Chen, J.; Fang, Z.; Guo, K.; Chen, Y.; Wang, L. Dynamic Surface Adapts to Multiple Service Stages by Orchestrating Responsive Polymers and Functional Peptides. Biomaterials 2023, 301, 122200. [Google Scholar] [CrossRef]
- Wang, T.; Bai, J.; Lu, M.; Huang, C.; Geng, D.; Chen, G.; Wang, L.; Qi, J.; Cui, W.; Deng, L. Engineering Immunomodulatory and Osteoinductive Implant Surfaces via Mussel Adhesion-Mediated Ion Coordination and Molecular Clicking. Nat. Commun. 2022, 13, 160. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, M.; Wang, X.; Zhou, Z.; Liu, Y. Design and Evaluation of Europium Containing Mesoporous Bioactive Glass Nanospheres: Doxorubicin Release Kinetics and Inhibitory Effect on Osteosarcoma MG-63 Cells. Nanomaterials 2018, 8, 961. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, M.; Labbaf, S.; Karimzadeh, F.; Pinna, A.; Houreh, A.B.; Nasr-Esfahani, M.H. Mesoporous Bioactive Glasses for the Combined Application of Osteosarcoma Treatment and Bone Regeneration. Mater. Sci. Eng. C 2019, 104, 109994. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, L.; Lin, Z.; Li, M.; Hu, X.; Zhang, Y.; Peng, M. Enhancing Osteosarcoma Killing and CT Imaging Using Ultrahigh Drug Loading and NIR-Responsive Bismuth Sulfide@Mesoporous Silica Nanoparticles. Adv. Healthc. Mater. 2018, 7, 1800602. [Google Scholar] [CrossRef]
- Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Phosphorus-Containing SBA-15 Materials as Bisphosphonate Carriers for Osteoporosis Treatment. Microporous Mesoporous Mater. 2010, 135, 51–59. [Google Scholar] [CrossRef]
- Zhou, X.; Weng, W.; Chen, B.; Feng, W.; Wang, W.; Nie, W.; Chen, L.; Mo, X.; Su, J.; He, C. Mesoporous Silica Nanoparticles/Gelatin Porous Composite Scaffolds with Localized and Sustained Release of Vancomycin for Treatment of Infected Bone Defects. J. Mater. Chem. B 2018, 6, 740–752. [Google Scholar] [CrossRef]
- Paris, J.L.; Lafuente-Gómez, N.; Cabañas, M.V.; Román, J.; Peña, J.; Vallet-Regí, M. Fabrication of a Nanoparticle-Containing 3D Porous Bone Scaffold with Proangiogenic and Antibacterial Properties. Acta Biomater. 2019, 86, 441–449. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The Possible “Proton Sponge” Effect of Polyethylenimine (PEI) Does Not Include Change in Lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef]
- Khorsand, B.; Nicholson, N.; Do, A.-V.; Femino, J.E.; Martin, J.A.; Petersen, E.; Guetschow, B.; Fredericks, D.C.; Salem, A.K. Regeneration of Bone Using Nanoplex Delivery of FGF-2 and BMP-2 Genes in Diaphyseal Long Bone Radial Defects in a Diabetic Rabbit Model. J. Control Release 2017, 248, 53–59. [Google Scholar] [CrossRef]
- Malek-Khatabi, A.; Javar, H.A.; Dashtimoghadam, E.; Ansari, S.; Hasani-Sadrabadi, M.M.; Moshaverinia, A. In situ bone tissue engineering using gene delivery nanocomplexes. Acta Biomater. 2020, 108, 326–336. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Chen, Y.E.; Chen, J.; Ma, P.X. Cell-Free 3D Scaffold with Two-Stage Delivery of miRNA-26a to Regenerate Critical-Sized Bone Defects. Nat. Commun. 2016, 7, 10376. [Google Scholar] [CrossRef]

| Reference | Aim of the Study | Type of Material | Field of Application | Type of Study | Outcomes of the Bone Substitute |
|---|---|---|---|---|---|
| Ferraz, M.P. [1] | To analyze and compare bone grafting materials used in dental and maxillofacial surgery, focusing on their properties, benefits, and clinical application challenges | Autologous bone, allografts, xenografts, synthetic or xenogeneic scaffolds with growth factors to enhance their osteogenic properties | Dentistry, oral and maxillofacial surgeries | In vivo and clinical research data | Osteogenic properties |
| Heitzer, M. et al. [2] | To investigate the osteogenic potential of dental pulp stem cells (DPSCs) on three types of bone graft substitutes—alloplastic (BCP), allogeneic (FDBA), and xenogeneic (DBBM)—under in vitro conditions | Alloplastic, allogeneic, and xenogeneic bone graft substitutes | Dentistry, oral and maxillofacial surgeries | In vitro | Osteogenic (bone-forming) potential |
| Fattahian, H. et al. [3] | To review current materials and methods for the repair and regeneration of bone defects, as well as new tissue engineering approaches to improve the treatment of bone injuries | Autografts, allografts, and xenografts | Orthopedics | Systematic reviews | Biocompatibility, osteoconductivity, osteoinductivity, mechanical strength, resorbability, availability, combinability with biofactors |
| Dahiya, U. et al. [4] | To analyze several bone substitutes, such as synthetics, bioceramics, and polymers, as a substitute for autologous or allogeneic bone for the treatment of bone defects. These bone substitutes should be biocompatible, bioresorbable, osteoconductive, osteoinductive, and promote new bone growth | Synthetics bone substitutes | Orthopedics, dentistry, oral and maxillofacial surgeries | Review | Biocompatibility, bioresorbability, osteoconductivity, osteoinductivity, support of new bone ingrowth |
| Manzini, B. et al. [5] | To provide an overview of the bone tissue, including the role of stem cells and some of the bioactive molecules associated with these processes | Synthetic bone substitutes, hydroxyapatite (HA), β-tricalcium phosphate (β-TCP), biphasic calcium phosphate (BCP), combinations of HA and TCP | Orthopedics, dentistry, oral and maxillofacial surgeries | Review | Stem cells and bioactive factors are key for regeneration |
| Miletić, M. et al. [6] | This in vivo study demonstrates for the superior bone regenerative capacity of CAP-pretreated β-TCP seeded with PDLSCs, highlighting the therapeutic potential of this combined approach in osteoregeneration | Beta-Tricalcium Phosphate | Orthopedics, dentistry, oral and maxillofacial surgeries | In vivo | Periodontal ligament stem cells with beta-tricalcium phosphate treated by cold atmospheric plasma significantly enhanced bone regeneration |
| Mukhlis, S. et al. [7] | To investigate the therapeutic potential of adipose-derived pericytes, in combination with biomaterials and growth factors, for enhancing bone tissue regeneration | Injectable hydrogels and polymer (gelatin, alginate, collagen, chitosan, poly-L-lactic acid, hyaluronic acid, fibrin, heparin, and polyethylene glycol) scaffolds with pericytes | Dentistry, oral and maxillofacial surgeries | In vivo | Preclinical studies show that pericytes combined with scaffolds or DBM enhance bone healing |
| Dőri, F. et al. [8] | To evaluate the histological findings of retrieved ePTFE membranes using PRP and bone substitutes, the effect of PRP on graft materials, and the correlation of the findings with the clinical outcomes | PRP + Beta-Tricalcium Phosphate + non-resorbable membrane (GTR) | Dentistry, oral and maxillofacial surgeries | In vivo | Application of β-TCP and PRP may enhance membrane integration and periodontal healing |
| Zhang, Q. et al. [9] | To analyze tissue engineering and regenerative therapies targeting cell senescence in bone and cartilage, with a focus on treating osteoporosis and age-related degenerative conditions through the use of biomaterials, growth factors, and cell-based strategies | Bone grafts with antiosteoporotic growth factors | Orthopedics | Review | Bone grafts combined with osteoporosis therapies—including scaffolds releasing anti-osteoporotic drugs or implants with therapeutic surface modifications—can improve low bone density and enhance impaired bone regeneration |
| Rupp, M. et al. [10] | To evaluate the overall use of bone graft substitutes, autografts and allografts, of different types of bone graft substitutes (calcium sulphate, calcium phosphate, calcium phosphate ceramics or polymethyl methacrylate) and of different bone grafts (cancellous vs. cortical), and the use of antibiotic-loading of bone graft substitutes in orthopedic surgery in Germany | Autografts and allografts, synthetic bone substitutes (calcium sulphate, calcium phosphate, calcium phosphate ceramics or polymethyl methacrylate) | Orthopedics | In vivo | Increasing use of bone graft substitutes and antibiotic-loaded bone graft substitutes |
| Laubach, M. et al. [11] | To review and evaluate current methods for the treatment of bone defects, with a particular focus on the potential and challenges of using personalized 3D-printed implants for long bone regeneration | 3D-printed scaffolds | Orthopedics | Review | 3D-printed scaffolds provide osteoconductive surfaces, mechanical support, and containment for bone grafts, enhancing bone ingrowth |
| Santoro, A. et al. [12] | To analyze current natural and synthetic bone substitutes used in orthopedic surgery, with a focus on innovations and challenges in the treatment of complex bone defects | Natural grafts—comprising autologous, allogeneic, and xenogeneic materials; synthetic alternatives, including biodegradable and non-biodegradable biomaterials | Regenerative medicine and orthopedic surgery | Review | Natural grafts (autologous, allogeneic, xenogeneic) offer biological advantages, whereas synthetic substitutes (biodegradable and non-biodegradable) provide structural versatility and lower immunogenicity |
| Tournier, P. et al. [13] | To evaluate the regenerative potential of a novel partially demineralized allogeneic bone paste as an alternative to traditional allogeneic grafts, focusing on enhanced bone healing and user-friendly application in challenging skeletal defect areas | Demineralized allogenic bone graft in the form of a paste | Orthopedic surgery | In vivo | The bone paste supported bone healing in guided bone regeneration and critical-size defect models |
| Parikh, S. et al. [14] | To present the ethical and clinical approach in a case of triple bone marrow donation from a single pediatric donor to three HLA-identical siblings with the same primary immunodeficiency, aiming to guide future decision-making in similar cases | Allogeneic bone grafts | Regenerative medicine | In vivo | Multiple harvests from pediatric donors can be performed safely with careful clinical and ethical oversight |
| Sharifi, M. et al. [15] | To explore the sources, advantages, challenges, and techniques of acellularization and recellularization in allogeneic and xenogeneic bone grafts, aiming to advance future bone defect treatment strategies and support product commercialization | Allogeneic and xenogeneic bone grafts | Regenerative medicine | Review | Allogeneic and xenogeneic acellularized bone grafts mimic native bone structure and support osteoconduction and osteoinduction |
| Ana, I. [16] | To review the biological aspects, advantages, and disadvantages of various bone graft substitutes used in dental implantology, with a focus on calcium phosphate-based materials | Calcium phosphate-based bone substitutes | Dentistry, oral and maxillofacial surgeries | Review | Advantages of calcium phosphate-based bone substitutes in dentistry |
| Janjua, O.S. et al. [17] | To investigate the potential and applications of autogenous tooth grafts for regenerating maxillary and mandibular bone defects, including sinus and ridge augmentations and socket preservation before implant placement | Autogenous tooth graft | Dentistry, oral and maxillofacial surgeries | Review | Autogenous tooth bone grafts exhibit osteoconductivity and osteoinductivity, similar to natural bone |
| Malik, S. et al. [18] | To evaluate the efficacy of adjuvant therapy with Concentrated Growth Factor (CGF) along with xenograft and autograft in mandible fractures, in terms of bone density gain and healing over the period of 6 months | Xenograft and autograft with Concentrated Growth Factor | Dentistry, oral and maxillofacial surgeries | In vivo | CGF enhances bone regeneration with both autograft and xenograft |
| Black, C. et al. [19] | To evaluate the effectiveness of Laponite™ nanoclay gel as a carrier for localized BMP-2 delivery in bone regeneration, using a relevant large animal preclinical model with femoral condyle defects in aged sheep | Synthetics Smectite nanoclay gel (Laponite™) combined with absorbable collagen sponge and BMP-2 (Bone Morphogenetic Protein-2) | Bone tissue regeneration | In vivo and clinical research data | Autograft showed superior bone formation, nanoclay gels exhibited excellent biocompatibility and potential for delivering bone morphogenetic protein-2 locally |
| Jin, Y. et al. [20] | To investigate the effectiveness of demineralized bone matrix (DBM) as a carrier for Escherichia coli-derived recombinant human BMP-2 (ErhBMP-2) in bone regeneration | Autograft, demineralized bone matrix (DBM) as a bone graft substitute and growth factor carrier | Orthopedic surgery, bone tissue regeneration | In vivo | DBM as the carrier showed significantly higher bone volume and bone thickness than the groups with HA as the carrier |
| Tasdemir, U. et al. [21] | To investigate the effect of combining autogenous bone graft with recombinant human vascular endothelial growth factor (rhVEGF) on bone regeneration in a rat mandibular defect model | Autogenous bone graft, gelatin sponge plus rhVEGF, autogenous bone graft plus rhVEGF | Dentistry, oral and maxillofacial surgeries | In vivo | Combining autogenous bone grafts with rhVEGF could potentially improve neovascularization and enhance bone regeneration. |
| Taşdemir, U. et al. [22] | To evaluate all the tooth layers mixed with simvastatin without any demineralization process effect on bone formation | Autogenous tooth bone grafts (ATGM) with simvastatin, xenogenic bone graft | Dentistry, oral and maxillofacial surgeries | In vivo | Autogenous mineralized tooth bone graft should be mixed with simvastatin for bone regeneration |
| Kunze, K. et al. [23] | To perform a meta-analysis of RCTs evaluating donor site morbidity after bone-patellar tendon-bone (BTB), hamstring tendon (HT) and quadriceps tendon (QT) autograft harvest for anterior cruciate ligament reconstruction (ACLR) | BTB autograft | Orthopedic surgery | Systematic reviews | Autograft selection should be personalized through considering differential rates of donor-site morbidity |
| Attia, A. et al. [24] | To evaluate the safety and donor site morbidity of autologous cancellous bone grafts harvested from the distal lower extremity (calcaneus, proximal, and distal tibia) through a meta-analysis of published research | Autologous cancellous bone grafts | Orthopedic surgery | Systematic reviews | Calcaneal, distal tibial, and proximal tibial bone autografts are safe with a low rate of overall and major complications |
| Khalid, M. et al. [25] | To assess the donor site morbidity in patients having anterior cruciate ligament reconstruction (ACLR) using peroneus longus tendon (PLT) autograft | Autograft | Orthopedic surgery | In vivo | ACLR using the PLT autograft resulted in a good functional outcome, smooth rehabilitation with an early return to sports, and minimal complications at the donor site |
| Sun, H. et al. [26] | This paper comprehensively explores the composition, mechanisms underlying osteoinductivity, preparation methods, and clinical applications of ADM with the aim of establishing a fundamental reference for future studies on this subject | Autogenous bone | Dentistry, oral and maxillofacial surgeries | Review | ADM has a low rejection rate, possesses osteoinductive properties, and is characterized by easy preparation |
| Liu, J. et al. [27] | To explore the potential effect of three allogenic bone substitute configurations on the viability, adhesion, and spreading of osteoblasts in vitro | Allogenic bone substitute | Orthopedic | In vitro | Bone powder and bone granule promote cell adhesion and spreading compared to bone fibre group |
| European Parliament and Council [28] | Setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells | Allogeneic bone tissue | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | In vivo | Not applicapable |
| European Commission [29] | To lay down technical requirements for the coding, processing, preservation, storage and distribution of human tissues and cells | Allogeneic bone tissue | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | In vivo | Not applicapable |
| Ilyas, I. et al. [30] | To present the 10-year experience of the bone bank at KFSH&RC, detailing its structure, donor/recipient protocols, graft processing and safety systems, and the clinical use of allografts in surgeries such as joint revision, spine, and tumour procedures | Allografts | Orthopedics, neurosurgery | Clinical, preclinical | Administrative structure, donor and recipient testing protocols, allograft retrieval, processing procedures, and safety arrangements |
| Regmi, A. et al. [31] | To present the establishment and management of an orthopedic bone bank in India, focusing on the legal, medical, and organizational aspects of using allogenic bone for treating bone defects | Allografts | Orthopedics | Review | Allogenic bone grafts, supported by bone banking systems, provide a safe alternative to autografts, though they require strict legal, medical, and organizational regulation |
| Asamoto, T. et al. [32] | To analyze the 15-year trends in the supply and use of bone allografts in a regional bone bank in Japan and to assess the effectiveness of the donor screening process | Bone allografts | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinical | The study highlights the importance of thorough screening processes for allograft donors to minimize the number of discarded grafts |
| American Association of Tissue Banks. AATB Standards Rebuild [33] | These regulations and practices underpin modern bone grafting procedures, aiming to optimize clinical outcomes while minimizing associated risks | Allografts | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinical | Standards for Tissue Banking |
| Um, I. et al. [34] | To summarize preclinical studies on the osteoinductive potential and antigenicity of allogeneic demineralized dentin matrix (Allo-DDM), in order to evaluate its viability as a reliable bone graft substitute, particularly at extraskeletal sites | Allogeneic demineralized dentin matrix | Dentistry, oral and maxillofacial surgeries | Review | Allo-DDM showed great potential for osteoinductivity in extraskeletal sites with low antigenicity |
| Waletzko-Hellwig, J. et al. [35] | To evaluate high hydrostatic pressure (HHP) as a gentle sterilization method for allogenic bone substitutes by assessing its effects on the mechanical properties of treated bone granules and trabecular bone, in comparison to untreated samples | Allogenic bone substitutes | Orthopedics | Preclinical | HHP treatment is suitable alternative to current processing techniques for allogenic bone substitutes since with no negative effects on mechanical properties occurred |
| Shao, A. et al. [36] | To investigate whether GGTA1/iGb3S double knockout (G/i DKO) mice are sensitive to Gal antigen-positive xenoimplants and assess their suitability as a model for studying α-Gal-mediated immunogenicity in xenotransplantation | Xenogeneic bone grafts | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinical | Evaluating the α-Gal-mediated immunogenic risk of xenogeneic grafts |
| Amid, R. et al. [37] | To systematically review and evaluate the structural and chemical properties of various xenograft bone substitutes based on in vitro studies | Xenogeneic bone grafts | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Systematic reviews | Xenograft bone substitutes, mainly bovine-derived, show structural similarity and biocompatibility with human bone, but their properties (porosity, crystallinity, Ca/P ratio, and osteogenesis) vary significantly depending on preparation methods. Proper pore size and interconnectivity are crucial for effective bone regeneration |
| Jerbić Radetić, A.T. et al. [38] | To investigate the biological properties of a new bovine xenogeneic biomaterial enriched with magnesium alloy in a 5 mm critical-sized bone defect (CSBD) model, and to compare its osteoconductive potential with existing xenogeneic biomaterials (Cerabone®, Cerabone® + autologous bone, and OsteoBiol®) for possible use in oral implantology | Xenogeneic bone grafts | Dentistry, oral and maxillofacial surgeries | Preclinical, in vivo | Novel bovine xenogeneic biomaterial enriched with magnesium alloy in a 5 mm CSBD model, compared to Cerabone® and OsteoBiol®, the Cerabone® + Mg group showed higher bone volume, faster biodegradation, and strong osteoinductive properties |
| Pröhl, A. et al. [39] | To evaluate the inflammatory tissue response and bone healing capacity of a newly developed xenogeneic bone substitute material (BSM) combined with hyaluronate (HY), compared to a control BSM without HY and a sham operation group, using a rat calvaria model over 2, 8, and 16 weeks | Xenogeneic bone substitute | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinical, clinical | Both materials proved biocompatible and supported gradual osteoconductive bone regeneration |
| Denner, J. [40] | To argue that although porcine cytomegalovirus (PCMV) is an important safety concern in xenotransplantation, there are effective methods for its elimination, and therefore, it does not represent an insurmountable obstacle to the advancement of this medical technology | Xenogeneic bone substitute | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinical, clinical | To apply methods for eliminating viruses in xenotransplantations |
| Groenendaal, H. et al. [41] | To develop a risk-based framework for identifying and evaluating porcine microorganisms and parasites (MP) that could pose health risks in xenotransplantation. | Xenogeneic bone substitute | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinical, clinical | Provides a structured way to categorize and mitigate the risks posed by porcine microorganisms in xenotransplantation, highlighting that most risks can be addressed with strict biosecurity and monitoring |
| U.S. Food and Drug Administration [42] | To provide guidance to the industry on the safe development and use of xenotransplantation products in humans | Xenotransplantation products in humans | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinical | Guidelines |
| European Medicines Agency [43] | To provide scientific and regulatory guidance on the quality, safety, and efficacy requirements for xenogeneic cell-based medicinal products intended for human use | Xenogeneic cell-based medicinal products | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinical | Guidelines |
| Hawthorne, W.J. et al. [44] | To support the safe and ethically responsible development of xenotransplantation by promoting international cooperation and coordination | Xenografts | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinical | Confirmed the significant progress and the need for global collaboration between science, technology and regulations in the field of xenotransplantation to harmonize practices and rules in different countries |
| Sánchez-Labrador, L. et al. [45] | To evaluate the feasibility of xenogeneic bone blocks for ridge augmentation compared with autogenous blocks by analyzing block survival rates, block resorption, subsequent implant survival rate, post-surgical complications, and histomorphometric findings | Xenogeneic bone blocks, autogenous blocks | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Systematic reviews | Histological and histomorphometric analysis observed more bone formation and less residual bone substitute with autogenous bone blocks than xenogeneic bone blocks |
| Maevskaia, E. et al. [46] | To evaluate a new design of synthetic bone substitutes based on the ADMS algorithm, which provides high mechanical stability and good osteoconductivity, comparable to traditional lattice structures, and is suitable for clinical use in treating bone defects | Synthetic bone substitutes | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinical, in vivo | The study found these substitutes, made from hydroxyapatite (a bone mineral), are highly osteoconductive, meaning they effectively promote the growth of new bone |
| Ding, Y. et al. [47] | To analyze the advantages and performance requirements of 3D-printed bone scaffolds compared to traditional ones. It reviews key 3D printing technologies used for bone scaffold fabrication, highlighting their benefits and limitations. Although 3D printing shows promise in improving recovery and scaffold performance, material-related challenges remain, and future research directions are suggested | 3D-printed bone scaffolds | Orthopedics | Clinical | 3D-printed bone scaffolds show clear advantages over traditional ones, offering better performance and faster recovery, though material issues remain |
| Li, X. et al. [48] | This paper reviews current optical 3D printing methods for tissue engineering scaffold fabrication, highlighting techniques like stereolithography, two-photon polymerization, and others | 3D-printed bone scaffolds | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinical | Each technique—extrusion-based printing, selective laser sintering, stereolithography, and two-photon polymerization—offers distinct advantages and limitations regarding precision, fabrication speed, material compatibility, and application suitability |
| Nifant’ev, I. et al. [49] | The study develops bone substitute composites using biodegradable polyesters (PLLA, PCL) and carbonated hydroxyapatite. It shows that adding special compatibilizers improves the composites’ strength, thermal stability, and overall performance, making them suitable for bone surgery and orthopedics | Synthetic bone substitutes | Bone surgery and orthopedics | Preclinical | PLLA composites with 25 wt% pCAp exhibited the best mechanical performance and thermal stability |
| Kang, T.Y. et al. [50] | This study enhanced the mechanical strength of bioactive glass (BAG) by incorporating varying amounts of zirconia (ZrO2) while preserving its bioactive properties. ZrO2-BAG showed improved toughness, slower degradation, good biocompatibility, and promoted cell growth and bone formation. In vivo tests confirmed its potential as a strong and effective bone graft substitute | Synthetic bone graft substitute | Bone surgery and orthopedics | Preclinical, In vivo | Incorporation of zirconium oxide (ZrO2) into bioactive glasses improved their mechanical strength while preserving porosity |
| Ferraz, M.P. et al. [51] | This review focuses on methods to produce nanophased HA through precipitation and discusses both traditional and recent advances in HA nanoparticle applications | Synthetic bone graft | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Review | Nanophased HA obtained via precipitation methods shows strong promise for improving the bioactivity and functionality of synthetic ceramics |
| Cheng, L. et al. [52] | This article reviews recent advances in 3D printing for bone repair, highlighting its ability to create custom scaffolds using biomaterials and living cells. It covers key printing techniques (SLA, SLS, FDM, inkjet) and their clinical potential in regenerating large bone defects | Synthetic 3D printing for bone repair | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Review | Various biomaterials and nanomaterials have been successfully applied in 3D-printed scaffolds, while major printing methods—including SLA, SLS, FDM, and ink-jet printing—have been utilized to create constructs with structural and functional similarities to bone tissue |
| Liang, D. et al. [53] | To present a simple method for fabricating porous tantalum (Ta) with controlled pore sizes and bone-like mechanical properties, demonstrating its excellent biocompatibility and effectiveness in supporting bone repair in vitro and in vivo | Synthetic bone graft | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | In vitro, in vivo | The porous tantalum demonstrated excellent biocompatibility in vitro and effective bone repair in vivo in a rat femur defect model, validating it as a viable bone substitute |
| Kumar, A. et al. [54] | To developed biodegradable bone scaffolds using a blend of polycaprolactone (PCL) | Synthetic bone graft | Orthopedics, entistry, oral and maxillofacial surgeries, neurosurgery | Preclinic | Increasing PLGA content from 25 wt.% to 75 wt.% accelerated the biodegradation rate threefold within 2 weeks in phosphate-buffered saline |
| Celik, D. et al. [55] | To developed a hybrid hydroxyapatite (HA) scaffold mimicking both cortical and cancellous bone using slip casting and freeze-drying | Synthetic bone graft | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinic | Hybrid scaffold demonstrated 70% open porosity, adequate mechanical strength, and structural resemblance to natural bone. Analyses confirmed its suitability for supporting vascularization, osteoinduction, and osteoconduction |
| Marongiu, G. et al. [56] | To evaluate the effectiveness of cements, bone substitutes, and other augmentation techniques in the surgical treatment of proximal humeral fractures | Synthetic bone graft | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Systematic review | Calcium phosphate and calcium sulphate injectable composites provided good biocompatibility, osteoconductivity, and lower mechanical failure rate when compared to non-augmented fractures |
| Kang, H. et al. [57] | To fabricate and compare multichannel biphasic calcium phosphate (BCP) and β-tricalcium phosphate (TCP) scaffolds in terms of their biodegradation and bone regeneration capabilities over time | Synthetic bone graft- biphasic calcium phosphate, β-tricalcium phosphate | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | Preclinic | BCP exhibited higher compressive strength, while TCP showed greater calcium and phosphorus ion release in SBF. Both scaffolds demonstrated excellent in vitro biocompatibility |
| Kowalewicz, K. et al. [58] | To evaluate the in vivo degradation, osseointegration, and biocompatibility of 3D powder-printed calcium magnesium phosphate cement (CMPC) scaffolds (Mg225 and Mg225d) compared to tricalcium phosphate (TCP), assessing their potential as bone substitutes | Synthetic bone graft | Orthopedics, dentistry, oral and maxillofacial surgeries, neurosurgery | In vivo | All scaffolds showed excellent biocompatibility and gradual osseointegration over 6 weeks |
| Heitzer, M. et al. [59] | To evaluate the osteogenic potential of dental pulp stem cells (DPSCs) and their extracellular vesicles (EVs) on different types of bone grafts (alloplastic, allogeneic, and xenogeneic), and to determine if EVs enhance DPSC proliferation and differentiation for potential use in bone regeneration therapies | Alloplastic, allogeneic, and xenogeneic bone grafts | Dentistry, oral and maxillofacial surgeries, neurosurgery | In vitro | Addition of EVs significantly enhances the biocompatibility of human pulp stem cells with xenogeneic bone grafts, leading to superior cell proliferation, attachment, and osteogenic differentiation compared to other bone grafts tested |
| Sallent, I. et al. [60] | To highlight successful clinical cases and key factors in the translation of innovative bone substitutes—such as biological glues, stem cell-seeded scaffolds, and gene-functionalized grafts—from research to clinical use, and to inform future strategies for effective bone graft development | Synthetic bone graft- biological glues, stem cell-seeded scaffolds, gene-functionalized grafts | Dentistry, oral and maxillofacial surgeries, neurosurgery | Review | Highlights important ideas and aspects in the field of bone grafting |
| Massari, L. et al. [61] | To evaluate the safety of porous hydroxyapatite bone substitutes in trauma orthopedic surgery by analyzing adverse events in a retrospective cohort of 114 patients | Synthetic bone graft- porous hydroxyapatite bone substitutes | Orthopedics | Clinical | Porous hydroxyapatite bone substitutes appear safe for use in trauma orthopedic surgery when applied following proper biomechanical principles |
| Mahardawi, B. et al. [62] | To evaluate the available literature on the Allogenic Demineralized Dentin Matrix (Allo-DDM), revealing its clinical performance when used for implant place-ment procedures | Allogenic demineralized dentin matrix graft | Dentistry, oral and maxillofacial surgeries | Systematic review | Allo-DDM could be a possible alternative to other grafting materials used for bone augmentation and implant placement |
| Batra, C. et al. [63] | To evaluate the clinical effectiveness of combining recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with xenogeneic bone substitutes in treating severe intrabony periodontal defects | Xenogeneic bone substitutes with platelet-derived growth factor-BB | Dentistry, oral and maxillofacial surgeries | Clinical | Combination of rhPDGF-BB and xenogeneic bone substitutes to be a safe and effective graft for severe periodontal intrabony defects |
| Zhang, S. et al. [64] | To highlight the potential of autogenous odontogenic materials as a novel and biocompatible bone substitute for jaw restoration, comparing their composition and osteogenic properties with other conventional bone grafts | Autogenous odontogenic materials | Dentistry, oral and maxillofacial surgeries | Review | Autogenous odontogenic materials represent a novel and promising class of bone substitutes, combining scaffold functionality with intrinsic osteogenic stimulation |
| Jung, K. et al. [65] | To demnstrate the clinical applicability and effectiveness of multichannel granular bone substitutes in treating various orthopedic conditions, including bone tumours, fractures, and defects associated with arthroplasty | Synthetic bone substitutes | Orthopedics | Clinical, in vivo | Bone substitute stabilized the bone defect without any complications, and the defect regenerated slowly during the postoperative period |
| Baldwin, P. et al. [66] | To review and compare the properties, benefits, and clinical applications of autografts, allografts, and synthetic bone graft substitutes in orthopedic trauma surgery, aiming to guide treatment decisions for bone grafting | Autografts, allografts, and synthetic bone graft substitutes | Orthopedics | Review | Autograft, allograft, and bone graft substitutes all possess their own varying degrees of osteogenic, osteoconductive, and osteoinductive properties that make them better suited for different procedures |
| Meesuk L. et al. [67] | To evaluate the proliferation and osteogenic differentiation potential of bone marrow and umbilical cord mesenchymal stem cells cultured on 3D-printed hydroxyapatite scaffolds, with or without biomimetic calcium phosphate coating, for applications in bone tissue engineering | Synthetic bone substitutes-3D-printed hydroxyapatite scaffolds, with or without biomimetic calcium phosphate coating | Orthopedics, dentistry, oral and maxillofacial surgeries | Preclinic | Osteogenic differentiation: Cultivation on HA scaffolds resulted in increased alkaline phosphatase ALP activity and increased expression of osteogenic genes and proteins compared to control 2D culture cells |
| Adamska, P. et al. [68] | To review the literature on teeth autotransplantation, supported by a case report involving the autotransplantation of a third mandibular molar into the site of an extracted infraoccluded first mandibular molar, as well as the utilization of advanced platelet-rich fibrin (A-PRF) alongside autogenous dentin grafts for bone tissue regeneration | Teeth autotransplantation | Dentistry, oral and maxillofacial surgeries | Systematic review | Evaluate the efficacy of autotransplantation, the application of growth factors, and the integration of autogenous dentin grafts in remedying dental deficiencies resulting from reinclusion |
| Source of Origin | Structural Features | Clinical Benefits | Main Disadvantages | Indications For Use |
|---|---|---|---|---|
| Bull | Mineral structure is as similar as possible to human bone; high crystallinity of hydroxyapatite; preserved trabecular architecture | Excellent osteoconductivity; long-term structural support; predictable resorption (12–24 months); broad clinical evidence base | Slow resorption may delay natural remodelling; potential risk of prion disease transmission (theoretical); religious restrictions for some patients | Dental implantology; sinus lift; alveolar ridge augmentation; filling of small bone defects |
| Pig | Lower density compared to bovine; higher porosity (60–70%); increased resorption capacity | Better remodelling properties; faster integration with native bone (6–12 months); optimal for young patients with active metabolism | Lower mechanical strength; risk of transmission of PERVs and PCMV; cultural and religious restrictions (Islam, Judaism); higher immunogenicity | Periodontal regeneration; filling of defects after tooth extraction; pediatric maxillofacial surgery |
| Horse | Intermediate characteristics between bovine and porcine; moderate density; balanced porosity | Alternative for patients with religious restrictions; moderate resorption rate (9–18 months); good biocompatibility after processing | Limited market availability; fewer clinical studies; higher cost due to lower prevalence; potential allergic reactions | Orthopedic surgery (limited use); alternative in case of intolerance to other xenografts |
| Substitute Type | Source | Osteogenicity | Osteoinductivity | Osteoconductivity | Biocompatibility | Main Advantages | Main Disadvantages |
|---|---|---|---|---|---|---|---|
| Autogenous | Patient’s own tissues | High | High | High | Missing | No rejection, presence of living cells, natural growth factors | Limited availability, donor site morbidity, additional pain |
| Allogeneic | Human donors | Missing | Limited | Moderate | Good after processing | Large volumes, no donor site morbidity | Risk of infection transmission, need for treatment, slow integration |
| Xenogenic | Animal tissues | Missing | Low | Moderate | Good after processing | Unlimited availability, structural similarity to human bone | Zoonoses risk, need for careful processing, slow remodelling |
| Synthetic | Artificial materials | Missing | Missing/Low | Good | Excellent | Controlled properties, no biological risks, 3D printing capabilities | Lack of biological activity, need for additional growth factors |
| Material Type | Porosity (%) | Compressive Strength (MPa) | Elastic Modulus (GPa) | Degradation Rate | Clinical Application |
|---|---|---|---|---|---|
| β-tricalcium phosphate (β-TCP) | 60–80 | 2–12 | 0.5–3.5 | Moderate (6–24 months) | Filling defects carrying moderate loads |
| Calcium sulphate | 30–50 | 10–30 | 1–6 | Fast (4–8 weeks) | Short-term applications, drug delivery |
| Hydroxyapatite | 55–75 | 20–80 | 7–13 | Very slow (>24 months) | Long-term structural support, spinal surgery |
| Bioactive glass with ZrO2 | 40–60 | 30–140 | 35–45 | Supervised (3–12 months) | Load-bearing areas, dental implantology |
| 3D printed frames with TPMS | 65–85 | 5–30 | 0.3–2.5 | Supervised (6–18 months) | Tissue engineering, individual implants |
| Medical Specialty | Main Indications | Recommended Types of Substitutes | Specific Requirements | Clinical Results |
|---|---|---|---|---|
| Orthopedic traumatology | Fractures, non-union, large defects | Autogenous, allogenic, synthetic (Ca-phosphate, Ca-sulphate) | High strength, osteoinduction, no immune reaction | High union rates (94% success rate), 6% of fractures are complicated by non-union out of 1,090,167 procedures; donor site morbidity 20–30% with autografts |
| Spinal surgery | Spondylodesis, segmental stabilization | Allogeneic, synthetic (β-TCP, BMP-2) | Structural support, stimulation of fusion | High fusion rates (85–95% when using BMP-2), β-TCP demonstrates optimal degradation profiles over 6–12 months |
| Oncological surgery | Tumour resection, restoration of integrity | Combined with growth factors, allogeneic materials | Preservation of anatomy and limb function | Effective restoration of functionality in 9–12 months, multi-channel cylindrical granular substitutes have shown successful regeneration |
| Maxillofacial surgery and dentistry | Augmentation, sinus lift, implantation | Xenogenic (bovine), Ca-phosphate synthetic, allogenic | Biocompatibility, volumetric stability, implant compatibility | Excellent implantation success rate (92–96%) with autogenous dentin matrices; improved soft tissue healing when combined with PRF |
| Neurosurgery | Cranioplasty, post-tumour defects | Autogenous dentin, synthetic polymers | Biocompatibility with nervous tissue, controlled degradation | High osteoconductive potential of autogenous dentin (85–90% integration in 6 months); 70% open porosity provides high strength |
| Revision surgery | Bone restoration, implant integration | Porous hydroxyapatite, combined synthetics | High biocompatibility, long-term stability | Study of 114 patients notes 100% safety with no side effects when using porous hydroxyapatite |
| Feature | Inkjet Bioprinting | Extrusion-Based Bioprinting | Pressure-Assisted Bioprinting | Laser-Assisted Bioprinting |
|---|---|---|---|---|
| Mechanism | Droplet ejection via thermal, electrostatic, or piezoelectric forces | Continuous extrusion (SSE/FDM) | Continuous extrusion under pneumatic, plunger, or screw pressure | Laser-induced droplet transfer |
| Materials | Low-viscosity hydrogels: alginate, gelatin, collagen, chitosan, PEG | Natural (alginate, gelatin, collagen, nanocellulose) and synthetic (PCL, PVA), often blended | Hydrogels, collagen, chitosan, ceramics, PEG, PLA, PCL | Natural polymers (collagen, gelatin, alginate, fibrin), PEG, dECM, nHA, cell-laden bioinks |
| Cell Compatibility | High | Moderate | High | High |
| Resolution | High | Moderate | Moderate | Very high |
| Viscosity Range | Low | High | High | Moderate |
| Advantages | Low cost, high-throughput, non-contact | High-viscosity bioinks, mechanically robust, multi-material | Cell-friendly, customizable geometry, high-viscosity | Non-contact, precise, complex architectures, high spatial resolution |
| Limitations | Limited viscosity, low cell density tolerance | Shear stress, lower resolution, slower | Shear stress, bioink optimization required | Equipment cost, thermal stress, viscosity constraints |
| Applications | Soft tissue models, high-throughput screening | Soft tissue, bone scaffolds, implants | Tissue constructs, high-viscosity bioinks | Complex tissue constructs, bone and vascular models |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, N.; Ivanov, S.; Peev, S.; Dikova, T. Types of Bone Substitutes and Their Application in Regenerative Medicine: A Systematic Review. J. Funct. Biomater. 2025, 16, 341. https://doi.org/10.3390/jfb16090341
Ivanova N, Ivanov S, Peev S, Dikova T. Types of Bone Substitutes and Their Application in Regenerative Medicine: A Systematic Review. Journal of Functional Biomaterials. 2025; 16(9):341. https://doi.org/10.3390/jfb16090341
Chicago/Turabian StyleIvanova, Nikoleta, Stoyan Ivanov, Stefan Peev, and Tsanka Dikova. 2025. "Types of Bone Substitutes and Their Application in Regenerative Medicine: A Systematic Review" Journal of Functional Biomaterials 16, no. 9: 341. https://doi.org/10.3390/jfb16090341
APA StyleIvanova, N., Ivanov, S., Peev, S., & Dikova, T. (2025). Types of Bone Substitutes and Their Application in Regenerative Medicine: A Systematic Review. Journal of Functional Biomaterials, 16(9), 341. https://doi.org/10.3390/jfb16090341









