Intraoperative Biologization of β-TCP and PCL-TCP by Autologous Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sample Collection
2.3. Removal of the Adsorbed Protein Layer
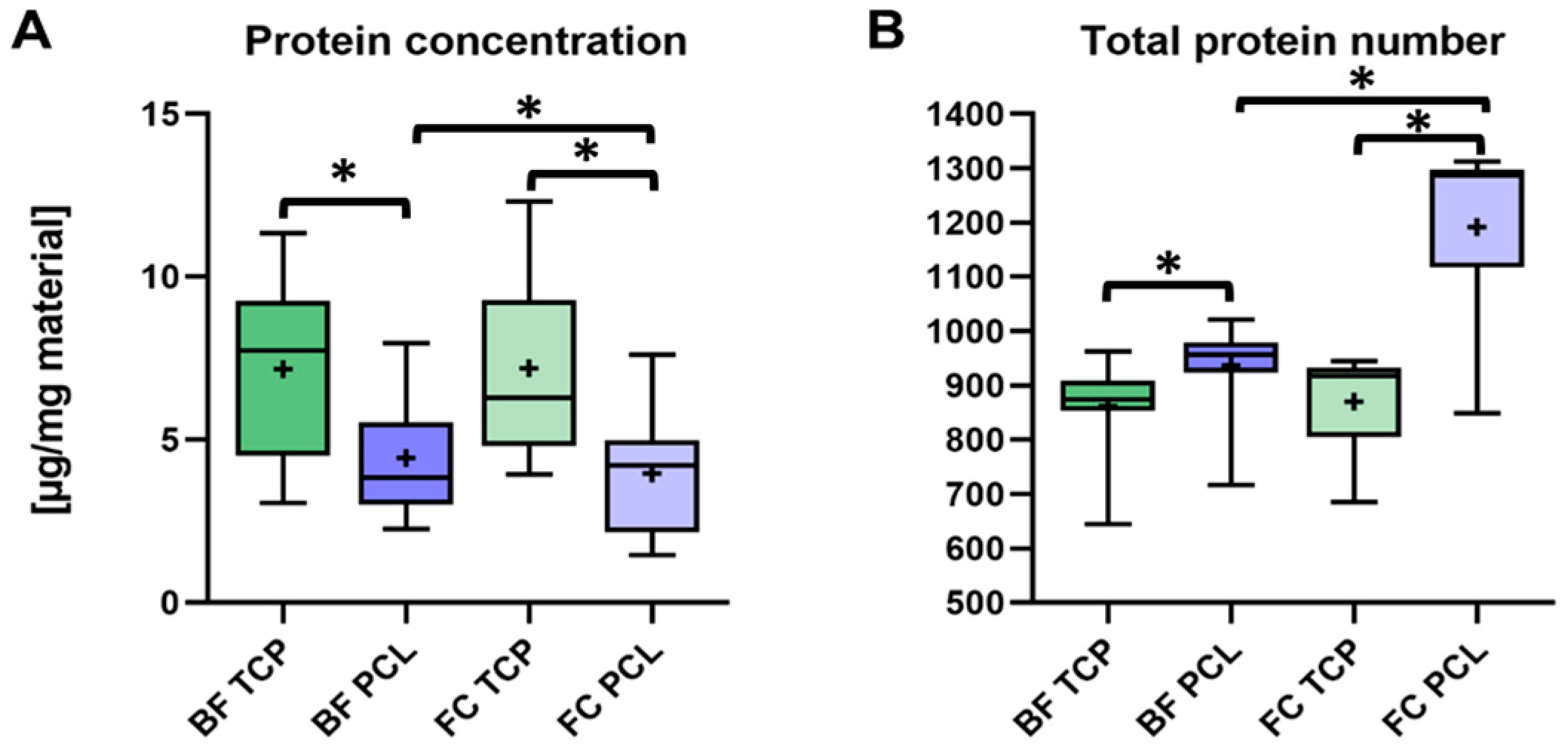
2.4. Protein Quantification and Proteome Analysis
2.5. Proteomic Data Annotation and Bioinformatics Analysis
2.6. Statistics and Data Visualization
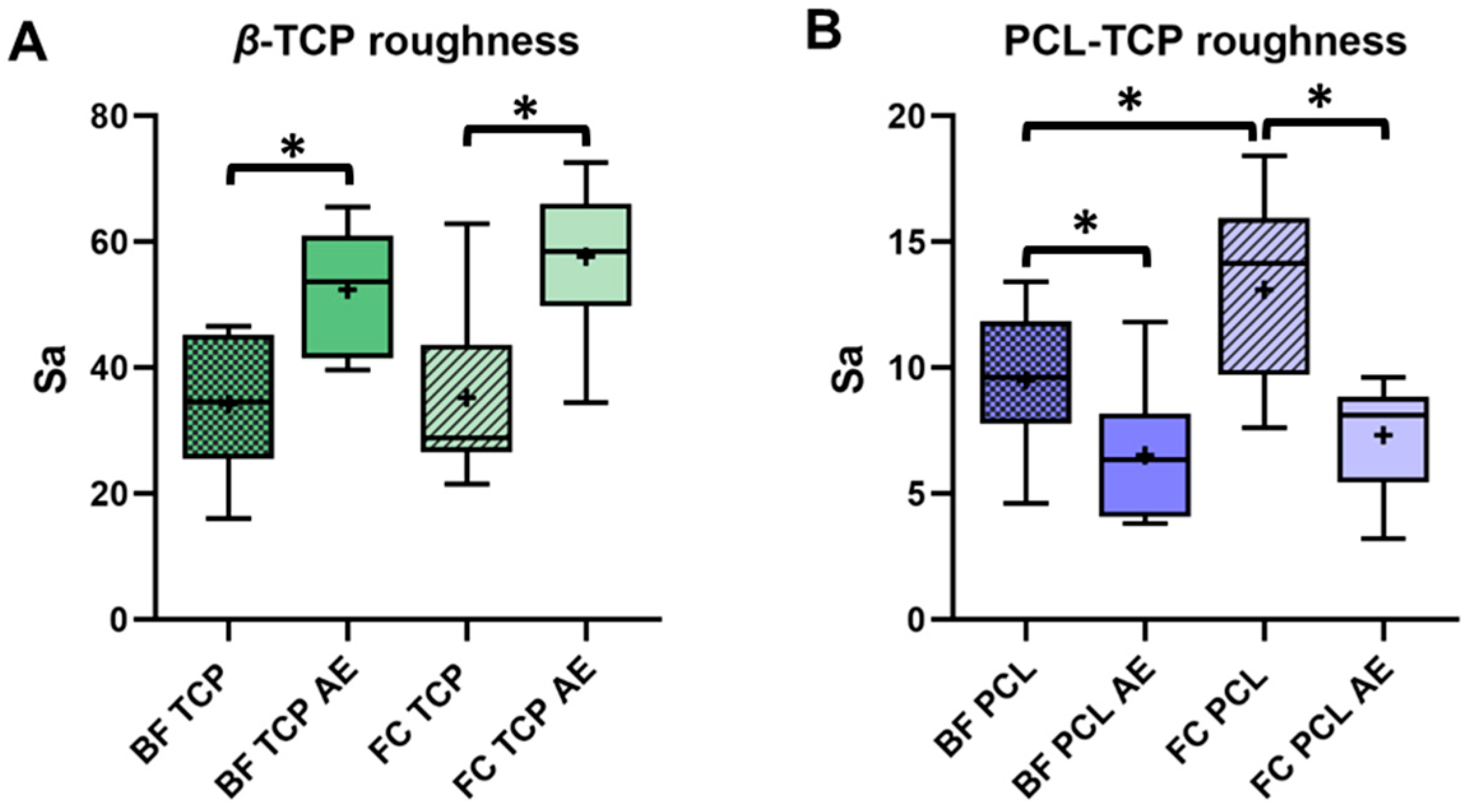
2.7. Surface Roughness Analysis
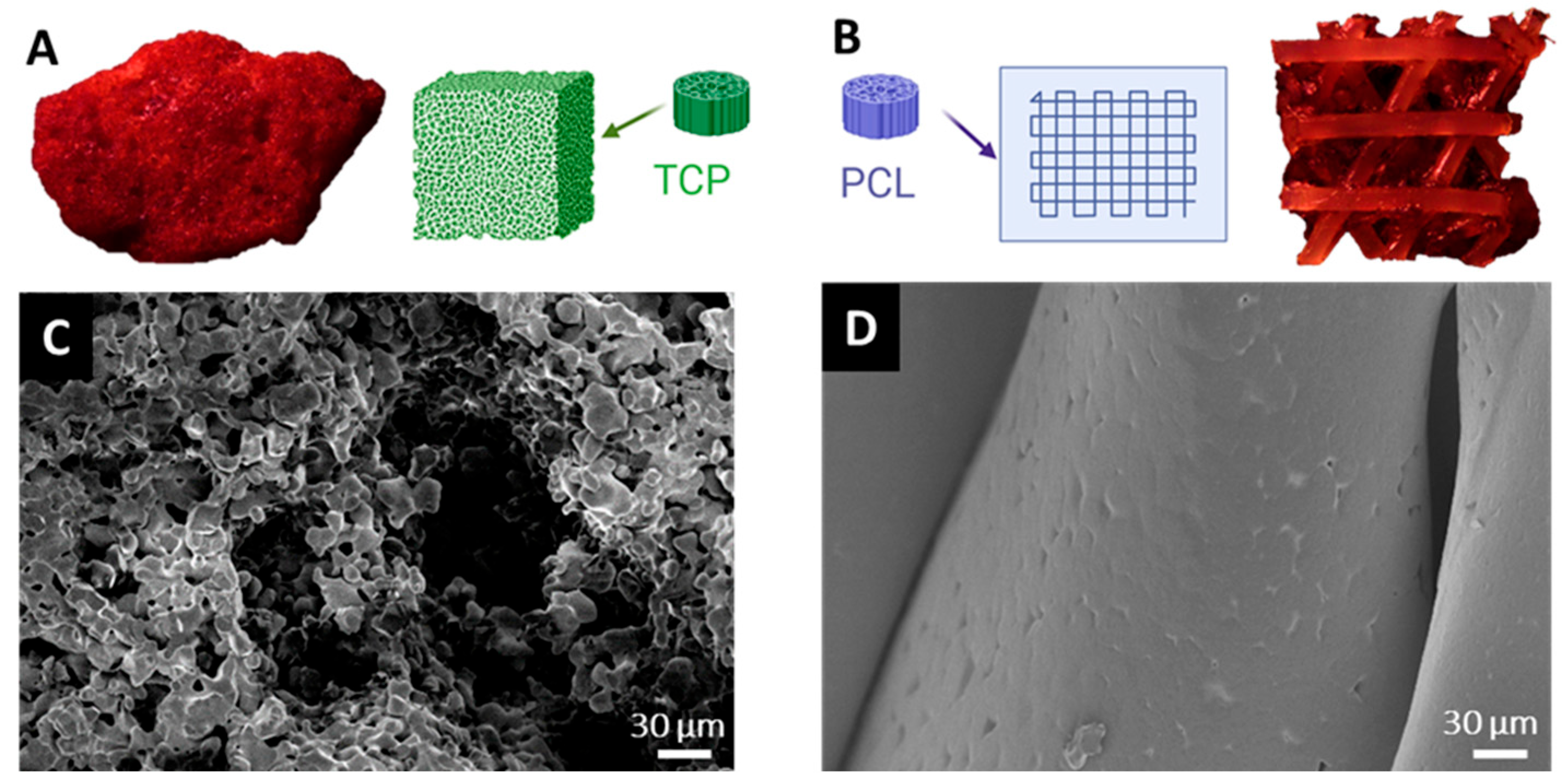
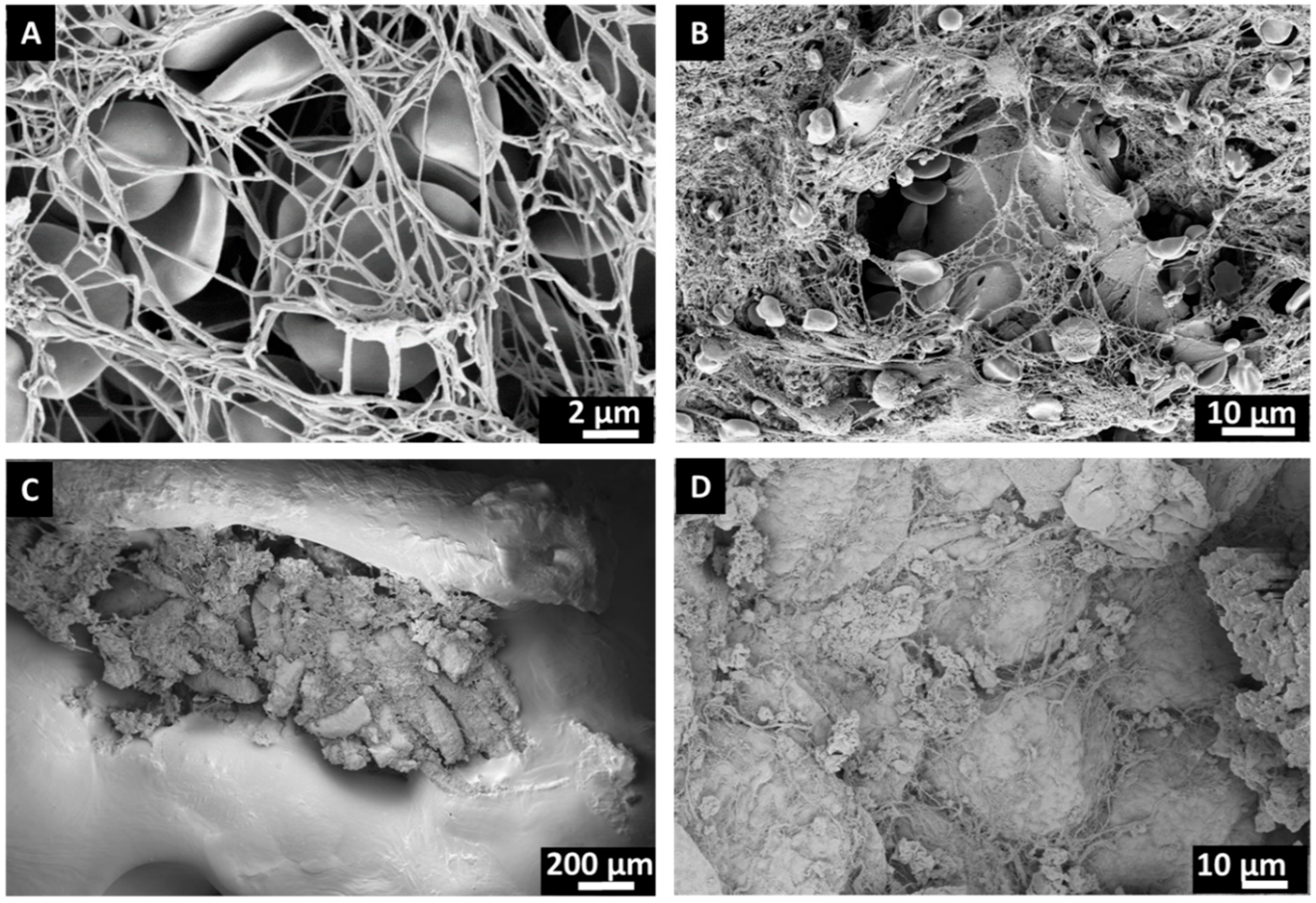
2.8. Scanning Electron Microscopy (SEM)
3. Results
3.1. Surface Characteristics of Bone Substitutes After Intraoperative Exposure
3.2. Impact of Protein Elution and Incubation Method on Bone Substitute Surface Roughness
3.3. Quantification of the Adsorbed Protein Layer on β-TCP and PCL-TCP
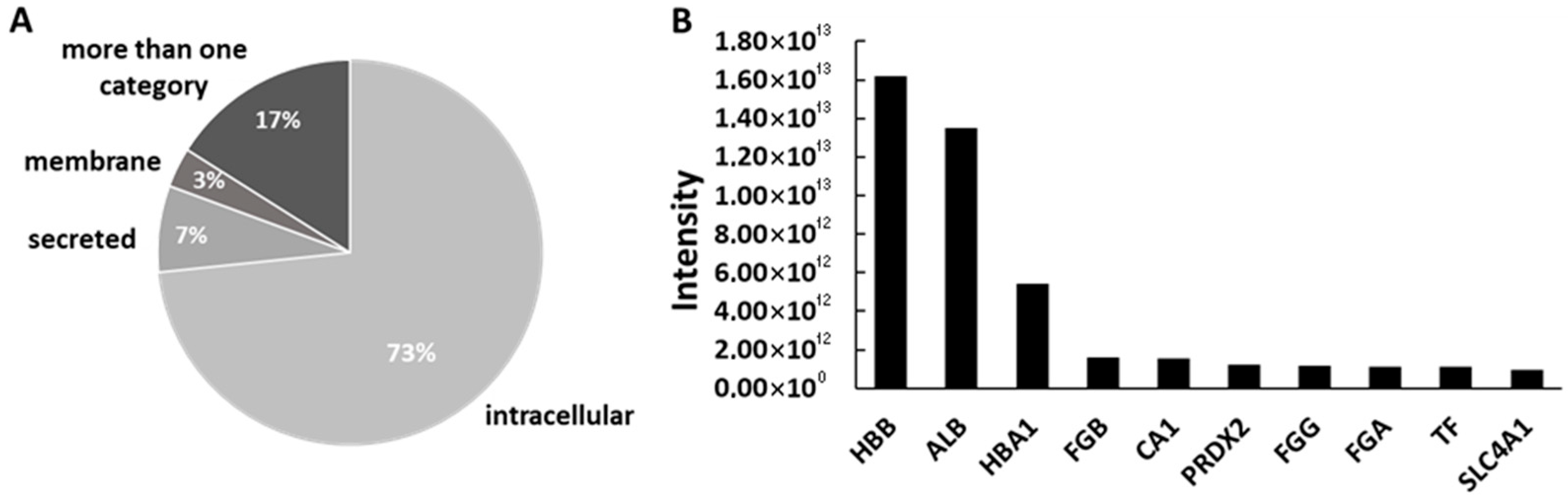
3.4. Proteomic Profile and Protein Origin in β-TCP and PCL-TCP Samples
3.5. Material-Specific Differences in Protein Adsorption Profiles
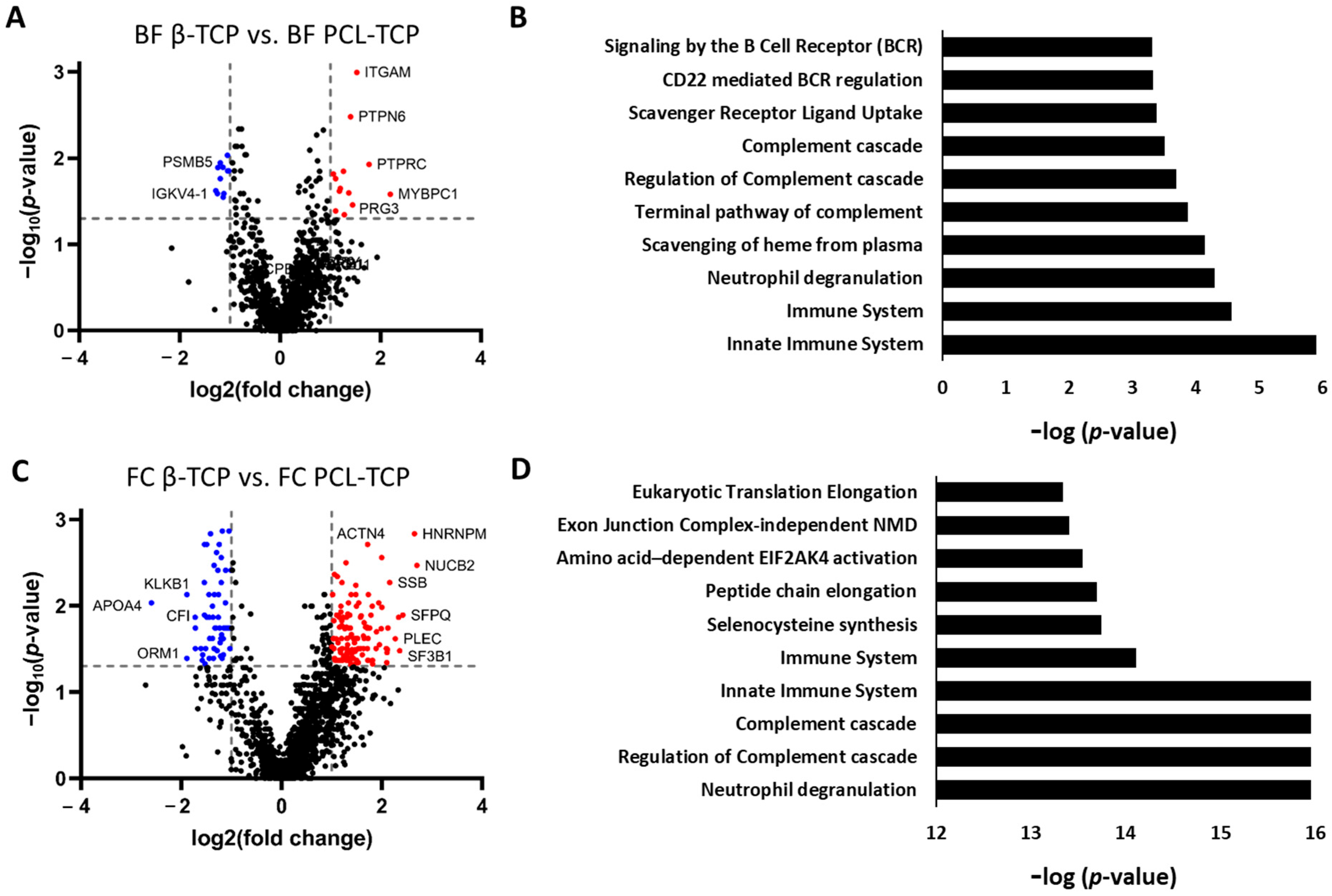
3.5.1. Selective Protein Adsorption on β-TCP and PCL-TCP Following Surgical Tissue Collector (BF) Incubation
3.5.2. Pronounced Material-Dependent Adsorption Differences After Femoral Medullary Cavity (FC) Incubation
3.6. Incubation-Dependent Protein Adsorption Profiles on Bone Substitutes
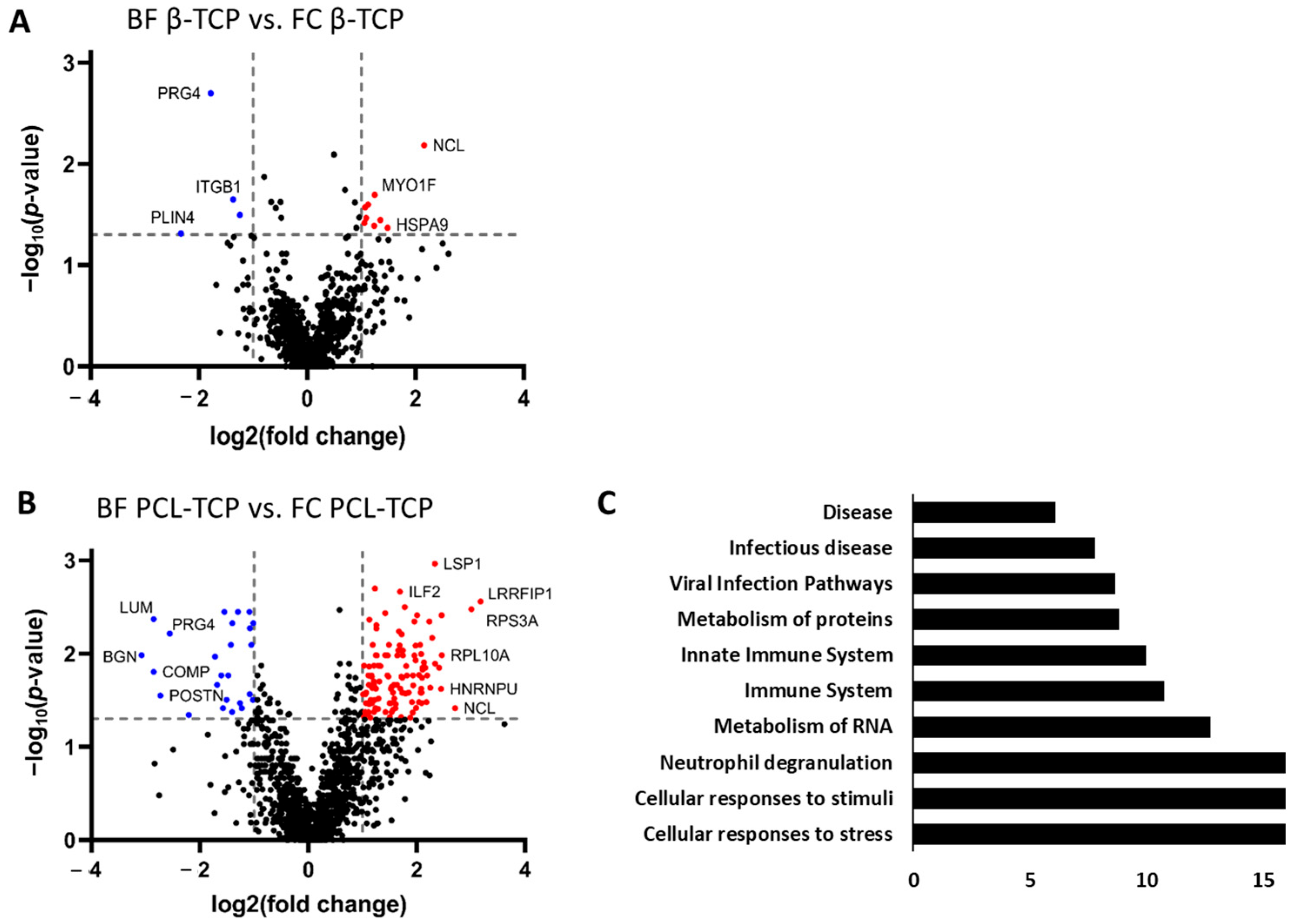
3.6.1. Protein Adsorption on β-TCP Is Minimally Affected by Incubation Method
3.6.2. Incubation Method Strongly Alters Protein Adsorption on PCL-TCP
4. Discussion
4.1. Protein Adsorption as a Key Regulator of Implant Integration
4.2. Material-Dependent Protein Adsorption: β-TCP vs. PCL-TCP
4.3. Impact of Incubation Conditions on Protein Adsorption
4.4. Comparative Insights from Intraoperative Proteomes of Bone Substitutes and Hip Stems
4.5. Study Limitations and Future Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medhat, D.; Rodríguez, C.I.; Infante, A. Immunomodulatory Effects of MSCs in Bone Healing. Int. J. Mol. Sci. 2019, 20, 5467. [Google Scholar] [CrossRef] [PubMed]
- Sterling, J.A.; Guelcher, S.A. Biomaterial scaffolds for treating osteoporotic bone. Curr. Osteoporos. Rep. 2014, 12, 48–54. [Google Scholar] [CrossRef]
- Lee, S.S.; Huang, B.J.; Kaltz, S.R.; Sur, S.; Newcomb, C.J.; Stock, S.R.; Shah, R.N.; Stupp, S.I. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials 2013, 34, 452–459. [Google Scholar] [CrossRef]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31 (Suppl. S5), S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, A.S.; Boden, S.D.; Goldberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N. Bone-graft substitutes: Facts, fictions, and applications. J. Bone Jt. Surg. Am. 2001, 83-A Pt 2 (Suppl. S2), 98–103. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar] [CrossRef]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, T.J.; Calori, G.M.; Schmidmaier, G. Autograft versus BMPs for the treatment of non-unions: What is the evidence? Injury 2013, 44 (Suppl. S1), S40–S42. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Bigham-Sadegh, A. Bone morphogenetic proteins: A powerful osteoinductive compound with non-negligible side effects and limitations. BioFactors 2014, 40, 459–481. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Jin, P.; Liu, L.; Cheng, L.; Chen, X.; Xi, S.; Jiang, T. Calcium-to-phosphorus releasing ratio affects osteoinductivity and osteoconductivity of calcium phosphate bioceramics in bone tissue engineering. Biomed. Eng. Online 2023, 22, 12. [Google Scholar] [CrossRef]
- Jasser, R.A.; AlSubaie, A.; AlShehri, F. Effectiveness of beta-tricalcium phosphate in comparison with other materials in treating periodontal infra-bony defects around natural teeth: A systematic review and meta-analysis. BMC Oral Health 2021, 21, 219. [Google Scholar] [CrossRef]
- Bohner, M.; Le Santoni, B.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Abtahi, S.; Chen, X.; Shahabi, S.; Nasiri, N. Resorbable Membranes for Guided Bone Regeneration: Critical Features, Potentials, and Limitations. ACS Mater. Au 2023, 3, 394–417. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Sheikh, F.A.; Gómez-Cerezo, N.; Alneairi, A.; Luqman, M.; Pant, H.R.; Ivanovski, S. A review of protein adsorption and bioactivity characteristics of poly ε-caprolactone scaffolds in regenerative medicine. Eur. Polym. J. 2022, 162, 110892. [Google Scholar] [CrossRef]
- Hiob, M.A.; She, S.; Muiznieks, L.D.; Weiss, A.S. Biomaterials and Modifications in the Development of Small-Diameter Vascular Grafts. ACS Biomater. Sci. Eng. 2017, 3, 712–723. [Google Scholar] [CrossRef]
- Garimella, A.; Ghosh, S.B.; Bandyopadhyay-Ghosh, S. Biomaterials for bone tissue engineering: Achievements to date and future directions. Biomed. Mater. 2024, 20, 012001. [Google Scholar] [CrossRef]
- Qasim, M.; Chae, D.S.; Lee, N.Y. Bioengineering strategies for bone and cartilage tissue regeneration using growth factors and stem cells. J. Biomed. Mater. Res. A 2020, 108, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Laubach, M.; Hildebrand, F.; Suresh, S.; Wagels, M.; Kobbe, P.; Gilbert, F.; Kneser, U.; Holzapfel, B.M.; Hutmacher, D.W. The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective. J. Funct. Biomater. 2023, 14, 341. [Google Scholar] [CrossRef]
- Yeo, A.; Rai, B.; Sju, E.; Cheong, J.J.; Teoh, S.H. The degradation profile of novel, bioresorbable PCL-TCP scaffolds: An in vitro and in vivo study. J. Biomed. Mater. Res. A 2008, 84, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, M.-R.; Oh, J.-S.; Han, I.; Shin, S.-W. Effects of polycaprolactone-tricalcium phosphate, recombinant human bone morphogenetic protein-2 and dog mesenchymal stem cells on bone formation: Pilot study in dogs. Yonsei Med. J. 2009, 50, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.T.; Chanchareonsook, N.; Tideman, H.; Teoh, S.H.; Chow, J.K.F.; Jansen, J.A. The use of a polycaprolactone-tricalcium phosphate scaffold for bone regeneration of tooth socket facial wall defects and simultaneous immediate dental implant placement in Macaca fascicularis. J. Biomed. Mater. Res. A 2014, 102, 1379–1388. [Google Scholar] [CrossRef]
- Henze, K.; Herten, M.; Haversath, M.; Busch, A.; Brandau, S.; Hackel, A.; Flohé, S.B.; Jäger, M. Surgical vacuum filter-derived stromal cells are superior in proliferation to human bone marrow aspirate. Stem Cell Res. Ther. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Herten, M.; Haversath, M.; Kaiser, C.; Brandau, S.; Jäger, M. Ceramic Scaffolds in a Vacuum Suction Handle for Intraoperative Stromal Cell Enrichment. Int. J. Mol. Sci. 2020, 21, 6393. [Google Scholar] [CrossRef]
- Rehage, E.; Sowislok, A.; Busch, A.; Papaeleftheriou, E.; Jansen, M.; Jäger, M. Surgical Site-Released Tissue is Potent to Generate Bone onto TCP and PCL-TCP Scaffolds In Vitro. Int. J. Mol. Sci. 2023, 24, 15877. [Google Scholar] [CrossRef]
- Groven, R.V.M.; Blokhuis, J.T.; Poeze, M.; van Griensven, M.; Blokhuis, T.J. Surgical suction filter-derived bone graft displays osteogenic miRNA and mRNA patterns. Eur. J. Trauma Emerg. Surg. 2024, 50, 315–326. [Google Scholar] [CrossRef]
- Hardinge, K. The direct lateral approach to the hip. J. Bone Joint Surg. Br. 1982, 64, 17–19. [Google Scholar] [CrossRef]
- Bauer, R.; Kerschbaumer, F.; Poisel, S.; Oberthaler, W. The transgluteal approach to the hip joint. Arch. Orthop. Trauma. Surg. 1979, 95, 47–49. [Google Scholar] [CrossRef]
- Jäger, M.; Jennissen, H.P.; Haversath, M.; Busch, A.; Grupp, T.; Sowislok, A.; Herten, M. Intrasurgical Protein Layer on Titanium Arthroplasty Explants: From the Big Twelve to the Implant Proteome. Proteomics Clin. Appl. 2019, 13, e1800168. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Latosinska, A.; Herten, M.; Busch, A.; Grupp, T.; Sowislok, A. The Implant Proteome-The Right Surgical Glue to Fix Titanium Implants In Situ. J. Funct. Biomater. 2022, 13, 44. [Google Scholar] [CrossRef]
- Sowislok, A.; Busch, A.; Kaschani, F.; Kaiser, M.; Jäger, M. Differences in the Synovial Fluid Proteome of Septic and Aseptic Implant Failure. Antibiotics 2024, 13, 346. [Google Scholar] [CrossRef]
- Farrah, T.; Deutsch, E.W.; Omenn, G.S.; Campbell, D.S.; Sun, Z.; Bletz, J.A.; Mallick, P.; Katz, J.E.; Malmström, J.; Ossola, R.; et al. A high-confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol. Cell. Proteom. 2011, 10, M110.006353. [Google Scholar] [CrossRef]
- Anderson, N.L.; Polanski, M.; Pieper, R.; Gatlin, T.; Tirumalai, R.S.; Conrads, T.P.; Veenstra, T.D.; Adkins, J.N.; Pounds, J.G.; Fagan, R.; et al. The human plasma proteome: A nonredundant list developed by combination of four separate sources. Mol. Cell. Proteom. 2004, 3, 311–326. [Google Scholar] [CrossRef]
- Geyer, P.E.; Kulak, N.A.; Pichler, G.; Holdt, L.M.; Teupser, D.; Mann, M. Plasma Proteome Profiling to Assess Human Health and Disease. Cell Syst. 2016, 2, 185–195. [Google Scholar] [CrossRef]
- Bryk, A.H.; Wiśniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Proteome Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, A.; Sidiropoulos, K.; Viteri, G.; Forner, O.; Marin-Garcia, P.; Arnau, V.; D’Eustachio, P.; Stein, L.; Hermjakob, H. Reactome pathway analysis: A high-performance in-memory approach. BMC Bioinform. 2017, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef]
- Rosentritt, M.; Schneider-Feyrer, S.; Kurzendorfer, L. Comparison of surface roughness parameters Ra/Sa and Rz/Sz with different measuring devices. J. Mech. Behav. Biomed. Mater. 2024, 150, 106349. [Google Scholar] [CrossRef]
- Davies, J.E. Understanding Peri-Implant Endosseous Healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Jennissen, H.P.; Dittrich, F.; Fischer, A.; Köhling, H.L. Antimicrobial and Osseointegration Properties of Nanostructured Titanium Orthopaedic Implants. Materials 2017, 10, 1302. [Google Scholar] [CrossRef]
- Mitra, S.P. Protein Adsorption on Biomaterial Surfaces: Subsequent Conformational and Biological Consequences—A Review. JSST 2020, 36, 7–38. [Google Scholar] [CrossRef]
- Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Waldeck, H.; Kao, W.J. Protein Adsorption to Biomaterials. In Biological Interactions on Materials Surfaces; Puleo, D.A., Bizios, R., Eds.; Springer: New York, NY, USA, 2009; pp. 1–18. [Google Scholar] [CrossRef]
- Akkas, T.; Citak, C.; Sirkecioglu, A.; Güner, F.S. Which is more effective for protein adsorption: Surface roughness, surface wettability or swelling? Case study of polyurethane films prepared from castor oil and poly(ethylene glycol). Polym. Int. 2013, 62, 1202–1209. [Google Scholar] [CrossRef]
- Song, W.; Chen, H. Protein adsorption on materials surfaces with nano-topography. Chin. Sci. Bull. 2007, 52, 3169–3173. [Google Scholar] [CrossRef]
- Han, M.; Sethuraman, A.; Kane, R.S.; Belfort, G. Nanometer-Scale Roughness Having Little Effect on the Amount or Structure of Adsorbed Protein. Langmuir 2003, 19, 9868–9872. [Google Scholar] [CrossRef]
- Cai, K.; Bossert, J.; Jandt, K.D. Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation? Colloids Surf. B Biointerfaces 2006, 49, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Piloni, A.; Wong, C.K.; Chen, F.; Lord, M.; Walther, A.; Stenzel, M.H. Surface roughness influences the protein corona formation of glycosylated nanoparticles and alter their cellular uptake. Nanoscale 2019, 11, 23259–23267. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, P.E.; Borgonovo, A.; Indrieri, M.; Giorgetti, L.; Bongiorno, G.; Carbone, R.; Podestà, A.; Milani, P. The effect of surface nanometre-scale morphology on protein adsorption. PLoS ONE 2010, 5, e11862. [Google Scholar] [CrossRef]
- Rechendorff, K.; Hovgaard, M.B.; Foss, M.; Zhdanov, V.P.; Besenbacher, F. Enhancement of protein adsorption induced by surface roughness. Langmuir 2006, 22, 10885–10888. [Google Scholar] [CrossRef]
- Dolatshahi-Pirouz, A.; Skeldal, S.; Hovgaard, M.B.; Jensen, T.; Foss, M.; Chevallier, J.; Besenbacher, F. Influence of Nanoroughness and Detailed Surface Morphology on Structural Properties and Water-Coupling Capabilities of Surface-Bound Fibrinogen Films. J. Phys. Chem. C 2009, 113, 4406–4412. [Google Scholar] [CrossRef]
- Boehm, R.D.; Skoog, S.A.; Diaz-Diestra, D.M.; Goering, P.L.; Dair, B.J. Influence of titanium nanoscale surface roughness on fibrinogen and albumin protein adsorption kinetics and platelet responses. J. Biomed. Mater. Res. A 2024, 112, 373–389. [Google Scholar] [CrossRef]
- Clemments, A.M.; Botella, P.; Landry, C.C. Protein Adsorption From Biofluids on Silica Nanoparticles: Corona Analysis as a Function of Particle Diameter and Porosity. ACS Appl. Mater. Interfaces 2015, 7, 21682–21689. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, C.; Hong, Y.; Zhang, X. A review of protein adsorption on bioceramics. Interface Focus 2012, 2, 259–277. [Google Scholar] [CrossRef]
- Pang, D.; He, L.; Wei, L.; Zheng, H.; Deng, C. Preparation of a beta-tricalcium phosphate nanocoating and its protein adsorption behaviour by quartz crystal microbalance with dissipation technique. Colloids Surf. B Biointerfaces 2018, 162, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Pomorska, A.; Nattich-Rak, M.; Wytrwal-Sarna, M.; Bernasik, A. Protein adsorption mechanisms at rough surfaces: Serum albumin at a gold substrate. J. Colloid Interface Sci. 2018, 530, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Norde, W. Adsorption of proteins from solution at the solid-liquid interface. Adv. Colloid Interface Sci. 1986, 25, 267–340. [Google Scholar] [CrossRef]
- Bronowska, A.K. Thermodynamics of Ligand-Protein Interactions: Implications for Molecular Design. In Thermodynamics—Interaction Studies—Solids, Liquids and Gases, 1st ed.; Moreno-Pirajan, J.C., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Latour, R.A. Fundamental Principles of the Thermodynamics and Kinetics of Protein Adsorption to Material Surfaces. Colloids Surf. B Biointerfaces 2020, 191, 110992. [Google Scholar] [CrossRef]
- Baszkin, A.; Lyman, D.J. The interaction of plasma proteins with polymers. I. Relationship between polymer surface energy and protein adsorption/desorption. J. Biomed. Mater. Res. 1980, 14, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef]
- Valero Vidal, C.; Olmo Juan, A.; Igual Muñoz, A. Adsorption of bovine serum albumin on CoCrMo surface: Effect of temperature and protein concentration. Colloids Surf. B Biointerfaces 2010, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kopac, T.; Bozgeyik, K.; Yener, J. Effect of pH and temperature on the adsorption of bovine serum albumin onto titanium dioxide. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 19–28. [Google Scholar] [CrossRef]
- Zhong, E.D.; Shirts, M.R. Thermodynamics of coupled protein adsorption and stability using hybrid Monte Carlo simulations. Langmuir 2014, 30, 4952–4961. [Google Scholar] [CrossRef] [PubMed]
- Vroman, L.; Adams, A.L. Identification of rapid changes at plasma-solid interfaces. J. Biomed. Mater. Res. 1969, 3, 43–67. [Google Scholar] [CrossRef]
- Horbett, T.A. Fibrinogen adsorption to biomaterials. J. Biomed. Mater. Res. A 2018, 106, 2777–2788. [Google Scholar] [CrossRef]
- Richter-Bisson, Z.W.; Hedberg, Y.S. Revisiting the Vroman effect: Mechanisms of competitive protein exchange on surfaces. Colloids Surf. B Biointerfaces 2025, 255, 114927. [Google Scholar] [CrossRef]
- Fang, F.; Szleifer, I. Kinetics and thermodynamics of protein adsorption: A generalized molecular theoretical approach. Biophys. J. 2001, 80, 2568–2589. [Google Scholar] [CrossRef]
- Reed, G.F.; Lynn, F.; Meade, B.D. Use of coefficient of variation in assessing variability of quantitative assays. Clin. Diagn. Lab. Immunol. 2002, 9, 1235–1239. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowislok, A.; Gruber, G.; Kaschani, F.; Kaiser, M.; Papaeleftheriou, E.; Jäger, M. Intraoperative Biologization of β-TCP and PCL-TCP by Autologous Proteins. J. Funct. Biomater. 2025, 16, 340. https://doi.org/10.3390/jfb16090340
Sowislok A, Gruber G, Kaschani F, Kaiser M, Papaeleftheriou E, Jäger M. Intraoperative Biologization of β-TCP and PCL-TCP by Autologous Proteins. Journal of Functional Biomaterials. 2025; 16(9):340. https://doi.org/10.3390/jfb16090340
Chicago/Turabian StyleSowislok, Andrea, Gerrit Gruber, Farnusch Kaschani, Markus Kaiser, Eleftherios Papaeleftheriou, and Marcus Jäger. 2025. "Intraoperative Biologization of β-TCP and PCL-TCP by Autologous Proteins" Journal of Functional Biomaterials 16, no. 9: 340. https://doi.org/10.3390/jfb16090340
APA StyleSowislok, A., Gruber, G., Kaschani, F., Kaiser, M., Papaeleftheriou, E., & Jäger, M. (2025). Intraoperative Biologization of β-TCP and PCL-TCP by Autologous Proteins. Journal of Functional Biomaterials, 16(9), 340. https://doi.org/10.3390/jfb16090340








