Chlorogenic Acid–Strontium-Containing Dual-Functional Bioresorbable External Stent Suppresses Venous Graft Restenosis via Hippo-YAP Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. SrCA Fabrication
2.2. SrCA eStent Fabrication
2.3. Characterization of SrCA and SrCA eStents
2.4. Cell Culture and SrCA eStent Extract Preparation
2.5. Cell Counting Kit-8 (CCK-8) Assay
2.6. Live/Dead Assay
2.7. Cell Migration Assay
2.8. Quantitative Real-Time PCR
2.9. Western Blot
2.10. Surgical Procedure
2.11. Histomorphology
2.12. Statistical Analysis
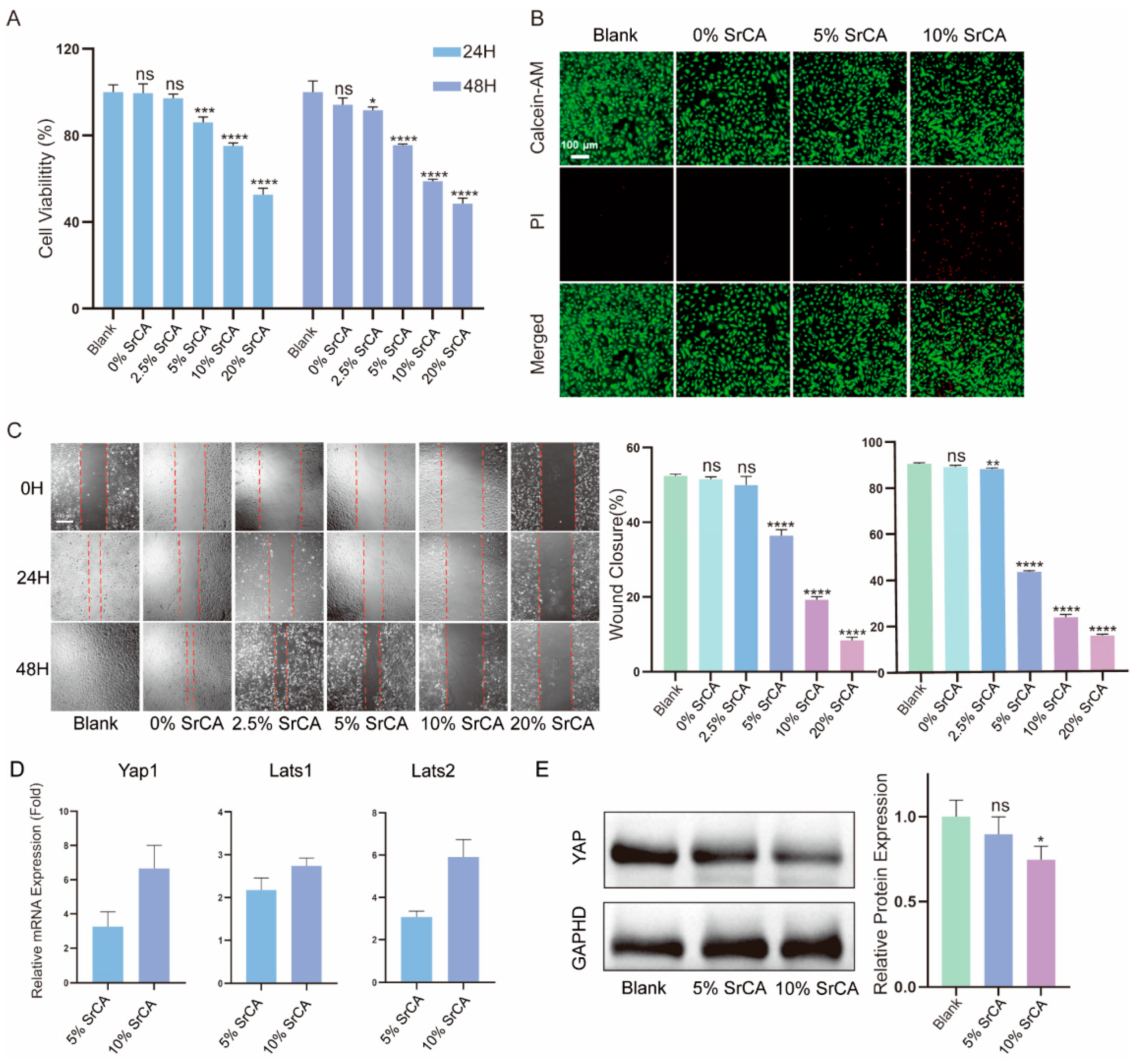
3. Results
3.1. Fabrication and Characterization of SrCA
3.2. Fabrication and Characterization of SrCA eStents
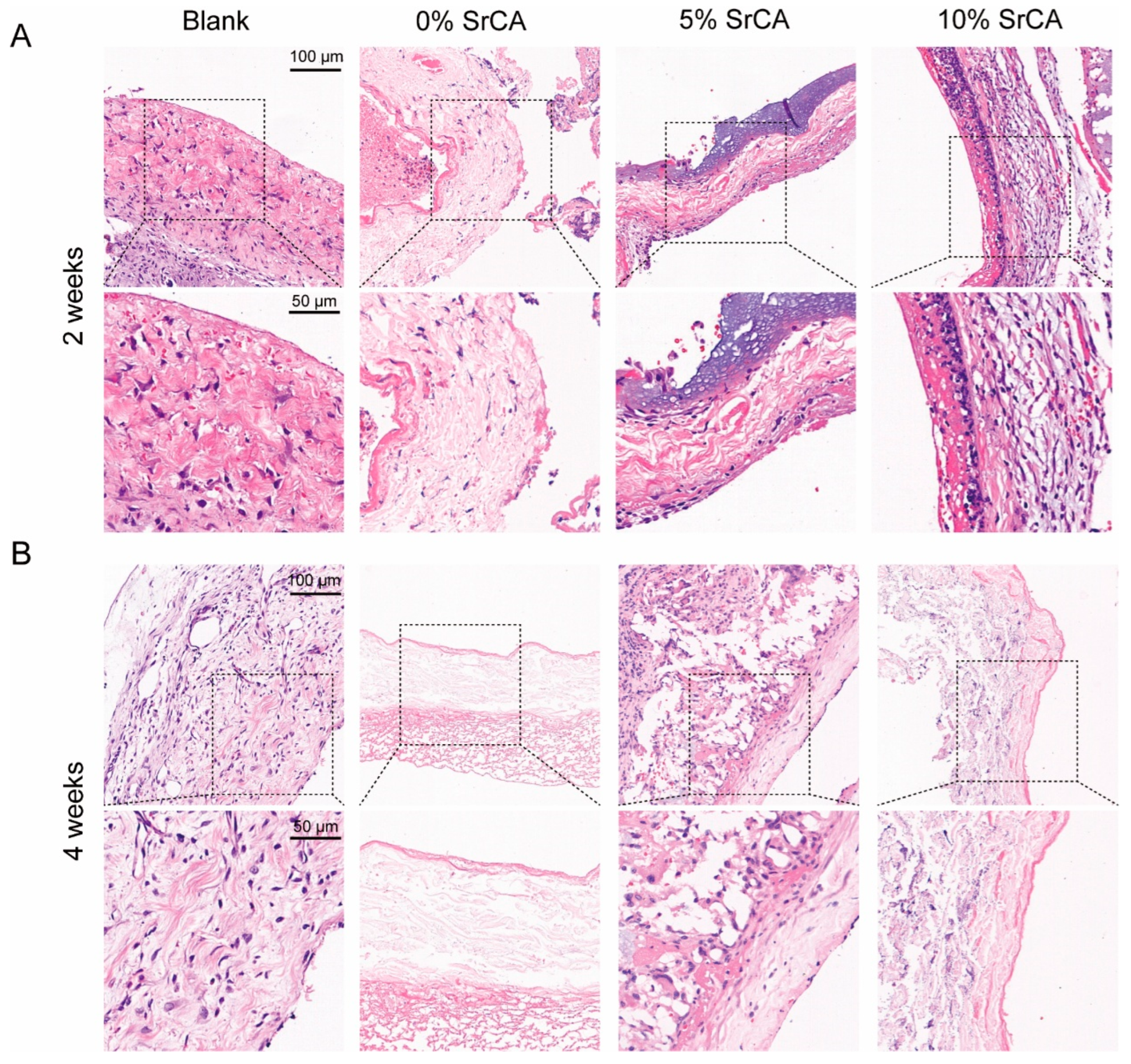
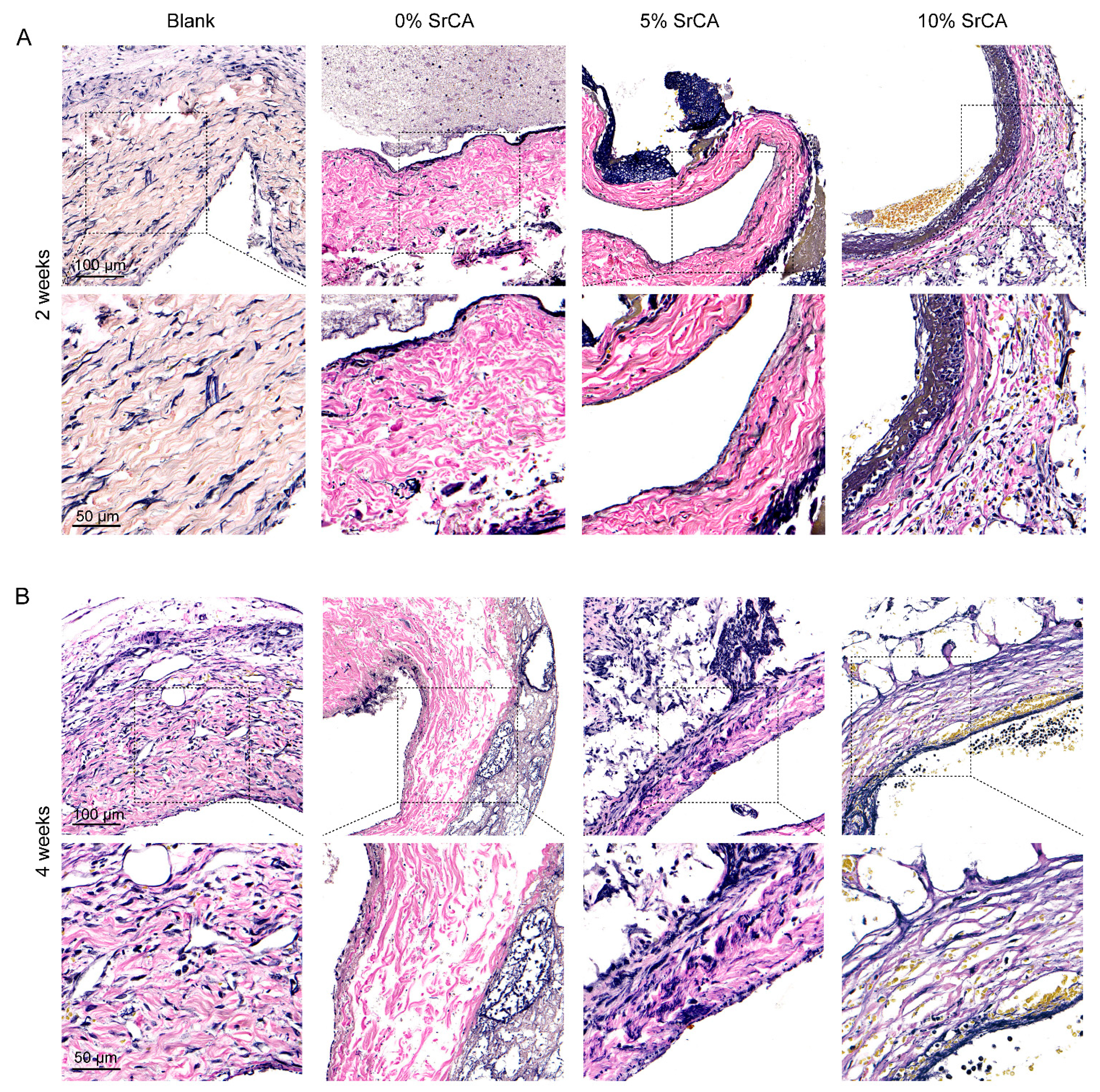
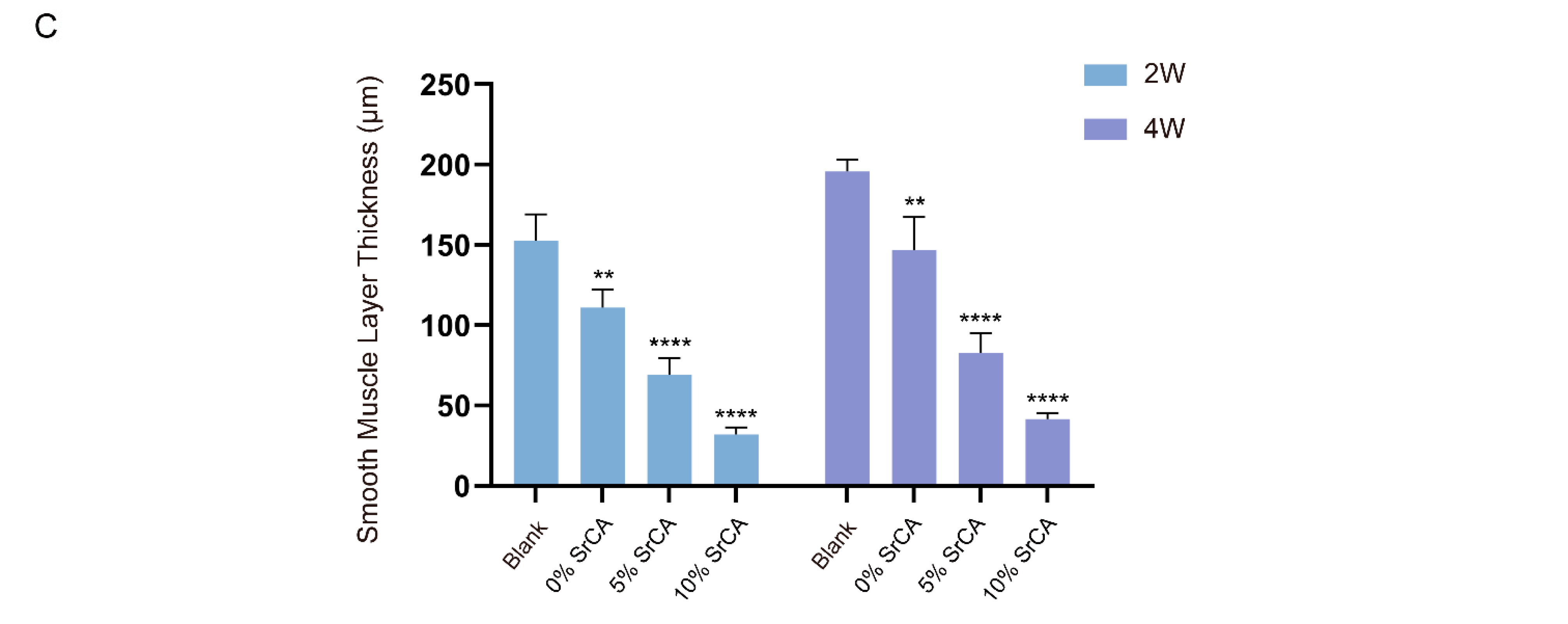
3.3. eStent Inhibited Intimal Hyperplasia of Vein Grafts and Regulated Vascular Remodeling
3.4. In Vivo Efficacy of EStents in Vein Grafts
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalen, J.E.; Alpert, J.S.; Goldberg, R.J.; Weinstein, R.S. The epidemic of the 20(th) century: Coronary heart disease. Am. J. Med. 2014, 127, 807–812. [Google Scholar] [CrossRef]
- Naghavi, M.; Wang, H.; Lozano, R.; Davis, A.; Liang, X.; Zhou, M.; Vollset, S.E.; Abbasoglu Ozgoren, A.; Abdalla, S.; Abd-Allah, F.; et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Mohr, F.W.; Morice, M.-C.; Kappetein, A.P.; E Feldman, T.; Ståhle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R.; Morel, M.-A.; Van Dyck, N.; et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013, 381, 629–638. [Google Scholar] [CrossRef]
- Brener, S.J.; Lytle, B.W.; Casserly, I.P.; Schneider, J.P.; Topol, E.J.; Lauer, M.S. Propensity Analysis of Long-Term Survival After Surgical or Percutaneous Revascularization in Patients With Multivessel Coronary Artery Disease and High-Risk Features. Circulation 2004, 109, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Benedetto, U.; Fremes, S.; Biondi-Zoccai, G.; Sedrakyan, A.; Puskas, J.D.; Angelini, G.D.; Buxton, B.; Frati, G.; Hare, D.L.; et al. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N. Engl. J. Med. 2018, 378, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Mounsey, C.A.; Mawhinney, J.A.; Werner, R.S.; Taggart, D.P. Does Previous Transradial Catheterization Preclude Use of the Radial Artery as a Conduit in Coronary Artery Bypass Surgery? Circulation 2016, 134, 681–688. [Google Scholar] [CrossRef]
- De Vries, M.R.; Simons, K.H.; Jukema, J.W.; Braun, J.; Quax, P.H.A. Vein graft failure: From pathophysiology to clinical outcomes. Nat. Rev. Cardiol. 2016, 13, 451–470. [Google Scholar] [CrossRef]
- Harskamp, R.E.; Lopes, R.D.; Baisden, C.E.; de Winter, R.J.; Alexander, J.H. Saphenous Vein Graft Failure After Coronary Artery Bypass Surgery. Ann. Surg. 2013, 257, 824–833. [Google Scholar] [CrossRef]
- Yahagi, K.; Kolodgie, F.D.; Otsuka, F.; Finn, A.V.; Davis, H.R.; Joner, M.; Virmani, R. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat. Rev. Cardiol. 2015, 13, 79–98. [Google Scholar] [CrossRef]
- Zilla, P.; Human, P.; Wolf, M.; Lichtenberg, W.; Rafiee, N.; Bezuidenhout, D.; Samodien, N.; Schmidt, C.; Franz, T. Constrictive external nitinol meshes inhibit vein graft intimal hyperplasia in nonhuman primates. J. Thorac. Cardiovasc. Surg. 2008, 136, 717–725. [Google Scholar] [CrossRef]
- Sato, A.; Kawamoto, S.; Watanabe, M.; Suzuki, Y.; Takahashi, G.; Masaki, N.; Kumagai, K.; Saijo, Y.; Tabayashi, K.; Saiki, Y. A novel biodegradable external mesh stent improved long-term patency of vein grafts by inhibiting intimal–medial hyperplasia in an experimental canine model. Gen. Thorac. Cardiovasc. Surg. 2015, 64, 1–9. [Google Scholar] [CrossRef]
- Chu, T.; Li, Q.; Dai, C.; Li, X.; Kong, X.; Fan, Y.; Yin, H.; Ge, J. A novel Nanocellulose-Gelatin-AS-IV external stent resists EndMT by activating autophagy to prevent restenosis of grafts. Bioact. Mater. 2023, 22, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Ben-Gal, Y.; Taggart, D.P.; Williams, M.R.; Orion, E.; Uretzky, G.; Shofti, R.; Banai, S.; Yosef, L.; Bolotin, G. Expandable external support device to improve Saphenous Vein Graft Patency after CABG. J. Cardiothorac. Surg. 2013, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.J.; Newby, A.C.; Jeremy, J.Y.; Baumbach, A.; Angelini, G.D. A randomized trial of an external Dacron sheath for the prevention of vein graft disease: The Extent study. J. Thorac. Cardiovasc. Surg. 2007, 134, 504–505. [Google Scholar] [CrossRef] [PubMed]
- Gharamti, A.; Kanafani, Z.A. Vascular Graft Infections. Infect. Dis. Clin. N. Am. 2018, 32, 789–809. [Google Scholar] [CrossRef]
- Taggart, D.P.; Ben Gal, Y.; Lees, B.; Patel, N.; Webb, C.; Rehman, S.M.; Desouza, A.; Yadav, R.; De Robertis, F.; Dalby, M.; et al. A Randomized Trial of External Stenting for Saphenous Vein Grafts in Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2015, 99, 2039–2045. [Google Scholar] [CrossRef]
- Taggart, D.P.; Gavrilov, Y.; Krasopoulos, G.; Rajakaruna, C.; Zacharias, J.; De Silva, R.; Channon, K.M.; Gehrig, T.; Donovan, T.J.; Friedrich, I.; et al. External stenting and disease progression in saphenous vein grafts two years after coronary artery bypass grafting: A multicenter randomized trial. J. Thorac. Cardiovasc. Surg. 2022, 164, 1532–1541.e2. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- He, J.; Bao, Q.; Yan, M.; Liang, J.; Zhu, Y.; Wang, C.; Ai, D. The role of Hippo/yes-associated protein signalling in vascular remodelling associated with cardiovascular disease. Br. J. Pharmacol. 2017, 175, 1354–1361. [Google Scholar] [CrossRef]
- Ruiter, M.S.; Pesce, M. Mechanotransduction in Coronary Vein Graft Disease. Front. Cardiovasc. Med. 2018, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Guo, Y.; Zhu, T.; Zhang, J.; Ma, P.X.; Chen, Y.E. Yap1 Protein Regulates Vascular Smooth Muscle Cell Phenotypic Switch by Interaction with Myocardin. J. Biol. Chem. 2012, 287, 14598–14605. [Google Scholar] [CrossRef]
- Amin, R.P.; Kunaparaju, N.; Kumar, S.; Taldone, T.; Barletta, M.A.; Zito, S.W. Structure elucidation and inhibitory effects on human platelet aggregation of chlorogenic acid from Wrightia tinctoria. J. Complement. Integr. Med. 2013, 10, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, D.; Tang, X.; Li, X.; Wo, D.; Yan, H.; Song, R.; Feng, J.; Li, P.; Zhang, J.; et al. Chlorogenic acid prevents isoproterenol-induced hypertrophy in neonatal rat myocytes. Toxicol. Lett. 2014, 226, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Akila, P.; Vennila, L. Chlorogenic acid a dietary polyphenol attenuates isoproterenol induced myocardial oxidative stress in rat myocardium: An in vivo study. Biomed. Pharmacother. 2016, 84, 208–214. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.; Lio, C.; Sun, W.; Lai, K.; Liu, Y.; Zhang, Z.; Liang, J.; Zhou, H.; Liu, L.; et al. A chlorogenic acid-phospholipid complex ameliorates post-myocardial infarction inflammatory response mediated by mitochondrial reactive oxygen species in SAMP8 mice. Pharmacol. Res. 2018, 130, 110–122. [Google Scholar] [CrossRef]
- Akila, P.; Asaikumar, L.; Vennila, L. Chlorogenic acid ameliorates isoproterenol-induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes. Biomed. Pharmacother. 2017, 85, 582–591. [Google Scholar] [CrossRef]
- Gu, Z.; Xie, H.; Li, L.; Zhang, X.; Liu, F.; Yu, X. Application of strontium-doped calcium polyphosphate scaffold on angiogenesis for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 1251–1260. [Google Scholar] [CrossRef]
- Xing, M.; Jiang, Y.; Bi, W.; Gao, L.; Zhou, Y.-L.; Rao, S.-L.; Ma, L.-L.; Zhang, Z.-W.; Yang, H.-T.; Chang, J. Strontium ions protect hearts against myocardial ischemia/reperfusion injury. Sci. Adv. 2021, 7, eabe0726. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, D.; Huang, S.; Yang, Q.; Song, B.; Guo, Y.; Shen, A.; Yuan, Z.; Li, S.; Qing, F.-L.; et al. Elastic 3D-Printed Hybrid Polymeric Scaffold Improves Cardiac Remodeling after Myocardial Infarction. Adv. Healthc. Mater. 2019, 8, e1900065. [Google Scholar] [CrossRef]
- No. 85-23; Guide for the Care and Use of Laboratory Animals. National Academies Press (US) Copyright 1996 by the National Academy of Sciences: Washington, DC, USA, 1996.
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. J. Thorac. Cardiovasc. Surg. 2015, 149, e5–e23. [Google Scholar] [CrossRef]
- Bates, E.R. Review: In CAD, CABG reduced 5-year mortality more than PCI in multivessel but not left main disease. Ann. Intern. Med. 2018, 168, Jc66. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Taggart, D.; Suma, H.; Puskas, J.D.; Crea, F.; Massetti, M. The Choice of Conduits in Coronary Artery Bypass Surgery. J. Am. Coll. Cardiol. 2015, 66, 1729–1737. [Google Scholar] [CrossRef]
- Wadey, K.; Lopes, J.; Bendeck, M.; George, S. Role of smooth muscle cells in coronary artery bypass grafting failure. Cardiovasc. Res. 2018, 114, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yang, X.; Che, X.; Yang, M.; Zhai, G. Biomedical application and controlled drug release of electrospun fibrous materials. Mater. Sci. Eng. C 2018, 90, 750–763. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef]
- Yang, Q.; Lei, D.; Huang, S.; Yang, Y.; Jiang, C.; Shi, H.; Chen, W.; Zhao, Q.; You, Z.; Ye, X. A novel biodegradable external stent regulates vein graft remodeling via the Hippo-YAP and mTOR signaling pathways. Biomaterials 2020, 258, 120254. [Google Scholar] [CrossRef]
- Giacoppo, D.; Alfonso, F.; Xu, B.; Claessen, B.E.; Adriaenssens, T.; Jensen, C.; Pérez-Vizcayno, M.J.; Kang, D.-Y.; Degenhardt, R.; Pleva, L.; et al. Drug-Coated Balloon Angioplasty Versus Drug-Eluting Stent Implantation in Patients with Coronary Stent Restenosis. J. Am. Coll. Cardiol. 2020, 75, 2664–2678. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Liu, J.; Xu, M.; Tong, X.; Wang, J. Chlorogenic acid prevents isoproterenol-induced DNA damage in vascular smooth muscle cells. Mol. Med. Rep. 2016, 14, 4063–4068. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, M.; Wang, X.; Li, W.; Chen, W. Functionalized gelatin/strontium hydrogel bearing endothelial progenitor cells for accelerating angiogenesis in wound tissue regeneration. Biomater. Adv. 2022, 136, 212803. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.R.; Hart, I.R.; Watson, A.R.; Welti, J.C.; Silva, R.G.; Robinson, S.D.; Da Violante, G.; Gourlaouen, M.; Salih, M.; Jones, M.C.; et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat. Med. 2009, 15, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, T.; Kong, Q.; Xu, H.; Zhao, Z.; Li, G.; Fan, H.; Wang, Y. A chlorogenic acid functional strategy of anti-inflammation, anti-coagulation and promoted endothelial proliferation for bioprosthetic artificial heart valves. J. Mater. Chem. B 2023, 11, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Jin, X.; Xu, B.; Nan, L.; Liu, S.; Wang, J.; Niu, N.; Wu, Z.; Chen, F.; Liu, J. Chlorogenic acid releasing microspheres enhanced electrospun conduits to promote peripheral nerve regeneration. Biomater. Sci. 2023, 11, 7909–7925. [Google Scholar] [CrossRef]
- Dutta, S.; Khan, R.; Prakash, N.S.; Gupta, S.; Ghosh, D.; Nandi, S.K.; Roy, M. In Vitro Degradation and In Vivo Biocompatibility of Strontium-Doped Magnesium Phosphate-Reinforced Magnesium Composites. ACS Biomater. Sci. Eng. 2022, 8, 4236–4248. [Google Scholar] [CrossRef]
- Sahoo, S.; Sinha, A.; Das, M. Synthesis, characterization and in vitro biocompatibility study of strontium titanate ceramic: A potential biomaterial. J. Mech. Behav. Biomed. Mater. 2020, 102, 103494. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Wang, J.; Jia, F.; Shen, Z.-D.; Zhang, W.-B.; Wang, Y.-X.; Ren, K.-F.; Fu, G.-S.; Ji, J. Bioinspired NO release coating enhances endothelial cells and inhibits smooth muscle cells. J. Mater. Chem. B 2022, 10, 2454–2462. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, Y.; Yin, F.; Yu, J.; Silverman, N.; Pan, D. Toll Receptor-Mediated Hippo Signaling Controls Innate Immunity in Drosophila. Cell 2016, 164, 406–419. [Google Scholar] [CrossRef]
- Rauskolb, C.; Sun, S.; Sun, G.; Pan, Y.; Irvine, K.D. Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell 2014, 158, 143–156. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.-L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Wang, X.; Hu, G.; Gao, X.; Wang, Y.; Zhang, W.; Harmon, E.Y.; Zhi, X.; Xu, Z.; Lennartz, M.R.; Barroso, M.; et al. The induction of yes-associated protein expression after arterial injury is crucial for smooth muscle phenotypic modulation and neointima formation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.E.; Duggirala, A.; Smith, M.C.; White, S.; Sala-Newby, G.B.; Newby, A.C.; Bond, M. The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP. J. Mol. Cell Cardiol. 2016, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Wang, S.; Liu, Z.; Gu, S.; Chen, F.; Zang, W. Chlorogenic Acid–Strontium-Containing Dual-Functional Bioresorbable External Stent Suppresses Venous Graft Restenosis via Hippo-YAP Signaling Pathway. J. Funct. Biomater. 2025, 16, 259. https://doi.org/10.3390/jfb16070259
Zhu G, Wang S, Liu Z, Gu S, Chen F, Zang W. Chlorogenic Acid–Strontium-Containing Dual-Functional Bioresorbable External Stent Suppresses Venous Graft Restenosis via Hippo-YAP Signaling Pathway. Journal of Functional Biomaterials. 2025; 16(7):259. https://doi.org/10.3390/jfb16070259
Chicago/Turabian StyleZhu, Ge, Su Wang, Zhang Liu, Shengji Gu, Feng Chen, and Wangfu Zang. 2025. "Chlorogenic Acid–Strontium-Containing Dual-Functional Bioresorbable External Stent Suppresses Venous Graft Restenosis via Hippo-YAP Signaling Pathway" Journal of Functional Biomaterials 16, no. 7: 259. https://doi.org/10.3390/jfb16070259
APA StyleZhu, G., Wang, S., Liu, Z., Gu, S., Chen, F., & Zang, W. (2025). Chlorogenic Acid–Strontium-Containing Dual-Functional Bioresorbable External Stent Suppresses Venous Graft Restenosis via Hippo-YAP Signaling Pathway. Journal of Functional Biomaterials, 16(7), 259. https://doi.org/10.3390/jfb16070259









