Impact of 45S5-Bioactive Glass on Chondrocytes in Knee Osteoarthritis—In Vitro Study Exploring Cellular Responses
Abstract
1. Introduction
2. Materials and Methods
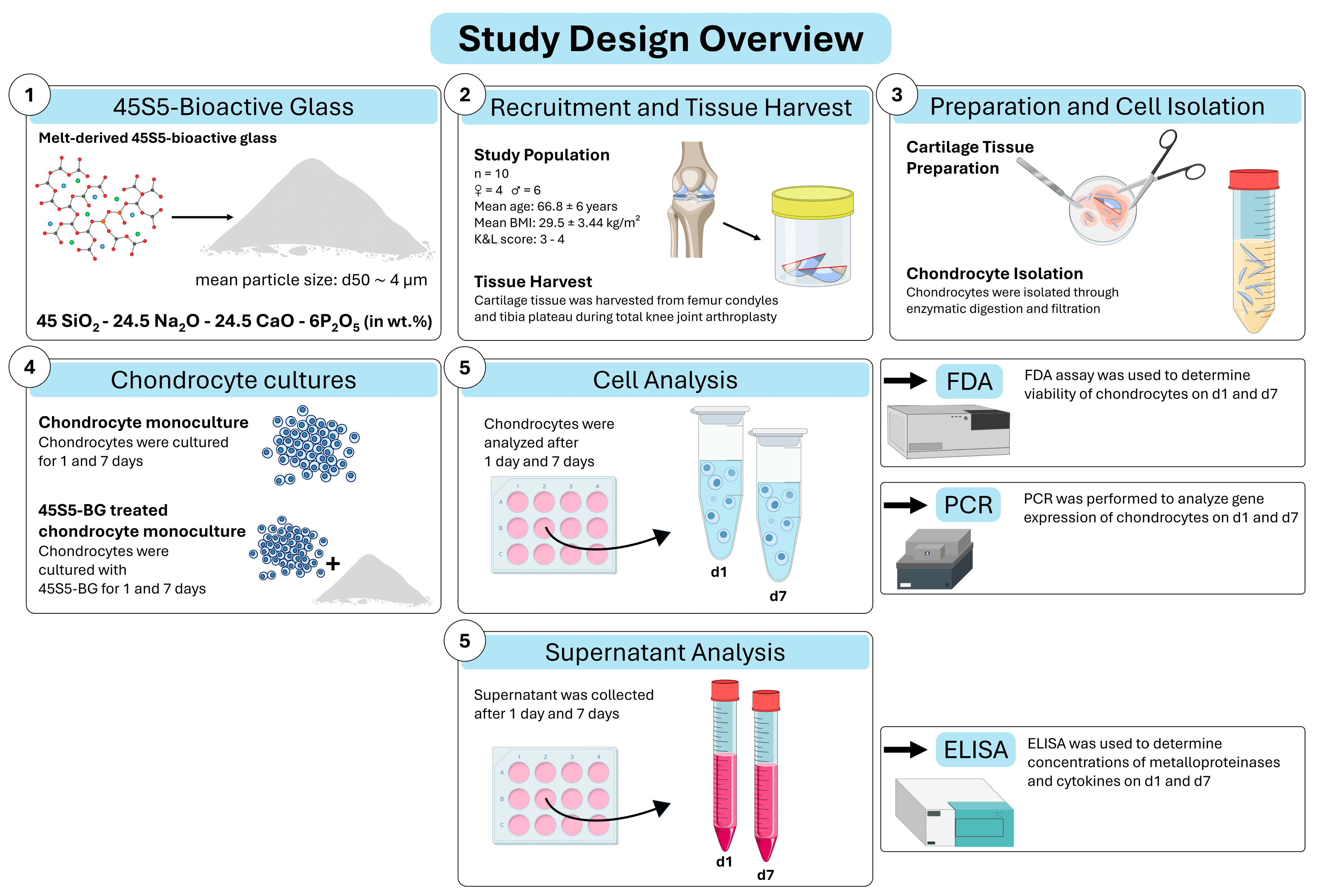
2.1. Study Design Overview
2.2. Study Population
2.3. Sample Collection
2.4. Bioactive Glass
2.5. Chondrocyte Isolation
2.6. Cell Expansion and Cell Culture
2.7. Cell Viability Assay
2.8. RNA Extraction, cDNA Synthesis, Real-Time Quantitative PCR
2.9. ELISA
2.10. Statistical Analysis
3. Results
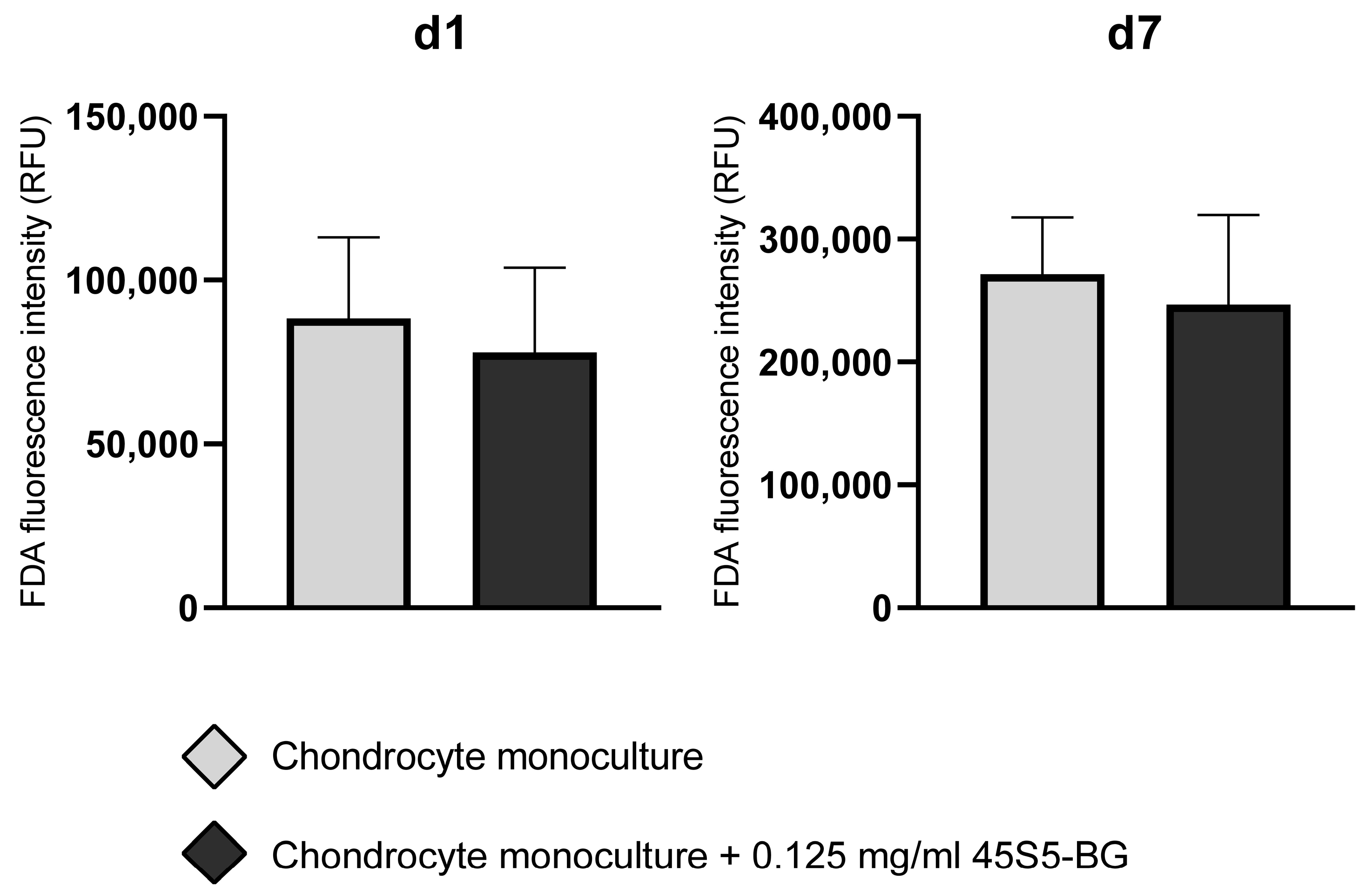
3.1. The Impact of 45S5-BG on Chondrocyte Viability
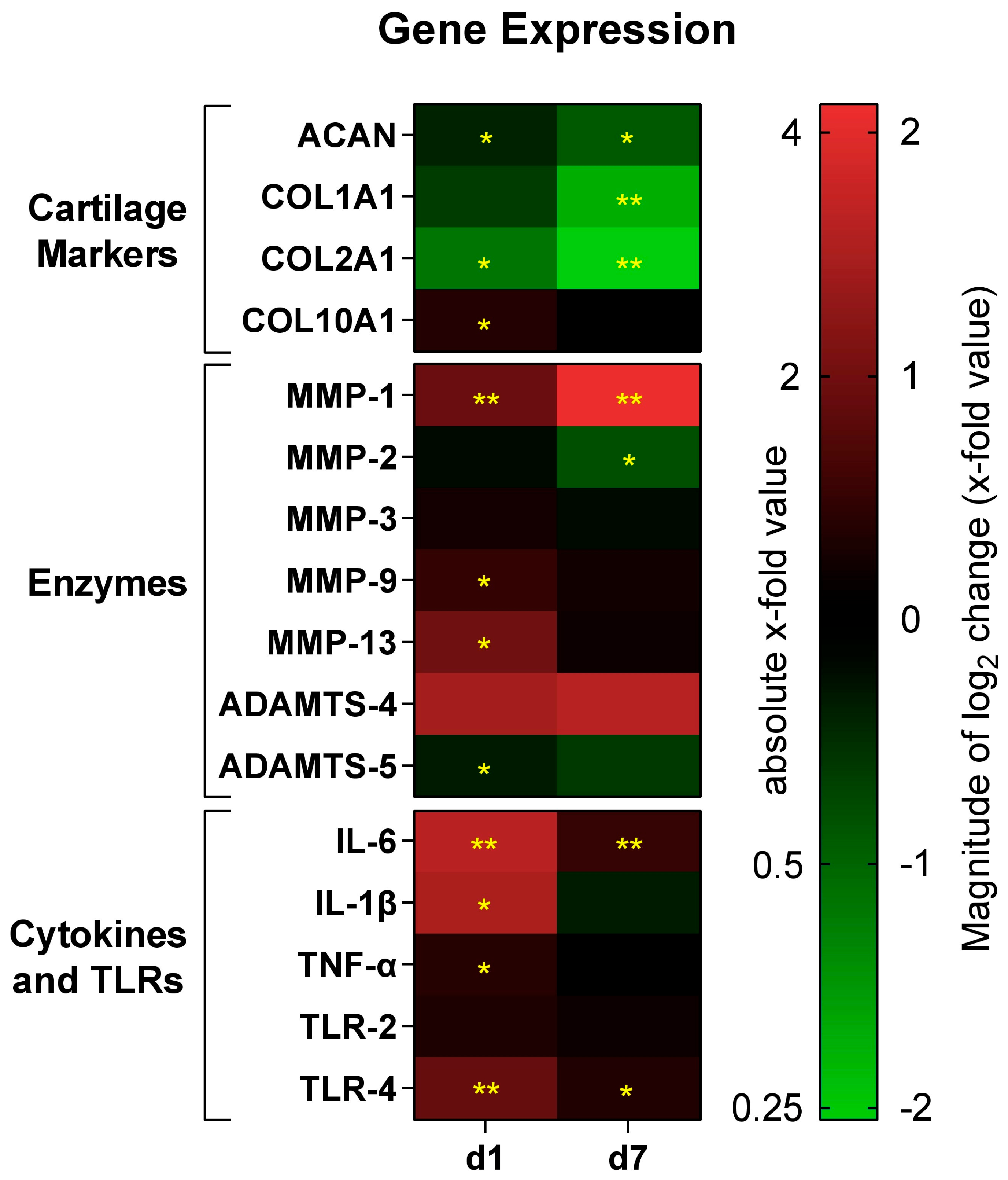
3.2. The Impact of 45S5-BG on Chondrocyte Gene Expression
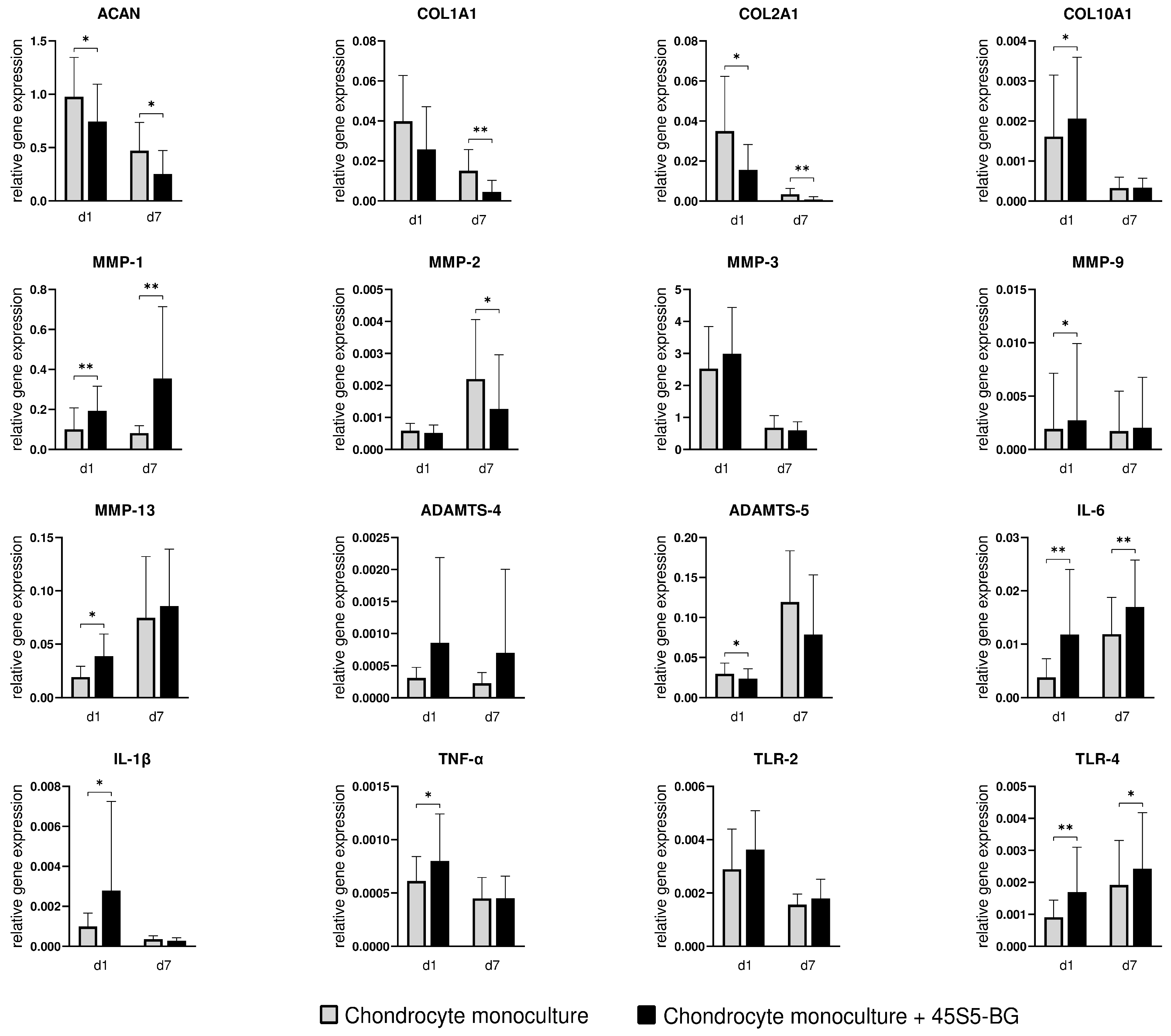
3.2.1. Cartilage Markers
3.2.2. Enzymes
3.2.3. Inflammatory Cytokines and Toll-like Receptors
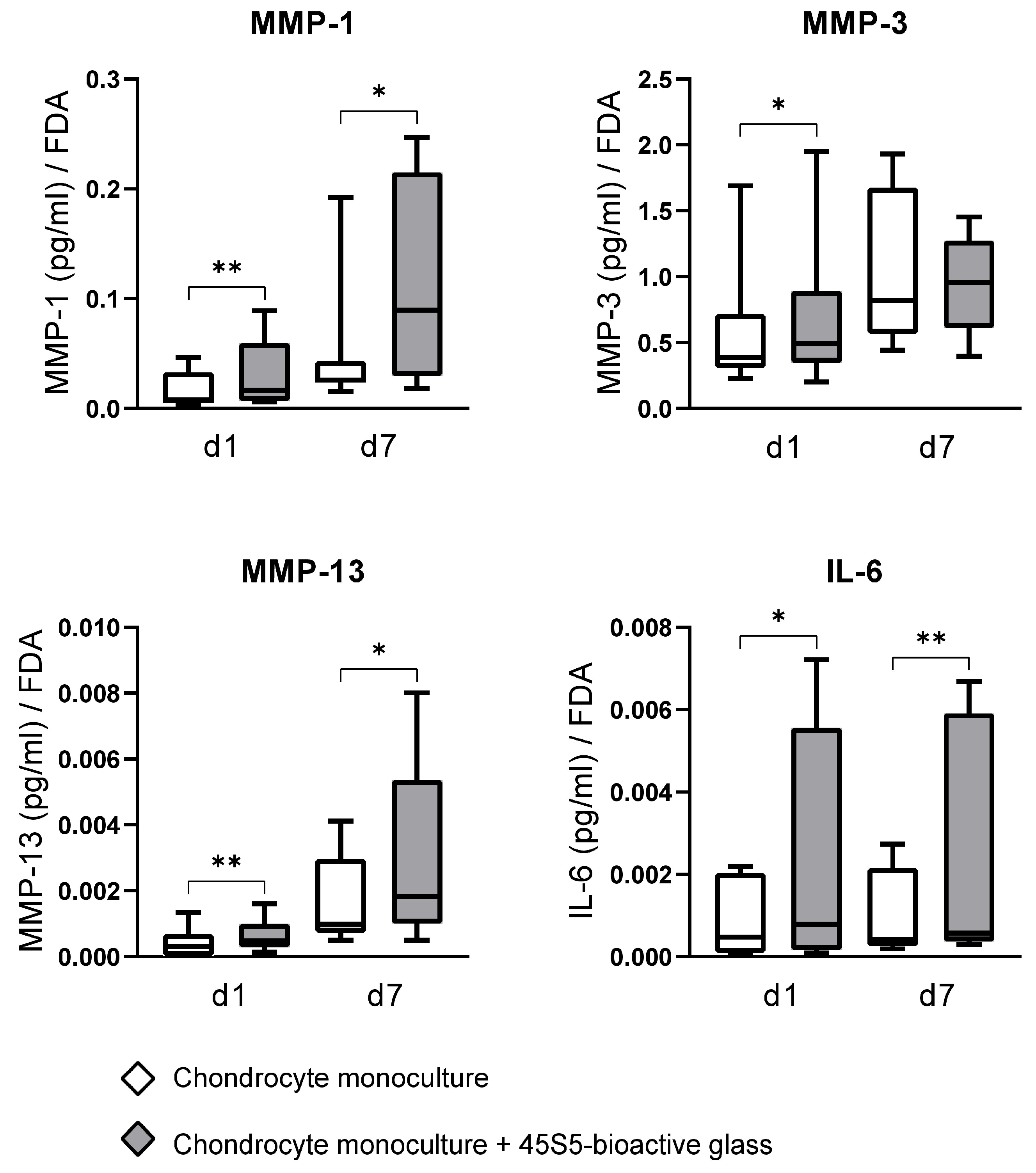
3.3. The Impact of 45S5-BG on Chondrocyte MMP and Cytokine Production
4. Discussion
4.1. The Impact of 45S5-BG on Cartilage Marker Expression
4.2. The Impact of 45S5-BG on Metalloproteinases
4.3. Collagenases (MMP-1, MMP-13) and Stromelysin-1 (MMP-3)
4.4. The Impact of 45S5-BG on Inflammatory Cytokines and the Toll-like Receptor Profile
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 45S5-BG | 45S5-bioactive glass |
| ΔΔCt | Delta–delta Ct |
| µm | Micrometers |
| °C | Celsius |
| ACAN | Aggrecan |
| ADAMTS | A disintegrin and metalloprotease with thrombospondin motifs |
| BMI | Body mass index |
| cDNA | Complementary deoxyribonucleic acid |
| cm2 | Centimeter squared |
| COL | Collagen |
| CO2 | Carbon dioxide |
| CRP | C-reactive protein |
| d1/7 | Day 1/7 |
| DMARDs | Disease-modifying antirheumatic drugs |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMOAD | Disease-modifying osteoarthritis drug |
| ELISA | Enzyme-linked immunosorbent assay |
| FCS | Fetal calf serum |
| FDA | Fluorescein diacetate |
| h | Hours |
| IL | Interleukin |
| K&L | Kellgren and Lawrence |
| mg | Milligrams |
| mL | Milliliters |
| MMP | Matrix metalloproteinase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| nm | Nanometers |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| OA | Osteoarthritis |
| P/S | Penicillin–Streptomycin |
| PBS | Phosphate-buffered saline |
| pg | Picograms |
| PRR | Pattern recognition receptor |
| qPCR | Quantitative polymerase chain reaction |
| RNA | Ribonucleic acid |
| RPS13 | Ribosomal protein S13 |
| s | Seconds |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
References
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, M.H.J.; Blom, A.B.; van der Kraan, P.M. Inflammation in osteoarthritis: Our view on its presence and involvement in disease development over the years. Osteoarthr. Cartil. 2024, 32, 355–364. [Google Scholar]
- Yunus, M.H.M.; Nordin, A.; Kamal, H. Pathophysiological Perspective of Osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef]
- Felson, D.T.; Niu, J.; Neogi, T.; Goggins, J.; Nevitt, M.C.; Roemer, F.; Torner, J.; Lewis, C.E.; Guermazi, A. Synovitis and the risk of knee osteoarthritis: The MOST Study. Osteoarthr. Cartil. 2016, 24, 458–464. [Google Scholar] [CrossRef]
- Platzer, H.; Nees, T.A.; Reiner, T.; Tripel, E.; Gantz, S.; Hagmann, S.; Moradi, B.; Rosshirt, N. Impact of Mononuclear Cell Infiltration on Chondrodestructive MMP/ADAMTS Production in Osteoarthritic Knee Joints-An Ex Vivo Study. J. Clin. Med. 2020, 9, 1279. [Google Scholar]
- Goldring, M.B. Articular cartilage degradation in osteoarthritis. Hss J. 2012, 8, 7–9. [Google Scholar]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Jeong, S.; Kim, H.; Kang, D.; Lee, J.; Kang, S.; Kim, J. Disease-modifying therapeutic strategies in osteoarthritis: Current status and future directions. Exp. Mol. Med. 2021, 53, 1689–1696. [Google Scholar] [CrossRef]
- Oo WM, L.C.; Duong, V.; Hunter, D.J. The Development of Disease-Modifying Therapies for Osteoarthritis (DMOADs): The Evidence to Date. Drug Des. Devel. Ther. 2021, 15, 2921–2945. [Google Scholar]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive Glass Applications: A Literature Review of Human Clinical Trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef]
- Shearer, A.; Montazerian, M.; Sly, J.J.; Hill, R.G.; Mauro, J.C. Trends and perspectives on the commercialization of bioactive glasses. Acta Biomater. 2023, 160, 14–31. [Google Scholar] [CrossRef]
- Fellenberg, J.; Losch, S.; Marinescu, M.R.; Frey, B.; Lehner, B.; Arango-Ospina, M.; Hadzhieva, Z.; Boccaccini, A.R.; Westhauser, F. Bioactive Glass Inhibits Tumor Development from Giant Cell Tumor of Bone-Derived Neoplastic Stromal Cells in a Chicken Chorioallantoic Membrane Assay. Cancers 2023, 15, 1868. [Google Scholar] [CrossRef]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review. Materials 2018, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, T.; Mesgar, A.S.; Mohammadi, Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5399–5430. [Google Scholar] [CrossRef]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.; Pelosine, A.M.; de Souza, A.C.S.; Machado, J., Jr.; Justo, G.Z.; Gamarra, L.F.; Marchi, J. Bioactive Glasses as Carriers of Cancer-Targeted Drugs: Challenges and Opportunities in Bone Cancer Treatment. Materials 2022, 15, 9082. [Google Scholar] [CrossRef]
- Naseri, S.; Lepry, W.C.; Nazhat, S.N. Bioactive glasses in wound healing: Hope or hype? J. Mater. Chem. B 2017, 5, 6167–6174. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Torre, E.; Bari, A.; Taccardi, N.; Cassinelli, C.; Morra, M.; Fiorilli, S.; Vitale-Brovarone, C.; Iviglia, G.; Boccaccini, A.R. Antioxidant mesoporous Ce-doped bioactive glass nanoparticles with anti-inflammatory and pro-osteogenic activities. Mater. Today Bio. 2020, 5, 100041. [Google Scholar] [CrossRef]
- Hammami, I.; Gavinho, S.R.; Jakka, S.K.; Valente, M.A.; Graça, M.P.F.; Pádua, A.S.; Silva, J.C.; Sá-Nogueira, I.; Borges, J.P. Antibacterial Biomaterial Based on Bioglass Modified with Copper for Implants Coating. J. Funct. Biomater. 2023, 14, 369. [Google Scholar] [CrossRef]
- Ciołek, L.; Zaczyńska, E.; Krok-Borkowicz, M.; Biernat, M.; Pamuła, E. Chitosan and Sodium Hyaluronate Hydrogels Supplemented with Bioglass for Bone Tissue Engineering. Gels 2024, 10, 128. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Pádua, A.S.; Holz, L.I.V.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Valente, M.A.; Graça, M.P.F. Bioactive Glasses Containing Strontium or Magnesium Ions to Enhance the Biological Response in Bone Regeneration. Nanomaterials 2023, 13, 2717. [Google Scholar] [CrossRef]
- Temel-Soylu, T.M.; Keçeciler-Emir, C.; Rababah, T.; Özel, C.; Yücel, S.; Basaran-Elalmis, Y.; Altan, D.; Kirgiz, Ö.; Seçinti İ, E.; Kaya, U.; et al. Green Electrospun Poly(vinyl alcohol)/Gelatin-Based Nanofibrous Membrane by Incorporating 45S5 Bioglass Nanoparticles and Urea for Wound Dressing Applications: Characterization and In Vitro and In Vivo Evaluations. ACS Omega 2024, 9, 21187–21203. [Google Scholar] [PubMed]
- Majumdar, S.; Hira, S.K.; Tripathi, H.; Kumar, A.S.; Manna, P.P.; Singh, S.P.; Krishnamurthy, S. Synthesis and characterization of barium-doped bioactive glass with potential anti-inflammatory activity. Ceram. Int. 2021, 47, 7143–7158. [Google Scholar] [CrossRef]
- Eberhard, J.; Reimers, N.; Dommisch, H.; Hacker, J.; Freitag, S.; Acil, Y.; Albers, H.; Jepsen, S. The effect of the topical administration of bioactive glass on inflammatory markers of human experimental gingivitis. Biomaterials 2005, 26, 1545–1551. [Google Scholar] [CrossRef]
- Day, R.M.; Boccaccini, A.R. Effect of particulate bioactive glasses on human macrophages and monocytes in vitro. J. Biomed. Mater. Res. Part A 2005, 73, 73–79. [Google Scholar]
- Platzer, H.; Marinescu, M.; Nawaz, Q.; Tripel, E.; Gantz, S.; Horsch, A.; Daniel, V.; Boccaccini, A.R.; Hagmann, S.; Moradi, B.; et al. The Impact of 45S5-Bioactive Glass on Synovial Cells in Knee Osteoarthritis-An In Vitro Study. Materials 2023, 16, 7594. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Zhang, S.; Ge, J.; Wang, Q.; Qi, L.; Liu, K. Integration of Bioglass Into PHBV-Constructed Tissue-Engineered Cartilages to Improve Chondrogenic Properties of Cartilage Progenitor Cells. Front. Bioeng. Biotechnol. 2022, 10, 868719. [Google Scholar] [CrossRef]
- Suominen, E.; Aho, A.J.; Vedel, E.; Kangasniemi, I.; Uusipaikka, E.; Yli-Urpo, A. Subchondral bone and cartilage repair with bioactive glasses, hydroxyapatite, and hydroxyapatite-glass composite. J. Biomed. Mater. Res. 1996, 32, 543–551. [Google Scholar] [CrossRef]
- Asselin, A.; Hattar, S.; Oboeuf, M.; Greenspan, D.; Berdal, A.; Sautier, J. The modulation of tissue-specific gene expression in rat nasal chondrocyte cultures by bioactive glasses. Biomaterials 2004, 25, 5621–5630. [Google Scholar] [CrossRef]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef]
- Davidson, R.K.; Waters, J.G.; Kevorkian, L.; Darrah, C.; Cooper, A.; Donell, S.T.; Clark, I.M. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res. Ther. 2006, 8, R124. [Google Scholar] [CrossRef]
- Zeng, G.Q.; Chen, A.B.; Li, W.; Song, J.H.; Gao, C.Y. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 2015, 14, 14811–14822. [Google Scholar] [CrossRef] [PubMed]
- Mehana, E.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Kunisch, E.; Kinne, R.W.; Alsalameh, R.J.; Alsalameh, S. Pro-inflammatory IL-1beta and/or TNF-alpha up-regulate matrix metalloproteases-1 and -3 mRNA in chondrocyte subpopulations potentially pathogenic in osteoarthritis: In situ hybridization studies on a single cell level. Int. J. Rheum. Dis. 2016, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- van de Loo, F.A.; Joosten, L.A.; van Lent, P.L.; Arntz, O.J.; van den Berg, W.B. Role of interleukin-1, tumor necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction. Effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum. 1995, 38, 164–172. [Google Scholar]
- Henrotin, Y.E.; Zheng, S.X.; Labasse, A.H.; Deby, G.P.; Crielaard, J.M.; Reginster, J.Y. Modulation of human chondrocyte metabolism by recombinant human interferon. Osteoarthr. Cartil. 2000, 8, 474–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Li, T.; Peng, J.; Li, Q.; Shu, Y.; Zhu, P.; Hao, L. The Mechanism and Role of ADAMTS Protein Family in Osteoarthritis. Biomolecules 2022, 12, 959. [Google Scholar] [CrossRef]
- Verma, P.; Dalal, K. ADAMTS-4 and ADAMTS-5: Key enzymes in osteoarthritis. J. Cell Biochem. 2011, 112, 3507–3514. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Zaucke, F. Osteoarthritis year in review 2024: Biology. Osteoarthr. Cartil. 2025, 33, 58–66. [Google Scholar] [CrossRef]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef]
- Eyre, D.R. The collagens of articular cartilage. Semin. Arthritis Rheum. 1991, 21 (Suppl. S2), 2–11. [Google Scholar] [CrossRef] [PubMed]
- Styczynska-Soczka, K.; Amin, A.K.; Hall, A.C. Cell-associated type I collagen in nondegenerate and degenerate human articular cartilage. J. Cell Physiol. 2021, 236, 7672–7681. [Google Scholar] [CrossRef]
- Qazi, T.H.; Hafeez, S.; Schmidt, J.; Duda, G.N.; Boccaccini, A.R.; Lippens, E. Comparison of the effects of 45S5 and 1393 bioactive glass microparticles on hMSC behavior. J. Biomed. Mater. Res. A 2017, 105, 2772–2782. [Google Scholar] [CrossRef] [PubMed]
- Galusková, D.; Kaňková, H.; Švančárková, A.; Galusek, D. Early-Stage Dissolution Kinetics of Silicate-Based Bioactive Glass under Dynamic Conditions: Critical Evaluation. Materials 2021, 14, 3384. [Google Scholar] [CrossRef]
- Wilkesmann, S.; Westhauser, F.; Fellenberg, J. Combined Fluorescence-Based in Vitro Assay for the Simultaneous Detection of Cell Viability and Alkaline Phosphatase Activity during Osteogenic Differentiation of Osteoblast Precursor Cells. Methods Protoc. 2020, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wu, L.D. Aggrecanase and aggrecan degradation in osteoarthritis: A review. J. Int. Med. Res. 2008, 36, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Alcaide-Ruggiero, L.; Molina-Hernández, V.; Granados, M.M.; Domínguez, J.M. Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects. Int. J. Mol. Sci. 2021, 22, 13329. [Google Scholar] [CrossRef]
- Luckman, S.P.; Rees, E.; Kwan, A.P. Partial characterization of cell-type X collagen interactions. Biochem. J. 2003, 372 Pt 2, 485–493. [Google Scholar] [CrossRef]
- Mixon, A.; Bahar-Moni, A.S.; Faisal, T.R. Mechanical characterization of articular cartilage degraded combinedly with MMP-1 and MMP-9. J. Mech. Behav. Biomed. Mater. 2022, 129, 105131. [Google Scholar] [CrossRef]
- Cawston, T.E.; Wilson, A.J. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best. Pr. Res. Clin. Rheumatol. 2006, 20, 983–1002. [Google Scholar] [CrossRef]
- Chin, J.R.; Murphy, G.; Werb, Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J. Biol. Chem. 1985, 260, 12367–12376. [Google Scholar] [CrossRef]
- Unemori, E.N.; Bair, M.J.; Bauer, E.A.; Amento, E.P. Stromelysin expression regulates collagenase activation in human fibroblasts. Dissociable control of two metalloproteinases by interferon-gamma. J. Biol. Chem. 1991, 266, 23477–23482. [Google Scholar] [CrossRef]
- Milner, J.M.; Elliott, S.F.; Cawston, T.E. Activation of procollagenases is a key control point in cartilage collagen degradation: Interaction of serine and metalloproteinase pathways. Arthritis Rheum. 2001, 44, 2084–2096. [Google Scholar] [CrossRef]
- Tetlow, L.C.; Adlam, D.J.; Woolley, D.E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: Associations with degenerative changes. Arthritis Rheum. 2001, 44, 585–594. [Google Scholar] [PubMed]
- Wiegertjes, R.; Fvan de Loo, A.J.; Davidson, E.N.B. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology 2020, 59, 2681–2694. [Google Scholar] [CrossRef]
- Haseeb, A.; Haqqi, T.M. Immunopathogenesis of osteoarthritis. Clin. Immunol. 2013, 146, 185–196. [Google Scholar] [CrossRef]
- Sengprasert, P.; Kamenkit, O.; Tanavalee, A.; Reantragoon, R. The Immunological Facets of Chondrocytes in Osteoarthritis: A Narrative Review. J. Rheumatol. 2024, 51, 13–24. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Liu-Bryan, R. Role of TLR2 and TLR4 in regulation of articular chondrocyte homeostasis. Osteoarthr. Cartil. 2020, 28, 669–674. [Google Scholar] [CrossRef]
- Barreto, G.; Manninen, M.; Eklund, K.K. Osteoarthritis and Toll-Like Receptors: When Innate Immunity Meets Chondrocyte Apoptosis. Biology 2020, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X.; Wang, G.; Wang, X.; Zhang, Y.; Zhang, T. Effects of TLR-2/NF-κB signaling pathway on the occurrence of degenerative knee osteoarthritis: An in vivo and in vitro study. Oncotarget 2017, 8, 38602–38617. [Google Scholar] [CrossRef]
- Sengprasert, P.; Waitayangkoon, P.; Kamenkit, O.; Sawatpanich, A.; Chaichana, T.; Wongphoom, J.; Ngarmukos, S.; Taweevisit, M.; Lotinun, S.; Tumwasorn, S.; et al. Catabolic mediators from TLR2-mediated proteoglycan aggrecan peptide-stimulated chondrocytes are reduced by Lactobacillus-conditioned media. Sci. Rep. 2024, 14, 18043. [Google Scholar] [CrossRef]
- Lin, R.; Deng, C.; Li, X.; Liu, Y.; Zhang, M.; Qin, C.; Yao, Q.; Wang, L.; Wu, C. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics 2019, 9, 6300–6313. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zheng, K.; Zhou, T.; Boccaccini, A.R. Incorporation of Zinc into Binary SiO2-CaO Mesoporous Bioactive Glass Nanoparticles Enhances Anti-Inflammatory and Osteogenic Activities. Pharmaceutics 2021, 13, 2124. [Google Scholar] [CrossRef]
- Lin, Z.; Fitzgerald, J.B.; Xu, J.; Willers, C.; Wood, D.; Grodzinsky, A.J.; Zheng, M.H. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J. Orthop. Res. 2008, 26, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
| Total Study Population (n = 10) | |
|---|---|
| Sex, n (%) | |
| Female | 4 (40%) |
| Male | 6 (60%) |
| Age (years), mean ± SD; IQR | 66.8 ± 6.1; 10.8 |
| BMI (kg/m2), mean ± SD; IQR | 29.5 ± 3.4; 6.4 |
| Leukocytes (cells/nL), mean ± SD; IQR | 6.4 ± 1.1; 1.5 |
| C-reactive protein (mg/L), mean ± SD; IQR | 1.8 ± 3.3; 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinescu, M.; Hagmann, S.; Fellenberg, J.; Tripel, E.; Gantz, S.; Mayakrishnan, R.; Boccaccini, A.R.; Renkawitz, T.; Moradi, B.; Westhauser, F.; et al. Impact of 45S5-Bioactive Glass on Chondrocytes in Knee Osteoarthritis—In Vitro Study Exploring Cellular Responses. J. Funct. Biomater. 2025, 16, 339. https://doi.org/10.3390/jfb16090339
Marinescu M, Hagmann S, Fellenberg J, Tripel E, Gantz S, Mayakrishnan R, Boccaccini AR, Renkawitz T, Moradi B, Westhauser F, et al. Impact of 45S5-Bioactive Glass on Chondrocytes in Knee Osteoarthritis—In Vitro Study Exploring Cellular Responses. Journal of Functional Biomaterials. 2025; 16(9):339. https://doi.org/10.3390/jfb16090339
Chicago/Turabian StyleMarinescu, Max, Sébastien Hagmann, Jörg Fellenberg, Elena Tripel, Simone Gantz, Ravikumar Mayakrishnan, Aldo R. Boccaccini, Tobias Renkawitz, Babak Moradi, Fabian Westhauser, and et al. 2025. "Impact of 45S5-Bioactive Glass on Chondrocytes in Knee Osteoarthritis—In Vitro Study Exploring Cellular Responses" Journal of Functional Biomaterials 16, no. 9: 339. https://doi.org/10.3390/jfb16090339
APA StyleMarinescu, M., Hagmann, S., Fellenberg, J., Tripel, E., Gantz, S., Mayakrishnan, R., Boccaccini, A. R., Renkawitz, T., Moradi, B., Westhauser, F., & Platzer, H. (2025). Impact of 45S5-Bioactive Glass on Chondrocytes in Knee Osteoarthritis—In Vitro Study Exploring Cellular Responses. Journal of Functional Biomaterials, 16(9), 339. https://doi.org/10.3390/jfb16090339







