Polyphosphazene-Based Nanotherapeutics
Abstract
1. Introduction
2. Chemistry
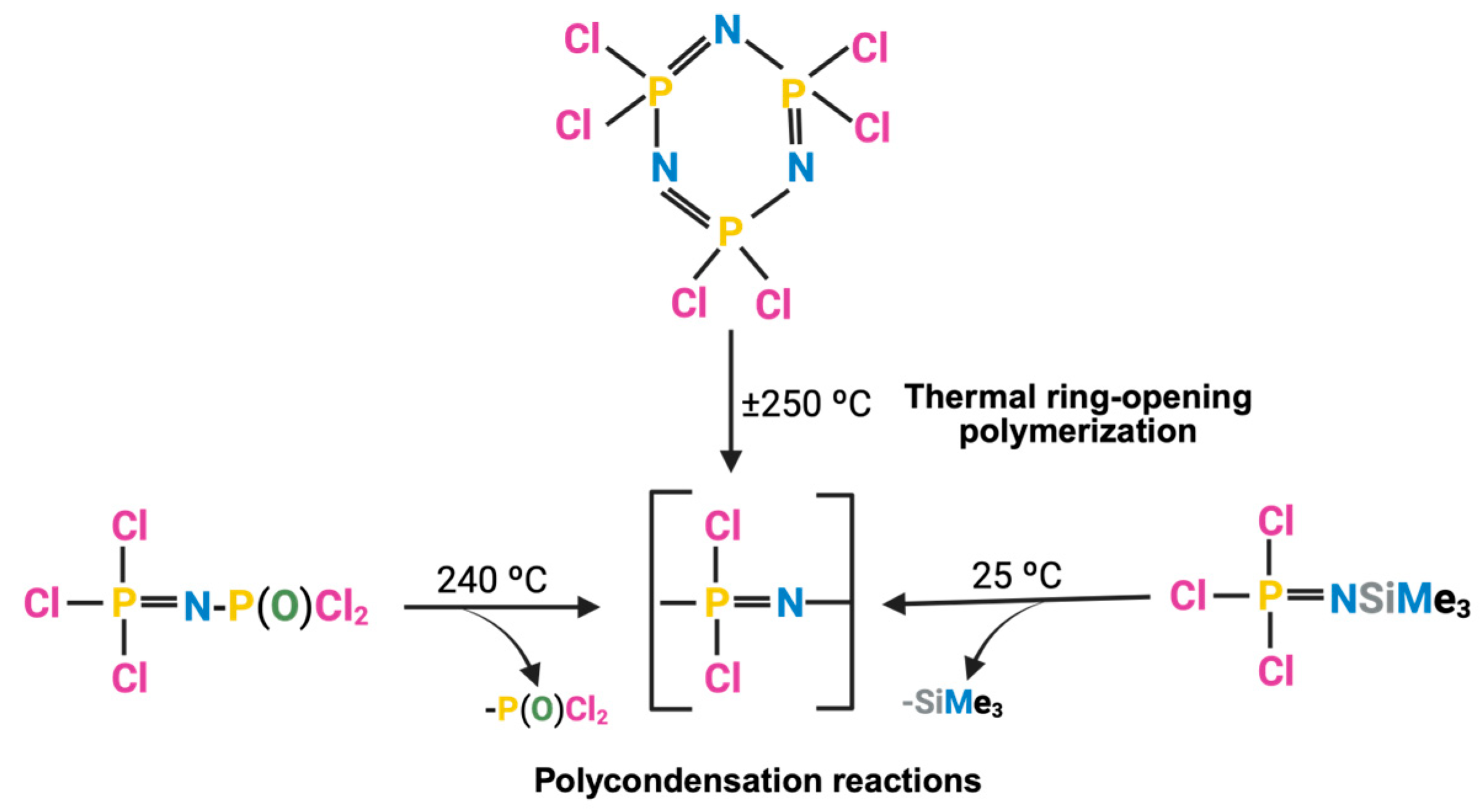
2.1. PPZ Two-Step Synthesis
2.1.1. Synthesis of Linear Polyphosphazene Precursor
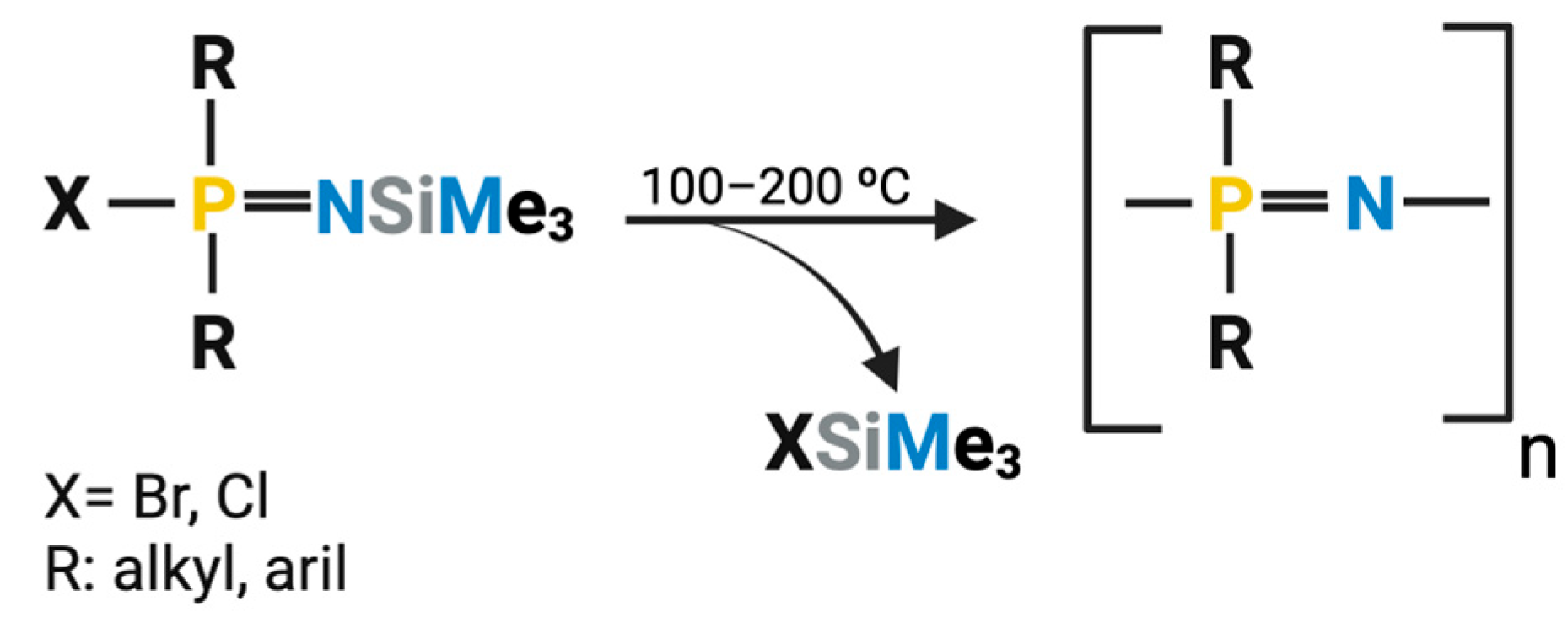
2.1.2. Macro-Substitution
2.2. One-Step Synthesis
3. Key Pharmaceutical Properties
3.1. Biodegradability and Tolerability of the Degradation Products
3.2. Stimuli-Responsive Behavior
4. PPZ-Based Nanosystems
4.1. Nanocarriers Based on Polyelectrolytic Complexation

| Therapeutic Application | PPZ Derivative | Cargo | In Vitro/In Vivo Model | Main Finding and Ref. |
|---|---|---|---|---|
| Gene delivery | Imidazole/DMAEA-PPZ | pDNA | In vitro (293T) | Lower toxicity than DMAEA-PPZ [20] |
| Gene delivery | Cysteamine-PPZ/ Mercaptohexanoic-PPZ | pDNA/siRNA | In vitro (U87) In vivo (U87) | High transfection; low toxicity; antitumor effect in vitro/in vivo [21] |
| Gene delivery | Cationic/aliphatic-PPZ Mercaptohexanoic acid-PPZ | pDNA (pBMP4) | In vitro (U87/U251), In vivo (U87) | Improved gene transfer in vitro and a potent antitumoral effect in vivo [22] |
| Vaccine adjuvant | PCPP/PCEP | Various proteins | In vitro (protein solution) | Strong antigen binding. Induces dendritic cells maturation and Th2 cytokine production [87,88] |
| Vaccine adjuvant | PCPP | EBOV glycoprotein | In vivo (mice) | Efficient immunization through MN-patches and IM administration in mice [89] |
| Vaccine adjuvant | PCPP | ogp160 | Phase I clinical trial | Higher T-cell proliferation than alum-adjuvanted [90] |
| Vaccine adjuvant | PCPP/PCEP | E2 | In vivo (mice) | PCEP PECs produced higher neutralizing antibody titers than Addavax, alum, and PCPP PECs [91] |
| Vaccine adjuvant | PCEP | H1N1 | In vivo (pig) | Strong anti-H1N1 immunogenicity; high IFN-γ, IL-13, IL-17A; no cross-protection against H3N2 [86] |
| Vaccine adjuvant | PCMP | VLP | In vitro In vivo (mice) | Greater stability and neutralizing titers than alum or Gardasil-9 [92] |
| Protein delivery | PEGylated-PPZ derivatives | L-asparaginase | In vitro | Improved thermostability and proteolytic resistance without loss of activity [83] |
| Protein delivery | PEGylated-PCPP + spermine | Lysozyme | In vitro | High antibacterial activity; low polydispersity; membrane-disruptive effect [93] |
| Protein delivery | Protamine-PPZ | Exendin-4 | In vivo (diabetic mouse) | Hydrogel formation at 37 °C; sustained release; improved glycemic control vs. free protein [94] |
4.1.1. Gene Therapy
4.1.2. Vaccines
4.1.3. Protein Delivery
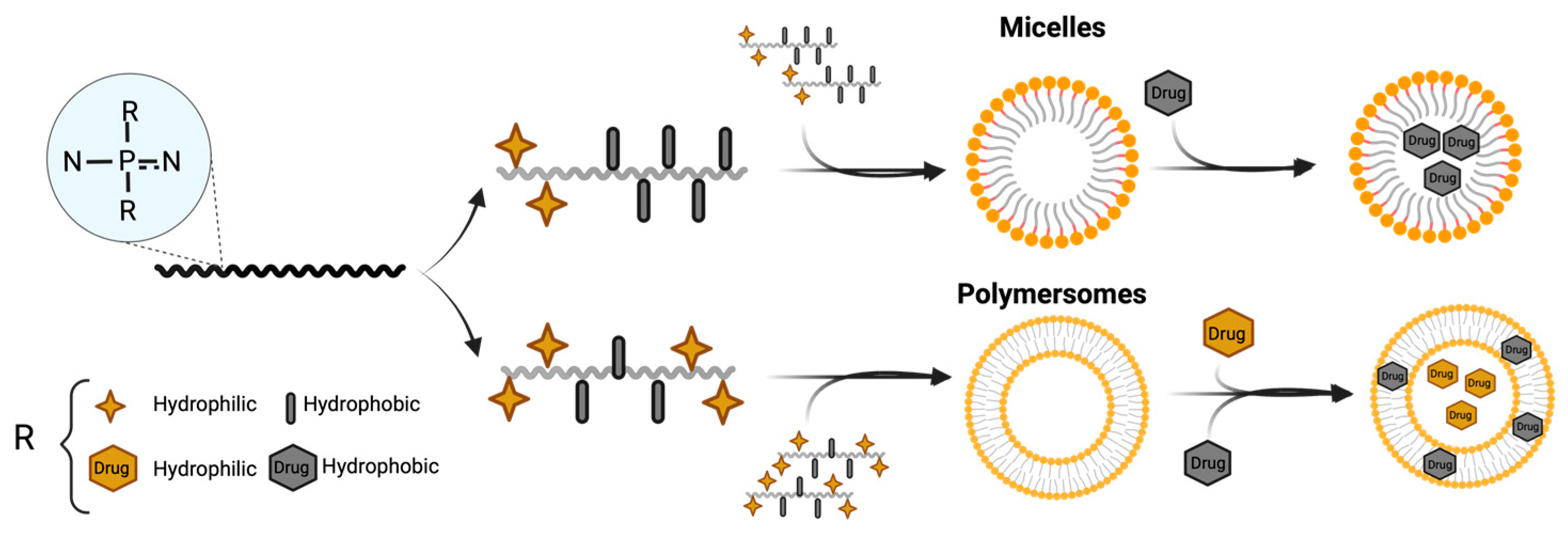
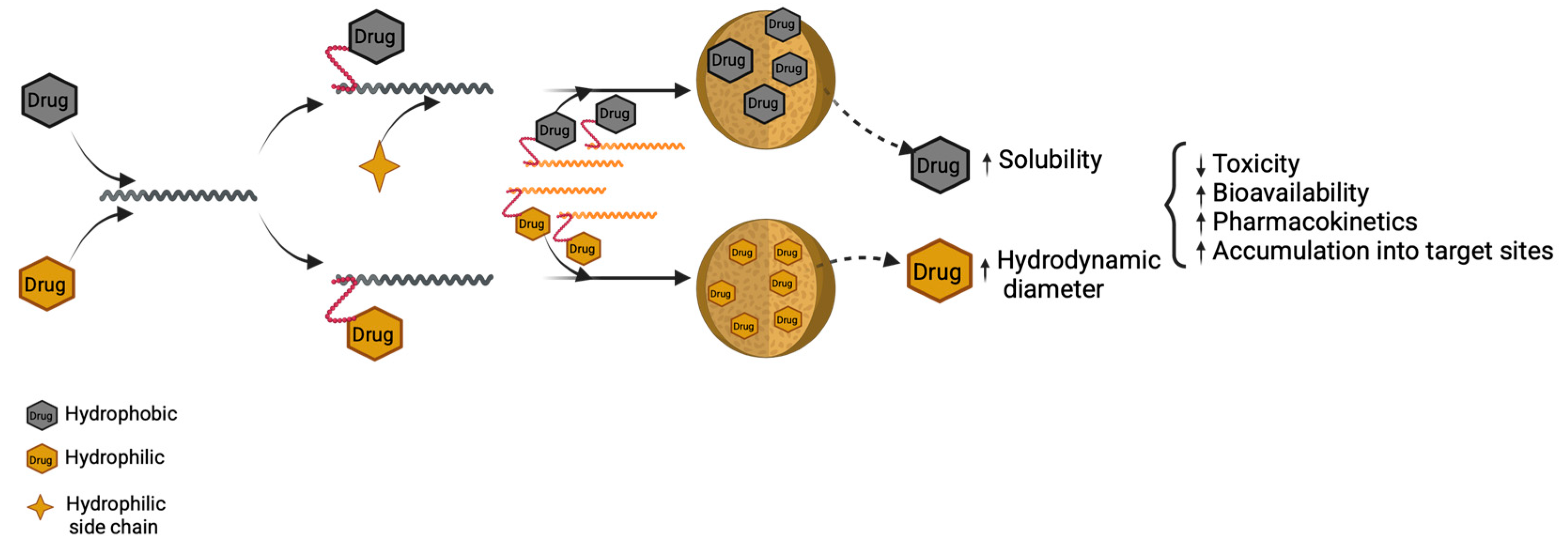
4.2. Nanocarriers Based on Hydrophilic/Hydrophobic Interactions
4.2.1. Polymeric Micelles for Hydrophobic Drugs
4.2.1.1. Anticancer Drugs
4.2.2. Polymersomes for Hydrophilic Drugs
4.2.2.1. Anticancer Drugs
4.2.2.2. Nucleic Acids
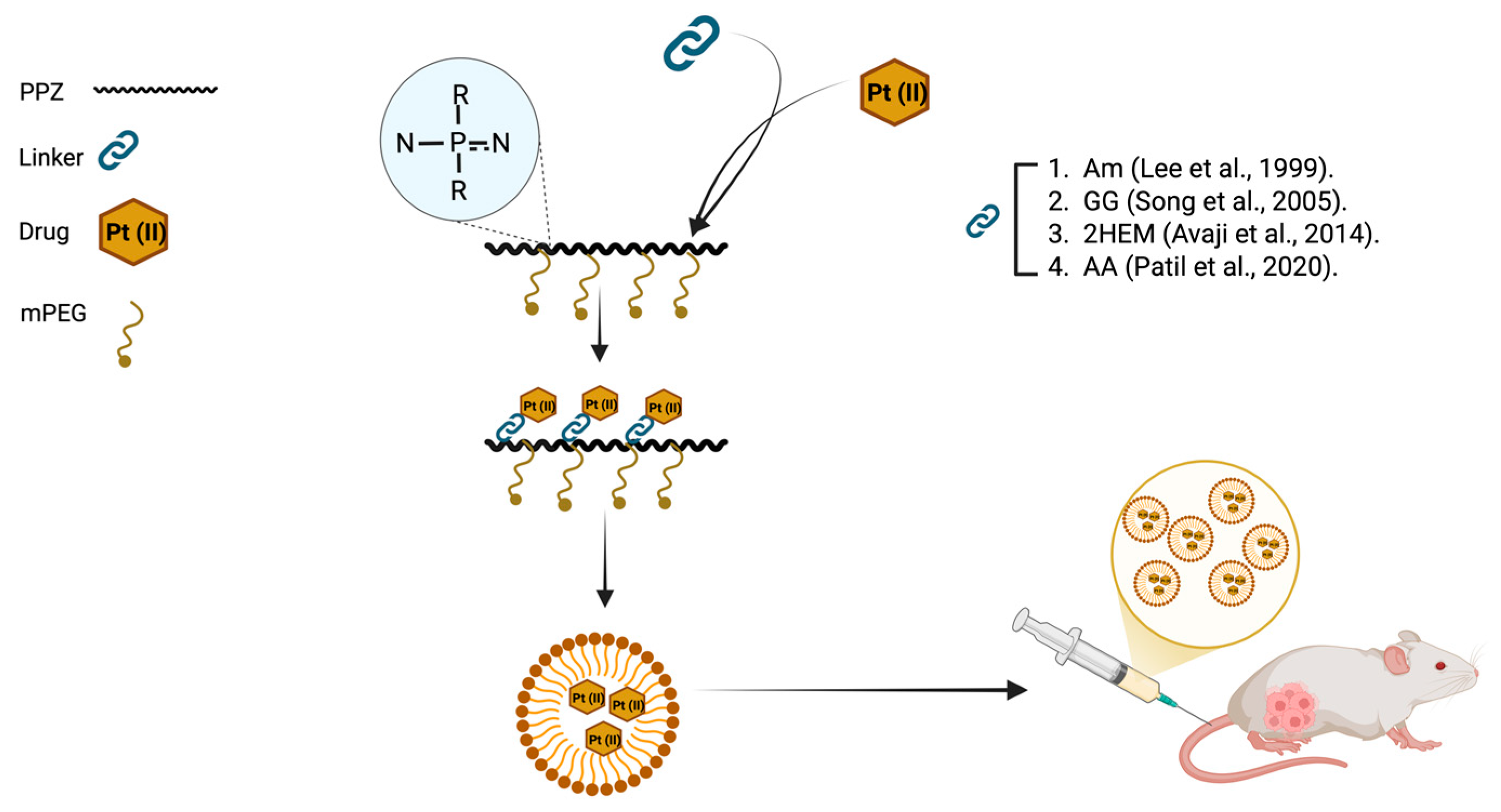
4.3. Polymer–Drug Conjugates
4.3.1. Anticancer Drugs
4.3.2. Other Drugs
5. Closing Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Cis-aconitic anhydride. |
| ABD | 4-aminomethyl-2-benzyloxy-[1,3]-dioxolan |
| AF4 | Asymmetric flow field-flow fractionation |
| ALVAC-HIV | Canarypox virus vector vaccine for HIV |
| AS1411 | Nucleolin-binding aptamer |
| AuNPs | Gold nanoparticles |
| BEL-7402 | Hepatocellular carcinoma cell line (human) |
| CG | Cytosine–phosphate–Guanine |
| CQ | Chloroquine |
| CT-26 | Colon carcinoma cell line (mouse) |
| DMAE | 2-Dimethylaminoethanol |
| DMAEA | 2-Dimethylaminoethylamine |
| DPA | Diisopropylamino |
| DPEA | N, N-Diisopropylethylenediamino |
| Dox | Doxorubicin |
| Dox-HCl | Doxorubicin hydrochloride |
| EAB | Ethyl 4-aminobenzoate |
| EBOV | Ebola virus |
| GG | Glycyl-L-glutamate |
| GlyEE | Glycine ethyl ester |
| GP | Glycoprotein |
| H1N1 | A subtype of the Influenza A virus (swine flu) |
| H3N2 | A subtype of the Influenza A virus |
| H5N1 | A subtype of Influenza A virus (avian influenza) |
| HCCP | Hexachlorocyclotriphosphazene |
| HepG2 | Liver cancer cell line (human) |
| HPV-VLP | Human papillomavirus virus-like particle |
| IFN-γ | Interferon gamma |
| IgM/IgG/IgG1 | Immunoglobulin M/G/G1 |
| IL-4 | Interleukin 4 |
| LCST | Lower critical solution temperature |
| MCF-7 | Breast cancer cell line (human) |
| MCF-7/adr | Adriamycin-resistant MCF-7 breast cancer cell line |
| mPEG | Methoxy-polyethylene glycol |
| OVCAR 3 | Ovarian cancer cell line 3 |
| PCPP | Poly[di(carboxylatophenoxy)phosphazene] |
| PCEP | Poly[di(carboxylatoethylphenoxy)phosphazene] |
| PBS | Phosphate-buffered saline |
| P-C | Phosphorus-Carbon (bond) |
| PDADM | Poly (diallyl dimethyl) ammonium chloride |
| PDI | Polydispersity Index |
| PEC(s) | Polyelectrolytic complex(es) |
| PEG | Polyethylene glycol |
| PEG-folate | Polyethylene Glycol conjugated with Folate |
| PEGylated | Modified with Polyethylene Glycol |
| PEI | Polyethyleneimine |
| PLA | Poly (lactic acid) |
| PLGA | Poly (lactic-co-glycolic acid) |
| PLC | Poly(ε-caprolactone) |
| pDNA | Plasmid DNA |
| pIL12 | IL-12 plasmid |
| PPD | Propyl pyrrolidone |
| PPA | Phenoxy propionic acid |
| PPZ(s) | Poly(organo)phosphazene(s) |
| ProPPZ | Protamine-conjugated polyphosphazene |
| P-N | Phosphorus–Nitrogen (bond) |
| P-O | Phosphorus–Oxygen (bond) |
| SIV | Swine influenza virus |
| Si-N | Silicon–Nitrogen (bond) |
| siRNA | Small Interfering RNA |
| THF | Tetrahydrofuran |
| Th1/Th2 | T helper type 1/type 2 |
| TLR(s) | Toll-like receptor(s) |
| TLR7/8 | Toll-Like Receptors 7 and 8 |
| TROP | Thermal ring-opening polymerization |
| XTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide |
| Pt (II) | Platinum in oxidation state +2 |
| Pt (IV) | Platinum in oxidation state +4 |
References
- Minko, T.; Khandare, J.J.; Vetcher, A.A.; Soldatenkov, V.A.; Garbuzenko, O.B.; Saad, M.; Pozharov, V.P. Multifunctional Nanotherapeutics for Cancer. In Multifunctional Pharmaceutical Nanocarriers; Springer Nature: New York, NY, USA, 2008; pp. 309–336. [Google Scholar] [CrossRef]
- Pepic, I.; Hafner, A.; Lovric, J.; Perina Lakos, G. Nanotherapeutics in the EU: An Overview on Current State and Future Directions. Int. J. Nanomed. 2014, 9, 1005–1023. [Google Scholar] [CrossRef]
- Andrianov, A.K. Polyphosphazenes for Biomedical Applications; Andrianov, A.K., Ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- De Jaeger, R.; Gleria, M. Poly(organophosphazene)s and Related Compounds: Synthesis, Properties and Applications. Prog. Polym. Sci. 1998, 23, 179–276. [Google Scholar] [CrossRef]
- Ahmad, M.; Nawaz, T.; Hussain, I.; Chen, X.; Imran, M.; Hussain, R.; Assiri, M.A.; Ali, S.; Wu, Z. Phosphazene Cyclomatrix Network-Based Polymer: Chemistry, Synthesis, and Applications. ACS Omega 2022, 7, 28694–28707. [Google Scholar] [CrossRef]
- Teasdale, I.; Brüggemann, O. Polyphosphazenes: Multifunctional, Biodegradable Vehicles for Drug and Gene Delivery. Polymers 2013, 5, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Allcock, H.R. Poly(organophosphazenes): Synthesis, Unique Properties, and Applications. Macromol. Symp. 1986, 6, 101–108. [Google Scholar] [CrossRef]
- Ni, Z.; Yu, H.; Wang, L.; Shen, D.; Elshaarani, T.; Fahad, S.; Khan, A.; Haq, F.; Teng, L. Recent Research Progress on Polyphosphazene-Based Drug Delivery Systems. J. Mater. Chem. B 2020, 8, 1555–1575. [Google Scholar] [CrossRef] [PubMed]
- Ogueri, K.S.; Allcock, H.R.; Laurencin, C.T. Generational Biodegradable and Regenerative Polyphosphazene Polymers and Their Blends with poly (lactic-co-glycolic acid). Prog. Polym. Sci. 2019, 98, 101146. [Google Scholar] [CrossRef]
- Cornelissen, A.; Sakamoto, A.; Sato, Y.; Kawakami, R.; Mori, M.; Kawai, K.; Kutyna, M.; Fernandez, R.; Ghosh, S.; Barakat, M.; et al. COBRA PzFTM Coronary Stent in Clinical and Preclinical Studies: Setting the Stage for New Antithrombotic Strategies? Future Cardiol. 2022, 18, 207–217. [Google Scholar] [CrossRef]
- Crommen, J.H.L.; Schacht, E.H.; Mense, E.H.G. Biodegradable Polymers II. Degradation Characteristics of Hydrolysis-Sensitive Poly[(Organo)Phosphazenes]. Biomaterials 1991, 13, 601–611. [Google Scholar] [CrossRef]
- Kopeček, J. Smart and Genetically Engineered Biomaterials and Drug Delivery Systems. Eur. J. Pharm. Sci. 2003, 20, 1–16. [Google Scholar] [CrossRef]
- Lakshmi, S.; Katti, D.S.; Laurencin, C.T. Biodegradable Polyphosphazenes for Drug Delivery Applications. Adv. Drug Deliv. Rev. 2003, 55, 467–482. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Ivirico, J.L.E.; Nair, L.S.; Allcock, H.R.; Laurencin, C.T. Biodegradable Polyphosphazene-Based Blends for Regenerative Engineering. Regen. Eng. Transl. Med. 2017, 3, 15–31. [Google Scholar] [CrossRef]
- Singh, A.; Krogman, N.R.; Sethuraman, S.; Nair, L.S.; Sturgeon, J.L.; Brown, P.W.; Laurencin, C.T.; Allcock, H.R. Effect of Side Group Chemistry on the Properties of Biodegradable -Alanine Cosubstituted Polyphosphazenes. Biomacromolecules 2006, 7, 914–918. [Google Scholar] [CrossRef]
- Payne, L.G.; Van Nest, G.; Barchfeld, G.L.; Siber, G.R.; Gupta, R.K.; Jenkins, S.A. PCPP as a Parenteral Adjuvant for Diverse Antigens. Dev. Biol. Stand. 1998, 92, 79–87. [Google Scholar]
- Roshan, J.; Meng, D.; Kumbar, S.G.; Laurencin, C.T. Polyphosphazenes. In Naturals and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 193–206. [Google Scholar] [CrossRef]
- Murthy, S.K. Nanoparticles in Modern Medicine: State of the Art and Future Challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Wang, L.; Liu, H.; Zhuang, J.; Wang, D. Small-Scale Big Science: From Nano- to Atomically Dispersed Catalytic Materials. Small Sci. 2022, 2, 2200036. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, X.; Du, C.; Zhao, B.; He, C.; Li, C.; Qiao, R. Water-Soluble Cationic Polyphosphazenes Grafted with Cyclic Polyamine and Imidazole as an Effective Gene Delivery Vector. Bioconjugate Chem. 2016, 27, 1005–1012. [Google Scholar] [CrossRef]
- Hsu, W.H.; Sánchez-Gómez, P.; Gomez-Ibarlucea, E.; Ivanov, D.P.; Rahman, R.; Grabowska, A.M.; Csaba, N.; Alexander, C.; Garcia-Fuentes, M. Structure-Optimized Interpolymer Polyphosphazene Complexes for Effective Gene Delivery against Glioblastoma. Adv. Ther. 2019, 2, 1800126. [Google Scholar] [CrossRef]
- Garcia-Mazas, C.; Bozzato, E.; Araujo Fernandez, J.A.; Quattrini, F.; Preat, V.; Sanchez, L.; Csaba, N.; Garcia-Fuentes, M. Designing Polyphosphazene Derivatives for Gene Delivery in Glioblastoma Treatment. Mater. Today Bio 2025, 33, 102010. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Huang, Z.; Zhang, J.; Wu, W.-X.; Liu, Y.-H.; Yu, X.-Q. Amphiphilic Polymers Formed from Ring-Opening Polymerization: A Strategy for the Enhancement of Gene Delivery. Biomater. Sci. 2017, 5, 718–729. [Google Scholar] [CrossRef]
- Mehnath, S.; Arjama, M.; Rajan, M.; Jeyaraj, M. Development of Cholate Conjugated Hybrid Polymeric Micelles for FXR Receptor Mediated Effective Site-Specific Delivery of Paclitaxel. New J. Chem. 2018, 42, 17021–17032. [Google Scholar] [CrossRef]
- Zhang, J.X.; Qiu, L.Y.; Zhu, K.J.; Jin, Y. Thermosensitive Micelles Self-Assembled by Novel N-Isopropylacrylamide Oligomer Grafted Polyphosphazene. Macromol. Rapid Commun. 2004, 25, 1563–1567. [Google Scholar] [CrossRef]
- Khan, R.U.; Yu, H.; Wang, L.; Zhang, Q.; Xiong, W.; Zain-ul-Abdin; Nazir, A.; Fahad, S.; Chen, X.; Elsharaarani, T. Synthesis of Polyorganophosphazenes and Preparation of Their Polymersomes for Reductive/Acidic Dual-Responsive Anticancer Drugs Release. J. Mater. Sci. 2020, 55, 8264–8284. [Google Scholar] [CrossRef]
- Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liang, L.; Qiu, L. In Situ Generated Gold Nanoparticle Hybrid Polymersomes for Water-Soluble Chemotherapeutics: Inhibited Leakage and PH-Responsive Intracellular Release. Adv. Funct. Mater. 2017, 27, 1604981. [Google Scholar] [CrossRef]
- Avaji, P.G.; Joo, H.I.; Park, J.H.; Park, K.S.; Jun, Y.J.; Lee, H.J.; Sohn, Y.S. Synthesis and Properties of a New Micellar Polyphosphazene-Platinum (II) Conjugate Drug. J. Inorg. Biochem. 2014, 140, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.J.; Park, J.H.; Park, K.S.; Avaji, P.G.; Lee, K.E.; Lee, H.J.; Sohn, Y.S. Design of Theranostic Nanomedicine (Ii): Synthesis and Physicochemical Properties of a Biocompatible Polyphosphazene–Docetaxel Conjugate. Int. J. Nanomed. 2017, 12, 5373–5386. [Google Scholar] [CrossRef]
- Patil, B.R.; Kang, S.Y.; Jung, D.H.; Avaji, P.G.; Jun, Y.J.; Lee, H.J.; Sohn, Y.S. Design of a Novel Theranostic Nanomedicine (Iii): Synthesis and Physicochemical Properties of Tumor-Targeting Cisplatin Conjugated to a Hydrophilic Polyphosphazene. Int. J. Nanomed. 2020, 15, 981–990. [Google Scholar] [CrossRef]
- Gleria, M.; De Jaeger, R. Phosphazenes: A Worldwide Insight; Gleria, M., De Jaeger, R., Eds.; Nova Publishers: New York, NY, USA, 2004. [Google Scholar]
- Allcock, H.R. Chemistry and Applications of Polyphosphazenes; Wiley-Interscienc: Hoboken, NJ, USA, 2003. [Google Scholar]
- Hagnauer, G.L. Polydichlorophosphazene Polymerization Studies. J. Macromol. Sci. Part A-Chem. 1981, 16, 385–408. [Google Scholar] [CrossRef]
- Blackstone, V.; Presa Soto, A.; Manners, I. Polymeric Materials Based on Main Group Elements: The Recent Development of Ambient Temperature and Controlled Routes to Polyphosphazenes. Dalton Trans. 2008, 33, 4363–4371. [Google Scholar] [CrossRef]
- Elayan, A.S.; Allen, C.W.; Peterson, E.S. Synthesis of Poly(dichlorophosphazene) by the Melt Phase Polymerization of P-Trichloro-N-(Dichlorophosphoryl)Monophosphazene. J. Inorg. Organomet. Polym. Mater. 2017, 27, 119–123. [Google Scholar] [CrossRef]
- De Jaeger, R.; Potin, P. Poly(dichlorophosphazene) from p-trichloro-n-dichlorophosphoryl monophosphazene Cl3P=N-POCl2. In Polyphosphazenes: A Worldwide Insight; Mario, G., Roger, D.J., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2004; Volume 1, pp. 25–48. [Google Scholar]
- Allcock, H.R.; Crane, C.A.; Morrissey, C.T.; Olshavsky, M.A. A New Route to the Phosphazene Polymerization Precursors, Cl3P=NSiMe3 and (NPCl2)3. Inorg. Chem. 1999, 38, 280–283. [Google Scholar] [CrossRef]
- Allcock, H.R.; Crane, C.A.; Morrissey, C.T.; Nelson, J.M.; Reeves, S.D.; Honeyman, C.H.; Manners, I. “Living” Cationic Polymerization of Phosphoranimines as an Ambient Temperature Route to Polyphosphazenes with Controlled Molecular Weights. Macromolecules 1996, 29, 7740–7747. [Google Scholar] [CrossRef]
- Allcock, H.R.; Reeves, S.D.; De Denus, C.R.; Crane, C.A. Influence of Reaction Parameters on the Living Cationic Polymerization of Phosphoranimines to Polyphosphazenes. Macromolecules 2001, 34, 748–754. [Google Scholar] [CrossRef]
- Scheer, M. The Coordination Chemistry of Group 15 Element Ligand Complexes–A Developing Area. Dalton Trans. 2008, 33, 4372–4386. [Google Scholar] [CrossRef]
- Blackstone, V.; Lough, A.J.; Murray, M.; Manners, I. Probing the Mechanism of the PCl5-Initiated Living Cationic Polymerization of the Phosphoranimine Cl3P=NSiMe3 Using Model Compound Chemistry. J. Am. Chem. Soc. 2009, 131, 3658–3667. [Google Scholar] [CrossRef]
- Blackstone, V.; Pfirrmann, S.; Helten, H.; Staubitz, A.; Presa Soto, A.; Whittell, G.R.; Manners, I. A Cooperative Role for the Counteranion in the PCl5-Initiated Living, Cationic Chain Growth Polycondensation of the Phosphoranimine Cl3P=NSiMe3. J. Am. Chem. Soc. 2012, 134, 15293–15296. [Google Scholar] [CrossRef]
- Henke, H.; Wilfert, S.; Iturmendi, A.; Brüggemann, O.; Teasdale, I. Branched Polyphosphazenes with Controlled Dimensions. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4467–4473. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; Chen, J.; LeGolvan, M.P. Poly(dichlorophosphazene) as a Precursor for Biologically Active Polyphosphazenes: Synthesis, Characterization, and Stabilization. Macromolecules 2004, 37, 414–420. [Google Scholar] [CrossRef]
- Hamilton, E.J.M. Synthetic Inorganic Chemistry–New Perspectives; Hosmane, N.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Vinogrodova, S.V.; Tur, D.R.; Vanesem, V.A. Open-Chain Poly(organophosphazenes). Synth. Prop. 1988, 67, 515–534. Available online: http://iopscience.iop.org/0036-021X/67/6/R04 (accessed on 29 July 2025).
- Allcock, H.R. Synthesis, Structures, and Emerging Uses for Poly(organophosphazenes). In Polyphosphazenes in Biomedicine, Engineering, and Pioneering Synthesis; American Chemical Society: Washington, WA, USA, 2018; Volume 1298, pp. 3–26. [Google Scholar] [CrossRef]
- Pertici, P.; Vitulli, G.; Gleria, M.; Facchin, G.; Milani, R.; Bertani, R. Metal-Containing Poly(organophosphazenes). Macromol. Symp. 2006, 235, 98–114. [Google Scholar] [CrossRef]
- Allcock, H.R. The Synthesis of Functional Polyphosphazenes and Their Surfaces. Appl. Organomet. Chem. 1998, 12, 659–666. Available online: https://deepblue.lib.umich.edu/bitstream/handle/2027.42/38302/773_ftp.pdf?sequence=1 (accessed on 29 July 2025). [CrossRef]
- Miranda, L.P.; Meldal, M. Unichemo Protection: A Concept for Chemical Synthesis. Angew. Chem. 2001, 40, 3655. [Google Scholar] [CrossRef]
- Wisian-Neilson, P. Polyphosphazenes from Condensation Polymerization. In Polyphosphazenes for Biomedical Applications; Andrianov, K.A., Ed.; Wiley: Hoboken, NJ, USA, 2008; pp. 155–167. [Google Scholar]
- Allcock, H.R.; Fuller, T.J.; Matsumura, K. Hydrolysis Pathways for Aminophosphazenes. Inorg. Chem. 1982, 21, 515–521. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A. Degradation of Polyaminophosphazenes: Effects of Hydrolytic Environment and Polymer Processing. Biomacromolecules 2006, 7, 1581–1586. [Google Scholar] [CrossRef]
- Allcock, H.R.; Kwon, S. Glyceryl Polyphosphazenes: Synthesis, Properties, and Hydrolysis. Macromolecules 1988, 21, 1980–1985. [Google Scholar] [CrossRef]
- Allcock, H.R.; Pucher, S.R. Polyphosphazenes with Glucosyl and Methylamino, Trifluoroethoxy, Phenoxy, or (Methoxyethoxy)Ethoxy Side Groups. Macromolecules 1991, 24, 23–34. [Google Scholar] [CrossRef]
- Allcock, H.R.; Pucher, S.R.; Scopelianos, A.G. Synthesis of Poly(orgnaophosphazenes) with Glycolic Acid Ester and Lactic Acid Ester Side Groups: Prototypes for New Bioerodible Polymers. Macromolecules 1994, 27, 1–4. [Google Scholar] [CrossRef]
- Baillargeon, A.L.; Mequanint, K. Biodegradable Polyphosphazene Biomaterials for Tissue Engineering and Delivery of Therapeutics. Biomed. Res. Int. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; Koh, H.J.; Neenan, T.X.; Allcock, H.R.; Langer, R. Controlled Release Using a New Bioerodible Polyphosphazene Matrix System. J. Biomed. Mater. Res. 1987, 21, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, A.M.A.; Allcock, H.R.; Katti, D.S.; Laurencin, C.T. Degradable Polyphosphazene/Poly(a-Hydroxyester) Blends: Degradation Studies. Biomaterials 2002, 23, 1667–1672. [Google Scholar] [CrossRef]
- Zhou, N.; Zhi, Z.; Liu, D.; Wang, D.; Shao, Y.; Yan, K.; Meng, L.; Yu, D. Acid-Responsive and Biologically Degradable Polyphosphazene Nanodrugs for Efficient Drug Delivery. ACS Biomater. Sci. Eng. 2020, 6, 4285–4293. [Google Scholar] [CrossRef]
- Hackl, C.M.; Schoenhacker-Alte, B.; Klose, M.H.M.; Henke, H.; Legina, M.S.; Jakupec, M.A.; Berger, W.; Keppler, B.K.; Brüggemann, O.; Teasdale, I.; et al. Synthesis and In Vivo Anticancer Evaluation of Poly(organo)Phosphazene-Based Metallodrug Conjugates. Dalton Trans. 2017, 46, 12114–12124. [Google Scholar] [CrossRef]
- Aichhorn, S.; Linhardt, A.; Halfmann, A.; Nadlinger, M.; Kirchberger, S.; Stadler, M.; Dillinger, B.; Distel, M.; Dohnal, A.; Teasdale, I.; et al. A PH-sensitive Macromolecular Prodrug as TLR7/8 Targeting Immune Response Modifier. Chem.—A Eur. J. 2017, 23, 17721–17726. [Google Scholar] [CrossRef]
- Teasdale, I. Stimuli-Responsive Phosphorus-Based Polymers. Eur. J. Inorg. Chem. 2019, 2019, 1445–1456. [Google Scholar] [CrossRef]
- Hou, S.-L.; Chen, S.-S.; Huang, Z.-J.; Lu, Q.-H. Dual-Responsive Polyphosphazene as a Common Platform for Highly Efficient Drug Self-Delivery. J. Mater. Chem. B 2019, 7, 4319–4327. [Google Scholar] [CrossRef]
- Iturmendi, A.; Monkowius, U.; Teasdale, I. Oxidation Responsive Polymers with a Triggered Degradation via Arylboronate Self-Immolative Motifs on a Polyphosphazene Backbone. ACS Macro Lett. 2017, 6, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Linhardt, A.; König, M.; Iturmendi, A.; Henke, H.; Brüggemann, O.; Teasdale, I. Degradable, Dendritic Polyols on a Branched Polyphosphazene Backbone. Ind. Eng. Chem. Res. 2018, 57, 3602–3609. [Google Scholar] [CrossRef] [PubMed]
- Salinas, Y.; Kneidinger, M.; Fornaguera, C.; Borrós, S.; Brüggemann, O.; Teasdale, I. Dual Stimuli-Responsive Polyphosphazene-Based Molecular Gates for Controlled Drug Delivery in Lung Cancer Cells. RSC Adv. 2020, 10, 27305–27314. [Google Scholar] [CrossRef]
- Ullah, R.S.; Wang, L.; Yu, H.; Abbasi, N.M.; Akram, M.; -ul-Abdin, Z.; Saleem, M.; Haroon, M.; Khan, R.U. Synthesis of Polyphosphazenes with Different Side Groups and Various Tactics for Drug Delivery. RSC Adv. 2017, 7, 23363–23391. [Google Scholar] [CrossRef]
- Iturmendi, A.; Theis, S.; Maderegger, D.; Monkowius, U.; Teasdale, I. Coumarin-Caged Polyphosphazenes with a Visible-Light Driven On-Demand Degradation. Macromol. Rapid Commun. 2018, 39, e1800377. [Google Scholar] [CrossRef]
- Feinweber, D.; Verwanger, T.; Brüggemann, O.; Teasdale, I.; Krammer, B. Applicability of New Degradable Hypericin-Polymer-Conjugates as Photosensitizers: Principal Mode of Action Demonstrated by in Vitro Models. Photochem. Photobiol. Sci. 2014, 13, 1607–1620. [Google Scholar] [CrossRef]
- Couffin-Hoarau, A.-C.; Leroux, J.-C. Report on the Use of Poly(organophosphazenes) for the Design of Stimuli-Responsive Vesicles. Biomacromolecules 2004, 5, 2082–2087. [Google Scholar] [CrossRef]
- Jagtap, P.; Patil, K.; Dhatrak, P. Polyelectrolyte Complex for Drug Delivery in Biomedical Applications: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1183, 012007. [Google Scholar] [CrossRef]
- Zhao, L.; Skwarczynski, M.; Toth, I. Polyelectrolyte-Based Platforms for the Delivery of Peptides and Proteins. ACS Biomater. Sci. Eng. 2019, 5, 4937–4950. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Singh, D.; Saraf, S.A.S.S.; Saraf, S. Nanocarriers: Promising Vehicle for Bioactive Drugs. Biol. Pharm. Bull. 2006, 29, 1790–1798. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric Nanomaterials: Recent Developments, Properties and Medical Applications. In Polymeric Nanomaterials in Nanotherapeutics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–66. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Roberts, B.E. Polyphosphazene Polyelectrolytes: A Link between the Formation of Noncovalent Complexes with Antigenic Proteins and Immunostimulating Activity. Biomacromolecules 2005, 6, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- De Wolf, H.K.; Luten, J.; Snel, C.J.; Oussoren, C.; Hennink, W.E.; Storm, G. In Vivo Tumor Transfection Mediated by Polyplexes Based on Biodegradable Poly(DMAEA)-Phosphazene. J. Control. Release 2005, 109, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.P.; Qamar, B.; Fuerst, T.R.; Muro, S.; Andrianov, A.K. Biodegradable “Smart” Polyphosphazenes with Intrinsic Multifunctionality as Intracellular Protein Delivery Vehicles. Biomacromolecules 2017, 18, 2000–2011. [Google Scholar] [CrossRef]
- Powell, B.S.; Andrianov, A.K.; Fusco, P.C. Polyionic Vaccine Adjuvants: Another Look at Aluminum Salts and Polyelectrolytes. Clin. Exp. Vaccine Res. 2015, 4, 23. [Google Scholar] [CrossRef]
- Luten, J.; Van Steenis, J.H.; Van Someren, R.; Kemmink, J.; Schuurmans-Nieuwenbroek, N.M.E.; Koning, G.A.; Crommelin, D.J.A.; Van Nostrum, C.F.; Hennink, W.E. Water-Soluble Biodegradable Cationic Polyphosphazenes for Gene Delivery. J. Control. Release 2003, 89, 483–497. [Google Scholar] [CrossRef]
- Luten, J.; van Steenbergen, M.J.; Lok, M.C.; de Graaff, A.M.; van Nostrum, C.F.; Talsma, H.; Hennink, W.E. Degradable PEG-Folate Coated Poly(DMAEA-Co-BA) Phosphazene-Based Polyplexes Exhibit Receptor-Specific Gene Expression. Eur. J. Pharm. Sci. 2008, 33, 241–251. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Martinez, A.P.; Weidman, J.L.; Fuerst, T.R. Hydrolytically Degradable PEGylated Polyelectrolyte Nanocomplexes for Protein Delivery. Biomacromolecules 2018, 19, 3467–3478. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Chen, L.; Gu, W.; Li, Y. Galactosylated Poly(2-(2-Aminoethyoxy)Ethoxy)Phosphazene/DNA Complex Nanoparticles: In Vitro and in Vivo Evaluation for Gene Delivery. Biomacromolecules 2010, 11, 927–933. [Google Scholar] [CrossRef]
- Payne, L.G.; Jenkins, S.A.; Woods, A.L.; Grund, E.M.; Geribo, W.E.; Loebelenz, J.R.; Andrianov, A.K.; Roberts, B.E. Poly[Di(Carboxylatophenoxy)Phosphazene] (PCPP) Is a Potent Immunoadjuvant for an Influenza. Vaccine 1998, 16, 92–98. [Google Scholar] [CrossRef]
- Magiri, R.B.; Lai, K.J.; Mutwiri, G.K.; Wilson, H.L. Experimental PCEP-Adjuvanted Swine Influenza H1N1 Vaccine Induced Strong Immune Responses but Did Not Protect Piglets against Heterologous H3N2 Virus Challenge. Vaccines 2020, 8, 235. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Fuerst, T.R. Molecular-Level Interactions of Polyphosphazene Immunoadjuvants and Their Potential Role in Antigen Presentation and Cell Stimulation. Biomacromolecules 2016, 17, 3732–3742. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.D.; Ninković, J.; Prokopowicz, Z.M.; Mancuso, C.J.; Marin, A.; Andrianov, A.K.; Dowling, D.J.; Levy, O. The Effect of Stable Macromolecular Complexes of Ionic Polyphosphazene on HIV Gag Antigen and on Activation of Human Dendritic Cells and Presentation to T-Cells. Biomaterials 2014, 35, 8876–8886. [Google Scholar] [CrossRef] [PubMed]
- Romanyuk, A.; Wang, R.; Marin, A.; Janus, B.M.; Felner, E.I.; Xia, D.; Goez-Gazi, Y.; Alfson, K.J.; Yunus, A.S.; Toth, E.A.; et al. Skin Vaccination with Ebola Virus Glycoprotein Using a Polyphosphazene-Based Microneedle Patch Protects Mice against Lethal Challenge. J. Funct. Biomater. 2022, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.J.; Excler, J.L.; Polonis, V.R.; Ratto-Kim, S.; Cox, J.; Jagodzinski, L.L.; Liu, M.; Wieczorek, L.; McNeil, J.G.; El-Habib, R.; et al. Safety and Immunogenicity of a Randomized Phase 1 Prime-Boost Trial with ALVAC-HIV (VCP205) and Oligomeric Glycoprotein 160 from HIV-1 Strains MN and LAI-2 Adjuvanted in Alum or Polyphosphazene. J. Infect. Dis. 2016, 213, 1946–1954. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Wang, R.; Chowdhury, A.; Agnihotri, P.; Yunus, A.S.; Pierce, B.G.; Mariuzza, R.A.; Fuerst, T.R. In Vivo and In Vitro Potency of Polyphosphazene Immunoadjuvants with Hepatitis C Virus Antigen and the Role of Their Supramolecular Assembly. Mol. Pharm. 2021, 18, 726–734. [Google Scholar] [CrossRef]
- Marin, A.; Chowdhury, A.; Valencia, S.M.; Zacharia, A.; Kirnbauer, R.; Roden, R.B.S.; Pinto, L.A.; Shoemaker, R.H.; Marshall, J.D.; Andrianov, A.K. Next Generation Polyphosphazene Immunoadjuvant: Synthesis, Self-Assembly and in Vivo Potency with Human Papillomavirus VLPs-Based Vaccine. Nanomedicine 2021, 33, 102359. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; Marin, A.; Deng, J.; Fuerst, T.R. Protein-Loaded Soluble and Nanoparticulate Formulations of Ionic Polyphosphazenes and Their Interactions on Molecular and Cellular Levels. Mater. Sci. Eng. C 2020, 106, 110179. [Google Scholar] [CrossRef]
- Seo, B.B.; Park, M.R.; Song, S.C. Sustained Release of Exendin 4 Using Injectable and Ionic-Nano-Complex Forming Polymer Hydrogel System for Long-Term Treatment of Type 2 Diabetes Mellitus. ACS Appl. Mater. Interfaces 2019, 11, 15201–15211. [Google Scholar] [CrossRef]
- Van den Berg, A.I.S.; Yun, C.O.; Schiffelers, R.M.; Hennink, W.E. Polymeric Delivery Systems for Nucleic Acid Therapeutics: Approaching the Clinic. J. Control. Release 2021, 331, 121–141. [Google Scholar] [CrossRef]
- Chollet, P.; Favrot, M.C.; Hurbin, A.; Coll, J.L. Side-Effects of a Systemic Injection of Linear Polyethylenimine-DNA Complexes. J. Gene Med. 2002, 4, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Spain, S.G.; Yasayan, G.; Soliman, M.; Heath, F.; Saeed, A.; Alexander, C. Nanoparticles for Nucleic Acid Delivery. In Comprehensive Biomaterials; Elsevier: Amsterdam, The Netherlands, 2011; pp. 389–410. [Google Scholar]
- De Wolf, H.K.; De Raad, M.; Snel, C.; Van Steenbergen, M.J.; Fens, M.H.A.M.; Storm, G.; Hennink, W.E. Biodegradable Poly(2-Dimethylamino Ethylamino)Phosphazene for in Vivo Gene Delivery to Tumor Cells. Effect of Polymer Molecular Weight. Pharm. Res. 2007, 24, 1572–1580. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Z.; Jiang, J.; Gao, Y.; Gu, W.; Chen, L.; Tang, X.; Li, Y. Poly(Imidazole/DMAEA)Phosphazene/DNA Self-Assembled Nanoparticles for Gene Delivery: Synthesis and in Vitro Transfection. J. Control. Release 2008, 127, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.; Chen, L.; Gu, W.; Li, Y. Urocanic Acid Improves Transfection Efficiency of Polyphosphazene with Primary Amino Groups for Gene Delivery. Bioconjugate Chem. 2010, 21, 419–426. [Google Scholar] [CrossRef]
- Barry, M. Single-Cycle Adenovirus Vectors in the Current Vaccine Landscape. Expert Rev. Vaccines 2018, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Nazarali, A.; Foldvari, M. Non-Viral Nucleic Acid Delivery: Key Challenges and Future Directions. Curr. Drug Deliv. 2011, 8, 235–244. [Google Scholar] [CrossRef]
- Saylor, K.; Gillam, F.; Lohneis, T.; Zhang, C. Designs of Antigen Structure and Composition for Improved Protein-Based Vaccine Efficacy. Front. Immunol. 2020, 11, 283. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key Roles of Adjuvants in Modern Vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of Adjuvanticity: A Review on Adjuvants in Licensed Vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Burova, T.V.; Grinberg, N.V.; Dubovik, A.S.; Grinberg, V.Y. Conformational Stability of Bovine Serum Albumin in Complexes with Poly[Di(Carboxylatophenoxy)Phosphazene]. Polym. Sci. Ser. A 2015, 57, 761–772. [Google Scholar] [CrossRef]
- Lueckheide, M.; Marin, A.; Tagad, H.D.; Posey, N.D.; Prabhu, V.M.; Andrianov, A.K. Monitoring Protein Complexation with Polyphosphazene Polyelectrolyte Using Automated Dynamic Light Scattering Titration and Asymmetric Flow Field Flow Fractionation and Protein Recognition Immunoassay. ACS Polym. Au 2023, 3, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; Decollibus, D.P.; Marin, A.; Webb, A.; Griffin, Y.; Webby, R.J. PCPP-Formulated H5N1 Influenza Vaccine Displays Improved Stability and Dose-Sparing Effect in Lethal Challenge Studies. J. Pharm. Sci. 2011, 100, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Wilson, H.L.; Singh, B.; Babiuk, L.A.; Mutwiri, G. The Adjuvant PCEP Induces Recruitment of Myeloid and Lymphoid Cells at the Injection Site and Draining Lymph Node. Vaccine 2014, 32, 2420–2427. [Google Scholar] [CrossRef]
- Mutwiri, G.; Benjamin, P.; Soita, H.; Townsend, H.; Yost, R.; Roberts, B.; Andrianov, A.K.; Babiuk, L.A. Poly[Di(Sodium Carboxylatoethylphenoxy)Phosphazene] (PCEP) Is a Potent Enhancer of Mixed Th1/Th2 Immune Responses in Mice Immunized with Influenza Virus Antigens. Vaccine 2007, 25, 1204–1213. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and PH-Responsive Polymers in Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Yessine, M.A.; Leroux, J.C. Membrane-Destabilizing Polyanions: Interaction with Lipid Bilayers and Endosomal Escape of Biomacromolecules. Adv. Drug Deliv. Rev. 2004, 56, 999–1021. [Google Scholar] [CrossRef]
- Andrianov, A.K.; DeCollibus, D.P.; Gillis, H.A.; Kha, H.H.; Marin, A.; Prausnitz, M.R.; Babiuk, L.A.; Townsend, H.; Mutwiri, G. Poly[Di(Carboxylatophenoxy)Phosphazene] Is a Potent Adjuvant for Intradermal Immunization. Proc. Natl. Acad. Sci. USA 2009, 106, 18936–18941. [Google Scholar] [CrossRef]
- Rawat, S.; Raman Suri, C.; Sahoo, D.K. Molecular Mechanism of Polyethylene Glycol Mediated Stabilization of Protein. Biochem. Biophys. Res. Commun. 2010, 392, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Aibani, N.; Callan, J.F.; Callan, B. Recent Advances in Amphiphilic Polymers for Simultaneous Delivery of Hydrophobic and Hydrophilic Drugs. Therapeutic Delivery 2015, 7, 15–31. [Google Scholar] [CrossRef]
- Perin, F.; Motta, A.; Maniglio, D. Amphiphilic Copolymers in Biomedical Applications: Synthesis Routes and Property Control. Mater. Sci. Eng. C 2021, 123, 111952. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block Copolymer Micelles: Preparation, Characterization and Application in Drug Delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef]
- Liu, Y.; Ghassemi, A.H.; Hennink, W.E.; Schwendeman, S.P. The Microclimate PH in Poly(d,l-Lactide-Co-Hydroxymethyl Glycolide) Microspheres during Biodegradation. Biomaterials 2012, 33, 7584–7593. [Google Scholar] [CrossRef]
- Wiseman, D.; Kost, J.; Domb, A.J. (Eds.) Handbook of Biodegradable Polymers; CRC Press: London, UK, 1998. [Google Scholar] [CrossRef]
- Wang, R.; Hughes, T.; Beck, S.; Vakil, S.; Li, S.; Pantano, P.; Draper, R.K. Generation of Toxic Degradation Products by Sonication of Pluronic® Dispersants: Implications for Nanotoxicity Testing. Nanotoxicology 2013, 7, 1272–1281. [Google Scholar] [CrossRef]
- Qiu, L.; Fu, J. Applications of Self-Assembled Polyphosphazene Nano-Aggregates in Drug Delivery. In Polyphosphazenes in Biomedicine, Engineering, and Pioneering Synthesis; American Chemical Society: Washington, WA, USA, 2018; pp. 143–164. [Google Scholar] [CrossRef]
- Chun, C.J.; Lee, S.M.; Kim, S.Y.; Yang, H.K.; Song, S.C. Thermosensitive Poly(organophosphazene)-Paclitaxel Conjugate Gels for Antitumor Applications. Biomaterials 2009, 30, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Mehnath, S.; Rajan, M.; Sathishkumar, G.; Amarnath Praphakar, R.; Jeyaraj, M. Thermoresponsive and PH Triggered Drug Release of Cholate Functionalized Poly(organophosphazene)—Polylactic Acid Co-Polymeric Nanostructure Integrated with ICG. Polymer 2017, 133, 119–128. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Wu, X.L.; Jin, Y. Doxorubicin-Loaded Polymeric Micelles Based on Amphiphilic Polyphosphazenes with Poly(N-Isopropylacrylamide-Co-N, N-Dimethylacrylamide) and Ethyl Glycinate as Side Groups: Synthesis, Preparation and in Vitro Evaluation. Pharm. Res. 2009, 26, 946–957. [Google Scholar] [CrossRef]
- Zheng, C.; Qiu, L.; Zhu, K. Novel Polymersomes Based on Amphiphilic Graft Polyphosphazenes and Their Encapsulation of Water-Soluble Anti-Cancer Drug. Polymer 2009, 50, 1173–1177. [Google Scholar] [CrossRef]
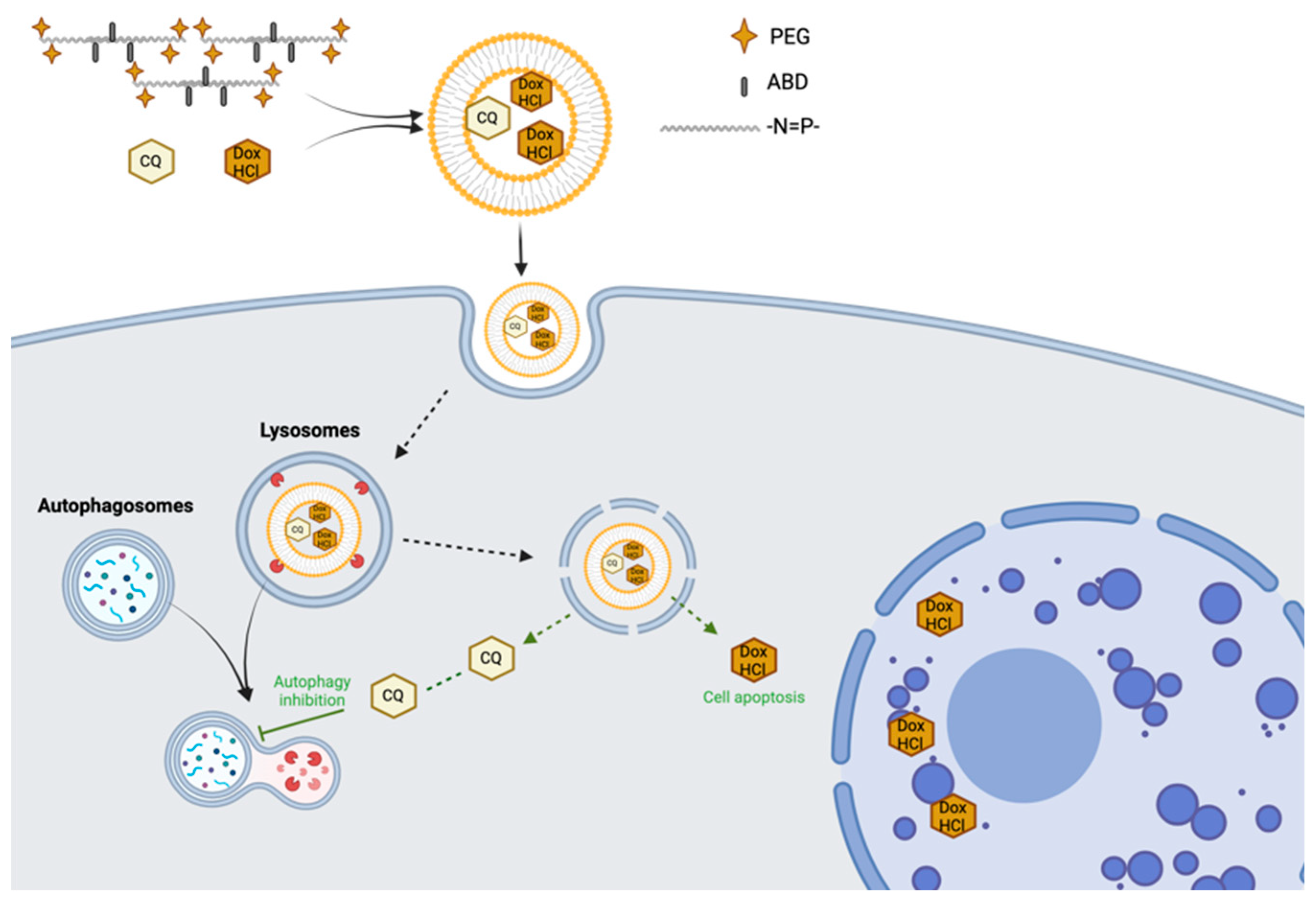
- Wang, J.; Qiu, L. Drug-Induced Self-Assembled Nanovesicles for Doxorubicin Resistance Reversal via Autophagy Inhibition and Delivery Synchronism. Theranostics 2022, 12, 3977–3994. [Google Scholar] [CrossRef]
- Li, X.; Zhu, X.; Qiu, L. Constructing Aptamer Anchored Nanovesicles for Enhanced Tumor Penetration and Cellular Uptake of Water-Soluble Chemotherapeutics. Acta Biomater. 2016, 35, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Peng, Y.; Qiu, L. Amino-Functionalized Nano-Vesicles for Enhanced Anticancer Efficacy and Reduced Myelotoxicity of Carboplatin. Colloids Surf. B Biointerfaces 2017, 157, 56–64. [Google Scholar] [CrossRef]
- Peng, Y.; Zhu, X.; Qiu, L. Electroneutral Composite Polymersomes Self-Assembled by Amphiphilic Polyphosphazenes for Effective MiR-200c In Vivo Delivery to Inhibit Drug-Resistant Lung Cancer. Biomaterials 2016, 106, 1–12. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, X.; Wu, L.; Qiu, L. Cationic Polyphosphazene Vesicles for Cancer Immunotherapy by Efficient in Vivo Cytokine IL-12 Plasmid Delivery. Biomacromolecules 2016, 17, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.Z.; Li, Y.Q.; Jin, Y.; Kang, J.Z. Physicochemical Characterization of Polymeric Micelles Constructed from Novel Amphiphilic Polyphosphazene with Poly(N-Isopropylacrylamide) and Ethyl 4-Aminobenzoate as Side Groups. Colloids Surf. B Biointerfaces 2005, 43, 123–130. [Google Scholar] [CrossRef]
- Zheng, C.; Qiu, L.; Yao, X.; Zhu, K. Novel Micelles from Graft Polyphosphazenes as Potential Anti-Cancer Drug Delivery Systems: Drug Encapsulation and in Vitro Evaluation. Int. J. Pharm. 2009, 373, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zheng, C.; Zhao, Q. Mechanisms of Drug Resistance Reversal in Dox-Resistant MCF-7 Cells by PH-Responsive Amphiphilic Polyphosphazene Containing Diisopropylamino Side Groups. Mol. Pharm. 2012, 9, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Yao, X.; Qiu, L. Novel Polymeric Vesicles with PH-Induced Deformation Character for Advanced Drug Delivery. Macromol. Biosci. 2011, 11, 338–343. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Q.; Jin, Y.; Qiu, L. High Loading of Hydrophilic/Hydrophobic Doxorubicin into Polyphosphazene Polymersome for Breast Cancer Therapy. Nanomedicine 2014, 10, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer–Drug Conjugate Therapeutics: Advances, Insights and Prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Marasini, N.; Haque, S.; Kaminskas, L.M. Polymer-Drug Conjugates as Inhalable Drug Delivery Systems: A Review. Curr. Opin. Colloid. Interface Sci. 2017, 31, 18–29. [Google Scholar]
- Dragojevic, S.; Ryu, J.S.; Raucher, D. Polymer-Based Prodrugs: Improving Tumor Targeting and the Solubility of Small Molecule Drugs in Cancer Therapy. Molecules 2015, 20, 21750–21769. [Google Scholar] [CrossRef]
- Henke, H.; Kryeziu, K.; Banfić, J.; Theiner, S.; Körner, W.; Brüggemann, O.; Berger, W.; Keppler, B.K.; Heffeter, P.; Teasdale, I. Macromolecular Pt(IV) Prodrugs from Poly(organo)Phosphazenes. Macromol. Biosci. 2016, 16, 1239–1249. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K.; Sharma, R.; Murthy, R.S.R.; Bhardwaj, T.R. Design, Synthesis and Evaluation of Antimalarial Potential of Polyphosphazene Linked Combination Therapy of Primaquine and Dihydroartemisinin. Eur. J. Pharm. Sci. 2015, 66, 123–137. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-Based Drugs for Cancer Therapy and Anti-Tumor Strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Sohn, Y.S.; Baek, H.; Cho, Y.H.; Lee, Y.-A.; Jung, O.-S.; Lee, C.O.; Kim, Y.S. Synthesis and Antitumor Activity of Novel Polyphosphazene-(Diamine)Platinum (II) Conjugates. Int. J. Pharm. 1997, 153, 79–91. [Google Scholar] [CrossRef]
- Lee, S.B.; Song, S.-C.; Jin, J.-I.; Sohn, Y.S. Synthesis and Antitumor Activity of Polyphosphazene Methoxy-Poly (Ethylene Glycol)/(Diamine)Platinum(II) Conjugates. Polym. J. 1999, 31, 1247–1252. [Google Scholar] [CrossRef]
- Song, R.; Yong, J.J.; Ju, I.K.; Jin, C.; Youn, S.S. Synthesis, Characterization, and Tumor Selectivity of a Polyphosphazene-Platinum(II) Conjugate. J. Control. Release 2005, 105, 142–150. [Google Scholar] [CrossRef]
- Teasdale, I.; Wilfert, S.; Nischang, I.; Brüggemann, O. Multifunctional and Biodegradable Polyphosphazenes for Use as Macromolecular Anti-Cancer Drug Carriers. Polym. Chem. 2011, 2, 828–834. [Google Scholar] [CrossRef]
- Albright, V.; Penarete-Acosta, D.; Stack, M.; Zheng, J.; Marin, A.; Hlushko, H.; Wang, H.; Jayaraman, A.; Andrianov, A.K.; Sukhishvili, S.A. Polyphosphazenes Enable Durable, Hemocompatible, Highly Efficient Antibacterial Coatings. Biomaterials 2021, 268, 120586. [Google Scholar] [CrossRef]
- Zhou, S.; Hou, S.; Lu, Q. Polyphosphazene Microparticles with High Free Radical Scavenging Activity for Skin Photoprotection. ACS Appl. Mater. Interfaces 2024, 16, 32649–32661. [Google Scholar] [CrossRef] [PubMed]
- Alwine, S.; Chen, C.; Shen, L.; Allcock, H.R.; Siedlecki, C.A.; Xu, L. Crosslinkable [Fluorophenoxy-substituted Poly[Bis(Octafluoropentoxy) Phosphazene] Biomaterials with Improved Antimicrobial Effect and Hemocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 1533–1545. [Google Scholar] [CrossRef] [PubMed]



| PPZ Type | Side Group | Degradation Products | Ref. | |
|---|---|---|---|---|
| Amino-PPZ | Imidazole | Amino acid | + Phosphate + Ammonia | [53,54] |
| Amino acid ester | Amino acid/alcohol | [53,54] | ||
| Alkoxy-PPZ | Glyceryl | Glycerol | [55] | |
| Glucosyl | Glucose | [56] | ||
| Methyl amino | Methylamine | [56] | ||
| Glycolic acid ester | Glycolic acid Benzyl alcohol/ethanol | [57] | ||
| Lactic acid ester | Lactic acid Benzyl alcohol/ethanol | [57] | ||
| Therapeutic Application | PPZ Derivative | Cargo | In Vitro/In Vivo Model | Main Finding and Ref. |
|---|---|---|---|---|
| Hydrophobic anticancer drug delivery | Colic acid-PLA- PCPP | Paclitaxel/indocyanine green | In vitro (MCF-7) | Sustained release; accelerated in acidic cancerous conditions [24] |
| Hydrophobic anticancer drug delivery | Colic acid-PDADM-PCPP | Paclitaxel | In vitro (MCF-7) | Improved drug delivery and cytotoxicity vs. free drug [124] |
| Hydrophilic anticancer drug delivery | mPEG-ABD-PPZ | Dox-HCl/CQ | In vivo (K562/ADR) | Prolonged circulation and greater antitumor effects [127] |
| Hydrophilic anticancer drug delivery | mPEG-EAB-PPZ + AuNPs | Dox-HCl | In vitro (S180) In vivo (S180) | pH-responsive release; greater antitumor effects than free drug [28] |
| Hydrophilic anticancer drug delivery | mPEG-EAB-PPZ + AS1411 | Dox-HCl | In vitro (MCF-7) In vivo (MCF-7) | Greater antiproliferative and antitumoral effect than free drug [128] |
| Hydrophilic anticancer drug delivery | mPEG-EAB-PPZ + tris(2-aminoethyl) amine | Carboplatin | In vitro (CT-26) In vivo (CT-26) | Greater antiproliferative activity and tumor targeting than free drug [129] |
| Gene delivery | mPEG-DPA-PPZ | miR-200c | In vitro (A549/T) In vivo (A549/T) | Antiproliferative and pro-apoptotic activity [130] |
| Gene delivery | DPEA-mPEG-PPZ | pIL12 | In vitro (B6/CT-26) In vivo (CT-26) | Good tolerance, high internalization and tumor suppression [131] |
| Therapeutic Application | PPZ Derivative | Cargo | In Vitro/In Vivo Model | Main Finding and Ref. |
|---|---|---|---|---|
| Anticancer drugs | mPEG-PPZ | Pt (II) | In vivo (MKN-28) | Greater antitumor effect and lower cytotoxicity [31] |
| Anticancer drugs | Jeffamine-PPZ | Pt (IV) | In vitro (A2780/HCT116) In vivo (CT-26) | Enhanced cellular uptake and cytotoxicity; modest in vivo efficacy [141] |
| Anticancer drugs | Jeffamine-PPZ | Rhodium | In vivo (CT-26) | Extended survival and reduced local toxicity [62] |
| Anticancer drugs | mPEG-PPZ | Docetaxel | In vitro In vivo (MKN-28) | Tumor regression with minimal systemic toxicity [30] |
| Antibiotics | Highly branched PPZ | Dihydroartemisinin/ primaquine | In vivo (Plasmodium berghei-infected mice) | Sustained drug release for 35 days; greater antimalarial effect [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Gutierrez, S.; Mellid-Carballal, R.; Csaba, N.; Garcia-Fuentes, M. Polyphosphazene-Based Nanotherapeutics. J. Funct. Biomater. 2025, 16, 285. https://doi.org/10.3390/jfb16080285
Gutierrez-Gutierrez S, Mellid-Carballal R, Csaba N, Garcia-Fuentes M. Polyphosphazene-Based Nanotherapeutics. Journal of Functional Biomaterials. 2025; 16(8):285. https://doi.org/10.3390/jfb16080285
Chicago/Turabian StyleGutierrez-Gutierrez, Sara, Rocio Mellid-Carballal, Noemi Csaba, and Marcos Garcia-Fuentes. 2025. "Polyphosphazene-Based Nanotherapeutics" Journal of Functional Biomaterials 16, no. 8: 285. https://doi.org/10.3390/jfb16080285
APA StyleGutierrez-Gutierrez, S., Mellid-Carballal, R., Csaba, N., & Garcia-Fuentes, M. (2025). Polyphosphazene-Based Nanotherapeutics. Journal of Functional Biomaterials, 16(8), 285. https://doi.org/10.3390/jfb16080285







