Evaluation of Hydroxyapatite–β-Tricalcium Phosphate Collagen Composites for Socket Preservation in a Canine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
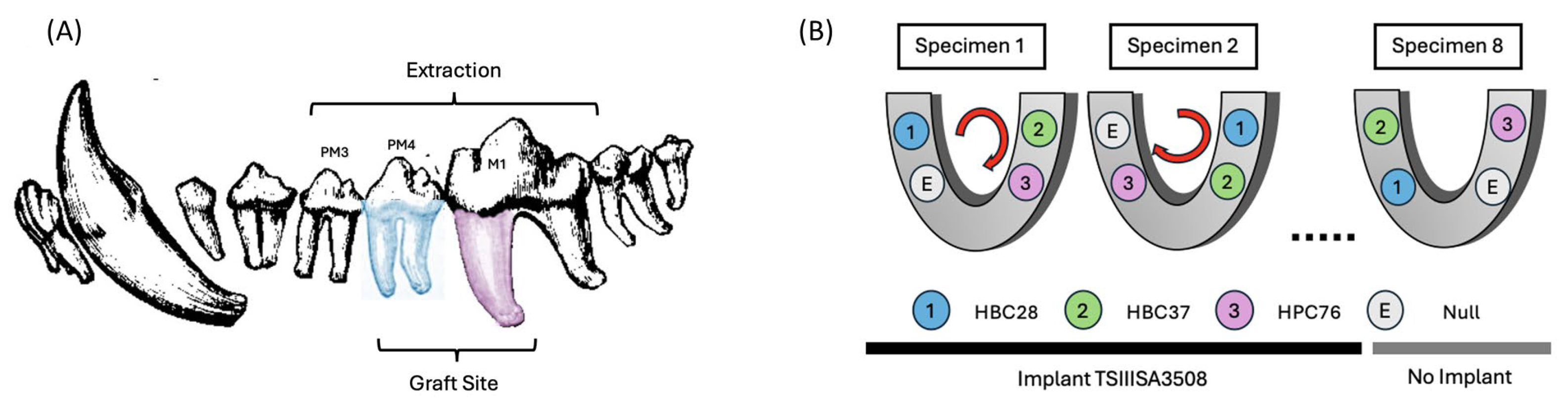
2.2. Experimental Design
2.3. Bone Substitutes
2.4. Surgical Procedures
2.5. Experimental Timeline and Evaluation Points
- 12 weeks postoperation:
- ○
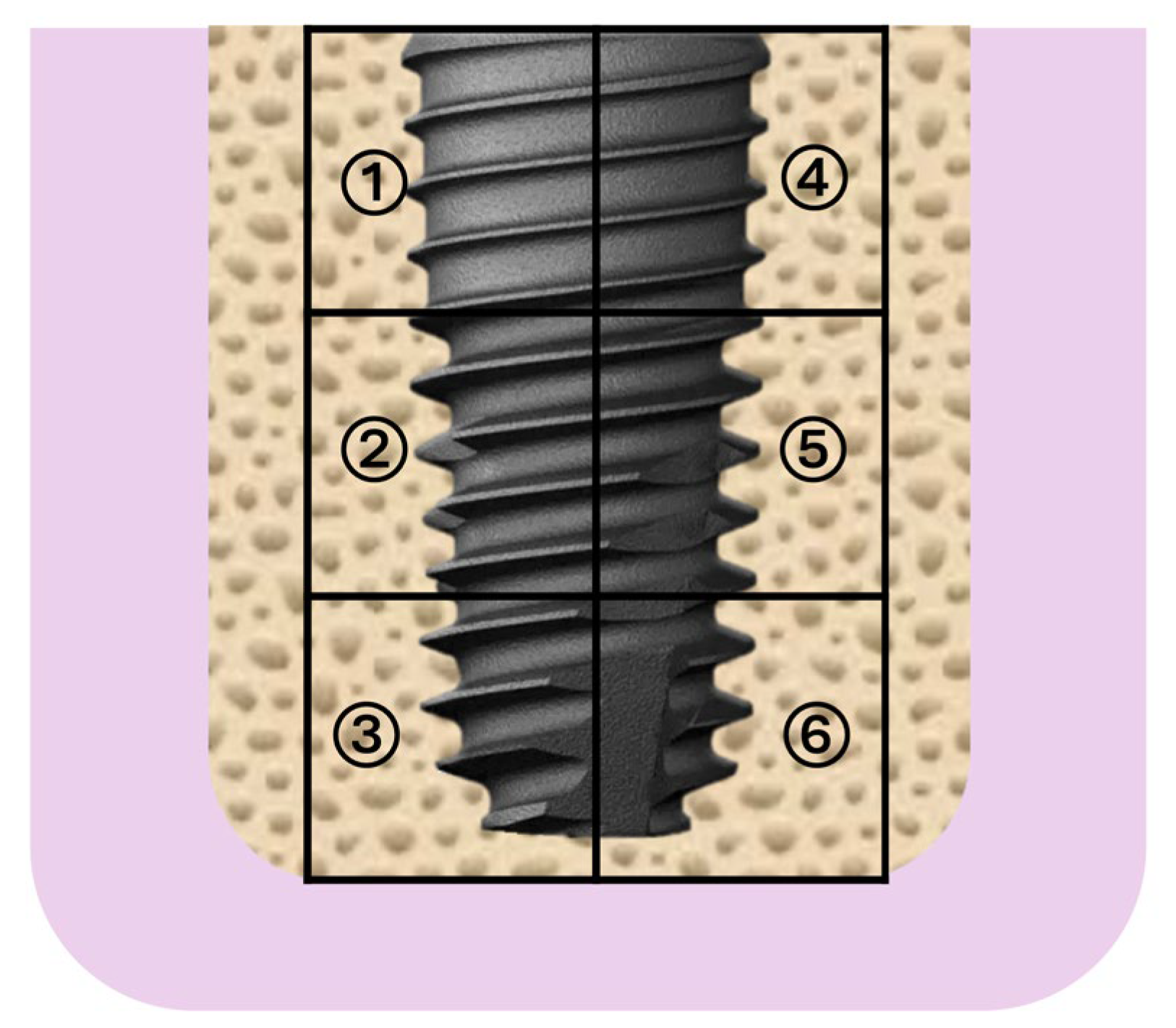
- Implant placement (TSIII SA implants (Ø3.5 mm × 8.0 mm, OSSTEM IMPLANT, Seoul, Republic of Korea).
- ○
- Measurement of drilling torque (DT) and insertion torque (IT) to assess primary stability and initial bone regeneration quality.
- 18 weeks post-operation:
- ○
- Measurement of removal torque (RT) to evaluate the strength of bone-to-implant integration (secondary stability).
- ○
- Implant stability quotient (ISQ) measurement using resonance frequency analysis (RFA) to objectively determine implant osseointegration and secondary stability.
- ○
- Euthanasia and tissue harvesting for histological and histomorphometric analysis.
2.6. Histological and Quantitative Analysis
2.7. Statistical Analysis
3. Results
3.1. Surgical Outcome and Clinical Observations
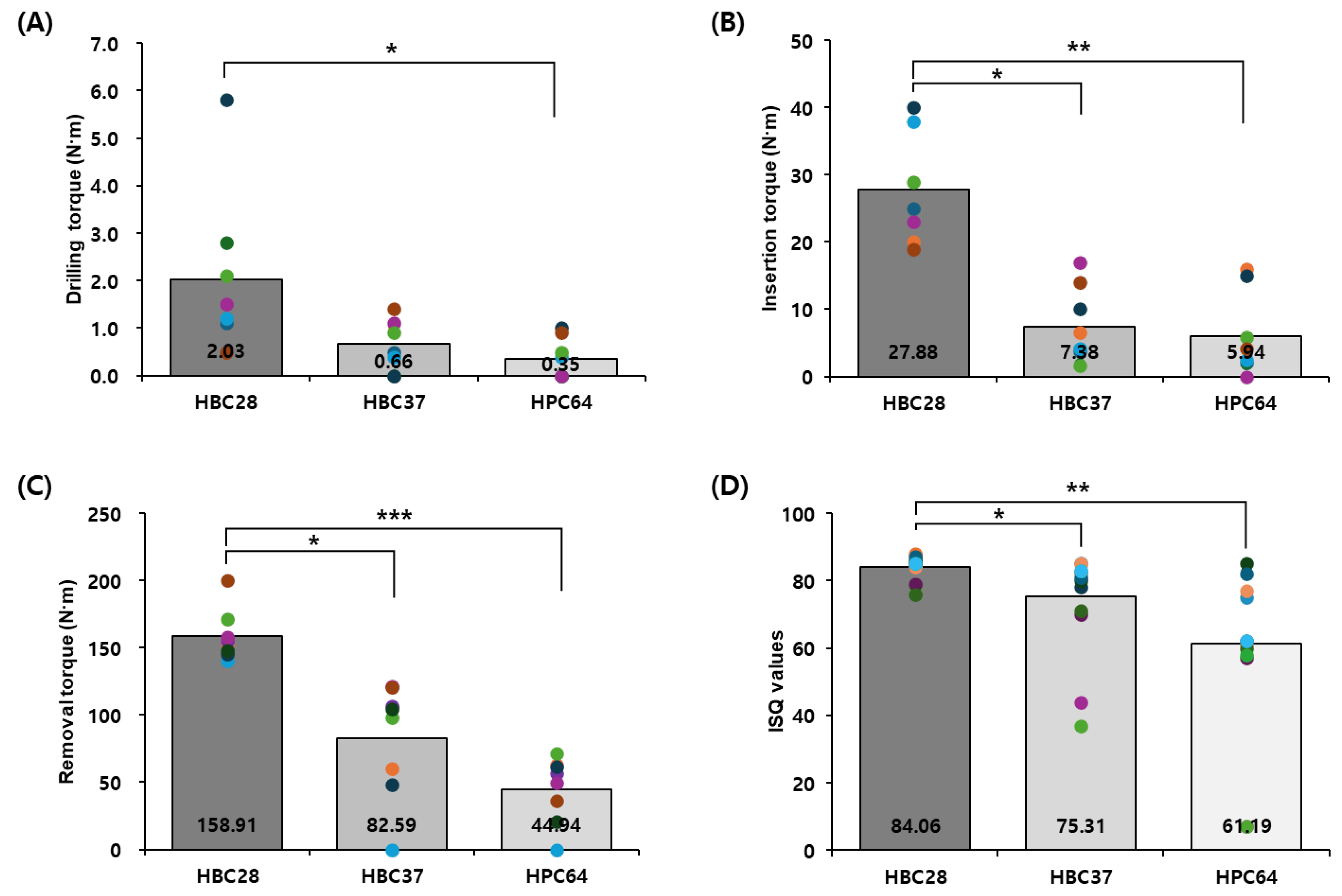
3.2. Mechanical Testing
3.3. Quantitative Histomorphometry
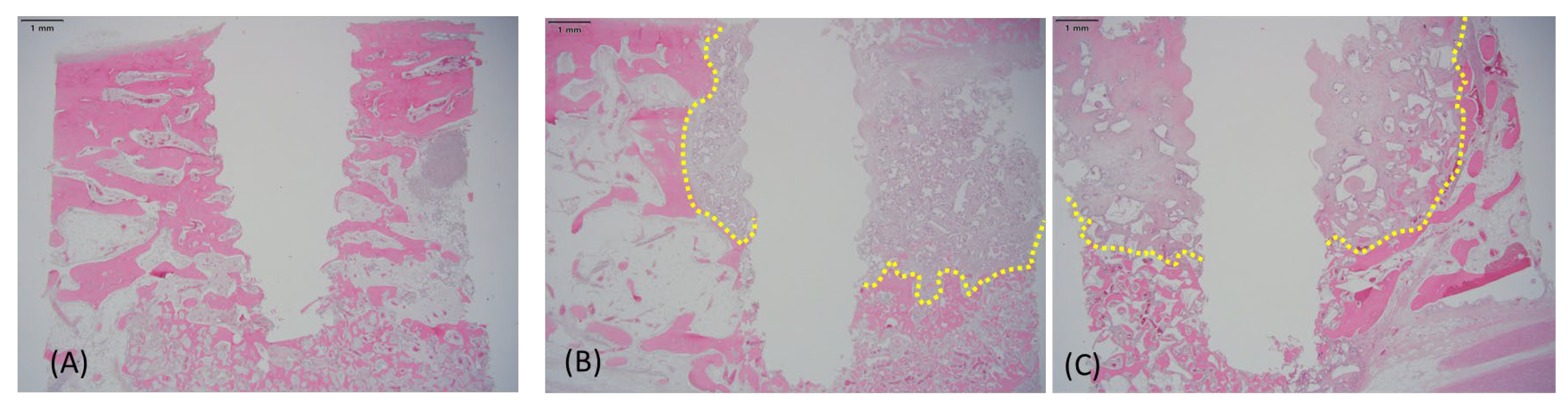
3.4. Histological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HA | Hydroxyapatite |
| β-TCP | Beta-Tricalcium Phosphate |
| BV/TV | Bone Volume to Total Volume |
| RGV/TV | Residual Graft Volume to Total Volume |
| ST/TV | Soft Tissue to Total Volume |
| BM/TV | Bone Marrow to Total Volume |
| ISQ | Implant Stability Quotient |
| DT | Drilling Torque |
| IT | Insertion Torque |
| RT | Removal Torque |
| ROI | Region of Interest |
References
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. Eur. J. Oral Sci. 1998, 106, 721–764. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E. Contemporary Implant Dentistry, 3rd ed.; Mosby Elsevier: St. Louis, MO, USA, 2008. [Google Scholar]
- Chiapasco, M.; Zaniboni, M.; Boisco, M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin. Oral Implants Res. 2006, 17 (Suppl. 2), 136–159. [Google Scholar] [CrossRef] [PubMed]
- Lekovic, V.; Camargo, P.M.; Klokkevold, P.R.; Weinlaender, M.; Kenney, E.B.; Dimitrijevic, B.; Nedic, M. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J. Periodontol. 1998, 69, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Darby, I. Periodontal materials. Aust. Dent. J. 2011, 56 (Suppl. 1), 107–118. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. 2), S96–S101. [Google Scholar] [CrossRef]
- Marx, R.E. Bone and bone graft healing. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 455–466. [Google Scholar] [CrossRef]
- Nkenke, E.; Schultze-Mosgau, S.; Radespiel-Tröger, M.; Kloss, F.; Neukam, F.W. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: A prospective study. Int. J. Oral Maxillofac. Surg. 2001, 30, 157–163. [Google Scholar] [CrossRef]
- Sbordone, L.; Bortolaia, C. Clinical outcomes of sinus augmentation with autogenous bone versus bone substitutes. Int. J. Oral Maxillofac. Implants 2006, 21, 943–950. [Google Scholar]
- Misch, C.M. Autogenous bone: Is it still the gold standard? Implant Dent. 2010, 19, 361. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, Y.C.; Wang, H.L.; Kao, C.Y. Prevention of bone resorption by HA/β-TCP collagen composite after tooth extraction: A case series. Int. J. Environ. Res. Public Health 2019, 16, 4616. [Google Scholar] [CrossRef] [PubMed]
- Pires, F.R.; Silva, G.A.B.; de Almeida, R.A.B.; Silva, D.N.; Guerra, E.N.S. Use of biomaterials in alveolar ridge preservation: A systematic review. J. Prosthodont. 2020, 29, 599–606. [Google Scholar]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Horch, H.H.; Sader, R.; Pautke, C.; Neff, A.; Deppe, H.; Kolk, A. Synthetic, pure-phase beta-tricalcium phosphate ceramic granules (Cerasorb®) for bone regeneration in the reconstructive surgery of the jaws. Int. J. Oral Maxillofac. Surg. 2006, 35, 708–713. [Google Scholar] [CrossRef]
- Jensen, S.S.; Broggini, N.; Hjorting-Hansen, E.; Schenk, R.; Buser, D. Bone healing and graft resorption of autograft, anorganic bovine bone, and beta-tricalcium phosphate: A histologic and histomorphometric study in the mandibles of minipigs. Clin. Oral Implants Res. 2006, 17, 237–243. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F.; et al. Influence of β-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010, 6, 4476–4487. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y.F. Osteoinduction: A review of old concepts with new standards. J. Dent. Res. 2012, 91, 736–744. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, S.G. Membranes for periodontal regeneration: A review. Dent. Clin. N. Am. 2017, 61, 467–487. [Google Scholar]
- Wang, H.L.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Pabst, A.M.; Happe, A.; Callaway, A.; Ziebart, T.; Stratul, S.I.; Ackermann, M.; Willershausen, B.; Kasaj, A. In vitro and in vivo characterization of porcine acellular dermal matrix for gingival augmentation procedures. J. Periodontal Res. 2014, 49, 371–381. [Google Scholar] [CrossRef]
- Kim, J.W.; Jung, I.H.; Lee, K.I.; Jung, U.W.; Kim, C.S.; Choi, S.H.; Cho, K.S. Volumetric bone regenerative efficacy of biphasic calcium phosphate-collagen composite block. Sci. Rep. 2019, 9, 5300. [Google Scholar]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. 3), S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Fukuba, S.; Okada, M.; Nohara, K.; Iwata, T. Alloplastic Bone Substitutes for Periodontal and Bone Regeneration in Dentistry: Current Status and Prospects. Materials 2021, 14, 1096. [Google Scholar] [CrossRef] [PubMed]
- Maté-Sánchez de Val, J.E.; Figueiredo, R.; Gómez, C.M.; Fernández, V.; Muñoz, F.; Mareque-Bueno, S. Behavior of three different bone substitute materials in bone regeneration: An experimental study in the rabbit calvaria. Clin. Oral Implants Res. 2014, 25, e145–e153. [Google Scholar]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D.V. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef]
- Horváth, A.; Mardas, N.; Mezzomo, L.A.; Needleman, I.G.; Donos, N. Alveolar ridge preservation: A systematic review. Clin. Oral Investig. 2013, 17, 341–363. [Google Scholar] [CrossRef]
- Silva, R.V.; Camilli, J.A.; Bertran, C.A.; Moreira, N.H. The use of autogenous bone grafts versus bone substitutes in sinus augmentation: A systematic review and meta-analysis. Implant Dent. 2017, 26, 715–726. [Google Scholar]
- Traini, T.; Degidi, M.; Sammons, R.L.; Piattelli, A. Histologic and ultrastructural analysis of anorganic bovine bone (Bio-Oss®) used in sinus augmentation procedures in humans. J. Periodontol. 2008, 79, 123–130. [Google Scholar]
- Zerbo, I.R.; Bronckers, A.L.; de Lange, G.L.; van Beek, G.J.; Burger, E.H. Histology of human alveolar bone regeneration with a porous tricalcium phosphate: Report of two cases. Clin. Oral Implants Res. 2001, 12, 379–384. [Google Scholar] [CrossRef]
- Sbordone, L.; Toti, P.; Menchini-Fabris, G.B.; Guidetti, M.; Califano, L. Long-term evaluation of the use of anorganic bovine bone in sinus augmentation. J. Craniofac. Surg. 2015, 26, 1391–1396. [Google Scholar]
- Kim, Y.K.; Yun, P.Y.; Lim, S.C.; Kim, S.G.; Lee, H.J.; Ong, J.L. Clinical evaluations of OSTEON® as a new alloplastic material in sinus bone grafting and its effect on bone healing. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 270–277. [Google Scholar] [CrossRef]

| Material | HBC28 | HBC37 | HPC64 |
|---|---|---|---|
| HA 1 (%) | 20 | 30 | 60 |
| β-TCP 1 (%) | 80 | 70 | 40 |
| Collagen Source 1 (%) | Bovine type 1 (10%) | Bovine type 1 (4–6%) | Porcine type 1 (6%) |
| Additional Features | Micron-sized crystal | Porosity 70% | Porosity 80% |
| Microcrystal Structure 2 |  |  |  |
| Collagen Attachment to Graft Material 2 |  |  |  |
| Material | DT (N) 1 | IT (N) 1 | RT (N) 2 | ISQ 2 | p-Value vs. HBC28 (DT) | p-Value vs. HBC28 (IT) | p-Value vs. HBC28 (RT) | p-Value vs. HBC28 (ISQ) |
|---|---|---|---|---|---|---|---|---|
| HBC28 | 2.03 ± 1.6 | 27.88 ± 7.3 | 158.91 ± 17.8 | 84.06 ± 3.3 | — | — | — | — |
| HBC37 | 0.66 ± 0.5 | 7.38 ± 5.4 | 82.59 ± 40.0 | 75.31 ± 13.9 | 0.1054 | 0.001 | 0.0004 | 0.016 |
| HPC64 | 0.35 ± 0.4 | 5.94 ± 5.8 | 44.94 ± 22.8 | 61.19 ± 22.7 | 0.0181 | 0.00027 | <0.00001 | 0.0009 |
| Material | BV/TV (%) | RGV/TV (%) | ST/TV (%) | BM/TV (%) |
|---|---|---|---|---|
| HBC28 | 51.23 ± 12.9 | 6.83 ± 6.3 | 4.08 ± 5.1 | 37.86 ± 11.6 |
| HBC37 | 22.91 ± 9.2 | 21.95 ± 6.4 | 47.55 ± 14.7 | 7.60 ± 8.6 |
| HPC64 | 12.47 ± 8.2 | 35.27 ± 8.3 | 49.07 ± 10.4 | 3.19 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.W.; Lee, D.; Ryu, J.; Kook, M.-S.; Park, H.-J.; Jung, S. Evaluation of Hydroxyapatite–β-Tricalcium Phosphate Collagen Composites for Socket Preservation in a Canine Model. J. Funct. Biomater. 2025, 16, 286. https://doi.org/10.3390/jfb16080286
Kim DW, Lee D, Ryu J, Kook M-S, Park H-J, Jung S. Evaluation of Hydroxyapatite–β-Tricalcium Phosphate Collagen Composites for Socket Preservation in a Canine Model. Journal of Functional Biomaterials. 2025; 16(8):286. https://doi.org/10.3390/jfb16080286
Chicago/Turabian StyleKim, Dong Woo, Donghyun Lee, Jaeyoung Ryu, Min-Suk Kook, Hong-Ju Park, and Seunggon Jung. 2025. "Evaluation of Hydroxyapatite–β-Tricalcium Phosphate Collagen Composites for Socket Preservation in a Canine Model" Journal of Functional Biomaterials 16, no. 8: 286. https://doi.org/10.3390/jfb16080286
APA StyleKim, D. W., Lee, D., Ryu, J., Kook, M.-S., Park, H.-J., & Jung, S. (2025). Evaluation of Hydroxyapatite–β-Tricalcium Phosphate Collagen Composites for Socket Preservation in a Canine Model. Journal of Functional Biomaterials, 16(8), 286. https://doi.org/10.3390/jfb16080286






