The Influence of Insertion Torque on Stress Distribution in Peri-Implant Bones Around Ultra-Short Implants: An FEA Study
Abstract
1. Introduction
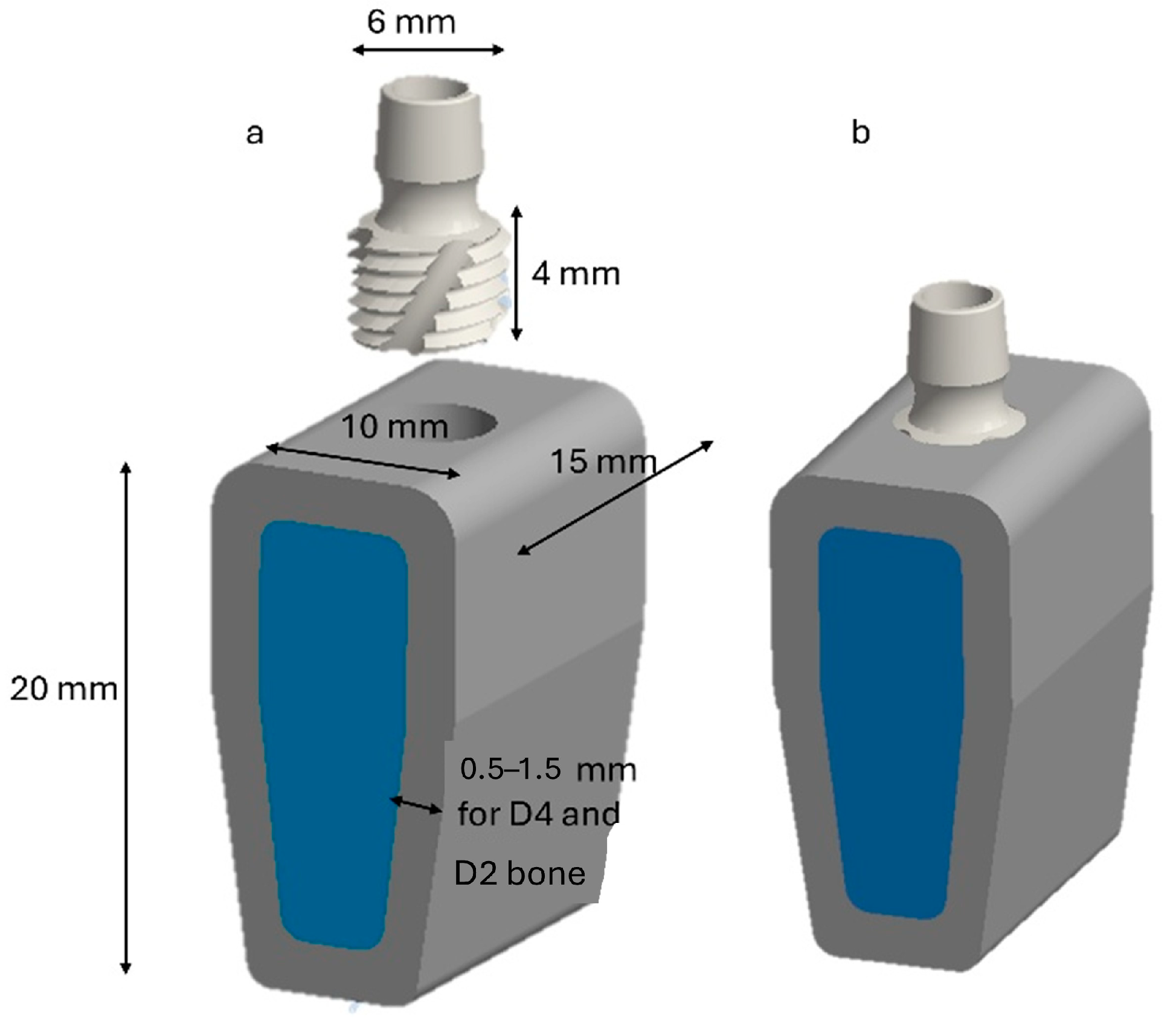
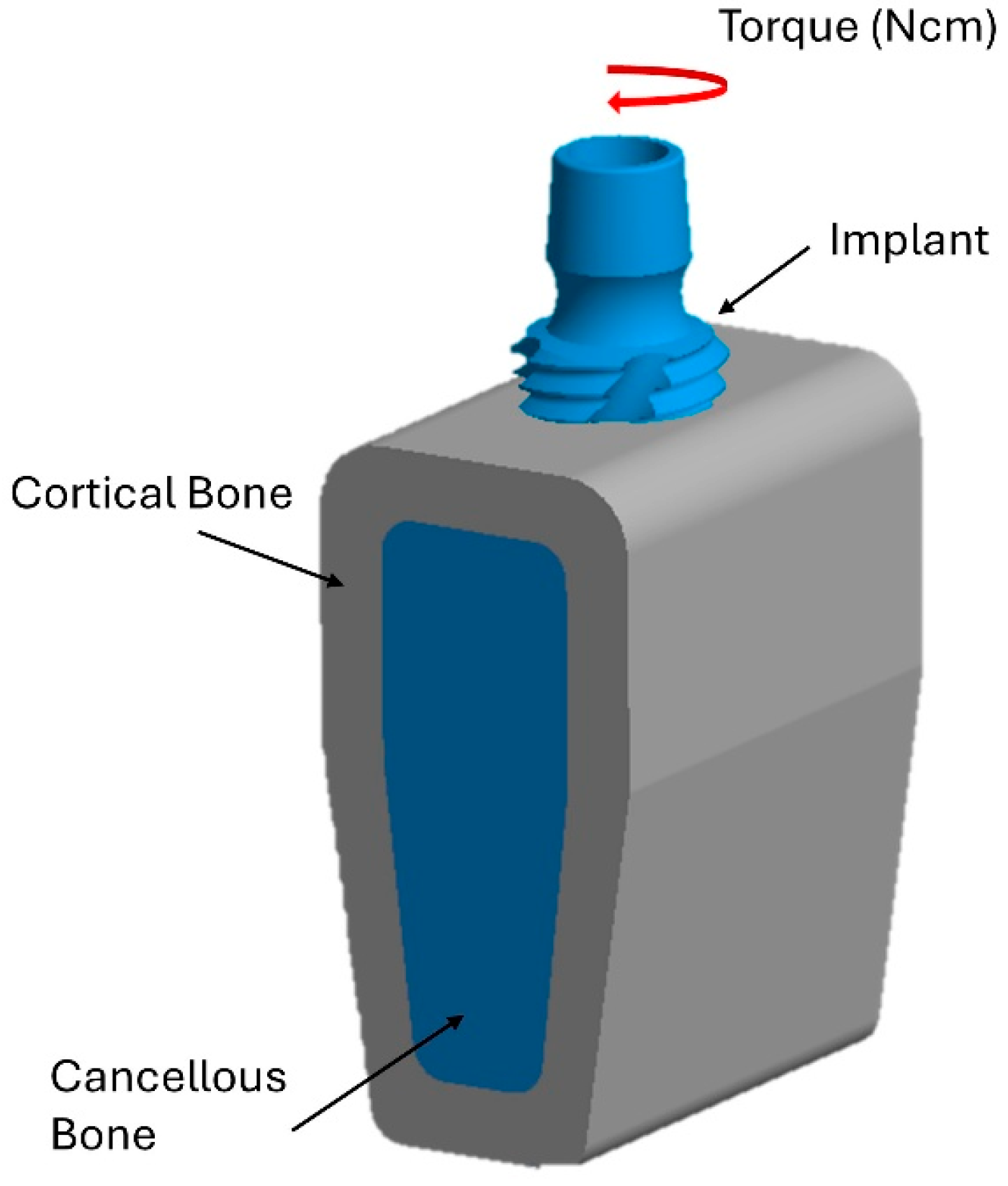
2. Materials and Methods
2.1. Boundary and Load Conditions
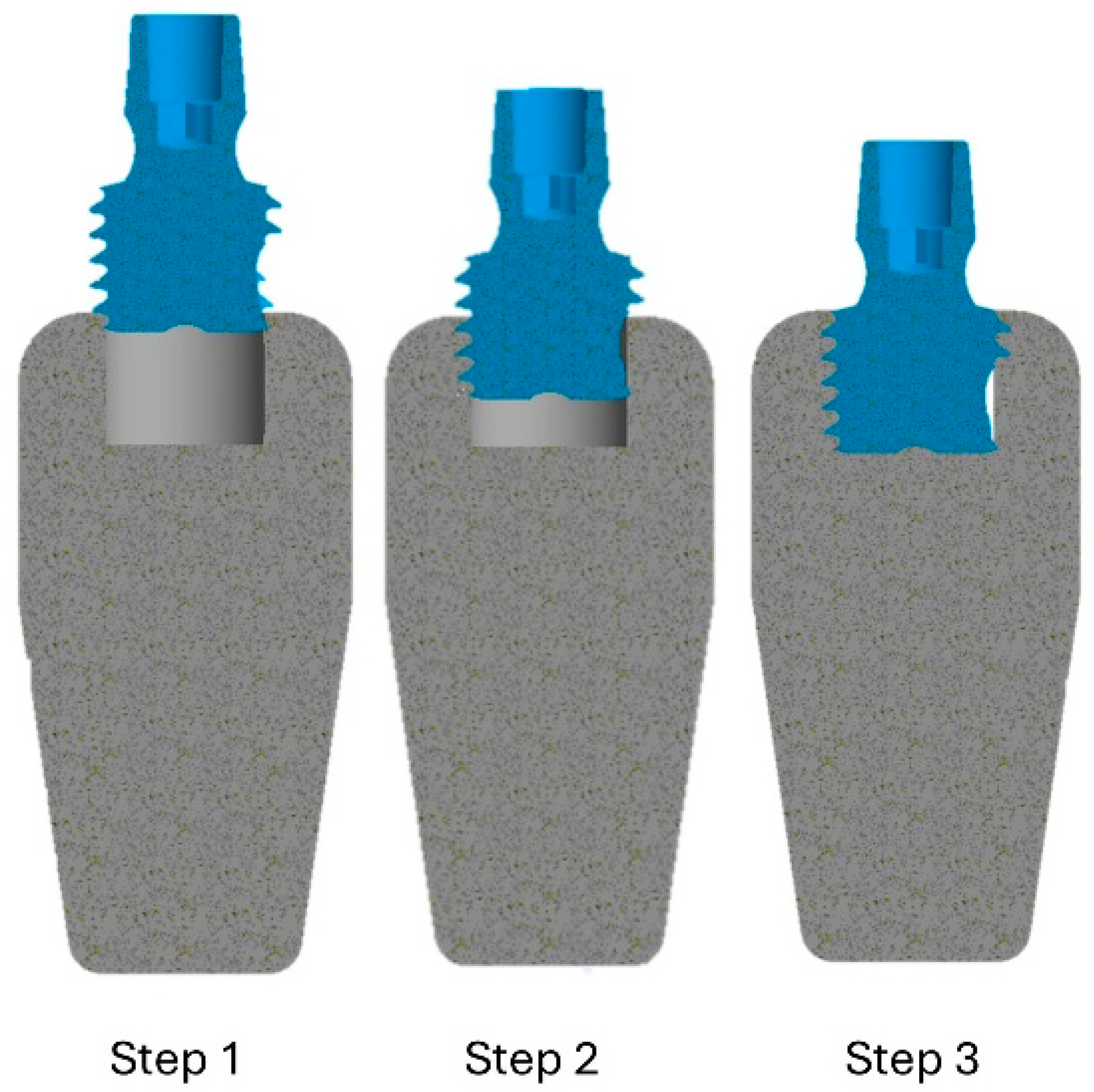
2.2. Simulation of the Insertion Technique
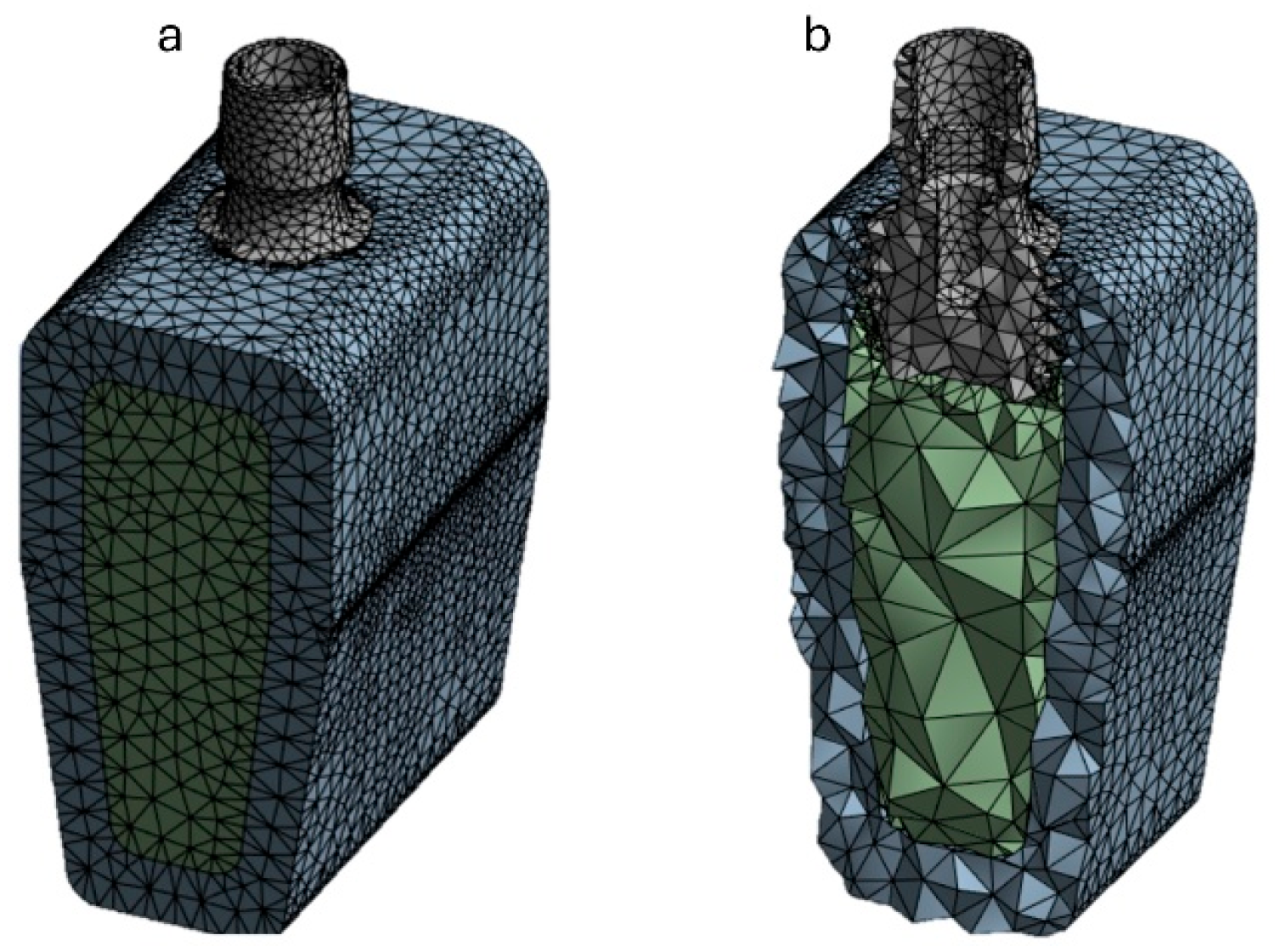
2.3. FEA Modeling
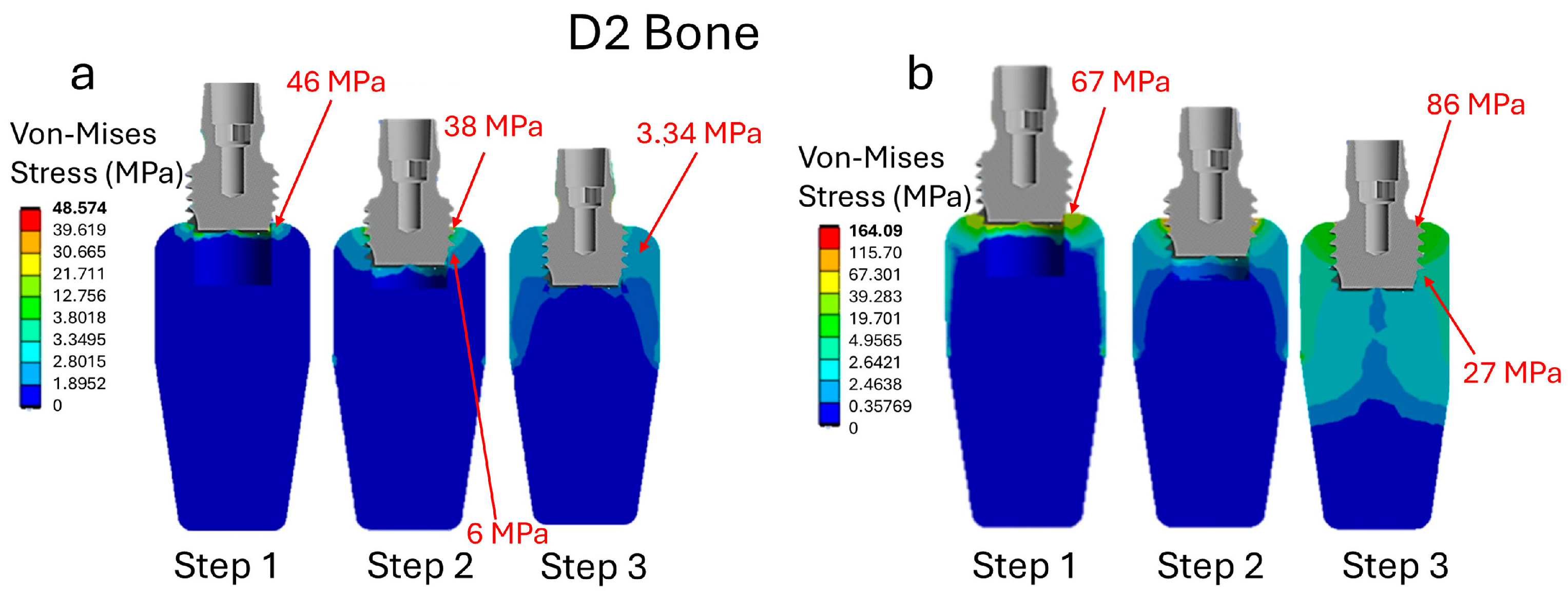
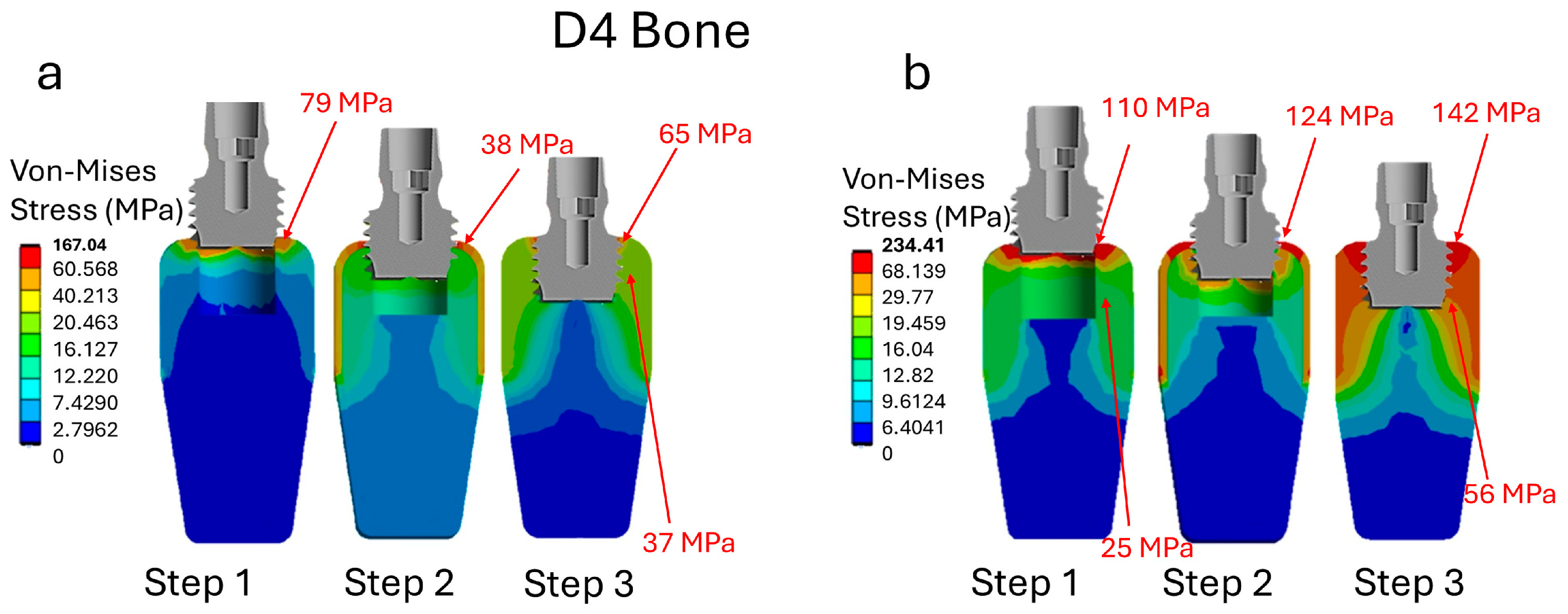
3. Results
3.1. Analysis of Stress in Bone D2
3.2. Analysis of Stress in Bone D4
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, edentulism, and risk of mortality: A systematic review with meta-analyses. J. Dent. Res. 2021, 100, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Coşgunarslan, A.; Soydan Çabuk, D.; Canger, E.M. Effect of total edentulism on the internal bone structure of mandibular condyle: A preliminary study. Oral Radiol. 2021, 37, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Stanbouly, D.; Zeng, Q.; Jou, Y.T.; Chuang, S.K. Edentulism (missing teeth) and brain central nervous system (CNS) deafferentation: A narrative review. Front. Oral Maxillofac. Med. 2024, 6, 8. [Google Scholar] [CrossRef]
- Emfietzoglou, R.; Dereka, X. Survival rates of short dental implants (≤6 mm) used as an alternative to longer (>6 mm) implants for the rehabilitation of posterior partial edentulism: A systematic review of RCTs. Dent. J. 2024, 12, 185. [Google Scholar] [CrossRef]
- Robert, L.; Aloy-Prósper, A.; Arias-Herrera, S. Vertical augmentation of the atrofic posterior mandibular ridges with onlay grafts: Intraoral blocks vs. guided bone regeneration. Systematic review. J. Clin. Exp. Dent. 2023, 15, e357. [Google Scholar] [CrossRef]
- Ewers, R.; Marincola, M.; Perpetuini, P.; Morina, A.; Bergamo, E.T.; Cheng, Y.C.; Bonfante, E.A. Severely atrophic mandibles restored with fiber-reinforced composite prostheses supported by 5.0-mm ultra-short implants present high survival rates up to eight years. J. Oral Maxillofac. Surg. 2022, 80, 81–92. [Google Scholar] [CrossRef]
- Yang, Q.; Guan, X.; Wang, B.; Zhang, D.; Bai, J.; Zhang, X.; Zhou, Y. Implant survival rate and marginal bone loss with the all-on-4 immediate-loading strategy: A clinical retrospective study with 1 to 4 years of follow-up. J. Prosthet. Dent. 2023, 130, 849–857. [Google Scholar] [CrossRef]
- Zhang, Y.; Gulati, K.; Li, Z.; Di, P.; Liu, Y. Dental implant nano-engineering: Advances, limitations and future directions. Nanomaterials 2021, 11, 2489. [Google Scholar] [CrossRef]
- Taghizadeh, E.; Negargar, S.; Larki, K.N.; Haghighi, R.S.; Shahoon, H. The Role of Guided Bone Regeneration in Enhancing Dental Implant Success: Guided Bone Regeneration in Dental Implant. Galen Med. J. 2024, 13, e3681. [Google Scholar] [CrossRef]
- Bizzi, I.H.; Menezes da Silveira, T.; Bitencourt, F.V.; Gomes Muniz, F.W.M.; Cavagni, J.; Fiorini, T. Primary stability of immediate implants placed in fresh sockets in comparison with healed sites: A systematic review and meta-analysis. Int. J. Oral Implantol. 2025, 18, 33. [Google Scholar]
- Abiram Bala, M.D.; Maloney, W.J. Cementless Acetabular Revision for Severe Acetabular Bone Loss without Pelvic Discontinuity (Type IIIA and B Defects). In Treatment of Acetabular Bone Loss and Chronic Pelvic Discontinuity-E-Book; Elsevier: Amsterdam, The Netherlands, 2023; p. 45. [Google Scholar]
- Sakdejayont, S.; Chobpenthai, T.; Suksirivecharuk, P.; Ninatkiattikul, I.F.; Poosiripinyo, T. A Review on Bone Tumor Management: Cutting-Edge Strategies in Bone Grafting, Bone Graft Substitute, and Growth Factors for Defect Reconstruction. Orthop. Res. Rev. 2025, 17, 175–188. [Google Scholar] [CrossRef]
- Laubach, M.; Hildebrand, F.; Suresh, S.; Wagels, M.; Kobbe, P.; Gilbert, F.; Kneser, U.; Holzapfel, B.M.; Hutmacher, D.W. The concept of scaffold-guided bone regeneration for the treatment of long bone defects: Current clinical application and future perspective. J. Funct. Biomater. 2023, 14, 341. [Google Scholar] [CrossRef]
- Ferraz, M.P. Bone grafts in dental medicine: An overview of autografts, allografts and synthetic materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef]
- Inchingolo, F.; Hazballa, D.; Inchingolo, A.D.; Malcangi, G.; Marinelli, G.; Mancini, A.; Maggiore, M.E.; Bordea, I.R.; Scarano, A.; Farronato, M.; et al. Innovative concepts and recent breakthrough for engineered graft and constructs for bone regeneration: A literature systematic review. Materials 2022, 15, 1120. [Google Scholar] [CrossRef]
- Tomasetti, B.J.; Ewers, R. (Eds.) Short Implants; No. 180175; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Esposito, M.; Barausse, C.; Pistilli, R.; Sammartino, G.; Grandi, G.; Felice, P. Short implants versus bone augmentation for placing longer implants in atrophic maxillae: One-year post-loading results of a pilot randomised controlled trial. Eur. J. Oral Implantol. 2015, 8, 257–268. [Google Scholar]
- Berzaghi, A.; Testori, T.; Scaini, R.; Bortolini, S. Occlusion and Biomechanical Risk Factors in Implant-Supported Full-Arch Fixed Dental Prostheses—Narrative Review. J. Pers. Med. 2025, 15, 65. [Google Scholar] [CrossRef]
- D’Amico, C.; Bocchieri, S.; Sambataro, S.; Surace, G.; Stumpo, C.; Fiorillo, L. Occlusal load considerations in implant-supported fixed restorations. Prosthesis 2020, 2, 252–265. [Google Scholar] [CrossRef]
- Cannizzaro, G.; Felice, P.; Leone, M.; Viola, P.; Esposito, M. Early loading of implants in the atrophic posterior maxilla: Lateral sinus lift with autogenous bone and Bio-Oss versus crestal mini sinus lift and 8-mm hydroxyapatite-coated implants. A randomised controlled clinical trial. Eur. J. Oral Implantol. 2009, 2, 25–38. [Google Scholar]
- Felice, P.; Checchi, V.; Pistilli, R.; Scarano, A.; Pellegrino, G.; Esposito, M. Bone augmentation versus 5-mm dental implants in posterior atrophic jaws. Four-month post-loading results from a randomised controlled clinical trial. Eur. J. Oral Implantol. 2009, 2, 267–281. [Google Scholar]
- Dias, F.D.N.; Pecorari, V.G.A.; Martins, C.B.; Del Fabbro, M.; Casati, M.Z. Short implants versus bone augmentation in combination with standard-length implants in posterior atrophic partially edentulous mandibles: Systematic review and meta-analysis with the Bayesian approach. Int. J. Oral Maxillofac. Surg. 2019, 48, 90–96. [Google Scholar] [CrossRef]
- Felice, P.; Cannizzaro, G.; Barausse, C.; Pistilli, R.; Esposito, M. Short implants versus longer implants in vertically augmented posterior mandibles: A randomised controlled trial with 5-year after loading follow-up. Eur. J. Oral Implantol. 2014, 7, 359–369. [Google Scholar]
- Al-Johany, S.S.; Al Amri, M.D.; Alsaeed, S.; Alalola, B. Dental implant length and diameter: A proposed classification scheme. J. Prosthodont. 2017, 26, 252–260. [Google Scholar] [CrossRef]
- Lombardo, G.; Signoriello, A.; Marincola, M.; Liboni, P.; Faccioni, P.; Zangani, A.; D’agostino, A.; Nocini, P.F. Short and ultra-short implants, in association with simultaneous internal sinus lift in the atrophic posterior maxilla: A five-year retrospective study. Materials 2022, 15, 7995. [Google Scholar] [CrossRef]
- Felice, P.; Barausse, C.; Pistilli, R.; Buti, J.; Gessaroli, M.; Esposito, M. Short Implants Versus Bone Augmentation and Longer Implants in Atrophic Maxillae. Five Year Post Loading Results of a Randomised Controlled Trial. Clin. Trials Dent. 2020, 2, 35–48. [Google Scholar] [CrossRef]
- Faegh, S.; Müftü, S. Load transfer along the bone–dental implant interface. J. Biomech. 2010, 43, 1761–1770. [Google Scholar] [CrossRef]
- Lee, C.H.; Mukundan, A.; Chang, S.C.; Wang, Y.L.; Lu, S.H.; Huang, Y.C.; Wang, H.C. Comparative analysis of stress and deformation between one-fenced and three-fenced dental implants using finite element analysis. J. Clin. Med. 2021, 10, 3986. [Google Scholar] [CrossRef]
- Nimbalkar, S.; Dhatrak, P.; Gherde, C.; Joshi, S. A review article on factors affecting bone loss in dental implants. Mater. Today Proc. 2021, 43, 970–976. [Google Scholar] [CrossRef]
- Lan, T.H.; Du, J.K.; Pan, C.Y.; Lee, H.E.; Chung, W.H. Biomechanical analysis of alveolar bone stress around implants with different thread designs and pitches in the mandibular molar area. Clin. Oral Investig. 2012, 16, 363–369. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, M.; Yeo, I.S.L. Three interfaces of the dental implant system and their clinical effects on hard and soft tissues. Mater. Horiz. 2022, 9, 1387–1411. [Google Scholar] [CrossRef]
- Falcinelli, C.; Valente, F.; Vasta, M.; Traini, T. Finite element analysis in implant dentistry: State of the art and future directions. Dent. Mater. 2023, 39, 539–556. [Google Scholar] [CrossRef]
- Anitua, E.; Larrazabal Saez de Ibarra, N.; Morales Martín, I.; Saracho Rotaeche, L. Influence of dental implant diameter and bone quality on the biomechanics of single-crown restoration. A finite element analysis. Dent. J. 2021, 9, 103. [Google Scholar] [CrossRef]
- Chen, Z.H.; Wu, J.J.; Guo, D.Y.; Li, Y.Y.; Chen, M.N.; Zhang, Z.Y.; Yuan, Z.D.; Zhang, K.W.; Chen, W.W.; Tian, F.; et al. Physiological functions of podosomes: From structure and function to therapy implications in osteoclast biology of bone resorption. Ageing Res. Rev. 2023, 85, 101842. [Google Scholar] [CrossRef]
- Hsueh, P.Y.; Yamaguchi, Y.; Yajima, Y. Effect of insertion load on insertion torque value. J. Dent. Sci. 2025, 20, 1861–1868. [Google Scholar] [CrossRef]
- Amari, Y.; Piattelli, A.; Apaza Alccayhuaman, K.A.; Mesa, N.F.; Ferri, M.; Iezzi, G.; Botticelli, D. Bone healing at non-submerged implants installed with different insertion torques: A split-mouth histomorphometric randomized controlled trial. Int. J. Implant Dent. 2019, 5, 39. [Google Scholar] [CrossRef]
- Trisi, P.; De Benedittis, S.; Perfetti, G.; Berardi, D. Primary stability, insertion torque and bone density of cylindric implant ad modum Branemark: Is there a relationship? An in vitro study. Clin. Oral Implant. Res. 2011, 22, 567–570. [Google Scholar] [CrossRef]
- Alfonsi, F.; Borgia, V.; Barbato, L.; Tonelli, P.; Giammarinaro, E.; Marconcini, S.; Romeggio, S.; Barone, A. The clinical effects of insertion torque for implants placed in healed ridges: A two-year randomized controlled clinical trial. J. Oral Sci. Rehabil. 2016, 2, 62–73. [Google Scholar]
- Ruppin, J.M.; Stimmelmayr, M. High insertion torque versus regular insertion torque: Early crestal bone changes on dental implants in relation to primary stability—A retrospective clinical study. Int. J. Implant Dent. 2024, 10, 22. [Google Scholar] [CrossRef]
- Beer, A.; Gahleitner, A.; Holm, A.; Tschabitscher, M.; Homolka, P. Correlation of insertion torques with bone mineral density from dental quantitative CT in the mandible. Clin. Oral Implant. Res. 2003, 14, 616–620. [Google Scholar] [CrossRef]
- Lemos, C.A.; Verri, F.R.; de Oliveira Neto, O.B.; Cruz, R.S.; Gomes, J.M.L.; da Silva Casado, B.G.; Pellizzer, E.P. Clinical effect of the high insertion torque on dental implants: A systematic review and meta-analysis. J. Prosthet. Dent. 2021, 126, 490–496. [Google Scholar] [CrossRef]
- Van Staden, R.C.; Guan, H.; Loo, Y.C. Application of the finite element method in dental implant research. Comput. Methods Biomech. Biomed. Eng. 2006, 9, 257–270. [Google Scholar] [CrossRef]
- Rubo, J.H.; Capello Souza, E.A. Finite-element analysis of stress on dental implant prosthesis. Clin. Implant Dent. Relat. Res. 2010, 12, 105–113. [Google Scholar] [CrossRef]
- Callea, C.; Ceddia, M.; Piattelli, A.; Specchiulli, A.; Trentadue, B. Finite element analysis (FEA) for a different type of cono-in dental implant. Appl. Sci. 2023, 13, 5313. [Google Scholar] [CrossRef]
- DeTolla, D.H.; Andreana, S.; Patra, A.; Buhite, R.; Comella, B. The role of the finite element model in dental implants. J. Oral Implantol. 2000, 26, 77–81. [Google Scholar] [CrossRef]
- Kayabaşı, O.; Yüzbasıoğlu, E.; Erzincanlı, F. Static, dynamic and fatigue behaviors of dental implant using finite element method. Adv. Eng. Softw. 2006, 37, 649–658. [Google Scholar] [CrossRef]
- Holmgren, E.P.; Seckinger, R.J.; Kilgren, L.M.; Mante, F. Evaluating Parameters of osseointegrated dental implants using finite element analysis a two-dimensional comparative study examining the effects of implant diameter, implant shape, and load direction. J. Oral Implantol. 1998, 24, 80–88. [Google Scholar] [CrossRef]
- Qiu, P.; Cao, R.; Li, Z.; Fan, Z. A comprehensive biomechanical evaluation of length and diameter of dental implants using finite element analyses: A systematic review. Heliyon 2024, 10, e26876. [Google Scholar] [CrossRef]
- Hosseini-Faradonbeh, S.A.; Katoozian, H.R. Biomechanical evaluations of the long-term stability of dental implant using finite element modeling method: a systematic review. J. Adv. Prosthodont. 2022, 14, 182. [Google Scholar] [CrossRef]
- Büyük, F.N.; Savran, E.; Karpat, F. Review on finite element analysis of dental implants. J. Dent. Implant Res. 2022, 41, 50–63. [Google Scholar] [CrossRef]
- Hasan, I.; Heinemann, F.; Aitlahrach, M.; Bourauel, C. Biomechanical finite element analysis of small diameter and short dental implant. Biomed. Eng./Biomed. Tech. 2010, 55, 341–350. [Google Scholar] [CrossRef]
- Li, R.; Wu, Z.; Chen, S.; Li, X.; Wan, Q.; Xie, G.; Pei, X. Biomechanical behavior analysis of four types of short implants with different placement depths using the finite element method. J. Prosthet. Dent. 2023, 129, 447.e1–447.e10. [Google Scholar] [CrossRef]
- Alqahtani, A.R.; Desai, S.R.; Patel, J.R.; Alqhtani, N.R.; Alqahtani, A.S.; Heboyan, A.; Fernandes, G.V.O.; Mustafa, M.; Karobari, M.I. Investigating the impact of diameters and thread designs on the Biomechanics of short implants placed in D4 bone: A 3D finite element analysis. BMC Oral Health 2023, 23, 686. [Google Scholar] [CrossRef]
- Desai, S.R.; Desai, M.S.; Katti, G.; Karthikeyan, I. Evaluation of design parameters of eight dental implant designs: A two-dimensional finite element analysis. Niger. J. Clin. Pract. 2012, 15, 176–181. [Google Scholar] [CrossRef]
- Misch, C.E. Bone density: A key determinant for treatment planning. Contemp. Implant Dent. 2007, 3, 2008–2130. [Google Scholar]
- Pammer, D. Evaluation of postoperative dental implant primary stability using 3D finite element analysis. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 280–287. [Google Scholar] [CrossRef]
- Alemayehu, D.B.; Jeng, Y.R. Three-dimensional finite element investigation into effects of implant thread design and loading rate on stress distribution in dental implants and anisotropic bone. Materials 2021, 14, 6974. [Google Scholar] [CrossRef]
- Reznikov, N.; Liang, H.; McKee, M.D.; Piché, N. Mapping of trabecular bone anisotropy and volume fraction in 3D using μCT images of the human calcaneus. Am. J. Biol. Anthropol. 2022, 177, 566–580. [Google Scholar] [CrossRef]
- Taheri, R.A.; Jarrahi, A.; Farnoosh, G.; Karimi, A. A comparative finite element simulation of stress in dental implant–bone interface using isotropic and orthotropic material models in three mastication cycles. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 489. [Google Scholar] [CrossRef]
- Gasik, M.; Lambert, F.; Bacevic, M. Biomechanical properties of bone and mucosa for design and application of dental implants. Materials 2021, 14, 2845. [Google Scholar] [CrossRef]
- Nobakhti, S.; Shefelbine, S.J. On the relation of bone mineral density and the elastic modulus in healthy and pathologic bone. Curr. Osteoporos. Rep. 2018, 16, 404–410. [Google Scholar] [CrossRef]
- Helgason, B.; Perilli, E.; Schileo, E.; Taddei, F.; Brynjólfsson, S.; Viceconti, M. Mathematical relationships between bone density and mechanical properties: A literature review. Clin. Biomech. 2008, 23, 135–146. [Google Scholar] [CrossRef]
- Ceddia, M.; Lamberti, L.; Trentadue, B. FEA comparison of the mechanical behavior of three dental crown materials: Enamel, ceramic, and zirconia. Materials 2024, 17, 673. [Google Scholar] [CrossRef]
- Nikam, N.; Shenoy, B.S.; KN, C.; Keni, L.G.; Shetty, S.; Bhat, N.S. Advancements in Surface Coatings for Enhancing Longevity in Hip Implants: A Review. Prosthesis 2025, 7, 21. [Google Scholar] [CrossRef]
- Ceddia, M.; Solarino, G.; Pulcrano, A.; Benedetto, A.; Trentadue, B. Finite Element Analysis of a 3D-Printed Acetabular Prosthesis for an Acetabular Defect According to the Paprosky Classification. Materials 2025, 18, 1295. [Google Scholar] [CrossRef]
- Kitagawa, T.; Tanimoto, Y.; Iida, T.; Murakami, H. Effects of material and coefficient of friction on taper joint dental implants. J. Prosthodont. Res. 2020, 64, 359–367. [Google Scholar] [CrossRef]
- Mosekilde, L.; Ebbesen, E.N.; Tornvig, L.; Thomsen, J.S. Trabecular bone structure and strength-remodelling and repair. J. Musculoskelet. Neuronal Interact. 2000, 1, 25–30. [Google Scholar]
- Wolfram, U.; Schwiedrzik, J. Post-yield and failure properties of cortical bone. BoneKEy Rep. 2016, 5, 829. [Google Scholar] [CrossRef]
- Elias, C.N. Factors affecting the success of dental implants. In Implant Dentistry: A Rapidly Evolving Practice; InTech: Rijeka, Croatia, 2011; pp. 319–364. [Google Scholar]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Factors influencing the fracture of dental implants. Clin. Implant Dent. Relat. Res. 2018, 20, 58–67. [Google Scholar] [CrossRef]
- Cook, S.D.; Klawitter, J.J.; Weinstein, A.M. The influence of implant geometry on the stress distribution around dental implants. J. Biomed. Mater. Res. 1982, 16, 369–379. [Google Scholar] [CrossRef]
- Gherde, C.; Dhatrak, P.; Nimbalkar, S.; Joshi, S. A comprehensive review of factors affecting fatigue life of dental implants. Mater. Today Proc. 2021, 43, 1117–1123. [Google Scholar] [CrossRef]
- Privitzer, E.; Widera, O.; Tesk, J.A. Some factors affecting dental implant design. J. Biomed. Mater. Res. 1975, 9, 251–255. [Google Scholar] [CrossRef]
- Zanardi, P.R.; Stegun, R.C.; Sesma, N.; Costa, B.; Shibli, J.A.; Laganá, D.C. Stress distribution around dental implants placed at different depths. J. Craniofacial Surg. 2015, 26, 2163–2166. [Google Scholar] [CrossRef] [PubMed]
- Albanchez-Gonzalez, M.I.; Brinkmann, J.C.B.; Pelaez-Rico, J.; Lopez-Suarez, C.; Rodriguez-Alonso, V.; Suarez-Garcia, M.J. Accuracy of digital dental implants impression taking with intraoral scanners compared with conventional impression techniques: A systematic review of in vitro studies. Int. J. Environ. Res. Public Health 2022, 19, 2026. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, Y.; Zhou, L.; Lin, Y.; Su, E.; Chen, J.; Wu, D. Accuracy and efficiency of robotic dental implant surgery with different human-robot interactions: An in vitro study. J. Dent. 2023, 137, 104642. [Google Scholar] [CrossRef]
- Storelli, S.; Caputo, A.; Palandrani, G.; Peditto, M.; Del Fabbro, M.; Romeo, E.; Oteri, G. Use of Narrow-Diameter Implants in Completely Edentulous Patients as a Prosthetic Option: A Systematic Review of the Literature. BioMed Res. Int. 2021, 2021, 5571793. [Google Scholar] [CrossRef]
- Khabadze, Z.; Stolov, L.; Wehbe, A.; Bakaev, Y.; Balashova, M.; Kulikova, A.; Generalova, Y.; Dashtieva, M.; Omarova, K.; Umarov, A.; et al. Implementation of Ultra-Short Implants into Dental Practice: A Systematic Review. J. Int. Dent. Med. Res. 2023, 16, 348–356. [Google Scholar]
- Goswami, R.; Trivedi, A.; Kumar, A. Evaluation of short and ultra-short dental implants in challenging clinical situations of resorbed ridges: A narrative review. SRM J. Res. Dent. Sci. 2024, 15, 45–49. [Google Scholar] [CrossRef]
- Magdy, M.; Abdelkader, M.A.; Alloush, S.; Fawzy El-Sayed, K.M.; Nawwar, A.A.; Shoeib, M.; ElNahass, H. Ultra-short versus standard-length dental implants in conjunction with osteotome-mediated sinus floor elevation: A randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 2021, 23, 520–529. [Google Scholar] [CrossRef]
- Wang, M.; Liu, F.; Ulm, C.; Shen, H.; Rausch-Fan, X. Short Implants versus Longer Implants with Sinus Floor Elevation: A Systemic Review and Meta-Analysis of Randomized Controlled Trials with a Post-Loading Follow-Up Duration of 5 Years. Materials 2022, 15, 4722. [Google Scholar] [CrossRef]
- Toledano, M.; Fernandez-Romero, E.; Vallecillo, C.; Toledano, R.; Osorio, M.T.; Vallecillo-Rivas, M. Short versus standard implants at sinus augmented sites: A systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 6681–6698. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, Y.; Yang, Y.; Ren, M.; Luo, L.; Zheng, L.; Liu, Y. Effect of short implant crown-to-implant ratio on stress distribution in anisotropic bone with different osseointegration rates. BMC Oral Health 2023, 23, 683. [Google Scholar] [CrossRef]
- Vargas-Moreno, V.F.; de Oliveira Ribeiro, M.C.; Gomes, R.S.; Faot, F.; Cury, A.A.D.B.; Marcello-Machado, R.M. Clinical performance of short and extrashort dental implants with wide diameter: A systematic review with meta-analysis. J. Prosthet. Dent. 2024, 132, 1260.e1–1260.e13. [Google Scholar] [CrossRef] [PubMed]
- Comuzzi, L.; Tumedei, M.; Romasco, T.; Petrini, M.; Afrashtehfar, K.I.; Inchingolo, F.; Piattelli, A.; Di Pietro, N. Insertion torque, removal torque, and resonance frequency analysis values of ultrashort, short, and standard dental implants: An in vitro study on polyurethane foam sheets. J. Funct. Biomater. 2022, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, M.; Romasco, T.; Marchioli, G.; Comuzzi, L.; Cipollina, A.; Piattelli, A.; Lamberti, L.; Di Pietro, N.; Trentadue, B. Finite Element Analysis of Implant Stability Quotient (ISQ) and Bone Stresses for Implant Inclinations of 0°, 15°, and 20°. Materials 2025, 18, 1625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Comuzzi, L.; Romasco, T.; Mourão, C.F.; Marchioli, G.; Piattelli, A.; Di Pietro, N. Biomechanical Analysis of Truncated Cone Implants for Maxillary Sinus Lift: An In Vitro Study on Polyurethane Laminas. Bioengineering 2025, 12, 53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ceddia, M.; Trentadue, B. Evaluation of rotational stability and stress shielding of a stem optimized for hip replacements—A finite element study. Prosthesis 2023, 5, 678–693. [Google Scholar] [CrossRef]
- Ficarella, E.; Minooei, M.; Santoro, L.; Toma, E.; Trentadue, B.; De Spirito, M.; Papi, M.; Pruncu, C.I.; Lamberti, L. Visco-hyperelastic characterization of the equine immature zona pellucida. Materials 2021, 14, 1223. [Google Scholar] [CrossRef]
- Ceddia, M.; Solarino, G.; Tucci, M.; Lamberti, L.; Trentadue, B. Stress analysis of tibial bone using three different materials for bone fixation plates. J. Compos. Sci. 2024, 8, 334. [Google Scholar] [CrossRef]
- Ceddia, M.; Solarino, G.; Giannini, G.; De Giosa, G.; Tucci, M.; Trentadue, B. A Finite Element Analysis Study of Influence of Femoral Stem Material in Stress Shielding in a Model of Uncemented Total Hip Arthroplasty: Ti-6Al-4V versus Carbon Fibre-Reinforced PEEK Composite. J. Compos. Sci. 2024, 8, 254. [Google Scholar] [CrossRef]
- Demirbas, A.E.; Ekici, R.; Karakaya, M.; Alkan, A. Bone stress and damage distributions during dental implant insertion: A novel dynamic FEM analysis. Comput. Methods Biomech. Biomed. Eng. 2022, 25, 1381–1392. [Google Scholar] [CrossRef]
- Van Staden, R.C.; Guan, H.; Johnson, N.W.; Loo, Y.C.; Meredith, N. Stepwise analysis of the dental implant insertion process using the finite element technique. Clin. Oral Implant. Res. 2008, 19, 303–313. [Google Scholar] [CrossRef]
- Joshi, S.; Dhatrak, P.; Nimbalkar, S.; Gherde, C. An effect of various parameters on insertion torque to improve the success rate of dental implantation: A review. Mater. Today Proc. 2021, 43, 928–934. [Google Scholar] [CrossRef]
- Torabzadeh, M.; Amid, R.; Farhad, S.Z. Maximum Insertion Torque, Stress, and Strain in Bone during Insertion of Three Dental Implants with Different Macroscopic Designs: A Dynamic Finite-Element Analysis. J. Long-Term Eff. Med. Implant. 2021, 31, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Romasco, T.; Pignatelli, P.; Tumedei, M.; Hossein, H.H.S.; Cipollina, A.; Piattelli, A.; Inchingolo, F.; Di Pietro, N. The influence of truncated-conical implant length on primary stability in maxillary and mandibular regions: An in vitro study using polyurethane blocks. Clin. Oral Investig. 2023, 28, 28. [Google Scholar] [CrossRef]
- Ovesy, M.; Indermaur, M.; Zysset, P.K. Prediction of insertion torque and stiffness of a dental implant in bovine trabecular bone using explicit micro-finite element analysis. J. Mech. Behav. Biomed. Mater. 2019, 98, 301–310. [Google Scholar] [CrossRef]
- Alsabeeha, N.H.; Duncan, W.J. Insertion torque of immediate wide-diameter implants: A finite element analysis. Quintessence Int 2012, 43, e115–e126. [Google Scholar]
- Bardyn, T.; Gédet, P.; Hallermann, W.; Büchler, P. Prediction of dental implant torque with a fast and automatic finite element analysis: A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 109, 594–603. [Google Scholar] [CrossRef]
- Roca-Millan, E.; Gonzalez-Navarro, B.; Dominguez-Minger, J.; Mari-Roig, A.; Jane-Salas, E.; Lopez-Lopez, J. Implant insertion torque and marginal bone loss: A systematic review and meta-analysis. Int. J. Oral Implantol. 2020, 13, 345–353. [Google Scholar]
- Norton, M.R. The Influence of Low Insertion Torque on Primary Stability, Implant Survival, and Maintenance of Marginal Bone Levels: A Closed-Cohort Prospective Study. Int. J. Oral Maxillofac. Implant. 2017, 32, 849–857. [Google Scholar] [CrossRef]
- McComb, D. Conservative operative management strategies. Dent. Clin. 2005, 49, 847–865. [Google Scholar] [CrossRef]
- Heimes, D.; Becker, P.; Pabst, A.; Smeets, R.; Kraus, A.; Hartmann, A.; Sagheb, K.; Kämmerer, P.W. How does dental implant macrogeometry affect primary implant stability? A narrative review. Int. J. Implant Dent. 2023, 9, 20. [Google Scholar] [CrossRef]
| Mechanical Properties | Cortical | D2 | D4 |
|---|---|---|---|
| Density ρ (kg/cm3) | 1.6 | 0.64 | 0.32 |
| Young’s modulus, E (GPa) | 16 | 5.5 | 0.690 |
| Poisson’s ratio, v | 0.3 | 0.3 | 0.3 |
| Young’s Modulus (GPa) | Poisson’s Ratio | Tensile Strength (MPa) | |
|---|---|---|---|
| Ti6Al4V | 114 | 0.34 | 900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceddia, M.; Montesani, L.; Comuzzi, L.; Cipollina, A.; Deporter, D.A.; Di Pietro, N.; Trentadue, B. The Influence of Insertion Torque on Stress Distribution in Peri-Implant Bones Around Ultra-Short Implants: An FEA Study. J. Funct. Biomater. 2025, 16, 260. https://doi.org/10.3390/jfb16070260
Ceddia M, Montesani L, Comuzzi L, Cipollina A, Deporter DA, Di Pietro N, Trentadue B. The Influence of Insertion Torque on Stress Distribution in Peri-Implant Bones Around Ultra-Short Implants: An FEA Study. Journal of Functional Biomaterials. 2025; 16(7):260. https://doi.org/10.3390/jfb16070260
Chicago/Turabian StyleCeddia, Mario, Lorenzo Montesani, Luca Comuzzi, Alessandro Cipollina, Douglas A. Deporter, Natalia Di Pietro, and Bartolomeo Trentadue. 2025. "The Influence of Insertion Torque on Stress Distribution in Peri-Implant Bones Around Ultra-Short Implants: An FEA Study" Journal of Functional Biomaterials 16, no. 7: 260. https://doi.org/10.3390/jfb16070260
APA StyleCeddia, M., Montesani, L., Comuzzi, L., Cipollina, A., Deporter, D. A., Di Pietro, N., & Trentadue, B. (2025). The Influence of Insertion Torque on Stress Distribution in Peri-Implant Bones Around Ultra-Short Implants: An FEA Study. Journal of Functional Biomaterials, 16(7), 260. https://doi.org/10.3390/jfb16070260






