Ultrasound-Enhanced Tumor Penetration of Carrier-Free Nanodrugs for High-Efficiency Chemo-Photodynamic Therapy of Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
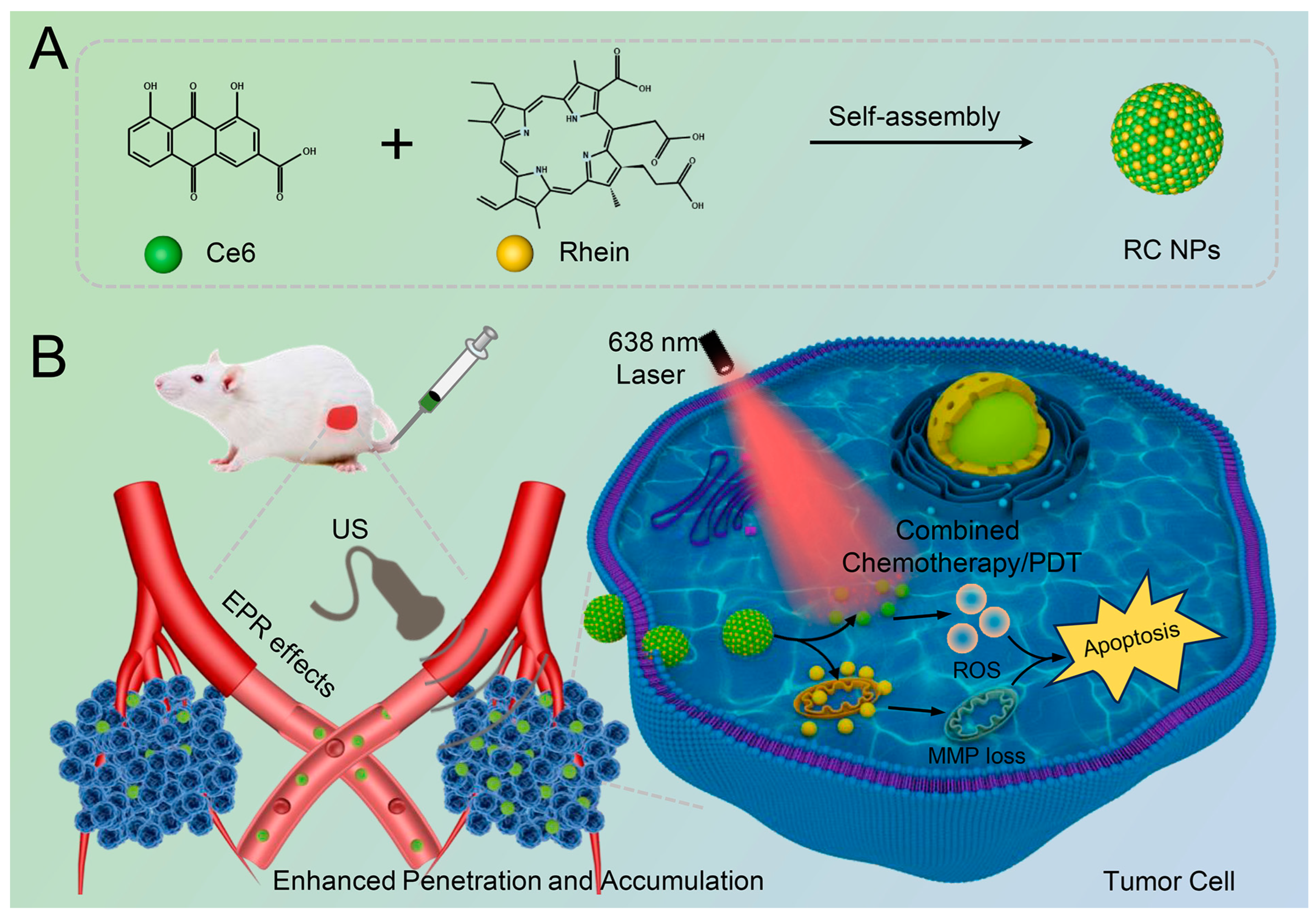
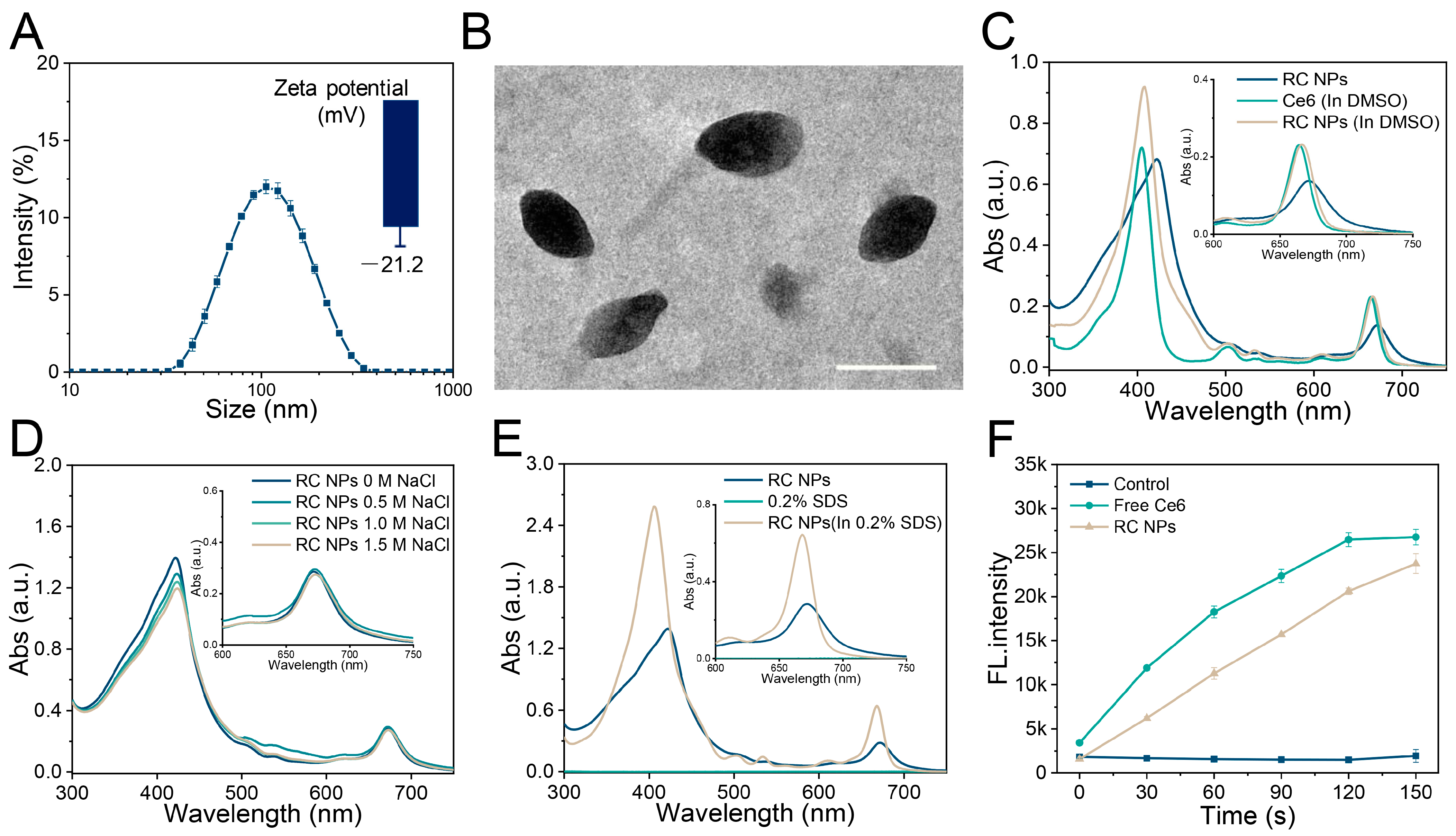
2.2. Preparation and Characterization of RC NPs
2.3. Exploration of Self-Assembly Mechanism
2.4. Cell Culture
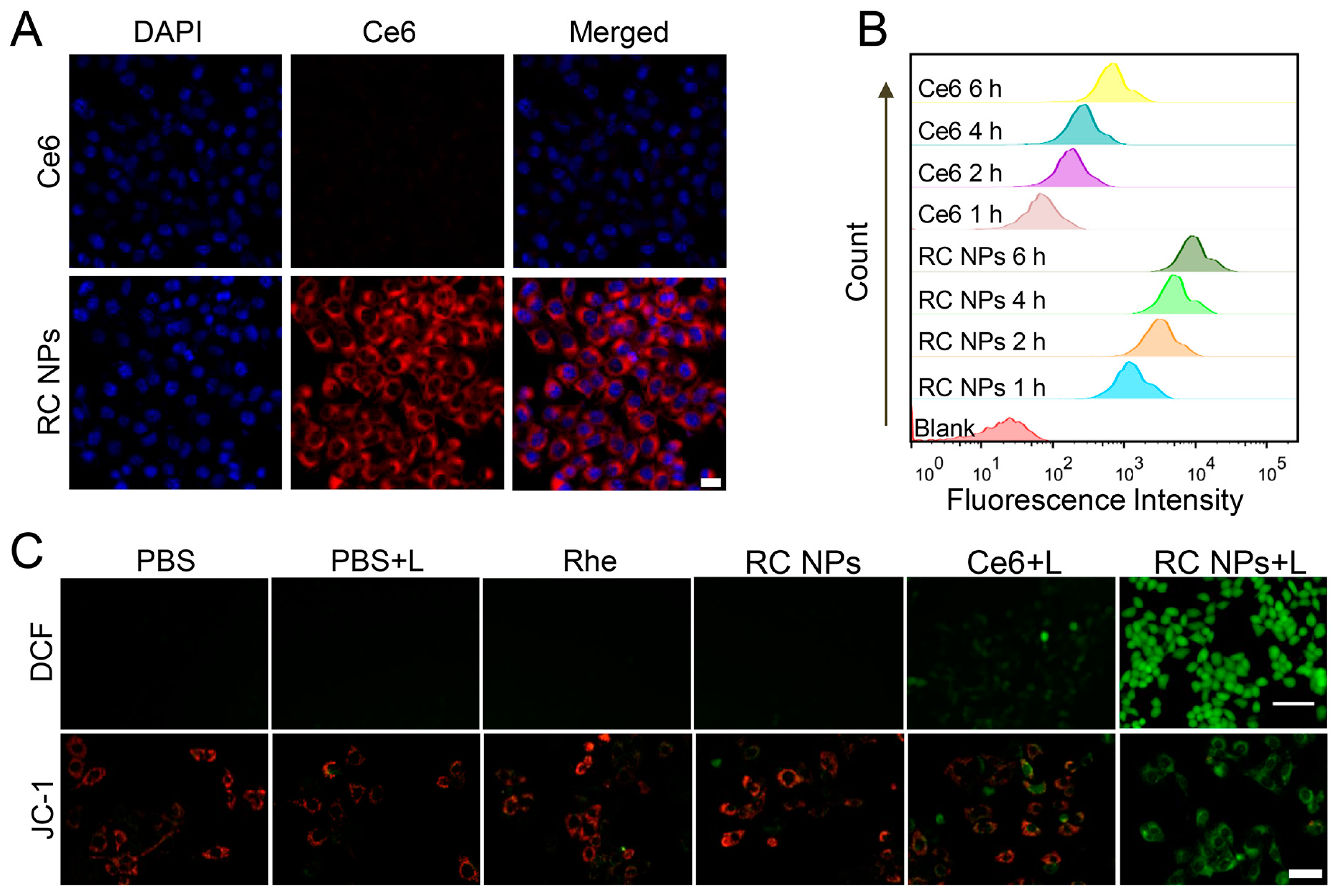
2.5. Cellular Uptake
2.6. Intracellular ROS Generation
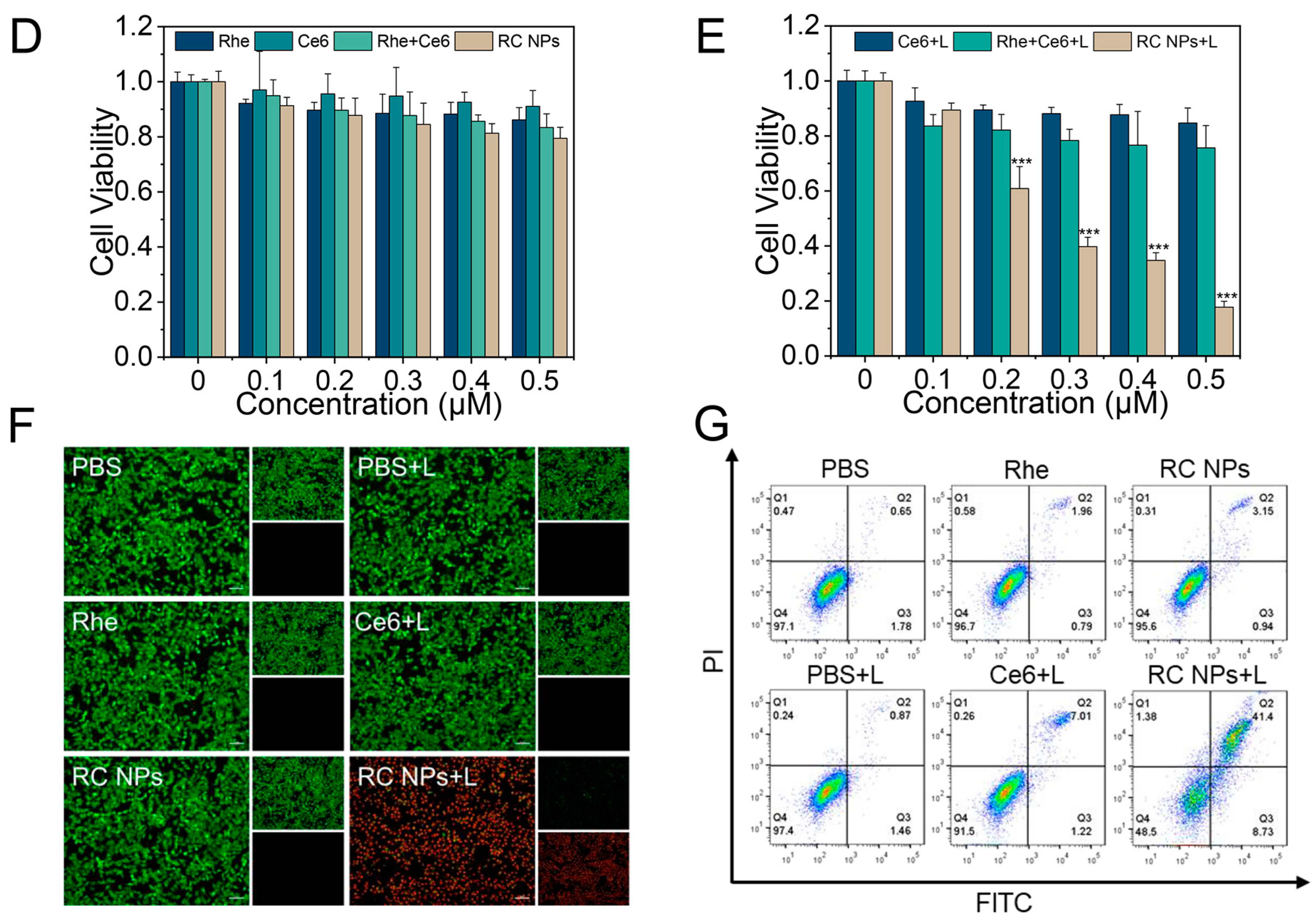
2.7. In Vitro Cytotoxicity
2.8. Live/Dead Cell Staining Assay
2.9. Apoptosis Assay
2.10. Detection of Mitochondrial Membrane Potential
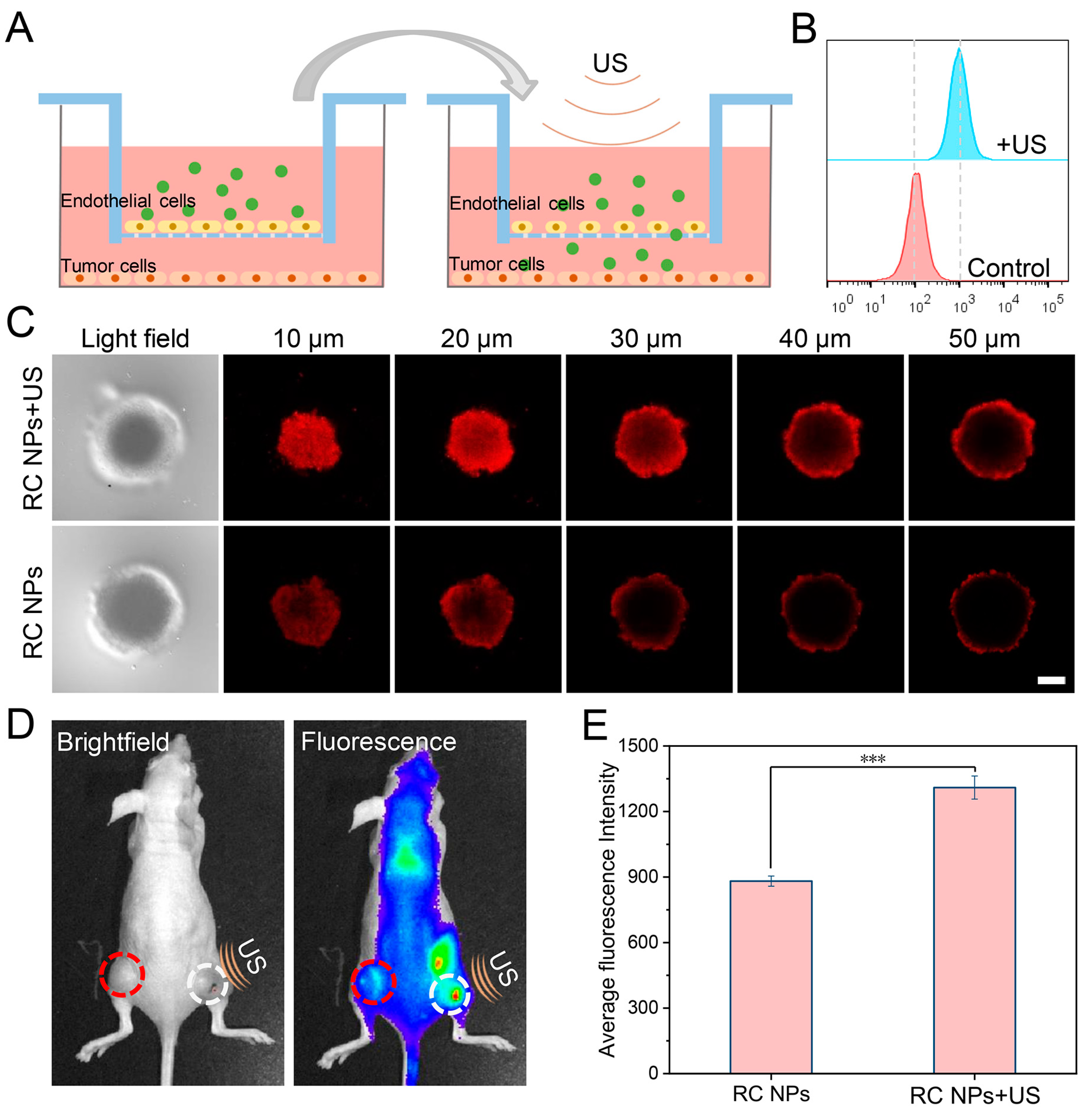
2.11. Ultrasonic Facilitated Penetration Detection In Vitro
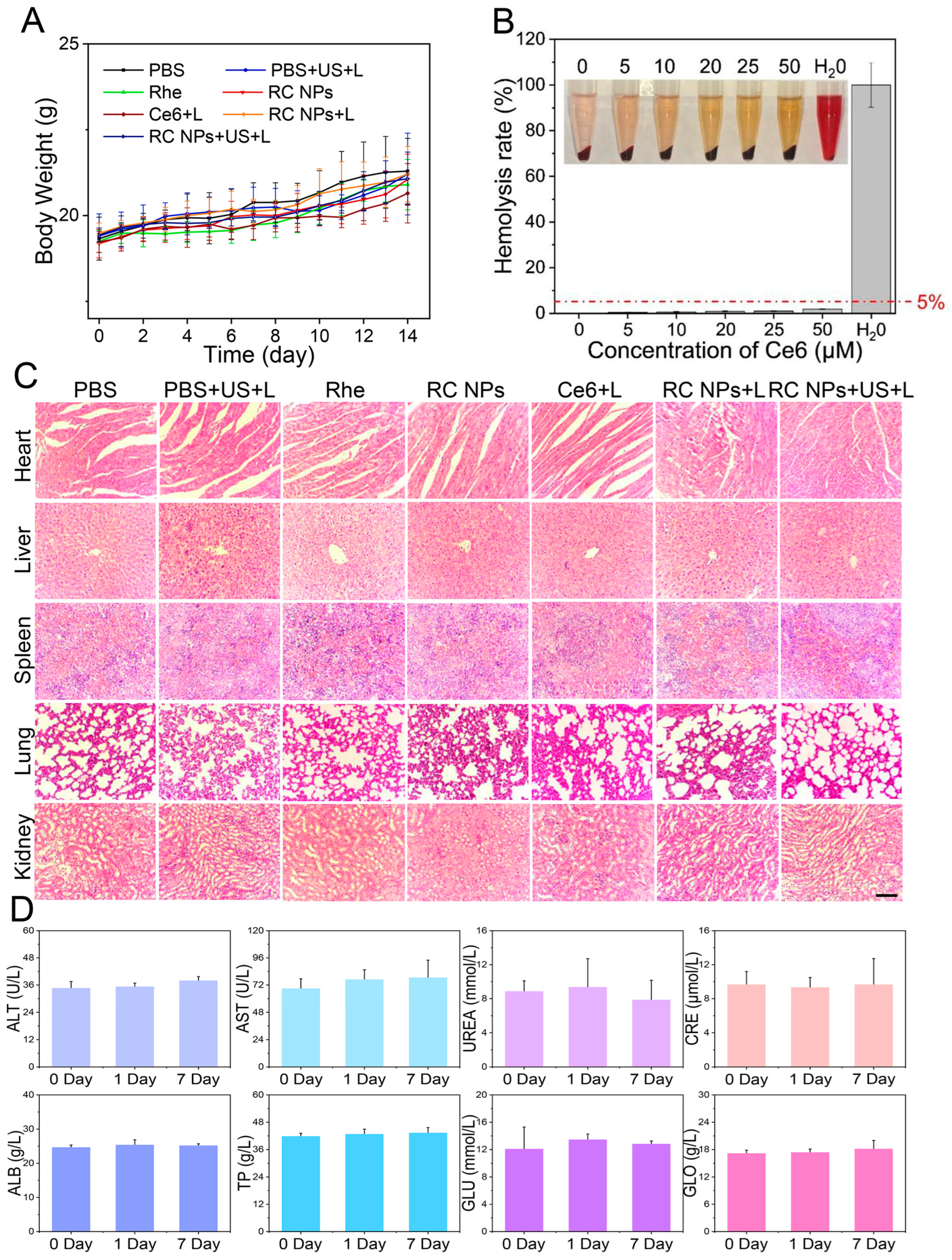
2.12. Hemolysis Test of RC NPs
2.13. Fluorescence Imaging In Vivo
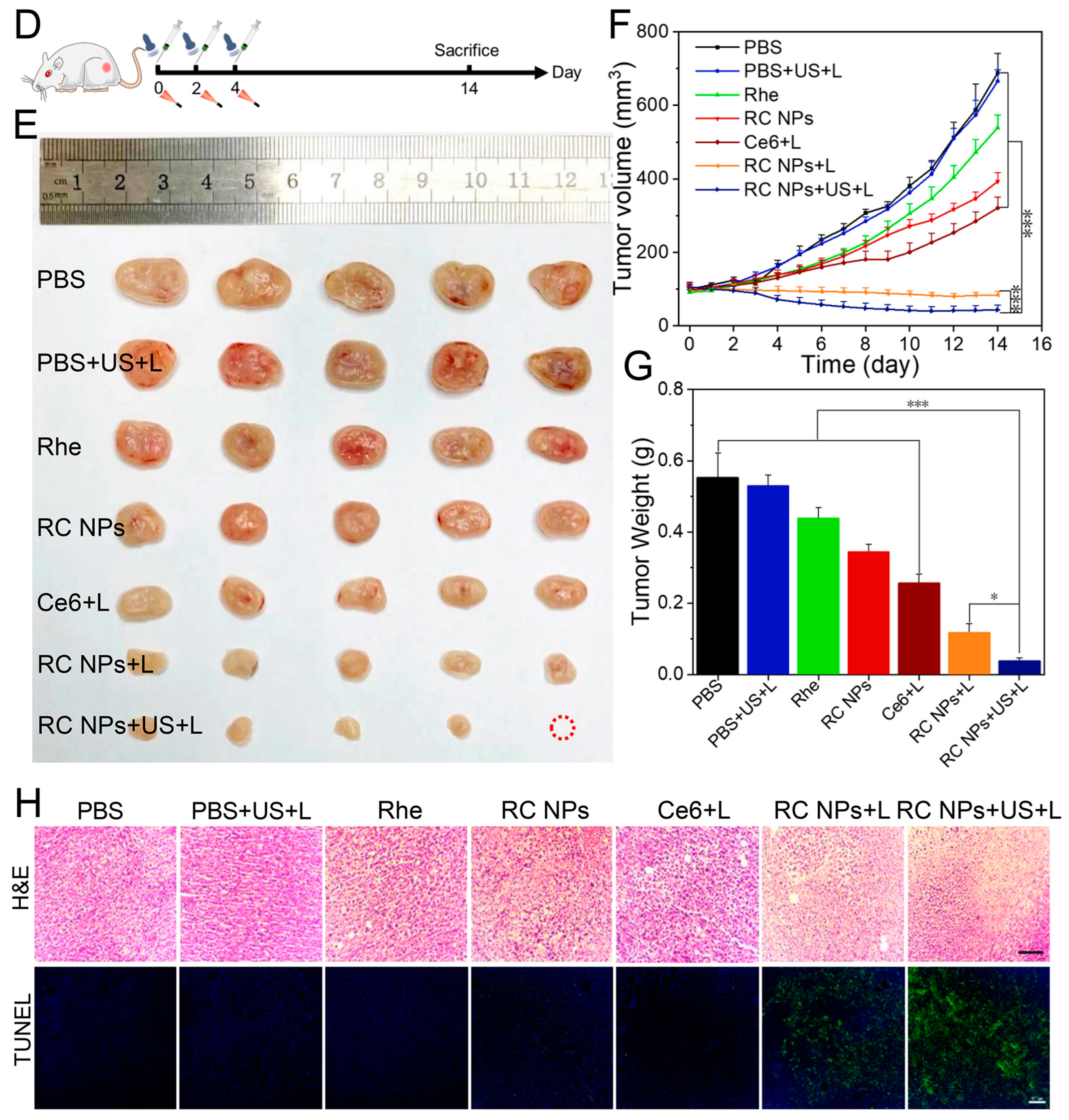
2.14. Antitumor Study In Vivo and Biosafety Assay
2.15. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of RC NPs
3.2. Cellular Uptake and In Vitro Antitumor Efficiency of RC NPs
3.3. Evaluation of US-Enhanced Tumor Penetration of RC NPs
3.4. In Vivo Antitumor Study
3.5. Biosafety Evaluation of RC NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Cent. 2022, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast Cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef] [PubMed]
- Oladeru, O.T.; Singh, A.K.; Ma, S.J. Association of Adjuvant Chemotherapy With Overall Survival Among Women With Small, Node-Negative, Triple-Negative Breast Cancer. JAMA Netw. Open 2020, 3, e2016247. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Kartal-Yandim, M.; Adan-Gokbulut, A.; Baran, Y. Molecular mechanisms of drug resistance and its reversal in cancer. Crit. Rev. Biotechnol. 2015, 36, 716–726. [Google Scholar] [CrossRef]
- Feng, B.; Hou, B.; Xu, Z.; Saeed, M.; Yu, H.; Li, Y. Self-Amplified Drug Delivery with Light-Inducible Nanocargoes to Enhance Cancer Immunotherapy. Adv. Mater. 2019, 31, e1902960. [Google Scholar] [CrossRef]
- Shu, X.; Chen, Y.; Yan, P.; Xiang, Y.; Shi, Q.-Y.; Yin, T.; Wang, P.; Liu, L.-H.; Shuai, X. Biomimetic nanoparticles for effective mild temperature photothermal therapy and multimodal imaging. J. Control. Release 2022, 347, 270–281. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, P.; Yan, P.; Chen, P.; Shi, Q.; Zhao, Z.; Chen, J.; Shu, X.; Wang, P.; Yang, B.; et al. Versatile Nanodrugs Containing Glutathione and Heme Oxygenase 1 Inhibitors Enable Suppression of Antioxidant Defense System in a Two-Pronged Manner for Enhanced Photodynamic Therapy. Adv. Heal. Mater. 2021, 10, 2100770. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, X.; Yuan, Y.; Yin, L.; He, W. A drug-delivering-drug strategy for combined treatment of metastatic breast cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2678–2688. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Z.; Yang, Y.; Shi, H.; Chen, H.; Gao, Y. A nanosensitizer self-assembled from oleanolic acid and chlorin e6 for synergistic chemo/sono-photodynamic cancer therapy. Phytomedicine 2021, 93, 153788. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, Y.; Yang, M.; Yuan, D.; Huang, J.; Chen, R.; Wang, H.; Hu, L.; Di, L.; Li, J. Synergistic inhibition of metastatic breast cancer by dual-chemotherapy with excipient-free rhein/DOX nanodispersions. J. Nanobiotechnology 2020, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ling, M.; Yu, K.; Yang, W.; Liu, Q.; He, L.; Cai, X.; Zhong, M.; Mai, Z.; Sun, R.; et al. Synergetic delivery of artesunate and isosorbide 5-mononitrate with reduction-sensitive polymer nanoparticles for ovarian cancer chemotherapy. J. Nanobiotechnology 2022, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zheng, J.; Wang, M.; Li, Y.; Ruan, J.; Zhang, H. Metabolic Activation of Rhein: Insights into the Potential Toxicity Induced by Rhein-Containing Herbs. J. Agric. Food Chem. 2016, 64, 5742–5750. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chiang, S.Y.; Lin, J.G.; Yang, J.S.; Ma, Y.S.; Liao, C.L.; Lai, T.Y.; Tang, N.Y.; Chung, J.G. Emodin, aloe-emodin and rhein induced DNA damage and inhibited DNA repair gene expression in SCC-4 human tongue cancer cells. Anticancer Res. 2010, 30, 945–951. [Google Scholar]
- Hsia, T.-C.; Yang, J.-S.; Chen, G.-W.; Chiu, T.-H.; Lu, H.-F.; Yang, M.-D.; Yu, F.-S.; Liu, K.-C.; Lai, K.-C.; Lin, C.-C.; et al. The Roles of Endoplasmic Reticulum Stress and Ca2+ on Rhein-induced apoptosis in A-549 Human Lung Cancer cells. Anticancer Res. 2009, 29, 309–318. [Google Scholar]
- Lin, M.-L.; Chung, J.-G.; Lu, Y.-C.; Yang, C.-Y.; Chen, S.-S. Rhein inhibits invasion and migration of human nasopharyngeal carcinoma cells in vitro by down-regulation of matrix metalloproteinases-9 and vascular endothelial growth factor. Oral Oncol. 2009, 45, 531–537. [Google Scholar] [CrossRef]
- Lin, S.; Fujii, M.; Hou, D.-X. Rhein induces apoptosis in HL-60 cells via reactive oxygen species-independent mitochondrial death pathway. Arch. Biochem. Biophys. 2003, 418, 99–107. [Google Scholar] [CrossRef]
- Shen, Z.; Zhu, B.; Li, J.; Qin, L. Rhein Augments Antiproliferative Effects of Atezolizumab Based on Breast Cancer (4T1) Regression. Planta Medica 2019, 85, 1143–1149. [Google Scholar] [CrossRef]
- Zhang, Y.; Khan, A.R.; Yang, X.; Shi, Y.; Zhao, X.; Zhai, G. A sonosensitiser-based polymeric nanoplatform for chemo-sonodynamic combination therapy of lung cancer. J. Nanobiotechnology 2021, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Dong, X.; Yin, X.; Yang, C.; Leng, X.; Wang, W.; Ni, J. Rhein Induces Cell Death in HepaRG Cells through Cell Cycle Arrest and Apoptotic Pathway. Int. J. Mol. Sci. 2018, 19, 1060. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Li, Z.; Dai, C.; Zhao, D.; Wang, Y.; Ma, C.; Liu, C. Chrysophanol inhibits proliferation and induces apoptosis through NF-B/cyclin D1 and NF-B/Bcl-2 signaling cascade in breast cancer cell lines. Mol. Med. Rep. 2018, 17, 4376–4382. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoglu, S.; Zhou, M.; Shi, B.; Zhang, X.; Williams, G.R.; Chen, X. Carrier-free nanodrugs for safe and effective cancer treatment. J. Control. Release 2020, 329, 805–832. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Zhou, X.; Liu, L.; Zheng, R.; Deng, F.; Liu, Y.; Yu, X.; Li, S.; Cheng, H. Carrier Free O2-Economizer for Photodynamic Therapy Against Hypoxic Tumor by Inhibiting Cell Respiration. Small 2022, 18, e2107467. [Google Scholar] [CrossRef]
- Liu, L.-H.; Zhang, X.-Z. Carrier-free nanomedicines for cancer treatment. Prog. Mater. Sci. 2022, 125, 100919. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Gong, H.; Chao, Y.; Xiang, J.; Han, X.; Song, G.; Feng, L.; Liu, J.; Yang, G.; Chen, Q.; Liu, Z. Hyaluronidase To Enhance Nanoparticle-Based Photodynamic Tumor Therapy. Nano Lett. 2016, 16, 2512–2521. [Google Scholar] [CrossRef]
- Gao, J.; Qin, H.; Wang, F.; Liu, L.; Tian, H.; Wang, H.; Wang, S.; Ou, J.; Ye, Y.; Peng, F.; et al. Hyperthermia-triggered biomimetic bubble nanomachines. Nat. Commun. 2023, 14, 1–15. [Google Scholar] [CrossRef]
- Cho, M.H.; Li, Y.; Lo, P.C.; Lee, H.; Choi, Y. Fucoidan-Based Theranostic Nanogel for Enhancing Imaging and Photodynamic Therapy of Cancer. Nanomicro Lett. 2020, 12, 47. [Google Scholar] [CrossRef]
- Li, H.; Ma, M.; Zhang, J.; Hou, W.; Chen, H.; Zeng, D.; Wang, Z. Ultrasound-Enhanced Delivery of Doxorubicin-Loaded Nanodiamonds from Pullulan-all-trans-Retinal Nanoparticles for Effective Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 20341–20349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, Y.; Chen, Y.; Blum, N.T.; Lin, J.; Huang, P. Ultrasound-Enhanced Chemo-Photodynamic Combination Therapy by Using Albumin “Nanoglue”-Based Nanotheranostics. ACS Nano 2020, 14, 5560–5569. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Q.; Hou, W.; Zhang, J.; Ye, H.; Li, H.; Zeng, D.; Bai, J. A redox-sensitive core-crosslinked nanosystem combined with ultrasound for enhanced deep penetration of nanodiamonds into tumors. RSC Adv. 2020, 10, 15252–15263. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Song, R.-J.; Lu, P.; Chen, J.-C.; Zhou, Y.-Q.; Shen, G.; Jiang, M.-J.; Zhang, W. pH-triggered charge-reversal and redox-sensitive drug release polymer micelles co-deliver doxorubicin and triptolide for prostate tumor therapy. Int. J. Nanomed. 2018, 13, 7229–7249. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Qiao, Y.; Gao, B.; Zheng, M.; Xie, Z. Controlled synthesis of spindle-shaped terrylenediimide nanoparticles for enhanced tumor accumulation and treatment. Chem. Eng. J. 2021, 419, 129552. [Google Scholar] [CrossRef]
- Mai, Z.; Zhong, J.; Zhang, J.; Chen, G.; Tang, Y.; Ma, W.; Li, G.; Feng, Z.; Li, F.; Liang, X.-J.; et al. Carrier-Free Immunotherapeutic Nano-Booster with Dual Synergistic Effects Based on Glutaminase Inhibition Combined with Photodynamic Therapy. ACS Nano 2023, 17, 1583–1596. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, P.; Guo, C.; Wang, D.; Zhang, X.; Chen, M.; Wang, Q.; Sun, M.; Zeng, P.; Lu, F.; et al. Folate-mediated and pH-responsive chidamide-bound micelles encapsulating photosensitizers for tumor-targeting photodynamic therapy. Int. J. Nanomed. 2019, ume 14, 5527–5540. [Google Scholar] [CrossRef]
- Xie, L.; Wang, L.; Li, L.; Liu, C.; Guo, L.; Liao, Y.; Zhou, S.; Wu, W.; Duo, Y.; Shi, L.; et al. Novel Carrier-Free Nanodrug Enhances Photodynamic Effects by Blocking the Autophagy Pathway and Synergistically Triggers Immunogenic Cell Death for the Efficient Treatment of Breast Cancer. ACS Appl. Mater. Interfaces 2024, 16, 5683–5695. [Google Scholar] [CrossRef]
- Li, X.; Deng, F.; Zheng, R.; Liu, L.; Liu, Y.; Kong, R.; Chen, A.; Yu, X.; Li, S.; Cheng, H. Carrier Free Photodynamic Synergists for Oxidative Damage Amplified Tumor Therapy. Small 2021, 17, 2102470. [Google Scholar] [CrossRef]
- Heo, S.-K.; Yun, H.-J.; Park, W.-H.; Park, S.-D. Rhein Inhibits TNF-α-Induced Human Aortic Smooth Muscle Cell Proliferation via Mitochondrial-Dependent Apoptosis. J. Vasc. Res. 2009, 46, 375–386. [Google Scholar] [CrossRef]
- Wu, L.; Shi, Y.; Ni, Z.; Yu, T.; Chen, Z. Preparation of a Self-Assembled Rhein–Doxorubicin Nanogel Targeting Mitochondria and Investigation on Its Antihepatoma Activity. Mol. Pharm. 2021, 19, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Maishi, N.; Sakurai, Y.; Hida, Y.; Harashima, H. Heterogeneity of tumor endothelial cells and drug delivery. Adv. Drug Deliv. Rev. 2016, 99, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Keller, E.T.; Garfield, D.H.; Shen, K.; Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2012, 32, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Nomikou, N.; Li, Y.S.; McHale, A.P. Ultrasound-enhanced drug dispersion through solid tumours and its possible role in aiding ultrasound-targeted cancer chemotherapy. Cancer Lett. 2010, 288, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zeng, D.; Wang, Z.; Fang, L.; Li, F.; Wang, Z. Ultrasound-Enhanced Delivery of Doxorubicin/All-Trans Retinoic Acid-Loaded Nanodiamonds Into Tumors. Nanomedicine 2018, 13, 981–996. [Google Scholar] [CrossRef]
- Quinlan, J.A.; Inglut, C.T.; Srivastava, P.; Rahman, I.; Stabile, J.; Gaitan, B.; Del Valle, C.A.; Baumiller, K.; Gaur, A.; Chiou, W.; et al. Carrier-Free, Amorphous Verteporfin Nanodrug for Enhanced Photodynamic Cancer Therapy and Brain Drug Delivery. Adv. Sci. 2024, 11, e2302872. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, Y.; Wang, M.; Younis, M.R.; Yi, H.; Zhao, X.; Chang, J.; Zheng, Y.; Guo, W.; Yu, X. Self-Assembled Carrier-Free Nanodrugs for Starvation Therapy-Amplified Photodynamic Therapy of Cancer. Adv. Healthc. Mater. 2023, 12, e2203177. [Google Scholar] [CrossRef]
- Liu, J.; Lyu, Q.; Wu, M.; Zhou, Y.; Wang, T.; Zhang, Y.; Fan, N.; Yang, C.; Wang, W. Integrating mTOR Inhibition and Photodynamic Therapy Based on Carrier-Free Nanodrugs for Breast Cancer Immunotherapy. Adv. Healthc. Mater. 2024, e2402357. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Y.; Liang, S.; Wang, P. Ultrasound-Enhanced Tumor Penetration of Carrier-Free Nanodrugs for High-Efficiency Chemo-Photodynamic Therapy of Breast Cancer. J. Funct. Biomater. 2025, 16, 206. https://doi.org/10.3390/jfb16060206
Xiang Y, Liang S, Wang P. Ultrasound-Enhanced Tumor Penetration of Carrier-Free Nanodrugs for High-Efficiency Chemo-Photodynamic Therapy of Breast Cancer. Journal of Functional Biomaterials. 2025; 16(6):206. https://doi.org/10.3390/jfb16060206
Chicago/Turabian StyleXiang, Yun, Shiyu Liang, and Ping Wang. 2025. "Ultrasound-Enhanced Tumor Penetration of Carrier-Free Nanodrugs for High-Efficiency Chemo-Photodynamic Therapy of Breast Cancer" Journal of Functional Biomaterials 16, no. 6: 206. https://doi.org/10.3390/jfb16060206
APA StyleXiang, Y., Liang, S., & Wang, P. (2025). Ultrasound-Enhanced Tumor Penetration of Carrier-Free Nanodrugs for High-Efficiency Chemo-Photodynamic Therapy of Breast Cancer. Journal of Functional Biomaterials, 16(6), 206. https://doi.org/10.3390/jfb16060206






