Polycaprolactone/Doped Bioactive Glass Composite Scaffolds for Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PCL and BG/PCL Scaffolds
2.2. Morphological Characterization
2.3. Chemical Characterization
2.4. Mechanical Characterization
2.5. Cell Culture
2.5.1. Cytotoxicity Assay
2.5.2. Adhesion and Proliferation
2.5.3. Alkaline Phosphatase (ALP) Activity
2.5.4. Immunofluorescence Staining
2.6. Statistical Analysis
3. Results and Discussion
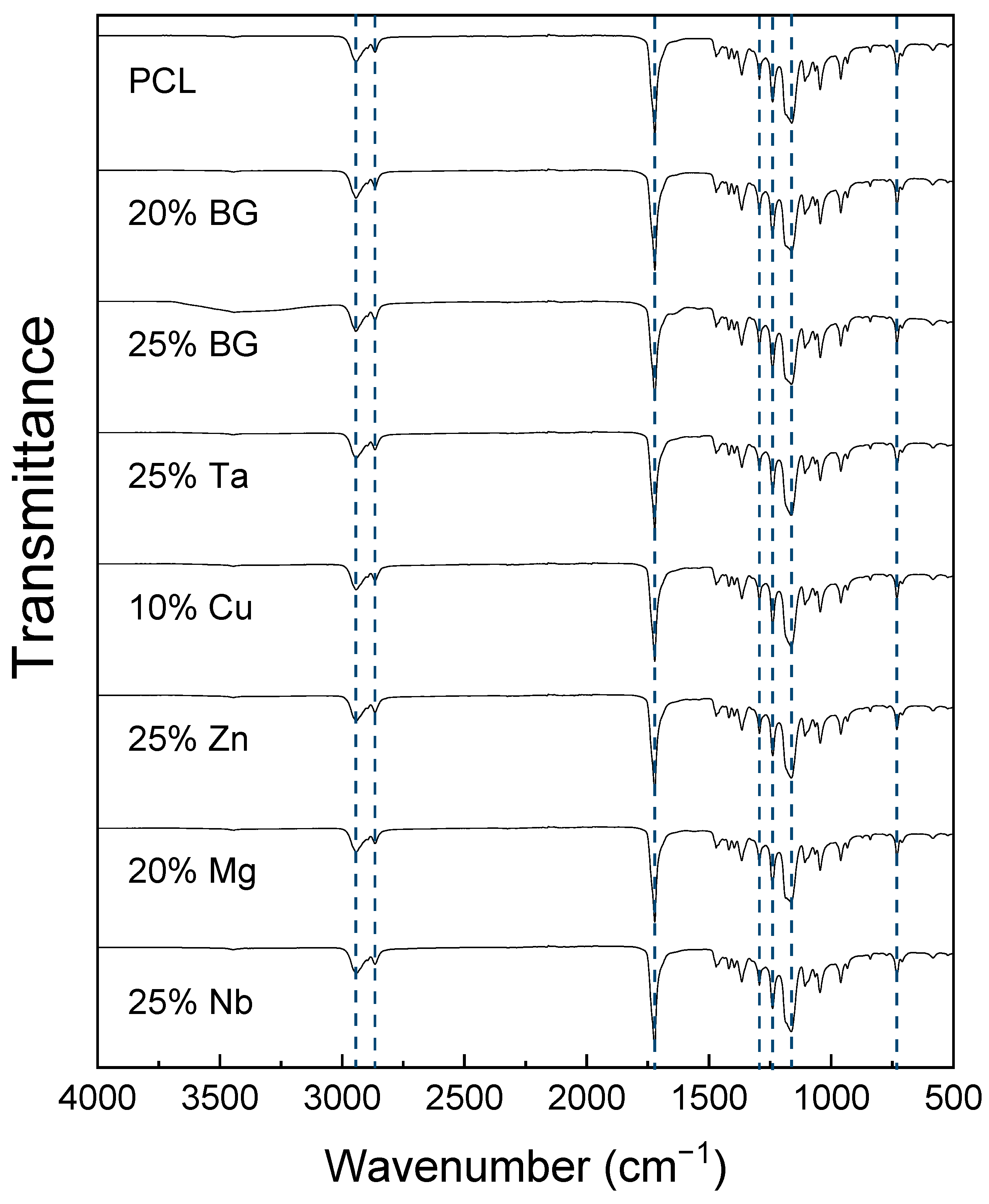
3.1. Chemical Characterization
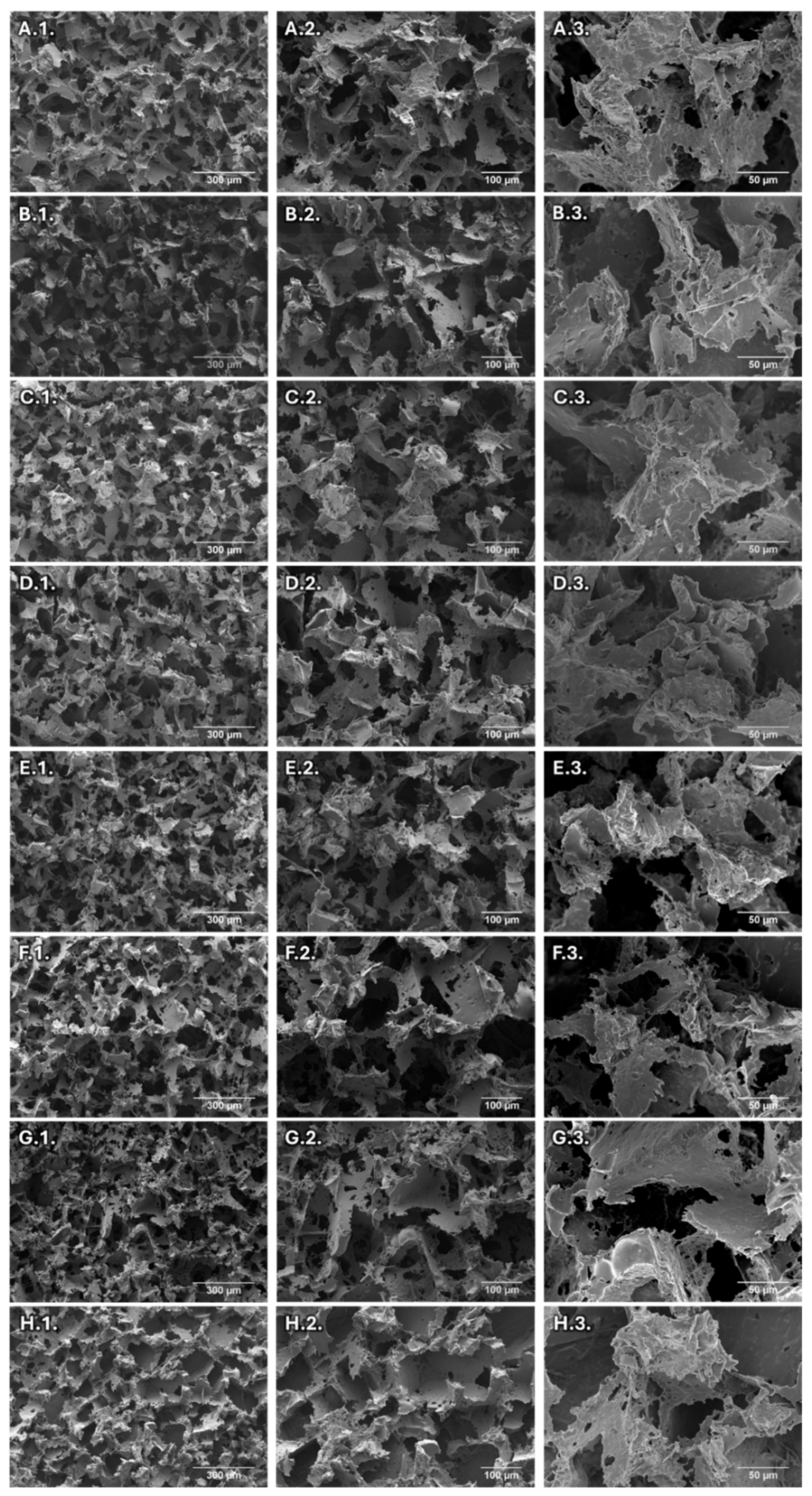
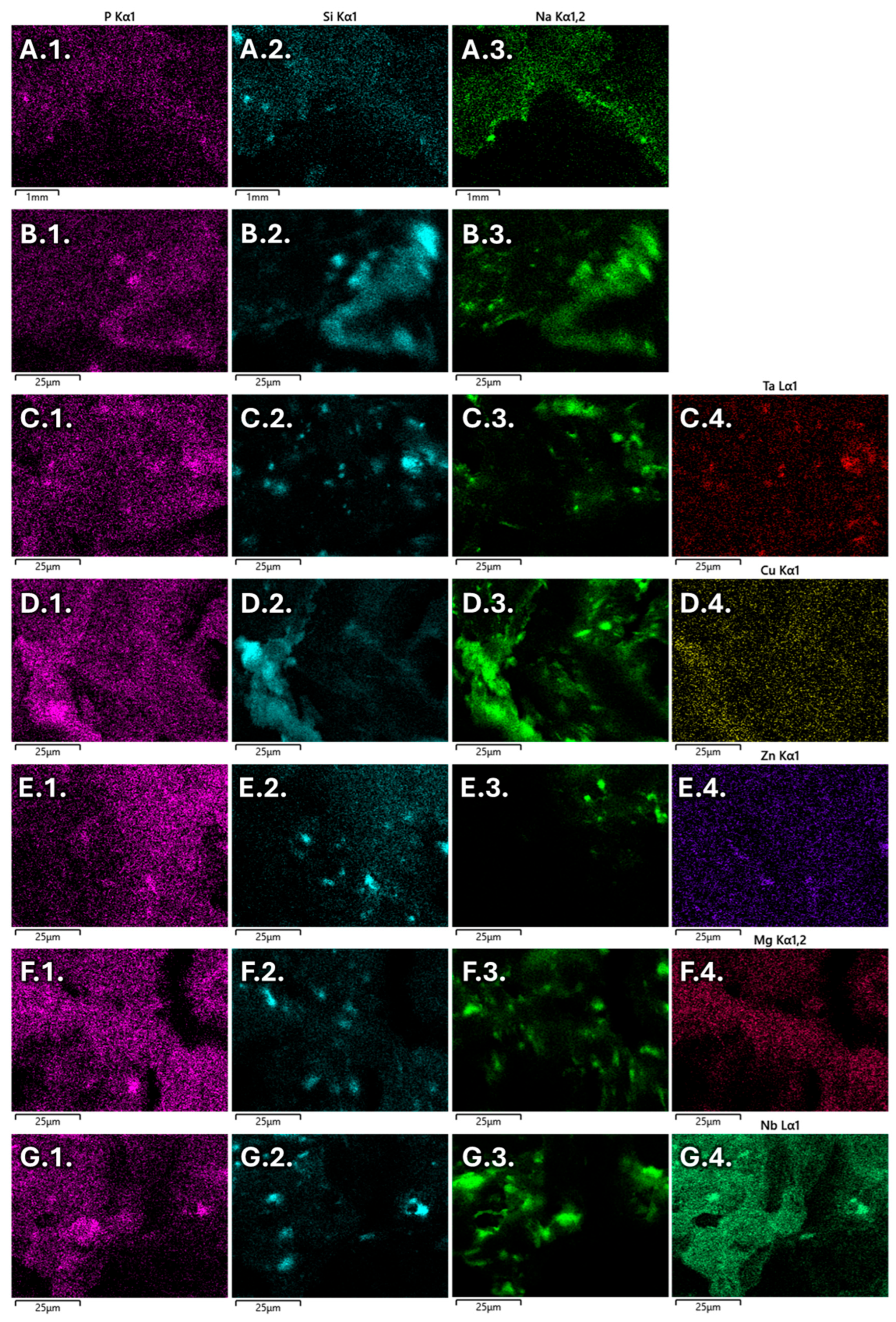
3.2. Morphological Characterization
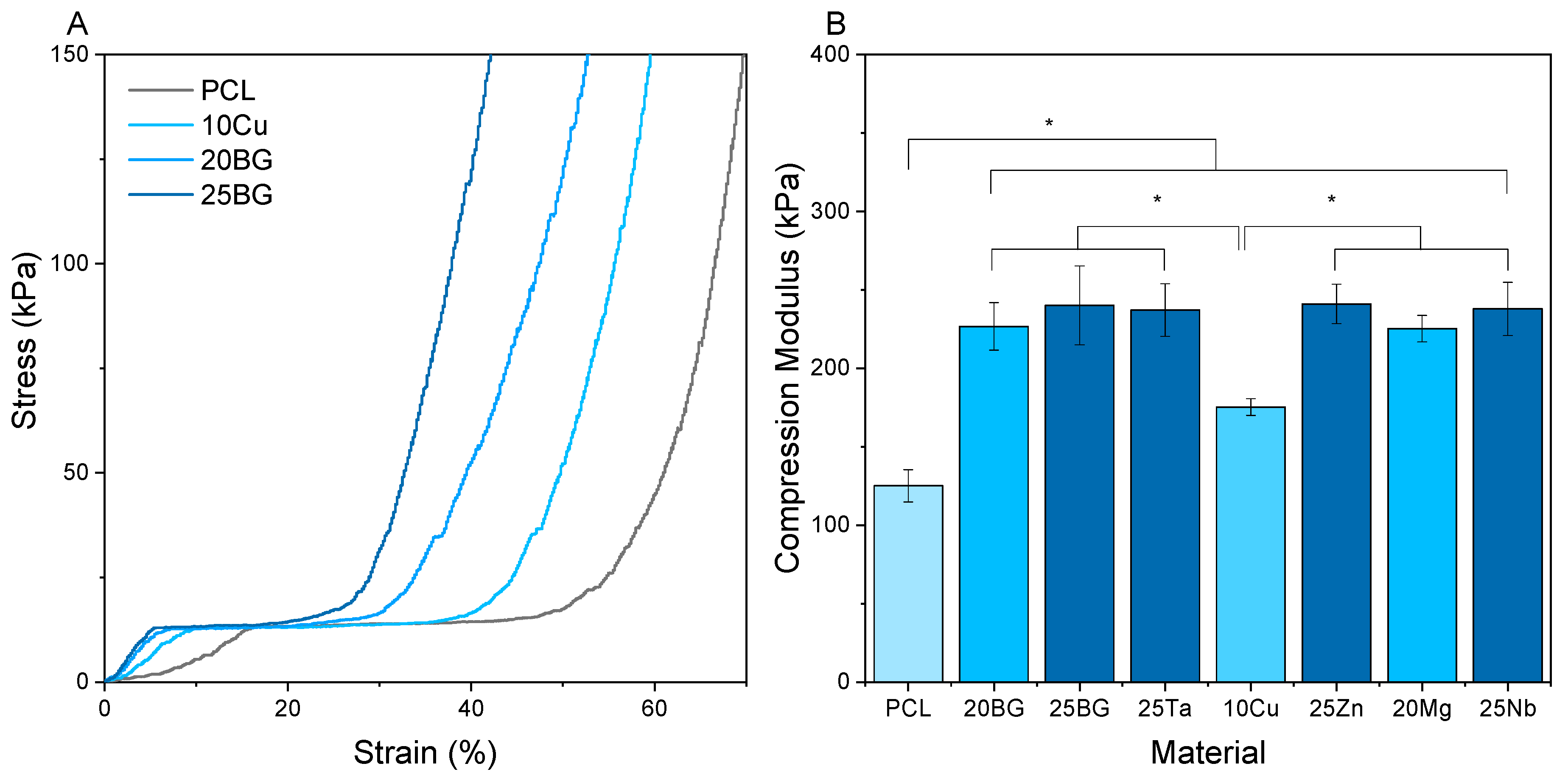
3.3. Compression Modulus
3.4. Cell Culture
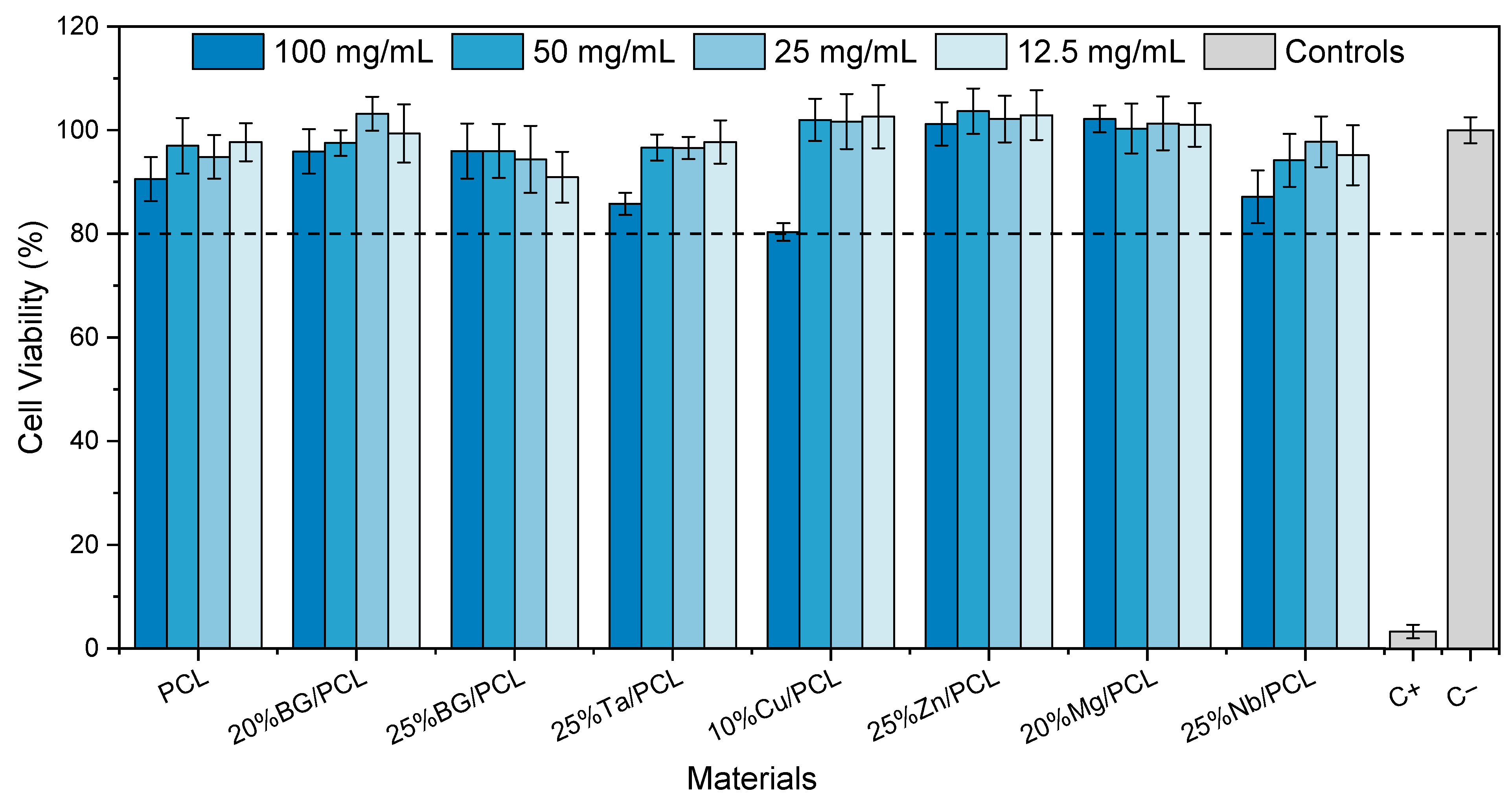
3.4.1. Cytotoxicity Assay
3.4.2. Cell Adhesion and Proliferation
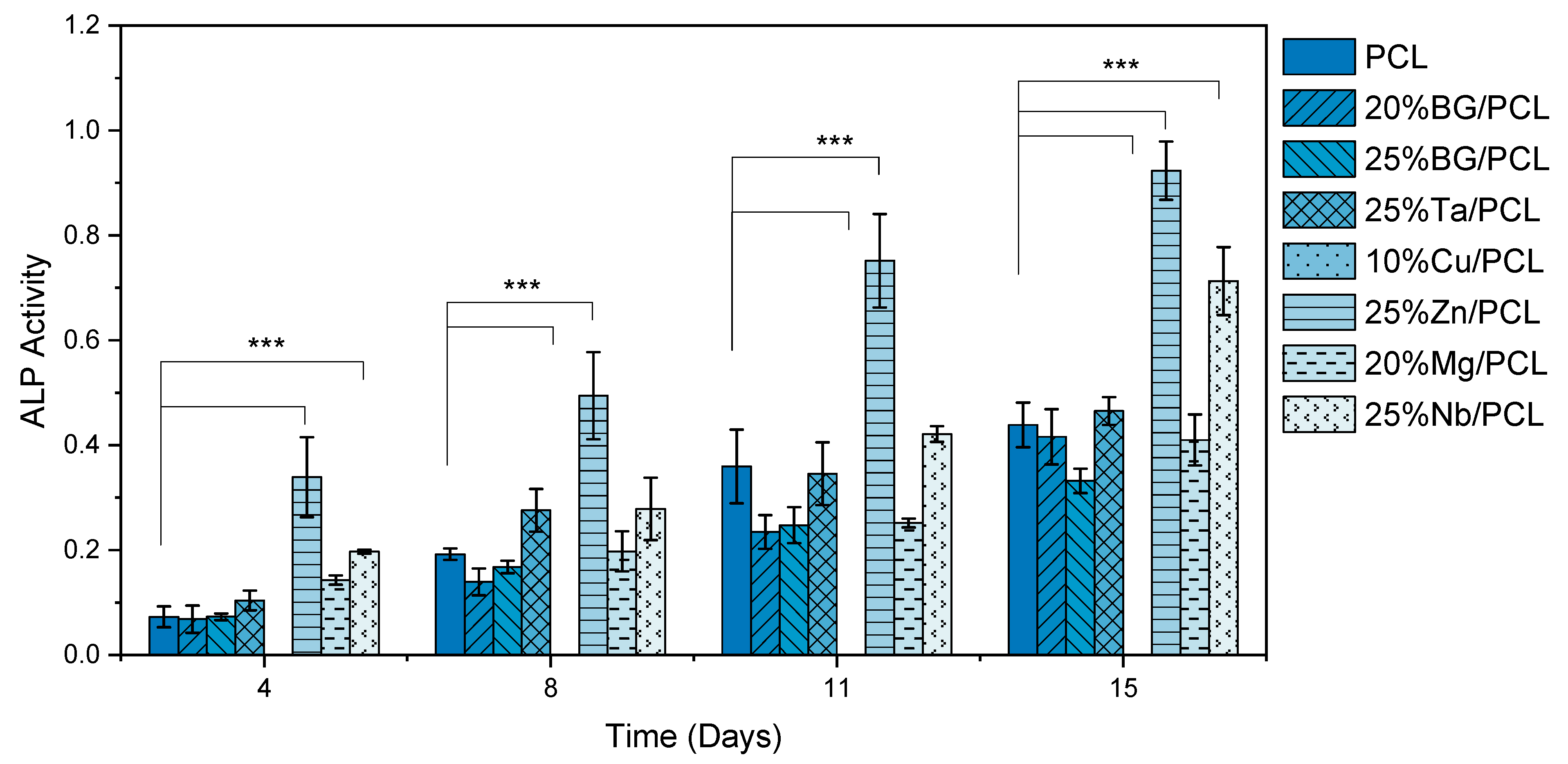
3.4.3. ALP Activity
3.4.4. Immunofluorescence Staining
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ATR-FTIR | Attenuated total reflectance Fourier-transform infrared |
| BG | Bioactive glass |
| Col I | Collagen type I |
| PRF | Platelet-rich fibrin |
| SEM/EDS | Scanning electron microscopy/energy dispersive X-ray spectroscopy |
| MBG | Mesoporous bioactive glass |
| NFC | Nanofibrillated cellulose |
| OCN | Osteocalcin |
| PBS | Phosphate-buffered saline |
| PCL | Polycaprolactone |
| PDLSCs | Periodontal ligament stem cells |
| PRF | Platelet-rich fibrin |
| RUNX2 | Runt-related transcription factor 2 |
| TII | Therapeutic inorganic ions |
References
- Mouriño, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 2010, 7, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic Approaches in Bone Tissue Engineering: Integrating Biological and Physicomechanical Strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone Tissue Engineering: A Review in Bone Biomimetics and Drug Delivery Strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of Bone Grafting Materials for Bone Repair and Regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Kaing, L.; Grubor, D.; Chandu, A. Assessment of Bone Grafts Placed within an Oral and Maxillofacial Training Programme for Implant Rehabilitation. Aust. Dent. J. 2011, 56, 406–411. [Google Scholar] [CrossRef]
- de Melo Pereira, D.; Habibovic, P. Biomineralization-Inspired Material Design for Bone Regeneration. Adv. Healthc. Mater. 2018, 7, 1800700. [Google Scholar] [CrossRef]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone Tissue Engineering via Growth Factor Delivery: From Scaffolds to Complex Matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Balice, G.; Paolantonio, M.; De Ninis, P.; Rexhepi, I.; Serroni, M.; Frisone, A.; Romano, L.; Sinjari, B.; Murmura, G.; Femminella, B. Treatment of Unfavorable Intrabony Defects with Autogenous Bone Graft in Combination with Leukocyte- and Platelet-Rich Fibrin or Collagen Membranes: A Non-Inferiority Study. Medicina 2024, 60, 1091. [Google Scholar] [CrossRef]
- Lin, S.; Yang, G.; Jiang, F.; Zhou, M.; Yin, S.; Tang, Y.; Tang, T.; Zhang, Z.; Zhang, W.; Jiang, X. A Magnesium-Enriched 3D Culture System That Mimics the Bone Development Microenvironment for Vascularized Bone Regeneration. Adv. Sci. 2019, 6, 1900209. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, e1901495. [Google Scholar] [CrossRef]
- Whitlow, J.; Paul, A.; Polini, A. Bioactive Materials: Definitions and Application in Tissue Engineering and Regeneration Therapy. Adv. Struct. Mater. 2016, 53, 1–17. [Google Scholar] [CrossRef]
- Jell, G.; Stevens, M.M. Gene Activation by Bioactive Glasses. J. Mater. Sci. Mater. Med. 2006, 17, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive Glasses: Sprouting Angiogenesis in Tissue Engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Björkenheim, R.; Jämsen, E.; Eriksson, E.; Uppstu, P.; Aalto-Setälä, L.; Hupa, L.; Eklund, K.; Ainola, M.; Lindfors, N.; Pajarinen, J. Sintered S53P4 Bioactive Glass Scaffolds Have Anti-Inflammatory Properties and Stimulate Osteogenesis in Vitro. Eur. Cell Mater. 2021, 41, 15–30. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Dong, Y. Regeneration of Pulp-Dentine Complex-like Tissue in a Rat Experimental Model under an Inflammatory Microenvironment Using High Phosphorous-Containing Bioactive Glasses. Int. Endod. J. 2021, 54, 1129–1141. [Google Scholar] [CrossRef]
- Bosetti, M.; Hench, L.; Cannas, M. Interaction of Bioactive Glasses with Peritoneal Macrophages and Monocytes in Vitro. J. Biomed. Mater. Res. 2002, 60, 79–85. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Q.; Lin, C.; Li, X.; Chen, X.; Hu, Q. Facile Synthesis and Characterization of Novel Rapid-Setting Spherical Sub-Micron Bioactive Glasses Cements and Their Biocompatibility in Vitro. Mater. Sci. Eng. C 2017, 75, 646–652. [Google Scholar] [CrossRef]
- Habraken, W.J.E.M.; Wolke, J.G.C.; Jansen, J.A. Ceramic Composites as Matrices and Scaffolds for Drug Delivery in Tissue Engineering. Adv. Drug Deliv. Rev. 2007, 59, 234–248. [Google Scholar] [CrossRef]
- Martelli, A.; Bellucci, D.; Cannillo, V. Additive Manufacturing of Polymer/Bioactive Glass Scaffolds for Regenerative Medicine: A Review. Polymers 2023, 15, 2473. [Google Scholar] [CrossRef]
- Tulyaganov, D.U.; Akbarov, A.; Ziyadullaeva, N.; Khabilov, B.; Baino, F. Injectable Bioactive Glass-Based Pastes for Potential Use in Bone Tissue Repair. Biomed. Glas. 2020, 6, 23–33. [Google Scholar] [CrossRef]
- Profeta, A.C.; Prucher, G.M. Bioactive-Glass in Periodontal Surgery and Implant Dentistry. Dent. Mater. J. 2015, 34, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.J.; Vallet-Regí, M. Glasses in Bone Regeneration: A Multiscale Issue. J. Non Cryst. Solids 2016, 432, 9–14. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Barberi, J.; Kargozar, S.; Marchi, J.; Massera, J.; Verné, E. Processing Methods for Making Porous Bioactive Glass-based Scaffolds—A State-of-the-art Review. Int. J. Appl. Ceram. Technol. 2019, 16, 1762–1796. [Google Scholar] [CrossRef]
- Qin, X.; Wu, D. Effect of Different Solvents on Poly(Caprolactone)(PCL) Electrospun Nonwoven Membranes. J. Therm. Anal. Calorim. 2012, 107, 1007–1013. [Google Scholar] [CrossRef]
- Pádua, A.S.; Figueiredo, L.; Silva, J.C.; Borges, J.P. Chitosan Scaffolds with Mesoporous Hydroxyapatite and Mesoporous Bioactive Glass. Prog. Biomater. 2023, 12, 137–153. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mitra, I.; Bose, S. 3D Printing for Bone Regeneration. Curr. Osteoporos. Rep. 2020, 18, 505–514. [Google Scholar] [CrossRef]
- Ivanovski, S.; Breik, O.; Carluccio, D.; Alayan, J.; Staples, R.; Vaquette, C. 3D Printing for Bone Regeneration: Challenges and Opportunities for Achieving Predictability. Periodontol. 2000 2023, 93, 358–384. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Casarrubios, L.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F.M.; Portolés, M.T.; et al. Mesoporous Bioactive Glass/ɛ-Polycaprolactone Scaffolds Promote Bone Regeneration in Osteoporotic Sheep. Acta Biomater. 2019, 90, 393–402. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Sánchez-Salcedo, S.; Izquierdo-Barba, I.; Arcos, D.; Vallet-Regí, M. In Vitro Colonization of Stratified Bioactive Scaffolds by Pre-Osteoblast Cells. Acta Biomater. 2016, 44, 73–84. [Google Scholar] [CrossRef]
- Wang, C.; Meng, C.; Zhang, Z.; Zhu, Q. 3D Printing of Polycaprolactone/Bioactive Glass Composite Scaffolds for in Situ Bone Repair. Ceram. Int. 2022, 48, 7491–7499. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Pádua, A.S.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Costa, L.C.; Graça, M.P.F. Biocompatibility, Bioactivity, and Antibacterial Behaviour of Cerium-Containing Bioglass®. Nanomaterials 2022, 12, 4479. [Google Scholar] [CrossRef]
- Hammami, I.; Gavinho, S.R.; Pádua, A.S.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Valente, M.A.; Graça, M.P.F. Bioactive Glass Modified with Zirconium Incorporation for Dental Implant Applications: Fabrication, Structural, Electrical, and Biological Analysis. Int. J. Mol. Sci. 2023, 24, 10571. [Google Scholar] [CrossRef]
- Mozafari, M.; Rajadas, J.; Kaplan, D.L. Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128133552. [Google Scholar]
- Nagrath, M.; Gallant, R.; Yazdi, A.R.; Mendonca, A.; Rahman, S.; Chiu, L.; Waldman, S.D.; Ni, H.; Towler, M.R. Tantalum-Containing Mesoporous Bioactive Glass Powder for Hemostasis. J. Biomater. Appl. 2021, 35, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Nagrath, M.; Bince, D.; Rowsell, C.; Polintan, D.; Rezende-Neto, J.; Towler, M. Porcine Liver Injury Model to Assess Tantalum-Containing Bioactive Glass Powders for Hemostasis. J. Mater. Sci. Mater. Med. 2022, 33, 53. [Google Scholar] [CrossRef] [PubMed]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive Glasses Incorporating Less-Common Ions to Improve Biological and Physical Properties. J. Mater. Sci. Mater. Med. 2022, 33, 3. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zheng, K.; Zhou, T.; Boccaccini, A.R. Incorporation of Zinc into Binary SiO2-CaO Mesoporous Bioactive Glass Nanoparticles Enhances Anti-Inflammatory and Osteogenic Activities. Pharmaceutics 2021, 13, 2124. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, F.; Liu, J.; Smått, J.H.; Gepperth, D.; Lastusaari, M.; Xu, C.; Hupa, L. Biocomposites of Copper-Containing Mesoporous Bioactive Glass and Nanofibrillated Cellulose: Biocompatibility and Angiogenic Promotion in Chronic Wound Healing Application. Acta Biomater. 2016, 46, 286–298. [Google Scholar] [CrossRef]
- Li, J.; Zhai, D.; Lv, F.; Yu, Q.; Ma, H.; Yin, J.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Preparation of Copper-Containing Bioactive Glass/Eggshell Membrane Nanocomposites for Improving Angiogenesis, Antibacterial Activity and Wound Healing. Acta Biomater. 2016, 36, 254–266. [Google Scholar] [CrossRef]
- Ryan, E.J.; Ryan, A.J.; González-Vázquez, A.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.J.; O’Brien, F.J. Collagen Scaffolds Functionalised with Copper-Eluting Bioactive Glass Reduce Infection and Enhance Osteogenesis and Angiogenesis Both in Vitro and in Vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Gavinho, S.R.; Jakka, S.K.; Valente, M.A.; Graça, M.P.F.; Pádua, A.S.; Silva, J.C.; Sá-Nogueira, I.; Borges, J.P. Antibacterial Biomaterial Based on Bioglass Modified with Copper for Implants Coating. J. Funct. Biomater. 2023, 14, 369. [Google Scholar] [CrossRef]
- Dziadek, M.; Zagrajczuk, B.; Menaszek, E.; Dziadek, K.; Cholewa-Kowalska, K. A Simple Way of Modulating in Vitro Angiogenic Response Using Cu and Co-Doped Bioactive Glasses. Mater. Lett. 2018, 215, 87–90. [Google Scholar] [CrossRef]
- Sergi, R.; Cannillo, V.; Boccaccini, A.R.; Liverani, L. Incorporation of Bioactive Glasses Containing Mg, Sr, and Zn in Electrospun PCL Fibers by Using Benign Solvents. Appl. Sci. 2020, 10, 5530. [Google Scholar] [CrossRef]
- Fathi, A.; Kermani, F.; Behnamghader, A.; Banijamali, S.; Mozafari, M.; Baino, F.; Kargozar, S. Three-Dimensionally Printed Polycaprolactone/Multicomponent Bioactive Glass Scaffolds for Potential Application in Bone Tissue Engineering. Biomed. Glas. 2021, 6, 57–69. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Baciu, D.; Gounari, E.; Steriotis, T.; Charalambopoulou, G.; Tzetzis, D.; Bikiaris, D. Composite Membranes of Poly(ε-Caprolactone) with Bisphosphonate-Loaded Bioactive Glasses for Potential Bone Tissue Engineering Applications. Molecules 2019, 24, 3067. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.K.; Lali, F.V.; Autefage, H.; Agarwal, S.; Nommeots-Nomm, A.; Metcalfe, A.D.; Stevens, M.M.; Jones, J.R. Bioactive Glasses and Electrospun Composites That Release Cobalt to Stimulate the HIF Pathway for Wound Healing Applications. Biomater. Res. 2021, 25, 1–16. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Pádua, A.S.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Costa, L.C.; Graça, M.P.F. Fabrication, Structural and Biological Characterization of Zinc-Containing Bioactive Glasses and Their Use in Membranes for Guided Bone Regeneration. Materials 2023, 16, 956. [Google Scholar] [CrossRef]
- Bai, X.; Liu, W.; Xu, L.; Ye, Q.; Zhou, H.; Berg, C.; Yuan, H.; Li, J.; Xia, W. Sequential Macrophage Transition Facilitates Endogenous Bone Regeneration Induced by Zn-Doped Porous Microcrystalline Bioactive Glass. J. Mater. Chem. B 2021, 9, 2885–2898. [Google Scholar] [CrossRef]
- Li, Z.; Xie, K.; Yang, S.; Yu, T.; Xiao, Y.; Zhou, Y. Multifunctional Ca-Zn-Si-Based Micro-Nano Spheres with Anti-Infective, Anti-Inflammatory, and Dentin Regenerative Properties for Pulp Capping Application. J. Mater. Chem. B 2021, 9, 8289–8299. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Pádua, A.S.; Holz, L.I.V.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Valente, M.A.; Graça, M.P.F. Bioactive Glasses Containing Strontium or Magnesium Ions to Enhance the Biological Response in Bone Regeneration. Nanomaterials 2023, 13, 2717. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Gavinho, S.R.; Pádua, A.S.; Graça, M.P.F.; Silva, J.C. Synthesis and Characterization of Iron Containing Bioactive Glass for Implants. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–18 November 2022; pp. 45–48. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Tavares, F.J.T.M.; Soares, P.I.P.; Silva, J.C.; Borges, J.P. Preparation and In Vitro Characterization of Magnetic CS/PVA/HA/PSPIONs Scaffolds for Magnetic Hyperthermia and Bone Regeneration. Int. J. Mol. Sci. 2023, 24, 1128. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun Poly(ε-Caprolactone)/Gelatin Nanofibrous Scaffolds for Nerve Tissue Engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and Characterization of PCL/Gelatin Composite Nanofibrous Scaffold for Tissue Engineering Applications by Electrospinning Method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef]
- Peng, H.; Han, Y.; Liu, T.; Tjiu, W.C.; He, C. Morphology and Thermal Degradation Behavior of Highly Exfoliated CoAl-Layered Double Hydroxide/Polycaprolactone Nanocomposites Prepared by Simple Solution Intercalation. Thermochim. Acta 2010, 502, 1–7. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Ito, A.; Sogo, Y. Synthesis and Characterization of Hierarchically Macroporous and Mesoporous CaO–MO–SiO2–P2O5 (M=Mg, Zn, Sr) Bioactive Glass Scaffolds. Acta Biomater. 2011, 7, 3638–3644. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Casarrubios, L.; Morales, I.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Effects of a Mesoporous Bioactive Glass on Osteoblasts, Osteoclasts and Macrophages. J. Colloid Interface Sci. 2018, 528, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Moghanian, A.; Sedghi, A.; Ghorbanoghli, A.; Salari, E. The Effect of Magnesium Content on in Vitro Bioactivity, Biological Behavior and Antibacterial Activity of Sol–Gel Derived 58S Bioactive Glass. Ceram. Int. 2018, 44, 9422–9432. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Kang, Y.; Chang, J. Channels in a Porous Scaffold: A New Player for Vascularization. Regen. Med. 2018, 13, 705–715. [Google Scholar] [CrossRef]
- Floriano, J.F.; Emanueli, C.; Vega, S.; Barbosa, A.M.P.; de Oliveira, R.G.; Floriano, E.A.F.; de Oliveira Graeff, C.F.; Abbade, J.F.; Herculano, R.D.; Sobrevia, L.; et al. Pro-Angiogenic Approach for Skeletal Muscle Regeneration. Biochim. Biophys. Acta (BBiophys. Acta (BBA)-Gen. Subj. 2021, 1866, 130059. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X.; Choi, J. Biodegradable Polymer Scaffolds with Well-Defined Interconnected Spherical Pore Network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef]
- Kanungo, B.P.; Silva, E.; Van Vliet, K.; Gibson, L.J. Characterization of Mineralized Collagen-Glycosaminoglycan Scaffolds for Bone Regeneration. Acta Biomater. 2008, 4, 490–503. [Google Scholar] [CrossRef]
- Dorj, B.; Won, J.E.; Kim, J.H.; Choi, S.J.; Shin, U.S.; Kim, H.W. Robocasting Nanocomposite Scaffolds of Poly(Caprolactone)/Hydroxyapatite Incorporating Modified Carbon Nanotubes for Hard Tissue Reconstruction. J. Biomed. Mater. Res. A 2013, 101 A, 1670–1681. [Google Scholar] [CrossRef]
- Cui, N.; Qian, J.; Wang, J.; Ji, C.; Xu, W.; Wang, H. Preparation, Physicochemical Properties and Biocompatibility of PBLG/PLGA/Bioglass Composite Scaffolds. Mater. Sci. Eng. C 2017, 71, 118–124. [Google Scholar] [CrossRef]
- Lores, N.J.; Aráoz, B.; Hung, X.; Talou, M.H.; Boccaccini, A.R.; Abraham, G.A.; Hermida, É.B.; Caracciolo, P.C. 3D-Printed Poly(Ester Urethane)/Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Bioglass Scaffolds for Tissue Engineering Applications. Polymers 2024, 16, 3355. [Google Scholar] [CrossRef]
- Obata, A.; Takahashi, Y.; Miyajima, T.; Ueda, K.; Narushima, T.; Kasuga, T. Effects of Niobium Ions Released from Calcium Phosphate Invert Glasses Containing Nb 2 O 5 on Osteoblast-Like Cell Functions. ACS Appl. Mater. Interfaces 2012, 4, 5684–5690. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.H.; Souza, L.P.; Domingues, J.A.; Ferreira, F.V.; de Alencar Hausen, M.; Camilli, J.A.; Martin, R.A.; de Rezende Duek, E.A.; Mazali, I.O.; Bertran, C.A. In Vitro and In Vivo Osteogenic Potential of Niobium-Doped 45S5 Bioactive Glass: A Comparative Study. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1372–1387. [Google Scholar] [CrossRef] [PubMed]
- Alhalawani, A.M.; Mehrvar, C.; Stone, W.; Waldman, S.D.; Towler, M.R. A Novel Tantalum-Containing Bioglass. Part II. Development of a Bioadhesive for Sternal Fixation and Repair. Mater. Sci. Eng. C 2017, 71, 401–411. [Google Scholar] [CrossRef]
- Westhauser, F.; Decker, S.; Nawaz, Q.; Rehder, F.; Wilkesmann, S.; Moghaddam, A.; Kunisch, E.; Boccaccini, A.R. Impact of Zinc-or Copper-Doped Mesoporous Bioactive Glass Nanoparticles on the Osteogenic Differentiation and Matrix Formation of Mesenchymal Stromal Cells. Materials 2021, 14, 1864. [Google Scholar] [CrossRef]

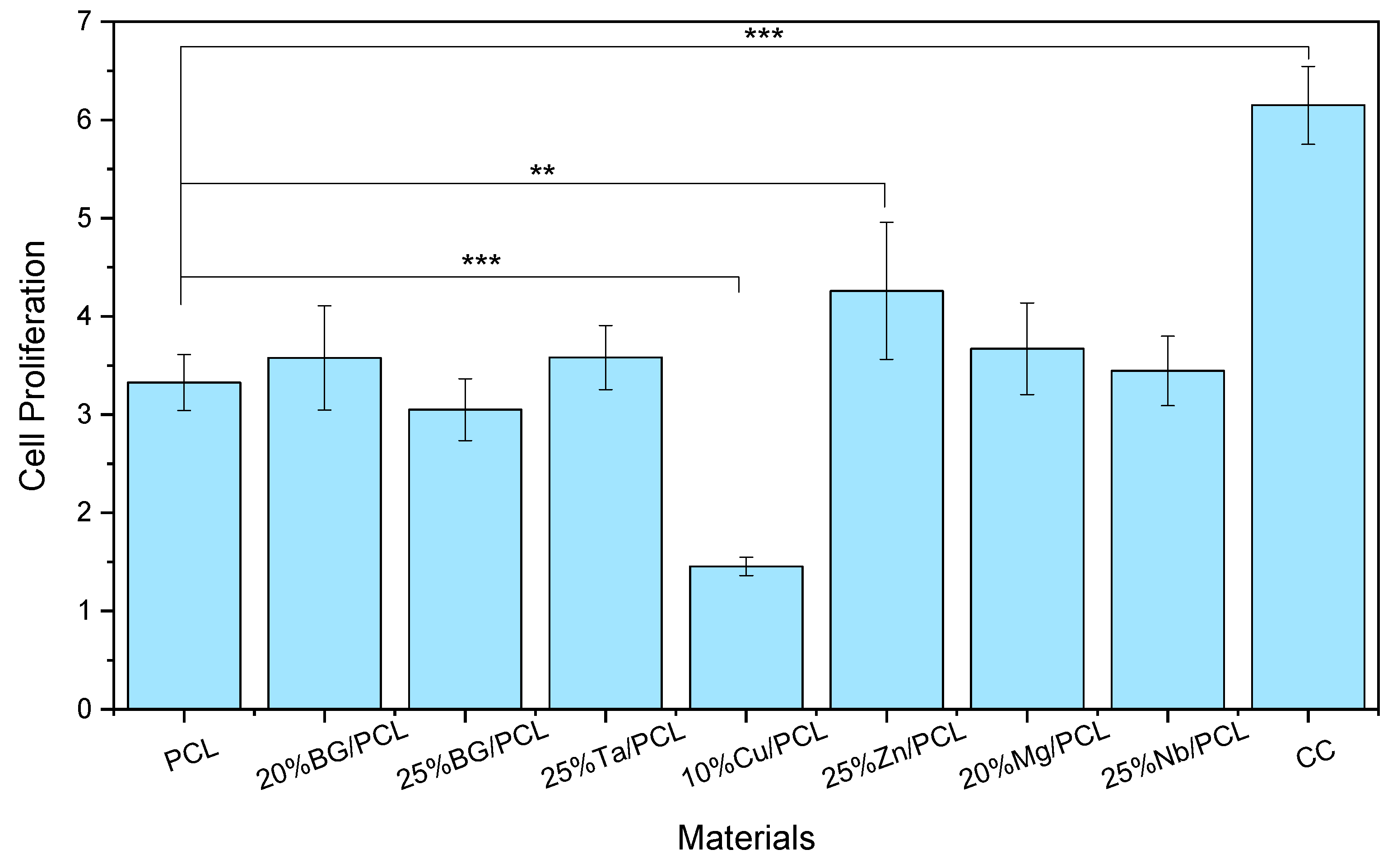
| Sample Name | PCL | NaCl | BG | BG/PCL Ratio |
|---|---|---|---|---|
| PCL | 10% | 5% | - | 0% |
| 20BG | 45S5 BG | 20% | ||
| 25BG | 45S5 BG | 25% | ||
| 25Ta | 4 mol% Ta doped BG | 25% | ||
| 10Cu | 1 mol% Cu doped BG | 10% | ||
| 25Zn | 4 mol% Zn doped BG | 25% | ||
| 20Mg | 4 mol% Mg doped BG | 20% | ||
| 25Nb | 4 mol% Nb doped BG | 25% |
| DAPI | Phaloidin | Merge | |
|---|---|---|---|
| PCL |  | 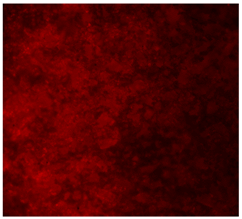 | 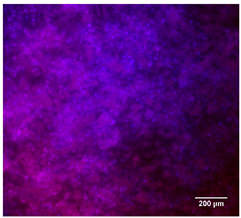 |
| 20%BG/PCL | 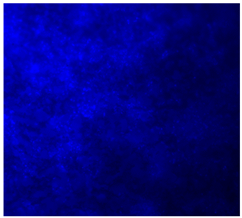 | 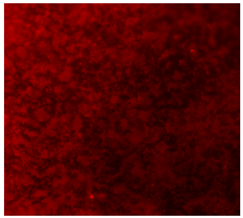 | 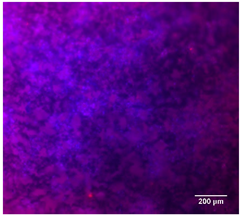 |
| 25%BG/PCL | 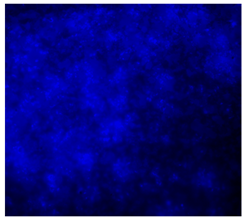 | 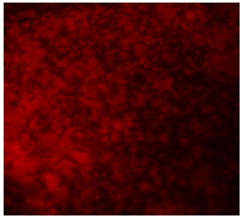 | 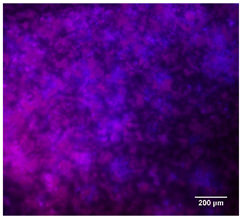 |
| 25%Ta/PCL | 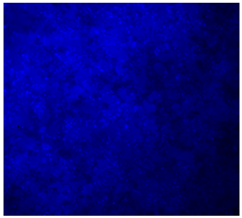 | 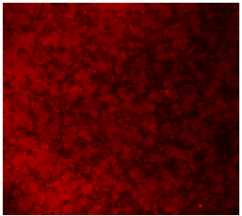 | 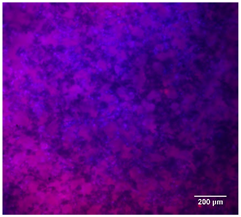 |
| 10%Cu/PCL | 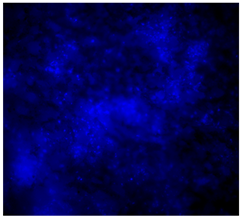 | 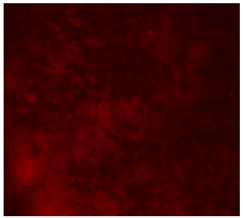 | 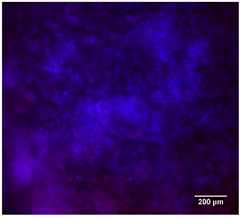 |
| 25%Zn/PCL | 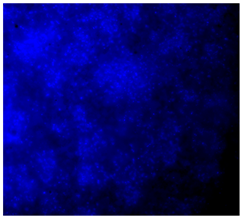 | 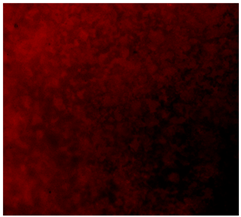 | 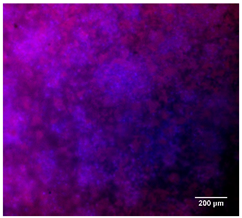 |
| 20%Mg/PCL | 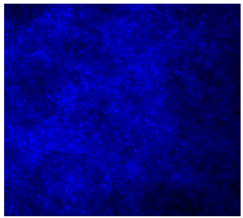 | 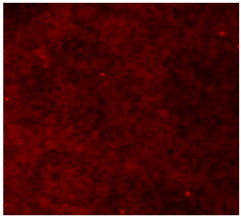 |  |
| 25%Nb/PCL |  | 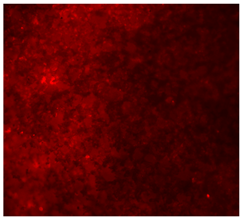 | 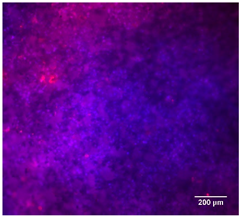 |
| Cell Control | 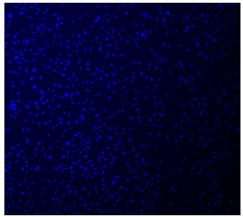 | 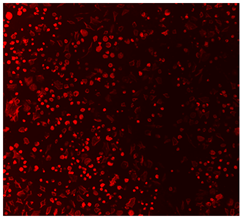 | 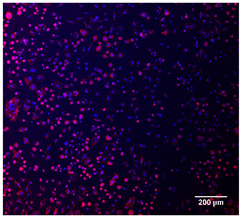 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pádua, A.S.; Graça, M.P.F.; Silva, J.C. Polycaprolactone/Doped Bioactive Glass Composite Scaffolds for Bone Regeneration. J. Funct. Biomater. 2025, 16, 200. https://doi.org/10.3390/jfb16060200
Pádua AS, Graça MPF, Silva JC. Polycaprolactone/Doped Bioactive Glass Composite Scaffolds for Bone Regeneration. Journal of Functional Biomaterials. 2025; 16(6):200. https://doi.org/10.3390/jfb16060200
Chicago/Turabian StylePádua, Ana Sofia, Manuel Pedro Fernandes Graça, and Jorge Carvalho Silva. 2025. "Polycaprolactone/Doped Bioactive Glass Composite Scaffolds for Bone Regeneration" Journal of Functional Biomaterials 16, no. 6: 200. https://doi.org/10.3390/jfb16060200
APA StylePádua, A. S., Graça, M. P. F., & Silva, J. C. (2025). Polycaprolactone/Doped Bioactive Glass Composite Scaffolds for Bone Regeneration. Journal of Functional Biomaterials, 16(6), 200. https://doi.org/10.3390/jfb16060200







