In Vivo Cell Migration and Growth Within Electrospun Porous Nanofibrous Scaffolds with Different Pore Sizes in a Mouse Pouch Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
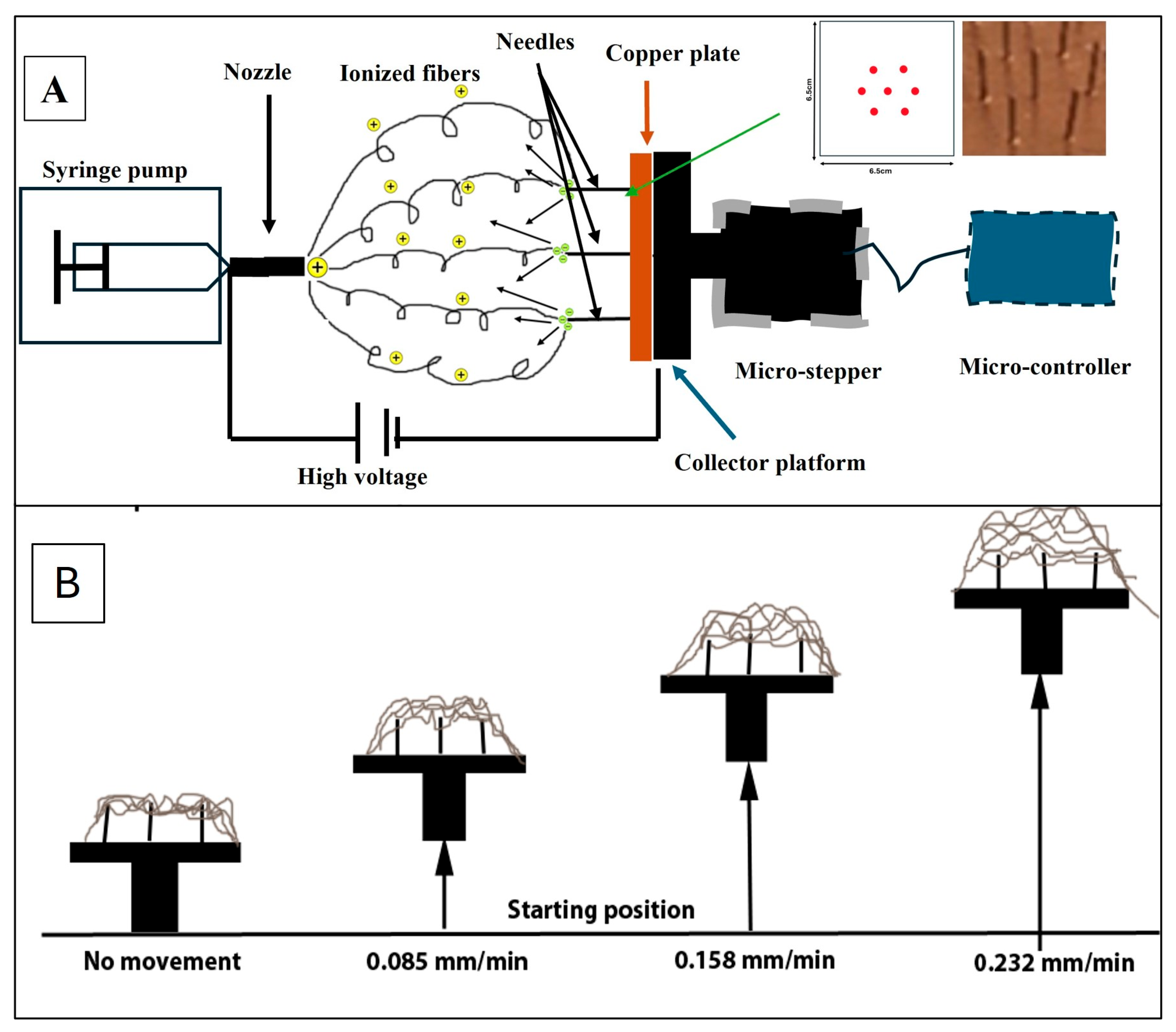
2.2. Electrospun PCL NF Sheets Preparation
2.3. Scanning Electron Microscopy (SEM)
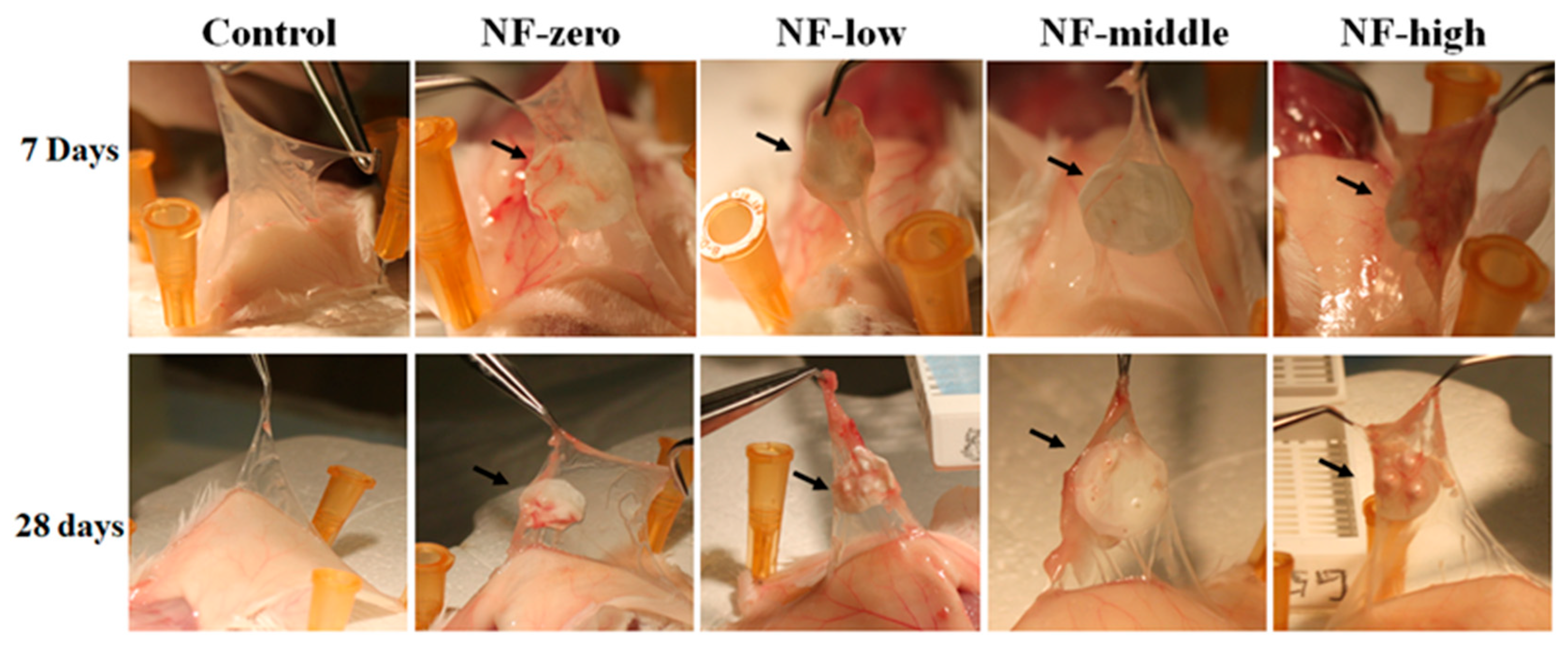
2.4. Mouse Air Pouch Model
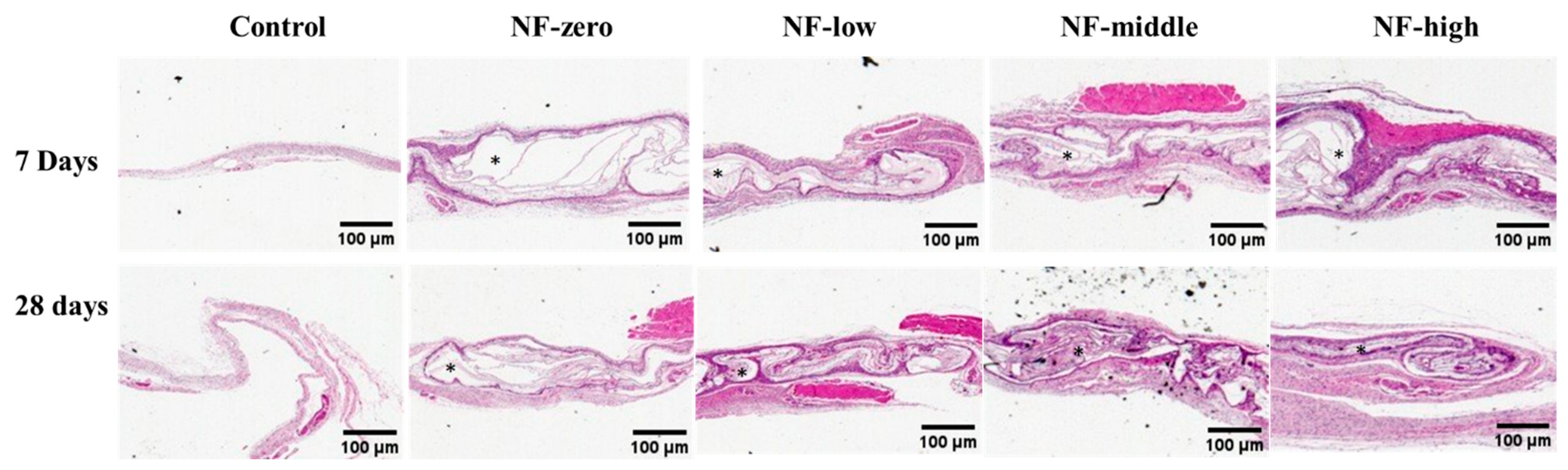
2.5. Histological Analysis
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Jonge, L.T.; Leeuwenburgh, S.C.; Wolke, J.G.; Jansen, J.A. Organic–inorganic surface modifications for titanium implant surfaces. Pharm. Res. 2008, 25, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Handorf, A.M.; Ionescu, L.C.; Li, W.J.; Mauck, R.L. New directions in nanofibrous scaffolds for soft tissue engineering and regeneration. Expert Rev. Med. Devices 2009, 6, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 12, 9622–9629. [Google Scholar] [CrossRef] [PubMed]
- Ifkovits, J.L.; Sundararaghavan, H.G.; Burdick, J.A. Electrospinning fibrous polymer scaffolds for tissue engineering and cell culture. J. Vis. Exp. 2009, 32, 1589–1593. [Google Scholar]
- Skotak, M.; Ragusa, J.; Gonzalez, D.; Subramanian, A. Improved cellular infiltration into nanofibrous electrospun cross-linked gelatin scaffolds templated with micrometer-sized polyethylene glycol fibers. Biomed. Mater. 2011, 6, 055012. [Google Scholar] [CrossRef]
- Yang, A.; Huang, Z.; Yin, G.; Pu, X. Fabrication of aligned, porous and conductive fibers and their effects on cell adhesion and guidance. Colloids Surf. B Biointerfaces 2015, 134, 469–474. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Woo, K.M.; Chen, V.J.; Ma, P.X. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J. Biomed. Mater. Res. A 2003, 11, 531–537. [Google Scholar] [CrossRef]
- Lowery, J.L.; Datta, N.; Rutledge, G.C. Effect of fiber diameter, pore size and seeding method on growth of human dermal fibroblasts in electrospun poly (ε-caprolactone) fibrous mats. Biomaterials 2010, 31, 491–504. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Chauhan, N.S.; Yadav, G.; Goswami, M.; Packirisamy, G. Design and fabrication of a dual protein-based trilayered nanofibrous scaffold for efficient wound healing. ACS Appl. Bio Mater. 2022, 6, 2726–2740. [Google Scholar] [CrossRef]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.J.; Dean, D.R.; Jun, H.W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials 2011, 32, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Schofer, M.D.; Roessler, P.P.; Schaefer, J.; Theisen, C.; Schlimme, S.; Heverhagen, J.T.; Voelker, M.; Dersch, R.; Agarwal, S.; Fuchs-Winkelmann, S.; et al. Electrospun PLLA nanofiber scaffolds and their use in combination with BMP-2 for reconstruction of bone defects. PLoS ONE 2011, 6, e25462. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthi, D.; Vasanthan, K.S.; Kuppan, P.; Krishnan, U.M.; Sethuraman, S. Electrospun nanostructured chitosan–poly (vinyl alcohol) scaffolds: A biomimetic extracellular matrix as dermal substitute. Biomed. Mater. 2012, 8, 045005. [Google Scholar] [CrossRef]
- Gaydhane, M.K.; Sharma, C.S.; Majumdar, S. Electrospun nanofibres in drug delivery: Advances in controlled release strategies. RSC Adv. 2023, 3, 7312–7328. [Google Scholar] [CrossRef]
- Agarwal, A.; Rao, G.K.; Majumder, S.; Shandilya, M.; Rawat, V.; Purwar, R.; Verma, M.; Srivastava, C.M. Natural protein-based electrospun nanofibers for advanced healthcare applications: Progress and challenges. 3 Biotech 2022, 12, 92. [Google Scholar] [CrossRef]
- Sasmal, P.; Datta, P. Tranexamic acid-loaded chitosan electrospun nanofibers as drug delivery system for hemorrhage control applications. J. Drug Deliv. Sci. Technol. 2019, 52, 559–567. [Google Scholar] [CrossRef]
- Liu, W.; Lipner, J.; Moran, C.H.; Feng, L.; Li, X.; Thomopoulos, S.; Xia, Y. Generation of electrospun nanofibers with controllable degrees of crimping through a simple, plasticizer-based treatment. Adv. Mater. 2015, 27, 2583–2588. [Google Scholar] [CrossRef]
- Weightman, A.; Jenkins, S.; Pickard, M.; Chari, D.; Yang, Y. Alignment of multiple glial cell populations in 3D nanofiber scaffolds: Toward the development of multicellular implantable scaffolds for repair of neural injury. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 291–295. [Google Scholar] [CrossRef]
- Lee, H.; Yeo, M.; Ahn, S.; Kang, D.O.; Jang, C.H.; Lee, H.; Park, G.M.; Kim, G.H. Designed hybrid scaffolds consisting of polycaprolactone microstrands and electrospun collagen-nanofibers for bone tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 5, 263–270. [Google Scholar] [CrossRef]
- Teo, W.E.; Inai, R.; Ramakrishna, S. Technological advances in electrospinning of nanofibers. Sci. Technol. Adv. Mater. 2011, 12, 013002. [Google Scholar] [CrossRef]
- Baker, B.M.; Gee, A.O.; Metter, R.B.; Nathan, A.S.; Marklein, R.A.; Burdick, J.A.; Mauck, R.L. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 2008, 29, 2348–2358. [Google Scholar] [CrossRef]
- Phipps, M.C.; Clem, W.C.; Grunda, J.M.; Clines, G.A.; Bellis, S.L. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 2012, 33, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007, 13, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Simonet, M.; Schneider, O.D.; Neuenschwander, P.; Stark, W.J. Ultraporous 3D polymer meshes by low-temperature electrospinning: Use of ice crystals as a removable void template. Polym. Eng. Sci. 2007, 47, 2020–2026. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Metter, R.B.; Burdick, J.A. Electrospun fibrous scaffolds with multiscale and photopatterned porosity. Macromol. Biosci. 2010, 10, 265–270. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Hattori, S.; Yoshikawa, C.; Yasuda, Y.; Koyama, H.; Takato, T.; Kobayashi, H. Novel wet electrospinning system for fabrication of spongiform nanofiber 3-dimensional fabric. Mater. Lett. 2009, 63, 754–756. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef]
- Chvojka, J.; Hinestroza, J.P.; Lukas, D. Production of poly (vinylalcohol) nanoyarns using a special saw-like collector. Fibres Text. East. Eur. 2013, 21, 28–31. [Google Scholar]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly (ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef]
- Shim, I.K.; Suh, W.H.; Lee, S.Y.; Lee, S.H.; Heo, S.J.; Lee, M.C.; Lee, S.J. Chitosan nano-/microfibrous double-layered membrane with rolled-up three-dimensional structures for chondrocyte cultivation. J. Biomed. Mater. Res. Part A 2009, 90, 595–602. [Google Scholar] [CrossRef]
- Thorvaldsson, A.; Stenhamre, H.; Gatenholm, P.; Walkenström, P. Electrospinning of highly porous scaffolds for cartilage regeneration. Biomacromolecules 2008, 9, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.; Bűttner, U.; Sanderson, R.D. Continuous yarns from electrospun fibers. Polymer 2005, 46, 2419–2423. [Google Scholar] [CrossRef]
- Song, W.; Chen, L.; Seta, J.; Markel, D.C.; Yu, X.; Ren, W. Corona discharge: A novel approach to fabricate three-dimensional electrospun nanofibers for bone tissue engineering. ACS Biomater. Sci. Eng. 2017, 3, 1146–1153. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Simon, C.G., Jr.; Yaszemski, M.J.; Ratcliffe, A.; Tomlins, P.; Luginbuehl, R.; Tesk, J.A. ASTM international workshop on standards and measurements for tissue engineering scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 949–959. [Google Scholar] [CrossRef]
- Chen, L.; Ameer, A.S.; Rea, C.; Mazeh, H.; Wu, X.; Chen, W.; Li, Y.; Song, W.; Markel, D.C.; Ren, W. Preparation of electrospun nanofibers with desired microstructures using a programmed three-dimensional (3D) nanofiber collector. Mater. Sci. Eng. C 2020, 106, 110188. [Google Scholar] [CrossRef]
- Song, W.; Yu, X.; Markel, D.C.; Shi, T.; Ren, W. Coaxial PCL/PVA electrospun nanofibers: Osseointegration enhancer and controlled drug release device. Biofabrication 2013, 5, 035006. [Google Scholar] [CrossRef]
- Markel, D.C.; Jackson, N.M.; Esquivel, A.O.; Ren, W.; Flynn, J.C. Immunological response to bolus versus multiple injections of hylan G-F 20 in a murine biocompatibility model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1375–1380. [Google Scholar] [CrossRef]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-based composite scaffold matrices for tissue engineering applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Jang, J.H.; Castano, O.; Kim, H.W. Electrospun materials as potential platforms for bone tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1065–1083. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Rampichová, M.; Chvojka, J.; Buzgo, M.; Prosecká, E.; Mikeš, P.; Vysloužilová, L.; Tvrdik, D.; Kochová, P.; Gregor, T.; Lukáš, D.; et al. Elastic three-dimensional poly (ε-caprolactone) nanofibre scaffold enhances migration, proliferation and osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2013, 46, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Leong, M.F.; Rasheed, M.Z.; Lim, T.C.; Chian, K.S. In vitro cell infiltration and in vivo cell infiltration and vascularization in a fibrous, highly porous poly (d, l-lactide) scaffold fabricated by cryogenic electrospinning technique. J. Biomed. Mater. Res. Part A 2009, 91, 231–240. [Google Scholar] [CrossRef] [PubMed]
- OLee, O.J.; Ju, H.W.; Kim, J.H.; Lee, J.M.; Ki, C.S.; Kim, J.H.; Moon, B.M.; Park, H.J.; Sheikh, F.A.; Park, C.H. Development of artificial dermis using 3D electrospun silk fibroin nanofiber matrix. J. Biomed. Nanotechnol. 2014, 10, 1294–1303. [Google Scholar]
- Chogan, F.; Mirmajidi, T.; Rezayan, A.H.; Sharifi, A.M.; Ghahary, A.; Nourmohammadi, J.; Kamali, A.; Rahaie, M. Design, fabrication, and optimization of a dual function three-layer scaffold for controlled release of metformin hydrochloride to alleviate fibrosis and accelerate wound healing. Acta Biomater. 2020, 113, 144–163. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kocbek, P.; Govedarica, B.; Rošic, R.; Baumgartner, S.; Kristl, J. The topography of electrospun nanofibers and its impact on the growth and mobility of keratinocytes. Eur. J. Pharm. Biopharm. 2013, 84, 401–411. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 5, 355–369. [Google Scholar] [CrossRef]
- Beniwal, G.; Saxena, K.K. A review on pore and porosity in tissue engineering. Mater. Today Proc. 2021, 44, 2623–2628. [Google Scholar]
- Persson, M.; Lehenkari, P.P.; Berglin, L.; Turunen, S.; Finnilä, M.A.; Risteli, J.; Skrifvars, M.; Tuukkanen, J. Osteogenic differentiation of human mesenchymal stem cells in a 3D woven scaffold. Sci. Rep. 2018, 7, 10457. [Google Scholar] [CrossRef]
- Edwards, J.C.W.; Sedgwick, A.D.; Willoughby, D.A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: An in vivo tissue culture system. J. Pathol. 1981, 134, 147–156. [Google Scholar] [CrossRef]
- Ren, W.; Yang, S.Y.; Fang, H.W.; Hsu, S.; Wooley, P.H. Distinct gene expression of receptor activator of nuclear factor-κB and rank ligand in the inflammatory response to variant morphologies of UHMWPE particles. Biomaterials 2003, 24, 4819–4826. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.P.; Song, W.; Esquivel, A.O.; Jackson, N.M.; Nelson, M.; Flynn, J.C.; Markel, D.C. Effect of erythromycin-doped calcium polyphosphate scaffold composite in a mouse pouch infection model. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhang, R.; Markel, D.C.; Wu, B.; Peng, X.; Hawkins, M.; Wooley, P.H. Blockade of vascular endothelial growth factor activity suppresses wear debris-induced inflammatory osteolysis. J. Rheumatol. 2007, 34, 27–35. [Google Scholar] [PubMed]
- Bai, L.; Li, Q.; Duo, X.; Hao, X.; Zhang, W.; Shi, C.; Guo, J.; Ren, X.; Feng, Y. Electrospun PCL-PIBMD/SF blend scaffolds with plasmid complexes for endothelial cell proliferation. RSC Adv. 2017, 7, 39452–39464. [Google Scholar] [CrossRef]
- Xue, J.; He, M.; Niu, Y.; Liu, H.; Crawford, A.; Coates, P.; Chen, D.; Shi, R.; Zhang, L. Preparation and in vivo efficient anti-infection property of GTR/GBR implant made by metronidazole loaded electrospun polycaprolactone nanofiber membrane. Int. J. Pharm. 2014, 475, 566–577. [Google Scholar] [CrossRef]
- Rujitanaroj, P.O.; Jao, B.; Yang, J.; Wang, F.; Anderson, J.M.; Wang, J.; Chew, S.Y. Controlling fibrous capsule formation through long-term down-regulation of collagen type I (COL1A1) expression by nanofiber-mediated siRNA gene silencing. Acta Biomater. 2013, 9, 4513–4524. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.Y.W.; Zheng, R.; Zhang, Y.; Cui, W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017, 49, 66–77. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Z.; Wang, H.; Wang, Y.; Carlson, M.A. Expanded 3D nanofiber scaffolds: Cell penetration, neovascularization, and host response. Adv. Healthc. Mater. 2016, 5, 2993–3003. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, S.; Wang, H.; Carlson, M.A.; Gombart, A.F.; Xie, J. CO2—Expanded nanofiber scaffolds maintain activity of encapsulated bioactive materials and promote cellular infiltration and positive host response. Acta Biomater. 2018, 68, 237–248. [Google Scholar] [CrossRef]
- Wu, J.; Huang, C.; Liu, W.; Yin, A.; Chen, W.; He, C.; Wang, H.; Liu, S.; Fan, C.; Bowlin, G.L.; et al. Cell infiltration and vascularization in porous nanoyarn scaffolds prepared by dynamic liquid electrospinning. J. Biomed. Nanotechnol. 2014, 10, 603–614. [Google Scholar] [CrossRef]
- Klumpp, D.; Rudisile, M.; Kühnle, R.I.; Hess, A.; Bitto, F.F.; Arkudas, A.; Bleiziffer, O.; Boos, A.M.; Kneser, U.; Horch, R.E.; et al. Three-dimensional vascularization of electrospun PCL/collagen-blend nanofibrous scaffolds in vivo. J. Biomed. Mater. Res. Part A 2012, 100, 2302–2311. [Google Scholar] [CrossRef]


| Group | n * | NF Discs (2 mm Thick and 0.5 cm in Diameter) |
|---|---|---|
| 1 | 6 | No PCL NF (control) |
| 2 | 6 | PCL NFs (NF-0 sheet) |
| 3 | 6 | PCL NFs (NF-low sheet) |
| 4 | 6 | PCL NFs (NF-middle sheet) |
| 5 | 6 | PCL NFs (NF-high sheet) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markel, D.C.; Bou-Akl, T.; Wu, B.; Pawlitz, P.; Yu, X.; Chen, L.; Shi, T.; Ren, W. In Vivo Cell Migration and Growth Within Electrospun Porous Nanofibrous Scaffolds with Different Pore Sizes in a Mouse Pouch Model. J. Funct. Biomater. 2025, 16, 181. https://doi.org/10.3390/jfb16050181
Markel DC, Bou-Akl T, Wu B, Pawlitz P, Yu X, Chen L, Shi T, Ren W. In Vivo Cell Migration and Growth Within Electrospun Porous Nanofibrous Scaffolds with Different Pore Sizes in a Mouse Pouch Model. Journal of Functional Biomaterials. 2025; 16(5):181. https://doi.org/10.3390/jfb16050181
Chicago/Turabian StyleMarkel, David C., Therese Bou-Akl, Bin Wu, Pawla Pawlitz, Xiaowei Yu, Liang Chen, Tong Shi, and Weiping Ren. 2025. "In Vivo Cell Migration and Growth Within Electrospun Porous Nanofibrous Scaffolds with Different Pore Sizes in a Mouse Pouch Model" Journal of Functional Biomaterials 16, no. 5: 181. https://doi.org/10.3390/jfb16050181
APA StyleMarkel, D. C., Bou-Akl, T., Wu, B., Pawlitz, P., Yu, X., Chen, L., Shi, T., & Ren, W. (2025). In Vivo Cell Migration and Growth Within Electrospun Porous Nanofibrous Scaffolds with Different Pore Sizes in a Mouse Pouch Model. Journal of Functional Biomaterials, 16(5), 181. https://doi.org/10.3390/jfb16050181






